Abstract
Background
Mislabeling patients as allergic to beta lactams poses an increased risk of morbidity, healthcare costs, and even mortality. This study aimed to define the accuracy of medical history, taken by a specialist, in diagnosing immediate reaction to beta lactams.
Methods
All patients labeled as allergic to beta lactam were interviewed by a specialist in allergy and clinical immunology and defined as suspected of having a history of immediate or non-immediate reaction. When indicated, skin tests to major and minor determinants and oral graded challenge to the culprit drug were performed.
Results
A total of 909 patients were evaluated. A total of 798 (87.7%) were labeled as allergic to penicillin. In 108 (11.9%) cases, the allergist suspected an immediate reaction based on clinical history. Skin test or challenge proven diagnosis of IgE-mediated allergy to beta lactam were significantly more prevalent in the group with an allergist's suspicion of an immediate allergy (23.1% vs. 5%, p < 0.01). The sensitivity and negative predictive values of an anamnesis of immediate reaction were high (0.9 and 0.95, respectively), but the specificity and positive predictive value were low (0.37 and 0.23, respectively).
Conclusion
Medical history taken by an allergist can exclude immediate hypersensitivity reaction, but it is not specific enough to confirm the diagnosis. Skin testing and graded challenge in suspected cases of immediate hypersensitivity reaction are indicated.
Keywords: Beta lactam allergy, Penicillin allergy, Immediate allergy, Clinical history, IgE mediated allergy
Introduction
Mislabeling patients as allergic to beta lactam (BL) antibiotics has a major impact on morbidity, health economics and even mortality.1,2 In order to properly diagnose or exclude penicillin allergy, a thorough clinical history, skin tests (ST) to BLs major and minor determinants, and/or graded challenge tests are needed.3 Although mandatory, these tests, are technically demanding, time consuming and not commonly available.4 In cases where the clinical history is compatible with non-immediate allergy, the negative predictive value is very high and oral challenge can be performed safely with no need for ST.5, 6, 7 Nevertheless, the accuracy of focused clinical history, when immediate reaction to BL is suspected, has not been evaluated previously.
The aim of this study was to determine the predictive value of a clinical anamnesis, performed by specialists in allergy and clinical immunology, in the diagnosis of immediate allergy to BL.
Methods
Patients and skin tests
All patients referred for evaluation of BL hypersensitivity in our clinic underwent a thorough anamnesis, performed by a physician specializing in allergy and clinical immunology, which consisted of a series of predefined clinical questions. According to the information obtained from the interview, the patients were defined as possibly having immediate, late benign, or late severe reactions.
Patients with suspected immediate or late benign reactions underwent prick and intradermal ST with 0.04 mg/ml penicilloyl-poly-lysine (1:10 and 1:1), 0.5 mg/ml minor determinants mixture (1:10 and 1:1), 20 mg/ml amoxicillin (1:10 and 1:1) (all produced by Diater, Madrid, Spain), and 10,000 U/ml penicillin G (Teva, Israel). If the culprit BL was different, patients were also tested (prick and intra-dermally) with the relevant drug: 20 mg/ml amoxicillin-clavulanic acid (Augmentin by GSK, Brentford, UK), 2 mg/ml cefuroxime (Zinnat by GSK), 2.8 mg/ml ceftriaxone (Rocephin, Hoffman-La Roche, Basel, Switzerland), and 1 mg/ml cefazolin (Kefazin, Vitamed, Israel). Histamine phosphate (histatrol 2.75 mg/ml for intradermal ST and 0.275 mg/ml for prick ST, by ALK, Washington, NY) and phenol saline (ALK) served as positive and negative controls, respectively. ST was considered positive when the largest diameter wheal was ≥3 mm of the negative control in the presence of flare.
Patients who had a clinical history consistent with a late severe reaction (ie, Stevens-Johnson syndrome, toxic epidermal necrolysis, drug-related eosinophilia with systemic symptoms, or acute generalized exanthematous pustulosis) or fixed drug eruption were excluded from the study. They did not undergo ST or oral challenge and were advised to avoid BL.
Oral challenges
After written consent from the patient or caregiver was received, challenge was done with the culprit BL. In cases where the initial culprit BL was unknown, challenge was done with amoxicillin.
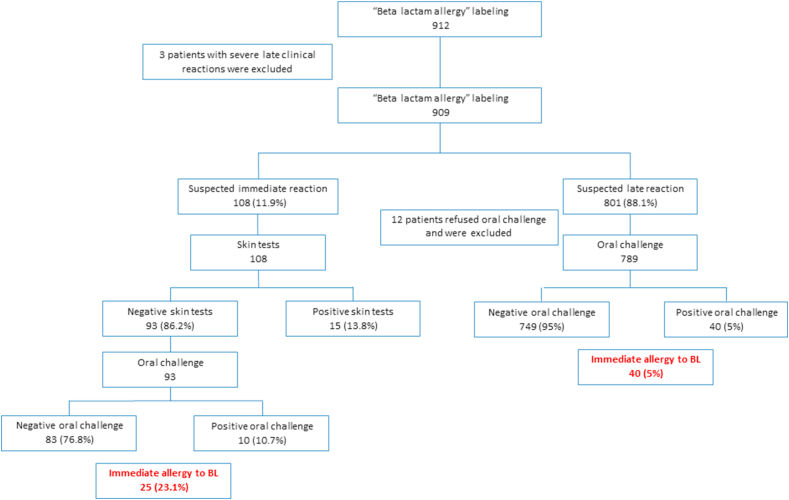
According to their weight, patients were given 1/10 of the calculated single dose. One hour after the initial dose, they were administered the full calculated single dose and were observed for 2 h. When clinical history was compatible with a non-immediate reaction, oral challenge was performed regardless of ST results (Fig. 1). When the clinical history was suspicious for immediate allergic reaction and positive ST, oral challenge was not performed.
Fig. 1.
Study design
Challenges and ST were performed in the Allergy Unit where trained personnel, as well as medication and equipment to treat anaphylactic reactions, were available at all times.
The study was approved by the local ethics committee.
Statistical analysis
Results are expressed as frequency and percentage or mean and standard deviation, as appropriate. Differences between groups were analyzed using chi-square test for categorical data, t-test for continuous, normally distributed variables and Mann-Whitney test for continuous parameters that did not have a normal distribution (for comparison between two groups). Differences among 3 groups were analyzed with Kruskal-Wallis non-parametric test. P values <0.05 were considered statistically significant. Data were analyzed using SPSS-23 software (IBM SPSS, Armonk, NY).
Results
Demographic and clinical characteristics
A total of 912 patients referred for BL allergy evaluation to the Allergy and Clinical Immunology Unit at Meir Medical Center from 2011 to 2018 were evaluated. Three patients with suspected late severe reactions to BL were excluded (Fig. 1). Demographic data are summarized in Table 1. The mean age was 23.8 ± 27.7 years. Eighteen patients (1.8%) were younger than 1 year old and 319 (35.1%) were over 18. Most patients reported reactions to penicillins (798, 87.7%) and 80 (8.8%) reported sensitivity to cephalosporins. Forty-two patients (4.6%) did not remember the culprit drug and 12 (1.3%) reported allergy to more than one drug. Mean interval from the reaction to clinical evaluation was 7.2 ± 12.6 years. However, 375 patients (41.3%) were referred for evaluation less than 1 year after the suspected allergic reaction. The most common clinical complaint (760, 83.6%) was a rash after taking a BL.
Table 1.
Demographics, clinical data and outcomes (N = 909)
| Age | 23.8 ± 27.70 |
| <1 | 18 (1.9%) |
| 1–18 | 572 (63%) |
| >18 | 319 (35.1%) |
| Male sex | 421 (46.3%) |
| Culprit drug | |
| Penicillin | (76.2%)693 |
| Amoxicillin-clavulanic acid | (11.5%)105 |
| First generation cephalosporin | (3.7%)34 |
| Second generation cephalosporin | (4.1%)38 |
| Third generation cephalosporin | (0.9%)8 |
| Unknown | (4.6%)42 |
| More than one drug | (1.3%)12 |
| Time from reaction to evaluation (years) | |
| Mean ± SD | 7.2 ± 12.6 |
| <1 | (41.3%) 375 |
| 1-10 | 299 (32.9%) |
| 10> | (25.8%) 235 |
| Type of reaction by clinical history | |
| Immediate | (11.8%)108 |
| Late or unknown | 801 (88.2%) |
| Clinical signs and symptoms | |
| Rash | 760 (83.6%) |
| Gastrointestinal | 16 (1.7%) |
| Dyspnea | 45 (4.9%) |
| Loss of consciousness | 3 (0.3%) |
| Unknown | 102 (11.2%) |
| Immediate allergy to beta lactam | 65 (7.1%) |
| Delayed type allergy to beta lactam | 123/780 (15.7%) |
Based on clinical history, the physician who examined the patient defined the response as immediate in 108 cases (11.8%). All were referred for ST and when ST did not show sensitivity, they proceeded to challenge. Skin tests showed sensitivity in 15 (13.8%) and immediate reaction to challenge was observed in an additional 10 patients (10.7%) (Fig. 1).
In 801 patients suspected by history of experiencing a non-immediate reaction, oral challenge was recommended regardless of ST results. A total of 789 patients consented and were challenged. Among them, an immediate reaction was diagnosed in 40 (5%) (Fig. 1).
Overall, immediate allergy to beta lactam was diagnosed in 65 cases (7.1%). A total of 780 patients (85.8%) completed a 5-day challenge. Of these, 123 (15.7%) had a late rash and were diagnosed with a delayed allergy to beta lactam.
Comparison between patients with immediate or late response based on clinical history
Based on clinical history, the response was defined as immediate in 108 (11.9%) patients and non-immediate in the remaining 801. The difference between these 2 groups is summarized in Table 2. Patients with an immediate-response type of reaction according to clinical history tended to be older, but this difference did not reach statistical significance (24.2 ± 24.6 vs. 20.2 ± 23.8 years old, p = 0.1). Symptoms of shortness of breath (24% vs. 2.3%, p < 0.01) or gastrointestinal complaints (4.6% vs. 1.3%, p = 0.03) prompted the physician to suspect an immediate reaction. Likewise, when the clinical history was not clear enough, and when the culprit drug was a cephalosporin, the physician tended to define the reaction as immediate (25% vs. 9.3%, and 23% vs. 6.8%, respectively, p < 0.01 for both comparisons).
Table 2.
Comparison according to clinical history findings.
| Variable | Immediate reaction by clinical history N = 108 |
Late reaction by clinical history N = 801 |
P- value |
|---|---|---|---|
| Age | 24.2 ± 24.6 | 20.2 ± 23.8 | 0.1 |
| Male sex | 44 (40.7%) | 377 (47%) | 0.2 |
| Culprit drug | |||
| Penicillin | 75 (69.4%) | 618 (76.4%) | <0.01 |
| Amoxicillin-clavulanic acid | 14 (12.9%) | 91 (11.3%) | |
| First generation cephalosporin | 9 (8.3%) | 25 (3.1%) | |
| Second generation cephalosporin | 13 (12.0%) | 25 (3.1%) | |
| Third generation cephalosporin | 3 (2.7%) | 5 (0.6%) | |
| Unknown | 5 (4.6%) | 37 (4.6%) | |
| Time from reaction to evaluation | 5.8 ± 11.1 | 7.3 ± 12.8 | |
| Clinical signs and symptoms | |||
| Rash | 86 (79.6%) | 674 (84.1%) | 0.2 |
| Gastrointestinal | 5 (4.6%) | 11 (1.3%) | 0.03 |
| Dyspnea | 26 (24.0%) | 19 (2.3%) | <0.01 |
| Loss of consciousness | 1 (0.9%) | 2 (0.2%) | 0.3 |
| Unknown | 27 (25%) | 75 (9.3%) | <0.01 |
| Positive skin test | 15 (13.8%) | ||
| Positive oral challengea | 10/93b (10.7%) | 40/789c (5%) | 0.03 |
| Immediate allergy to beta lactam | 25 (23.1%) | 40 (5%) | <0.01 |
When clinical history was not compatible with an immediate reaction, oral challenge test was performed regardless of skin test results.
In patients with clinical history of immediate reaction and positive ST, oral challenge was not performed.
12 patients refused oral challenge
Positive ST and oral challenge were significantly more common in the group that the specialist defined as having a history of an immediate reaction (13.8% vs. 4.1%, and 10.7% vs. 5%, respectively, p < 0.01). When combining the results of the 2 procedures, a diagnosis of IgE mediated allergy to beta lactam was significantly more prevalent in the group with a clinical history of immediate allergy (23.1% vs. 5%, p < 0.01).
Comparison between immediate response by clinical history and results of evaluation
We compared the 108 patients with a clinical history compatible with an immediate reaction according to the final diagnosis obtained after skin test and oral challenge (Table 3). As noted, 25 (23.1%) with suspected immediate response by history were eventually diagnosed as having IgE mediated allergy to BL. The sensitivity of the clinical history was 0.37, with a specificity of 0.9. The positive predictive value of immediate response by anamnesis was 0.23, while the negative predictive value was 0.95. In patients over the age of 18 (60% vs. 36.1%, p = 0.03), and when the suspected drug was amoxicillin-clavulanic acid (32% vs. 7.2%, p = 0.003), the physician's impression of immediate reaction was more accurate. When less than a year had passed from the reaction to the clinical evaluation, the physician's conclusion according to the anamnesis was more accurate, but this difference did not reach statistical significance (60% vs. 39.7%, p = 0.1).
Table 3.
Immediate reaction by clinical history compared to diagnosis after testing.
| Variable | Positive skin test/oral challenge N = 25 |
Negative skin test/oral challenge N = 83 |
P-value |
|---|---|---|---|
| Male sex | 9 (36%) | 35 (42.1%) | 0.6 |
| Age >18 | 15 (60%) | 30 (36.1%) | 0.03 |
| Culprit druga | |||
| Penicillin | 16 (52%) | 59 (71%) | 0.6 |
| Cephalosporin | 9 (36%) | 16 (19.2%) | 0.1 |
| Amoxicillin-clavulanic acid | 8 (32%) | 6 (7.2%) | 0.003 |
| Unknown | 0 | 5 (6%) | 1 |
| Time from reaction to evaluation <1 year | 15 (60%) | 33 (39.7%) | 0.1 |
| Clinical signs and symptoms | |||
| Rash | 22 (88%) | 64 (77.1%) | 0.4 |
| Gastrointestinal | 1 (4%) | 4 (4.8%) | 1 |
| Dyspnea | 9 (36%) | 17 (20.4%) | 0.1 |
| Loss of consciousness | 0 | 1 (1.2%) | 1 |
| Unknown | 1 (4%) | 26 (31.3%) | 0.004 |
Five patients in each group reported having allergic reactions to more than one drug
Discussion
This study focused on the efficacy of the initial clinical history intake taken by a specialist in allergy and clinical immunology for the diagnosis of immediate allergy to BL. When the allergy expert did not suspect an immediate reaction, in most cases the diagnosis was in accordance with the objective results of ST and challenge. On the other hand, not surprisingly, the percentage of cases in which the test results were positive for IgE mediated allergy, although low, was found to be significantly higher when the physician suspected that it was an immediate allergy.
To date, few studies have discussed the efficacy of clinical history intake by a specialist, regarding drug allergies in general, and BL allergy in specific. Green et al found poor efficacy of anamnesis for allergy to penicillin in a large group of patients with penicillin allergy labeling.8 However, they did not differentiate between immediate and non-immediate allergy, and in most patients, the suspicion was not proven with an oral challenge. Another retrospective work found no correlation between focused anamnesis and ST results or oral challenges in a small group of patients with penicillin allergy.9 Accordingly, the accepted approach was that in cases of BL allergy labeling, the clinician cannot rely on the clinical history alone, and ST followed by oral challenge are mandatory.3 Lately, this approach has been reevaluated, especially in the pediatric population with a clinical history of non-immediate reactions to BL.5, 6, 7,10,11 Results of a large prospective study conducted in our institution that focused on patients with a clinical history of non-immediate reaction to BL were recently published. In this population of children and adults, we found that there is no need to perform ST, and a direct oral challenge is safe and efficient.5 In line with these findings, Gruchalla and Pirmohamed proposed performing a direct graded oral challenge in cases compatible with a delayed maculopapular rash by clinical history.12 In a recent report, Krishna and Misbah suggested using a computerized system to evaluate the risk of patients with suspected penicillin allergy according to a predefined algorithm,13,14 which differentiates between low- and high-risk patients, based on clinical history. It has been suggested that this system will assist non-specialist healthcare workers. The proposed algorithm was based on a cohort study with 231 patients that showed, as we did in the current study, high negative predictive values — 94% in low-risk patients and 83% in high-risk patients.
In accordance with our findings, a recent review on penicillin allergy in the New England Journal of Medicine, noted that a detailed history, taken by physicians specializing in allergy and clinical immunology, is proposed as a pivotal step in the diagnosis and evaluation of BL allergy.15
In this paper, we focused on a group of patients about whom a physician specialist in allergy and clinical immunology was convinced their clinical history is compatible with immediate reaction to BL. We found that the positive predictive value and specificity related to the physician's conclusion were very low. However, the 0.95 negative predictive value and the 0.9 sensitivity were very high.
The findings presented here support the notion that while a clinical history compatible with an immediate allergy to BL is not sufficient for diagnosis, the absence of such anamnesis almost completely negates the likelihood that the patient has an immediate type allergy to BLs.
The current study reinforces our previous recommendations5 and shows that in cases where the clinical history does not indicate the likelihood of an immediate response, the risk of performing a direct challenge is minimal.
The low positive predictive value and specificity found in our study can be explained in several ways. The clinical impression was found to be less accurate when a longer period had elapsed from the initial reaction to the clinical workup. The passage of time tends to obscure the details the patient delivers to the physician. It is also known that allergic reactions to BL tend to decrease with time.16 The clinical conclusion was found to be more accurate in adults. It can be hypothesized that, when anamnesis is delivered directly by the patient and not by the caregiver, clinical data tend to be more exact. In addition, when the culprit drug was amoxicillin-clavulanate or a cephalosporin, the clinical impression was more precise. One can assume that when the culprit drug is a less commonly prescribed medication, the impression of the reaction is more memorable and clinical data tend to be more accurate.
In order to confirm an allergy to drugs in general and to define its specific nature, a graded challenge must be performed. In this study, patients with a clinical history of an immediate reaction and positive ST, were not challenged due to safety and ethical reasons. One can assume that a portion of these patients may not be allergic, which might bias our results. However, because only a few patients had a positive ST, this is unlikely to substantially affect our conclusions.
The main advantages of the data presented here are that we prospectively evaluated a large cohort of patients labeled as allergic to BL and that the evaluation was done systematically, by specialists in allergy and clinical immunology.
In conclusion, this study presents the results of an evaluation of a large cohort of patients labeled as allergic to BL. A focused clinical intake, done by a specialist in allergy and clinical immunology, is not sufficient for diagnosis but can safely exclude patients with an immediate allergy to BL. Skin testing and graded oral challenges are still the gold standard methods for diagnosing an immediate reaction to BL.
Abbreviations
BL, Beta lactam; ST, Skin tests.
Funding sources
The authors declare that there are no funding sources to declare.
Author contributions
Yossi Rosman: Substantial contributions to the conception or design of the work, drafting the work, final approval of the version to be published and agreement to be accountable for all aspects of the work.
Mohamad Elmalek: Acquisition, analysis and interpretation of data for the work, drafting the work, final approval of the version to be published and agreement to be accountable for all aspects of the work.
Meir-Shafrir Keren: Acquisition, analysis and interpretation of data for the work, revising it critically for important intellectual content, final approval of the version to be published and agreement to be accountable for all aspects of the work.
Lachover-Roth Idit: Acquisition, analysis and interpretation of data for the work, revising it critically for important intellectual content, final approval of the version to be published and agreement to be accountable for all aspects of the work.
Cohen-Engler Anat: Acquisition, analysis and interpretation of data for the work, revising it critically for important intellectual content, final approval of the version to be published and agreement to be accountable for all aspects of the work.
Confino-Cohen Ronit: Substantial contributions to the conception or design of the work, revising it critically for important intellectual content, final approval of the version to be published and agreement to be accountable for all aspects of the work.
Statement of ethics
The study protocol was approved by the institute's committee on human research.
Submission declaration
The above article is original and has not been submitted for publication elsewhere. Our article has been written read and approved by all authors. All requirements for authorship have been met and we declare no conflicts of interest for each named author.
Consent for publication
We consent that the manuscript will be published in the “World Allergy Organization Journal”.
Availability of data and materials
All data and materials are available upon request.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Footnotes
Full list of author information is available at the end of the article https://doi.org/10.1016/j.waojou.2020.100506
References
- 1.Blumenthal K.G., Lu N., Zhang Y., Li Y., Walensky R.P., Choi H.K. Risk of meticillin resistant Staphylococcus aureus and Clostridium difficile in patients with a documented penicillin allergy: population based matched cohort study. BMJ. 2018 Jun 27;361 doi: 10.1136/bmj.k2400. k2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Macy E., Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol. 2014 Mar;133:790–796. doi: 10.1016/j.jaci.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 3.Romano A., Atanaskovic-Markovic M., Barbaud A. Towards a more precise diagnosis of hypersensitivity to beta-lactams - an EAACI position paper. Allergy. 2019 Nov 21 doi: 10.1111/all.14122. [DOI] [PubMed] [Google Scholar]
- 4.Confino-Cohen R., Rosman Y., Lachover I., Meir Shafrir K., Goldberg A. The importance of amoxicillin and amoxicillin-clavulanate determinants in the diagnosis of immediate allergic reactions to β-lactams. Int Arch Allergy Immunol. 2016 Jul;8170:62–66. doi: 10.1159/000446961. [DOI] [PubMed] [Google Scholar]
- 5.Confino-Cohen R., Rosman Y., Meir-Shafrir K., Stauber T., Lachover-Roth I., Hershko A. Oral challenge without skin testing safely excludes clinically significant delayed-onset penicillin hypersensitivity. J Allergy Clin Immunol Pract. 2017;5:669–675. doi: 10.1016/j.jaip.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 6.Tucker M.H., Lomas C.M., Ramchandar N., Waldram J.D. Amoxicillin challenge without penicillin skin testing in evaluation of penicillin allergy in a cohort of Marine recruits. J Allergy Clin Immunol Pract. 2017 Mar 21;5:813–815. doi: 10.1016/j.jaip.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 7.Macy E., Ensina L.F. Controversies in allergy: is skin testing required prior to drug challenges? J Allergy Clin Immunol Pract. 2019 Feb;7:412–417. doi: 10.1016/j.jaip.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Green G.R., Rosenblum A.H., Sweet L.C. Evaluation of penicillin hypersensitivity: value of clinical history and skin testing with penicilloyl-polylysine and penicillin G. A cooperative prospective study of the penicillin study group of the American Academy of Allergy. J Allergy Clin Immunol. 1977 Dec;60:339–345. doi: 10.1016/0091-6749(77)90064-1. [DOI] [PubMed] [Google Scholar]
- 9.Wong B.B.L., Keith P.K., Waserman S. Clinical history as a predictor of penicillin skin test outcome. Ann Allergy Asthma Immunol. 2006 Aug;97:169–174. doi: 10.1016/S1081-1206(10)60008-7. [DOI] [PubMed] [Google Scholar]
- 10.Mill C., Primeau M.-N., Medoff E. Assessing the diagnostic properties of a graded oral provocation challenge for the diagnosis of immediate and nonimmediate reactions to amoxicillin in children. JAMA Pediatr. 2016 Jun 6;170 doi: 10.1001/jamapediatrics.2016.0033. [DOI] [PubMed] [Google Scholar]
- 11.Vezir E., Dibek Misirlioglu E., Civelek E. Direct oral provocation tests in non-immediate mild cutaneous reactions related to beta-lactam antibiotics. Pediatr Allergy Immunol. 2016 Feb;27:50–54. doi: 10.1111/pai.12493. [DOI] [PubMed] [Google Scholar]
- 12.Gruchalla R.S., Pirmohamed M. Clinical practice. Antibiotic allergy. N Engl J Med. 2006 Feb 9;354:601–609. doi: 10.1056/NEJMcp043986. [DOI] [PubMed] [Google Scholar]
- 13.Krishna M.T., Misbah S.A. Is direct oral amoxicillin challenge a viable approach for “low-risk” patients labelled with penicillin allergy? J Antimicrob Chemother. 2019 Sep 1;74:2475–2479. doi: 10.1093/jac/dkz229. [DOI] [PubMed] [Google Scholar]
- 14.Mohamed O.E., Beck S., Huissoon A. A retrospective critical analysis and risk stratification of penicillin allergy delabeling in a UK specialist regional allergy service. J Allergy Clin Immunol Pract. 2019 Jan;7:251–258. doi: 10.1016/j.jaip.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 15.Castells M., Khan D.A., Phillips E.J. Penicillin allergy. N Engl J Med. 2019 Dec 12;381:2338–2351. doi: 10.1056/NEJMra1807761. [DOI] [PubMed] [Google Scholar]
- 16.Solensky R., Earl H.S., Gruchalla R.S. Lack of penicillin resensitization in patients with a history of penicillin allergy after receiving repeated penicillin courses. Arch Intern Med. 2002 Apr 8;162:822–826. doi: 10.1001/archinte.162.7.822. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials are available upon request.