Abstract
Tolvaptan, a vasopressin type-2 receptor antagonist, is indicated for fluid retention. It is considered that the response to tolvaptan reduces as renal function deteriorates, whereas we sometimes experience “non-responders” to tolvaptan despite well-preserved renal function. While the expression of aquaporin-2 might be a key to response to tolvaptan, detailed mechanism of refractoriness to tolvaptan remains unknown. We experienced two patients with congestive heart failure and diabetic nephropathy, in whom the responses to tolvaptan were uniquely opposite. In one case, immunohistochemical staining showed expression of aquaporin-2 in the collecting duct despite severely reduced renal function, followed by the good response to tolvaptan with increased urine output. In another case, immunohistochemical staining showed absence of aquaporin-2 with infiltration of inflammatory cells in the kidney medulla despite relatively preserved renal function, followed by refractoriness to tolvaptan without any increase in urine output. Inactivated aquaporin-2 expression in the collecting duct, which was for example caused by pre-clinical urinary infection as our latter case, might have an association with refractoriness to tolvaptan.
Keywords: Arginine vasopressin, Responder, Diuretics
Introduction
Arginine vasopressin stimulates the activity of aquaporin-2, which is transferred on the surface of collecting duct and reabsorbs free water, followed by concentrated urine (i.e., increased urine osmolality) and excretion of aquaporin-2 in urine [1]. Tolvaptan, one of the vasopressin type-2 receptor antagonists, is indicated for the fluid retention due to congestion and inhibits re-absorption of free water in the collecting duct [2].
Given the above mechanism, our team hypothesized that residual function of collecting duct, which would be indirectly assessed by elevated baseline urine osmolality and urine aquaporin-2 excretion at fasting condition, might be essential to respond to tolvaptan, and proposed these indices as predictive markers for responders [3, 4].
It is considered in general that the response to tolvaptan reduces as renal function deteriorates [4], whereas we sometimes experience “non-responders” to tolvaptan despite well-preserved renal function. Detailed mechanism of refractoriness to tolvaptan remains unknown thus far. We present here two patients, in whom the activity of aquaporin-2 in the collecting duct was immunohistochemically investigated and tolvaptan was administered to treat their symptomatic congestion.
Case report
Case 1 (responder)
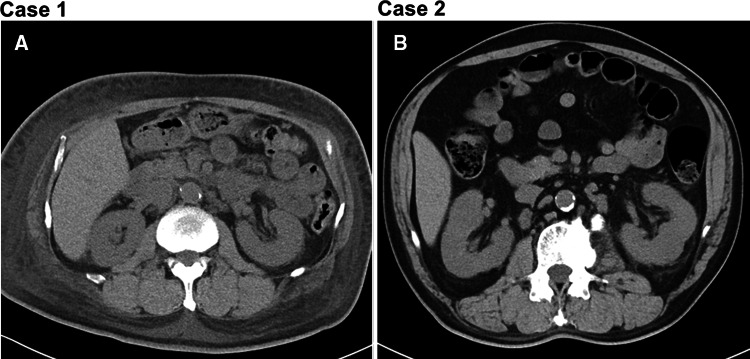
The patient was 51-year-old man without any medical histories, who admitted presenting dyspnea on effort and bilateral peripheral edema, diagnosed as congestive heart failure. Estimated glomerular filtration rate was 15.7 mL/min/1.73 m2, plasma B-type natriuretic peptide was 817 pg/mL, and urine protein was 12.5 g/g of creatinine. Laboratory data are shown in Table 1. The funduscopy showed diabetic retinopathy despite HbA1c 5.9%. Computed tomography showed bilateral pleural effusion and subcutaneous edema as well as mild atrophy of kidney (Fig 1a).
Table 1.
Laboratory data on admission
| Laboratory test | Case 1 (responder) | Case 2 (non-responder) |
|---|---|---|
| Urinalysis | ||
| Urine specific gravity | 1.015 | 1.007 |
| Urine protein | (3 +) | (2 +) |
| Urine occult blood | (–) | (1 +) |
| Urine sedimentation | ||
| Red blood cells, high power field | 1–4 | 5–9 |
| White blood cells, high power field | 1–4 | 50–99 |
| Urine biochemistry | ||
| Urine osmolality, mOsm/L | 366 | 208 |
| Urine aquaporin-2, ng/mL | 3.51 | < 0.35 |
| Complete blood cell counts | ||
| White blood cells, µL | 10,540 | 8300 |
| Red blood cells, µL | 347 × 104 | 375 × 104 |
| Hemoglobin, g/dL | 9.2 | 11.3 |
| Platelets, µL | 44.5 × 104 | 21.1 × 104 |
| Serum biochemistry | ||
| Total protein, g/dL | 6.0 | 6.7 |
| Albumin, g/dL | 2.6 | 3.1 |
| Blood urea nitrogen, mg/dL | 55 | 23 |
| Creatinine, mg/dL | 4.41 | 1.98 |
| Uric acid, mg/dL | 8.2 | 9.0 |
| Total cholesterol, mg/dL | 196 | 236 |
| Low density lipoprotein cholesterol, mg/dL | 109 | 117 |
| High density lipoprotein cholesterol, mg/dL | 59 | 33 |
| Triglyceride, mg/dL | 123 | 442 |
| Sodium, mEq/L | 138 | 137 |
| Potassium, mEq/L | 5.4 | 4.8 |
| Chlorine, mEq/L | 108 | 101 |
| Calcium, mg/dL | 8.1 | 8.8 |
| Inorganic phosphorus, mg/dL | 5.6 | 4.0 |
| Carbohydrate metabolism test | ||
| Hemoglobin A1c, % | 5.9 | 7.4 |
| Fasting blood glucose, mg/dL | 100 | 120 |
| Serum immunological test | ||
| C-reactive protein, mg/dL | 0.16 | 0.51 |
| Endocrine test | ||
| Plasma aldosterone concentration, pg/mL | 82 | 101 |
| Plasma renin activity, ng/mL/h | 1.7 | 0.6 |
| Plasma arginine vasopressin, pg/mL | 6.8 | 1.0 |
Fig. 1.
Findings of abdominal computed tomography
Baseline urine osmolality and urine aquaporin-2 were well preserved (366 mOsm/L and 3.51 ng/mL, respectively). Given refractoriness to loop diuretics (intravenous administration of furosemide 40 mg/day), 15 mg/day of tolvaptan was administered, resulting in increased urine output and improved clinical course (blue bar and line in Fig. 2).
Fig. 2.
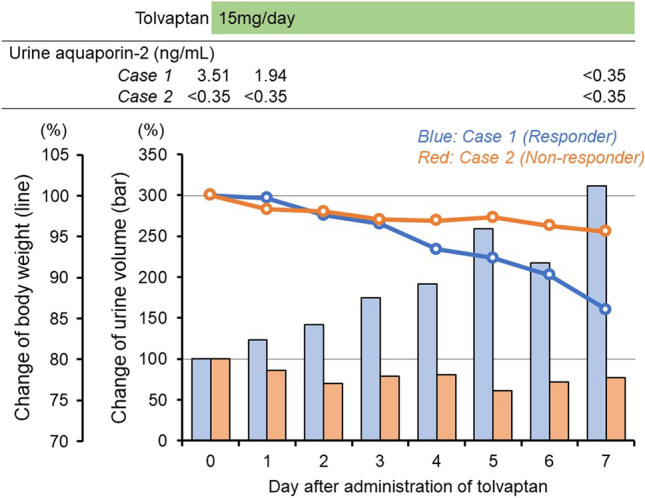
Clinical course and serial changes of body weight (line) and urine volume (bar) after administration of tolvaptan in responder (case 1 [blue]) and non-responder (case 2 [red])
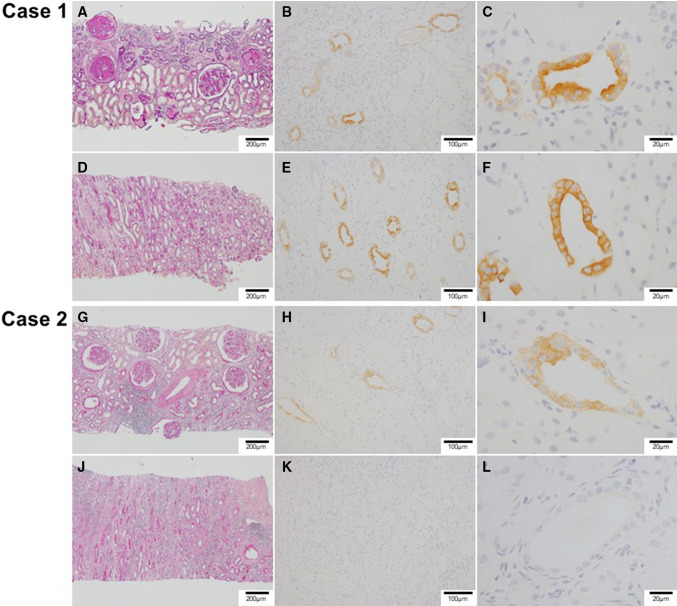
Oral glucose tolerance test did not satisfy the criteria of diabetes mellitus. To further investigate the etiologies of renal impairment and proteinuria, we performed a kidney biopsy, which showed severe glomerular sclerosis (Fig. 3a), indicating diabetic nephropathy. Despite severe atrophy of tubule in the cortex, they were relatively preserved in the medulla (Fig. 3d). Immunohistochemical staining showed expression of aquaporin-2 in the collecting duct (Fig. 3b, c, e, f). Written informed consent was obtained for histopathological staining of aquaporin-2 and measurement of urine aquaporin-2 concentration.
Fig. 3.
Microscopic findings of kidney samples in responder (case 1 [a–f]) and non-responder (case 2 [g–l]). a Case 1: severe glomerular sclerosis, indicating diabetic nephropathy, and severe tubular atrophy in the renal cortex. PAS (Periodic Acid-Schiff) stain. b Case 1: immunohistrochemical staining showing expression of aquapolin-2 in the collecting duct of the renal cortex. c Case 1: high-power filed of the renal cortex showing stronger staining of aquapolin-2 in the lumen side. d Case 1: relatively preserved structures of tubules in the renal medulla. PAS stain. e Case 1: immunohistrochemical staining showing expression of aquapolin-2 in the medullary collecting duct. f Case 1: high-power filed of the renal medulla showing stronger staining of aquapolin-2 in the lumen side. g Case 2: slight tubular atrophy and interstitial inflammatory cell infiltration in the renal cortex. Relatively preserved structures of glomeruli, indicating diabetic nephropathy. PAS stain. h Case 2: immunohistrochemical staining showing weak expression of aquapolin-2 in the renal cortex collecting duct. i Case 2: high-power field of renal cortex showing weak staining of aquapolin-2. j Case 2: severe interstitial inflammatory cell infiltration and tubular atrophy in the renal medulla. PAS stain. k Case 2: aquapolin-2 immunostaining was negative in the renal medulla. l Case 2: high-power field of renal medulla showing no staining of aquapolin-2
Case 2 (non-responder)
The patient was 66-year-old man with a history of 8-year diabetes mellitus who admitted with peripheral edema, diagnosed as congestive heart failure and diabetic nephropathy. He received dipeptidyl-peptidase 4 inhibitor (sitagliptin 50 mg/day), angiotensin 2 receptor blocker (candesartan 8 mg/day), and calcium channel blocker (amlodipine 10 mg/day) on admission. Renal function was relatively preserved (estimated glomerular filtration rate 32.4 mL/min/1.73 m2) whereas urine protein was 7.9 g/g of creatinine. Laboratory data are summarized in Table 1. White blood cells were observed in urine (50–99/high power filed) without any sign or symptom of urinary infection. Computed tomography showed no kidney atrophy (Fig. 1b).
Baseline urine osmolality and urine aquaporin-2 were remarkably low (208 mOsm/L and < 0.35 ng/mL, respectively). Despite 15 mg/day of tolvaptan was administered given refractoriness to loop diuretics (azosemide 60 mg/day), urine output did not increase (red bar and line in Fig. 2).
We performed kidney biopsy to exclude other diseases including tubulointerstitial nephritis. Renal biopsy showed slight atrophy of renal cortex (Fig. 3g). On the contrary, infiltration of inflammatory cells and severe atrophy of tubule were observed in the medulla (Fig. 3j) without any staining of aquaporin-2 (Fig. 3k, j). Witten informed consent was obtained for histopathological staining of aquaporin-2 and measurement of urine aquaporin-2 concentration.
Discussion
We presented two patients with congestive heart failure and diabetic nephropathy, who showed opposite responses to tolvaptan. One patient had an expression of aquaporin-2 despite severe renal dysfunction and showed good response to tolvaptan; whereas another did not have an expression of aquaporin-2 despite relatively preserved renal function, showing refractoriness to tolvaptan.
Intra-cellular expression and urine excretion of aquaporin-2
In these cases, we confirmed our previous findings that elevated baseline urine osmolality and baseline urine aquaporin-2 level, which might indicate the preserved function of collecting duct [3, 4], were associated with good response to tolvaptan with increased urine output, irrespective of baseline level of glomerular filtration rate [5]. For further investigation of the relationship between aquaporin-2 and response to tolvaptan, we performed immunohistochemical staining of aquaporin-2 in the renal tissue obtained by biopsy, as Tanaka and colleagues reported previously [6, 7]. We performed renal biopsies for the purpose of clinical necessity following obtaining written informed consents. Given that a urine aquaporin-2 level is a good non-invasive marker to predict responsiveness to tolvaptan, we do not recommend at all renal biopsy for such a purpose.
As expected, a responder (case 1) showed expression of aquaporin-2 in the collecting duct with preserved ability to concentrate urine (i.e., baseline elevated urine osmolality) and excretion of aquaporin-2 in urine, indicating the preserved function of the collecting duct. Following the administration of tolvaptan, excretion of aquapolin-2 in urine decreased immediately, indicating appropriate suppression of aquapolin-2 expression by antagonizing arginine vasopressin type-2 receptor.
On the contrary, a non-responder (case 2) showed no expression of aquaporin-2 with the inability to concentrate urine (i.e., baseline low urine osmolality) and no excretion of aquaporin-2 in urine, indicating dysfunction of the collecting duct. Given the severe interstitial impairment, an ability to concentrate urine would have been reduced. This is a typical case of non-responder to tolvaptan, in whom aquapolin-2 signal cascade is already deteriorated irrespective of the tolvaptan signal.
Next concern would be the correlation between the intracellular expression of aquaporin-2 and the excretion of aquaporin-2 in the urine. Interestingly, the glomerular filtration rate did not correlate with the expression of aquaporin-2 as well as response to tolvaptan.
Mechanism of refractoriness to tolvaptan
While it has been mentioned that the expression of aquaporin-2 might be a key to response to tolvaptan [3, 6], detailed mechanism lying between the absence of aquapoinr-2 expression and refractoriness to tolvaptan remains unknown. In the non-responder (case 2), severe infiltration of inflammatory cells and atrophic tubule were observed in the medulla despite only mild glomerular sclerosis. Given asymptomatic pyuria, the patient might have had a pre-clinical urinary infection, which might have chronically injured the function of collecting duct located in the medulla, while relatively preserving cortex. Further large-scale study is warranted to demonstrate the mechanism of refractoriness to tolvaptan and innovate novel therapeutic strategy to manage non-responders.
Conclusion
We experienced two patients with congestive heart failure and diabetic nephropathy, showing opposite responses to tolvaptan. We confirmed histopathologically that the expression of aquaporin-2 is not observed in the non-responders to tolvaptan. Further studies are warranted to investigate the relationship between chronic inflammation of medulla, dysfunction of collecting duct, and refractoriness to tolvaptan.
Compliance with ethical standards
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Urine aquaporin-2 measurements and immunostaining of aquaporin-2 were approved in the local ethical committee and written informed consents were obtained beforehand from each patient.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Imamura T, Kinugawa K. Urine aquaporin-2: a Promising marker of response to the arginine vasopressin type-2 antagonist, tolvaptan in patients with congestive heart failure. Int J Mol Sci. 2016;17:105. doi: 10.3390/ijms17010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imamura T, Kinugawa K. Update of acute and long-term tolvaptan therapy. J Cardiol. 2019;73:102–107. doi: 10.1016/j.jjcc.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Imamura T, Kinugawa K, Fujino T, Inaba T, Maki H, Hatano M, Yao A, Komuro I. Increased urine aquaporin-2 relative to plasma arginine vasopressin is a novel marker of response to tolvaptan in patients with decompensated heart failure. Circ J. 2014;78:2240–2249. doi: 10.1253/circj.CJ-14-0244. [DOI] [PubMed] [Google Scholar]
- 4.Imamura T, Kinugawa K, Shiga T, Kato N, Muraoka H, Minatsuki S, Inaba T, Maki H, Hatano M, Yao A, Kyo S, Nagai R. Novel criteria of urine osmolality effectively predict response to tolvaptan in decompensated heart failure patients–association between non-responders and chronic kidney disease. Circ J. 2013;77:397–404. doi: 10.1253/circj.CJ-12-0971. [DOI] [PubMed] [Google Scholar]
- 5.Bricker NS, Dewey RR, Lubowitz H, Stokes J, Kirkensgaard T. Observations on the concentrating and diluting mechanisms of the diseased kidney. J Clin Investig. 1959;38:516–523. doi: 10.1172/JCI103829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka A, Nakamura T, Sato E, Node K. Aquaporin-2 is a potential biomarker for tolvaptan efficacy in decompensated heart failure complicated by diabetic nephrotic syndrome. Int J Cardiol. 2016;210:1–3. doi: 10.1016/j.ijcard.2016.02.106. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka A, Nakamura T, Sato E, Ueda Y, Node K. Different effects of tolvaptan in patients with idiopathic membranous nephropathy with nephrotic syndrome. Intern Med. 2017;56:191–196. doi: 10.2169/internalmedicine.56.7539. [DOI] [PMC free article] [PubMed] [Google Scholar]