Abstract
Peritoneal dialysis (PD)-related peritonitis is a common complication of PD. Nonocclusive mesenteric ischemia (NOMI) is a rare complication of PD-related peritonitis, has a high mortality rate, and therefore should be detected early once it occurs. We describe a case of a 70-year-old woman on PD presented with moderate abdominal pain and low blood pressure, which contributed to the early diagnosis of PD-related peritonitis complicated with NOMI. Increased white cell count of 7150/μL (neutrophil, 84%) in dialysate effluent was diagnostic of PD-related peritonitis, which was later found to be caused by Pseudomonas putida. Computed tomography with contrast performed after administering crystalloids revealed hepatic portal venous gas, pneumatosis intestinalis in the ascending colon, and normal enhancement of the bowel wall and mesenteric arteries, which suggested a reperfusion of the previously ischemic ascending colon. Colonoscopy on hospital day seventeen revealed mucosal hemorrhage and ulcers in the entire right colon and the terminal ileum while the remaining colon was normal. These findings are compatible with the consequence of NOMI. Increased peak systolic velocity of the superior mesenteric artery (SMA) implied its stenosis. Past studies show that ischemia of the colon in patients with chronic kidney disease commonly occurs in the right colon. Arteriosclerosis of the SMA due to the long history of chronic kidney disease and diabetes might have caused its vulnerability to low blood pressure. Abdominal complications including NOMI should be screened for when a patient presents with low blood pressure and strong abdominal pain. This is the first case report that shows colonoscopy images of the colonic ulcers post-NOMI and PD-related peritonitis.
Keywords: Peritoneal dialysis, Peritonitis, Nonocclusive mesenteric ischemia, Hepatic portal venous gas, Superior mesenteric artery, Diabetic nephropathy
Introduction
Although peritoneal dialysis (PD)-related peritonitis is a common complication in patients on PD, PD-related peritonitis is rarely complicated with nonocclusive mesenteric ischemia (NOMI). Herein, we present a case of PD-related peritonitis presented with moderate abdominal pain and low blood pressure, which led to an early detection of NOMI that was later confirmed with computed tomography (CT) and colonoscopy, and discuss its etiology with a review of literature.
Case report
A 70-year-old woman with type 2 diabetes mellitus, chronic kidney disease (CKD) due to diabetic nephropathy, hypertension and dyslipidemia presented to our emergency department with twelve days of diarrhea and two days of worsening abdominal pain. She had started PD ten months before presentation and had been successfully continuing dialysis without complications. She was at her normal state of health until eleven days before presentation, when she realized diarrhea and chills. On the day before presentation, she started to feel intermittent abdominal pain and lost her appetite. On the next day, her abdominal pain worsened, called the emergency medical service, and presented to the hospital.
Her past medical history was otherwise notable for polyvascular disease: cardiovascular disease, cerebral infarction, carotid artery stenosis, peripheral artery disease, and diabetic foot ulcer status post amputation of the left foot. She did not have atrial fibrillation and was taking clopidogrel and aspirin for polyvascular disease. She used to use insulin in the past but was only on oral hypoglycemic agents (linagliptin 5 mg and voglibose 0.9 mg) at the time of presentation due to improved glycemic control. Her hypertension had been controlled well with amlodipine 10 mg, olmesartan 40 mg, and carvedirol 5 mg per day.
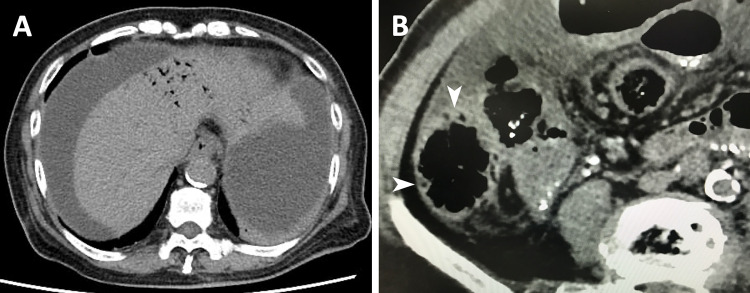
On examination, temperature was 37.1 °C, pulse 74/min, and blood pressure 86/50 mmHg. Abdominal examination revealed moderate diffuse tenderness but no rigidity. White debris were found within the PD catheter lumen, through which white cloudy dialysate effluent was collected. Exit-site and catheter-tunnel infections were absent. Laboratory testing showed the following: white blood cell, 29,100/μL (neutrophil, 94%); C-reactive protein, 17.5 mg/dL; lactate, 4.1 mmol/L; white cell in dialysate effluent, 7,150/μL (neutrophil, 84%). Echocardiography revealed normal left ventricle wall motion. Although these findings could be explained by PD-related peritonitis and sepsis, screening for abdominal complications was performed due to low blood pressure and moderate abdominal pain. After administering crystalloids, CT was performed with a contrast agent and revealed hepatic portal venous gas (Fig. 1a), pneumatosis intestinalis in the ascending colon (Fig. 1b), and normal enhancement of the bowel wall and mesenteric arteries, which suggested a reperfusion of the previously ischemic ascending colon. PD-related peritonitis complicated with NOMI was suspected, and hence resuscitation fluids, intraperitoneal ceftazidime, intravenous vancomycin, and intravenous meropenem were started. Oral intake was withheld. The patient was admitted to the intensive care unit.
Fig. 1.
Computed tomography on admission revealed hepatic portal venous gas (a), pneumatosis intestinalis in the ascending colon (b, arrowheads), and severe calcification of the aorta (a, b). Each image in a, b was obtained before and after administering a contrast agent, respectively
Loose and orange stool with blood was seen from hospital day two and was considered to be the consequence of transient ischemia of the colon. Although the care team wanted to avoid using vasopressors, which may worsen the mesenteric ischemia, blood pressure remained low despite sufficient fluid resuscitation, and therefore minimum necessary amount of noradrenalin was administered from hospital day two to six. Peritoneal dialysis was switched to continuous hemodiafiltration on hospital day five and resumed on hospital day eight. Abdominal CT scan on hospital day four revealed disappearance of hepatic portal venous gas and pneumatosis intestinalis. Pseudomonas putida, a Gram-negative rod-shaped bacterium frequently found in soil and water, was detected in the dialysate effluent. Two sets of blood cultures obtained on admission were both negative. Touch contamination was suspected to be the cause, because exit-site and catheter-tunnel infections were not detected, PD catheter and dialysate bags were not damaged, bacterial translocation is unlikely caused by Pseudomonas putida, and primary gastrointestinal diseases that may cause peritonitis secondarily were not found. Intravenous vancomycin and meropenem were de-escalated to piperacillin/tazobactam based on susceptibility results. White blood cell count, serum C-reactive protein level, and white cell count in dialysis effluent normalized in two weeks.
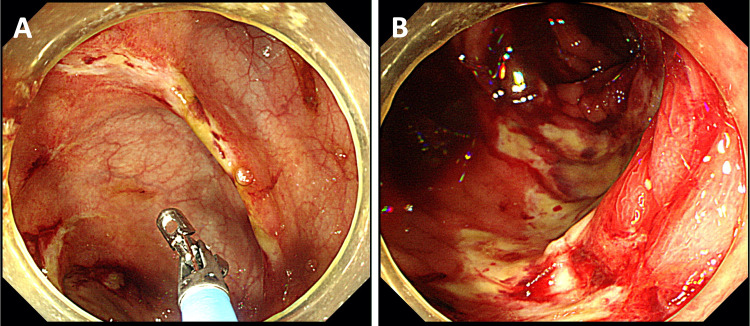
Orange loose stool turned green in five days, and remained loose even after another week. Although Clostridium difficile toxin was negative, oral metronidazole was started considering the possibility of Clostridium difficile colitis given the character of the stool. Colonoscopy on hospital day seventeen revealed mucosal hemorrhage and ulcers in the entire right colon and the terminal ileum (Fig. 2), which are supplied by the superior mesenteric artery (SMA), while the remaining colon was normal. These findings were compatible with the consequence of NOMI. Differential diagnosis of the mucosal hemorrhage and ulcers in the right colon included cytomegalovirus colitis and intestinal tuberculosis, both of which were denied serologically and histologically. Stool culture was negative for Salmonella, Campylobacter, Shigella, Vibrio parahaemolyticus, Clostridium difficile, and enteropathogenic Escherichia coli.
Fig. 2.
Colonoscopy revealed mucosal hemorrhage and ulcers—some of which were annular (a)—in the entire right colon and the terminal ileum (b) but not in other regions
Ultrasound imaging of the SMA showed the peak systolic velocity of 237 cm/s, which implied its stenosis [1]. Three weeks after admission, her diarrhea ceased, and she resumed oral intake without recurrent abdominal pain or diarrhea (Fig. 3).
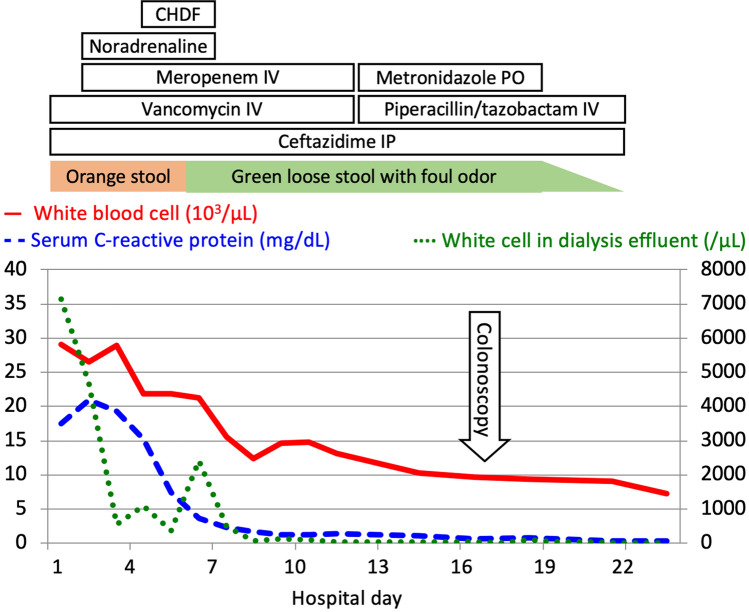
Fig. 3.
Clinical course of the patient is described. After 3 weeks of antibiotic treatment, her diarrhea ceased, and she resumed oral intake without recurrent abdominal pain or diarrhea. CHDF continuous hemodiafiltration, IP intraperitoneal, IV intravenous, PO per oral
Discussion
NOMI is a rare complication in PD patients [2] and has a high mortality rate once it occurs [3]. In the presented case, the early detection of NOMI led to close hemodynamic monitoring in the intensive care unit, sufficient fluid resuscitation, minimal use of vasopressors, and bowel rest, which contributed to her survival.
Although ischemia of the colon commonly occurs in the left colon in the general population, past studies show that patients with CKD experience right colon ischemia more often than left colon ischemia. A retrospective study of 313 patients with colon ischemia revealed that right colon was the major site of ischemia (20%) after pancolonic ischemia (30%) in patients with CKD stage 5. While the left colon was the most affected site in the entire population (33%), it accounted for only 5% in patients with CKD stage 5 with colon ischemia [4]. Left colon ischemia often occurs due to decreased blood flow of the watershed area between the SMA and the inferior mesenteric artery (IMA): the splenic flexure (Griffiths’ point) and sigmoid colon (Sudeck’s point) are anatomically weak to decreased blood flow [4]. The disease entity of ischemic colitis coincides with this etiology. In contrast, right colon ischemia is often due to insufficient decreased blood flow of the SMA, which normally supplies the terminal ileum and the ascending colon. NOMI is caused by decreased blood flow of the SMA or IMA often due to circulatory failure and vasoconstriction [5]. Sakai et al. reported that the left colon is more often affected in spontaneous colon ischemia than the right colon, whereas the right colon is more often damaged in shock-associated colon ischemia than the left colon [6]. Landreneau et al. proposed that the right colon is the most severely affected segment of the colon in cases with NOMI, since it has little collateral blood flow to compensate the deprived blood supply when vasoconstriction occurs [7]. This may well explain why right colon involvement is associated with severe colon ischemia and occurs frequently in patients with CKD on hemodialysis [8]. In the presented case, low blood pressure due to systemic inflammation caused by peritonitis had likely induced decreased blood flow of the SMA.
In summary, we described a case of a 70-year-old woman on PD due to CKD secondary to diabetic nephropathy presented with moderate abdominal pain and low blood pressure, which turned out to be due to PD-related peritonitis caused by Pseudomonas putida complicated with NOMI. The long history of CKD and diabetes of the presented case might have caused the arteriosclerosis of the SMA, which made it vulnerable to the decreased blood flow due to sepsis and vasoconstriction. Low blood pressure and strong abdominal pain may be a clue to detect abdominal complications including NOMI. To the best of our knowledge, this is the first case report that shows colonoscopy images of the colonic ulcers post-NOMI and PD-related peritonitis.
Acknowledgements
The authors thank Dr. Akiko Sasaki and others at the Gastroenterology Medicine Center, Shonan Kamakura General Hospital for their support in performing colonoscopy and investigating the etiology of the disease.
Funding
This study was not supported by any funding.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed, voluntary, and written consent has been obtained from the next of kin of the patient for publication of this case report.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.AbuRahma AF, Stone PA, Srivastava M, et al. Mesenteric/celiac duplex ultrasound interpretation criteria revisited. J Vasc Surg. 2012;55:428–436. doi: 10.1016/j.jvs.2011.08.052. [DOI] [PubMed] [Google Scholar]
- 2.Archodovassilis F, Lagoudiannakis EE, Tsekouras DK, et al. Nonocclusive mesenteric ischemia: a lethal complication in peritoneal dialysis patients. Perit Dial Int. 2007;27:136–141. doi: 10.1177/089686080702700206. [DOI] [PubMed] [Google Scholar]
- 3.Korzets Z, Ben-Chitrit S, Bernheim J. Nonocclusive mesenteric infarction in continuous ambulatory peritoneal dialysis. Nephron. 1996;74:415–418. doi: 10.1159/000189345. [DOI] [PubMed] [Google Scholar]
- 4.Brandt LJ, Feuerstadt P, Blaszka MC. Anatomic patterns, patient characteristics, and clinical outcomes in ischemic colitis: a study of 313 cases supported by histology. Am J Gastroenterol. 2010;105:2245–2252. doi: 10.1038/ajg.2010.217. [DOI] [PubMed] [Google Scholar]
- 5.Wilcox MG, Howard TJ, Plaskon LA, Unthank JL, Madura JA. Current theories of pathogenesis and treatment of nonocclusive mesenteric ischemia. Dig Dis Sci. 1995;40:709–716. doi: 10.1007/BF02064966. [DOI] [PubMed] [Google Scholar]
- 6.Sakai L, Keltner R, Kaminski D. Spontaneous and shock-associated ischemic colitis. Am J Surg. 1980;140:755–760. doi: 10.1016/0002-9610(80)90111-7. [DOI] [PubMed] [Google Scholar]
- 7.Landreneau RJ, Fry WJ. The right colon as a target organ of nonocclusive mesenteric ischemia. Case report and review of the literature. Arch Surg. 1990;125:591–594. doi: 10.1001/archsurg.1990.01410170037007. [DOI] [PubMed] [Google Scholar]
- 8.Flobert C, Cellier C, Berger A, et al. Right colonic involvement is associated with severe forms of ischemic colitis and occurs frequently in patients with chronic renal failure requiring hemodialysis. Am J Gastroenterol. 2000;95:195–198. doi: 10.1111/j.1572-0241.2000.01644.x. [DOI] [PubMed] [Google Scholar]