Abstract
Hypercalcemia is usually secondary to one etiology, although two coexisting etiologies can rarely cause hypercalcemia. Here, we report a 47-year-old woman with hypercalcemia caused by comorbid parathyroid adenoma and pulmonary tuberculosis. Primary hyperparathyroidism is the most common cause of hypercalcemia. Tuberculosis is a rare cause of hypercalcemia, but Japan continues to have an intermediate tuberculosis burden. Therefore, tuberculosis should be considered as a cause of hypercalcemia in Japan. Patients with tuberculosis are often asymptomatic, making the diagnosis difficult. In the previous cases in which these diseases coexisted, one disease was diagnosed after treatment of the other. In our case, the very high 1,25-dihydroxyvitamin D level (162 pg/mL) helped us to diagnose asymptomatic tuberculosis and both diseases were diagnosed promptly. It is necessary to consider comorbidities, including tuberculosis in a case with a very high 1,25-dihydroxyvitamin D level. We report a valuable case in which the early diagnosis and treatment of tuberculosis and primary hyperparathyroidism prevented the spread of tuberculosis.
Keywords: Hypercalcemia; Primary hyperparathyroidism; Tuberculosis; 1,25-Dihydroxyvitamin D
Introduction
Hypercalcemia is a common electrolyte disorder with various etiologies, of which primary hyperparathyroidism (PHPT) and malignancy are the most frequent (ca. 90%) [1]. Coexisting tuberculosis (TB) and PHPT is a rare cause of hypercalcemia, with three reported cases [2–4]. In all previous cases, one disease was diagnosed after treating the other. While TB is not a main cause of hypercalcemia, Japan still has an intermediate-TB burden [5]. Therefore, TB should be considered as a cause of hypercalcemia. Here, we report a woman with coinciding PHPT and asymptomatic pulmonary TB, which were both diagnosed promptly and treated adequately.
Case report
A 47-year-old woman was referred for hypertension detected at a check-up. She had no symptoms and her physical examination was normal. Her blood pressure was 158/115 mmHg and her resting heart rate was 97 bpm. Biochemical studies showed hypercalcemia (11.0 mg/dL) and a low phosphate level (2.5 mg/dL). The fractional excretion of calcium (FECa) based on examining spot-urine samples was 0.66%, indicating hypocalciuria. Table 1 shows the detailed biochemical data. She had a history of depression, lumbar fracture, osteoporotic fracture of the right ankle, and urolithiasis. There was no family history of hypercalcemia or cancer. She was already in remission for depression at the first visit and was not taking any oral medication. She had not taken lithium carbonate, vitamin A or D supplements, or thiazide diuretics. Her serum intact parathyroid hormone (PTH) level was 100 (reference range 10–65) pg/dL. Ultrasonography revealed an enlarged left upper parathyroid gland measuring 16.4 × 7.2 mm. Based on these findings, PHPT was diagnosed. The 1,25-dihydroxyvitamin D (1,25[OH]2D) level was markedly elevated at 162 (reference range 20–60) pg/mL, which exceeded the reported average 1,25(OH)2D level in PHPT patients [6], suggesting comorbidities in addition to PHPT. Granulomatous diseases such as sarcoidosis and TB, or tumors such as lymphomas can increase 1,25(OH)2D [7]. A chest X-ray was normal (Fig. 1a), but a detailed work-up was performed due to a strong suspicion of a complication like granulomatous disease or neoplastic lesions. Computed tomography (CT) of the chest revealed multiple nodules in both lungs. Neither pleural effusion nor significant lymphadenopathy was observed on CT (Fig. 1b). An interferon gamma releasing assay (T-SPOT®) was positive and Mycobacterium tuberculosis was detected in gastric fluid from a solid medium (Ogawa medium) culture test after 3 weeks. Thus, pulmonary TB was diagnosed. These data indicate that PHPT and pulmonary TB caused the hypercalcemia in the present case. The patient had osteoporosis diagnosed based on a history of fracture and a low femoral neck bone mineral density (BMD) measured by DXA; therefore, bisphosphonate treatment was used early and a parathyroidectomy was scheduled.
Table 1.
Biochemical data
| Parameters | Parameters | Reference range |
|---|---|---|
| Hemoglobin (g/dL) | 14.2 | 11.5–14.5 |
| White blood cell count (/mm3) | 5500 | 3300–8600 |
| Albumin (g/dL) | 4.1 | 3.5–5.2 |
| Creatinine (mg/dL) | 0.69 | 0.4–0.8 |
| Urea (mg/dL) | 15 | 8.0–20.0 |
| Sodium (mEq/L) | 139 | 136–146 |
| Potassium (mEq/L) | 4.3 | 3.6–4.8 |
| Chloride (mEq/L) | 105 | 98–109 |
| Calcium (mgl/dL) | 11.0 | 8.6–10.2 |
| Phosphate (mg/dL) | 2.5 | 2.6–4.6 |
| CRP (mg/dL) | < 0.04 | < 0.3 |
| Alkaline phosphatase (IU/L) | 213 | 96–300 |
| TSH (μIU/mL) | 0.75 | 0.34–4.04 |
| PTH (pg/mL) | 100 | 10–65 |
| PTHrP (pmol/mL) | < 1.0 | < 1.0 |
| 1,25(OH)2D (pg/mL) | 162 | 20.0–60.0 |
| ACE (U/L) | 8.6 | 8.3–21.4 |
| Urine TP (mg/dL) | 6 | |
| Urine creatine (mg/dL) | 88.39 | |
| Urine urea (mg/dL) | 623 | |
| Urine calcium (mg/dL) | 9.3 | |
| Urine phosphate (mg/dL) | 45.5 | |
| FECa (%) | 0.66 | |
| FEP (%) | 14.21 | |
| TmP/GFR (mg/dL) | 2.14 |
1,25(OH)2D 1,25-dihydroxyvitamin D3, ACE angiotensin-converting enzyme, PTH parathyroid hormone, PTHrP parathyroid hormone-related protein, TP total protein, TSH thyroid-stimulating hormone, TmP/GFR the ratio of tubular maximum reabsorption of phosphate (TmP) to glomerular filtration rate (GFR)
Fig. 1.
a The chest X-ray findings were normal. b Chest computed tomography scan shows multiple nodular shadows (dotted circle) in both lungs
She had neither upper respiratory symptoms nor fever. Her sputum samples were smear- and PCR negative for M. tuberculosis, and M. tuberculosis was detected only in the “Ogawa medium” culture test, which is highly sensitive. Thus, infectivity was low; however, it was considered that performing endotracheal intubation during general anesthesia would place the medical staff at risk. To prevent the spread of TB, she was given anti-tuberculous therapy with isoniazid, ethambutol, rifampicin, and pyrazinamide for 2 months, followed by isoniazid and rifampicin for 4 months before the parathyroidectomy. She was treated as an outpatient and did not use the negative pressure room. The pulmonary TB treatment increased her FECa to 1.28%, but it did not change the elevated serum calcium level. The PTH level after TB treatment and before parathyroidectomy was 185 pg/dL.
After the parathyroidectomy, the serum calcium and intact PTH and 1,25(OH)2D levels normalized (42 and 47.1 pg/mL, respectively). Histologically, the removed parathyroid gland showed diffuse proliferation of chief cells without malignancy, which was compatible with parathyroid adenoma. Oral daily alfacalcidol (0.25 μg) and calcium lactate (1 g) were initiated, and her calcium level was controlled. The patient’s BMD was preserved from the first visit. Figure 2 summarizes the clinical course of this case.
Fig. 2.
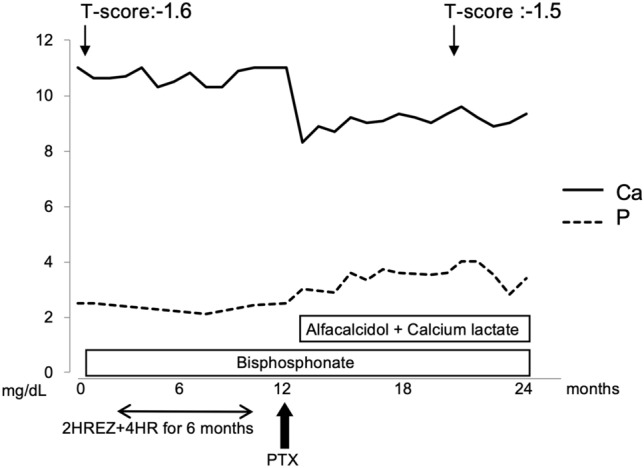
The clinical course of the patient. HREZ H: isoniazid + R: rifampicin + E: ethambutol + Z: pyrazinamide, HR H: isoniazid + R: rifampicin, T-score T-score of femoral neck bone mineral density
Discussion
This is a rare case of hypercalcemia caused by comorbid parathyroid adenoma and pulmonary TB. In this case, although the TB was asymptomatic and difficult to diagnose, the diagnoses of PHPT and TB were established promptly because of the high 1,25(OH)2D level, which was higher in this case than in PHPT patients (162 vs. 72.7 ± 27.1 [mean value ± standard deviation] pg/mL) [6]. Therefore, the existence of a disease contributing to the elevated 1,25(OH)2D other than PHPT was suspected. In patients with granulomatous disease, such as sarcoidosis or TB, and tumors such as lymphomas, hypercalcemia results from excessive 1,25(OH)2D induced by ectopically expressed 25(OH)D-1-hydroxylase in macrophages or tumor cells [7, 8]. Detailed examinations revealed the existence of TB. The reported average 1,25(OH)2D level in TB patients is 96.18 pg/mL [9], which is lower than the level in this case. Therefore, the markedly high 1,25(OH)2D level in this case could have been caused by the comorbid PHPT and TB. In cases of a very high 1,25(OH)2D level, it is necessary to consider comorbidities, including TB. Measuring 1,25(OH)2D is expensive and not appropriate for all patients with hypercalcemia. However, measuring 1,25(OH)2D should be considered in cases where urinary calcium excretion decreases and the findings are not consistent with PHPT or when there are complications suspected of tumorous disease or granulomatous disease that promote 1,25(OH)2D synthesis, such as the present case.
PHPT is the most common cause of hypercalcemia and is most frequently identified in postmenopausal women [10, 11]. Although less frequent than PHPT, TB is also an important cause of hypercalcemia [8]. TB remains an important global public health problem [12]. Patients with TB are often asymptomatic, making the diagnosis difficult [13]. Japan is still an intermediate-TB burden country, with a TB incidence of 12.3 cases per 100,000 population in 2018 [5]. Therefore, hypercalcemia is associated with concurrent TB in Japan.
The coexistence of TB and PHPT is a rare cause of hypercalcemia, with only three previously reported cases [2–4] (Table 2). In these three cases and our case, hypercalcemia with an elevated PTH level was observed. No symptoms were observed in our case, and a high 1,25(OH)2D level, which was not measured in the other cases, was found. Despite the absence of symptoms, this case is the first in which coincident PHPT and TB were diagnosed at an early stage. Pulmonary TB should be diagnosed promptly to prevent the spread of TB. In two previous cases [3, 4], TB was found incidentally in resected parathyroid tissue. In the third case [2], PHPT was diagnosed late after subsequent examinations for persistent hypercalcemia after treating peritonitis TB. In the present case, the calcium level did not decrease after the TB treatment alone, as in previous cases [2]. The PTH level after TB treatment and before parathyroidectomy was 185 pg/dL, which was higher than that before the TB treatment, and the high PTH level maintained the calcium level. However, the 1,25(OH)2D level after TB treatment and before parathyroidectomy was not measured at another hospital where perioperative management was performed. A higher PTH level may have been maintained due to the decrease of 1,25(OH)2D from the TB treatment, reducing the PTH-inhibitory effect of 1,25(OH)2D. Alternatively, PTH, which is a driver of 1,25(OH)2D, was high and that may have maintained 1,25(OH)2D to some extent.
Table 2.
Comparison between reported cases of hypercalcemia caused by comorbid PHPT and TB
| Ref | Age | Sex | Symptom | PE | Location of TB | First Dx | Ca before Tx (mg/dL) | IP before Tx (mg/dL) | PTH (pg/mL) | 1,25(OH)2D (pg/mL) | Ca after TB Tx (mg/dL) | Ca after TB Tx and PTX (mg/dL) | Tx |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (2) | 51 | F | Diffuse abdominal fullness and lower abdominal pain | Abdominal resonant percussion and shifting dullness | Peritoneal | TB peritonitis | 10.7 | 1.8 | 175 (after TB Tx) | ND | 10.6–13.1 | 9.3–9.7 | PTX after TB Tx for 18 months |
| (3) | 36 | F | Bone pain, proximal muscular weakness, and difficulty in walking | Neck examination revealed two enlarged, firm, non-tender lymph nodes in the right supraclavicular and mid-jugular regions | Parathyroid | PHPT | 12.2–13 | 1.8–2.5 | 2000 | ND | ND | ND | TB Tx for 9 months after PTX |
| (4) | 35 | F | Generalized body ache | Examination of the neck did not detect any lump or cervical lymphadenopathy | Parathyroid | PHPT | Elevated | ND | Elevated | ND | ND | ND | TB Tx for 6 months after PTX |
| Our case | 46 | F | None | None | Pulmonary | Pulmonary TB and PHPT | 11.0 | 2.5 |
100 (before TB Tx) 185 (after TB Tx) |
168 | 10.3–11 | 8.3–9.6 | PTX after TB Tx for 6 months |
Dx diagnosis, F female, ND no data, PE physical examination, PHPT primary hyperparathyroidism, PTH parathyroid hormone, Ref reference, TB tuberculosis, Tx treatment
In our case, the low FECa indicated hypocalciuria. Familial hypocalciuric hypercalcemia (FHH) is suspected when hypercalcemia and low urinary calcium excretion coexist. FHH often involves adenomas in the parathyroid glands, and the serum calcium level does not improve after parathyroidectomy [14, 15]. Despite an elevated PTH level, patients with FHH typically have a normal BMD [16]; while, PHPT patients often have a low BMD, particularly at the cortical site [17]. In the present case, there was no family history of FHH, a parathyroid adenoma was found only in one parathyroid gland, the BMD was low despite the patient’s young age, and urinary calcium excretion increased (FECa > 1%) after the TB treatment. These findings ruled out FHH, and the patient underwent a parathyroidectomy for PHPT. Serum calcium level decreased after the parathyroidectomy, which is not generally observed in patients with FHH. A low urinary calcium may have been associated with the increase in the 1,25(OH)2D level caused by TB. 1,25(OH)2D increases calcium reabsorption in the distal tubules via TRPV5; therefore, urinary calcium excretion decreases [18].
In summary, this case highlights the coexistence of PHPT and TB as a cause of hypercalcemia. A markedly high 1,25(OH)2D level prompted the diagnosis of the comorbidities, despite the patient being asymptomatic. The treatment of pulmonary TB before a parathyroidectomy should prevent the spread of TB.
Compliance with ethical standards
Conflict of interest
None of the authors declare any competing interests.
Informed consent
Informed consent was obtained from the patient included in this case report.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lafferty FW. Differential diagnosis of hypercalcemia. J Bone Miner Res. 1991;6(Suppl 2):S51–S59. doi: 10.1002/jbmr.5650061413. [DOI] [PubMed] [Google Scholar]
- 2.Hung KH, Chen FC, Hu YH, Chen JB, Hsu KT. Incidental primary hyperparathyroidism in a hypercalcaemic woman with tuberculous peritonitis. Int J Clin Pract. 2005;59(Suppl. 147):64–66. doi: 10.1111/j.1368-504X.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- 3.Kar DK, Agarwal G, Mehta B, Agarwal J, Gupta RK, Dhole TN, et al. Tuberculous granulomatous inflammation associated with adenoma of parathyroid gland manifesting as primary hyperparathyroidism. Endocr Pathol. 2001;12:355–359. doi: 10.1385/EP:12:3:355. [DOI] [PubMed] [Google Scholar]
- 4.Jacob PM, Sukumar GC, Nair A, Thomas S. Parathyroid adenoma with necrotizing granulomatous inflammation presenting as primary hyperparathyroidism. Endocr Pathol. 2005;16:157–160. doi: 10.1385/EP:16:2:157. [DOI] [PubMed] [Google Scholar]
- 5.Tuberculosis Surveillance Center. Tuberculosis in Japan—annual report 2018. Tokyo: Department of Epidemiology and Clinical Research, the Research Institute of Tuberculosis; 2018.
- 6.Christensen SE, Nissen PH, Vestergaard P, Heickendorff L, Rejnmark L, Brixen K, et al. Plasma 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D, and parathyroid hormone in familial hypocalciuric hypercalcemia and primary hyperparathyroidism. Eur J Endocrinol. 2008;159:719–727. doi: 10.1530/EJE-08-0440. [DOI] [PubMed] [Google Scholar]
- 7.Tebben PJ, Singh RJ, Kumar R. Vitamin D-mediated hypercalcemia: mechanisms, diagnosis, and treatment. Endocr Rev. 2016;37:521–547. doi: 10.1210/er.2016-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanai S, Shinzato T, Inazu D, Tokuda Y. Hypercalcaemia caused by active pulmonary tuberculosis in an elderly person without fever or pulmonary symptoms. BMJ Case Rep. 2017;2017:bcr2016217797. doi: 10.1136/bcr-2016-217797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ralph AP, Rashid Ali MRS, William T, Piera K, Parameswaran U, Bird E, et al. Vitamin D and activated vitamin D in tuberculosis in equatorial Malaysia: a prospective clinical study. BMC Infect Dis. 2017;17:312. doi: 10.1186/s12879-017-2314-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bilezikian JP. Primary hyperparathyroidism. J Clin Endocrinol Metab. 2018;103:3993–4004. doi: 10.1210/jc.2018-01225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker MD, Silverberg SJ. Primary hyperparathyroidism. Nat Rev Endocrinol. 2018;14:115–125. doi: 10.1038/nrendo.2017.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Global tuberculosis report 2019. Geneva: World Health Organization; 2019. Licence:CCBY-NC-SA3.0IGO.
- 13.Lawn SD, Zumla AI. Tuberculosis. Lancet (Lond, Engl) 2011;378:57–72. doi: 10.1016/S0140-6736(10)62173-3. [DOI] [PubMed] [Google Scholar]
- 14.Komaba H, Ikeda K, Fukagawa M. Familial hypocalciuric hypercalcemia. Nihon Naika Gakkai Zasshi (J Jpn Soc Intern Med) 2007;96:681–687. doi: 10.2169/naika.96.681. [DOI] [PubMed] [Google Scholar]
- 15.Christensen SE, Nissen PH, Vestergaard P, Mosekilde L. Familial hypocalciuric hypercalcaemia: a review. Curr Opin Endocrinol Diabetes Obes. 2011;18:359–370. doi: 10.1097/MED.0b013e32834c3c7c. [DOI] [PubMed] [Google Scholar]
- 16.Isaksen T, Nielsen CS, Christensen SE, Nissen PH, Heickendorff L, Mosekilde L. Forearm bone mineral density in familial hypocalciuric hypercalcemia and primary hyperparathyroidism: a comparative study. Calcif Tissue Int. 2011;89:285–294. doi: 10.1007/s00223-011-9517-x. [DOI] [PubMed] [Google Scholar]
- 17.Eller-Vainicher C, Falchetti A, Gennari L, Cairoli E, Bertoldo F, Vescini F, et al. Diagnosis of endocrine disease: evaluation of bone fragility in endocrine disorders. Eur J Endocrinol. 2019;180:R213–R232. doi: 10.1530/EJE-18-0991. [DOI] [PubMed] [Google Scholar]
- 18.Schlatter E. Who wins the competition: TRPV5 or calbindin-D28K? J Am Soc Nephrol. 2006;17:2954–2956. doi: 10.1681/ASN.2006080935. [DOI] [PubMed] [Google Scholar]



