Abstract
A 52-year-old woman had been found to have hematuria at her annual checkup 5 years in a row. She hoped to donate her kidney to her husband, so we performed a percutaneous kidney biopsy at our department. It was difficult for us to detect apparent abnormalities under a light microscopic examination, and she was determined to meet the eligibility criteria for living kidney transplantation. However, the sample for electron microscopy was not evaluated before kidney donation. She subsequently underwent living kidney transplantation as a donor. A 1-h biopsy revealed swelling and obvious vacuolation of the glomerular podocytes, which were characteristic of Fabry disease. Her medical history and examinations were reviewed. No findings or episodes were observed. Pre-donation electronmicroscopy revealed numerous zebra bodies in the podocytes. A definite diagnosis of heterozygous Fabry disease was made based on the GLA gene mutation despite the normal range of leukocyte α-Gal A activity. Based on the pathological deposition of GL-3, chaperone therapy was initiated to suppress the progression of organ damage. In this case, we could not confirm a diagnosis of Fabry disease despite performing a renal biopsy prior to kidney donation. Kidney donor candidates may sometimes have factors that cannot be assumed based on medical or family history. Thus, it is important to perform a renal biopsy before kidney donation when necessary, and to always conduct a detailed evaluation including electron microscopy.
Keywords: Heterozygous Fabry disease, α-Galactosidase A, GLA gene mutation, Migalastat, Chaperone therapy, Kidney transplantation
Introduction
Fabry disease is a rare X-linked multisystemic lysosomal storage disorder caused by reduction in activity of leukocyte α-galactosidase A due to the genetic deletion, resulting in the accumulation of glycolipids, such as globotriaosylceramide (GL-3) [1, 2]. Early signs and symptoms typically manifest during childhood and adolescence and may include neuropathic pain, impaired hearing, corneal opacities, angiokeratoma, and hypohidrosis. Disease progression leads to kidney dysfunction and cardiac and cerebrovascular complications in adulthood, which causes a poor quality of life and increased risk of premature death [3]. Not only men but also heterozygous women may develop and present with variable phenotypes [4, 5]. It is necessary for all suspected women to have their GLA gene evaluated, as the α-Gal A activity may be in the normal range [3, 6, 7].
We herein report a case of unrecognized heterozygous Fabry disease in a patient who underwent kidney donation as an instructive case study.
Case presentation
A 52-year-old woman had been taking an antihypertensive agent for 7 years, but her weight loss brought her blood pressure under control, so drugs were no longer needed. There was no remarkable family history. Her husband had been on hemodialysis due to diabetic nephropathy for 10 years, and she hoped to donate her kidney to her husband. However, hematuria had been noted on her annual checkup for the past 5 years. Therefore, she was referred to our hospital for a detailed examination of her kidneys.
On admission, her height was 164 cm, weight was 63 kg, and blood pressure was 116/73 mmHg. Laboratory studies showed the following: white blood cells 4.730/μL, red blood cells 425 × 104/μL, hemoglobin 13.5 g/dL, hematocrit 39.2%, platelets 208,000/μL, serum total protein 7.3 g/dL, albumin 4.2 g/dL, blood urea nitrogen 13.6 mg/dL, creatinine 0.76 mg/dL, 24-h creatinine clearance 103 mL/min (mGFR 105.7 mL/min/1.73 m2), sodium 140 mEq/L, potassium 4.0 mEq/L, chloride 106 mEq/L, calcium 9.3 mg/dL, phosphorus 3.4 mg/dL, and cystatin C 0.84 mg/L. A urinalysis showed hematuria (2 +). A urinary sediment examination found hematuria at 1–4/high-power field and no urinary casts. Neither evident proteinuria nor albuminuria was noted. The bilateral kidneys were of normal size and form on computed tomography. The electrocardiography showed no arrhythmia. The echocardiography revealed no valvular disease or cardiac hypertrophy. The lung function was normal.
She underwent a percutaneous renal biopsy to ascertain the etiology of her hematuria. Light microscopy contained only seven glomeruli, none of which showed sclerosis. On immunofluorescence, there was no significant positive staining for IgG, IgA, IgM, C1q, C3d, or fibrinogen. It was difficult for us to detect latent abnormalities under light microscopy (Fig. 1a) before kidney donation, and she was determined to meet the eligibility criteria for living kidney transplantation. However, the sample for electron microscopy was not evaluated before kidney donation. She subsequently underwent living kidney donation to her husband.
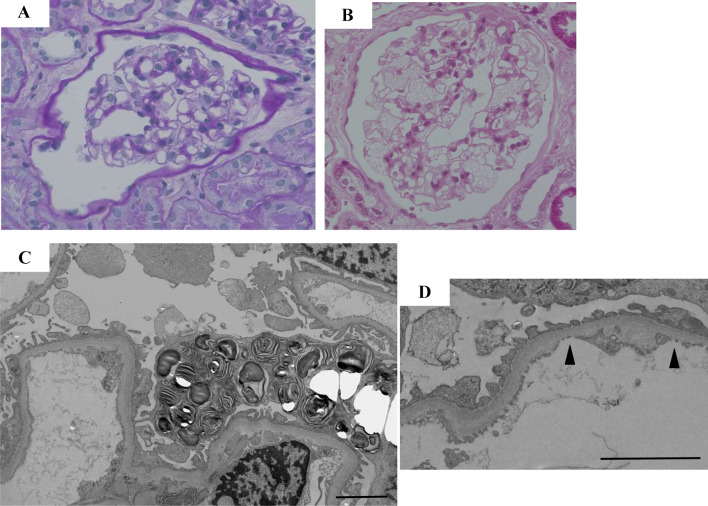
Fig. 1.
a, c, d Renal pathology prior to kidney donation; b a 1-h biopsy. a No apparent change was detected in the glomeruli under a light microscopic examination before kidney donation (PAS staining × 400). b A 1-h biopsy revealed remarkable swelling and obvious vacuolation of the glomerular podocytes (PAS staining × 400). c Pre-donation electron microscopy revealed numerous zebra bodies in the podocytes (× 3000). d Basement membrane thinning (180 nm) was observed in part of the specimen (Arrowhead). Scale bars 2 μm
The ABO-incompatible protocol of our hospital was initiated, and basiliximab, steroids, tacrolimus, and mycophenolatemofetil were used as primary immunosuppressive agents after desensitization by plasma exchange. Her husband was discharged with an acceptable graft function (serum creatinine 1.69 mg/dL) However, a 1-h biopsy revealed swelling and obvious vacuolation of the glomerular podocytes, characteristic of Fabry disease (Fig. 1b), which prompted us to suspect Fabry disease for the first time.
Her medical history and examinations were reviewed. No findings or episodes, such as impaired hearing, pain in the limbs, hypohidrosis, abdominal pain, cornea verticillata, or angiokeratoma, were observed. Although the leukocyte α-Gal A activity was 50.9 nmol/h/mg protein within a normal lower limit (controls 49.8–116.4 nmol/h/mg protein), pre-donation electron microscopy revealed numerous zebra bodies, consistent with Fabry disease, in the podocytes (Fig. 1c). In addition, basement membrane thinning was observed (Fig. 1d). Multiple microinfarctions of cerebral white matter were detected on brain magnetic resonance imaging. A definitive diagnosis of heterozygous Fabry disease was made based on GLA gene testing (missense mutation, c.888G > A; p. Met296Ile).
Although her renal function has been stable with no albuminuria observed either before or after kidney donation, based on the accumulation of glycolipids in kidney cells, migalastat as chaperone therapy was initiated to suppress the progression of organ damage due to Fabry disease. Mild headache was the only adverse effect; however, the symptom gradually improved and administration could be continued.
Discussion
In the present case, Fabry disease was not considered as a differential diagnosis because most patients with hematuria without overt proteinuria are, in general, pathologically diagnosed as IgA nephropathy or thin basement membrane disease [8], and there was no characteristic medical or family history. However, a kidney biopsy was performed before kidney donation, and there should have been an opportunity to diagnose the underlying disease. Actually, on a detailed review of pre-donation light microscopy specimens, slight swelling and vacuolation of the glomerular podocytes were observed. The major cause of failure to diagnose Fabry disease was that kidney donation was performed without confirming the electron microscopic findings before kidney donation. An opportunity to reconsider the indication of the donor might have been obtained if Fabry nephropathy had been suspected based on the detection of zebra bodies by electron microscopic observation and a definitive diagnosis had been made by genetic testing.
Furthermore, basement membrane thinning was observed in part of the specimen by electron microscopy, which was considered to be a cause of hematuria. Thus, a thorough evaluation for basement membrane disease should be performed before donation. In recent years, cases of benign familial hematuria including thin basement membrane disease have been reported to cause focal segmental glomerulosclerosis and lead to renal failure [9]. In particular, it is noted that urinary protein tends to appear rapidly after the 30 s in patients with COL4A3/COL4A4 mutations, as is observed in patients with Alport syndrome. Although the risk of renal failure due to the natural course of thin basement membrane disease may have been low in this patient in her 50 s, genetic testing for basement membrane disease as well as Fabry disease should be performed before donation.
A recent report indicated that kidney donors had an increased risk of end-stage kidney disease in comparison with a matched cohort of healthy nondonors [10]. In addition to Fabry disease, the patient had risk factors for kidney failure, such as thin basement membrane disease and donor nephrectomy, thus, the care taken for chronic kidney disease management should have been greater in comparison with that for other donors.
There are only a few reports of unrecognized kidney donation from heterozygous patients to recipients (Table 1) [11–17], suggesting that the renal prognosis after kidney donation appears to be stable in the short term, however, the long-term prognosis is unclear. The renal complications of Fabry disease are key contributors to the morbidity and mortality associated with the disorder [18]. Fabry nephropathy proven by a renal biopsy was demonstrated in this case, and minimizing the effects of Fabry nephropathy may improve the patient’s renal outcome. General guidelines recommend that specific treatment be initiated as soon as symptoms occur, regardless of a classical or heterozygous status, since patients in whom treatment is initiated at an appropriate timing benefit the most and have more favorable long-term renal outcomes [19, 20]. While she was asymptomatic before and after donation, based on the pathological deposition of GL-3, we determined that migalastat should be administered to prevent the development of organ damage.
Table 1.
Living-donor kidney transplantations from patients with unrecognized Fabry disease
| Donor | Recipient | Timing of the diagnosis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Age (years) | Relationship | Prognosis | Therapy for FD | Sex | Age (years) | FD/not FD | Prognosis | Therapy for FD | ||
| Grünfeld JP, 1975 | F | 46 | Mother | N/A | N/A | F | 20 | Not FD | Stable | Unnecessary | 8 years later |
| Popli S, 1987 | F | N/A | Sister | N/A | N/A | M | 29 | FD | Worsen | Unnecessary | 5 years later |
| Puliyanda DP, 2003 | F | 31 | Sister | Stable | N/A | F | 31 | FD | Stable | None | 5 years later |
| Paull LS, 2012 | F | 34 | Sister | Stable | N/A | F | 29 | Not FD | Loss | Unnecessary | 8 years later |
| Taneda S, 2013 | M | N/A | Brother | N/A | None | M | 46 | FD | Worsen | ERT | 3 years later |
| Nishioka R, 2014 | F | 62 | Mother | Stable | ERT | M | 35 | FD | Stable | ERT | At KTx |
| Odani K, 2016 | F | N/A | Mother | Stable | None | M | 40 | FD | Stable | None | At KTx |
| This case, 2019 | F | 52 | Wife | Stable | Chaperone | M | 62 | Not FD | Stable | Unnecessary | At KTx |
FD Fabry disease, ERT enzyme replacement therapy
Enzyme replacement therapy (ERT) has been conventionally shown to have a good therapeutic effect [21], however, ERT must be administered as an intravenous infusion every other week. In addition, complications such as infusion reaction can affect the treatment compliance [22]. Migalastat, the first-in-class pharmacologic chaperone administered in this case, was approved in Japan in 2018. The ATTRACT study indicated that the therapeutic effect of migalastat on the renal function is equivalent that of ERT [23], and the greatest advantage is of migalastat is that it can be administered orally. However, it is necessary to confirm that the patient has a migalastat-amenable form of α-Gal A mutation [24–26]. The present patient was fortunate to have a migalastat-amenable mutation, despite the fact that such mutations account for < 30% of currently identified gene mutations in the Japanese population [27]. The main adverse effect is headache, which can be treated symptomatically in the majority of cases. Additionally, migalastat is not recommended for patients with severe renal dysfunction, and attention should be paid to changes in the renal function after renal donation in the future.
In summary, we encountered a case of latent heterozygous Fabry disease in a female living kidney donor candidate. Kidney donor candidates may sometimes have factors that cannot be assumed based on medical or family history and may be at risk for renal failure in the future. Thus, it is important to perform a renal biopsy before kidney donation when necessary, and to always conduct a detailed evaluation, including electron microscopy.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brady RO, Gal AE, Bradley RM, Martensson E, Warshaw AL, Laster L. Enzymatic defect in Fabry’s disease. N Engl J Med. 1967;27:1163–1167. doi: 10.1056/NEJM196705252762101. [DOI] [PubMed] [Google Scholar]
- 2.Kint JA. Fabry’s disease: alpha-galactosidase deficiency. Science. 1970;167:1268–1269. doi: 10.1126/science.167.3922.1268. [DOI] [PubMed] [Google Scholar]
- 3.Germain DP. Fabry disease. Orphanet J Rare Dis. 2010;5:30. doi: 10.1186/1750-1172-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacDermot KD, Holmes A, Miners AH, Rowland HS. Anderson-Fabry disease: clinical manifestations and impact of disease in a cohort of 98 hemizygous males. J Med Genet. 2001;38(11):750–760. doi: 10.1136/jmg.38.11.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacDermot KD, Holmes A, Miners AH, Rowland HS. Anderson-Fabry disease: clinical manifestations and impact of disease in a cohort of 60 obligate carrier females. J Med Genet. 2001;38(11):769–775. doi: 10.1136/jmg.38.11.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linthorst GE, Vedder AC, Aerts JMFG, Hollak CEM. Screening for Fabry disease using whole blood spots fails to identify one-third of female carriers. Clin Chim Acta. 2005;353(1–2):201–203. doi: 10.1016/j.cccn.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Linthorst GE, Poorthuis BJHM, Hollak CEM. Letters to the editor enzyme activity for determination of presence of Fabry disease in women results in 40 % false-negative results. J Am Coll Cardiol. 2008;51(21):2082–2083. doi: 10.1016/j.jacc.2008.02.050. [DOI] [PubMed] [Google Scholar]
- 8.Hoshino Y, Kaga T, Abe Y. Renal biopsy findings and clinical indicators of patients with hematuria without overt proteinuria. Clin Exp Nephrol. 2015;19(5):918–924. doi: 10.1007/s10157-015-1090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pierides A, Voskarides K, Athanasiou Y, Ioannou K, Damianou L, Arsali M, et al. Clinico-pathological correlations in 127 patients in 11 large pedigrees, segregating one of three heterozygous mutations in the COL4A3 COL4A4 genes associated with famikial hematuria and significant late progression to proteinuria and chronic kidney disease from focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2009;24(9):2721–2729. doi: 10.1093/ndt/gfp158. [DOI] [PubMed] [Google Scholar]
- 10.Muzaale AD, Massie AB, Wan MC, Montgomery RA, McBride MA, Wainright JL, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311(6):579–586. doi: 10.1001/jama.2013.285141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grünfeld JP, Le Porrier M, Droz D, Bensaude I, Hinglais N, Crosnier J. Renal transplantation in patients suffering from Fabry's disease. Kidney transplantation from an heterozygote subject to a subject without Fabry's disease. NouvPresse Med. 1975;4(29):2081–2085. [PubMed] [Google Scholar]
- 12.Popli S, Molnar ZV, Leehey DJ, Daugirdas JT, Roth DA, Adams MB, et al. Involvement of renal allograft by Fabry's disease. Am J Nephrol. 1987;7(4):316–318. doi: 10.1159/000167493. [DOI] [PubMed] [Google Scholar]
- 13.Puliyanda DP, Wilcox WR, Bunnapradist S, Nast CC, Jordan SC. Case report Fabry disease in a renal allograft. Am J Transplant. 2003;3:1030–1032. doi: 10.1034/j.1600-6143.2003.00139.x. [DOI] [PubMed] [Google Scholar]
- 14.Paull LS, Lipinski MJ, Wilson WG, Lipinski SE. Female with Fabry disease unknowingly donates affected kidney to sister: a call for pre-transplant genetic testing. JIMD Rep. 2012;4:1–4. doi: 10.1007/8904_2011_108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taneda S, Honda K, Nakajima I, Huchinoue S, Oda H. Renal transplantation between siblings with unrecognized Fabry disease. Transplant Proc. 2013;45(1):115–118. doi: 10.1016/j.transproceed.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 16.Nishioka R, Sofue T, Moritoki M, Nishijima Y, Nishioka S, Hara T, et al. Case report: a case of living-donor kidney transplantation from a heterozygote mother to a hemizygote son of Fabry disease diagnosed by donated allograft biopsy. Nihon NaikaGakkaiZasshi. 2015;104(4):775–780. doi: 10.2169/naika.104.775. [DOI] [PubMed] [Google Scholar]
- 17.Odani K, Okumi M, Honda K, Ishida H, Tanabe K. Kidney transplantation from a mother with unrecognized Fabry disease to her son with low α-galactosidase A activity: a 14-year follow-up without enzyme replacement therapy. Nephrology. 2016;21(Suppl. 1):57–59. doi: 10.1111/nep.12771. [DOI] [PubMed] [Google Scholar]
- 18.Ortiz A, Oliveira J, Waldek S, Warnock DG, Cianciaruso B, Wanner C. Nephropathy in males and females with Fabry disease: cross-sectional description of patients before treatment with enzyme replacement therapy. Nephrol Dial Transplant. 2008;23(5):1600–1607. doi: 10.1093/ndt/gfm848. [DOI] [PubMed] [Google Scholar]
- 19.Biegstraaten M, Arngrímsson R, Barbey F, Boks L, Cecchi F, Deegan PB, et al. Recommendations for initiation and cessation of enzyme replacement therapy in patients with Fabry disease: the European Fabry Working Group consensus document. Orphanet J Rare Diseases. 2015;10:36. doi: 10.1186/s13023-015-0253-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wanner C, Arad M, Baron R, Burlina A, Elliott PM, Feldt-Rasmussen U, et al. European expert consensus statement on therapeutic goals in Fabry disease. Mol Genet Metab. 2018;124(3):189–203. doi: 10.1016/j.ymgme.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Tøndel C, Bostad L, Larsen KK, Hirth A, Vikse BE, Houge G, et al. Agalsidase benefits renal histology in young patients with Fabry disease. J Am Soc Nephrol. 2013;24:137–148. doi: 10.1681/ASN.2012030316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka A, Takeda T, Hoshina T, Fukai K, Yamano T. Enzyme replacement therapy in a patient with Fabry disease and the development of IgE antibodies against agalsidase beta but not agalsidase alpha. J Inherit Metab Dis. 2010;33(Suppl 3):249–252. doi: 10.1007/s10545-010-9136-0. [DOI] [PubMed] [Google Scholar]
- 23.Hughes DA, Nicholls K, Shankar SP, Sunder-Plassmann G, Koeller D, Nedd K. Oral pharmacological chaperone migalastat compared with enzyme replacement therapy in Fabry disease: 18-month results from the randomised phase III ATTRACT study. J Med Genet. 2017;54:288–296. doi: 10.1136/jmedgenet-2016-104178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan JQ, Ishii S, Asano N, Suzuki Y. Accelelated transport and maturation of lysosomal α–galactosidase A in Fabrylymphoblasts by an enzyme inhibitor. Nat Med. 1999;5(1):112–115. doi: 10.1038/4801. [DOI] [PubMed] [Google Scholar]
- 25.Yam GH, Zuber C, Roth J. A synthetic chaperone corrects the trafficking defect and disease phenotype in a protein misfolding disorder. FASEB J. 2005;19(1):12–18. doi: 10.1096/fj.04-2375com. [DOI] [PubMed] [Google Scholar]
- 26.Asano N, Kizu H, Ikeda K, Yasuda K, Ishii S, Martin OR, et al. In vitro inhibition and intracellular enhancement of lysosomal α-galactosidasea activity in fabrylymphoblasts by 1-deoxygalactonojirimycin and its derivatives. Eur J Biochem. 2000;267(13):4179–4186. doi: 10.1046/j.1432-1327.2000.01457.x. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi M, Ohashi T, Kaneshiro E, Higuchi T, Ida H. Mutation spectrum of α-galactosidase gene in Japanese patients with Fabry disease. J Hum Genet. 2019;64(7):695–699. doi: 10.1038/s10038-019-0599-z. [DOI] [PubMed] [Google Scholar]