Abstract
Fabry disease is an X-linked recessive disease of glycosphingolipid metabolism caused by deficiency or reduced activity of α-galactosidase A. Fabry disease phenotypes are known to consist of a classic variant and a late-onset variant. In patients with Fabry disease, the phenotype is generally considered to be defined (at least partially) by the genotype. However, patients with the classic variant have been encountered in families with mutations that are expected to produce the late-onset variant. Here, we describe a 4-year-old boy with a classic variant of Fabry disease in a family with the M296I late-onset variant. The patient’s grandfather, mother, and aunt experienced late-onset disease, characteristic of the M296I variant. Conversely, the patient experienced typical disease symptoms in childhood. He had symptoms of hypohidrosis and associated heat accumulation. He cried at night due to the occurrence of severe acroparaesthesia. This symptom became more pronounced in warmer climates. Although the patient’s family had a late-onset variant mutation of Fabry disease, we determined that the patient’s symptoms were similar to those of classic Fabry disease. Therefore, the patient began enzyme replacement therapy, which alleviated his symptoms. Notably, enzyme replacement therapy led to rapid improvement of the patient’s subjective symptoms. Thus, we presumed that the patient’s symptoms supported a diagnosis of classic Fabry disease. These findings suggest that childhood symptoms may occur in patients with Fabry disease, even in families with late-onset variant mutations. The genotype–phenotype correlation in Fabry disease remains controversial.
Keywords: Fabry disease, M296I mutation, Classic variant, Late-onset variant, Genotype, Phenotype
Introduction
Fabry disease is a disease of glycosphingolipid metabolism with X-linked recessive inheritance caused by deficiency or reduced activity of α-galactosidase A (α-gal A). This disease constitutes a lysosomal storage disorder caused by mutations in the α-gal A-encoding gene on the X chromosome (Xq22.1) [1]. Storage of glycosphingolipids within the lysosomes of various organs results in fatal complications, such as progressive hypertrophic cardiomyopathy, severe heart failure, end-stage kidney disease, and stroke [2]. More than 700 Fabry disease-causing mutations have been identified in the human α-gal A-encoding gene. In patients with Fabry disease, the range of α-gal A mutation phenotypes can vary widely, according to individual genotype [3]. Moreover, the spectrum of Fabry disease phenotypes can vary widely among patients with the same α-gal A mutation, as observed in patients with many other genetic diseases [2, 4]. Fabry disease includes a classic variant with systemic symptoms that begin in childhood, as well as late-onset variants that develop in adulthood and affect limited organs [1, 5]. A missense mutation in exon 6 (i.e. the M296I mutation) has been reported to cause a late-onset variant of Fabry disease, characterised by late onset and manifestations limited to the heart [6]. This mutation causes partial retention of enzyme activity; symptom onset occurs in adulthood. Notably, in some patients with Fabry disease, genotype and phenotype may not be clearly related. A previous report described a patient with classic Fabry disease symptoms in a family with a late-onset mutation [7]. To the best of our knowledge, there have been no reports of patients with a classic variant of Fabry disease in a family with the M296I late-onset variant of Fabry disease. Here, we describe a 4-year-old boy with Fabry disease. His grandfather, mother, and aunt were known to carry the M296I mutation in the human α-gal A-encoding gene. The patient had symptoms of severe acroparaesthesia, which worsened as his temperature increased. Initially, we were not unsure whether these symptoms were associated with classic Fabry disease, because the patient’s family exhibited a late-onset variant of Fabry disease. However, we found that the patient’s α-gal A activity was extremely low; thus, we presumed that his phenotype might differ from his genotype. After administration of enzyme replacement therapy (ERT), the patient’s subjective symptoms improved and his quality of life was restored. Accordingly, we considered this patient to exhibit a classic variant of Fabry disease in a family with the M296I late-onset variant. This report was written to illustrate that some patients may exhibit differences between genotype and phenotype that require careful consideration during treatment. To the best of our knowledge, this is the first report of a patient with the classic Fabry disease variant in a family with the M296I late-onset variant.
Case report
A 4-year-old boy was referred to our hospital with lower limb pain during exercise. He had begun to complain of pain in his toes at 3 years of age. At 4 years of age, he had begun to cry at night, due to the onset of severe acroparaesthesia; this symptom became more pronounced in warmer climates and was reduced by exposure to cooling. In winter, the use of heating exacerbated the patient’s acroparaesthesia. During playtime in school, his body temperature increased, which led to worsened acroparaesthesia. Furthermore, he began to exhibit an aversion to bathing because of the increased temperature. The patient also exhibited hypohidrosis during exercise, which caused skin and scalp dryness and itchiness. However, he did not exhibit corneal haemangioma, corneal opacities, skin angiokeratoma, electrocardiogram abnormalities, abnormal cardiac function, or cardiac morphology abnormalities, as determined by echocardiography.
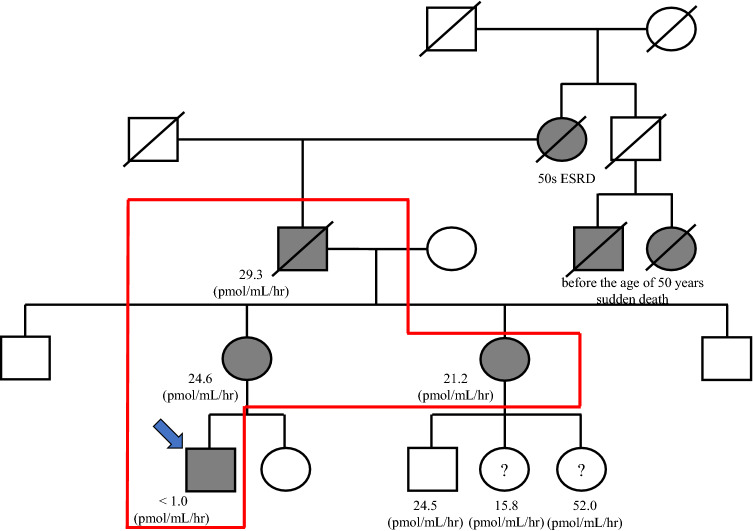
The patient’s grandfather had been diagnosed with Fabry disease at 55 years of age, based on the results of three renal biopsy procedures; the renal biopsy findings revealed changes that appeared to be glomerular and tubulointerstitial injuries, mainly due to ischaemia. In the third renal biopsy, the presence of vacuolation was confirmed in glomeruli (Fig. 1). The patient’s grandfather then received ERT until 60 years of age. Genetic tests of the patient’s grandfather showed that the causative disease mutation was M296I (i.e. the ‘cardiac variant’ [3]). However, the patient’s grandfather had died suddenly at 60 years of age; notably, he had been diagnosed with cardiac hypertrophy and proteinuria before death. The patient’s mother and aunt both had no symptoms of Fabry disease; they had undergone measurement of α-gal A enzyme activity, which revealed results within the almost normal range. The patient’s grandfather had shown maintenance of α-gal A enzyme activity, even at 55 years of age. Furthermore, the grandfather’s cousin had also died before the age of 50 years, presumably as a result of sudden cardiac death (Fig. 2).
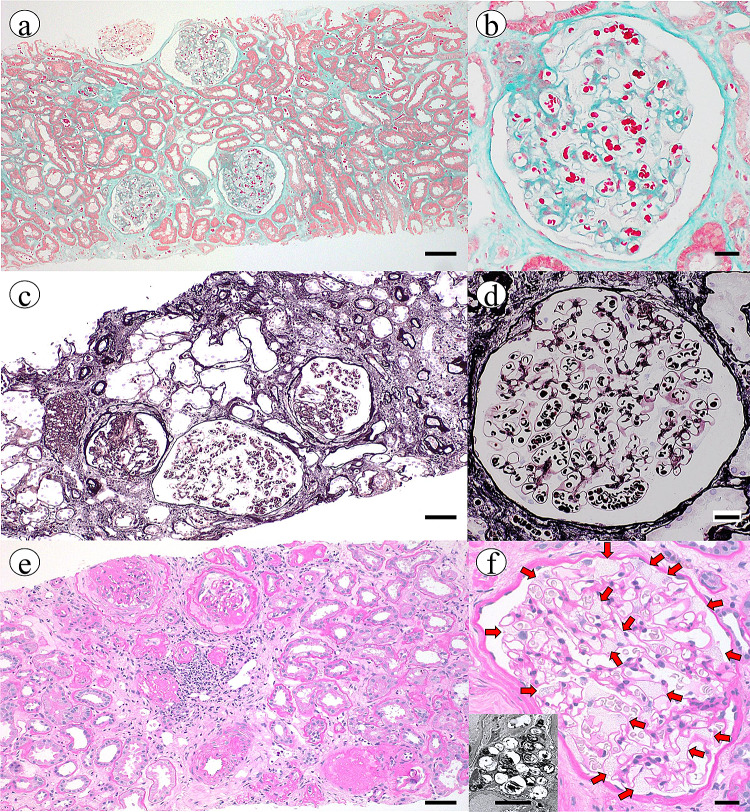
Fig. 1.
Findings of renal biopsy in the patient’s grandfather. a, b First renal biopsy (47 years of age). There is no evidence to suggest hardening of the glomeruli. There are no findings of vacuolation or foamy appearance in glomeruli. Tubulointerstitial fibrosis is also inconspicuous. There are few signs of arteriosclerosis. Scale bars indicate 50 and 20 µm, respectively. (Masson’s trichrome stain). c, d Second renal biopsy (53 years of age). Segmental nephrosclerosis and hypertrophy of glomeruli are noticeable. The presence of vacuolation or foamy appearance in glomeruli remains unclear. Tubulointerstitial fibrosis is present. These findings are similar to ischaemic changes due to nephrosclerosis. Scale bars indicate 50 and 20 µm, respectively. (Periodic acid-methenamine-silver stain). e,f. Third renal biopsy (55 years of age). Many glomeruli have collapsed. Remaining glomeruli clearly exhibit vacuolation and foamy appearance (panel f, red arrows). Electron microscopy findings confirm the presence of Zebra bodies (panel f, small window, scale bars indicate 5 µm). Significant arteriosclerosis is present. Tubulointerstitial lesions show minimal progression. Scale bars indicate 50 and 20 µm, respectively. (Periodic acid–Schiff stain)
Fig. 2.
Pedigree of the patient’s family. Blue arrow indicates proband. The enzyme activities of the proband, his mother, his aunt, and his grandfather are shown below their locations in the pedigree. Some of the proband’s relatives died suddenly of an unknown cause between 30 and 40 years of age, while another relative died of end-stage renal disease between 50 and 60 years of age. Abbreviation: ESKD, end-stage kidney disease
These findings suggested that typical M296I-subtype Fabry disease was present among members of the patient’s family. To confirm the presence of Fabry disease in our patient, a blood sample was collected, frozen quickly, and assessed by fluorescence spectroscopy. In addition, enzyme activity was measured by the filter paper method at another facility; however, the result was below the sensitivity limit of < 1 pmol/hr/disk. Because the patient’s reduced α-gal A activity was a reproducible result, the observed enzyme activity level was regarded as a valid finding. Notably, our patient had very low α-gal A enzyme activity and experienced extremity pain in childhood. His disease presentation differed from that of other family members. Accordingly, genetic examination was performed, comprising analysis of all exons of the α-gal A-encoding gene by direct sequencing of DNA from peripheral blood leukocytes. The results revealed a hemizygous G > A point mutation at nucleotide 888 (c.888G > A) in codon 296 (p.M296I) of exon 6 in our patient. No other mutations were identified in the gene. Patients with classic variants exhibit Fabry disease in childhood or adolescence; this disease typically includes various symptoms, which lead to a high rate of end-stage renal disease morbidity. Conversely, patients with the late-onset variant of Fabry disease typically have milder clinical symptoms, mostly limited to the heart or kidneys [3]. Our patient exhibited a classic variant of Fabry disease in a family with the M296I late-onset variant. Unlike other members of his family, he began to receive ERT at 4 years of age. The application of ERT alleviated the patient’s pain symptoms and led to improvement of anhidrosis. His lyso-Gb3 level in plasma was 2.92 ng/mL before treatment; it decreased to 1.65 ng/mL after 2 months of treatment.
Discussion
Patients with Fabry disease exhibit varying residual enzyme activities, depending on their genotype. Therefore, the genotype of these patients may be used to guide their examination and treatment. Patients with late-onset variants of Fabry disease exhibit partial maintenance of α-gal A enzyme activity; subjective symptoms are less likely to appear in childhood. However, in some patients with Fabry disease, there is not a clear relationship between genotype and phenotype. A prior report described a patient with the late-onset variant R301Q mutation who had a family member with the classic variant of the disease [7]; notably, only one member of that family had classic Fabry disease symptoms. In patients with Fabry disease, the phenotypic severity may differ despite the presence of an identical mutation, even in patients with similar genetic backgrounds (i.e. siblings) [8]. The amount of enzyme–substrate produced may differ among affected patients, and gene modifications may affect α-gal A activity. For mutations that show relatively high residual activity (i.e. the M296I mutation in our patient), such events are expected to occur. However, the specific underlying mechanism remains unknown. Thus, phenotype cannot be clearly predicted on the basis of genotype information in patients with Fabry disease.
The M296I mutation was identified during differential diagnosis of adult patients with unexplained left ventricular hypertrophy in Japan. The M296I mutation reportedly resulted in moderately reduced expression of α-galactosidase mRNA; residual α-galactosidase appeared to exhibit normal enzyme activity, but might be degraded more rapidly than in individuals without the mutation [6]. Based on that report, the M296I mutation has been known as a late-onset, cardiac variant. However, to the best of our knowledge, there have been no reports regarding the development of a classic variant of Fabry disease in a family with the M296I mutation.
Among family members with the same Fabry disease mutation, some phenotypic variability has been reported; these findings indicate that genotype may interact with other factors to produce the phenotypes observed in patients with Fabry disease [7, 9, 10]. These other factors may include the rate of substrate production, rate of cell division, and tolerance to accumulated substrate in each organ or tissue [7]. Some acquired factors, such as obesity, and lifestyle-related diseases that cause obesity, have also been identified [11]. However, because our patient was young (4 years of age), his exposure to relevant acquired factors was presumably limited.
Because patients with late-onset variants retain a degree of α-gal A activity, the difference in extent of residual α-gal A activity, combined with other factors, may determine the phenotype in these patients [7]. A report regarding a family with the L415P mutation, a typical classic variant, revealed that members of the family exhibited identical L415P mutations; however, they showed different degrees of organ damage and had distinct therapeutic responses to ERT [12]. The rate of X chromosome inactivation in patients with Fabry disease may underlie the variability of Fabry disease phenotype, especially in women [13]; this phenomenon can be explained by the Lyon hypothesis of random X chromosomal inactivation [14]. This may be one of the mechanisms by which phenotype differs among patients, despite the presence of identical gene mutations. Because α-gal A activity varied widely in our patient’s family, we speculate that the Lyon hypothesis may explain this variation.
Furthermore, Yamamoto et al. suggested that epigenetic mechanisms may contribute to the disparity between genotype and phenotype in patients with Fabry disease [7]. Epigenetics is regarded as ‘mitotically and meiotically heritable changes in gene expression not caused by changes in the primary DNA sequence’ [15]. Because most lysosomal storage disorders exhibit wide phenotypic heterogeneity, epigenetic mechanisms may contribute to the spectrum of clinical variability [15]. Epigenetic changes constitute uninvolved genetic factors, which may explain the lack of tight genotype–phenotype correlation in patients with Fabry disease. Therefore, future assessment of patients with Fabry disease should involve both genetic and epigenetic analyses.
If treatment is initiated early, ERT can slow the progression of heart failure due to myocardial hypertrophy [13] and delay the progression of renal dysfunction due to Fabry disease [16]. However, the patient’s grandfather died of sudden cardiac death after 5 years of treatment. If the patient’s grandfather had received ERT before the age at which he first underwent kidney biopsy (47 years), he might not have died suddenly at 60 years of age. In addition, because M296I is mainly characterised as a late-onset, cardiac variant, the findings of kidney biopsy in the patient’s grandfather may have resembled the normal course of renal sclerosis, rather than nephropathy typical of Fabry disease [17]. Notably, the patient’s grandfather underwent three renal biopsies over a period of 8 years, which revealed Fabry disease-related morphological changes that were not modified by ERT. Therefore, the findings in the patient’s grandfather present rare insights regarding the course of Fabry disease.
In conclusion, a clear phenotype–genotype correlation was difficult to establish in our patient. The key points in this report are that, in a family with a late-onset variant of Fabry disease, a patient developed symptoms in childhood; this phenomenon can occur for multiple types of Fabry disease-related mutations. The genotype–phenotype correlation remains controversial in patients with Fabry disease. However, after a diagnosis of Fabry disease is confirmed, all organs in an affected patient’s body should be thoroughly examined to clearly establish disease-related changes in these patients.
Acknowledgements
The authors thank Ryan Chastain-Gross, Ph.D., from Edanz Group (https://en-author-services.edanzgroup.com) for editing a draft of this manuscript and helping to draft the abstract. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Compliance with ethical standards
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical approval
All procedures performed in these studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Written consent to publish this information was obtained from the patient’s parent.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shuma Hirashio, Email: shuma.hirashio@gmail.com.
Reiko Kagawa, Email: ykagawa@ja2.so-net.ne.jp.
Go Tajima, Email: tajima-g@ncchd.go.jp.
Takao Masaki, Email: masakit@hiroshima-u.ac.jp.
References
- 1.Ries M, Ramaswami U, Parini R, Lindblad B, Whybra C, Willers I, et al. The early clinical phenotype of Fabry disease: a study on 35 European children and adolescents. Eur J Pediatr. 2003;162:767–772. doi: 10.1007/s00431-003-1299-3. [DOI] [PubMed] [Google Scholar]
- 2.Ortiz A, Abiose A, Bichet DG, Cabrera G, Charrow J, Germain DP, et al. Time to treatment benefit for adult patients with Fabry disease receiving agalsidase: data from the Fabry Registry. J Med Genet. 2016;53:495–502. doi: 10.1136/jmedgenet-2015-103486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Čerkauskaite A, Čerkauskiene R, Miglinas M, Laurinavičius A, Ding C, Rolfs A, et al. Genotype-phenotype correlation in a new fabry-disease-causing mutation. Medicina (Kaunas) 2019;55:122. doi: 10.3390/medicina55050122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishino T, Obata Y, Furusu A, Hirose M, Shinzato K, Hattori K, et al. Identification of a novel mutation and prevalence study for fabry disease in Japanese dialysis patients. Ren Fail. 2012;34:566–570. doi: 10.3109/0886022X.2012.669300. [DOI] [PubMed] [Google Scholar]
- 5.Peng H, Xu X, Zhang L, Zhang X, Peng H, Zheng Y, et al. GLA variation pE66Q identified as the genetic etiology of Fabry disease using exome sequencing. Gene. 2016;575:363–367. doi: 10.1016/j.gene.2015.09.088. [DOI] [PubMed] [Google Scholar]
- 6.Nakao S, Takenaka T, Maeda M, Kodama C, Tanaka A, Tahara M, et al. An late-onset variant of Fabry’s disease in men with left ventricular hypertrophy. N Engl J Med. 1995;333:288–293. doi: 10.1056/NEJM199508033330504. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto S, Nagasawa T, Sugimura K, Kanno A, Tatebe S, Aoki T, et al. Clinical diversity in patients with anderson-fabry disease with the R301Q mutation. Intern Med. 2019;58:603–607. doi: 10.2169/internalmedicine.0959-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mignani R, Moschella M, Cenacchi GA, Donati I, Flachi M, Grimaldi D, et al. Different renal phenotypes in related adult males with Fabry disease with the same classic genotype. Mol Genet Genomic Med. 2017;5:438–442. doi: 10.1002/mgg3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cammarata G, Fatuzzo P, Rodolico MS, Colomba P, Sicurella L, Iemolo F, et al. High variability of fabry disease manifestations in an extended italian family. Biomed Res Int. 2015;2015:504784. doi: 10.1155/2015/504784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rigoldi M, Concolino D, Morrone A, Pieruzzi F, Ravaglia R, Furlan F, et al. Intrafamilial phenotypic variability in four families with anderson-fabry disease. Clin Genet. 2014;86:258–263. doi: 10.1111/cge.12261. [DOI] [PubMed] [Google Scholar]
- 11.Saito A, Kimura T, Takeuchi Y, Matsuda K, Fukami H, Sato H, et al. A case of rapid progression of Fabry nephropathy with remarkable glomerulomegaly: a case report and mini literature review of weak response to enzyme replacement therapy. Renal Replacement Ther. 2016;2:69–78. doi: 10.1186/s41100-016-0081-8. [DOI] [Google Scholar]
- 12.Politei J, Schenone AB, Cabrera G, Heguilen R, Szlago M. Fabry Disease and Enzyme Replacement Therapy in Classic Patients With Same Mutation: different Formulations-Different Outcome? Clin Genet. 2016;89:88–92. doi: 10.1111/cge.12590. [DOI] [PubMed] [Google Scholar]
- 13.Schiffmann R, Hughes DA, Linthorst GE, Ortiz A, Svarstad E, Warnock DG, et al. Screening, diagnosis, and management of patients with Fabry disease: conclusions from a “kidney disease: improving global outcomes” (KDIGO) controversies conference. Kidney Int. 2017;91:284–293. doi: 10.1016/j.kint.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Anderson JR. The lyon hypothesis. Br Med J. 1963;2:1215–1216. doi: 10.1136/bmj.2.5367.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hassan S, Sidransky E, Tayebi N. The role of epigenetics in lysosomal strage disorder: uncharted territory. Mol Genet Metab. 2017;122:10–18. doi: 10.1016/j.ymgme.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Skrunes R, Tøndel C, Leh S, Larsen KK, Houge G, Davidsen ES, et al. Long-term dose-dependent agalsidase effects on kidney histology in fabry disease. Clin J Am Soc Nephrol. 2017;12:1470–1479. doi: 10.2215/CJN.01820217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abe M, Okada K, Maruyama N, Takashima H, Oikawa O, Soma M. Comparison of clinical trajectories before initiation of renal replacement therapy between diabetic nephropathy and nephrosclerosis on the KDIGO guidelines heat map. J Diabetes Res. 2016;2016:5374746. doi: 10.1155/2016/5374746. [DOI] [PMC free article] [PubMed] [Google Scholar]