Abstract
Convalescent plasma (CP) therapy is rapidly becoming an established consideration in the treatment of COVID-19 patients though there is a need to critically review this area for proof of efficacy. Neutralizing antibodies (NAb) present in CP generated in response to SARS-CoV-2 infection directed against the receptor-binding domain (RBD) of the spike protein are considered to play main role in viral clearance. CP infusion may also help in the modulation of immune response by its immunomodulatory effect. The FDA allows for administration of CP to COVID-19 patients. The present published literature in COVID-19 is limited to case series and randomised controlled trial where plasma therapy was used in moderate, severe and critically ill patients. Though multiple uncertainties exist regarding to its efficacy, appropriate donor selection and NAb titres, the efficacy data of CP use inCOVID-19 is limited having shown hope with early and severe to critically ill COVID-19 patients.
Keywords: Convalescent plasma (CP ), Neutralising antibody (NAbs) , COVID-19
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) originating in China is now a pandemic of unprecedented proportions. While no single agent has stood out as a specific therapeutic strategy, a combination of antivirals, steroids and tocilizumab has gained the confidence of the medical fraternity in mitigating the morbidity from the disease. Strategies beyond these are still needed in a proportion of patients to mitigate mortality or the need for prolonged intensive care. Convalescent plasma (CP) therapy is rapidly becoming an established consideration in the treatment of COVID-19 patients. There is need to critically review this area for proof of efficacy.
Plasma collected from healthy, voluntary donors who have recovered from a recent infection contains pathogen-specific antibodies with neutralising activity in varied concentrations and plasma with high titre of these neutralising antibodies is expected to provide immediate passive short-term immunisation. Though the exact mechanism of how these antibodies in plasma clear the viral load is still not clear, multiple mechanisms including antiviral and immunomodulatory actions have been hypothesized.
Antiviral action of neutralising antibodies in the CP
The spike protein is responsible for entry of the virus into the cell. Neutralising antibodies (NAb) generated in response to SARS-CoV-2 infection are directed against the receptor-binding domain (RBD) of the spike protein. Plasma of mice immunised with the RBD of SARS-CoV are also able to inhibit the entry of SARS-CoV-2 into Vero 6 cells [1]. The neutralising ability of plasma for SARS-CoV-2 is measured by the ability of its highest dilution to reduce 50% of viral plaques in culture compared to control plasma [2]. This is correlated with the neutralising antibody titre measured by a sandwich ELISA and a titre of 1:80 in plasma correlates with a titre of 1:1280 for the S-RBD IgG measured by ELISA [3].
Salazar et al. studied the relationship between two antibodies, the anti-spike ectodomain (ECD) and the anti-RBD IgG titres, and SARS-CoV-2 virus neutralisation (VN) titres using convalescent plasma samples obtained from 68 COVID-19 patients. There was strong positive correlation between plasma anti-RBD and anti-ECD IgG titres, and in vitro VN titre. Anti-RBD plasma IgG correlated slightly better than anti-ECD IgG titre with VN titre. Anti-RBD or anti-ECD titres of ≥ 1:1350 were most often associated with a probability of a VN titre ≥ 160.
Plasma samples from 63% (43/68) of patients had a VN titre of ≥ 1:160, and the FDA recommended VN antibody titre in convalescent plasma considered therapeutic. Thirty-seven percent (25/68) of convalescent plasma donors lacked adequate VN titres. Plasma from patients with dyspnoea, hospitalization and severe disease had significantly higher VN titre. Frequent donation of convalescent plasma did not significantly decrease either VN or IgG titres [4].
Shen et al. showed that COVID-19 convalescent donors had SARS-CoV-2–specific ELISA antibody titres ranging between 1800 and 16,200 and NAb titres between 80 and 480. The plasma obtained from the donors and transfused to the recipients at the same day led to decreased viral load and time-dependent increase in titres of IgG and IgM in the recipients [5].
In one study which used pseudotyped-lentiviral-vector-based neutralisation assay to measure specific NAbs in CP of SARS-CoV-2, the patients demonstrated variations in NAb titres with approximately 30% of patients not developing high NAb titres after infection [6]. In another study, a small number of even asymptomatic individuals were also found to have high NAb (> 1000). Recent studies have proved that IgG titres tend to remain high over a period of 3 months [7, 8].
In plasma, in addition to NAb, there are non-NAb that bind to the virus, but do not affect its capacity to replicate and might contribute to prophylaxis and/or recovery improvement [9].
CP immunomodulatory action
While it is hoped that the S-RBD IgG in convalescent serum because of its ability to neutralise viral entry into cells may lead to decrease in viral replication within the body, it is also well known by now that the pathophysiology of COVID-19 is more due to the inflammation triggered by the virus rather than the tissue cytopathic effect of the virus.
In SARS-CoV/macaque models, anti-spike IgG (S-IgG), in infected lungs, caused severe ALI by reducing the inflammation-resolving response. Patients who eventually died of SARS displayed presence of pulmonary pro-inflammatory macrophages, lower numbers of anti-inflammatory macrophages and higher and earlier anti-spike antibodies during infection than those who survived. Their sera enhanced SARS-CoV–induced MCP1 and IL-8 production by human monocyte–derived wound-healing macrophages, whereas blockade of FcγR reduced such effects [10]. During the SARS outbreak in Hong Kong, the development of ARDS coincided with IgG seroconversion [11]. The anti-spike NAb response developed significantly faster after the onset of clinical symptoms in deceased patients compared with recovered patients [12]. In recovered patients, it took an average of 20 days to reach their peak of NAb activities, as opposed to only 14.7 days for the deceased patients. The actual NAb titre was significantly higher in deceased patients compared to recovered patients during the same time period. These findings suggest a role of anti-S antibodies in SARS-CoV–mediated ALI during acute infection. Consistently, pre-existing serum antibodies against influenza antigens were found to associate with worse clinical severity and poor outcomes in patients during the 2009 influenza pandemic [13, 14].
Intranasal infection of SARS-CoV in C57BL/6J mouse model results in high virus replication within the lung, induction of inflammatory cytokines and chemokines and immune cell infiltration. Using this model, complement activation cascade was observed in the lung as early as day 1 following SARS-CoV infection. SARS-CoV-infected C3–/– mice exhibited significantly less weight loss and less respiratory dysfunction despite equivalent viral loads in the lung, along with fewer immune cells compared to C57BL/6J control mice. Lower cytokine and chemokine levels were present in the lungs of C3–/– mice than in C56BL/6J controls. These results suggest that complement activation largely contributes to systemic inflammation and migration of neutrophils to the lungs, perpetuating tissue damage [15]. Studies have shown that IgG transferred by plasma neutralise cytokines such as IL-1β and TNFα [16]. In this sense, passive immunity by infusion of CP-COVID-19 may limit the inflammatory cascade driven by pathogenic antibodies, as well as the cellular damage induced by the complement cascade activation in excessive inflammatory environments.
In acute viral infection, resident tissue macrophages are mobilised and polarised to a pro-inflammatory state which helps in viral clearance. As infection subsides, the anti-inflammatory prototype of macrophages is generated which promotes wound healing. The major immunological factor associated with inflammation and lung damage in COVID-19 is macrophage activation with migration to lung tissues [17]. Thereby, inhibition of macrophage activation pathway may help to control excessive cytokine production and prevent pulmonary damage (i.e. fibrosis). The beneficial effects of intravenous immunoglobulin in various autoimmune diseases arise partly from its action on dendritic cells [18]. IgG-induced Fcγ receptor activation induces upregulation of FCγRIIB which triggers inhibitory effects on lymphocytes. Therefore, CP infusion may help the modulation of immune response via Fcγ receptors.
CP efficacy in previous outbreaks
Plasma therapy is not a new idea and has been used effectively in the past to treat infections particularly in previous viral epidemics. Spanish flu pandemic ( 1918-1920) was the first viral infection where CP was found effective in clinical studies. It was effectively used as preventive therapy in measles outbreak (1934) and also proved its therapeutic efficacy in annual epidemics of Argentine haemorrhagic fever (1959-1983).
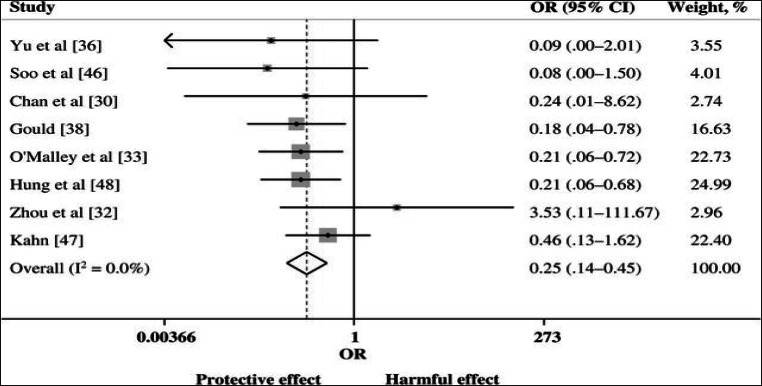
During the 2009 pandemic of influenza A H1N1 (H1N1pdm09), Hung and colleagues [19] analysed a prospective cohort of 93 patients who had clinical deterioration despite optimal antiviral treatment and required intensive care within 7 days of symptom onset. Twenty-one of these received CP treatment. Those who refused consent or were ineligible for non-influenza reasons were used as controls. Convalescent plasma with neutralising antibody titre of > 1:160 was used to treat patients. Mortality in the treatment group was significantly lower than in the nontreatment group (20.0% vs. 54.8%; p = .01). In multivariate analysis, CPT reduced mortality (odds ratio (OR) 0.20; 95% confidence interval (CI) = 0.06-.69; p = 0.011). In 44 of these patients who had estimation of serial respiratory tract viral load and cytokine levels, CP therapy was significantly associated with lower viral loads at days 3, 5 and 7 compared to controls (p < .05). The corresponding temporal levels of interleukin 6, interleukin 10 and TNF were also significantly lower in the treatment group. A meta-analysis by Mair-Jenkins J et al. [20] of 32 studies in SARS coronavirus and severe influenza showed a statistically significant reduction in mortality, reduced length of hospital stay and critical care support, decreased viral load and increased antibody level with no significant adverse effects following CP therapy compared with placebo. Another post hoc meta-analysis evaluated pooled data from 8 comparative studies: 2 studies on SARS-CoV infection, 2 on influenza A (H1N1pdm09), 1 on avian influenza A (H5N1) and 3 on Spanish influenza A (H1N1) and showed a significantly lower risk of mortality in the group treated with CP (pooled OR 0.25; 95% CI 0.14 to 0.45; p < .001; I2 = 0%) (Fig. 1).
Fig. 1.
Forest plot of pooled odds ratios (ORs) of mortality following treatment with CP in different viral infections [20]
In the West African Ebola virus disease (EVD) outbreak (2013-2016), the use of CP was recommended as an empirical treatment by the WHO under Blood Regulators Network [21]. Sahr et al. studied CP in 44 patients with Ebola infection, and showed decreased mortality rate in the CP-treated group compared to the control group (27.9% vs. 44%, respectively) (OR 2.3, 95% CI 0.8–6.5) [22].
A protocol for treatment of Middle East respiratory syndrome (MERS) coronavirus with CP was approved in June 2014 by the Ministry of the National Guard Health Affairs Institutional Review Board (IRB) for critically ill patients (in Saudi Arabia) [23]. It was found to be an attractive therapeutic option being biologically plausible, easy to obtain and administer, relatively inexpensive and almost safe.
Perceived need for CP therapy in COVID-19
The management of the current COVID-19 pandemic has mainly focused on infection prevention, case detection, monitoring and supportive care. Antiviral therapies in initial phase of viremia followed by the use of steroids and IL-6 blockers in the phase of hyperinflammation or cytokine storm are becoming well-established standard of care despite lack of high-quality evidence, based on expert opinion and perceived success. Hence, CP from patients who have recovered from viral infections is also being fiercely considered a treatment option since it is without significant safety concerns even though well-designed clinical trials are not yet available.
FDA guidelines
The FDA [24] allows for administration of CP to COVID-19 patients through three pathways. First is the Emergency Use Authorization (EUA) of CP for the treatment of patients with COVID-19. Health care providers (HCP) must maintain records and conduct a thorough investigation of adverse reactions after transfusion of COVID-19 convalescent plasma, and must report fatalities to the FDA. Second is the expanded access protocol for use of investigational CP for patients with serious or immediately life-threatening COVID-19 disease who are not eligible or who are unable to participate in randomised clinical trials. Third is through clinical trials. HCPs are encouraged to enrol patients in those trials and complete clinical trials to fully answer the questions about the effectiveness of convalescent plasma for the treatment of COVID-19.
The Indian Council of Medical Research (ICMR) has also published potential donor and recipient criteria for CP therapy. Potential donors include those between 18 and 60 years with body weight > 50 kg, COVID-19 RT-PCR or RAG positive with 14 days of symptom resolution (testing negative for COVID-19 is not necessary) and IgG antibody against COVID-19 titre of 1:640 (ELISA) or 13 AU (arbitrary unit)/ml9 (CLIA) or neutralising antibody (NAbs) titres of 1:80 (PRNT/MNT). Potential recipient criteria include patients with early stage of COVID-19 disease, 3-7 days from onset of symptoms, but not later than 10 days and no IgG antibody against COVID-19 [25].
Evidence of CP use in COVID-19
The present published literature in COVID-19 is limited to case series and randomised controlled trial where plasma therapy was used in moderate, severe and critically ill patients.
Search methodology
We used the mesh words like CP therapy, COVID-19 for searching purpose and searched the MEDLINE, Embase, PubMed for recently conducted case series, RCTs and ongoing trials, Cochrane COVID-19 Study Register, Centers for Disease Control and Prevention COVID-19 Research Article Database. Studies evaluating effectiveness of CP for people with moderate to severe COVID-19 are included, irrespective of study design, disease severity, age, gender or ethnicity.
Case series
Table 1 summarises the case series examining the use of CP therapy in the setting of SARS-CoV-2 critically ill patients
Table 1.
Case series on CP use in COVID-19
| Author | Shen et al. [5] | Duan et al. [2] | Zhang et al. [26] |
|---|---|---|---|
| No. of cases/controls | 5/0 | 10/10 | 4/0 |
| Disease status | Critically ill patient | Critically ill patient | Critically ill patient |
| Day of CP therapy given from symptom onset | 10-12 | 16.5 | 15.5 |
| Volume transfused | 400 ml, single dose | 200 ml, single dose |
900 ml in 3 divided doses 8 doses (2400 ml) 200 and 300 ml, single dose |
| Antibody titre in donor | SARS-CoV-2–specific antibody titre > 1:1000 and neutralising antibody titre > 1: 40 | Neutralising antibody titre > 1:640 | Not measured |
| Clinical outcome |
Body temperature normalized within 3 days in 4/5patients SOFA score decreased PaO2/FiO2 increased within 12 days ARDS resolved in 4 patients at 12 days |
Clinically improved within 3 days Increase in O2 saturation within 3 days Trend in increased lymphocyte counts and decreased CRP Imaging showed varying degrees of resolution of lung lesions within 7 days |
Imaging showed resolution or partial absorption, of lung lesions Discharged between days 18 and 43(3/4) Remained hospitalized with multiorgan failure (1/4) |
| Post transfusion viral load and passive NAb titre in recipient |
Viral load negative by 12 days Antibody titre increased to 80-320 by D7 (from 40 to 60) |
Serum Nabs in 9 patients – 1:640 RNA viral load (CT method) –negative |
Viral RT-PCR negative for all (by 3-22 days) |
| Adverse events | None | None | None |
Randomised clinical trials
In the RCT reported by Li et al. [3], 101 patients with severe COVID-19 received convalescent plasma with a NAb (S-RBD) titre of at least 1:640. They found no difference in time to clinical improvement measured at 28 days. There is a possibility that this may be a type-2 error, where no benefit was found despite there being one, due to low sample size and due to early termination of the trial. There was some clinical improvement in severely but not critically ill patients. The lack of efficacy of CP among critically ill patients receiving mechanical ventilation with multiorgan failure highlights that the pathologic process in these individuals becomes irreversible.
Early therapy
Administration of plasma therapy before SARS-CoV-2 seroconversion may be critical. In viremia peaks in the first week of infection and by days 10-14, the patient usually develops primary immune response followed by viral clearance [27]. Early administration of CP containing polyclonal neutralising Abs may assist in the inhibition of viral entry and replication and consequently blunt an early pro-inflammatory pathogenic endogenous antibody response. Based on the most recent data available, initiating treatment no later than day 5 may be the most appropriate.
Joyner et al. in their multicentre study included 35,322 hospitalized COVID-19 patients with (or at risk of) severe or life-threatening acute COVID-19 respiratory syndrome who had received at least one unit of convalescent plasma transfusion during hospitalization. Seven and 30-day mortality post CP transfusion was analysed. The 7-day mortality rate was 8.7% [95% CI 8.3-9.2] in patients transfused within 3 days of diagnosis but 11.9% [11.4-12.2] in patients transfused beyond 4 days after diagnosis. Patients who received CP with high antibody titres had significantly reduced mortality compared to those transfused with CP with medium/low antibody levels. The pooled relative risk of mortality among patients transfused with high antibody level plasma units was 0.65 [0.47-0.92] for 7 days and 0.77 [0.63-0.94] for 30 days compared to low antibody level plasma units [28].
In a recently conducted multicentre PlasmAR trial [29] which excluded patients with mild to moderate cases, 228 severe COVID-19 patients received CP therapy and 105 received placebo. At day 30, no significant difference was noted between the groups in the distribution of clinical outcomes according to the ordinal scale (odds ratio, 0.83 (95% confidence interval (CI), 0.52 to 1.35; p = 0.46)).
In the recently concluded multicentre PLACID trial [30] conducted in India by ICMR which included 464 adults with confirmed moderate COVID-19, 235 were assigned to CP (two doses of 200 ml plasma) with available best standard of care therapy, while 229 received only the latter. Progression to severe disease or all-cause mortality at 28 days after enrollment occurred in 44 (19%) participants in the intervention arm and 41 (18%) in the control arm (risk ratio 1.04, 95% confidence interval 0.71 to 1.54). However, priori measurement of NAb titres was not done in donors and participants in this study.
Ongoing randomised clinical trials
Many randomised clinical trials have been started throughout the world (Table 2). Many of them are in status of recruiting participants, some are yet to start recruitment and few are in the phase of enrolling by invitation. There are around 138 ongoing studies on CP therapy in COVID-19, of which 73 are RCTs [31].
Table 2.
A brief summary of large randomised clinical trials actively recruiting patients for CP treatment in COVID-19
| Sponsor | Participants | Population | Control | Primary outcome |
|---|---|---|---|---|
| Cristina AvendañoSo (Con-Plas 19 trial) | 278 | Hospitalized patients without mechanical ventilation | Hospitalized with standard of care treatment | Category changes in ordinal scale—proportion of patients in categories 5,6 or 7 of the 7-point ordinal scale at day 15. |
| Erasmus Medical Center(CoV-Early Trial) | 690 | 300 ml CP with a minimum of neutralising antibodies | 300 ml fresh frozen plasma | Highest disease status on the 5-point ordinal disease severity scale in the CP group will be compared with the FFP group. |
| Institute of Liver and Biliary Sciences, India | 400 | Two doses of CP + standard of care to severely sick COVID-19 patients | Standard of care treatment | Efficacy of CP in time to clinical improvement (clinical improvement: reduction of two points in ordinal scale or live discharge from the intensive care unit, whichever is earlier) (time frame: day 28) |
| Johns Hopkins University | 150 | Asymptomatic, with negative PCR test and high-risk exposure and higher risk for severe illness | Asymptomatic high-risk control treated with standard plasma |
1. Death 2. Requiring mechanical ventilation and/or in ICU 3. Non-ICU hospitalization, requiring supplemental oxygen 4. Non-ICU hospitalization, not requiring supplemental oxygen 5. Not hospitalized, but with clinical and laboratory evidence of COVID-19 infection 6. Not hospitalized, no clinical evidence of COVID-19 infection, but positive PCR for SARS-CoV-2 |
Meta-analysis
A recently updated Cochrane review on the effectiveness of CP on COVID-19 by Khai Li Chai et al. included 19 studies (2 RCTs, 8 controlled non randomised studies on intervention (NRSIs), 9 non-controlled NRSIs) with 38,160 participants, of whom 36,081 received CP. CP therapy might result in little to no difference in improvement of clinical symptoms (i.e. need for respiratory support) at 7 days but increase improvement of clinical symptoms at up to 15 days and at up to 30 days. It was uncertain whether CP decreases all-cause mortality at hospital discharge and time to event mortality. No study reported the outcome of quality of life [31].
Stephen A et al. did a systematic review on CP therapy in COVID-19 based on eighteen clinical trials including five RCTs, thirteen matched-control studies and twenty case series or case reports containing 10,436 COVID-19 patients. Aggregation of mortality data from all clinical trials including RCTs and matched-control trials indicated that patients transfused with CP exhibited a 51% reduction in mortality rate compared to patients receiving standard treatments (19% vs. 29% mortality; OR: 0.49, 95% CI: 0.37, 0.64, p < 0.001, I2 = 53%). These results favour the efficacy of CP as a therapeutic tool in COVID-19 [32].
In a meta-analysis of current medical treatment approach for COVID-19 published by Wang M et al., CP treatment showed to decrease the risk of mortality of patients with severe COVID-19 (RR = 0.65; 95% CI 0.42 to 1.02). The pooled result also showed that there was a higher viral nucleic acid negative rate in patients treated with CP therapy (RR = 2.47; 95% CI 1.70 to 3.57) [33].
Uncertainties
There are still numerous uncertainties regarding the CP therapy.
Pathogen related: Effectiveness of CP may vary with type of pathogen. The SARS-CoV-2 viral replication kinetics and host interactions are yet to be fully established.
Assays to determine viral neutralising antibody titres: They are not widely available, in part because they are labour intensive, cumbersome and require a biosafety level-3 laboratory if live virus is used. This may hamper identification of ideal donors.
Titre of neutralising antibodies in CP which will prove protective: Titres of protective neutralising antibody and kinetics of neutralising antibodies in infected COVID-19 patients over time are not well established hence precluding knowledge about the optimal time for plasma collection. Since asymptomatic COVID-19 patients have lower antibody titres which last for transient periods, uncertainty exists whether only symptomatic-recovered patients should be selected for plasma collection. A pseudotyped-lentiviral-vector-based neutralisation assay to measure specific NAbs in plasma from recovered patients with SARS-CoV-2 showed variations in NAb titres, where approximately 30% of patients did not develop high NAb titres after infection [6].
Antibody-dependent enhancement (ADE) is a mechanism in which the intensity of infection increases in the presence of pre-existing NAbs with poor neutralising ability, favouring the replication of virus into macrophages and other cells through interaction with Fc and/or complement receptors. In vitro assays with human promonocyte cell lines demonstrated that SARS-CoV ADE was primarily mediated by antibodies against spike proteins, significantly increasing the rate of apoptosis in these cells [34, 35].
Technical issues: Many obstacles may preclude successful donations, among which, failure to meet blood donation eligibility criteria, failed laboratory tests and inability to make the apheresis appointment are important. Availability of well-developed apheresis machines with safe apheresis practice is also limited in resource poor countries. Ideally after collection, plasma should be subjected to pathogen reduction treatment to reduce or eliminate detectable infectious organisms, including bacteria, viruses and parasites. But availability of such inactivation procedure is also scarce in resource-constrained laboratories [36].
Conclusion
CP therapy will still be considered for use in severe and critically ill patients based on evidence from systematic review and meta-analysis with resolution of clinical symptoms at early CP therapy, mortality benefit and higher viral clearance. However, randomised controlled trials of CP therapy with well-defined control groups still needed to have sufficient evidence of efficacy. Uncertainty exists regarding neutralising antibody testing assays, ideal titres and antibody-dependent enhancement mechanisms affecting the efficacy of CP therapy. Safety data of CP use in different viral pandemics in the past and recent data in COVID-19 have shown minimal adverse effects. However, CP will still be considered a therapeutic option for early course of severe or critical disease in this period until the goal of mass vaccination is achieved.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. P Natl Acad Sci Usa. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. Jama. 2020;324(2). [DOI] [PMC free article] [PubMed]
- 4.Salazar E, Kuchipudi SV, Christensen PA, Eagar TN, Yi X, Zhao P, et al. Relationship between anti-spike protein antibody titers and SARS-CoV-2 in vitro virus neutralization in convalescent plasma. BioRxiv. 2020;138990.
- 5.Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. Jama. 2020;323(16):1582–9. [DOI] [PMC free article] [PubMed]
- 6.Wu F, Wang A, Liu M, Wang Q, Chen J, Xia S, et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. MedRxiv. 2020.
- 7.Seow J, Graham C, Merrick B, Acors S, Steel KJ, Hemmings O, et al. Longitudinal evaluation and decline of antibody responses in SARS-CoV-2 infection. medRxiv. 2020.
- 8.Wajnberg A, Amanat F, Firpo A, Altman D, Bailey M, Mansour M, et al. SARS-CoV-2 infection induces robust, neutralizing antibody responses that are stable for at least three months. medRxiv. 2020.
- 9.Bloch EM, Shoham S, Casadevall A, Sachais BS, Shaz B, Winters JL, et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020;130:2757–65. [DOI] [PMC free article] [PubMed]
- 10.Liu L, Wei Q, Lin Q, et al. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 2019;4(4):e123158. doi: 10.1172/jci.insight.123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361(9371):1767–72. [DOI] [PMC free article] [PubMed]
- 12.Zhang L, et al. Antibody responses against SARS coronavirus are correlated with disease outcome of infected individuals. J Med Virol. 2005;78:1–8. doi: 10.1002/jmv.20499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.To KKW, Zhang AJX, Hung IFN, Xu T, Ip WCT, Wong RTY, et al. High titer and avidity of non-neutralizing antibodies against influenza vaccine antigen are associated with severe influenza. Clin Vaccine Immunol. 2012;19(7):1012–1018. doi: 10.1128/CVI.00081-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monsalve AC, Bataller JP, Lopez MF, et al. Severe pandemic 2009 H1N1 influenza disease due to pathogenic immune complexes. Nat Med. 2011;17(2):195–199. doi: 10.1038/nm.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gralinski LE, Sheahan TP, Morrison TE, Menachery VD, Jensen K, Leist SR, et al. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. Mbio. 2018;9(5):e01753–818. [DOI] [PMC free article] [PubMed]
- 16.Abe Y, Horiuchi A, Miyake M, Kimura S. Anti-cytokine nature of natural human immunoglobulin: one possible mechanism of the clinical effect of intravenous immunoglobulin therapy. Immunol Rev. 1994;139:5–19. doi: 10.1111/j.1600-065X.1994.tb00854.x. [DOI] [PubMed] [Google Scholar]
- 17.McGonagle D, Sharif K, O’Regan A, Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19(6):102537. [DOI] [PMC free article] [PubMed]
- 18.Tjon ASW, van Gent R, Jaadar H, Martin van Hagen P, Mancham S, van der Laan LJW, et al. Intravenous immunoglobulin treatment in humans suppresses dendritic cell function via stimulation of IL-4 and IL-13 production. J Immunol. 2014;192:5625–34. [DOI] [PubMed]
- 19.Hung IF, To KK, Lee CK, et al. CP treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52:447–456. doi: 10.1093/cid/ciq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mair-Jenkins J, Saavedra-Campos M, Baillie JK, Cleary P, Khaw FM, Lim WS, et al. The effectiveness of CP and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. [DOI] [PMC free article] [PubMed]
- 21.WHO? Use of convalescent whole blood or plasma collected from patients recovered from Ebola virus disease for transfusion, as an empirical treatment during outbreaks. 2014. http://apps.who.int/iris/rest/bitstreams/604045/retrieve. Accessed Feb 20, 2020.
- 22.Sahr F, Ansumana R. MassaquoiTA, et al. Evaluation of convalescent whole blood for treating Ebola virus disease in Freetown, Sierra Leone. J Inf Secur. 2017;74(3):302–309. doi: 10.1016/j.jinf.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arabi Y, Balkhy H, Hajeer AH. Feasibility, safety, clinical, and laboratory effects of CP therapy for patients with Middle East respiratory syndrome coronavirus infection: a study protocol. Springerplus. 2015;4:709. doi: 10.1186/s40064-015-1490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.USFDA. Investigational COVID-19 convalescent plasma: guidance for industry [Internet] 2020 Available from: https://www.fda.gov/media/13678/download. Accessed 1 Nov 2020.
- 25.ICMR. Evidence based advisory to address inappropriate use of convalescent plasma in COVID-19 patients. ICMR ADVISORY Convalescent plasma.17112020v1%20(1).pdf.
- 26.Zhang B, Liu S, Tan T, et al. Treatment with CP for critically ill patients with severe acute respiratory syndrome coronavirus 2 infection [published online ahead of print, 2020 Mar 31] Chest. 2020;S0012-3692(20):30571–30577. doi: 10.1016/j.chest.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng Y, Wong R, Soo YO, et al. Use of CP therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michael J, Scott Wright R, Fairweather DL, Senefeld J, Bruno K, et al. Early safety indicators of COVID-19 convalescent plasma in 5,000 patients. MedRxiv. 2020;05(12):20099879. [Google Scholar]
- 29.Simonovich VA, Pratx LDB, Scibona P, Beruto MV, Vallone MG, Vázquez C, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. New Engl J Med. 2020. [DOI] [PMC free article] [PubMed]
- 30.Agarwal A, Mukherjee A, Kumar G, et al. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial) BMJ. 2020;371:m3939. doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valk SJ, Piechotta V, Chai KL, Doree C, Monsef I, Wood EM, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID‐19: a rapid review. Cochrane Db Syst Rev. 2020;5(5):CD013600 [DOI] [PMC free article] [PubMed]
- 32.Klassen SA, Senefeld JW, Johnson PW, Carter RE, Wiggins CC, Shoham S, et al. Evidence favoring the efficacy of convalescent plasma for COVID-19 therapy. medRxiv. 2020:07.29.20162917. 10.1101/2020.07.29.20162917.
- 33.Wang M, Wu T, Zuo Z, et al. Evaluation of current medical approaches for COVID-19: a systematic review and meta-analysis. BMJ Support Palliat Care. Published Online First: 21 September 2020. [DOI] [PubMed]
- 34.Kulkarni R. Antibody-dependent enhancement of viral infections BT-dynamics of immune activation in viral diseases. In: Bramhachari PV, editor. Dyn. Immune Act. Viral Dis Singapore. Singapore: Springer; 2020. pp. 9–41. [Google Scholar]
- 35.Wang S-F, Tseng S-P, Yen C-H, Yang J-Y, Tsao C-H, Shen C-W, et al. Antibody dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem Biophys Res Commun. 2014;451:208–214. doi: 10.1016/j.bbrc.2014.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown BL, McCullough J. Treatment for emerging viruses: CP and COVID-19. Transfus Apher Sci. 2020;59(3):102790. doi: 10.1016/j.transci.2020.102790. [DOI] [PMC free article] [PubMed] [Google Scholar]