Abstract
Triple combination therapy is suggested in current pulmonary arterial hypertension guidelines in case of unsatisfactory treatment with oral double combination therapy. However, there is a lack of evidence concerning some of the drug combinations currently employed. We demonstrate the clinical and hemodynamical benefits of inhaled iloprost as third add-on therapy in idiopathic pulmonary arterial hypertension.
Keywords: inhaled iloprost, sequential triple combination therapy, pulmonary arterial hypertension, hemodynamics
Pulmonary arterial hypertension (PAH) is a progressive and life-threatening disease characterized by vascular proliferation and endothelial remodeling that leads to an increase in pulmonary vascular resistance (PVR) and ultimately right heart failure and death.1 Current pharmacological PAH treatment includes drugs from three distinct biochemical pathways: prostacyclin, endothelin, and nitric oxide.2 These drugs can be used in monotherapy or combination, determined by the PAH etiology and the severity of the disease.3 PAH guidelines suggest that treatment should be escalated according to prognostic risk in an attempt of improving symptoms and delaying the progression of the disease.4
Given their pharmacological characteristics, including shorter half-life and administration route, drugs from the prostacyclin pathway are less often used for initial PAH therapy.5 They are more commonly used as add-on therapy if the patient fails to achieve expected therapeutic goals with oral drugs from the other pathways.6 However, data evaluating their efficacy as third add-on therapy are scarce. A subgroup analysis from the GRIPHON trial evaluating the addition of selexipag as a third agent in patients receiving double combination therapy at baseline identified an incremental benefit to improve outcomes for patients with PAH.7 In line with these findings, the use of upfront triple combination therapy, including intravenous epoprostenol in combination with an endothelin receptor antagonist (ERA) and a phosphodiesterase 5 inhibitor (PDE5i), in a severe uncontrolled group of PAH patients significantly improved hemodynamics.8 Data accessing the use of other prostanoids are not as encouraging. The addition of oral treprostinil to background ERA and PDE5i therapy did not result in a statistically significant improvement in exercise capacity in the FREEDOM C2 study.9
Inhaled iloprost is another drug acting on the prostacyclin pathway. It has less severe side effects than other prostanoids10 and presents benefits if used as monotherapy11 or as an add-on to bosentan12 in PAH patients. Nevertheless, to the best of our knowledge, the effect of inhaled iloprost as add-on sequential triple combination therapy is still unknown.
This study aimed to investigate if add-on sequential triple therapy with inhaled iloprost in patients with idiopathic pulmonary arterial hypertension (IPAH) was effective in improving hemodynamics and decreasing patients’ risk of death.
This was a single-center retrospective study. All IPAH were evaluated between 2018 and 2020 and included in the analysis if they fulfilled three criteria:
IPAH diagnosis following current international guidelines.13
Use of double combination therapy (an ERA plus a PDE5i) without achieving low-risk status in European Society of Cardiology (ESC)/European Respiratory Society (ERS) guidelines risk stratification, evaluated as previously suggested.14
Availability of complete right heart catheterization data before and after iloprost treatment.
Clinical assessment for establishing the New York Heart Association functional class (FC), laboratory testing with brain natriuretic peptide (BNP), and the thresholds used to define risk stratification before and after at least 12 weeks of inhaled iloprost initiation were recorded. The study was reviewed and approved by our Internal Review Board (protocol 855475513.3.0000.0068).
The continuous variables were expressed as mean ± SD and the categorical variables as frequency and percentage. Differences between baseline and week 12 were compared with paired Student’s t-test. All statistical tests were considered to be significant with p-values < 0.05.
We identified eight eligible IPAH patients, who formed the population of the study. Patients were predominantly female (n = 7 (87.5%)) and with a mean age of 43.75 ± 10.62 years. About 12.5% of the patients were in FC II, 50% in FC III, and 37.5% in FC IV. Six patients were in intermediate risk and two at high risk, according to the ESC risk stratification. All patients were titrated to receive inhaled iloprost 45 mcg daily, for at least 12 weeks, utilizing an OMRON® nebulizer NE-U22. This device delivered aerosolized particles of 5 µm, at a rate of 0.25 ml/min. For inhalation, iloprost was diluted with saline to a concentration of 5 µg per milliliter, and 2 ml was added to the nebulizer. The patient was oriented to nebulize for 6–8 min and discharge the content of the nebulizer at the end of the inhalation.
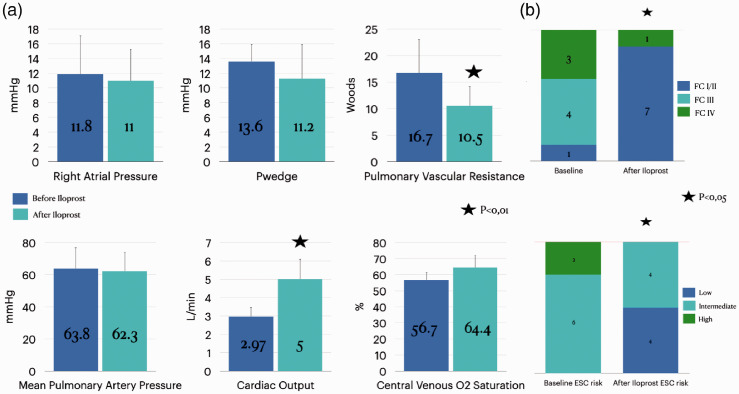
The mean interval between the baseline and follow-up evaluations was 19.4 ± 6 months. Inhaled iloprost add-on to dual combination therapy was associated with a statistically significant improvement in PVR (16.7 ± 6.3 to 10.6 ± 3.7 Woods), cardiac output, evaluated by thermodilution method (2.97 ± 0.53 to 5 ± 1.1 L/min) (Fig. 1a), FC, and ESC risk stratification (Fig. 1b). After the add-on therapy with inhaled iloprost, half of the patients reached the low-risk status. BNP levels were available for five patients and decreased after iloprost introduction (125 ± 107 to 65 ± 43.4 pg/ml), but it did not reach statistical significance (p = 0.153).
Fig. 1.
Effects of add-on sequential therapy with inhaled iloprost to a PDE5i and ERA, for at least 12 weeks. (a) Hemodynamic and BNP data. (b) Functional class and improvement in ESC/ERS risk stratification data.
RAP: right atrial pressure; PAP: pulmonary arterial pressure; Pwedge: Wedge pressure; PVR: pulmonary vascular resistance; BNP: brain natriuretic peptide.
To our knowledge, the present study represents the first systematic evaluation of the benefit of sequential triple therapy with inhaled iloprost in IPAH patients. Our findings show significant improvements in hemodynamics and risk stratification after inhaled iloprost use as third add-on therapy.
Previous reports focusing on sequential add-on triple combination therapy were scarce. A post hoc analysis of the GRIPHON study showed incremental benefit with the addition of selexipag to patients receiving double combination therapy. At the end of the study, there was a reduction in the number of PAH-related hospitalizations and disease progression events, but with no effect on mortality.7 On the other hand, sequential triple therapy with inhaled iloprost had not been evaluated up to now. Aerosolized Iloprost Randomized (AIR) Study11 and Safety and Pilot Efficacy Trial in combination with bosentan for Evaluation in Pulmonary Arterial Hypertension (STEP)12 studies were demonstrated its efficacy and safety for PAH treatment as monotherapy or in combination with bosentan, respectively.
The therapy with inhaled iloprost can be difficult for the patient, due to dose frequency (at least six times/day) and upper airway side effects symptoms. Despite that, in our cohort, there was no treatment failure. Our sample is comparable, in terms of disease severity, with the largest international records, such as the Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension,14 and the Swedish Pulmonary Arterial Hypertension Registry,15 with the majority of PAH patients in the intermediate-risk stratum of severity. Both registries demonstrated that overall survival in PAH patients in the intermediate or the high-risk severity status is unacceptable, and the low-risk status should be pursued. The most evident course of action in this setting is adding a third drug if the PAH patient is already receiving double oral combination therapy and did not reach low-risk status. As such, our data demonstrated that the low-risk status can be achieved in a relevant proportion of patients when iloprost is added as the third drug in sequential combination therapy.
Our study has several limitations that need to be acknowledged. The retrospective study nature, the single-center, and the small sample size are the main ones. However, systematic analysis of hemodynamic data and risk stratification based on the most recent ESC/ERS criteria,6 before and after inhaled iloprost provide useful data, despite the study limitations. Our case series is also similar in FC and hemodynamic parameters to the IPAH population previously described in our registry.16 Therefore, we believe that our case series is representative of an overall IPAH population that may require triple combination therapy.
In conclusion, sequential triple therapy with inhaled iloprost was efficient to improve hemodynamic parameters and risk stratification in a case series of IPAH patients.
Footnotes
Conflict of interest: The author(s) declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs: Caio J.C.D.S. Fernandes https://orcid.org/0000-0002-4912-021X
Rogerio de Souza https://orcid.org/0000-0003-2789-9143
References
- 1.Calderaro D, Alves Junior JL, Fernandes C, et al. Pulmonary hypertension in general cardiology practice. Arq Bras Cardiol 2019; 113: 419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Souza R, Fernandes CJ, Hoeper MM. Carbon monoxide diffusing capacity and the complexity of diagnosis in pulmonary arterial hypertension. Eur Respir J 2014; 43: 963–965. [DOI] [PubMed] [Google Scholar]
- 3.Fernandes CJCDS, Humbert M, Souza R. Challenging the concept of adding more drugs in pulmonary arterial hypertension. Eur Respir J 2017; 50: 1701527. [DOI] [PubMed] [Google Scholar]
- 4.Galie N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J 2019; 53: 1801889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olsson KM, Delcroix M, Ghofrani HA, et al. Anticoagulation and survival in pulmonary arterial hypertension: results from the Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA). Circulation 2014; 129: 57–65. [DOI] [PubMed] [Google Scholar]
- 6.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 7.Coghlan JG, Channick R, Chin K, et al. Targeting the prostacyclin pathway with selexipag in patients with pulmonary arterial hypertension receiving double combination therapy: insights from the randomized controlled GRIPHON study. Am J Cardiovasc Drugs 2018; 18: 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sitbon O, Jais X, Savale L, et al. Upfront triple combination therapy in pulmonary arterial hypertension: a pilot study. Eur Respir J 2014; 43: 1691–1697. [DOI] [PubMed] [Google Scholar]
- 9.Tapson VF, Jing ZC, Xu KF, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients receiving background endothelin receptor antagonist and phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C2 study): a randomized controlled trial. Chest 2013; 144: 952–958. [DOI] [PubMed] [Google Scholar]
- 10.Olschewski H. Inhaled iloprost for the treatment of pulmonary hypertension. Eur Respir Rev 2009; 18: 29–34. [DOI] [PubMed] [Google Scholar]
- 11.Olschewski H, Simonneau G, Galie N, et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med 2002; 347: 322–329. [DOI] [PubMed] [Google Scholar]
- 12.McLaughlin VV, Oudiz RJ, Frost A, et al. Randomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertension. Am J Respir Crit Care Med 2006; 174: 1257–1263. [DOI] [PubMed] [Google Scholar]
- 13.Frost A, Badesch D, Gibbs JSR, et al. Diagnosis of pulmonary hypertension. Eur Respir J 2019; 53: 1801904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoeper MM, Kramer T, Pan Z, et al. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J 2017; 50: 1700740. [DOI] [PubMed] [Google Scholar]
- 15.Kylhammar D, Kjellstrom B, Hjalmarsson C, et al. A comprehensive risk stratification at early follow-up determines prognosis in pulmonary arterial hypertension. Eur Heart J 2017; 39: 4175–4181. [DOI] [PubMed] [Google Scholar]
- 16.Alves JL, Gavilanes F, Jardim C, et al. Pulmonary arterial hypertension in the southern hemisphere: results from a registry of incident Brazilian cases. Chest 2015; 147: 495–501. [DOI] [PubMed] [Google Scholar]