Abstract
Macitentan is a safe and effective substance for treatment of adults with pulmonary arterial hypertension. Data on its use in paediatric patients are limited. In this single-centre prospective study, we report on our experience with macitentan in children focusing on applicability and practical aspects. Between December 2014 and July 2018, macitentan was introduced to paediatric patients according to a dosing protocol adjusted to body weight. Blood pressure, heart rate, saturation and clinical symptoms were recorded daily during introduction. Liver function parameters and haemoglobin levels were measured at baseline, four weeks and three months after initiation and after one year of treatment. Twenty-four patients (14 male, 10 female) were enrolled for treatment with macitentan. The mean age was 10.7 ± 7.6 years (range: 0.1 year–23 years). Fifteen out of 24 patients were World Health Organization functional class (FC) II, 7 patients in FC III and 2 patients in FC IV. Twenty out of 24 patients (83%) received additional advanced therapy with sildenafil and/or prostacyclines. We had two early discontinuations because of clinical relevant oedema. In the remaining 22 patients, macitentan was well tolerated. Liver function parameters and blood count levels remained stable during the observational time. The introduction of macitentan was feasible and mostly well tolerated in paediatric patients. Special attention should be paid to oedema during introduction of the drug. To the best of our knowledge, this is the first study to report on its applicability in infants and children. However, larger prospective trials are warranted to verify these preliminary findings.
Keywords: pulmonary arterial hypertension, paediatric, oedema, endothelin receptor antagonist, infants
Introduction
Pulmonary hypertensive vascular disease (PHVD) is a severe condition characterized by a progressive increase of pressure or vascular resistance leading to chronic right heart failure. In infancy and childhood, this condition can present isolated as idiopathic pulmonary arterial hypertension (iPAH) or as a consequence or part of many underlying diseases such as lung hypoplasia, complex heart defects and other conditions.1–7
Unlike adults, infants and children often present with markedly elevated pressures at diagnosis.8 Depending on the pathophysiological background in childhood, PHVD has variable outcomes with sometimes very poor prognosis. Therefore, in many cases, early initiation of medical therapy will be necessary.9–11
The main target of pharmacological treatment is to keep right ventricular (RV) function preserved by continuously lowering pulmonary artery (PA) pressure and/or resistance. Medical management is generally based on targeted therapies consisting of phosphodiesterase inhibitors (PDE-I), endothelin receptor antagonists (ERA) and prostacyclines (PGI) or its receptor agonists in addition to other supportive treatment.1,12
By reason of rapid disease progression in children with markedly elevated PA pressures, we often initiate early upfront combination therapy within the first days after diagnosis or rapid escalation of treatment during the following weeks.
Recently, macitentan, a novel ERA, has been shown to exert beneficial effects with better tissue properties and less hepatic side effects than its precursor bosentan.13–15 A huge multicentre study has proven its tolerability and favourable effects in adult PAH patients.16 Experience on its use in children is scarce.17,18 This issue is actually faced by a large randomized multicentre open label study (TOMORROW study AC-055-312), which hopefully will lead to license of this drug in childhood. However, in many cases, severity of the disease forces paediatricians to implement medications without approval, before escalating therapy to more invasive therapeutical options such as intravenous or subcutaneous prostacyclines, Potts shunt or lung transplantation.
Macitentan was approved for the treatment of adult PAH and chronic thromboembolic pulmonary hypertension (CTEPH) in 2013. Since then, we started to use this substance in an off-label manner on a compassionate base in children with PAH. The aim of this paper is to report on our first experience with macitentan in children.
Methods
Study design
This is a single-centre observational study conducted from December 2014 to July 2018, at the department of Paediatric Cardiology at the Medical University of Vienna. Macitentan was used as compassionate therapy in 24 paediatric patients with PHVD. Oral informed consent was obtained from all patients and their respective caregivers before treatment initiation. The study was approved by the local ethics committee (Local ethics Nr: 1619/2018)
Study endpoints
Primary endpoint of the study was safety and feasibility of treatment with macitentan. Secondary endpoints were the documentation of clinical side effects as well as laboratory abnormalities such as change in liver function parameter or changes in blood cell count.
Patients and inclusion/exclusion criteria
Patients diagnosed with PHVD based on measurements of transthoracic echocardiography and/or invasive catheterization were included. Classification of pulmonary hypertension (PH) was based on the consensus paper reported by the paeditatric taskforce of the Pulmonary vascular Research Institute (Panama Paper) and the updated classification proposed by the 6th World Symposium on Pulmonary Hypertension.19 Neonates (<1 month of age) and adults as well as patients with postcapillary PH were excluded from the study.
Side effects/safety
Blood pressure, oxygen saturation and heart rate were recorded in every patient before initiation of treatment, during uptitration, after four weeks and every three months after achieving the target dose. A significant drop of blood pressure was defined as a drop of >15% of systolic value and >10% of diastolic value compared to baseline.
Clinical side effects described in adults, such as cough, headache, nausea, vomiting, peripheral oedema, nasopharyngitis and bronchitis, were recorded daily during introduction and at each follow-up visit.
Laboratory values, such as liver function parameters (serum glutamic oxaloacetic transaminase (SGOT), serum glutamate-pyruvate transaminase (SGPT), gamma-glutamyltransferase (GGT), alkaline phosphatase (AP)), kidney parameters (creatinine (Crea) and uric acid (UA)) and blood cell count (hemoglobin (Hb), hematocrit (Hkt), white blood cells (WBC) and platelets (PLT)) were measured before, four weeks after initiation of treatment and subsequently every three months on the targeted dosage of the drug.
Treatment
Since no established paediatric protocols for dosing are reported, different starting doses depending on body weight (1 mg, 3 mg, 5 mg, 7.5 mg, 10 mg) were applied as single daily dose (Table 1).
Table1.
Dosing regimen according to body weight at initiation, after three months and after one year of treatment.
| Weight range (kg) | Age (years) | Weight (kg) | Starting dose (mg) | Target (mg) dose after 3 months | Dose (mg/kg) after 3 months | Dose (mg) after 1 year |
|---|---|---|---|---|---|---|
| 0–10 | 7.5 | 1 | 3 | 0.4 | 5 | |
| 0.8 | 7.5 | 1 | 3 | 0.4 | 5 | |
| 6 | 1 | Discontinued | 0.5 | Discontinued | ||
| 6.3 | 1 | 3 | 0.5 | 5 | ||
| 3.1 | 1 | 2 | 0.64 | 3 | ||
| 8.6 | 1 | 3 | 0.33 | 5 | ||
| 10–15 | 13 | 3 | 5 | 0.38 | 7.5 | |
| 14 | 3 | 5 | 0.35 | 7.5 | ||
| 15–20 | 15 | 3 | 7.5 | 0.33 | 10 | |
| >20 | 22 | 5 | 7.5 | 0.34 | 10 | |
| 22 | 5 | 7.5 | 0.34 | 10 | ||
| 26 | 5 | 7.5 | 0.28 | 10 | ||
| 28 | 5 | Discontinued | 0.26 | Discontinued | ||
| 32 | 5 | 7.5 | 0.23 | 10 | ||
| >35 | 39 | 5 | 10 | 0.26 | 10 | |
| 44 | 5 | 10 | 0.22 | 10 | ||
| 41 | 5 | 10 | 0.24 | 10 | ||
| 35 | 5 | 10 | 0.28 | 10 | ||
| 60 | 5 | 10 | 0.16 | 10 | ||
| 83 | 5 | 10 | 0.12 | 10 | ||
| 76 | 5 | 10 | 0.13 | 10 | ||
| 50 | 5 | 10 | 0.2 | 10 | ||
| 44 | 5 | 10 | 0.22 | 10 | ||
| 86 | 5 | 10 | 0.11 | 10 | ||
| 47 | 5 | 10 | 0.21 | 10 |
For dose adjustment in children with lower body weight, we used macitentan capsules containing lactose monohydrate. Older patients requiring higher doses received the original macitentan tablets (10 mg). Patients were uptitrated to target doses according to weight within a maximum of one week.
Infants with a body weight below 10 kg received a starting dose of 1 mg of macitentan, which was increased daily in 1 mg steps up to a maximum dose of 3 mg per day or a decrease of blood pressure.
Children with a body weight between 10 kg and 15 kg started with 3 mg of macitentan to reach a target dose of 5 mg (daily uptitration mode: 3 mg – 4 mg – 5 mg).
In children with a body weight between 15 kg and 20 kg, the starting dose was 3 mg, and this was increased to a maximum of 7.5 mg (uptitration mode: 3 mg – 5 mg – 7.5 mg).
Adolescents and all children with a body weight above 20 kg received 5 mg as starting dose. While older children were rapidly increased to a target dose of 10 mg (5 mg – 7.5 mg – 10 mg), children with a body weight between 20 and 30 kg remained on 7.5 mg until the first follow-up. They were then increased to the target dose of 10 mg, provided there was no significant drop of blood pressure or other side effects.
Thirteen patients were switched from bosentan to macitentan; in those, bosentan was stopped 24 h before start of the new treatment. Introduction and dosage escalation of macitentan was performed as described before.
Statistics
Descriptive statistic was used to characterize the clinical incidences of side effects. Continuous parameters were described as mean ± standard deviation, in case of normal distribution (Crea, Hb, Hct, WBC, PLT) and as median with first quartile in case of skewed distribution (SGOT, SGPT, GGT). Categorical variables were displayed as frequencies. Differences between baseline, three months and one year laboratory parameters with normal or skewed distribution were tested with one way Student’s t test (within group differences), or non-parametric test, respectively. Statistical significance was regarded as p <0.05. Statistical tests were performed by using IBM SPSS Statistics for Windows, version 25 (IBM Corp., Armonk, NY, USA).
Results
Twenty-four paediatric patients (14 male, 10 female) were enrolled for treatment with macitentan. PHVD was classified according to PANAMA criteria (3) as either isolated PAH (n = 7), associated with congenital heart defects (CHDs) (n = 10), as developmental pulmonary vascular hypertensive disease (n = 2), associated with bronchopulmonary dysplasia (n = 2) and associated with other systemic disorders (n = 2) or CTEPH (n = 1). Detailed demographic data are listed in Table 2.
Table 2.
Demographic and clinical data of enrolled patients.
| PHVD classification | PH group (6th WSPH) | Age (years) | Pulmonary pressure | Additional advanced therapy | Supportive therapy | Diagnosis | WHO FC | Follow-up time (months) | |
|---|---|---|---|---|---|---|---|---|---|
| 1. | IPAH | 1 | Suprasystemic | PDE5-I, inh.PGI | Y | Catheter + Echo | III | 42 | |
| 2. | IPAH | 1 | 12 | Suprasystemic | PDE5-I | Y | Catheter + Echo | II | 41 |
| 3. | IPAH | 1 | 22 | Systemic | iv PGI | Y | Catheter + Echo | II | 19 |
| 4. | IPAH | 1 | 1.5 | Suprasystemic | PDE5-I, inh. PGI | Y | Catheter + Echo | II | 36 |
| 5. | IPAH | 1 | 11 | Suprasystemic | PDE5-I, iv PGI | Y | Catheter + Echo | IV | – |
| 6. | IPAH | 1 | 16 | Suprasystemic | PDE5-I, inh.PGI | Y | Catheter + Echo | IV | 12 |
| 7. | PAH/PVOD | 1 | 0.5 | Suprasystemic | PDE5-I inh.PGI | Y | Catheter + Echo | III | – |
| 8. | CHD (ES) | 1 | 15 | Systemic | PDE5-I | N | Catheter + Echo | II | 43 |
| 9. | CHD (ES) | 1 | 10.5 | Systemic | PDE5-I | Y | Catheter + Echo | II | 42 |
| 10. | CHD postop | 1 | 23 | Systemic | sGC stimulator | N | Catheter + Echo | II | 49 |
| 11. | CHD (PA/VSD/MAPCAS) | 5 | 0.8 | Systemic | PDE5-I | Y | Catheter + Echo | II | 43 |
| 12. | CHD (PA/VSD/ MAPCAS) | 5 | 1.6 | Subsystemic | PDE5-I | Y | Catheter + Echo | II | 38 |
| 13. | CHD (PA/VSD/MAPCAS) | 5 | 19 | Systemic | PDE5-I | Y | Catheter + Echo | III | 49 |
| 14. | CHD (PA/VSD/MAPCAS) | 5 | 23 | Suprasystemic | PDE5-I, inh. PGI | Y | Catheter + Echo | III | 49 |
| 15. | CHD (PA/VSD/MAPCAS) | 5 | 10 | Subsystemic | PDE5-I | N | Catheter + Echo | II | 10 |
| 16. | CHD postop | 1 | 19 | Systemic | PDE5-I | Y | Echo | II | 44 |
| 17. | CHD postop | 1 | 11 | Subsystemic | – | Y | Catheter + Echo | II | 12 |
| 18. | CHD+ lung disease | 1 + 3 | 0.1 | Systemic | – | Y | Catheter + Echo | III | 11 |
| 19. | BPD, developmental PVHD | 3 | 8 | Suprasystemic | PDE5-I | N | Catheter + Echo | II | 33 |
| 20. | Developmental PVHD | 3 | 6 | Subsystemic | – | N | Catheter + Echo | II | 29 |
| 21. | Developmental PVHD | 3 | 4 | Subsystemic | PDE5-I | N | Echo | II | 16 |
| 22. | Systemic disorder (mitochondriopathy) | 5 | 0.5 | Suprasytemic | PDE5-I , inh.PGI | Y | Catheter + Echo | III | 15 |
| 23. | CTEPH | 4 | 16 | Systemic | PDE5-I, inh.PGI | Y | Catheter + Echo | III | 41 |
| 24. | Systemic disorder (malignancy, bone marrow transplantation) | 5 | 15 | Subsystemic | .– | Y | Catheter + Echo | II | 36 |
Conventional therapy included anticoagulation, diuretics, digoxin, and/or iron supplements.
CHD: congenital heart defect; PA/VSD/MAPCAS: pulmonary atresia with ventricular septal defect (VSD) and aortopulmonary collaterals; BPD: bronchopulmonary dysplasia; 6th WSPH: World Symposium on Pulmonary Hypertension; WHO FC: World Health Organization functional class; PHVD: pulmonary hypertensive vascular disease; CTEPH: chronic thromboembolic pulmonary hypertension; PH: pulmonary hypertension; IPAH: idiopathic pulmonary arterial hypertension; PGI: prostacyclin; PAH: pulmonary arterial hypertension; PVOD: pulmonary veno-occlusive disease; PDE5-I: phosphodiesterase type 5 inhibitor; sGC: soluble guanylate cyclase; ES: Eisenmenger Syndrome.
Mean age of the included patients was 10.7 ± 7.6 years (range: 0.1 year–23 years). Median observation period was 36 months (interquartile range: 15 and 43 months).
The first 10 patients and all infants below 10 kg of body weight or under 2 years of age were started on macitentan in hospital, where vital signs and clinical symptoms were documented daily.
After uneventful initiation of treatment in the first 10 patients, the following patients (beyond the age of 2 years) were started on macitentan in an outpatient manner. Measurement of saturation, blood pressure and heart rate monitoring were performed daily for the first five days, either in our outpatient clinic or by a caring nurse or physician and at each follow-up visit at our outpatient clinic.
Whenever macitentan was started in an ambulatory setting, symptoms, blood pressure, oxygen saturation and heart rate were inquired and reported to us on a daily basis.
Macitentan was started as monotherapy in six patients; two out of six patients were switched from bosentan, while the remaining four received macitentan as first pharmacological agent. In 10 patients, macitentan was used in addition to an ongoing treatment with either PDE-Is, soluble guanylate cyclase stimulator or PGIs. Eight patients were on a combined treatment with PDE-Is in addition to either inhalative or intravenous prostacyclines, when macitentan was initiated.
Eighteen out of 24 patients received conventional baseline therapy with diuretics, iron supplements, anticoagulation and/or digoxin (Table 2).
Switch from bosentan to macitentan
Thirteen out of 24 patients were switched from bosentan to macitentan. Although clinically stable, there were clear signs of disease progression documented by echocardiography, 6-min walking test and/or oxygen saturation. All except of two patients were treated with a combination of ERAs, PDE-Is and/or PGIs (see Table 2).
Patients were asked to take the last dose of bosentan 24 h before introduction of the new medication. The switch was well tolerated in all patients. We did not observe any adverse effects or increase of PH-specific symptoms during transition and the period of uptitration.
Tolerability
Twenty out of 24 patients showed good tolerability of the drug without any clinical side effects or change of blood parameters. Two out of 24 patients presented with mild cephalea and nasopharyngitis at the initiation of treatment, but these symptoms resolved spontaneously within the first four weeks on therapy. Both patients had previously been on bosentan. Two out of 24 patients presented with severe peripheral oedema, nasopharyngeal congestion and coughing at the beginning of treatment, leading to discontinuation of therapy. One patient (Table 2, No. 5) was a 12-year-old boy with iPAH and suprasystemic pulmonary pressure treated with epoprostenol (74 ng/kg/min), sildenafil and bosentan in addition to conventional therapy. He was switched uneventfully and started with 5 mg of macitentan, which was increased to 7.5 mg during the following week. He then presented with peripheral oedema and progredient nasopharyngeal congestion and coughing. Blood pressure, saturation and liver parameters remained unchanged. We therefore stopped macitentan and switched him back to bosentan whereby symptoms resolved gradually. The other patient (Table 2, No. 7) was a six-month-old boy newly diagnosed with severe PAH and suprasystemic pulmonary pressure and RV failure; he was admitted to our intensive care unit where he was treated with inhalative NO and intravenous prostacyclin. After stabilization and withdrawal of nitric oxide (iNO), he was started on sildenafil. Intravenous prostacycline was switched to inhalative application, and finally he received macitentan, starting with 1 mg. After uptitration to a target dose of 3 mg, he developed progressive oedema with a trend to decrease of blood pressure. After termination of macitentan, we observed a quick decline of symptoms. Repeated computer tomography scans of the lungs revealed pathomorphological features highly suspicious for pulmonary veno-occlusive disease. However, the patient remained stable in the follow-up with improved RV function on a combination of sildenafil and inhalative PGIs in addition to baseline supportive therapy. Another ERA (bosentan) was not introduced so far.
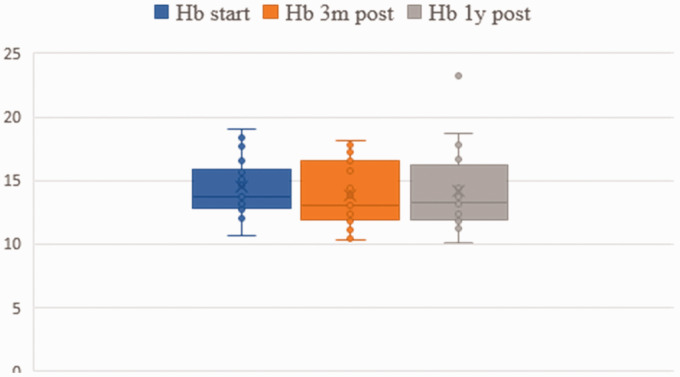
Liver and hematological data are presented in Figs 1 and 2. No statistical difference between baseline, three-month and one-year follow-up data was found. However, in one patient (Table 2, No. 18), we observed an increase of liver enzymes, which normalized after the dosage was reduced from 3 mg to 2 mg. In fact, this was the youngest child with 7 weeks of age and 3.1 kg of weight at the time of treatment. The girl was born small for gestational age and had a supracardiac type of total anomalous pulmonary venous return operated in the first days of life. She was mechanically ventilated for 51 days and developed bronchopulmonary dysplasia. After cardiac catheterization which revealed increased PA pressure in the absence of venous obstruction, she was started on macitentan beginning with 1 mg, which was gradually increased to 3 mg. Laboratory tests after one week revealed isolated increase of SGOP, SGPT and GGT to more than twice the normal range. After decreasing the dose of macitentan to 2 mg, liver enzymes normalized rapidly.
Fig. 1.
Liver function parameter (serum glutamic oxaloacetic transaminase (SGOT), serum glutamate-pyruvate transaminase (SGPT), gamma-glutamyltransferase (GGT), alkaline phosphatase (AP)) before treatment, three months after treatment and one year after treatment.
Fig. 2.
Hemoglobin (Hb) and hematocrit (HKT) before treatment, three months after treatment and one year after treatment.
Finally, we observed an insignificant decrease of blood pressure in all patients when reaching the target dose. However, the drop did not exceed >15% of the systolic baseline value and >10% of diastolic baseline value. At three-month follow-up, the median decrease of systolic blood pressure was 1 mmHg (Fig. 3).
Fig. 3.
Systolic and diastolic blood pressure (BP) levels before treatment, three months after treatment and one year after treatment.
Brain natriuretic peptide (BNP) levels before the start of treatment and three months after are listed in Fig. 4. There was a marked decrease, but this did not reach statistical significance.
Fig. 4.
proBNP levels before, three months and one year after treatment.
BNP: brain natriuretic peptide.
Mortality
We had one death during our observational period. The patient had complex heart disease with Eisenmenger physiology and was severely cyanotic and deceased by sudden cardiac death 4 years after treatment initiation at 23 years of age.
Overall, long-term follow-up of macitentan treatment was achieved in 22/24 patients with a maximum observation period of 49 months. Apart from two early discontinuations of macitentan during the first weeks of introduction, all patients remained on macitentan with good safety and tolerability profile.
Discussion
PHVD remains a severe condition leading to limited life expectancy and quality of life. However, the introduction of targeted therapies such as PDIs, ERAs and PGIs over the last 20 years has improved outcomes substantially.10,11,20 Macitentan is in fact a next-generation dual ERA; based on its enhanced pharmacological properties compared to other ERAs, its use gives hope for further improvement in PAH patients.13–15 Whereas clinical trials and case reports in adults support its efficacy and tolerability, data on children are limited.16–18,21–27
Our study is the first to report on its use in children during long-term follow-up with special emphasis on safety and tolerability. We used it on a compassionate base in our patients with relatively advanced disease progression. More than 50% of the treated patients had severe PHVD with at least systemic PA pressures, therefore receiving combination therapy including prostacyclines at the time of introduction of macitentan. Especially in those we felt it was clinically important to take advantage of the better pharmacological properties of this novel substance.
At the time of our study, there was very little information about optimal dosing of macitentan in children. With awareness of this limitation, the first 10 patients and all children below 2 years of age or 10 kg of weight were admitted into hospital for close monitoring during introduction and uptitration. We used a very strict protocol especially in younger infants, as treatment was started and uptitrated only in hospital with close monitoring of hemodynamic parameters as well as laboratory tests. In the majority of cases, we did not observe any significant side effects during introduction, and patients were discharged after a maximum of five days. Therefore, the following patients were started on macitentan in an outpatient manner. In this setting, symptoms and vital signs were recorded daily and reported to us.
A switch from bosentan to macitentan was performed in 13/24 patients. For this purpose, bosentan was stopped 24 h before treatment initiation. The switch was well tolerated in all patients without any clinical side effects and especially without any signs of deterioration of PAH symptoms. Our experience is similar to other reports published recently,16,27,28 one of those including two smaller children.18
We used macitentan in different dosages adjusted to body weight. About one third of the patients had a body weight lower than 15 kg. In these children, the applied dose ranged from 0.33 mg/kg/d to 0.6 mg/kg/d.
Recently, the TOMORROW study – a large multicentre placebo controlled trial – has been initiated to investigate macitentan in children. In this study, dosing regimens are quite similar to the dosages that we used. Notably, most of our treated children received macitentan as a compassionate therapy before this trial was initiated. Furthermore, many of our patients would not have been eligible for this trial as they were on additional prostacyclines at the time of treatment and/or had variable underlying diseases not eligible for the trial. However, currently we are including our patients in this big study. Its results will hopefully lead to approval of macitentan in paediatric PAH.
We had two early discontinuations (8%) due to peripheral oedema, both reversible after termination of treatment. This phenomenon seems to be an important issue, though the mechanism is not completely understood. Vercauteren et al.29 showed that in vitro endothelin A selective antagonism was associated with systemic vasodilation, transient norepinephrine and arginine vasopressin release, water retention and vascular leakage, whereas dual antagonism had significantly smaller effects on these variables. However, fluid retention exists also on dual ERAs, and this association was observed in several clinical trials.30–33 The suggested mechanisms responsible for this phenomenon include unopposed precapillary arteriolar vasodilation and changes in capillary permeability.34 A recently published meta-analysis that compared three ERAs (bosentan, ambrisentan and macitentan) regarding side effects showed that bosentan and ambrisentan had a significantly higher incidence of peripheral oedema compared to macitentan.35 The incidence of peripheral oedema according to the SERAPHIN trial was 18.2% in the 10 mg macitentan treatment group. Apart from that, macitentan has been associated with peripheral oedema in other smaller studies with one including complex older patients.19,36 In this study, more than half of the patients had to stop macitentan due to oedema.19 Growing experience with the use of macitentan will hopefully improve understanding of this associated side effect. Whereas paediatric data on side effects with macitentan are not available yet, the commonly used ERAs were associated with lower rates of side effects compared to adults.37–39 However, the incidence of peripheral oedema with bosentan was reported to be 8% in one larger paediatric study, which is quite comparable to our data on macitentan.40 In our experience, patients receiving bosentan never presented with oedema so far. Notably one of our patients on macitentan presenting with oedema had been on bosentan without any clinical side effects for several years.
Furthermore, we had two patients who presented with mild symptoms of nasopharyngitis and cephalea. Symptoms started at the beginning of uptitration and resolved within a period of four weeks on treatment. These side effects are commonly described, and in fact, we do see them in a number of our patients on sildenafil and other targeted therapies.16,35,37,38
The most common documented feature was a well-tolerated decrease of blood pressure, which was statistically not significant. However, this phenomenon was observed in almost all patients when reaching the target dose. Similarly, to other targeted therapies such as sildenafil and PGIs, lowering of blood pressure might reflect not only the effect of the drug on the systemic blood pressure but also on the pulmonary vasculature.
Finally, we had one patient with an increase of liver enzymes more than twofold of the normal range. In fact, this was the youngest infant with a body weight of 3.2 kg. This patient had been uptitrated to a dose of 3 mg when liver enzymes increased. After reduction of macitentan to 2 mg, values normalized completely, indicating a dose-dependent and reversible impact on liver function. Whether immaturity of her liver function or the recent cardiac surgery played an additional role remains unclear, but fortunately, we did not have to stop macitentan in this severely affected girl with PHVD. Apart from this case, liver enzymes and blood count remained unchanged in all of our treated patients. Importantly, anaemia was not observed.
With respect to efficacy, our analysis shows a decrease of BNP levels at the three-month follow-up indicating beneficial effects and ameliorated RV function. However, the change was statistically not significant which might be explained the relatively small number of patients (Fig. 4).
During long-term follow-up, there was one death not related to study treatment as it occurred as a sudden event almost four years after macitentan had been started. The young woman had pulmonary atresia with ventricular septal defect and hypoplastic pulmonary arteries and had been palliated with two BT shunts in childhood; she was severely cyanotic and refused further therapeutical interventions. She was treated with sildenafil and macitentan, which had been switched from bosentan in addition to baseline therapy. In fact, her death at the age of 23 years reflects the malignant course of palliated complex heart defects with PHVD.
However, the clinical efficacy of macitentan in CHD remains unclear. It seems to be reasonable that macitentan is at least as beneficial as its precursor bosentan in patients with Eisenmenger Syndrome (ES) patients.41–44 While there are several reports supporting its beneficial effects, this finding could not be verified by the recently published MAESTRO study (Clinical study to evaluate the effects of macitentan on exercise capacity in subjects with Eisenmenger Syndrome).45–48
As shown in Table 2, we used macitentan in several different entities associated with PHVD, provided that postcapillary PH was ruled out. The use of its precursor bosentan in paediatric PAH has been well studied in a series of paediatric-specific trials leading to its approval.40,49–54 Whereas the bigger trials mainly included children with iPAH and PAH associated with CHD, there are several smaller studies and case reports supporting the use of ERAs in other associated diseases.55–57 However so far, bigger trials on the use of ERAs in different entities do not exist. Given the huge variability of associated diseases and the relatively small numbers of paediatric patients, it might be difficult to conduct studies on each paediatric PHVD entity.
The fatal outcome of this disease is contrasted by a lack of randomized controlled trials in paediatric PH. Currently there is only limited experience on the use of macitentan in children. Available data include one smaller study in children older than 12 years17 and a recently published work by Flores et al.18 reporting on their initial experience of macitentan in two smaller children. Our observational study adds to the limited paediatric experience and indicates that macitentan might be safely applied in children under careful monitoring though, of course, randomized controlled trials – such as the currently ongoing TOMORROW study – are essential to further prove this experience and to introduce macitentan as treatment option in paediatric PAH.
By now, the use of macitentan in infants < 6 months of age has not yet been reported. The limited data on this age group in our study suggest that the use of macitentan in smaller infants might be associated with increased side effects, such as increase of liver enzymes or oedema. The use of this agent in this population should therefore not be encouraged unless it is performed within larger paediatric-specific trials.
Apart from that, our long-term experience on macitentan in children comprises a maximal clinical observational time of 49 months and is in accordance with study results available from adults with a good safety and tolerability profile.16,46
Limitations
Limitations include the small sample size and the heterogeneity of study population, which in fact reflects the shortcomings of a single-centre study in paediatric PAH patients. Another limitation is that the macitentan doses used were empiric and were extrapolated from adult studies. We did not perform a pharmacokinetic analysis, which is actually performed in a currently ongoing trial. However, we faced this important limitation by a strict monitoring protocol including in hospital introduction of this agent and close follow-up visits. Finally, the open label nature of our study constitutes a further limitation.
In summary, this single-centre prospective observational study provides valuable clinical information on the application of macitentan in paediatric PAH. Our data support the fact that macitentan can be used safely in childhood under careful monitoring of potential side effects. Overall, it is clinically well tolerated and not correlated with anaemia, significant increase of liver enzymes or hypotension. Special attention should be paid on oedema during introduction. So far, this is the first report on the use of macitentan in smaller children. Whether this drug, similar to its precursor bosentan, can be used safely in infants < 6 months of age cannot be ultimately answered. Our experience does not provide sufficient data to recommend its use in this age group. Larger prospective randomized trials are needed to verify our preliminary findings in children and to prove the use of macitentan in the very young.
Acknowledgements
The authors would like to thank Dr Manfred Marx for his comments and for proofreading of the manuscript.
Footnotes
Author contributions: SA wrote the manuscript and was responsible for study design and conduction. IP was responsible for data collection and analysis and contributed to the final manuscript. EK did the hemodynamic measurements and participated in research coordination. IM-B was responsible for study conduction, hemodynamic measurements and contributed to the writing of this manuscript. All authors reviewed the final manuscript.
Conflict of interest: The author(s) declare that there is no conflict of interest.
Guarantor: Sulaima Albinni
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Ivy DD, Abman SH, Barst RJ, et al. Pediatric pulmonary hypertension. J Am Coll Cardiol 2013; 62: D117–D126. [DOI] [PubMed] [Google Scholar]
- 2.Lang IM, Bonderman D, Kneussl M, et al. Paediatric pulmonary vascular disease. Paediatr Respir Rev 2004; 5: 238–248. [DOI] [PubMed] [Google Scholar]
- 3.del Cerro MJ, Abman S, Diaz G, et al. A consensus approach to the classification of pediatric pulmonary hypertensive vascular disease: report from the PVRI pediatric taskforce, Panama 2011. Pulm Circ 2011; 1: 286–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barst RJ, Ertel SI, Beghetti M, et al. Pulmonary arterial hypertension: a comparison between children and adults. Eur Respir J 2011; 37: 665–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beghetti M, Berger RMF. The challenges in paediatric pulmonary arterial hypertension. Eur Respir Rev 2014; 23: 498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53: 1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenzweig EB, Abman SH, Adatia I, et al. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J 2019; 53: 1801916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger RM, Beghetti M, Humpl T, et al. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet 2012; 379: 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zijlstra WMH, Douwes JM, Rosenzweig EB, et al. Survival differences in pediatric pulmonary arterial hypertension. J Am Coll Cardiol 2014; 63: 2159–2169. [DOI] [PubMed] [Google Scholar]
- 10.Moledina S, Hislop AA, Foster H, et al. Childhood idiopathic pulmonary arterial hypertension: a national cohort study. Heart 2010; 96: 1401–1406. [DOI] [PubMed] [Google Scholar]
- 11.Barst RJ, McGoon MD, Elliott CG, et al. Survival in childhood pulmonary arterial hypertension: insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Circulation 2012; 125: 113–122. [DOI] [PubMed] [Google Scholar]
- 12.Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS) Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 13.Sidharta PN, Krähenbühl S, Dingemanse J. Pharmacokinetic and pharmacodynamic evaluation of macitentan, a novel endothelin receptor antagonist for the treatment of pulmonary arterial hypertension. Expert Opin Drug Metab Toxicol 2015; 11: 437–449. [DOI] [PubMed] [Google Scholar]
- 14.Iglarz M, Binkert C, Morrison K, et al. Pharmacology of macitentan, an orally active tissue-targeting dual endothelin receptor antagonist. J Pharmacol Exp Ther 2008; 327: 736–745. [DOI] [PubMed] [Google Scholar]
- 15.Gatfield J, Mueller Grandjean C, Sasse T, et al. Slow receptor dissociation kinetics differentiate macitentan from other endothelin receptor antagonists in pulmonary arterial smooth muscle cells. PLoS One 2012; 7: e47662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pulido T, Adzerikho I, Channick RN, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med 2013; 369: 809–818. [DOI] [PubMed] [Google Scholar]
- 17.Aypar E, Alehan D, Karagöz T, et al. Clinical efficacy and safety of switch from bosentan to macitentan in children and young adults with pulmonary arterial hypertension. Cardiol Young 2018; 28: 542–547. [DOI] [PubMed] [Google Scholar]
- 18.Flores M, Caro AT, Mendoza A. Initial experience in children with the use of macitentan in pulmonary arterial hypertension after side effects with other endothelin receptor antagonists. Prog Pediatr Cardiol 2019; 52: 55–56. [Google Scholar]
- 19.Tahara N, Dobashi H, Fukuda K, et al. Efficacy and safety of a novel endothelin receptor antagonist, macitentan, in Japanese patients with pulmonary arterial hypertension. Circ J 2016; 80: 1478–1483. [DOI] [PubMed] [Google Scholar]
- 20.van Loon RLE, Roofthooft MTR, Delhaas T, et al. Outcome of pediatric patients with pulmonary arterial hypertension in the era of new medical therapies. Am J Cardiol 2010; 106: 117–124. [DOI] [PubMed] [Google Scholar]
- 21.Dingemanse J, Sidharta PN, Maddrey WC, et al. Efficacy, safety and clinical pharmacology of macitentan in comparison to other endothelin receptor antagonists in the treatment of pulmonary arterial hypertension. Expert Opin Drug Saf 2014; 13: 391–405. [DOI] [PubMed] [Google Scholar]
- 22.Mehta S, Sastry BKS, Souza R, et al. Macitentan improves health-related quality of life for patients with pulmonary arterial hypertension. Chest 2017; 151: 106–118. [DOI] [PubMed] [Google Scholar]
- 23.Cadenas-Menéndez S, Álvarez-Vega P, Martín-Moreiras J, et al. Macitentan in daily clinical practice: a single centre, 1-year experience. Pulmonology 2018; 24: 170–173. [DOI] [PubMed] [Google Scholar]
- 24.Ghofrani H-A, Simonneau G, D’Armini AM, et al. Macitentan for the treatment of inoperable chronic thromboembolic pulmonary hypertension (MERIT-1): results from the multicentre, phase 2, randomised, double-blind, placebo-controlled study. Lancet Respir Med 2017; 5: 785–794. [DOI] [PubMed] [Google Scholar]
- 25.Keating GM. Macitentan: a review in pulmonary arterial hypertension. Am J Cardiovasc Drugs Drugs Devices Interv 2016; 16: 453–460. [DOI] [PubMed] [Google Scholar]
- 26.Davila C, Monaco T. Safety, efficacy, and clinical utility of macitentan in the treatment of pulmonary arterial hypertension. Drug Des Devel Ther 2016; 10: 1675–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demetriades P, Aziz A, Condliffe R, et al. The use of macitentan in Fontan circulation: a case report. BMC Cardiovasc Disord 2017; 17: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Safdar Z, Thakur A, Frost A. Tolerability of switch to macitentan from bosentan in pulmonary arterial hypertension. South Med J 2017; 110: 223–228. [DOI] [PubMed] [Google Scholar]
- 29.Vercauteren M, Trensz F, Pasquali A, et al. Endothelin ET A receptor blockade, by activating et b receptors, increases vascular permeability and induces exaggerated fluid retention. J Pharmacol Exp Ther 2017; 361: 322–333. [DOI] [PubMed] [Google Scholar]
- 30.Aversa M, Porter S, Granton J. Comparative safety and tolerability of endothelin receptor antagonists in pulmonary arterial hypertension. Drug Saf 2015; 38: 419–435. [DOI] [PubMed] [Google Scholar]
- 31.Humbert M. Combination of bosentan with epoprostenol in pulmonary arterial hypertension: BREATHE-2. Eur Respir J 2004; 24: 353–359. [DOI] [PubMed] [Google Scholar]
- 32.Galiè N, Rubin L, Hoeper M, et al. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet 2008; 371: 2093–2100. [DOI] [PubMed] [Google Scholar]
- 33.Jaïs X, D’Armini AM, Jansa P, et al. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol 2008; 52: 2127–2134. [DOI] [PubMed] [Google Scholar]
- 34.Shapiro S, Pollock DM, Gillies H, et al. Frequency of edema in patients with pulmonary arterial hypertension receiving ambrisentan. Am J Cardiol 2012; 110: 1373–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei A, Gu Z, Li J, et al. Clinical adverse effects of endothelin receptor antagonists: insights from the meta-analysis of 4894 patients from 24 randomized double-blind placebo-controlled clinical trials. J Am Heart Assoc 2016; 5: e003896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khan A, Collins N, Reeves G, et al. Initial experience with macitentan in contemporary patient cohort. Heart Lung Circ 2015; 24: S127. [Google Scholar]
- 37.Roldan T, Deiros L, Romero JA, et al. Safety and tolerability of targeted therapies for pulmonary hypertension in children. Pediatr Cardiol 2014; 35: 490–498. [DOI] [PubMed] [Google Scholar]
- 38.Maxey DM, Ivy DD, Ogawa MT, et al. Food and Drug Administration (FDA) postmarket reported side effects and adverse events associated with pulmonary hypertension therapy in pediatric patients. Pediatr Cardiol 2013; 34: 1628–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beghetti M, Hoeper MM, Kiely DG, et al. Safety experience with bosentan in 146 children 2–11 years old with pulmonary arterial hypertension: results from the European postmarketing surveillance program. Pediatr Res 2008; 64: 200–204. [DOI] [PubMed] [Google Scholar]
- 40.Rosenzweig EB, Ivy DD, Widlitz A, et al. Effects of long-term bosentan in children with pulmonary arterial hypertension. J Am Coll Cardiol 2005; 46: 697–704. [DOI] [PubMed] [Google Scholar]
- 41.Arnott C, Strange G, Bullock A, et al. Pulmonary vasodilator therapy is associated with greater survival in Eisenmenger syndrome. Heart 2018; 104: 732–737. [DOI] [PubMed] [Google Scholar]
- 42.Schulze-Neick I, Gilbert N, Ewert R, et al. Adult patients with congenital heart disease and pulmonary arterial hypertension: first open prospective multicenter study of bosentan therapy. Am Heart J 2005; 150: 716.e7–716.e12. [DOI] [PubMed] [Google Scholar]
- 43.Apostolopoulou SC, Manginas A, Cokkinos DV, et al. Long-term oral bosentan treatment in patients with pulmonary arterial hypertension related to congenital heart disease: a 2-year study. Heart 2007; 93: 350–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galiè N, Beghetti M, Gatzoulis MA, et al. Bosentan therapy in patients with eisenmenger syndrome: a multicenter, double-blind, randomized, placebo-controlled study. Circulation 2006; 114: 48–54. [DOI] [PubMed] [Google Scholar]
- 45.Blok IM, van Riel ACMJ, van Dijk APJ, et al. From bosentan to macitentan for pulmonary arterial hypertension and adult congenital heart disease: further improvement? Int J Cardiol 2017; 227: 51–52. [DOI] [PubMed] [Google Scholar]
- 46.Herbert S, Gin-Sing W, Howard L, et al. Early experience of macitentan for pulmonary arterial hypertension in adult congenital heart disease. Heart Lung Circ 2017; 26: 1113–1116. [DOI] [PubMed] [Google Scholar]
- 47.Wacker J, Weintraub RG. Macitentan in pulmonary arterial hypertension associated with congenital heart defects. Heart Lung Circ 2017; 26: 1006–1007. [DOI] [PubMed] [Google Scholar]
- 48.Gatzoulis MA, Landzberg M, Beghetti M, et al. Evaluation of macitentan in patients with Eisenmenger syndrome: results from the randomized, controlled MAESTRO study. Circulation 2019; 139: 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beghetti M. Bosentan in pediatric patients with pulmonary arterial hypertension. Curr Vasc Pharmacol 2009; 7: 225–233. [DOI] [PubMed] [Google Scholar]
- 50.Barst RJ, Ivy D, Dingemanse J, et al. Pharmacokinetics, safety, and efficacy of bosentan in pediatric patients with pulmonary arterial hypertension. Clin Pharmacol Ther 2003; 73: 372–382. [DOI] [PubMed] [Google Scholar]
- 51.Simpson CM, Penny DJ, Cochrane AD, et al. Preliminary experience with bosentan as initial therapy in childhood idiopathic pulmonary arterial hypertension. J Heart Lung Transplant 2006; 25: 469–473. [DOI] [PubMed] [Google Scholar]
- 52.Beghetti M, Haworth SG, Bonnet D, et al. Pharmacokinetic and clinical profile of a novel formulation of bosentan in children with pulmonary arterial hypertension: the FUTURE-1 study. Br J Clin Pharmacol 2009; 68: 948–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maiya S. Response to bosentan in children with pulmonary hypertension. Heart 2006; 92: 664–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hislop AA, Moledina S, Foster H, et al. Long-term efficacy of bosentan in treatment of pulmonary arterial hypertension in children. Eur Respir J 2011; 38: 70–77. [DOI] [PubMed] [Google Scholar]
- 55.Yamamura K, Nagata H, Ikeda K, et al. Efficacy of bosentan therapy for segmental pulmonary artery hypertension due to major aortopulmonary collateral arteries in children. Int J Cardiol 2012; 161: e1–e3. [DOI] [PubMed] [Google Scholar]
- 56.Grant EK, Berger JT. Use of pulmonary hypertension medications in patients with tetralogy of Fallot with pulmonary atresia and multiple aortopulmonary collaterals. Pediatr Cardiol 2016; 37: 304–312. [DOI] [PubMed] [Google Scholar]
- 57.Kadmon G, Schiller O, Dagan T, et al. Pulmonary hypertension specific treatment in infants with bronchopulmonary dysplasia: treatment of BPD-associated PH. Pediatr Pulmonol 2017; 52: 77–83. [DOI] [PubMed] [Google Scholar]