Abstract
STUDY QUESTION
What is the impact of prolonged exposure to hyperandrogenemia (T), Western-style diet (WSD) and the combination on metabolic and reproductive function in female rhesus macaques, particularly in the post-partum period?
SUMMARY ANSWER
Combined T + WSD worsened measures of insulin sensitivity and parameters of cyclicity following prolonged (5 years) exposure, but there was no effect on post-partum metabolic function.
WHAT IS KNOWN ALREADY
Women with hyperandrogenemia due to polycystic ovary syndrome are at higher risk for gestational diabetes and Type 2 diabetes post-partum, but it is unknown if this is related to hyperandrogenemia. Hyperandrogenemia in the presence of a WSD worsens metabolic function in female nonhuman primates.
STUDY DESIGN, SIZE, DURATION
Female rhesus macaques began treatment near menarche (roughly 2.5 years of age) consisting of either cholesterol (control; C) or testosterone (T) implants (average serum levels 1.4 ng/ml) and exposure to standard monkey chow or a WSD (15 vs 36% of calories from fat, respectively). The four groups were maintained on treatment for 3 years, underwent a fertility trial in Year 4 and continued with treatments through Year 5.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Metabolic measurements (glucose tolerance tests and double X-ray absorptiometry scans) were performed yearly, and results from 5 years of treatment are reported for all animals. Animals were bled daily for 30 days at 5 years to capture changes in ovarian cycle hormones, and ultrasound measurements were performed during the early follicular and luteal phase.
MAIN RESULTS AND THE ROLE OF CHANCE
After 5 years of treatment, WSD exposure moderately increased body weight and body fat, although control animals also had a high body mass index due to ad libitum feeding. Animals in the T + WSD group had increased fasting insulin and insulin secretion during an intravenous glucose tolerance test. WSD exposure also altered ovarian cycles, delaying the time to the E2 surge, decreasing progesterone and anti-Müllerian hormone levels and increasing the number of antral follicles present by ultrasound. Longitudinal assessment of metabolic function for only those animals that became pregnant in Year 4 of treatment revealed no differences in post-partum metabolism between groups, although WSD resulted in overall elevated weights, body fat and measures of insulin resistance.
LARGE SCALE DATA
None.
LIMITATIONS, REASONS FOR CAUTION
The small sample size and heterogeneity in metabolic effects observed in the T + WSD group are limitations of the current study, with only a subset of animals in this group showing impaired insulin resistance relative to controls. In addition, obesity in the C group prevented comparisons to lean animals.
WIDER IMPLICATIONS OF THE FINDINGS
Hyperandrogenemia combined with WSD had a greater impact on insulin sensitivity and ovarian function than either treatment alone.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by NIH grant P50 HD071836 to C.T.R., J.H. and C.T. and P51 OD011092 for support of the Oregon National Primate Research Center. All authors declare no competing interests.
Keywords: hyperandrogenemia, polycystic ovary syndrome, obesity, high-fat diet, post-partum
Introduction
Polycystic ovary syndrome (PCOS) is a prevalent cause of subfertility, affecting an estimated 5–10% of reproductive-age women in the USA (Azziz et al., 2016). The most commonly used diagnostic criteria require that women have at least two of the following symptoms: oligo- or anovulation, polycystic ovaries upon ultrasound and hyperandrogenemia. In addition, patients with PCOS have higher rates of both obesity and insulin resistance, which also have a negative impact on fertility (Clark et al., 1995, 1998; Pasquali et al., 2003; Brewer and Balen, 2010; Rubin et al., 2017; Yao et al., 2017). Consistent with worsened measures of insulin sensitivity, PCOS women are at increased risk of developing gestational diabetes during pregnancy (Pan et al., 2015; Rubin et al., 2017; Yao et al., 2017). Gestational diabetes further increases the risk for the development of Type 2 diabetes post-partum (Kim et al., 2002; Bellamy et al., 2009). It is unclear from clinical data whether the risk for gestational diabetes and post-partum Type 2 diabetes in PCOS patients is secondary to PCOS-associated obesity, or if it may be inherent to the disease and elevated levels of androgens.
Our group has developed a nonhuman primate model to explore the independent and combined impact of elevated androgens and an obesogenic Western-style diet (WSD) on metabolic and reproductive health. Female macaques were exposed to elevated testosterone (T), WSD or the combination (T + WSD) beginning at puberty. The timing of treatment initiation was chosen to mimic the emergence of hyperandrogenemia in obese adolescent females at this developmental period (McCartney et al., 2006, 2007). Previous data have shown the most significant metabolic and reproductive impairments were present in the combination T + WSD group, consistent with the hypothesis that both factors may contribute to adverse health outcomes in PCOS patients (True et al., 2017; Bishop et al., 2018a, b). The T + WSD group had the greatest insulin resistance and lowest fertility rate, although significant heterogeneity in response to treatment was present in this group as well. Animals that failed to achieve pregnancy after three timed matings during the window of fertility, or that had unviable pregnancies, had higher levels of fasting insulin compared to those that conceived, suggesting that hyperinsulinemia and insulin resistance are associated with infertility. Because animals that became pregnant tended to have better indices of insulin sensitivity, only modest group differences during gestation were observed, including elevated fasting glucose levels and reduced glucose clearance during a second-trimester intravenous glucose tolerance test (ivGTT) in the T + WSD group compared to controls (Bishop et al., 2018a). It is unknown whether androgens, in isolation or combination with a WSD, can contribute to a further metabolic decline in the post-partum period, which is often observed in women who experience gestational diabetes (Kim et al., 2002; Bellamy et al., 2009). The current study sought to expand upon our previous reporting in this model into 5 years of continuous T, WSD and T + WSD treatment on overall metabolic and reproductive health. In addition, we examined whether those animals that experienced a pregnancy in Year 4 of treatment showed differential metabolic responses in the acute and long-term post-partum period.
Materials and methods
Animals
All animal procedures were approved by the Oregon National Primate Research Center (ONPRC) Institutional Animal Care and Use Committee and comply with the Animal Welfare Act and the APA Guidelines for Ethical Conduct in the Care and Use of Nonhuman Animals in Research. The model and treatments have been described previously (True et al., 2017). Female rhesus macaques, aged roughly 2.5 years at the time of treatment initiation, were in one of four treatment groups: control animals receiving cholesterol implants and a control diet (C), animals receiving T implants and a control diet (T), animals receiving cholesterol implants and a WSD and animals receiving T implants and a WSD (T + WSD). Average serum T levels in the T-treated group were 1.35 ng/ml compared to an average value of 0.27 ng/ml in cholesterol-treated animals (True et al., 2017). The WSD (Purina 5LOP) has roughly 36% of calories from fat compared to the 15% of calories from fat in standard monkey chow (Purina 5000/5052). Animals were typically pair-housed in the same treatment group and maintained on a 0700–1900 light cycle with ad libitum access to water. All animals underwent 3 years of treatment, and in the fourth year, underwent a fertility trial where they were mated with a proven male breeder over a maximum of three menstrual cycles during the window of fertility. A large subset of the animals became pregnant and had pregnancies terminated by C-section in the third trimester, as reported previously (Bishop et al., 2018a). T and/or WSD treatments were continued throughout and after the fertility trial up to 5 years of treatment.
Metabolic measurements
Body composition and ivGTTs were not performed at a specific menstrual cycle stage and have been described previously (True et al., 2017). Metabolic measurements for the entire cohort were performed at 3 years and were previously published (True et al., 2017), while a subset of these data for only those animals that went on to become pregnant in Year 4 of treatment is presented as shown below (C n = 9, T n = 9, WSD n = 7 and T + WSD n = 4). Additional metabolic measurements were made roughly 3–4 months after pregnancy termination. Metabolic measurements were repeated for all animals at 5 years of treatment (C n = 10, T n = 9, WSD n = 9 and T + WSD n = 7–8). Metabolic measurements at this time point corresponded to roughly 16–17 months post-pregnancy termination for those animals that became pregnant in Year 4 of treatment.
Body composition was determined by double X-ray absorptiometry scan (Horizon A QDR DXA System, Hologic Inc.), where the percent of fat coming from the android region calculated as described previously (True et al., 2017). Additional metabolic measurements included body weight and ivGTTs, where animals were fasted overnight, sedated with Telazol (3–5 mg/kg) and a glucose bolus (50% dextrose solution) was administered at a dose of 0.6 g/kg via the saphenous vein. Baseline blood samples were obtained before the glucose injection, and 1 ml blood samples were taken 1, 3, 5, 10, 20, 40 and 60 min later by venipuncture (typically saphenous vein). Glucose was measured immediately using a OneTouch Ultra Blood Glucose Monitor (LifeScan), and the remainder of the blood was placed in heparinized tubes on ice for insulin measurements. After ivGTTs, samples were centrifuged at 2400g for 20 min, and plasma was stored at −80°C until assay. Baseline ivGTT samples were used for determining fasting glucose, insulin and hemoglobin a1c (HbA1c) levels. Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) was determined as described previously (Matthews et al., 1985; Bonora et al., 2000) and calculated as fasting (serum insulin (U/ml) × fasting plasma glucose (mg/dl))/405.
Menstrual cycle analyses
Females were monitored for menses daily as previously reported (Bishop et al., 2018b), with weekly serum sampling for progesterone (P4) to detect presumably ovulatory cycles (C n = 10, T n = 10, WSD n = 10 and T + WSD n = 9). Dams who underwent pregnancy termination were allowed to recover a minimum of 5.8 months before more detailed menstrual cycle analyses began during Year 5 of treatment (average 8.1 ± 0.2 months post-partum). After recovery, a single menstrual cycle was analyzed, as previously published (Bishop et al., 2018b). Serum samples were collected daily for hormone assays (estradiol, E2; P4; and anti-Müllerian hormone, AMH) beginning at the onset of menses (Day 1) through to the onset of the subsequent menses. Ultrasound evaluation of ovarian structure was performed in the early follicular phase (Days 1–3 post-menses onset) and in the late follicular phase (the day after serum E2 levels reached above 150 pg/ml or at Day 10 post-menses, in the case of longer follicular phases) by 3D/4D ultrasound analyses (C n = 9, T n = 10, WSD n = 10 and T + WSD n = 8) (Bishop et al., 2018b). Similar to previous studies in this cohort, analyses included antral follicle counts and follicle diameters on the presumptive dominant ovary (ovary with the largest follicle in the cohort) and the non-dominant ovary in each female separately, with an additional analysis combining all follicle characteristics from both ovaries from an individual female. In the late follicular phase, the largest follicle ≥1 mm in diameter observed for each female was considered the preovulatory follicle.
Hormone assays
Plasma concentrations of insulin, as well as serum concentrations of E2, LH, FSH, AMH and P4, were measured by the ONPRC Endocrine Technologies Core (ETC) using assays previously validated for rhesus macaques. All quality controls and calibrations provided by the manufacturers, as well as ETC monkey serum controls, were analyzed with test samples. Insulin concentrations in monkey plasma, as well as serum concentrations of E2 and P4, were determined using a chemiluminescence-based automated clinical platform (Roche Diagnostics cobas e411, Indianapolis, IN). The range of the insulin assay is 0.2–1000 μIU/ml and the intra- and inter-assay coefficients of variation (CV) were <7%. Serum E2 and P4 assay ranges were between 5 and 3000 pg/ml and 0.05 and 60 ng/ml, respectively. E2 and P4 intra-assay CVs were 8.3% and 4.2%, whereas inter-assay CVs were 5.9% and 6.3%, respectively. Serum LH was measured by radioimmunoassay with a range of 0.01–5 ng/sample and an intra-assay CV of 5.7%; all samples were analyzed in the same assay. Serum FSH was also measured in a single radioimmunoassy, with a range 0.05–100 ng/ml and an inter-assay CV of 11.6%. Serum AMH was measured by ELISA (Roche Diagnostics) (Bishop et al., 2018b), with a range of 0.087–16.5 ng/ml and intra-assay CV of 4.0–4.2% and inter-assay CV of 6.7%. HbA1c was measured by Siemens DCA Vantage Analyzer, with a range of 0.5–14% (4–130 mmol/mol) and a manufacturer reported CV of 2–3%.
Statistics
All discrete variables were analyzed by Linear models function of SAS (version 9.4), similar to previous studies (Bishop et al., 2018a), analyzing main effects of Diet (chow and WSD) and Steroid (cholesterol and T), and interaction between Diet and Steroid. Repeated measurements from females across time were also analyzed similar to previous studies by the Mixed Models function of SAS, analyzing these same effects across time (repeated measures). When main effects were significant (P ≤ 0.05), pairwise comparisons were analyzed by the Least Squared Means function of SAS with Tukey–Kramer adjustment for multiple comparisons.
Results
Metabolic outcomes after 5 years of testosterone and/or WSD exposure
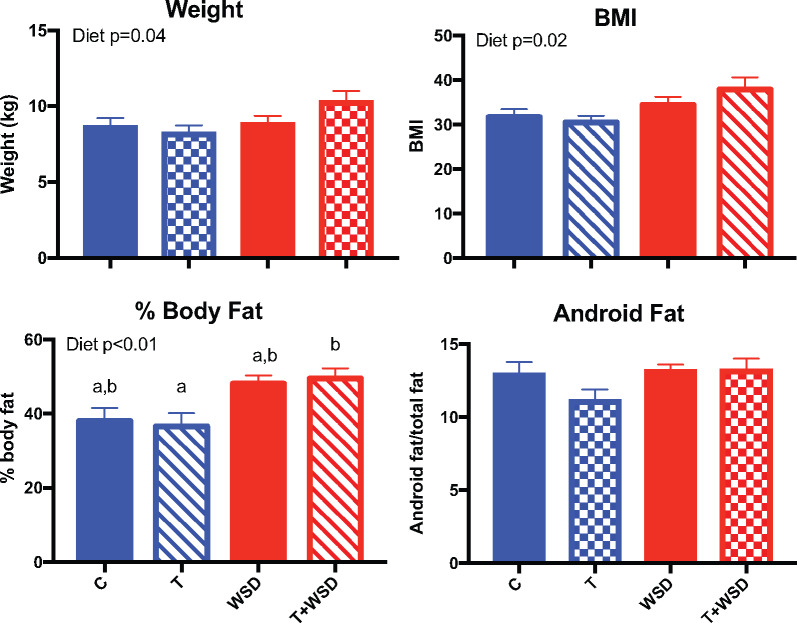
To assess the continued effect of treatment on metabolic parameters, we investigated measures of body composition and insulin sensitivity at 5 years of treatment. Some animals became pregnant in a fertility trial in Year 4, and differences in parity do exist between the groups. The 5-year metabolic measurements were done ∼16–17 months after pregnancies were terminated. WSD treatment significantly increased weight (WSD P = 0.04), BMI (WSD P = 0.02) and the percent of body fat (WSD P < 0.01; Fig. 1). However, the only difference between the four individual treatment groups was increased body fat in the T + WSD group compared to the T group (P = 0.04). There were no treatment effects or individual group differences in the percent of fat located in the abdominal android region (P > 0.1), suggesting no significant change in fat distribution occurred after 5 years of treatment.
Figure 1.
Effects of T, WSD and T + WSD treatment on body composition after 5 years of continuous exposure. Body weight (kg) was measured, along with crown-rump measurement for BMI calculation (BW/(CR)2) under sedation, followed by a double X-ray absorptiometry scan to assess the % body fat and the amount of fat coming from the android region. Data are presented as means ± SEM (C n = 10, T n = 9, WSD n = 9 and T + WSD n = 8). Significant effects (P < 0.05) of WSD and/or T, or interactions over time, as determined by linear models ANOVA are presented as text within the graphs. Different letters signify significant group differences as determined by Tukey–Kramer post hoc analysis. C, Control; T, testosterone; WSD, Western-style diet.
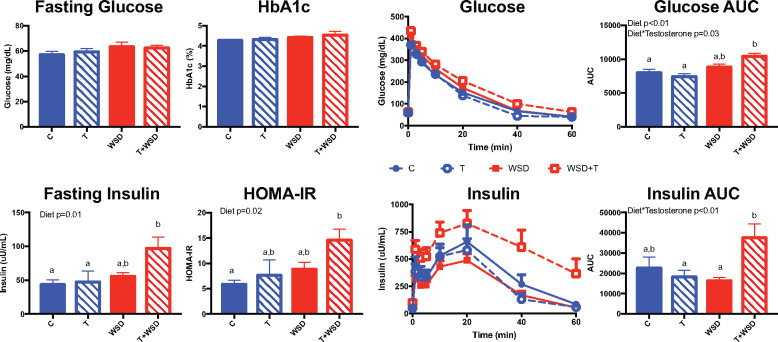
There were no effects of treatment on fasting glucose or HbA1c, indicating that 5 years of treatment did not result in significant hyperglycemia (Fig. 2). WSD did significantly increase fasting insulin (WSD P = 0.01), with the T + WSD group having significantly increased fasting insulin compared to both the C (P = 0.02) and T (P = 0.04) groups. Similarly, WSD increased HOMA-IR (WSD P = 0.02), with the T + WSD group demonstrating elevated insulin resistance compared to the C group (P = 0.03). To further assess changes in measures of insulin sensitivity, ivGTTs were performed. Glucose clearance was decreased, leading to a larger AUC (calculated from zero) with WSD exposure (WSD P < 0.01), with an interaction between T and WSD (T × WSD P = 0.03). A comparison between the four individual groups revealed that T + WSD animals had significantly increased glucose AUC compared to the C (P < 0.01) and T (P < 0.01) groups. Insulin secretion during the ivGTT also showed a significant interaction between T and WSD (T × WSD P < 0.01), with T + WSD animals having higher insulin AUC than the T (P = 0.04) and WSD (P = 0.02) groups.
Figure 2.
Measures of insulin sensitivity after 5 years of continuous exposure to T, WSD and T + WSD. Animals were fasted overnight to determine HbA1c as well as fasting glucose and insulin, which were used to calculate HOMA-IR (glucose (mg/dl) × insulin (µU/ml)/405). Animals then underwent an intravenous glucose tolerance test. Glucose and insulin AUC were calculated from zero. Data are presented as means SEM (C n = 10, T n = 9, WSD n = 9 and T + WSD n = 7). Significant effects (P < 0.05) of WSD and/or T or interactions over time as determined by linear models ANOVA are presented as text within the graphs. Different letters signify significant group differences as determined by Tukey–Kramer post hoc analysis. C, Control; HbA1c, hemoglobin A1c; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; T, testosterone; WSD, Western-style diet.
Reproductive outcomes after 5 years of T and/or WSD exposure
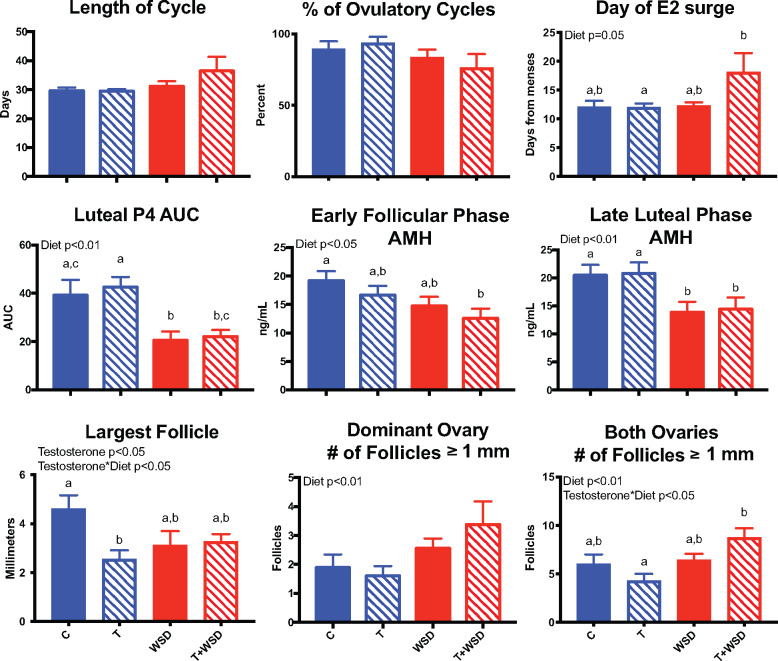
There was no significant impact of WSD and/or T on the number of days it took females to resume menstrual cyclicity post-partum (data not shown). T and/or WSD exposure did not impact menstrual cycle frequency at 4.5–5 years of treatment, regardless of previous parity (data not shown). However, there was a tendency for WSD to increase cycle length (WSD P = 0.095) as well as to decrease the number of ovulatory cycles (WSD P < 0.07; Fig. 3). WSD delayed the day of ovulation/E2 surge (WSD P = 0.05), with T + WSD exhibiting the most significant effect. Consumption of the WSD alone was associated with a slightly shorter luteal phase (C, T and T + WSD; 15 ± 0.5, 16 ± 0.4 and 15 ± 0.5 days, respectively, vs WSD 14 ± 0.7 days; P < 0.03). WSD consumption was associated with reduced P4 secretion (P < 0.01), with the WSD group having significantly lower P4 secretion than the C (P < 0.03) and T groups (P = 0.007), while the T + WSD group had significantly lower levels only compared to the T group (P < 0.02; Fig. 3).
Figure 3.
Reproductive function after 5 years of exposure to chronic T and/or WSD. The average length of cycle and percent ovulatory cycles were analyzed during Year 5 of treatment as detailed in Materials and Methods (C, T, WSD n = 10; T + WSD n = 9). At ∼5.8 months post-partum, females underwent more detailed analyses during one randomly chosen menstrual cycle (C n = 9, T, WSD n = 10 and T + WSD n = 8). Data are presented as means SEM. Significant effects (P < 0.05) of WSD and/or T or interactions between these treatments determined by linear models ANOVA are presented as text within the graphs. Different letters signify significant group differences as determined by Tukey–Kramer post hoc analysis. C, Control; T, testosterone; WSD, Western-style diet.
Consumption of WSD decreased AMH levels in the early follicular phase (WSD P < 0.02), which persisted into the late luteal phase (Fig. 3; WSD P = 0.002). Circulating AMH levels were significantly lower in the T + WSD group compared to controls during the early follicular phase (P < 0.01) and the late luteal phase (P = 0.04). Alterations in serum AMH levels occurred in parallel with treatment dependent changes in follicle size and numbers. In the early follicular phase, T treatment significantly decreased the size of the largest follicle present (T P = 0.048), with an interaction between T and WSD (T × WSD P = 0.025). Previous research in rhesus macaques demonstrated that the largest follicle during the early follicular phase is likely the dominant follicle (Bishop et al., 2009). Females in the T cohort had the smallest dominant antral follicle compared to controls (Fig. 3; P < 0.02). In the late follicular phase, females consuming the WSD had greater numbers of antral follicles ≥1 mm in diameter present on their ovaries (Fig. 3; WSD P < 0.01).
We compared ovarian follicle structure and function measurements at ∼5 years of treatment to those reported previously in this cohort at roughly 3.5 years of treatment (Bishop et al., 2018b) (Supplementary Fig. S1). In the early follicular phase, the number of follicles >1 mm in diameter at menses increased in C, T and WSD cohorts between 4 and 5 years of treatment (year P < 0.001). Luteal function as measured by P4 production in the WSD cohort worsened with further treatment, more closely resembling the T + WSD group (Supplementary Fig. S1; WSD P < 0.01). The E2 surge trended lower in the T cohort after 5 years of treatment (Supplementary Fig. S1; year P = 0.06, T Year 4 vs Year 5 P = 0.098).
Effects of testosterone and WSD on post-pregnancy metabolic outcomes
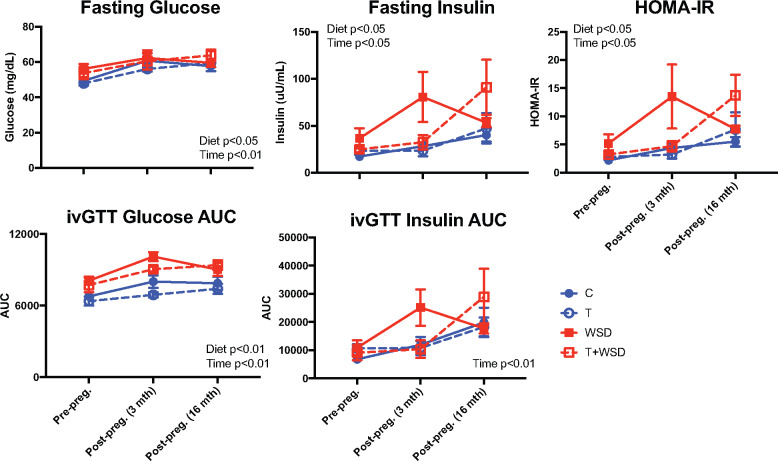
We previously reported that a subset of animals became pregnant during a fertility trial in Year 4 of treatment (Bishop et al., 2018a). Metabolic outcomes were assessed longitudinally at three time points, each approximately a year apart: pre-pregnancy (Year 3 of treatment; previously published results for the entire cohort detailed in (True et al., 2017)), acute post-pregnancy (3–4 months after third-trimester pregnancy termination) and long-term post-pregnancy (16–17 months after pregnancy termination). WSD exposure and years of treatment increased body weight (time P < 0.01, WSD P = 0.02), BMI (time P < 0.01, WSD P < 0.01) and body fat percentage (time P < 0.01, WSD P < 0.01; Fig. 4). No interaction between treatment and time was observed, indicating the treatments did not have a significant effect on post-pregnancy body composition. Years of treatment increased indices of insulin resistance, including fasting glucose (P < 0.01), fasting insulin (P < 0.05), HOMA-IR (P = 0.03), ivGTT glucose AUC (P < 0.01) and insulin AUC (P < 0.01; Fig. 5). WSD also increased indices of insulin resistance (fasting glucose P = 0.03; insulin P = 0.02; HOMA-IR P = 0.03; and ivGTT glucose AUC P < 0.01). Once again, no interactions between treatment and time were observed for T and WSD groups regarding insulin resistance after pregnancy. The limited sample size of nonpregnant animals prevented a statistical comparison between the metabolic status of parous and nulliparous animals; however, weight, body fat, fasting glucose and insulin levels of nulliparous animals tended to fall within the same range of values observed in pregnant animals (Supplementary Fig. S2).
Figure 4.
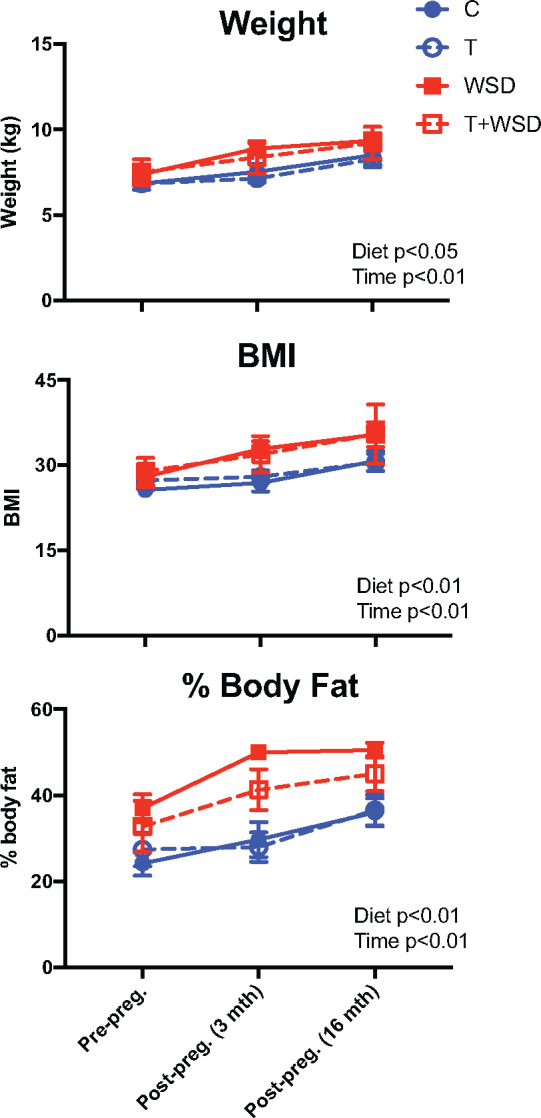
Longitudinal analysis of body composition before and after pregnancy. Body weight (kg), BMI (BW/(CR)2) and body fat (%) were compared for those animals that became pregnant at pre-pregnancy (∼6 months before mating) and at 3–4 months and 16–17 months post-pregnancy termination (third trimester). Data are presented as means ± SEM (C n = 9, T n = 9, WSD n = 7 and T + WSD n = 4). Significant effects of WSD and/or T or interactions over time as determined by a mixed models ANOVA are presented as text within the graphs. C, Control; T, testosterone; WSD, Western-style diet.
Figure 5.
Longitudinal analysis of insulin sensitivity before and after pregnancy. Fasting glucose, insulin, HOMA-IR and AUC of glucose and insulin during an ivGTT were compared for those animals that became pregnant at pre-pregnancy (∼6 months before mating), and then at 3–4 months and 16–17 months post-pregnancy termination (third trimester). Data are presented as means ± SEM (C n = 9, T n = 9, WSD n = 7 and T + WSD n = 3). Significant effects of WSD and/or T or interactions over time as determined by a mixed models ANOVA are presented as text within the graphs. C, Control; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; ivGTT, intravenous glucose tolerance test; T, testosterone; WSD, Western-style diet.
Discussion
PCOS is a complex heterogeneous disorder that is likely driven by multiple contributing factors, including hyperandrogenemia and metabolic dysfunction (Conway et al., 1989; Rosenfield and Ehrmann, 2016; Crespo et al., 2018; Escobar-Morreale, 2018). This study sought to explore the impact of elevated T and an obesogenic WSD, in isolation and in combination, on both reproductive function and whole-body metabolism. In a previous analysis of this cohort, following 3 years of treatment, the T + WSD group showed the worse metabolic outcomes, and, in several instances, more so than WSD alone (True et al., 2017). After continued exposure to these same treatments, we now show that, after 5 years of treatment differences, in body composition and metabolic parameters between the WSD and T + WSD groups became more limited. This is similar to studies analyzing women first at ∼16 years of age, and again at ∼21 years of age (McGee et al., 2014). Taken together, we hypothesize that T may accelerate the onset of metabolic dysfunction in the presence of a WSD, but weight and fat gain were similar between T + WSD and WSD groups with prolonged exposure. The one exception to this finding is that the T + WSD treatment consistently demonstrated the most significant degree of hyperinsulinemia, indicating that T may worsen the overall degree of insulin resistance in the presence of an obesogenic WSD. This hypothesis supports the observation that women with PCOS consistently have higher indices of insulin resistance than their weight-matched counterparts (Dumesic et al., 2019; Satyaraddi et al., 2019). However, data from the T + WSD group also mimics the heterogeneity within the human PCOS population, with greater variability in measures of insulin resistance (Al-Jefout et al., 2017). Moreover, after 5 years on study, all groups, including controls, demonstrated elevated body fat percentage and BMI, likely due to ad libitum feeding in all groups; therefore, treatment effects can no longer be compared to a ‘lean’ control group.
Women with PCOS are at increased risk of gestational diabetes (Pan et al., 2015; Yao et al., 2017), which, in turn, increases the risk for post-partum development of Type 2 diabetes (Bellamy et al., 2009). Previous work with this cohort found differences in glucose homeostasis across gestation; namely, an increase in fasting glucose and ivGTT glucose AUC in the T + WSD group during the latter half of pregnancy (Bishop et al., 2018a). To determine whether these treatments worsened post-partum glucose homeostasis, we investigated metabolic outcomes in the subset of animals that became pregnant before and after pregnancy. All animals displayed increasing fasting glucose, insulin and HOMA-IR over time, consistent with worsening glucose homeostasis with age and increased weight gain in all groups. However, there were no interactions with the treatment variables and time, suggesting that these treatments did not differentially affect metabolic health in the post-partum period. This outcome could be because those animals that did not become pregnant, or failed to carry a pregnancy to the third trimester, had on average higher fasting insulin and HOMA-IR (Bishop et al., 2018a). It is also possible that those animals that did become pregnant were more ‘metabolically healthy’, which might obscure differences between the groups. All pregnancies were conceived spontaneously (Bishop et al., 2018a), with no interventions to increase likelihood of pregnancy. Because use of assisted reproductive techniques is a known confounder of many studies on the impact of PCOS phenotypes and pregnancy (Palomba et al., 2015), this was avoided in the current cohort. Therefore, some potential impacts of T and WSD on metabolic dysfunction during this post-partum interval may be underappreciated in this study.
Similar to findings after 3 years of treatment, continued treatment for 5 years of either T, WSD or T + WSD did not result in cessation of ovarian cycles or amenorrhea. However, WSD exposure led to effects on ovarian hormone synthesis and cycle parameters. WSD consumption is associated with greater numbers of antral follicles in the late follicular phase. It is at this stage of the menstrual cycle when the selection of the dominant follicle occurs, and the subordinate follicles undergo atresia, resulting in the observation of a single preovulatory follicle by ultrasound (Bishop et al., 2009). Previous in vitro analysis of growth dynamics of macaque follicles isolated after in vivo short-term WSD exposure (14 months) reported a decline in follicle survival, but no differences in follicular size at the end of culture (Xu et al., 2015). The reduced follicle survival in vitro following in vivo WSD treatment may be due to length of exposure to the WSD, since analysis of follicle numbers at 4 and 5 years of treatment showed increased numbers of 1 mm or larger follicles at menses (Supplementary Fig. S1). Therefore, the effect of exposure to WSD on follicular dynamics may be related to the length of exposure.
In the preceding fertility trial, both the WSD and T + WSD groups displayed a lower pregnancy rate (Bishop et al., 2018a). One causative factor for this subfertility could be reduced P4 production due to luteal insufficiency. Lower P4 levels first manifested in the T + WSD group after 4 years of treatment, and now, at 5 years of treatment, is present in both the WSD and T + WSD groups (Supplementary Fig. S1). However, it is essential to note that P4 levels were, on average, ∼4 ng/ml in each group. In rhesus macaques, these levels are sufficient to induce a normal decidualization response by the endometrium (∼3 ng/ml (Slayden et al., 1993)), suggesting luteal insufficiency is unlikely to explain all infertility in the WSD and T + WSD groups. Lower intrafollicular P4 levels on the day of oocyte collection are associated with reduced rates of blastocyst formation in women (O’Brien et al., 2019). Therefore, lower levels of P4 may be associated with ovulation/luteinization of a follicle containing a poor-quality oocyte, which is currently under investigation in these animals.
AMH levels were previously reported to correlate with oocyte post-fertilization development, wherein women undergoing assisted reproduction with circulating AMH levels >15 pmol/l yielded oocytes with the highest blastocyst formation rates (O’Brien et al., 2019). Decreased serum AMH levels were also observed in the T + WSD group in the early follicular phase, near menses, and in the late luteal phase of the same cycle. The lower levels of AMH observed in the T + WSD group is in contrast to PCOS patients reported to possess elevated AMH levels (Pigny et al., 2003; Piltonen et al., 2005). However, the association between AMH levels and the specific PCOS phenotype can differ, whereby women with PCOS that continue to cycle, with an ovulatory phenotype, have lower AMH levels than those with oligoanovulation (Dewailly et al., 2010).T and/or WSD treatments did not significantly impact frequency of menstrual cycles, and WSD modestly decreased the number of ovulatory cycles within the study period due to a slightly increased cycle length. Therefore, these data indicate that increases in serum AMH levels may not be exclusively due to mild hyperandogenemia, at least in ovulatory nonhuman primates. It should be noted that the reduced AMH levels in the circulation observed in the present study are consistent with reported reduced production of AMH by follicles in culture removed from the ovaries of rhesus macaques that received a short-term (∼14 months) exposure to WSD compared to follicles obtained from animals consuming a standard low-fat diet (Xu et al., 2015). Consistent with this observation are findings in prenatally androgenized nonhuman primates that demonstrate increased adiposity and insulin resistance later in life, but reduced AMH similar to the current study’s T + WSD group (Dumesic et al., 2016). Lastly, a reduction in AMH is observed in female-to-male transsexuals when T treatment is used to drive virulization, albeit with male level of androgens unlike the mild level of exposure in this study (Caanen et al., 2015). Given these findings, it is possible that the elevated AMH levels observed in some cohorts of PCOS patients may be driven by factors outside of obesity and hyperandrogenemia.
Interestingly, gonadotropin data obtained after 5 years of treatment show the lowest values of LH and LH:FSH ratio in the T + WSD group (Supplementary Fig. S3), which is also in contrast to clinical findings of increased LH secretion in PCOS patients (Kazer et al., 1987; Waldstreicher et al., 1988; Malini and Roy George, 2018). In utero exposure to androgens in both NHPs and sheep leads to increased LH secretion in adulthood, suggesting that LH hypersecretion in individuals with PCOS may be programmed by androgen exposure earlier in life (Savabieasfahani et al., 2005; Abbott et al., 2018). Epigenetic alterations that occur in response to either androgen or diet may differ depending on when during development the exposure occurs. Thus, in utero or perinatal exposure to T and/or WSD could lead to epigenetic alterations in the fetus that ultimately yield similar changes in endocrine factors (i.e. AMH, LH/FSH ratio) observed in women with PCOS relative to our prepubertal exposure model. While PCOS is associated with elevated LH levels, obesity is known to reduce LH pulsatility in women (Pagan et al., 2006; Jain et al., 2007); therefore, our current findings could also indicate that the low LH in the T + WSD is secondary to the higher adiposity observed in this group. Lastly, as an alternative explanation, genetic influences are known to play a role in PCOS etiology outside of androgens and obesity may contribute to these hormonal changes in PCOS (Kosova and Urbanek, 2013; McAllister et al., 2015).
In conclusion, these data demonstrate continued exposure to chronic, mild hyperandrogenemia exacerbates the metabolic defects associated with the consumption of an obesogenic WSD. While females in the treatment groups did not experience cessation of menstrual cycles, previous data from this cohort indicated that combined hyperandrogenemia and WSD result in a high proportion of infertility/subfertility (Bishop et al., 2018a), possibly due to impaired endometrial receptivity (Bishop et al., 2018b) or ovulation of poor-quality oocytes (Bishop et al., 2019). These data also indicate that consumption of a WSD in the absence of hyperandrogenemia impairs reproductive parameters, but these effects take longer to manifest and may be secondary to alterations in metabolic function. The data reported here indicate prolonged exposure to these insults alters the metabolic status and reproductive phenotype of the female primate as she transitions into a young adult.
Data availability
The data underlying this article will be shared upon reasonable request to the corresponding author.
Supplementary Material
Acknowledgements
We would like to acknowledge the Department of Comparative Medicine and the Endocrine Technologies Core at the Oregon National Primate Research Center.
Authors’ roles
C.B. and C.T. compiled and analyzed the data and wrote the manuscript. D.T. and E.M. performed studies and analyzed the data. O.S., C.T.R. and J.H. contributed to study design and provided feedback on data interpretation and the manuscript.
Funding
National Institutes of Health (grant P50 HD071836 to C.T.R., J.H. and C.T. and P51 OD011092 for support of the Oregon National Primate Research Center). Grant P50 HD071836 is administered by the Eunice Kennedy Shriver National Institute of Child Health & Human Development, and P51 OD011092 is administered by the Office of The Director, National Institutes of Health
Conflict of interest
Authors declare no additional conflicts of interest.
References
- Abbott DH, Vepraskas SH, Horton TH, Terasawa E, Levine JE. Accelerated episodic luteinizing hormone release accompanies blunted progesterone regulation in PCOS-like female rhesus monkeys (Macaca mulatta) exposed to testosterone during early-to-mid gestation. Neuroendocrinology 2018;107:133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Jefout M, Alnawaiseh N, Al-Qtaitat A. Insulin resistance and obesity among infertile women with different polycystic ovary syndrome phenotypes. Sci Rep 2017;7:5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azziz R, Carmina E, Chen Z, Dunaif A, Laven JSE, Legro RS, Lizneva D, Natterson-Horowtiz B, Teede HJ, Yildiz BO. et al. Polycystic ovary syndrome. Nat Rev Dis Primers 2016;2:16057. [DOI] [PubMed] [Google Scholar]
- Bellamy L, Casas J-P, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 2009;373:1773–1779. [DOI] [PubMed] [Google Scholar]
- Bishop CV, Mishler EC, Takahashi DL, Reiter TE, Bond KR, True CA, Slayden OD, Stouffer RL. Chronic hyperandrogenemia in the presence and absence of a Western-style diet impairs ovarian and uterine structure/function in young adult rhesus monkeys. Hum Reprod 2018. b;33:128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop CV, Reiter TE, Erikson DW, Hanna CB, Daughtry BL, Chavez SL, Hennebold JD, Stouffer RL. Chronically elevated androgen and/or consumption of a Western-style diet impairs oocyte quality and granulosa cell function in the nonhuman primate periovulatory follicle. J Assist Reprod Genet 2019;36:1497–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop CV, Sparman ML, Stanley JE, Bahar A, Zelinski MB, Stouffer RL. Evaluation of antral follicle growth in the macaque ovary during the menstrual cycle and controlled ovarian stimulation by high-resolution ultrasonography. Am J Primatol 2009;71:384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop CV, Stouffer RL, Takahashi DL, Mishler EC, Wilcox MC, Slayden OD, True CA. Chronic hyperandrogenemia and Western-style diet beginning at puberty reduces fertility and increases metabolic dysfunction during pregnancy in young adult, female macaques. Hum Reprod 2018. a;33:694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, Monauni T, Muggeo M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 2000;23:57–63. [DOI] [PubMed] [Google Scholar]
- Brewer CJ, Balen AH. The adverse effects of obesity on conception and implantation. Reproduction 2010;140:347–364. [DOI] [PubMed] [Google Scholar]
- Caanen MR, Soleman RS, Kuijper EAM, Kreukels BPC, De Roo C, Tilleman K, De Sutter P, van Trotsenburg MAA, Broekmans FJ, Lambalk CB. et al. Antimullerian hormone levels decrease in female-to-male transsexuals using testosterone as cross-sex therapy. Fertil Steril 2015;103:1340–1345. [DOI] [PubMed] [Google Scholar]
- Clark AM, Ledger W, Galletly C, Tomlinson L, Blaney F, Wang X, Norman RJ. Weight loss results in significant improvement in pregnancy and ovulation rates in anovulatory obese women. Hum Reprod 1995;10:2705–2712. [DOI] [PubMed] [Google Scholar]
- Clark AM, Thornley B, Tomlinson L, Galletley C, Norman RJ. Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum Reprod 1998;13:1502–1505. [DOI] [PubMed] [Google Scholar]
- Conway GS, Honour JW, Jacobs HS. Heterogeneity of the polycystic ovary syndrome: clinical, endocrine and ultrasound features in 556 patients. Clin Endocrinol 1989;30:459–470.), [DOI] [PubMed] [Google Scholar]
- Crespo RP, Bachega TASS, Mendonça BB, Gomes LG. An update of genetic basis of PCOS pathogenesis. Arch Endocrinol Metab 2018;62:352–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewailly D, Pigny P, Soudan B, Catteau-Jonard S, Decanter C, Poncelet E, Duhamel A. Reconciling the definitions of polycystic ovary syndrome: the ovarian follicle number and serum anti-Mullerian hormone concentrations aggregate with the markers of hyperandrogenism. J Clin Endocrinol Metab 2010;95:4399–4405. [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Akopians AL, Madrigal VK, Ramirez E, Margolis DJ, Sarma MK, Thomas AM, Grogan TR, Haykal R, Schooler TA. et al. Hyperandrogenism accompanies increased intra-abdominal fat storage in normal weight polycystic ovary syndrome women. J Clin Endocrinol Metab 2016;101:4178–4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumesic DA, Phan JD, Leung KL, Grogan TR, Ding X, Li X, Hoyos LR, Abbott DH, Chazenbalk GD. Adipose insulin resistance in normal-weight women with polycystic ovary syndrome. J Clin Endocrinol Metab 2019;104:2171–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol 2018;14:270–284. [DOI] [PubMed] [Google Scholar]
- Jain A, Polotsky AJ, Rochester D, Berga SL, Loucks T, Zeitlian G, Gibbs K, Polotsky HN, Feng S, Isaac B. et al. Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J Clin Endocrinol Metab 2007;92:2468–2473. [DOI] [PubMed] [Google Scholar]
- Kazer RR, Kessel B, Yen SS. Circulating luteinizing hormone pulse frequency in women with polycystic ovary syndrome. J Clin Endocrinol Metab 1987;65:233–236. [DOI] [PubMed] [Google Scholar]
- Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 2002;25:1862–1868. [DOI] [PubMed] [Google Scholar]
- Kosova G, Urbanek M. Genetics of the polycystic ovary syndrome. Mol Cell Endocrinol 2013;373:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malini NA, Roy George K. Evaluation of different ranges of LH:FSH ratios in polycystic ovarian syndrome (PCOS) - clinical based case control study. Gen Comp Endocrinol 2018;260:51–57. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- McAllister JM, Legro RS, Modi BP, Strauss JF. Functional genomics of PCOS: from GWAS to molecular mechanisms. Trends Endocrinol Metab 2015;26:118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney CR, Blank SK, Prendergast KA, Chhabra S, Eagleson CA, Helm KD, Yoo R, Chang RJ, Foster CM, Caprio S. et al. Obesity and sex steroid changes across puberty: evidence for marked hyperandrogenemia in pre- and early pubertal obese girls. J Clin Endocrinol Metab 2007;92:430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney CR, Prendergast KA, Chhabra S, Eagleson CA, Yoo R, Chang RJ, Foster CM, Marshall JC. The association of obesity and hyperandrogenemia during the pubertal transition in girls: obesity as a potential factor in the genesis of postpubertal hyperandrogenism. J Clin Endocrinol Metab 2006;91:1714–1722. [DOI] [PubMed] [Google Scholar]
- McGee WK, Bishop CV, Pohl CR, Chang RJ, Marshall JC, Pau FK, Stouffer RL, Cameron JL. Effects of hyperandrogenemia and increased adiposity on reproductive and metabolic parameters in young adult female monkeys. Am J Physiol Endocrinol Metab 2014;306:E1292–E1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien Y, Wingfield M, O’Shea LC. Anti-Mullerian hormone and progesterone levels in human follicular fluid are predictors of embryonic development. Reprod Biol Endocrinol 2019;17:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagan YL, Srouji SS, Jimenez Y, Emerson A, Gill S, Hall JE. Inverse relationship between luteinizing hormone and body mass index in polycystic ovarian syndrome: investigation of hypothalamic and pituitary contributions. J Clin Endocrinol Metab 2006;91:1309–1316. [DOI] [PubMed] [Google Scholar]
- Palomba S, de Wilde MA, Falbo A, Koster MPH, La Sala GB, Fauser BCJM. Pregnancy complications in women with polycystic ovary syndrome. Hum Reprod Update 2015;21:575–592. [DOI] [PubMed] [Google Scholar]
- Pan M-L, Chen L-R, Tsao H-M, Chen K-H. Relationship between polycystic ovarian syndrome and subsequent gestational diabetes mellitus: a nationwide population-based study. PLoS One 2015;10:e0140544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali R, Pelusi C, Genghini S, Cacciari M, Gambineri A. Obesity and reproductive disorders in women. Hum Reprod Update 2003;9:359–372. [DOI] [PubMed] [Google Scholar]
- Pigny P, Merlen E, Robert Y, Cortet-Rudelli C, Decanter C, Jonard S, Dewailly D. Elevated serum level of anti-Mullerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab 2003;88:5957–5962. [DOI] [PubMed] [Google Scholar]
- Piltonen T, Morin-Papunen L, Koivunen R, Perheentupa A, Ruokonen A, Tapanainen JS. Serum anti-Mullerian hormone levels remain high until late reproductive age and decrease during metformin therapy in women with polycystic ovary syndrome. Hum Reprod 2005;20:1820–1826. [DOI] [PubMed] [Google Scholar]
- Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev 2016;37:467–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin KH, Glintborg D, Nybo M, Abrahamsen B, Andersen M. Development and risk factors of type 2 diabetes in a nationwide population of women with polycystic ovary syndrome. J Clin Endocrinol Metab 2017;102:3848–3857. [DOI] [PubMed] [Google Scholar]
- Satyaraddi A, Cherian K, Kapoor N, Kunjummen A, Kamath MS, Thomas N, Paul TV. Body composition, metabolic characteristics, and insulin resistance in obese and nonobese women with polycystic ovary syndrome. J Hum Reprod Sci 2019;12:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savabieasfahani M, Lee JS, Herkimer C, Sharma TP, Foster DL, Padmanabhan V. Fetal programming: testosterone exposure of the female sheep during midgestation disrupts the dynamics of its adult gonadotropin secretion during the periovulatory period. Biol Reprod 2005;72:221–229. [DOI] [PubMed] [Google Scholar]
- Slayden OD, Hirst JJ, Brenner RM. Estrogen action in the reproductive tract of rhesus monkeys during antiprogestin treatment. Endocrinology 1993;132:1845–1856. [DOI] [PubMed] [Google Scholar]
- True CA, Takahashi DL, Burns SE, Mishler EC, Bond KR, Wilcox MC, Calhoun AR, Bader LA, Dean TA, Ryan ND. et al. Chronic combined hyperandrogenemia and Western-style diet in young female rhesus macaques causes greater metabolic impairments compared to either treatment alone. Hum Reprod 2017;32:1880–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldstreicher J, Santoro NF, Hall JE, Filicori M, Crowley WF. Hyperfunction of the hypothalamic-pituitary axis in women with polycystic ovarian disease: indirect evidence for partial gonadotroph desensitization. J Clin Endocrinol Metab 1988;66:165–172. [DOI] [PubMed] [Google Scholar]
- Xu J, McGee WK, Bishop CV, Park BS, Cameron JL, Zelinski MB, Stouffer RL. Exposure of female macaques to Western-style diet with or without chronic T in vivo alters secondary follicle function during encapsulated 3-dimensional culture. Endocrinology 2015;156:1133–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao K, Bian C, Zhao X. Association of polycystic ovary syndrome with metabolic syndrome and gestational diabetes: aggravated complication of pregnancy. Exp Ther Med 2017;14:1271–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.