Abstract
[Purpose] We aimed to examine the accuracy of heart rate monitors worn on the wrist by patients with stroke. The wrist worn heart rate monitor could improve the quality of rehabilitation by monitoring exercise intensity during physical therapy. [Participants and Methods] Thirty inpatients with subacute hemiparetic stroke wore heart rate monitors on both (non-paretic and paretic) wrists, as well as a chest heart rate monitor. We recorded the heart rate values measured at the wrist and chest every minute during physical therapy sessions. The wrist monitors were an optical heart rate measurement device based on photoplethysmography, and the chest monitor was a traditional chest device based on electrocardiography. The relative and absolute reliabilities between the heart rate measurements from the wrist and chest monitors were calculated. [Results] The intraclass correlation coefficients for model 2.1 ranged from 0.75 to 0.79. Bland-Altman analysis revealed a very slight fixed bias; however, no significant proportional bias was observed. For the non-paretic and paretic sides, the lower and upper limits of agreement ranged from −21.8 to 23.8 beats/min and from −20.8 to 21.6 beats/min, and the mean absolute percentage errors were 6.7% and 5.9%, respectively. The Cohen’s d value was small. [Conclusion] The relative reliability of the wrist heart rate monitors was substantial. The absolute reliability as bias in wrist heart rate and chest heart rate was small, but heart rates estimated from wrist monitors were not particularly accurate.
Key words: Exercise intensity, Reliability, Wearable device
INTRODUCTION
Aerobic exercise and moderate-to-vigorous activity for patients with stroke are strongly recommended to improve motor function and physical fitness1,2,3). Experimental animal studies have shown that early aerobic exercise reduces lesion volume, oxidative damage, inflammation and cell death, and promotes neurogenesis and angiogenesis4). Neurotrophins such as brain-derived neurotrophic factor, insulin-like growth factor-I and nerve growth factor are secreted during aerobic exercise and promote neuroplasticity and recovery following stroke5, 6). Human studies have shown that aerobic exercise improves aerobic fitness, functional performance, cognitive function and quality of life7,8,9,10,11). In addition, exercise combined with cardiorespiratory training and muscle-strengthening effects tend to reduce mortality in patients with stroke, although the odds ratio is large12). Aerobic exercise thus appears important for patients with stroke.
Exercise intensity can be estimated using heart rates (HRs), rating of perceived exertion (RPE) and the Talk test13). However, RPE and the Talk test can vary in their ability to estimate exercise intensity. RPE and the Talk test are subjective methods to estimate exercise intensity. In addition, some patients with stroke have cognitive disabilities that could affect subjective perceptions of exercise intensity. However, HR is an objective marker for estimating exercise intensity and is unaffected by the cognitive condition of the patient. In the management of exercise intensity, exercise tolerance test by objective means is the best way to set aerobic exercise. HR thus remains the preferred means of monitoring and consequently prescribing exercise intensity14). Measuring HR is thus useful for monitoring aerobic exercise intensity.
The current gold standard for monitoring HR is electrocardiography (ECG), in which electrodes are placed on various sites of the upper body to measure electrical signals produced by the heart. However, ECG is expensive and electrical leads placed on the chest are attached to a telemeter and can thus impede physical therapy, which comprises various exercises. HR-monitoring devices should ideally be simple and lightweight. Optical blood flow sensing by photoplethysmography (PPG) is a popular optical means of monitoring HR by detecting changes in blood volume15) and devices can be conveniently worn on the upper arm or wrist.
Heart rate monitors worn on the wrist by healthy young adults are accurate and offer a valid means of monitoring HR at rest or while walking or running16,17,18,19,20). However, ageing is one factor associated with increased arterial stiffness, leading to changes in peripheral pulses propagation. In young adults, the characteristics of peripheral pulses in PPG are a steep rise and early peak21, 22). In elderly adults, the peripheral pulse wave in PPG shows a blunted slope and the systolic rise is more gradual21, 22). These characteristics of aging would affect the accuracy of HR measurements at the wrist, because HR measurements from the wrist are determined from the steep pulse intervals. In addition, arterial stiffness is higher in patients with stroke than in people without stroke23).
Patients with stroke showed reduced blood flow in the paretic lower limb24,25,26). In addition, vasomotor dysfunction was observed by flowmetry in patients with stroke27, 28). These responses indicate that HR measurement at the wrist using PPG may not be accurate. On the other hand, patients swing their arms irregularly and reach in various directions during exercise, and this can result in HR monitors worn on the wrist delivering variable results during physical therapy, because PPG signals are very susceptible by motion artifacts29). Comparing HR based on ECG, as the gold standard for monitoring HR, with HR measured at the wrist (wrist HR; WHR) based on PPG is important to investigate accuracy of HR monitors worn on the wrist.
The present study aimed to determine the accuracy of WHR monitors among patients with stroke. We postulated that WHR monitors should be reliable enough to monitor HR in such patients. If patients with stroke can wear wrist devices during physical therapy, aerobic exercise would be easier to manage, promoting physical and cognitive functions and overall health.
PARTICIPANTS AND METHODS
Inpatients with subacute hemiparetic stroke at hospital were recruited between April and September 2018. Patients were usually treated with acute care for ≥2 weeks in an acute stroke unit until physical condition was stable. Inclusion criteria comprised hemiparetic stroke regardless of severity of paralysis and walking independence levels, no exercise restriction due to medical condition and ability to perform exercise during physical therapy. All participants were undertaking a 40- to 80-min physical therapy program that comprised exercises for range of motion, muscle strength, postural balance, transferring, and walking. Exclusion criteria comprised bilateral hemiparesis or inability to understand the study protocol or instructions provided by physical therapists. Thirty-two participants were initially recruited to this study. One patient was excluded due to an inability to follow instructions provided by a physical therapist and another dropped out after the chest HR monitor caused an adverse reaction of itch on the chest. Data from 30 participants were thus analyzed. Mean (standard deviation; SD) age of participants was 69.7 (11.3) years, 11 and 19 patients had right and left paralysis, respectively, and 13, 5 and 12 patients had assisted, supervised and independent gait, respectively. Four patients had a history of heart disease, in the form of atrial fibrillation (AF; n=2), angina (n=1) and myocardial infarction (n=1) (Table 1).
Table 1. Characteristics of 30 participants.
| Mean ± SD | n | |||
| Age (years) | 69.7 ± 11.3 | |||
| Male/Female | 17/13 | |||
| Height (m) | 1.60 ± 0.09 | |||
| Weight (kg) | 55.8 ± 11.9 | |||
| Body mass index (kg/m²) | 21.7 ± 3.7 | |||
| Ischemic/hemorrhagic stroke | 15/15 | |||
| Left/right hemiparesis | 19/11 | |||
| Brunnstrom Recovery Stage, 1/2/3/4/5/6 | ||||
| Upper extremity | 2/7/2/3/7/9 | |||
| Finger | 4/6/1/0/8/11 | |||
| Lower extremity | 0/7/2/3/5/13 | |||
| Gait ability (assisted/supervised/independent) | 13/5/12 | |||
| mRS, 0/1/2/3/4/5/6 | 0/3/6/6/14/1 | |||
| History of heart disease, angina/myocardial infarction/atrial fibrillation | 1/1/2 | |||
mRS: modified Rankin scale; SD: standard deviation.
This cross-sectional study was undertaken at a hospital in Gunma, Japan. All patients wore WHR monitors on the non-paretic and paretic wrists and a chest HR (CHR) monitor. WHR and CHR were measured during physical therapy sessions three times within 1 week. Sample size was not calculated. STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines were followed to standardize the present study30).
We used Polar A370 WHR monitors (Polar Electro, Kempele, Finland) and Polar H10 CHR monitors (Polar Electro, Kempele, Finland), connected to an Actigraph-wGT3X-BT (Manufacturing Technology, Pensacola, United States) to monitor physical activity31). The Polar A370 optically senses blood flow and provides HR based on perfusion in wrist blood vessels using PPG. The Polar H10 is ECG-based and has been used in many studies with accuracy comparable to that of ECG18, 32,33,34). CHR was used as the measurement criterion. Sampling frequency was 1 Hz for both the Polar H10 and Polar A370. These devices provided time-stamped HR data. For our analyses, we extracted values from the 3 devices every minute, then time-matched all non-zero HR data from the Polar H10 and Polar A370.
We analyzed age, height, body weight, body mass index, type of stroke, history of heart disease, Brunnstrom Recovery Stage (BRS), gait independence and modified Rankin Scale (mRS) scores to define the characteristics of the participants. The BRS was used to assess paretic severity in upper and lower extremities and fingers. Scores range from 1 (flaccidity and no limb movement on the affected side) to 6 (near-normal to normal movement and coordination)35). Higher scores represent better motor function. Gait was assessed as “assisted”, “supervised” or “independent” during physical therapy sessions. The mRS assesses the severity of post-stroke disabilities based on the degree of disability or independence in activities of daily living, with scores ranging from 0 (asymptomatic, independent) to 5 (severely disabled, requiring constant nursing care)36).
Data were statistically analyzed using R version 3.6.1 software (R Foundation for Statistical Computing, Vienna, Austria). An HR dataset was excluded when CHR data were missing. To estimate relative reliability, we used intraclass correlation coefficients (ICCs) for model 2,1 with 95% confidence intervals (95%CIs) between WHR and CHR, using the psych package37). ICCs were interpreted as: 0.0–0.2, slight; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, substantial; and 0.81–1.0, excellent38). To estimate absolute reliability, we used the mean absolute percentage error (MAPE), mixed-model Bland-Altman plots, and Cohen’s d. MAPE was calculated to gauge general measurement errors of WHR and CHR (MAPE=absolute error/criterion ×100). We considered a MAPE <10% as indicative of accurate results39). Mixed-model Bland-Altman plots and limits of agreement (LOA) were constructed for WHR and CHR, including participant as a random effect, using the nlme package40,41,42). LOA were formulated as mean ± 1.96 SD. To investigate systematic bias, fixed bias was calculated from the LOA and 95%CI of the difference (Δ) between WHR and CHR. On the other hand, proportional bias was analyzed as Pearson’s correlations with 95%CI between the difference and mean of WHR and CHR. Cohen’s d were calculated for differences among non-paretic WHR, paretic WHR and CHR, using the effsize package43, 44). The level of significance was set at 0.05.
The institutional review boards at the Geriatrics Research Institute and hospital approved this study (approval no. 60) and all participants provided written informed consent to participate.
RESULTS
The WHR and CHR amassed a total of 5,129 data points, from which 69 missing data points were excluded. We finally analyzed 5,060 data points. Mean differences (SD; 95%CI) between non-paretic or paretic WHR and CHR were 1.0 (11.6; 0.7, 1.3) beats/min and 0.4 (10.8; 0.1, 0.7) beats/min, respectively (Table 2). Cohen’s d between WHR and CHR were 0.09 on the non-paretic side and 0.09 on the paretic side. Cohen’s d between non-paretic and paretic sides was 0.06.
Table 2. Comparison of chest and wrist HR (n=5,060).
| Mean | SD | Min | Max | 95%CI | |||
| Lower | Upper | ||||||
| CHR (beats/min) | 83.3 | 17.1 | 22.0 | 200.0 | |||
| Non-paretic WHR (beats/min) | 84.3 | 15.8 | 38.0 | 158.0 | |||
| Paretic WHR (beats/min) | 83.7 | 16.2 | 44.0 | 157.0 | |||
| Difference between non-paretic WHR and CHR (beats/min) | 1.0 | 11.6 | −103.0 | 79.0 | 0.7 | 1.3 | |
| Difference between paretic WHR and CHR (beats/min) | 0.4 | 10.8 | −98.0 | 76.0 | 0.1 | 0.7 | |
| Mean non-paretic WHR and CHR (beats/min) | 83.8 | 15.4 | 41.0 | 148.5 | |||
| Mean paretic WHR and CHR (beats/min) | 83.5 | 15.7 | 41.5 | 152.0 | |||
Mean MAPE (SD) on non-paretic and paretic sides were 6.7 (6.7) % and 5.9 (4.1) %, respectively. Cohen’s d between WHR and CHR were 0.09 for both non-paretic and paretic sides. Cohen’s d between non-paretic and paretic sides was 0.06.
CHR: chest heart rate; CI: confidence interval; MAPE: mean absolute percentage error; SD: standard deviation; WHR: wrist heart rate.
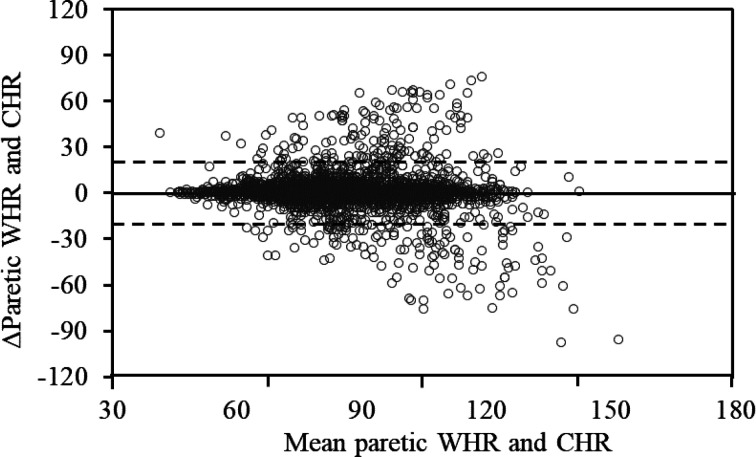
Bland-Altman plots showed the error distribution (Figs. 1, 2). On non-paretic and paretic sides, lower and upper LOA in mixed models were −21.8 beats/min and 23.8 beats/min and −20.8 beats/min and 21.6 beats/min, respectively.
Fig. 1.
Bland-Altman plots of WHR and CHR on the non-paretic side.
Solid line indicates mean ΔWHR and CHR. Dashed lines represent the upper and lower limits of agreement (LOA) for WHR and CHR. Lower and upper LOA were −21.8 beats/min and 23.8 beats/min, respectively. Pearson’s correlation coefficient [95%CI] for mean and difference (Δ) between WHR and CHR was −0.11 [−0.14, −0.08] (p<0.05).
CHR: chest heart rate; ΔWHR and CHR: difference between wrist heart rate and chest heart rate; CI: confidence interval; WHR: wrist heart rate.
Fig. 2.
Bland-Altman plots of WHR and CHR in paretic side.
Solid line indicates mean ΔWHR and CHR. Dashed lines represent upper and lower limits of agreement (LOA) of WHR and CHR. Lower and upper LOA were −20.8 beats/min and 21.6 beats/min, respectively. Pearson’s correlation coefficient [95%CI] for mean and difference (Δ) between WHR and CHR was −0.08 [−0.11, −0.06] (p<0.05).
CHR: chest heart rate; ΔWHR and CHR: difference between wrist heart rate and chest heart rate; CI: confidence interval; WHR: wrist heart rate.
Table 3 shows that the ICC (2.1; 95%CI) between WHR and CHR were 0.75 (0.74, 0.76) (p<0.05) and 0.79 (0.78, 0.80) (p<0.05) on the non-paretic and paretic sides, respectively.
Table 3. Intraclass correlation coefficients between WHR and CHR.
| ICC (2.1) | 95%CI | |||
| Lower | Upper | |||
| Non-paretic WHR and CHR | 0.75 | 0.74 | 0.76 | |
| Paretic WHR and CHR | 0.79 | 0.78 | 0.80 | |
| Non-paretic and paretic WHR | 0.81 | 0.81 | 0.82 | |
All ICCs were significant (p<0.05).
CHR: chest heart rate; CI: confidence interval; ICC: intraclass correlation coefficient; WHR: wrist heart rate.
DISCUSSION
We investigated the accuracy of WHR monitors that used PPG to continually measure HR during physical therapy sessions. Relative reliability of WHR compared to CHR was substantial by ICC. Very slight fixed bias was revealed, but no significant proportional bias was evident from Bland-Altman plots. LOAs on non-paretic and paretic sides ranged widely. MAPEs were small for both non-paretic and paretic sides.
In terms of relative reliability, the ICC of WHR and CHR was substantial. Correlations between WHR and CHR were close for walking, but moderate for other activities18, 45). The change in vascular volume was larger at peripheral sites such as the wrist and finger when motion artifacts occurred, which could cause a large effect on the volume of distributed blood46). This could explain the lower correlations between WHR and CHR in the present investigation, compared with previous studies.
In terms of absolute reliability as the precision of WHR, MAPEs were <10% on both non-paretic and paretic sides. This indicated that monitoring HR on non-paretic and paretic wrists offers a valid way to refer to cut-off points of MAPE <10%39). However, LOAs between WHR and CHR in Bland-Altman plots were large. These results indicate that the deviance of differences between WHR and CHR was large. Previous studies analyzing differences in WHR and CHR yielded similar differences to our values16,17,18,19,20, 47). In terms of the validity of LOA, the acceptance value was less 15–20% between the current reference and new technique48). In our study, LOAs ranged by ± 24–28% between WHR and CHR. This poor prediction ability might be attributable to motion artifacts29). In terms of systematic bias, fixed biases as differences in WHR and CHR were very small, and the 95%CI was associated with a small positive bias with WHR. Some reports have indicated that the mean difference between WHR and CHR ranged from −12.8 to 8.9 beats/min, with a standard deviation ranging from 14.4 to 33.5 during rest, resistance training, activities of daily living and treadmill walking18, 19). Compared with past studies, the difference was not large in our study. This small bias might indicate that vasomotor dysfunction in vessels is not severe enough to present measurement of WHR among hemiparetic inpatients with stroke.
On the other hand, no significant proportional bias was observed by associations between difference and mean of WHR and CHR. The values of Pearson’s correlations ranged from −0.11 to −0.08, which would be interpreted as showing little or no correlation. These very weak correlations would be acceptable to use WHR monitors during physical therapy.
Paretic WHR was slightly accurate than non-paretic WHR in MAPE and difference between WHR and CHR. Patients with stroke exercised for longer periods for gait, sit-to-stand and pre-gait activity, indicating that the duration of functional training of a paretic upper limb is shorter than that of locomotor exercise49, 50). In addition, patients with stroke were in the post-acute phase and might have been able to use their non-paretic arm to support balance by holding on to walking aids. Difference of accuracy in paretic side and non-paretic side could be related with use of arm and hand support causing motion artifact46).
We included two patients with AF in the analysis of WHR monitors. Measuring HR might be more difficult in patients with AF, due to the theoretical technique applied to interpret sampling frequency and delayed pressure waveforms51). However, measuring HR using PPG is practical, and AF could be detected52, 53). In addition, WHR monitors are useful to manage AF and could measure the HR of patients with AF54). The MAPE of two patients with AF in the present study were 5.8% and 12.5% on the non-paretic side and 5.86% and 17.4% on the paretic side. These values were not particularly high compared with previous studies18, 47). Atrial fibrillation is usually treated during the acute phase, indicating stable HR control in these patients with post-acute AF. In the present study, motion artifacts or active exercise might have had more effect on HR difference than AF. However, the influence of AF on WHR monitoring was not clear from our study.
One limitation of this study was the synchronization of WHR and CHR. Setting precisely the same time for each device is difficult, because they have different algorithms for setting time. The HR generated by each device was sampled at slightly different times. Moreover, the reliability of the Polar A370 WHR monitor is not generalizable, because the accuracy of WHR monitors differs across devices20, 48). We did not determine the accuracy of WHR monitors for patients with arrhythmias such as AF, or generalize its reliability for patients with heart disease. Further studies are needed to explore the reliability of other devices and the benefits they might offer to patients with arrhythmia.
In conclusions, this study investigated the accuracy of WHR monitors compared with CHR monitors. The relative reliability of WHR was substantial compared with CHR. On the other hand, the absolute reliability as bias in WHR and CHR was small, but estimation of HR measured at the wrist was not particularly accurate because of the wide LOA. The fixed bias was very slight, but no significant proportional bias was observed in WHR. The wrist worn heart rate monitor has potential of improving rehabilitation quality measuring exercise intensity. Physical therapist would easily manage various exercise or activity intensity by wrist worn monitor.
Conflict of interest
The authors have no conflicts of interest to declare.
Acknowledgments
We wish to thank all the participants, Dr. Yasujiro Sakai, Dr. Kohei Kurokawa, Kazuya Fuji, Saito Toru, Shiori Kasahara, Takahumi Shimura, and Chihiro Tajima for support with data collection.
REFERENCES
- 1.Hebert D, Lindsay MP, McIntyre A, et al. : Canadian stroke best practice recommendations: stroke rehabilitation practice guidelines, update 2015. Int J Stroke, 2016, 11: 459–484. [DOI] [PubMed] [Google Scholar]
- 2.Billinger SA, Arena R, Bernhardt J, et al. American Heart Association Stroke Council,Council on Cardiovascular and Stroke Nursing,Council on Lifestyle and Cardiometabolic Health,Council on Epidemiology and Prevention,Council on Clinical Cardiology: Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 2014, 45: 2532–2553. [DOI] [PubMed] [Google Scholar]
- 3.Khadilkar A, Phillips K, Jean N, et al. Ottawa Panel: Ottawa panel evidence-based clinical practice guidelines for post-stroke rehabilitation. Top Stroke Rehabil, 2006, 13: 1–269. [DOI] [PubMed] [Google Scholar]
- 4.Austin MW, Ploughman M, Glynn L, et al. : Aerobic exercise effects on neuroprotection and brain repair following stroke: a systematic review and perspective. Neurosci Res, 2014, 87: 8–15. [DOI] [PubMed] [Google Scholar]
- 5.Ploughman M, Austin MW, Glynn L, et al. : The effects of poststroke aerobic exercise on neuroplasticity: a systematic review of animal and clinical studies. Transl Stroke Res, 2015, 6: 13–28. [DOI] [PubMed] [Google Scholar]
- 6.Morais VA, Tourino MF, Almeida AC, et al. : A single session of moderate intensity walking increases brain-derived neurotrophic factor (BDNF) in the chronic post-stroke patients. Top Stroke Rehabil, 2018, 25: 1–5. [DOI] [PubMed] [Google Scholar]
- 7.Pang MY, Charlesworth SA, Lau RW, et al. : Using aerobic exercise to improve health outcomes and quality of life in stroke: evidence-based exercise prescription recommendations. Cerebrovasc Dis, 2013, 35: 7–22. [DOI] [PubMed] [Google Scholar]
- 8.Boyne P, Welge J, Kissela B, et al. : Factors influencing the efficacy of aerobic exercise for improving fitness and walking capacity after stroke: a meta-analysis with meta-regression. Arch Phys Med Rehabil, 2017, 98: 581–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kendall BJ, Gothe NP: Effect of aerobic exercise interventions on mobility among stroke patients: a systematic review. Am J Phys Med Rehabil, 2016, 95: 214–224. [DOI] [PubMed] [Google Scholar]
- 10.Zheng G, Zhou W, Xia R, et al. : Aerobic exercises for cognition rehabilitation following stroke: a systematic review. J Stroke Cerebrovasc Dis, 2016, 25: 2780–2789. [DOI] [PubMed] [Google Scholar]
- 11.McDonnell MN, Smith AE, Mackintosh SF: Aerobic exercise to improve cognitive function in adults with neurological disorders: a systematic review. Arch Phys Med Rehabil, 2011, 92: 1044–1052. [DOI] [PubMed] [Google Scholar]
- 12.Naci H, Ioannidis JP: Comparative effectiveness of exercise and drug interventions on mortality outcomes: metaepidemiological study. BMJ, 2013, 347: f5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canadian Partnership for Stroke Recovery: Aerobic exercise recommendations to optimize best practices in care after stroke: AEROBICS 2019 Update. https://www.strokengine.ca/wp-content/uploads/2019/03/AEROBICS-2019-last-revised-March.pdf. (Accessed Jan. 10. 2020) [DOI] [PMC free article] [PubMed]
- 14.Hills AP, Mokhtar N, Byrne NM: Assessment of physical activity and energy expenditure: an overview of objective measures. Front Nutr, 2014, 1: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen J: Photoplethysmography and its application in clinical physiological measurement. Physiol Meas, 2007, 28: R1–R39. [DOI] [PubMed] [Google Scholar]
- 16.Stahl SE, An HS, Dinkel DM, et al. : How accurate are the wrist-based heart rate monitors during walking and running activities? Are they accurate enough? BMJ Open Sport Exerc Med, 2016, 2: e000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thiebaud RS, Funk MD, Patton JC, et al. : Validity of wrist-worn consumer products to measure heart rate and energy expenditure. Digit Health, 2018, 4: 2055207618770322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddy RK, Pooni R, Zaharieva DP, et al. : Accuracy of wrist-worn activity monitors during common daily physical activities and types of structured exercise: evaluation study. JMIR Mhealth Uhealth, 2018, 6: e10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallen MP, Gomersall SR, Keating SE, et al. : Accuracy of heart rate watches: implications for weight management. PLoS One, 2016, 11: e0154420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Claes J, Buys R, Avila A, et al. : Validity of heart rate measurements by the Garmin Forerunner 225 at different walking intensities. J Med Eng Technol, 2017, 41: 480–485. [DOI] [PubMed] [Google Scholar]
- 21.Zahedi E, Chellappan K, Ali MA, et al. : Analysis of the effect of ageing on rising edge characteristics of the photoplethysmogram using a modified Windkessel model. Cardiovasc Eng, 2007, 7: 172–181. [DOI] [PubMed] [Google Scholar]
- 22.Yousef Q, Reaz MB, Ali MA: The analysis of PPG morphology: investigating the effects of aging on arterial compliance. Meas Sci Rev, 2012, 12: 266–271. [Google Scholar]
- 23.Tuttolomondo A, Di Sciacca R, Di Raimondo D, et al. : Arterial stiffness indexes in acute ischemic stroke: relationship with stroke subtype. Atherosclerosis, 2010, 211: 187–194. [DOI] [PubMed] [Google Scholar]
- 24.Landin S, Hagenfeldt L, Saltin B, et al. : Muscle metabolism during exercise in hemiparetic patients. Clin Sci Mol Med, 1977, 53: 257–269. [DOI] [PubMed] [Google Scholar]
- 25.Ivey FM, Gardner AW, Dobrovolny CL, et al. : Unilateral impairment of leg blood flow in chronic stroke patients. Cerebrovasc Dis, 2004, 18: 283–289. [DOI] [PubMed] [Google Scholar]
- 26.Adams WC, Imms FJ: Resting blood flow in the paretic and nonparetic lower legs of hemiplegic persons: relation to local skin temperature. Arch Phys Med Rehabil, 1983, 64: 423–428. [PubMed] [Google Scholar]
- 27.Naver H, Blomstrand C, Ekholm S, et al. : Autonomic and thermal sensory symptoms and dysfunction after stroke. Stroke, 1995, 26: 1379–1385. [DOI] [PubMed] [Google Scholar]
- 28.Robinson T, Potter J: Cardiopulmonary and arterial baroreflex-mediated control of forearm vasomotor tone is impaired after acute stroke. Stroke, 1997, 28: 2357–2362. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Liu B, Zhang Z: Combining ensemble empirical mode decomposition with spectrum subtraction technique for heart rate monitoring using wrist-type photoplethysmography. Biomed Signal Process Control, 2015, 21: 119–125. [Google Scholar]
- 30.Vandenbroucke JP, von Elm E, Altman DG, et al. STROBE Initiative: Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med, 2007, 4: e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campos C, DePaul VG, Knorr S, et al. : Validity of the ActiGraph activity monitor for individuals who walk slowly post-stroke. Top Stroke Rehabil, 2018, 25: 295–304. [DOI] [PubMed] [Google Scholar]
- 32.Léger L, Thivierge M: Heart rate monitors: validity, stability, and functionality. Phys Sportsmed, 1988, 16: 143–151. [DOI] [PubMed] [Google Scholar]
- 33.Goodie JL, Larkin KT, Schauss S: Validation of polar heart rate monitor for assessing heart rate during physical and mental stress. J Psychophysiol, 2000, 4: 159. [Google Scholar]
- 34.Gillinov S, Etiwy M, Wang R, et al. : Variable accuracy of wearable heart rate monitors during aerobic exercise. Med Sci Sports Exerc, 2017, 49: 1697–1703. [DOI] [PubMed] [Google Scholar]
- 35.Brunnstrom S: Motor testing procedures in hemiplegia: based on sequential recovery stages. Phys Ther, 1966, 46: 357–375. [DOI] [PubMed] [Google Scholar]
- 36.van Swieten JC, Koudstaal PJ, Visser MC, et al. : Interobserver agreement for the assessment of handicap in stroke patients. Stroke, 1988, 19: 604–607. [DOI] [PubMed] [Google Scholar]
- 37.Revelle W: psych: procedures for personality and psychological research, 2019. Version 1.9.12.
- 38.Landis JR, Koch GG: The measurement of observer agreement for categorical data. Biometrics, 1977, 33: 159–174. [PubMed] [Google Scholar]
- 39.Nelson MB, Kaminsky LA, Dickin DC, et al. : Validity of consumer-based physical activity monitors for specific activity types. Med Sci Sports Exerc, 2016, 48: 1619–1628. [DOI] [PubMed] [Google Scholar]
- 40.Bland JM, Altman DG: Statistical methods for assessing agreement between two methods of clinical measurement. Lancet, 1986, 1: 307–310. [PubMed] [Google Scholar]
- 41.Carstensen B, Simpson J, Gurrin LC: Statistical models for assessing agreement in method comparison studies with replicate measurements. Int J Biostat, 2008, 4: 16. [DOI] [PubMed] [Google Scholar]
- 42.Pinheiro J, Bates D, DebRoy S, et al.: nlme: linear and nonlinear mixed effects models. 2020. Version 3.1–148.
- 43.Faul F, Erdfelder E, Lang AG, et al. : G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods, 2007, 39: 175–191. [DOI] [PubMed] [Google Scholar]
- 44.Torchiano M: effsize: efficient effect size computation. 2020. Version 0.8.0.
- 45.Spierer DK, Rosen Z, Litman LL, et al. : Validation of photoplethysmography as a method to detect heart rate during rest and exercise. J Med Eng Technol, 2015, 39: 264–271. [DOI] [PubMed] [Google Scholar]
- 46.Maeda Y, Sekine M, Tamura T: Relationship between measurement site and motion artifacts in wearable reflected photoplethysmography. J Med Syst, 2011, 35: 969–976. [DOI] [PubMed] [Google Scholar]
- 47.Dooley EE, Golaszewski NM, Bartholomew JB: Estimating accuracy at exercise intensities: a comparative study of self-monitoring heart rate and physical activity wearable devices. JMIR mhealth uhealth, 2017, 5: e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Critchley LA, Critchley JA: A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Comput, 1999, 15: 85–91. [DOI] [PubMed] [Google Scholar]
- 49.Shinohara T, Usuda S: Are contents of physical therapy in nine Japanese hospitals for inpatients with stroke related to inpatients’ and physical therapists’ characteristics? J Phys Ther Sci, 2013, 25: 641–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeJong G, Hsieh CH, Putman K, et al. : Physical therapy activities in stroke, knee arthroplasty, and traumatic brain injury rehabilitation: their variation, similarities, and association with functional outcomes. Phys Ther, 2011, 91: 1826–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carpenter A, Frontera A: Smart-watches: a potential challenger to the implantable loop recorder? Europace, 2016, 18: 791–793. [DOI] [PubMed] [Google Scholar]
- 52.White RD, Flaker G: Smartphone-based arrhythmia detection: should we encourage patients to use the ECG in their pocket? J Atr Fibrillation, 2017, 9: 1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chan PH, Wong CK, Poh YC, et al. : Diagnostic performance of a smartphone-based photoplethysmographic application for atrial fibrillation screening in a primary care setting. J Am Heart Assoc, 2016, 5: e003428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rudner J, McDougall C, Sailam V, et al. : Interrogation of patient smartphone activity tracker to assist arrhythmia management. Ann Emerg Med, 2016, 68: 292–294. [DOI] [PubMed] [Google Scholar]