Abstract
Traditionally, cardiac and vascular surgeons have been treating diseases of the aorta as individual specialists. Neither cardiac nor vascular surgeons have ever considered the aorta as a whole, which can be diseased throughout its length at the same time requiring a more thoughtful and different approach. Aortic dissection and aneurysmal disease may well benefit from a multidisciplinary approach. In the context of this review, we discuss examples of joint operating between cardiac and vascular surgeons that may well become a more routine approach in more units in the future.
Keywords: aortic team, cardiac surgeons, vascular surgeons, aortic aneurysm, aortic dissection
Introduction
Traditionally, cardiac and vascular surgeons have been treating diseases of the aorta as individual specialists. Cardiac surgeons have addressed diseases of the aortic root and ascending aorta including the aortic arch while the descending thoracic and abdominal aorta has remained the domain of the vascular surgeons. The typical example is acute aortic dissection where type A has always been aggressively treated by the cardiac surgeon with immediate surgery while type B has been mostly managed conservatively or referred to the vascular colleague for assessment and treatment of complications through an endovascular approach. As a matter of fact, neither cardiac nor vascular surgeons have ever considered the aorta as a whole, which can be diseased throughout its length at the same time requiring a more thoughtful and different approach. Although immediate surgery has always been considered mandatory for type A aortic dissection, more recent evidence has questioned whether this is always the case and argued that it can also be addressed in a different and more elective manner.1,2 It is already known that patient selection and knowledge of risk factors play a significant role in the management of these patients.3-5
We feel that something more can be added.
Concerns over patient outcomes and variation in clinical practice have led to the development and evolution of the “Multi-Disciplinary Team Meetings” in cancer care. Subsequently, the same approach has become well established in other medical specialties.6 The “Heart Team” approach has become a more established setting in recent years with particular reference to the “Structural Heart Disease” multidisciplinary team meeting regarding the suitability of a surgical or interventional approach for certain category of patients.7
Aortic dissection and aneurysmal disease may well benefit from a multidisciplinary approach.
Proximal lesions, such as aortic valve disease with or without involvement of the root, ascending and descending thoracic aorta, often require open surgery for a definitive treatment. Nevertheless, aortic disease may well extend beyond these boundaries, and patients with complex disease involving the whole aorta may well benefit from the less invasive endovascular approach of the vascular colleagues. Then, why not merging the 2 disciplines as a strategic attempt to join forces against the same enemy? Although there has been some collaborative attitude in the past, the surgical philosophy has not always been a combined one: one team would operate and then hand off to the other one. Although acknowledged as a potential beneficial approach, joint operating between the 2 specialties remains not widely accepted by the majority.
Case Selection and Presentation
Here are some examples where a joint operating between cardiac and vascular surgeons may well become a more routine approach in more units in the future. Regardless of the outcome, which can be unpredictable and related to nonsurgical factors, the team approach is what we argue as an additional contribution to patient treatment.
Case 1
Acute aortic dissection in a 60-year-old patient involving the aortic arch, epiaortic vessels, and superior mesenteric artery (SMA) (Figures 1 and 2). Comorbidities included arterial hypertension, sickle cell anemia, and chronic renal impairment requiring dialysis for which a left brachiocephalic fistula had been created. Following transfer and further review of the available images, it was clear that the dissection extended to the SMA giving matter of concern for potential bowel ischemia postoperatively. Therefore, a decision was made to maintain blood pressure control and intervene 24 hours later. The aim would be to perform a digital subtraction angiography in our hybrid theater to further assess the visceral vessels and insert a wire in the SMA for stenting after the planned surgical procedure. Following contrast injection, it became evident that the features of the disease were more consistent with a type B dissection that had progressed in a retrograde manner with lower entry points in the descending thoracic aorta. These findings allowed planning of a definitive surgical strategy following a period of stabilization. A frozen elephant trunk with potential thoracic endovascular aortic repair (TEVAR) extension and mesenteric protection should the need arise to address the SMA was considered an appropriate delayed course of action on this occasion. In the meanwhile, the patient would be nursed in the high dependency unit with strict blood pressure control. Initial medical management was relatively successful although serial computed tomography (CT) scan imaging showed further dilatation of the ascending aorta, which triggered the final timing for intervention.
Figure 1.
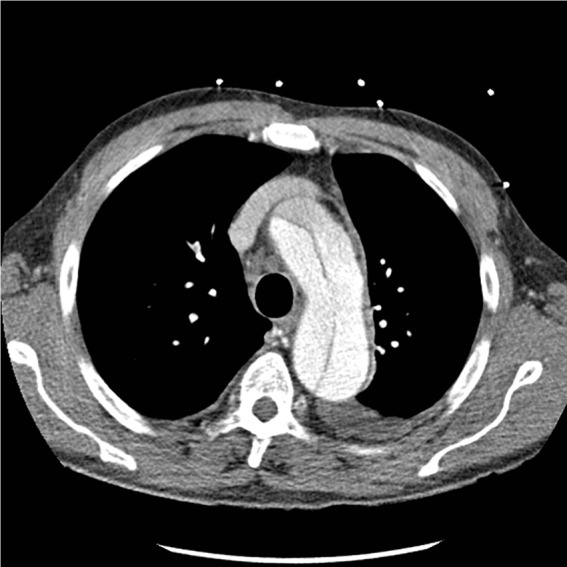
Preoperative contrast computed tomography scan showing arch involvement for patient 1.
Figure 2.
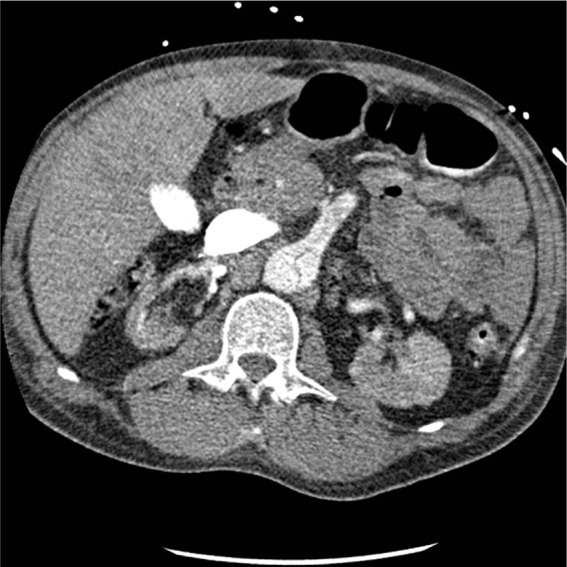
Preoperative contrast computed tomography scan showing involvement of the superior mesenteric artery for patient 1.
Plasmapheresis was required to address the sickle cell anemia preoperatively.
The patient was transferred once again to our hybrid theater and prepped in the supine position. Invasive arterial and venous monitoring was obtained through the left and right radial artery, left femoral artery, and right internal jugular vein. Cardiopulmonary bypass was established through right femoral and right atrial cannulation. Antegrade and retrograde cold blood cardioplegia was delivered for myocardial protection. Body temperature was lowered to 24 °C as a compromise in view of the sickle cell anemia. Antegrade cerebral perfusion was achieved with continuous, noninvasive monitoring of cerebral oxygen saturation using INVOS system. A frozen elephant trunk with a 28-mm Thoraflex device was performed with distal anastomosis within zone 2 sparing the left subclavian artery under circulatory arrest. Subsequently, distal reperfusion was commenced through the side arm of the Thoraflex. The true lumen placement of the stent of the Thoraflex was guaranteed by the presence of a stiff wire previously inserted through the left femoral artery under image intensifier. Then, the proximal anastomosis was completed maintaining further myocardial protection. Finally, the innominate and left common carotid arteries were debranched and anastomosed to the Thoraflex device. The patient was rewarmed and gradually weaned off cardiopulmonary bypass. Blood products, surgical sealants, and additional suturing were required to achieve satisfactory hemostasis. Atrial and ventricular pacing wires were used for temporary pacing as required. Three chest drains were inserted, and the chest closed in layers. An on-table angiogram was performed, which confirmed appropriate placement of the Thoraflex stent in the descending thoracic aorta with satisfactory occlusion of the distal entry point. Therefore, further stenting as previously planned was not required at this stage. Also the SMA did not require further attention. The postoperative course was complicated by temporary impairment of gas exchanges requiring aggressive physiotherapy and continuous positive airway pressure (CPAP) delivery. Regular filtration was used to address the renal impairment. Strict blood pressure control was maintained. Finally, the patient was discharged to the local hospital 19 days postoperatively for continuity of care. A postoperative echocardiographic and CT scan assessment was considered extremely satisfactory. The innominate and left common carotid arteries remained well perfused. The left subclavian artery still showed some residual dissection without obstruction. Residual degree of perfusion of the false lumen was observed with celiac axis, and SMA appropriately perfused from both true and false lumen as preoperatively.
Case 2
Severe aortic valve stenosis in a 43-year-old patient with bicuspid aortic valve and previous end-to-end repair of aortic coarctation (aged 3) followed by resection of subaortic stenosis (aged 7). Further coarctation repair (aged 18) with interposition graft through clam-shell approach complicated by rupture of a false aneurysm involving the previous Dacron graft and disconnection of subclavian arteries followed by stroke due to occlusion of left carotid artery requiring reconstruction with interposition graft between ascending and descending thoracic aorta (Figure 3). Referred for severe aortic valve stenosis and narrowing of the graft between ascending and descending thoracic aorta. After further assessment and discussion at our multidisciplinary meeting, a transcatheter aortic valve replacement (TAVI) option was considered, but given the anatomical features, it would not offer a long-lasting alternative. Therefore, high-risk surgical intervention was considered an appropriate course of action. The patient was admitted and transferred to the theater. After induction of anesthesia, central venous monitoring through the right internal jugular vein and blood pressure monitoring through bilateral radial and right femoral arteries was achieved. The patient was prepped and draped in the supine position. Exposure of the right carotid, left subclavian, and left femoral arteries followed by anastomosis of 10-mm and two 8-mm Dacron grafts to these vessels. Cardiopulmonary bypass was established between the right carotid, left subclavian, and left femoral arteries with left femoral venous return through a 25-Fr multistage venous cannula. Given the significant adhesions between the graft and the sternum as per previous imaging investigation, body temperature was lowered to 18 °C as a prelude to the likely requirement for circulatory arrest should the graft be damaged on chest entry. A repeat clam-shell incision was followed by a transverse and midline sternotomy. The graft was inevitably damaged as expected requiring a period of circulatory arrest to carry out full dissection and appropriately identify the anatomical relationship between the surrounding structures. Then, the 2 ends of the graft were clamped and the circulation restored. Following a transverse aortotomy, cold blood cardioplegia was delivered through the coronary ostia for additional myocardial protection. The native valve was excised, and the left ventricular outflow tract inspected for potential obstruction. Then, a 23-mm Top Hat mechanical prosthesis was inserted using multiple pledgeted interrupted sutures. Finally, the anastomosis between ascending and descending thoracic aorta was completed using an additional 30-mm interposition Dacron conduit. After rewarming, cardiopulmonary bypass was discontinued uneventfully. Two atrial and 2 ventricular pacing wires were placed. Prolonged hemostasis requiring blood products and surgical sealants was necessary. Two pleural and one mediastinal drains were placed, and the chest closed in layers with particular attention to the breast soft tissues to avoid seroma formation. The postoperative course was complicated by prolonged ventilation and inotropic support. Thereafter, slow recovery requiring aggressive anti-failure treatment. Finally, the patient was discharged home 30 days postoperatively with satisfactory imaging investigation confirming patency of the graft.
Figure 3.
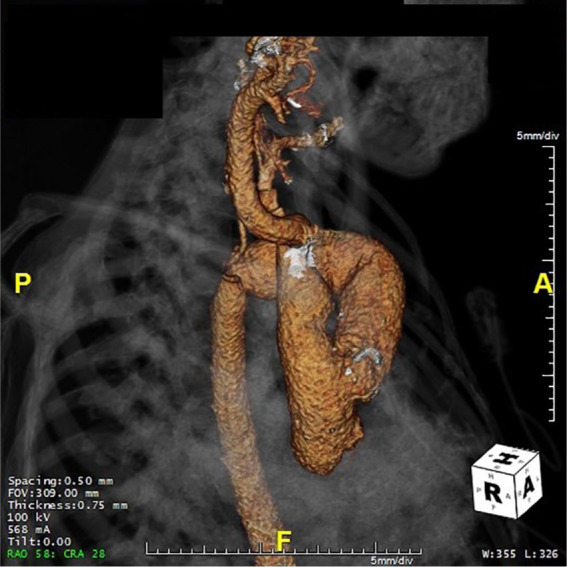
Preoperative 3-dimensional reconstruction for patient 2 to highlight the relationship between anatomical structures and previous graft.
Case 3
Chronic type B dissection in a 12-year-old patient with previous valve sparing aortic root replacement in the context of bicuspid aortic valve and Loeys-Dietz syndrome followed by replacement of the aortic valve with a bioprosthesis, ascending aorta and arch, and debranching of the neck vessels. Device closure of patent ductus arteriosus was required at 1 year of age. The development of significant aortic root dilatation and severe aortic regurgitation required a valve-sparing procedure with a 26-mm Gelweave Dacron graft (Vascutek) at the age of 9 years. Postoperative course was complicated by myocardial ischemia requiring patching of the left anterior descending coronary artery. The onset of further aortic regurgitation in the context of stenosis at the site of the distal anastomosis between the graft and ascending aorta with concomitant dilatation of the aortic arch required arch and ascending aorta replacement with a 28-mm Gelweave Lupiae Dacron interposition graft (Vascutek), debranching of the epiaortic vessels, and aortic valve replacement with a 21-mm bioprosthesis at the age of 11 years. The procedure was complicated by type B aortic dissection requiring referral to our center for further management and treatment. An initial conservative approach was considered based on blood pressure control and serial imaging investigation to monitor disease progression (Figure 4). A fast-expanding false lumen with near occlusion of the true one forced an urgent multidisciplinary team meeting where the unanimous decision was to proceed with surgical intervention in the context of a high-risk background including moderately impaired left ventricular systolic function and a certain degree of renal dysfunction. The patient was readmitted and transferred to the theater. After induction of anesthesia, invasive arterial and venous monitoring was established through the left and right radial artery, right femoral artery, and right central venous access. A Vascath was also inserted with a view to renal replacement therapy postoperatively as required. Motor and sensory evoked potentials monitoring was maintained throughout the whole procedure. Spinal drainage was also placed. After prepping and draping in the left semi recumbent position, the left neck was entered through an oblique incision anterior to the sternocleidomastoid muscle. A left subclavian to left carotid extra-anatomical bypass was performed with a T-shaped 8-mm interposition Dacron graft (Vascutek). The side of this graft was used to place a 14-Fr arterial cannula. Then, the left groin was entered through an oblique incision parallel to the inguinal ligament and the femoral vessels exposed. A 14-Fr arterial cannula was inserted in the left femoral artery, and a 17-mm multistage venous cannula inserted in the left femoral vein. After heparin administration, cardiopulmonary bypass was established through the left femoral artery and the extra-anatomical bypass with left femoral venous return. Body temperature was lowered to 28 °C. The chest was entered following a left posterolateral thoracotomy through the fifth intercostal space. After visual inspection, the distal arch and the descending thoracic aorta were dissected free. The proximal end of the left subclavian artery was resected and oversown. The distal aortic arch and the proximal descending thoracic aorta were replaced with a 22-mm Haemashield tubular graft followed by fenestration of the distal lumen to account for size mismatch between the graft and the native vessel. The patient was rewarmed and gradually weaned off cardiopulmonary bypass. Hemostasis was achieved, 2 basal and 1 apical drains were placed, and the chest closed in layers. The postoperative course was complicated by hemodynamic instability requiring prolonged inotropic support and mechanical ventilation. Subsequently, a lower respiratory tract infection required aggressive antibiotic treatment. Thereafter, slow but uneventful recovery. Imaging investigations revealed a laminar thrombus in the abdominal aorta requiring surveillance. He was finally discharged home 16 days postoperatively on anti-aggregant and antihypertensive treatment. Four months later, he was readmitted for the second-stage surgical intervention. Prepping and draping as previously described with central venous monitoring through the left internal jugular vein, bilateral radial, and right femoral arterial blood pressure monitoring. Again, motor and sensory evoked potentials monitoring was maintained throughout the whole procedure. Spinal drainage was also placed. The chest was entered through the previous thoracotomy, and the incision extended as a thoraco-phreno-laparotomy to access the abdomen. Previous adhesions were dissected free, the retroperitoneum was accessed, and the anatomy identified. Two 6-mm Dacron grafts were sutured to the left common iliac artery and the proximal descending thoracic aortic prosthesis. Cardiopulmonary bypass was established with two 14-Fr arterial cannulas placed in the 2 grafts and venous return through a 19-Fr multistage cannula inserted in the left femoral vein through a Seldinger technique. Beating heart technique and mild hypothermia were used. Replacement of the descending thoracic and abdominal aorta was carried out with a 20-mm Haemashield Dacron graft followed by re-implantation of the celiac trunk, superior mesenteric, and renal arteries. The intercostal vessels were debranched and oversown. Given the size mismatch between the native vessel and the Haemashield graft, the distal end was oversown and a 10-mm Dacron graft was used to join the native aorto-iliac bifurcation. Cardiopulmonary bypass was discontinued uneventfully. Hemostasis was achieved followed by the insertion of an apical and 2 basal chest drains. The chest and abdomen were closed in layers, and the patient transferred to the intensive care unit. The postoperative course was complicated by retroperitoneal hematoma requiring re-exploration; right-sided pleural effusion requiring drainage; fluctuating hemodynamic status requiring inotropic support. Thereafter, slow but steady progress. A CT scan showed a widely patent conduit in the descending thoracic aorta position. The celiac axis, the SMA, and the right renal artery remained well perfused from the new conduit with good runoff into the distal aorta and iliac arteries. Sadly, while on the ward, he suffered a cardiac arrest successfully resuscitated and followed by transfer to the intensive care unit. Finally, 2 further episodes of cardiac arrest in the context of abdominal distension with clinical and imaging features suggestive of new abdominal aortic rupture led to fatal outcome.
Figure 4.
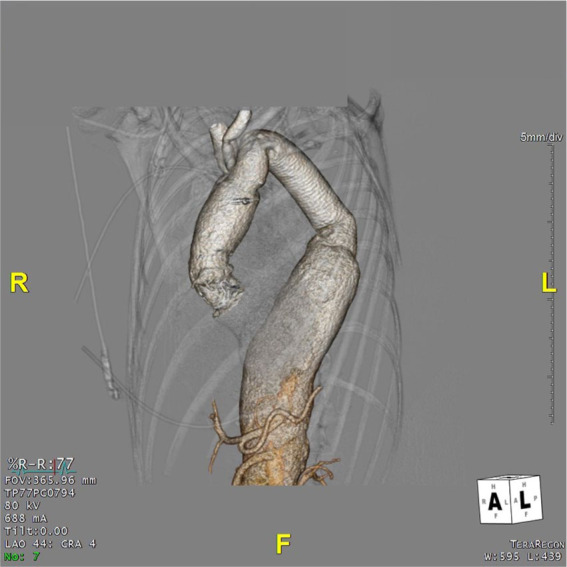
Preoperative 3-dimensional reconstruction for patient 3 showing the features of the abdominal aneurysm.
Case 4
Descending thoracic aortic aneurysm obstructing the left main bronchus in a 19-year-old patient with previous aortic valve replacement with a mechanical prosthesis and frozen elephant trunk to replace ascending aorta and arch in the context of Marfan syndrome (Figure 5). The patient was referred to our center in view of rapidly expanding descending thoracic aortic aneurysm requiring surgical intervention. The patient was admitted and transferred to the theater. After induction of anesthesia, central venous monitoring through the right internal jugular vein and arterial blood pressure monitoring through left radial and right femoral arteries was obtained. After prepping and draping in the supine position, the neck vessels were exposed through an oblique incision parallel to the anterior aspect of the sternocleidomastoid muscle. The left subclavian and carotid arteries were dissected free and looped. Debranching of the left subclavian artery was performed followed by an extra-anatomical bypass between the 2 vessels with 8-mm Dacron graft. Then, the left femoral vessels were exposed through an oblique incision in the left groin parallel to the inguinal ligament. The patient was repositioned in the left recumbent position, prepped, and draped again. The chest was entered through a left posterolateral thoracotomy through the fifth intercostal space. The anatomy was identified and a purse string placed at the origin of the left superior pulmonary vein. Left heart cardiopulmonary bypass was established between the left femoral artery and the left atrium. Extensive dissection was required to expose the aneurysm of the proximal descending thoracic aorta. Finally, replacement of the descending thoracic aorta with 34-mm Gelweave Dacron graft (Vascutek). The early postoperative course was uneventful leading to early extubation and weaning from inotropic support with successful mobilization and physiotherapy. Subsequently, sudden onset of neurological deterioration requiring reintubation on the third postoperative day. Neurological opinion was sought with further imaging investigation suggestive of cerebellar infarct secondary to basilar artery thrombosis in the absence of bleeding. He remained ventilated with antibiotic treatment in view of raised inflammatory markers. Surgical tracheostomy on the 20th postoperative day was performed to allow further progress and facilitate neurological assessment. Subsequently, clinical conditions remained stable but in need of close monitoring and intensive care nursing with very slow neurological progress in the context of locked-in syndrome. A CT scan showed satisfactory appearances of the interposition graft in the descending thoracic aorta. Finally, interhospital transfer was arranged for further neurorehabilitation and continuity of care.
Figure 5.
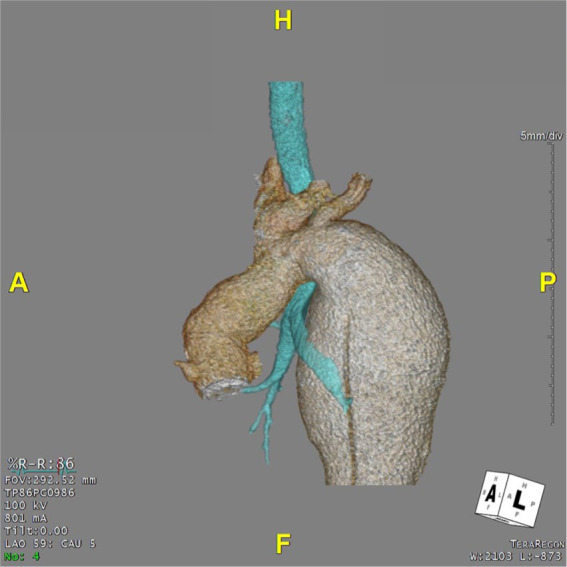
Preoperative 3-dimensional reconstruction for patient 4 showing the compression of the left main bronchus by the aneurysm.
Discussion
Acute aortic syndromes are closely related to chronic hypertension, often poorly controlled, and connective tissue disorders such as Marfan and Loeys-Dietz syndromes where a high level of clinical suspicion combined with imaging investigation is essential for appropriate differential diagnosis.8 Thoracic aortic aneurysms progressing to acute aortic dissection often lead to fatal outcome. Medical treatment with β-blockers and close imaging follow-up is usually acknowledged with surgical treatment being offered when the risk of dissection is higher than the one related to repair. Given that thoracic aortic enlargement occurs in the majority of patients before acute dissection, there is a window to initiate treatment that may slow enlargement and delay or ideally avoid the need for surgical intervention.9 Subsequently, the initial enthusiasm for At1r antagonists (angiotensin II type I receptor), such as losartan to be more effective than conventional treatment with β-blockers in the prevention of aneurysm development,10 has not been confirmed by large randomized clinical trials in the pediatric and adult population with Marfan syndrome.11-14 The management of patients with aortic disease can be very challenging and may well benefit from a combined cardiac and vascular approach. This is in line with the lesson learnt from cancer care where a multidisciplinary approach should be restricted to the more complex patients.6 Therefore, an aging cardiac surgical population with multiple comorbidities and increased surgical complexity in the context of growing health care costs and high expectations may well require a more targeted and multidisciplinary approach as an attempt to a long-lasting solution. The role of the aortic team is being acknowledged15 but still not completely and widely accepted. Nevertheless, preoperative malperfusion remains a critical factor in relation to early and late outcome,16-18 and current evidence suggests that acute aortic syndromes are best treated in dedicated, high-volume aortic centres.19 A close cooperation between the 2 disciplines allows sharing of different skills settings with a real-time joint operating by senior surgeons to shorten procedure time. The backup of the vascular colleagues may play a significant role should additional intervention be required such as stenting, on-table angiography, and visceral assessment and protection. The aim is not competition for patients, but on the contrary, the offer of every possible option to improve outcome. Although communication between teams takes time and may lead to treatment delay, it remains an important element when dealing with patients with complex background requiring the input of multiple specialists. The benefit of operating in the same room means that the original plan may evolve according to the difficulty of the procedure and patient’s need. We can do things together that could not be done individually. The cases here discussed are only an introduction to a different way of working toward a common aim where outcome may not always be successful, but a true team effort remains the key element. The type of work we do is certainly unique, but it may be adopted by other surgeons as long as the right set of skills is brought to the operating table and both parties feel comfortable with each other. In the current era, many vascular surgeons in training are mainly being exposed to stenting and endovascular repair. An appropriate balance between open and endovascular techniques would give them a broader set of skills. At the same time, many cardiothoracic surgeons are not exposed to endovascular techniques, which would give instead a different dimension to their approach to treatment. Needless to say, this unique type of work requires the right support from anesthetists, operating room technicians, and intensive care unit staff intraoperatively and postoperatively. There are not just surgeon-based factors: the system has to be built around what can be done to achieve the desired outcome.
Conclusion
Aortic aneurysm disease remains challenging with limited therapeutic options to delay or prevent it despite the significant progress made over the past years to understand its pathogenesis. Nevertheless, surgical intervention plays a key role in terms of prophylactic and prognostic reasons. We have discussed a series of cases based on a combined cardiac and vascular approach for the treatment of aortic disease. We advocate the role of the aortic team as a different and innovative way to achieve the desired outcome when dealing with complex patients.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Written informed consent was obtained from the patients for their anonymized information to be published in this article.
References
- 1. Estrera AL, Huynh TTT, Porat EE, Miller CC, 3rd, Smith JJ, Safi HJ. Is acute type A aortic dissection a true surgical emergency? Semin Vasc Surg. 2002;15:75-82. [DOI] [PubMed] [Google Scholar]
- 2. Fleck T, Hutschala D, Czerny M, et al. Combined surgical and endovascular treatment of acute aortic dissection type A: preliminary results. Ann Thorac Surg. 2002;74:761-766. [DOI] [PubMed] [Google Scholar]
- 3. Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897-903. [DOI] [PubMed] [Google Scholar]
- 4. Trimarchi S, Nienaber CA, Rampoldi V, et al. On behalf of the International Registry of Acute Aortic Dissection Investigators. Contemporary results of surgery in acute type A aortic dissection: the International Registry of Acute Aortic Dissection experience. J Thorac Cardiovasc Surg. 2005;129:112-122. [DOI] [PubMed] [Google Scholar]
- 5. LeMaire SA, Russell L. Epidemiology of thoracic aortic dissection. Nat Rev Cardiol. 2011;8:103-113. [DOI] [PubMed] [Google Scholar]
- 6. Whiteman AR, Dhesi JK, Walker D. The high-risk surgical patient: a role for a multi-disciplinary team approach? Br J Anaesth. 2016;116:311-314. [DOI] [PubMed] [Google Scholar]
- 7. Chu D, Anastacio MM, Mulukutla SR, et al. Safety and efficacy of implementing a multidisciplinary heart team approach for revascularisation in patients with complex coronary artery disease: an observational cohort pilot study. JAMA Surg. 2014; 149:1109-1112. [DOI] [PubMed] [Google Scholar]
- 8. Akin I, Kische S, Renders TC, et al. Acute aortic syndromes. Medicine (Baltimore). 2010;38:450-455. [Google Scholar]
- 9. Milewicz DM, Ramirez F. Therapies for thoracic aortic aneurysms and acute aortic dissections. Old controversies and new opportunities. Arterioscler Thromb Vasc Biol. 2019; 39:126-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Habashi JP, Judge DP, Holm TM, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Groenink M, den Hartog AW, Franken R, et al. Losartan reduces aortic dilatation rate in adults with Marfan syndrome: a randomized controlled trial. Eur Heart J. 2013;34:3491-3500. [DOI] [PubMed] [Google Scholar]
- 12. Lacro RV, Dietz HC, Sleeper LA, et al. ; Pediatric Heart Network Investigators. Atenolol versus losartan in children and young adults with Marfan’s syndrome. N Engl J Med. 2014;371:2061-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Milleron O, Arnoult F, Ropers J, et al. Marfan Sartan: a randomized, double-blind, placebo-controlled trial. Eur Heart J. 2015;36:2160-2166. [DOI] [PubMed] [Google Scholar]
- 14. Forteza A, Evangelista A, Sánchez V, et al. Efficacy of losartan vs atenolol for the prevention of aortic dilation in Marfan syndrome: a randomized clinical trial. Eur Heart J. 2016;37:978-985. [DOI] [PubMed] [Google Scholar]
- 15. Czerny M, Schmidli J, Adler S, et al. Current options and recommendations for the treatment of thoracic aortic pathologies involving the aortic arch: an expert consensus document of the European Association for Cardio-Thoracic Surgery (EACTS) and the European Society for Vascular Surgery (ESVS). Eur J Cardiothorac Surg. 2019;55:133-162. [DOI] [PubMed] [Google Scholar]
- 16. Girdauskas E, Kuntze T, Borger MA, Falk V, Mohr FW. Surgical risk of preoperative malperfusion in acute type A aortic dissection. J Thorac Cardiovasc Surg. 2009;138:1363-1369. [DOI] [PubMed] [Google Scholar]
- 17. Czerny M, Schoenhoff F, Etz C, et al. The impact of pre-operative malperfusion on outcome in acute type A aortic dissection: results from the GERAADA registry. J Am Coll Cardiol. 2015;65:2628-2635. [DOI] [PubMed] [Google Scholar]
- 18. Narayan P, Rogers CA, Benedetto U, Caputo M, Angelini GD, Bryan AJ. Malperfusion rather than merely timing of operative repair determines early and late outcome in type A aortic dissection. J Thorac Cardiovasc Surg. 2017;154:81-86. [DOI] [PubMed] [Google Scholar]
- 19. Mariscalco G, Maselli D, Zanobini M, et al. Aortic centres should represent the standard of care for acute aortic syndrome. Eur J Prev Cardiol. 2018;25(1 suppl):3-14. [DOI] [PubMed] [Google Scholar]


