Abstract
This paper presents novel insights into the archaeology of food in ancient South Asia by using lipid residue analysis to investigate what kinds of foodstuffs were used in ceramic vessels by populations of the Indus Civilisation in northwest India. It examines how vessels were used in urban and rural Indus settlements during the Mature Harappan period (c.2600/2500–1900 BC), the relationship between vessels and the products within them, and identifies whether changes in vessel use occurred from the Mature Harappan to Late Harappan periods, particularly during climatic instability after 4.2 ka BP (c.2100 BC). Despite low lipid concentrations, which highlight challenges with conducting residue analysis in arid, seasonally-wet and alkaline environments, 71% of the vessels yielded appreciable quantities of lipid. Lipid profiles revealed the use of animal fats in vessels, and contradictory to faunal evidence, a dominance of non-ruminant fats, with limited evidence of dairy processing. The absence of local modern reference fats makes this dataset challenging to interpret, and it is possible that plant products or mixtures of plant and animal products have led to ambiguous fatty acid-specific isotopic values. At the same time, it appears that urban and rural populations processed similar types of products in vessels, with limited evidence for change in vessel use from the urban to the post-urban period. This study is a systematic investigation into pot lipid residues from multiple sites, demonstrating the potential of the method for examining ancient Indus foodways and the need for the development of further research in ancient organic residues in South Asia.
Keywords: Foodways, Lipid residues, Indus civilisation, Vessel use, Pottery, Urbanism, Climate change
Highlights
-
•
Systematic study on ceramic lipid residues from rural and urban sites of the Indus Civilisation in northwest India.
-
•
Lipid residue analysis provides chemical evidence for milk, meat, and possible mixtures of products and/or plant consumption.
-
•
Similarities in vessel use across settlements suggest common regional culinary practices.
-
•
Evidence for continuity in vessel usage practices after the decline of urbanism.
1. Introduction
A great diversity of food traditions exists across South Asia, and the social role of food in the subcontinent is well-recognised (e.g. Appadurai, 1981; Nandy, 2004). However, investigations into the archaeology of food from prehistoric contexts are in a relatively nascent stage. For example, despite the strong preoccupation with research on subsistence in the Indus Civilisation (c.3000–1300 BC) (Murphy and Fuller, 2016), discussions about food production and variability have been focused on the crops that are grown (e.g. Madella and Fuller, 2006; Petrie et al., 2016; Petrie and Bates, 2017). The role of plants, animals and material culture is rarely discussed in tandem for a holistic understanding of foodways (e.g. Fuller, 2005).
Ceramic lipid residue analysis provides a powerful means by which the foodways of populations can be examined and has been used in a range of archaeological contexts around the world to extract and identify foodstuff within ancient vessels (Evershed, 2008; Regert, 2011; Roffet-Salque et al., 2017). Organic residue analysis can also provide a new understanding of vessel specialisation and use (Roffet-Salque et al., 2017; Dunne et al., 2020). Ceramics are one of the most ubiquitous artefacts recovered during archaeological excavations of proto- and historic South Asian sites. However, they are often embedded in typology- and form-based discussions alone and divorced from their cultural and culinary role. Until recently, only a single pottery sherd from South Asia had been studied via lipid residue analysis (Bourgeois and Gouin, 1995). More recently, a study investigated ceramic lipid residues from 59 vessels from a single site in Gujarat (Chakraborty et al., 2020). This paper presents the results of a much larger corpus of ceramic lipid residues across multiple Indus Civilisation sites in northwest India to investigate broader patterns of food consumption and vessel use. The results obtained are contextualised with existing archaeobotanical, zooarchaeological and isotopic evidence to have a fuller understanding of the culinary strategies adopted by the Indus settlements in question.
One of the major factors affecting the study of ancient food in South Asia, particularly northwest India, is the degree of organic preservation at archaeological sites. It is well-recognised that fluctuations in temperature and moisture, pH levels and mineralisation negatively affect the preservation of organic material in South Asian archaeological sites (e.g. Weber and Kashyap, 2016; García-Granero et al., 2017; Joglekar et al., 2017). However, the preservation of lipids is dependent on a range of environmental and cultural factors (Eglington and Logan, 1991; Evershed, 2008), and lipid preservation cannot be reliably predicted. Thus, this study also tested the degree of preservation of lipids in pottery from sites in northwest India.
1.1. Indus urban period: settlements and material culture
The Indus Civilisation was one of the first complex civilisations of the Old World, spread across large parts of modern Pakistan, northwest and western India and Afghanistan (Possehl, 2002; Agrawal, 2007; Wright, 2010). This region incorporates areas where winter rain or summer monsoonal rain predominate independently, and where they overlap (Petrie et al., 2017), and Indus settlements were located in diverse habitats and environmental contexts, including alluvial plains, foothills, deserts, scrubland, and coastal regions (Wright, 2010; Petrie et al., 2017). Between c.2600–1900 BC (Mature Harappan or urban period), five Indus Civilisation settlements developed into sizable cities, with a range of other medium-sized urban settlements, small settlements with specialised craft production/and or fortifications, and rural settlements (Petrie, 2013). Indus cities had multiple walled areas and three-dimensional, segregated spaces, as well as large public structures (Kenoyer, 1997; Eltsov, 2008; Vidale, 2010; Petrie, 2013). The urban period is best known for its iconic material cultural such as beads, bangles, standardised weights, and stamp steatite seals. Extensive exchange networks existed across the Indus Civilisation, which saw the movement of such iconic items as well as quotidian products like grinding stones (Law, 2011), and possibly foodstuffs (Madella, 2014). It has also been suggested that food supply to cities was derived from rural hinterlands (Kenoyer, 2008). The presence of highly valued material in small, likely rural settlements (Dales and Kenoyer, 1986:9; Petrie et al., 2009; 2017; Parikh and Petrie, 2009), suggests that long-distance networks of exchange were accessible by various populations (Petrie et al., 2009, 2017) and that relationships between settlements was complex; possibly governed by mutual economic dependence rather than overt control of cities over rural hinterlands (Wright, 2010; Parikh and Petrie, 2019). However, the dynamics between Indus cities and smaller settlements have not been systematically investigated (Petrie, 2013).
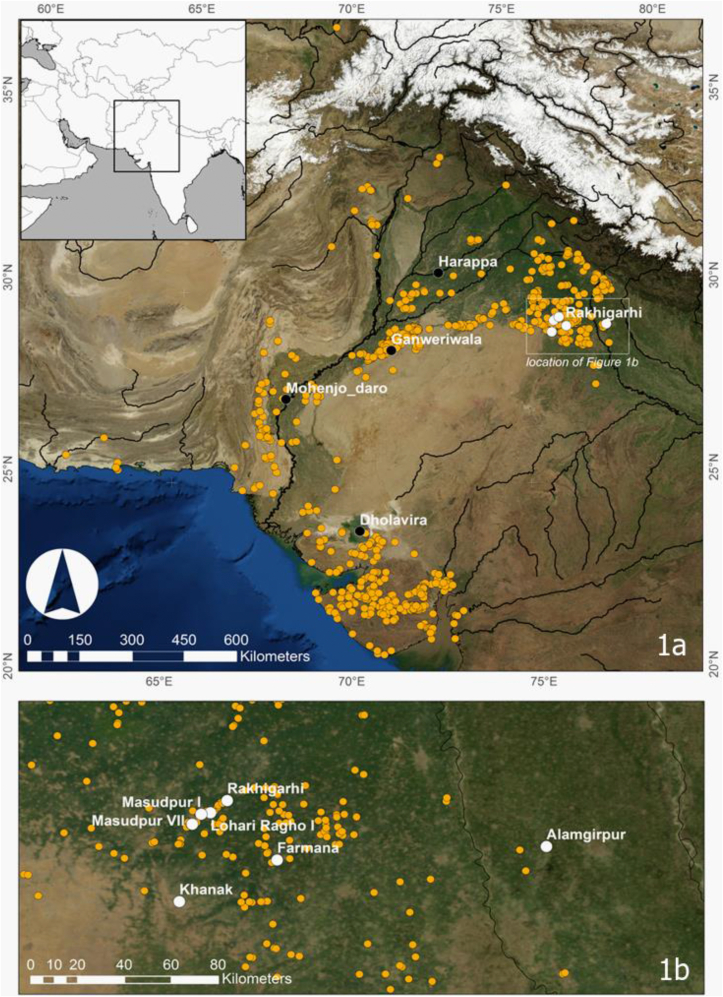
Rural settlements predominated in the Indus Civilisation (Petrie et al., 2017) and new evidence has revealed the immense complexity, diversity and distinctiveness of the rural character of the Indus Civilisation (Chase et al., 2014; García-Granero et al., 2016, 2017; Petrie et al., 2017; Lancelloti, 2018; Parikh and Petrie, 2019), particularly evinced by choices of pottery production and crop selection. For example, although regional variations in crop choices correspond to differential patterns of climate and rainfall, differences in cropping strategies have also been observed within regions experiencing similar environments, for example, within northwest India (Petrie et al., 2016; Petrie and Bates, 2017), suggesting unique food choices in different settlements. In this study, organic residues in pottery from one city, one town, and five rural settlements in northwest India (Fig. 1) are investigated to characterise any possible similarities or differences in foodstuffs used in vessels by urban and rural populations in a single region.
Fig. 1.
Extent of Indus settlements in the urban period with cities in black (1a) and study sites in white (1b). Other small- and medium-sized settlements are presented in yellow. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
1.2. Transformation from urbanism
After c.2100 BC, settlements located in the western part of the Indus Civilisation were progressively abandoned, but there was an increase in settlement density in the eastern regions (Possehl, 2002; Petrie, 2013). Many defining traits of Indus urbanism such as the use of the Indus script, seals, and weights were no longer evident in the Late Harappan period (Wright, 2010). This transition, included a clear shift toward more village-based settlements with significant reduction to the scale and extent of Indus exchange systems (Wright, 2010; Law, 2011), suggesting a dramatic alteration to the urban character of the Indus Civilisation (Wright, 2010). A range of causes have been proposed to explain the demise of the Indus urban period (see Raikes, 1964, 1968, 1979; Possehl, 1977, 1997, 2002; Wright, 2010), particularly the weakening of the Indian Summer Monsoon (ISM), which began around 4.2 ka BP or c. 2150 BC and lasted up to several centuries (Staubwasser et al., 2003; Sarkar et al., 2015). While several records provide evidence of abrupt monsoon weakening around this time (Gupta et al., 2003; Staubwasser et al., 2003; Dixit et al., 2014; Giesche et al., 2019); others indicate contrary evidence (Ponton et al., 2012; Tiwari et al., 2015). Significantly, there is clear evidence for step-wise monsoon weakening at Kotla Dahar around 4.2–4.1 ka BP (Dixit et al., 2014), which is an ephemeral lake that lies relatively close to the Indus sites investigated in this study. It is not clear, however, if the 4.2 ka BP weakening involved more than just a reduction in monsoon intensity, or also changes in seasonality and annual moisture availability, as well as a reduction in winter rainfall (Staubwasser et al., 2003; Giesche et al., 2019). Additionally, this shift must also be put within the context of an ongoing monsoon weakening; winter rainfall weakening after 4.3 ka (Giesche et al., 2019); and variability over decadal and centennial scales. Thus, it is still not possible to determine exactly what this event meant for rainfall patterns, nor how detrimental it may have been for human populations. Evidence from rural settlements in northwest India suggests the broad continuity of cropping patterns with a slight increase in summer crops (Petrie et al., 2016; Petrie and Bates, 2017), exemplifying the challenges in reconciling different types of archaeological evidence and data obtained from climate proxies. This paper examines whether changes in vessel usage are identifiable from the urban (Mature Harappan) to the post-urban (Late Harappan) period and after 4.2 ka BP in northwest India to assess the possible impact of cultural/climatic changes on quotidian practices of Indus populations.
1.3. The study region
The study sites lie on the semi-arid alluvial plains of northwest India also referred to as the ‘eastern domain’ of the Indus Civilisation (Possehl, 2002). Today, these sites experience mostly summer, but also winter rainfall; hot summers and cool winters (Table 1) and it is likely that a version of this pattern also existed in the past. The generally accepted chronological divisions of Mature Harappan (or Harappa phase c.2600/2500–1900 BC) and Late Harappan (c.1900–1300 BC) are used in this paper, with the Mature Harappan broken into sub-divisions (Mature Harappan i-ii: c.2600/2500–2200 BC and Mature Harappan iii: c.2200–1900 BC) fine-tuned through ceramic analysis within Indus sites in northwest India (Petrie et al., 2017; Parikh and Petrie, 2019). The use of this terminology emphasises the regionally distinct presence of Indus material culture in northwest India (Parikh and Petrie, 2019), but also maintains coherence with the chronology at Harappa (Kenoyer, 1991, 2008), which is the only Indus city to have a well-published chronological sequence (Table 1).
Table 1.
Details of study sites in northwest India. Periodisation of sites based on radiocarbon dates, corresponding to samples selected in this study. (Shinde, 2011; Singh et al., 2013; Petrie et al., 2016; Vahia et al., 2016; Petrie and Bates, 2017; Nath, 2018). MHi-ii: Mature Harappan i-ii, MHiii: Mature Harappan iii, LH: Late Harappan.
| Site code | Site name | Period | Estimated site size (ha) | Type of Indus settlement |
|---|---|---|---|---|
| ALM | Alamgirpur | MHiii, LH | 1 | small village |
| MSDVII | Masudpur VII | MHiii, LH | 1 | small village |
| MSDI | Masudpur I | MHiii | 6 | village |
| LHRI | Lohari Ragho | MHi-ii | 8 | village |
| KNK | Khanak | MHi-ii | 5 | village |
| FRN | Farmana | MHi-ii | 18 | town |
| RGR | Rakhigarhi | MHi-ii | 100–300 | city |
1.4. Indus ceramics and subsistence practices
There are several regional styles of pottery that were in use during the urban period, which have been recognised across the Indus Civilisation. Although several of these regional styles developed in the pre-urban or Early Harappan period (c.3000–2600 BC), many of them persisted and responded dynamically to what is considered the ‘Classical Harappan’ (Uesugi 2011a, 2013, 2017) or ‘Red Harappan Ware’ (Dales and Kenoyer, 1986) that dominated in the large settlements of Harappa and Mohenjo-daro. Far from being static archetypes as they are often described, these regional ceramic repertoires are visually distinctive in terms of their surface finish and decoration, and yet recognisably a part of the Indus material canon in terms of their forms (Parikh and Petrie, 2019; Ceccarelli, 2020). They are also found in association with other types of material culture that are typical to the Indus urban phase, which suggests that pottery was a unique medium that was materialised differently across the Indus Civilisation (Parikh and Petrie 2019; Ceccarelli, 2020). In northwest India, vessels are referred to as Sothi-Siswal ( Bhan, 1975; Bala, 2003; Garge, 2010), ‘Non-Harappan pottery’ (Uesugi 2011a, 2011b) or ‘Haryana Harappan’ pottery (Parikh and Petrie, 2019), and comprise a red fabric of medium texture and few inclusions with great variety in techniques and decoration (Uesudi, 2011a, 2011b; Parikh and Petrie, 2019). Most of the vessels in this study belong to this fabric type; the rest are ‘Classical Harappan’ (SI 3 for descriptions and images of all analysed vessels). Despite the unique visual and technical language used to produce ‘Haryana Harappan’ pottery, there are overlaps with ‘Classic Harappan’ forms, such as perforated jars, ‘cooking vessels’, and dish-on-stands, although the range of vessel forms is relatively limited (Parikh and Petrie, 2019).
Associations between vessel forms and specific culinary activities have been suggested, but not examined in detail (Dales and Kenoyer, 1986: 110; Wright, 1991; Kenoyer, 1998; Krishnan, 2018). Ledge-shouldered jars and large storage jars at Harappa have been linked to storage of liquids such as wine and oil (Wright, 1991: 83), and dish-on-stand vessels with the display and offering of food (Dales and Kenoyer, 1986). Additionally, ‘cooking jars’ or ‘ledged jars’ resemble handis, which are vessels made from clay and/or steel or copper that are used for cooking in contemporary contexts in Pakistan and northwest India. Finally, perforated vessels, characterised by a body pierced with holes and a large hole in the base, are found in various shapes and sizes (Dales and Kenoyer, 1986: 110) and documented at numerous Indus sites. The unique form of this vessel has prompted multiple interpretations of its purported uses, ranging from dairy processing (Gouin, 1990; Bourgeois and Gouin, 1995), to braziers for heating (Mackay, 1938: 207), colanders for draining or straining liquids, or sieves for preparing cereal pastes (Dales and Kenoyer, 1986: 108–109). In this study, the vessel forms analysed included jars of varying sizes, ledged jars, necked jars, perforated vessels, and bowls (Fig. 2).
Fig. 2.
Examples of Indus pottery fragments investigated in this study. A) Left: Classic Harappan; right: Haryana Harappan), B) Examples of vessel forms investigated. Drawings courtesy Danika Parikh and Alessandro Ceccerelli.
1.4.1. Macro- and microbotanical evidence
Diverse environmental and geographical conditions across the Indus Civilisation likely led to variable agricultural strategies, with varying proportions of summer and/or winter crops grown depending on access to rainfall (Possehl, 2002; Weber, 1999, 2003; Weber et al., 2010). Despite limitations related to the unsystematic collection of archaeobotanical data from Indus sites (Vishnu-Mittre and Savithri, 1982; Madella and Fuller, 2006), there is clear evidence of diversity of plant products, and regional variation in cropping practices (Fuller and Madella, 2002; Petrie and Bates, 2017; Petrie et al., 2017). In the study region, both summer- and winter-based cropping was practiced, with evidence of barley, wheat, rice (C3 crops) and millets (C4 crops). Apart from cereals, the archaeobotanical assemblage is extremely diverse, characterised by a range of winter and summer pulses, oilseeds and fruits (Petrie and Bates, 2017) (Table 2). Starches of cereals, pulses, vegetables and underground storage organs have also been identified on surfaces of stone tools, pottery and human and cattle teeth from the site of Farmana, one of the study sites (Kashyap and Weber, 2010; Weber et al., 2011: 820).
Table 2.
List of winter and summer crops found in the Indus Civilisation, based on approximate order of ubiquity. ‘A’ indicates annual, ‘P’ indicates perennial plant, and ‘A/P’ indicates a plant that can be either. All plants reported are C3 plants except millets, which are C4 (*) (after Petrie and Bates, 2017; Weber et al., 2011; Bates, 2019).
| Type | Winter (rabi) | Summer (kharif) |
|---|---|---|
| Cereals | Barley (Hordeum vulgare) A | Rice (Oryza cf. sativa) A |
| Wheat (Triticum sp.) A | Signalgrass millet (Brachiaria ramosa) A* | |
| Oats (Avena sativa) A | Sawa millet (Echinochloa colona) A* | |
| Little millet (Panicum sumatrense) A* | ||
| Broomcorn millet (Panicum miliaceum) A* | ||
| Foxtail millet (Setaria italica) A* | ||
| Yellow foxtail millet (Setaria pumila) A* | ||
| Pearl millet (Pennisetum glaucum) A* | ||
| Kodo millet (Paspalum scrobiculatum) A* | ||
| African finger millet (Eleusine coracana) A* | ||
| Sorghum millet (Sorghum bicolor) A* | ||
| Pulses | Chickpea (Cicer sp.) A/P | Horsegram (Macrotylma uniflorum) A |
| Vetching (Vicia/Lathyrus sp.) A | Black/Urad bean (Vigna mungo) A | |
| Lentil (Lens sp.) A | Mung bean (Vigna radiata) A/P | |
| Pea (Pisum sp.) A/P | African gram bean (Vigna cf. trilobata) A/P | |
| Fruits | Indian jujube (Ziziphus mauritiana) P | Grape (Vitis vinifera) P |
| Eggplant (Macrotylma solanum) P | Cucumber/melon (Cucumis sp.) P | |
| Date palm (Phoenix dactylifera) P | ||
| Oilseeds and Fibre | Sesame (Sesamum sp.) | Cotton (Gossypium sp.) A/P |
| Linseed/Flax (Linum sp.) A | Hemp (Cannabis cf. sativa) A/P | |
| Poppy (Papaver sp.) | Jute (Corchorus sp.) A/P | |
| Mustard (Brassica sp.) A/P | ||
| Underground storage organs | Ginger (Zingiber sp.) P Garlic (Allium sativum) P |
Turmeric (Curcuma sp.) P |
1.4.2. Zooarchaeological evidence and secondary-product exploitation
On average, about 80% of the faunal assemblage from various Indus sites belong to domestic animal species (Thomas and Joglekar, 1994; Thomas, 2002). Out of the domestic animals, cattle/buffalo are the most abundant, averaging between 50 and 60% of the animal bones found, with sheep/goat accounting for 10% of animal remains (Thomas, 2002; Miller, 2004; Joglekar et al., 2013; Chase, 2014). The high proportions of cattle bones may suggest a cultural preference for beef consumption across Indus populations, supplemented by the consumption of mutton/lamb. Animal kill-off profiles for Indus settlements reveal a general trend of the presence of older adults for bovine and caprine/ovine species suggesting that meat consumption was complemented with the widespread existence of secondary-product exploitation (Joglekar et al., 2013; Chase, 2010, 2014). According to Miller (2004), at Harappa, 90% of cattle were kept alive until the age of 3–3.5 years, suggesting that females were used for dairying production, whereas males were used for traction (Miller, 2003). Pigs make up about 2–3% of total faunal assemblages across Indus sites but the domestic status of the pig is not yet determined (Thomas, 2002; Chase, 2014). Wild animal species like deer, antelope, gazelle, hares, birds, and riverine/marine resources are also found in small proportions in the faunal assemblages of both rural and urban Indus sites (Belcher, 2003; Deshpande Mukherjee, 1998), suggesting that these diverse resources had a place in the Indus diet. The pattern is similar at the sites in northwest India, where domestic and wild mammals, and smaller proportions of birds, reptiles, riverine fish, and molluscs were consumed (Joglekar et al., 2013). In the study region, cattle make up the largest proportion of domestic species across sites (Joglekar et al., 2013), but not all excavated sites have information available (Table 3). Cattle size measurements suggest a dominance of castrated bulls or females present in the assemblages studied, a herding strategy likely adopted to practice dairying (Joglekar et al., 2013). No change across time with respect to species ratios has been observed.
Table 3.
Frequency of faunal remains from study sites (Joglekar et al. 2013, 2016, 2017, 2018). na: not available, nr: not reported.
| Faunal evidence (%NISP) |
NISP |
||||||
|---|---|---|---|---|---|---|---|
| Site name | Cattle/buffalo | Sheep/goat | Pig | Wild ruminants | Freshwater fish | Other | |
| Alamgirpur | 81 | 9 | 4 | 5 | nr | 1 | 493 |
| Masudpur VII | 80 | 17 | nr | 2 | 0.6 | 0.4 | 159 |
| Masudpur I | 83 | 10 | 2 | 3 | 0.2 | 1.8 | 1367 |
| Lohari Ragho I | na | na | na | na | na | na | na |
| Khanak | na | na | na | na | na | na | na |
| Farmana | 79 | 15 | 0.5 | 1 | 3.3 | 1.2 | 4208 |
| Rakhigarhi | na | na | na | na | na | na | na |
Available carbon isotopic results from tooth enamel of domestic animals suggest that cattle/buffalo had almost exclusively C4 diets, while sheep/goat had mixed consumption of C4 and C3 plants (Chase et al., 2014, Chase et al., 2018; Sarkar et al., 2016; Chakraborty et al., 2018; Lightfoot et al., 2020). The data suggests that humans may have controlled cow and water buffalo diets, feeding them millets or wild C4 vegetation (Chase et al., 2014; Lightfoot et al., 2020), while sheep and goats had less controlled diets, and were presumably roaming the landscape (Lightfoot et al., 2020). This likely involved the separation of tasks at an individual or group level (Lightfoot et al., 2020). Crucially, even though the information available for different time periods is limited, there appears to be no change in enamel δ13C values for most species across time in northwest India, suggesting continuity in foddering practices over time (Lightfoot et al., 2020).
2. Materials and methods
2.1. Sample selection
Analysis was conducted on 172 pottery fragments recovered from rural and urban settlements (n = 7) located in northwest India (Table 1; Fig. 1). Pottery fragments were generally selected from contexts that had radiocarbon dates associated with them, and/or from contexts that were indicative of occupational surfaces. Rims of vessels were preferentially selected as experimental evidence suggests that the boiling of products in vessels would lead to lipids accumulating here (Charters et al., 1993). However, body sherds of perforated vessels were analysed as they are easy to identify. Rim fragments were used to reconstruct the original shape and size of the mouth of vessels, but the volumetric capacity of vessels could not be determined.
2.2. Sample preparation and instrumental analyses
The external surface of ceramic sherds was removed with a modelling drill and a sample (1g) was obtained by drilling to a depth of 2–5 mm from the sherd surface (Craig et al., 2005, 2011). When available, upto 5g of sediment adhering to potsherds was collected with tweezers. Drill bits and tweezers were cleaned prior to use and in-between samples with dichloromethane.
Lipids were extracted and methylated in one step with the acidified methanol technique (Craig et al., 2011; Correa-Ascencio and Evershed, 2014). Methanol was added to the sample and sonicated for 15 min. Concentrated sulphuric acid (800 μl) was added to the mixture and heated in sealed tubes for 4 h at 70 °C. After cooling, the lipids were extracted with n-hexane (3 × 2 ml) and then analysed by gas chromatography-mass spectrometry (GC-MS) and gas chromatography-combustion-isotopic ratio mass spectrometry (GC-C-IRMS). Internal standards were added before and after the extraction process.
GC-MS was carried out on all samples using an Agilent 7890 B Series Gas Chromatograph attached to an Agilent 5977 B Mass Spectromer with a quadrupole mass analyser (Agilent technologies, Cheadle, Cheshire, UK). All samples were initially screened using a split/splitless injector in splitless mode which was maintained at 300 °C. The GC carrier gas was helium, configured at a constant flow rate of 1 ml min−1. The column (HP-5MS) was coated with 5% phenyl-methylpolysiloxane (30 m × 0.25 mm x 0.25 μm; Agilent technologies, Cheadle, Cheshire, UK). The oven temperature was set at 50 °C for 2 min, then raised by 10 °C min−1 until 325 °C was reached, where it was held for 15 min until the end of the run. The ionization energy of the mass spectrometer was 70 eV and spectra were obtained in scanning mode between m/z 50 and 800.
Of the total, 73 samples were selected for analysis with a GC-C-IRMS system comprising an Isoprime 100 (Isoprime, Cheadle, UK) linked to a Hewlett Packard 7890B series Gas Chromatograph (Agilent Technologies, Santa Clara, CA, USA) with an Isoprime GC5 interface (Isoprime, Cheadle, UK), according to previously described protocols (Lucquin et al., 2016). These samples were selected because they had relatively higher concentrations of C16:0 and C18:0 fatty acids. The results from the analyses are reported relative to an international scale (VPDB). Replicate measurements of the sample and a mixture of fatty acid methyl esters (FAMEs) with δ13C values comparable to international standards were used to determine instrument precision (±0.3‰ SE) and accuracy (±0.5‰ SE). Values were further corrected to account for the methylation of the carboxyl group (Lucquin et al., 2016). Reference fats from South Asia were not available except for two dairy references obtained from previous publications (Craig et al., 2005). Following previous publications (e.g. Evershed et al., 2008; Dunne et al., 2012), Δ13C (δ13C18:0-16:0) values obtained from fatty acids were compared to modern reference animal fats from Africa (Dunne et al., 2012), UK (animals raised on a pure C3 diet) (Dudd and Evershed, 1998), Kazakhstan (Outram et al., 2009), Switzerland (Spangenberg et al., 2006) and the Near East (Gregg et al., 2009).
2.3. Statistical tests
Non parametric Kruskal-Wallis tests were used to compare lipid yields from different vessel forms and across sites, as well as the δ13C values of fatty acids obtained from lipid extracts of vessels across sites. All statistical tests were conducted in R (Version 3.4.1). The R script for all statistical tests and figures is available in Supplementary Information (SI 1 and SI 2). Access to raw data files and R Markdown documents is available in a data repository at https://doi.org/10.17863/CAM.54273.
3. Results
3.1. Survival of absorbed residues
Interpretable concentrations of lipid (above 5 μg/g) (Heron et al., 1991; Evershed et al., 1999, 2008; Reber et al., 2019) were obtained from 122 sherds (71%). However, overall obtained lipid concentrations were relatively low, comparable with those obtained from pottery found in sites in the Near East and Mediterranean region (Evershed et al., 2008; Gregg et al., 2009; Spiteri et al., 2016; Drieu, 2017) (Table 4). All the study sites have alkaline soils and experience seasonal, heavy rainfall and hot temperatures (Neogi et al., 2019), and have been subject to major transformations in the recent past due to agricultural activity. Microbial activity is optimal in pH above 6.5 (DeLaune et al., 1981) and fatty acids present in the form of soluble salts are more easily removed by leaching (Evershed et al., 1997). The combination of these conditions likely creates an unfavourable environment for organic preservation, which is also reflected in poor preservation of seeds and bones at these sites.
Table 4.
Total vessel fragments analysed, samples with appreciable lipid concentration (above 5 μg/g), % of samples with appreciable lipid yield, and mean and median lipid concentrations for vessels per site.
| Site code |
|||||||
|---|---|---|---|---|---|---|---|
| ALM | MSDVII | MSDI | LHRI | KNK | FRN | RGR | |
| Total number of samples analysed | 15 | 31 | 30 | 28 | 7 | 30 | 30 |
| Samples with appreciable lipid concentrations (above 5 μg/g) | 14 | 28 | 24 | 16 | 4 | 18 | 18 |
| % of samples with appreciable lipid concentrations (above 5 μg/g) | 93% | 90% | 79% | 60% | 43% | 60% | 60% |
| Mean lipid concentration (μg/g) | 14.3 | 30.2 | 23.3 | 31.6 | 49.2 | 14.5 | 15.7 |
| Median lipid concentration (μg/g) | 10.5 | 17.3 | 12.3 | 20.2 | 28.6 | 9.8 | 11.8 |
3.2. Molecular characterisation
Lipid profiles from vessels from urban and rural Indus sites in northwest India typically contained saturated medium-chain fatty acids with even number of carbons (C12:0, C14:0, C16:0, C18:0), mainly dominated by palmitic acid. The ratio of palmitic and stearic acid ranged from 0 to 3 (SI 3). Long-chain fatty acids (LCFAs) (C22:0, C24:0) were present in 74 of 122 samples (60%), but were less abundant compared to medium-chain components.
Such profiles are characteristic of degraded animal fats (Dudd et al., 1998), and the LCFA distributions were indicative of being routed through animal diet (Halmemies-Beauchet-Filleau et al., 2014; Whelton et al., 2018). Most samples contained odd-chain fatty acids (such as C15:0 and C17:0), and 69 samples (56%) contained odd-branched-chain fatty acids (C15Br and C17Br), which are typical of ruminant fats, but may also have bacterial origin (Dudd et al., 1999).
In addition, dicarboxylic acids and n-alkanes were detected in some samples (see data repository).
Long-chain n-alkanes, with an odd-over-even carbon chain length predominance, typical of plant epicuticular waxes (Kolattukudy, 1970; Dunne et al., 2016, 2018a, 2018b) were absent. Instead, the n-alkane profiles were more likely derived from petroleum contamination (Freeman and Pancost, 2014; Whelton et al., 2018) or thermal alteration of organic matter in sediments (March et al., 2014; Wang et al., 2017; Bondetti et al., 2020).
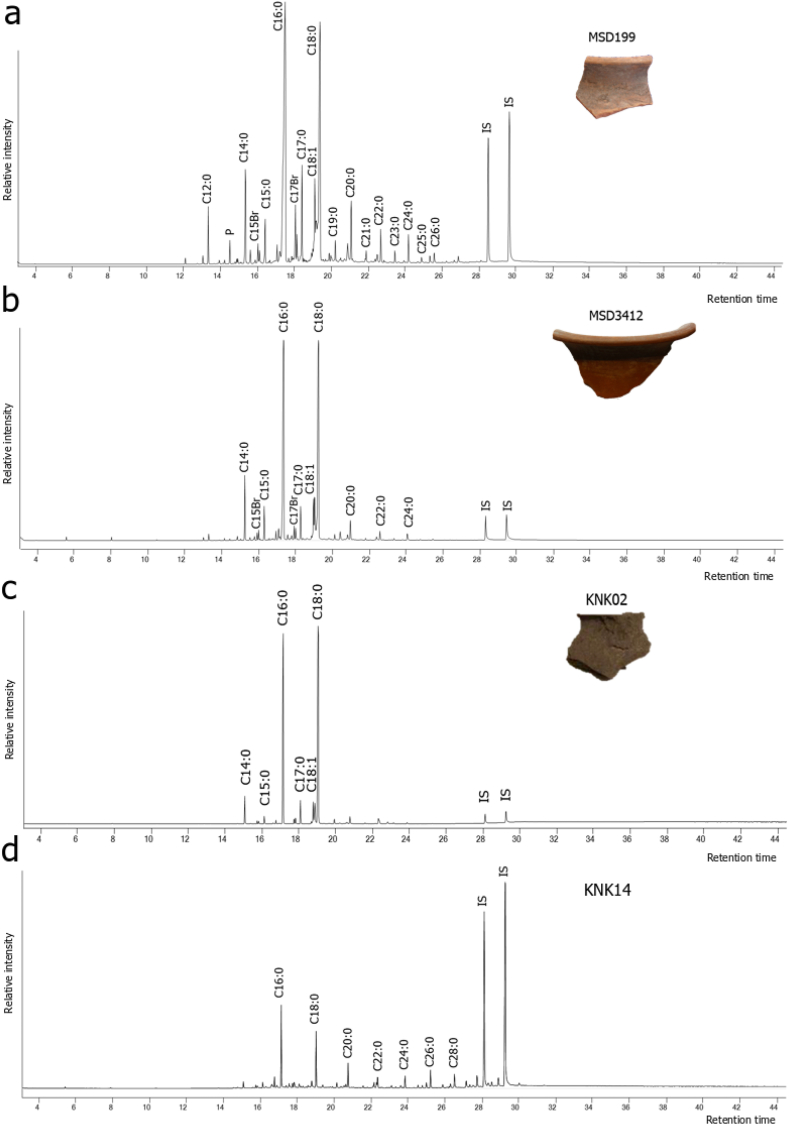
As oxidation products of unsaturated fatty acids, dicarboxylic acids may indicate the cooking of plant and animal resources, but they can also result from different degradation processes occurring during burial (Copley et al., 2005; Regert et al., 1998). However, despite their low concentration, comparison of lipids obtained from sediment adhering to potsherds revealed trace or minimal lipid yields, which suggest that the extracted lipids are derived from the use of vessels (Fig. 3), although this needs to be assessed on a case-by-case basis. Details of the relative contribution of fatty acids in each sample and presence/absence of compounds are provided in SI 3.
Fig. 3.
a) and b) Partial total ion chromatograms of vessel fragments from Masudpur I (MSD199) and Masudpur VII (MSD3412), c) Khanak (KNK02), and d) sediment adhering to potsherd KNK02 (KNK14). MSD199 has a profile with the distribution of LCFAs characteristic of degraded animal fats. MSD3412 has a lipid profile characteristic of degraded animal fats, typical of the analysed assemblage. The sediment sample KNK14 contains trace quantities of lipid and even LCFAs (C22:0-C30:0), not detected in KNK02. Cn:x indicates fatty acid with n carbon atoms and x double bonds. IS: Internal Standard, P: phthalate, Br: branched-chain fatty acid.
Overall, the lipid profiles of vessel fragments from all sites suggest the presence of degraded animal fats such as dairy or carcass fats. Aquatic biomarkers comprising ω-(o-alkylphenyl)alkanoic acids, vicinal dihydroxy fatty acids, and isoprenoid fatty acids (Cramp and Evershed, 2014) were not detected in the vessels. The biomarker miliacin, which is derived from the processing of millets such as Panicum miliaceum (Bossard et al., 2013) was also absent in all the vessels investigated. None of the vessel fragments analysed had any sooting or charring marks which suggests that they may not have been exposed to fire for long durations of time; but as most fragments analysed were rim-sherds, it was not possible to assess their exposure to fire.
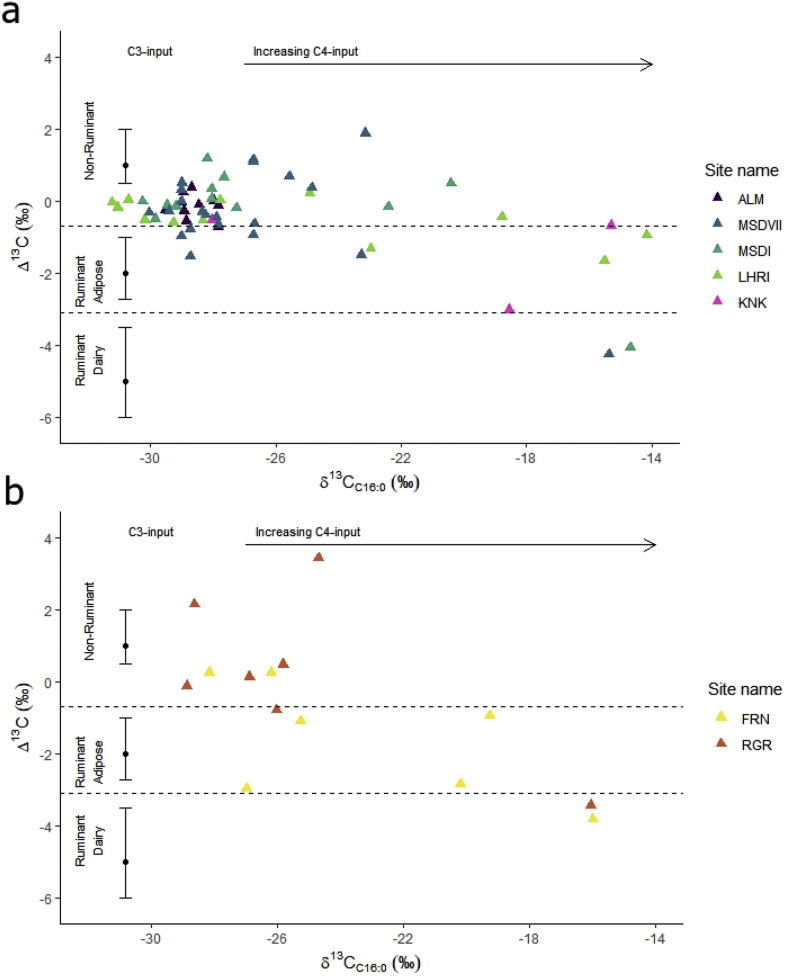
3.3. Compound-specific isotopic results
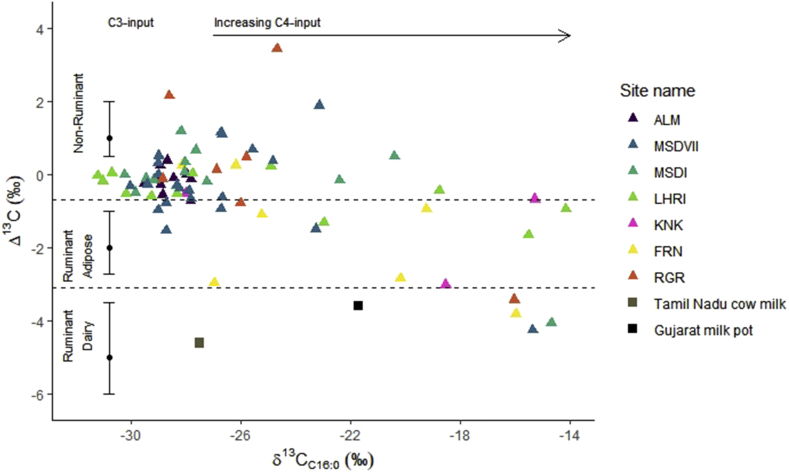
Most of the vessels analysed have Δ13C (δ13C18:0 - δ13C16:0) values ranging from −1‰ to 3.4‰ (n = 44, 60%), which places them within the global reference range (Evershed, 2008; Dunne et al., 2012) for non-ruminant products, with some vessels with Δ13C values falling between the ranges of non-ruminant and ruminant adipose fats, suggesting their mixtures (Figure 4). A smaller percentage have Δ13C values that fall within the range for ruminant adipose products. Only four vessels (5.5%, from Masudpur VII, Masudpur I, Farmana and Rakhigarhi) have values within the range for dairy products. A negative correlation was observed between the δ13C16:0 and Δ13C values, suggesting that samples with more negative Δ13C values produced fatty acids enriched in 13C, likely from tissues of ruminant animals that were consuming C4 plants , which was common in the region (Lightfoot et al., 2020). Comparison with available reference dairy fats from South Asia, which include a modern milk pot used to process milk from a C4 plant fed cow from Gujarat (Western India) and cow's milk from Tamil Nadu (South India) that was fed a mixed C3 and C4 diet (Craig et al., 2005: 886) confirms this suggestion.
Fig. 4.
Δ13C (δ13C18:0-16:0) values plotted against δ13C16:0 values of vessels (n = 73) across Indus sites in northwest India, colour-coded according to site and dairy references from South Asia (in squares) (Craig et al., 2005). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
4.1. Non-ruminant fats, plant products or mixtures
The obtained results were unexpected and challenging to interpret. Firstly, nearly 60% of the vessels analysed for compound-specific isotopic analysis fall within the range of non-ruminant fats, even though the lipid profiles of most vessels were characterised by odd, branched-chain fatty acids that are common in ruminant fats. Secondly, these results do not correlate with available faunal assemblages in the region. Only 2–3% NISP of faunal remains from the study sites comprised non-ruminant or omnivorous taxa (Table 3) (Singh et al., 2013; Joglekar et al., 2013, 2018) such as pigs, fowls/birds or hares. Although the Δ13C values of several vessels are consistent with established values of chicken adipose fats (Colonese et al., 2017), the presence of domesticated chicken (Gallus gallus domesticus) in the Indus Civilisation is uncertain, and early reports confirming its presence (Sewell and Guha, 1931; Prasad, 1936) are questionable, given recent genetic studies (Liu et al., 2006; Kanginakudru et al., 2008; Storey et al., 2012; Miao et al., 2013). Furthermore, chicken bones have not been conclusively identified from the study sites (Joglekar et al., 2013).
The observed discrepancy between faunal remains and compound specific isotopic results raises several hypotheses. It is possible that taphonomic conditions may have privileged the survival of large bones of cattle and buffalo; incomplete recovery practices that may have reduced the chances of collection of small bones of pigs or birds; or that animals may have been prepared away from sites and brought in for consumption (Mukherjee et al., 2008). However, high proportions of cattle bones in assemblages cannot be explained by coincidence or taphonomy alone. Similarly, sampling strategies for archaeobotany and zooarchaeology in the region have improved, and small remains such as fishbones and charcoal are collected during excavation. Furthermore, there is butchery evidence for a range of different animals at several sites including Masudpur I, Masudpur VII and Farmana (Joglekar et al., 2013; Joglekar et al., 2018), with no evidence for disarticulation and removal of low meat-bearing parts at a separate kill-site (Joglekar et al., 2018). It is possible, however, that specific methods of preparation were used for types of animals, such as spit-roasting for cattle/buffalo meat. It is also possible that the potsherds selected were not representative of the whole pottery assemblage.
A number of samples also have compound-specific isotopic results that fall within ranges reported for plant products, especially C3 oilseed plants such as sesame (Steele et al., 2010). Plants have a much higher C16:0 to C18:0 ratio than animal fats and produce significant deviations in Δ13C values depending on the absolute δ13C values of the end-members (Steele et al., 2010; Hendy et al., 2018). However, the ratios of palmitic/stearic acid in the study samples were not suggestive of plant products (SI 3). Despite this, plant products cannot be ruled out as they are often rendered ‘invisible’ due to their low-fat content (Hendy et al., 2018; Grillo et al., 2020).
Other interpretations include the possibility that low lipid yields or the burial environment may have affected compound specific isotopic values. However, this is ruled out by the lack of correlation between Δ13C values and lipid yields of vessels (SI 1, R2 = −0.05; p = 0.67). It is also possible that mixtures of products during cooking have resulted in ambiguous fatty acids values (e.g. Bondetti et al., 2020). For example, Hendy et al. (2018) demonstrated that mixtures of ruminant adipose products and C3 plants could produce Δ13C values similar to non-ruminant fats. A number of C3 plants, such as wheat, barley, and C3 pulses and oilseeds, were found at the study sites (see Table 2), which may have been mixed with ruminant adipose products during cooking. Additionally, the mixing of fatty acids from C4 plants and C3 fed ruminant adipose fats may result in Δ13C values that fall within the range for ruminant dairy fats (i.e. < −3.3‰) (Hendy et al., 2018). This effect occurs when C4 plant, enriched in 13C and with relatively high C16:0 fatty acid content, are mixed with C3 ruminant adipose fats that are relatively depleted in 13C and have a relatively greater C18:0 content. Hypothetical mixing curves suggest that the greater the difference in δ13C between the fatty acids in the two sources, the greater the effect on reducing the Δ13C value will be (see SI 1). However, there is little enamel stable isotope evidence that the ruminants were entirely fed on C3 plants (Lightfoot et al., 2020), therefore Indus vessels with Δ13C values below −3.3‰ can be confidently associated with dairy processing.
Given the diversity of resources that were available to Indus populations, it is possible that vessels were used for both plant and animal products to create foodstuffs throughout their life-histories. The availability of C3 and C4 plants and freshwater resources to Indus populations in northwest India further adds to the complexity in resolving isotopic mixtures. Without further knowledge of the isotopic end-members for different food products, interpretation or more accurate quantification using compound-specific stable isotope analysis is challenging. A dedicated programme involving the creation of theoretical models of mixtures of different products, experimental research, and building a database of modern reference fats with known diets from different regions of South Asia is essential for future research.
4.2. Limited dairy processing
Intriguingly, although cattle/buffalo and sheep/goat contribute to 80–90% NISP of faunal remains, and cattle size measurements suggest a dominance of females or castrated males at the study sites (Joglekar et al., 2013), the evidence for ruminant carcass and dairy products is extremely limited. Four vessels (MSD329, MSD3586, FRN04 and RGR20; jars with differences in shape and surface treatment) from four different sites have Δ13C values that fall within the established range for dairy products (below −3.3‰). Additionally, the vessels have δ13C16:0 values ranging between −16‰ and −14.7‰, which suggests that they are derived from ruminants consuming C4 plants. Given the evidence that Indus cattle/buffalo were consistently foddering on C4 plants throughout their lifetimes (Lightfoot et al., 2020), and comparison with previously published dairy reference fats from India (Craig et al., 2005), it is likely these four vessels were predominantly used for the processing of cattle/buffalo dairy products, although the presence of sheep/goat dairy products cannot be ruled out. Milk was either stored in its raw form in these vessels, or used to produce different types of dairy products, including yoghurt, clarified butter (ghee), or cream.
Cattle provide resources that have been intricately involved in the early urban economies (Zeder, 2006). These large animals yield great output in both meat and milk per animal compared to sheep and goat and also serve as beasts of burden in agricultural production and transport (Zeder, 2006). The importance of cattle in the Indus Civilisation has long been emphasised (e.g. Fairservis, 1967, 1986), but zooarchaeologists have mostly focused on early cattle domestication and breed differentiation (Meadow 1981, 1989, 1993, 1996). As there are high proportions of adult cattle found in Indus zooarchaeological assemblages, it is generally assumed they were used for secondary products utilisation (e.g. Channarayapatna, 2018; Chase et al., 2014, 2018), such as traction (Miller, 2003; Chase, 2010) and dairying (Gouin, 1990). Although the results suggest that ruminant milk may have been used in some Indus vessels, the percentage of vessels that are linked directly to dairy processing is very small.
Comparison with ceramic lipid residues found in prehistoric contexts around the world suggests that the minimal presence of dairy in Indus vessels from northwest India is highly unusual. Direct evidence for extensive dairy processing has been found at sites in northwest Anatolia as early as the seventh and sixth millennia BC (Dudd and Evershed, 1998; Evershed et al., 2008), in the sixth millennium BC in eastern Europe (Craig et al., 2005), and in Britain by the fourth millennium BC, with increased dairy processing in the Bronze and Iron Ages (Copley et al., 2005a, 2005b, 2005c). Even in arid regions such as Libyan Sahara, the independent inception of dairying practices by mobile pastoral groups has been dated to the fifth millennium BC (Dunne et al., 2012, 2018).
This marked difference raises questions about how widespread the practice of dairying in Indus settlements in northwest India may have been. It is possible that dairy consumption was limited to fewer groups, was not as widely practiced in these Indus settlements, or that dairy products were primarily used in vessels not analysed in this study, or used in vessels made from organic materials that have not survived (e.g. Joshi, 2016). The possibility of dairy being rare or ‘special’ at certain settlements suggests everyone may not have had access to specific animal products. Alternatively, it is also possible that vessels used for processing dairy were re-used for several years, as three out of the four vessels have relatively higher lipid concentrations compared to other analysed vessels from similar contexts (MSD329: 38.3 μg/g, MSD3586: 66.7 μg/g, RGR20: 36.7 μg/g), thus constraining the likelihood of other vessels demonstrating a strong dairy signal from contemporaneous contexts.
Interestingly, a recent paper reports dairy processing in 8 out of 21 vessels, out of which 4 are bowls and one is a perforated vessel, from a contemporaneous Sorath Harappan settlement in Gujarat, Western India (Chakraborty et al., 2020). Apart from the dairy evidence, the results obtained are similar in terms of the range of isotope values obtained in this study. Both studies demonstrate the importance of using ceramic lipid residues to understand the extent of dairy exploitation across the Indus and the need to expand the number of samples and vessel types analysed. A large-scale analysis of vessels may yield insight into regional variations in foodways or vessel-usage.
4.3. Differences between settlements
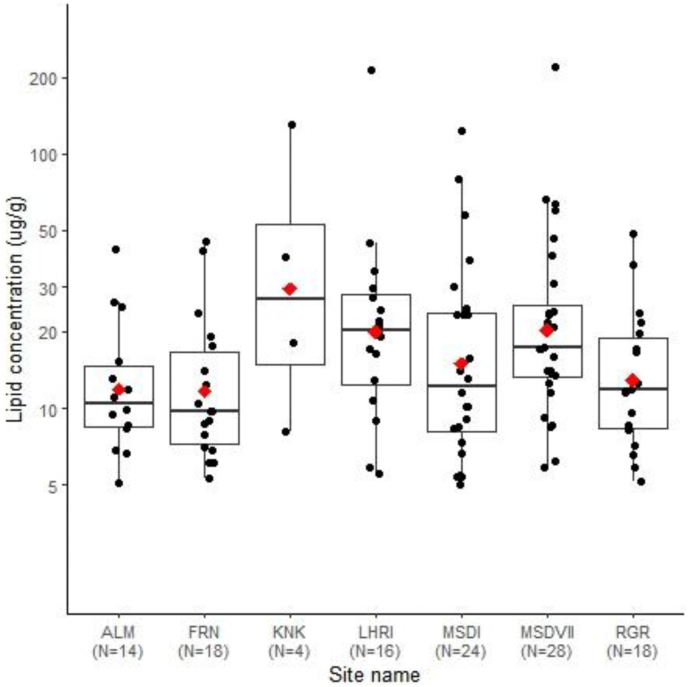
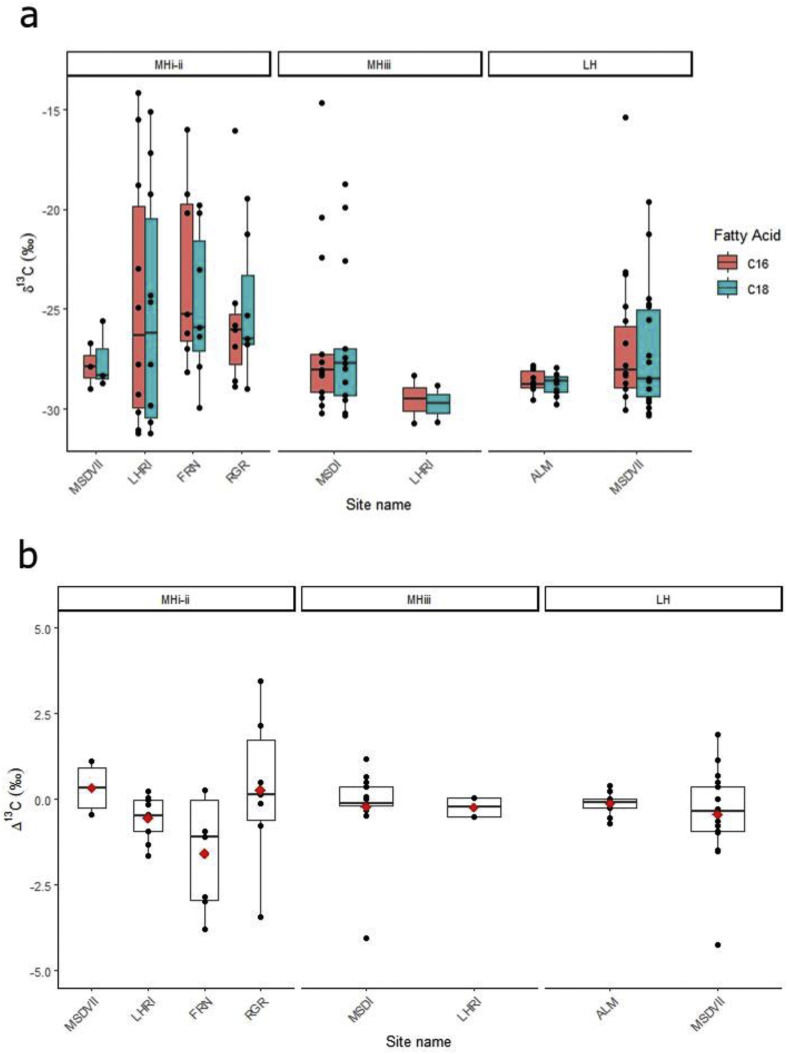
Comparisons of lipid yields suggest there are no differences in lipid yields across sites (Fig. 5; Kruskal-Wallis test of effect of site (χ2(6) = 11.8, p = 0.06). This pattern held when comparing all groups, or those for which n ≥ 10. Meanwhile, inter-site variations between compound-specific isotopic values of vessels were observed (Fig. 6), and there was a significant effect of settlement type (rural vs. urban) on δ13C16:0 (χ2(1) = 5.9, p = 0.01) and δ13C18:0 (χ2(1) = 5.3, p = 0.02) values. However, there were no effect of settlement type (rural vs. urban) on Δ13C values (χ2(1) = 2.1, p = 0.6), indicating that settlements used vessels in similar ways.
Fig. 5.
Lipid concentrations of vessels from different Indus sites in northwest India. Lipid concentrations are represented on a log scale for better visualisation; red diamonds are mean values. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 6.
Δ13C (δ13C18:0-16:0) values plotted against δ13C16:0 values of vessels from a) rural and b) urban Indus sites in northwest India, colour-coded according to site. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
However, there are some indications of variability between settlements (Fig. 6). For example, at the small village site of Alamgirpur (ALM), Δ13C values from ceramic lipid residues concentrate between −1‰ and 1‰, while other sites demonstrate wider ranges of values. Additionally, the village site of Lohari Ragho I (LHRI) demonstrates the largest range of δ13C16:0 values, suggesting that inhabitants used vessels for products from animals feeding on purely C4 plants as well as mixtures of C3 and C4 plants. The seasonal movement of animals to different pastures could also explain the wide range of δ13C values observed in adipose fats, but this is more difficult to explain for non-ruminants. As enamel isotopic evidence from cattle/buffalo at Indus sites suggests they were consistently fed C4 plants (Chase et al., 2014; Sarkar et al., 2016; Chakraborty et al., 2018; Lightfoot et al., 2020), vessels with the ruminant adipose range with δ13C16:0 values below −22‰ likely contained beef products. Additionally, samples from the city of Rakhigarhi (RGR) demonstrated the largest range in Δ13C values. Ranges in Δ13C values have been used to discuss differing pastoral modes of subsistence, such as vertical transhumance (Dunne et al., 2012, Dunne et al., 2018a). It is possible that animals/animal products from different regions and/or in different seasons were being brought to Rakhigarhi, which as a large city required foodstuff from other settlements to feed its population (e.g., Kenoyer, 2008).
However, a broad similarity in products is observed across both rural and urban sites, possibly indicating a degree of regional culinary unity. This similarity has implications for how we understand the dynamics between rural and urban populations in northwest India, where rural populations demonstrate distinctive material culture (Parikh and Petrie, 2019), and also appear to have used different cropping strategies compared to those seen at Harappa, particularly through the growing of millets and rice as opposed to wheat and barley (Petrie and Bates, 2017). Unfortunately, it is not presently possible to distinguish between different C3 plants in lipid extracts, and millets were not directly detected in the vessels. It is thus not possible to determine nuanced differences in specific plant products, or modes of food preparation.
4.4. Differences across cultural and climatic periods
An assessment of possible temporal changes from the urban (MHi-ii and MHiii) to the post-urban (LH) periods is challenging, as evidence that spans the periods of interest is limited to a small set of samples from the same sites. Nevertheless, results demonstrate that different types of animal meat and milk were likely processed within vessels during the urban period (MHi-ii and MHiii) (Fig. 7). Samples from Khanak (KNK) have been removed due to small sample size. Additionally, the wide range of Δ13C and δ13C16:0 values highlight the likely mobility of animals and diversity of animal management and feeding strategies, particularly in the MHi-ii period. No change was observed across cultural periods for δ13C16:0 values (χ2(2) = 4.2, p = 0.12), δ13C18:0 values (χ2(2) = 4.4, p = 0.1) or Δ13C values (χ2(2) = 0.78, p = 0.6).
Fig. 7.
Boxplots of a) δ13C16:0 and δ13C18:0 values and b) Δ13C (δ13C18:0-16:0) values of fatty acids from vessels across cultural time periods. MHi-ii: Mature Harappan i-ii, MHiii: Mature Harappan iii, LH: Late Harappan. Red diamonds represent mean values. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Out of the sites from which data before and after 4.2 ka BP is available, Alamgirpur has narrow ranges for δ13C values across time, suggesting no observable change in products processed in vessels during and after the period of climatic instability, and at Masudpur VII, although the sample sizes are small, the range of δ13C values of the fatty acids from vessels from after 4.2 ka BP is wider (SI 1). It is possible that the differences observed are due to uneven sample sizes, or that inhabitants at Masudpur VII diversified the products processed in vessels after 4.2 ka BP. Although there is considerable difficulty in reconciling climate ‘events’ and archaeological evidence as they operate on unique temporal scales, the evidence appears to suggest that specific culinary practices seem to have been practised for hundreds of years at Alamgirpur, with minor evidence for some change at Masudpur VII, matching patterns observed via archaeobotanical analyses (Petrie and Bates, 2017). Although more evidence is required, the faunal, archaeobotanical and lipid residue results suggest the continuation of daily practices at small rural sites over changing climatic conditions.
4.5. Patterns of vessel usage
Comparisons of lipid yields suggest there are no differences in lipid yields across vessel forms (χ2(10) = 11.24, p = 0.33), but the ranges of lipid yield are variable (SI 1). This pattern might suggest that certain vessels were used more frequently for processing of fatty-rich products or for longer periods of time. Lipid profiles suggest the consistent use of animal products (although it is difficult to comment on the presence of plant products), and the compound-specific isotopic results suggest the multi-functionality of vessels (Fig. 8). Studies have demonstrated how specific types of food processing in vessels, such as boiling or roasting, would lead to higher concentrations of lipid in specific parts of vessels (e.g. Charters et al., 1993, 1997). However, as different parts of the same vessel were not analysed, and overall lipid preservation was poor, it was not possible to make interpretations about the use of vessels for specific types of food-processing.
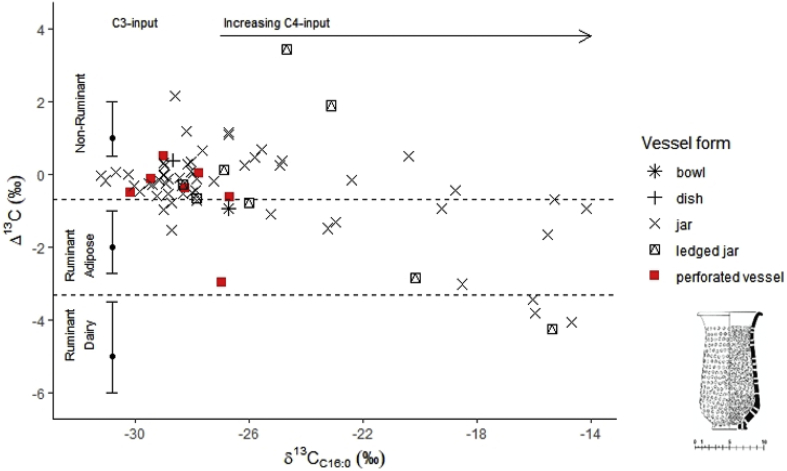
Fig. 8.
Δ13C (δ13C18:0-16:0) values plotted against δ13C16:0 values of different vessel forms across Indus sites in this study. Perforated vessels are marked in red. Example of a complete perforated vessel reproduced from Gouin (1990). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
This study revealed intriguing new information about the use of perforated vessels. Perforated vessels have been used for identifying dairy activities archaeologically in European contexts and are comparable to modern cheese strainers used for draining and separating curds during the hard cheese-making process (Salque et al., 2013). Although an early study suggested a similar use for them in Indus contexts (Gouin 1990; Bourgeois and Gouin, 1995), a hard cheese-making tradition has not been documented in the study region. Dairy curds and yoghurt are prepared daily in modern households, but cloth is preferentially used in the straining process. Ethnographic research of dairy practices in modern Punjab has, however, described the use of perforated lids used for heat regulation during dairy production (Miller, 2004: 216–217). A related example, although not linked to dairy processing, documents the use of a perforated vessel for the distilling of spirits from a variety of fermented liquors in the central states of modern India (Allchin, 1979: 57).
Out of the 23 analysed fragments of perforated vessels, 15 had mean lipid concentrations comparable to rim fragments of other vessels, which might indicate they were used for the processing of fat-rich products. Additionally, they had fatty acid-specific δ13C values that are consistent with either non-ruminant fats, plants, and/or mixtures of products (Fig. 8). Although the interpretations are presently ambiguous, these results suggest that perforated vessels were not primarily associated with dairy processing (contra Bourgeois and Gouin, 1995). Similarly, it is unlikely these vessels were used as braziers for heating (Mackay, 1938: 207) or simply as colanders for draining or straining liquids (Dales and Kenoyer, 1986: 108–109), as they contain fat-rich lipids. However, as 8 vessels contained less than 5 μg/g of lipid (SI 3, Table S15), it is possible that not all perforated vessels were used the same way. Although not conclusive, these results are exciting and have implications for how the function of Indus perforated vessels is interpreted in future research.
5. Conclusions
The organic residue analysis of Indus vessels presented here reveal that lipids are preserved in Indus vessels, but lipid concentrations are generally low. Dairy products, ruminant carcass meat, and either non-ruminant adipose fats, plants, or mixtures of these products constituted what was cooking in Indus vessels. The results presented here suggest a similarity in vessel usage across rural and urban settlements, and the multi-functionality of vessels. It is notable that evidence for direct plant-processing is limited, as are dairy products, although the interpretation of a large proportion of the data is presently ambiguous. Despite the limitations, this study constitutes an important starting point to broaden our thinking about Indus commensality. The priority of future research in the study of lipid residues in the region should be the building of reliable local isotopic references for fats and oils, which will clarify future interpretations. Assessing changes over cultural and climatic periods will require further sampling of pottery from well-dated contexts. The results demonstrate that the use of organic residue analysis in South Asia, combined with other bioarchaeological approaches, will facilitate a new understanding into the enormous diversity of prehistoric South Asian foodways and the relationship between pottery and foodstuff over time.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was based on AS's PhD dissertation that was funded by the Cambridge Commonwealth, European & International Trust and Nehru Trust for Cambridge University. Research funding was provided by the European Research Council (ERC) Consolidator Grant (2015–2020) under the Horizon 2020 research and innovation programme (TwoRains project: grant agreement number 648609), a UKIERI DST Thematic Partnership Award [DST-UKIERI-2016-17-0047] and by smaller grants awarded by Sidney Sussex College, Anthony Wilkin Fund, Smuts Memorial Fund and the McDonald Institute for Archaeological Research, University of Cambridge. CH thanks the Wellcome Trust for funding (Grant ref: 097365/Z/11/Z). The paper was written during AS's Fyssen Foundation postdoctoral fellowship (2020–2022) at CEPAM (UMR 7264 du CNRS), Université Nice Côte d’Azur, Nice.
AS thanks the Land, Water and Settlement and TwoRains teams for their contribution and support during this project, and Danika Parikh, Arun K. Pandey and Alessandro Ceccarelli for discussions about pottery and the drawings. Thanks are also due to the students and staff of the Department of Ancient Indian History, Culture and Archaeology, Banaras Hindu University, Varanasi, and Deccan College Post-Graduate and Research Institute, Pune for their support during sample collection; the Archaeological Survey of India (ASI) for granting permission to study the pottery; and members of BioArch, University of York, and Scientific Research, British Museum who supported her with the analyses. Thank you to Marco Madella and Martine Regert for their comments during the PhD viva, and to the anonymous reviewers whose suggestions greatly improved the paper.
All authors read and approved the final version of the paper. AS: Conceptualization, Data Analysis, Investigation, Visualisation, Writing- Original draft preparation, Writing- Reviewing and Editing; MC: Resources, Methodology, Validation; CPH: Resources, Methodology, Validation; OEC: Resources, Methodology, Validation, Writing- Reviewing and Editing; VS: Resources; RNS: Resources; TCO: Supervision, Resources, Methodology, Validation, Writing- Reviewing and Editing. CAP: Supervision, Resources, Writing- Reviewing and Editing.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jas.2020.105291.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- Agrawal D.P. Aryan Books International; New Delhi: 2007. The Indus Civilization: an Interdisciplinary Perspective. [Google Scholar]
- Allchin F.R. India: The Ancient Home of Distillation? Man. 1979;14(1):55–63. doi: 10.2307/2801640. [DOI] [Google Scholar]
- Appadurai A. Gastro-politics in Hindu South Asia. Am. Ethnol. 1981;8(3):494–511. [Google Scholar]
- Bala M. The pottery. In: Lal B.B., Joshi J.P., Thapar B.K., Bala M., editors. Excavations at Kalibangan: the Early Harappans 1961-1969, Memoirs of the Archaeological Survey of India No, 98 B. vols. 101–222. Archaeological Survey of India; New Delhi: 2003. [Google Scholar]
- Bates J. Oilseeds, spices, fruits and flavour in the Indus civilisation. J. Archaeol. Sci.: Report. 2019;24(April):879–887. doi: 10.1016/j.jasrep.2019.02.033. [DOI] [Google Scholar]
- Belcher W.R. Fish exploitation of the Indus valley tradition. In: Weber S., Belcher W.R., editors. Indus Ethnobiology. Lexington Books; Oxford: 2003. pp. 95–174. [Google Scholar]
- Bhan Suraj. Kurukshetra University; Kurukshetra: 1975. Excavation at Mitathal (1968) and Other Explorations in the Sutlej-Yamuna Divide. [Google Scholar]
- Bondetti M., Lucquin A., Savel’ev N.A., Weber A.W., Craig O.E., Jordan P.D. Resource processing, early pottery and the emergence of Kitoi culture in Cis-Baikal: insights from lipid residue analysis of an Early Neolithic ceramic assemblage from the Gorelyi Les habitaton site, Eastern Siberia. Archaeological Research in Asia. 2020;24:100225. doi: 10.1016/j.ara.2020.100225. [DOI] [Google Scholar]
- Bossard N., Jacob J., Le Milbeau C., Sauze J., Tervilliger V.T., Poissonnier B., Lallier-Vergès E. Distribution of miliacin (olean-18-en-3β-ol methyl ether) and related compounds in broomcorn millet (Panicum miliaceum) and other reputed sources: Implications for the use of sedimentary miliacin as a tracer of millet. Org. Geochem. 2013;63:48–55. doi: 10.1016/j.orggeochem.2013.07.012. [DOI] [Google Scholar]
- Bourgeois G., Gouin P. ‘Résultats d’une analyse de traces organiques fossiles dans une « faisselle » harappéenne’. Paleorient. 1995;21(1):125–128. [Google Scholar]
- Ceccarelli A. Unpublished PhD Thesis. University of Cambridge; Cambridge: 2020. Ceramic Traditions and Ceramic Landscapes of the Indus Civilisation: Investigating the Technologies and Socio-Economic Complexity of Rural Pottery Production in Bronze Age Northwest India. [Google Scholar]
- Chakraborty K.S., Chakraborty S., Le Roux P., Miller H.M.-L., Shirvalkar P., Rawat Y. Enamel isotopic data from the domesticated animals at Kotada Bhadli, Gujarat, reveals specialized animal husbandry during the Indus civilization. J. Archaeol. Sci.: Report. 2018;21(October):183–199. doi: 10.1016/j.jasrep.2018.06.031. [DOI] [Google Scholar]
- Chakraborty K.S., Slater G., Miller H.M.-L., Shirvalkar P., Rawat Y. Compound specific isotope analysis of lipid residues provides the earliest direct evidence of dairy product processing in South Asia. Nature: Sci. Rep. 2020;10:16095. doi: 10.1038/s41598-020-72963-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channarayapatna S. In: A Study of Animal Utilization Strategies From Early to Late Harappan Periods in Haryana. Uesugi A., editor. Research Group for South Asian Archaeology, Archaeological Research Institute,; Kansai University: 2018. (South Asian Archaeology 3). [Google Scholar]
- Charters S., Evershed R.P., Goad L.J., Leyden A., Blinkhorn P.W., Denham V. Quantification and distribution of lipid in archaeological ceramics: implications for sampling potsherds for organic residue analysis and classification of vessel use. Archaeometry. 1993;35(2):211–223. doi: 10.1111/j.1475-4754.1993.tb01036.x. [DOI] [Google Scholar]
- Charters S., Evershed R.P., Quye A., Blinkhorn P.W., Reeves V. Simulation experiments for determining the use of ancient pottery vessels: the behaviour of epicuticular leaf wax during boiling of a leafy vegetable. J. Archaeol. Sci. 1997;24(1):1–7. doi: 10.1006/jasc.1995.0091. [DOI] [Google Scholar]
- Chase B. Social change at the Harappan settlement of Gola Dhoro: a reading from animal bones. Antiquity. 2010;84(324):528–543. doi: 10.1017/S0003598X00066758. [DOI] [Google Scholar]
- Chase B. On the pastoral economies of Harappan Gujarat: faunal analyses at Shikarpur in context. Heritage: Journal of Multidisciplinary Studies in Archaeology. 2014;2:1–22. [Google Scholar]
- Chase B., Meiggs D., Ajithprasad P., Slater P.A. Pastoral land-use of the Indus Civilization in Gujarat: faunal analyses and biogenic isotopes at Bagasra. J. Archaeol. Sci. 2014;50(October):1–15. doi: 10.1016/j.jas.2014.06.013. [DOI] [Google Scholar]
- Chase B., Meiggs D., Ajithprasad P., Slater P.A. What is left behind: advancing interpretation of pastoral land-use in Harappan Gujarat using herbivore dung to examine biosphere strontium isotope (87Sr/86Sr) variation. J. Archaeol. Sci. 2018;92(April):1–12. doi: 10.1016/j.jas.2018.01.007. [DOI] [Google Scholar]
- Colonese A.C., Lucquin A., Guedes E.P., Thomas R., Best J., Fothergill B.T., Sykes N. The identification of poultry processing in archaeological ceramic vessels using in-situ isotope references for organic residue analysis. J. Archaeol. Sci. 2017;78(February):179–192. doi: 10.1016/j.jas.2016.12.006. [DOI] [Google Scholar]
- Copley M.S., Berstan R., Mukherjee A.J., Dudd S.N., Straker V., Payne S., Evershed R.P. Dairying in antiquity. III. Evidence from absorbed lipid residues dating to the British Neolithic. J. Archaeol. Sci. 2005;32(4):523–546. doi: 10.1016/j.jas.2004.08.006. [DOI] [Google Scholar]
- Copley M.S., Berstan R., Dudd S.N., Straker V., Payne S., Evershed R.P. Dairying in antiquity. I. Evidence from absorbed lipid residues dating to the British Iron Age. J. Archaeol. Sci. 2005;32(4):485–503. doi: 10.1016/j.jas.2004.07.004. [DOI] [Google Scholar]
- Copley M.S., Berstan R., Straker V., Payne S., Evershed R.P. Dairying in antiquity. II. Evidence from absorbed lipid residues dating to the British Bronze Age. J. Archaeol. Sci. 2005;32(4):505–521. doi: 10.1016/j.jas.2004.07.005. [DOI] [Google Scholar]
- Copley M.S., Bland H.A., Rose P., Horton M., Evershed R.P. Gas chromatographic, mass spectrometric and stable carbon isotopic investigations of organic residues of plant oils and animal fats employed as illuminants in archaeological lamps from Egypt. Analyst. 2005;130:860–871. doi: 10.1039/b500403a. [DOI] [PubMed] [Google Scholar]
- Correa-Ascencio M., Evershed R.P. High throughput screening of organic residues in archaeological potsherds using direct acidified methanol extraction. Analytical Methods. 2014;6(5):1330. doi: 10.1039/C3AY41678J. [DOI] [Google Scholar]
- Craig O.E., Chapman J., Heron C.P., Willis L.H., Bartosiewicz L., Taylor G., Whittle A., Collins M.J. Did the first farmers of central and eastern Europe produce dairy foods? Antiquity. 2005;79(306):882–894. doi: 10.1017/S0003598X00115017. [DOI] [Google Scholar]
- Craig O.E., Steele V.J., Fischer A., Hartz S., Andersen S.H., Donohoe P., Glykou A. Ancient lipids reveal continuity in culinary practices across the transition to agriculture in northern Europe. Proc. Natl. Acad. Sci. Unit. States Am. 2011;108(44):17910–17915. doi: 10.1073/pnas.1107202108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramp L., Evershed R.P. Reconstructing aquatic resource exploitation in human prehistory using lipid biomarkers and stable isotopes. In: Hollan H.D., Turekian K.K., editors. Treatise on Geochemistry. 2nd. Elsevier; Oxford: 2014. pp. 319–339. 12. [Google Scholar]
- Dales G.F., Kenoyer J.M. University of Pennsylvania Museum; Philadelphia, PA: 1986. Excavations at Mohenjo-Daro, Pakistan: the pottery, with an account of the pottery from the 1950 excavations of Sir Mortimer Wheeler. [Google Scholar]
- DeLaune R.D., Reddy C.N., Jr Patrick W.H. Organic matter decomposition in soil as influenced by pH and redox conditions. Soil Biol. Biochem. 1981;13(6):533–534. [Google Scholar]
- Deshpande-Mukherjee A. Shellfishing and shell crafts during the Harappan period in Gujarat. Man and Environment. 1998;23(1):63–80. [Google Scholar]
- Dixit Y., Hodell D.A., Petrie C.A. Abrupt weakening of the summer monsoon in northwest India ~4100 Yr ago. Geology. 2014;42(4):339–342. doi: 10.1130/G35236.1. [DOI] [Google Scholar]
- Drieu L. Unpublished PhD thesis. Université Côte d’Azur; Nice: 2017. Fabrication et usages des poteries durant le néolithique et la protohistoire en europe: les apports de l’archéologie biomoléculaire. [Google Scholar]
- Dudd S.N., Evershed R.P. Direct demonstration of milk as an element of archaeological economies. Science. 1998;282(5393):1478–1481. doi: 10.1126/science.282.5393.1478. [DOI] [PubMed] [Google Scholar]
- Dudd S.N., Evershed R.P., Gibson A.M. Evidence for varying patterns of exploitation of animal products in different prehistoric pottery traditions based on lipids preserved in surface and absorbed residues. J. Archaeol. Sci. 1999;26(12):1473–1482. doi: 10.1006/jasc.1998.0434. [DOI] [Google Scholar]
- Dudd S.N., Regert M., Evershed R.P. Assessing microbial lipid contributions during laboratory degradations of fats and oils and pure triacylglycerols absorbed in ceramic potsherds. Org. Geochem. 1998;29(5–7):1345–1354. doi: 10.1016/S0146-6380(98)00093-X. [DOI] [Google Scholar]
- Dunne J., di Lernia S., Chłodnicki M., Kherbouche F., Evershed R.P. Timing and pace of dairying inception and animal husbandry practices across Holocene North Africa. Quat. Int. 2018;471(March):147–159. doi: 10.1016/j.quaint.2017.06.062. [DOI] [Google Scholar]
- Dunne J., Evershed R.P., Salque M., Cramp L., Bruni S., Ryan K., Biagetti S., di Lernia S. First dairying in green saharan Africa in the fifth millennium BC. Nature. 2012;486(7403):390–394. doi: 10.1038/nature11186. [DOI] [PubMed] [Google Scholar]
- Dunne J., Grillo K.M., Casanova E., Whelton H.L., Evershed R.P. Pastoralist foodways recorded in organic residues from pottery vessels of modern communities in Samburu, Kenya. J. Archaeol. Method Theory. 2018;26:619–642. doi: 10.1007/s10816-018-9384-0. [DOI] [Google Scholar]
- Dunne J., Mercuri A.M., Evershed R.P., Bruni S., Di Lernia S. Earliest direct evidence of plant processing in prehistoric Saharan pottery. Nature Plants. 2016;3:16194. doi: 10.1038/nplants.2016.194. [DOI] [PubMed] [Google Scholar]
- Dunne J., Chapman A., Blinkhorn P., Evershed R.P. Fit for purpose? Organic residue analysis and vessel specialisation: the perfectly utilitarian medieval pottery assemblage from West Cotton, Raunds. J. Archaeol. Sci. 2020;120:105178. doi: 10.1016/j.jas.2020.105178. [DOI] [Google Scholar]
- Eglington G., Logan G.A. Molecular preservation. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1991;333(1268):315–328. doi: 10.1098/rstb.1991.0081. [DOI] [PubMed] [Google Scholar]
- Eltsov P.A. Brill; Boston: 2008. From Harappa to Hastinapura: A Study of the Earliest South Asian City and Civilization. [Google Scholar]
- Evershed R.P. Organic residue analysis in archaeology: the archaeological biomarker revolution. Archaeometry. 2008;50(6):895–924. doi: 10.1111/j.1475-4754.2008.00446.x. [DOI] [Google Scholar]
- Evershed R.P., Mottram H.R., Dudd S.N., Charters S., Stott A.W., Lawrence G.J., Gibson A.M., Conner A., Blinkhorn P.W., Reeves V. New criteria for the identification of animal fats preserved in archaeological pottery. Naturwissenschaften. 1997;84(9):402–406. doi: 10.1007/s001140050417. [DOI] [Google Scholar]
- Evershed R.P., Dudd S.N., Charters S., Mottram H., Stott A.W., Raven A., van Bergen P.F., Bland H.A., Jones M., Bada J. Lipids as carriers of anthropogenic signals from prehistory. Phil. Trans.: Biol. Sci. 1999;354(1379):19–31. doi: 10.1098/rstb.1999.0357. [DOI] [Google Scholar]
- Evershed R.P., Payne S., Sherratt A.G., Copley M.S., Coolidge J., Urem-Kotsu D., Kotsakis K. Earliest date for milk use in the Near East and southeastern Europe linked to cattle herding. Nature. 2008;455(7212):528–531. doi: 10.1038/nature07180. [DOI] [PubMed] [Google Scholar]
- Fairservis W.A. The Origin, Character and Decline of an Early Civilization. American Museum Novitates. 1967;(2302) [Google Scholar]
- Fairservis W.A. Cattle and the Harappan Chiefdoms of the Indus Valley. Expedition. 1986;28(2):43–49. [Google Scholar]
- Freeman K.H., Pancost R.D. Treatise on Geochemistry. second ed. Elsevier; Oxford: 2014. Biomarkers for terrestrial plants and climate; pp. 395–416. [Google Scholar]
- Fuller D. Ceramics, seeds and culinary change in prehistoric India. Antiquity. 2005;79(306):761–777. doi: 10.1017/S0003598X00114917. [DOI] [Google Scholar]
- García-Granero J.J, Lancelotti C., Madella M., Ajithprasad P. Millets and herders: the origins of plant cultivation in semiarid north Gujarat (India) Curr. Anthropol. 2016;57(2):149–173. doi: 10.1086/685775. [DOI] [Google Scholar]
- Fuller D.Q., Madella M. Issues in Harappan Archaeobotany: Retrospect and Prospect. In: Korisettar R., Settar S., editors. Indian Archaeology in Retrospect: Protohistory. Manohar; New Delhi: 2002. pp. 317–390. [Google Scholar]
- García-Granero J.J., Lancelotti C., Madella M. A methodological approach to the study of microbotanical remains from grinding stones: a case study in northern Gujarat (India) Veg. Hist. Archaeobotany. 2017;26(1):43–57. doi: 10.1007/s00334-016-0557-z. [DOI] [Google Scholar]
- Garge T. Sothi-siswal ceramic assemblage: a reappraisal. Ancient India. 2010;2:15–40. [Google Scholar]
- Giesche A., Staubwasser M., Petrie C.A., Hodell D.A. Indian winter and summer monsoon strength over the 4.2 BP event in foraminifer isotope records from the Indus River delta in the Arabian Sea. Clim. Past. 2019;15(1):73–90. doi: 10.5194/cp-15-73-2019. [DOI] [Google Scholar]
- Gouin P. Rapes, jarres et faisselles : la production et l’exportation des produits laitiers dans l’Indus du 3e millénaire. Paleorient. 1990;16(2):37–54. doi: 10.3406/paleo.1999.4694. [DOI] [Google Scholar]
- Gregg M.W., Banning E.B., Gibbs K., Slater G.F. Subsistence practices and pottery use in Neolithic Jordan: molecular and isotopic evidence. J. Archaeol. Sci. 2009;36(4):937–946. doi: 10.1016/j.jas.2008.09.009. [DOI] [Google Scholar]
- Grillo K.M., Dunne J., Marshall F., Prendergast M.E., Casanova E., Gidna A.O., Janzen A., Karega-Muneneg, Keuteh J., Mabullai A.Z.P., Robertshawj P., Gillard T., Walton-Doyleb C., Whelton H.L., Ryan K., Evershed R.P. Molecular and isotopic evidence for milk, meat, and plants in prehistoric eastern African herder food systems. PNAS. 2020:9793–9799. doi: 10.1073/pnas.1920309117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A.K., Anderson D.M., Overpeck J.T. Abrupt changes in the Asian southwest monsoon during the Holocene and their links to the North Atlantic Ocean. Nature. 2003;421(6921):354–357. doi: 10.1038/nature01340. [DOI] [PubMed] [Google Scholar]
- Halmemies-Beauchet-Filleau A., Vanhatalo A., Toivonen V., Heikkilä T., Lee M., Shingfield K. Effect of replacing grass silage with red clover silage on nutrient digestion, nitrogen metabolism, and milk fat composition in lactating cows fed diets containing a 60:40 forage-to-concentrate ratio. J. Dairy Sci. 2014;97(6):3761–3776. doi: 10.3168/jds.2013-7358. [DOI] [PubMed] [Google Scholar]
- Hendy J., Colonese A.C., Franz I., Fernandes R., Fischer R., Orton D., Lucquin A. Ancient proteins from ceramic vessels at Çatalhöyük West reveal the hidden cuisine of early farmers. Nat. Commun. 2018;9(1):4064. doi: 10.1038/s41467-018-06335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron C., Evershed R.P., Goad L.J. Effects of migration of soil lipids on organic residues associated with buried potsherds. J. Archaeol. Sci. 1991;18(6):641–659. doi: 10.1016/0305-4403(91)90027-M. [DOI] [Google Scholar]
- Joglekar P.P., Sharada C.V., Abhayan G.S. Faunal diversity during the Harappan period in Haryana’. Heritage: Journal of Multidisciplinary Studies in Archaeology. 2013;1:262–287. [Google Scholar]
- Joglekar P.P., Sharada C.V., Shinde V.S. Animal remains from the Mature Harappan contexts at Farmana, Rohtak District, Haryana, India. Puratattva. 2018;47:101–114. [Google Scholar]
- Joglekar P.P., Singh R.N., Petrie C.A. A preliminary report of animal remains from Bhimwada Jodha (Masudpur VII), Haryana. Bharati. 2016;39:1–9. [Google Scholar]
- Joglekar P.P., Singh R.N., Petrie C.A. Faunal remains from Sampolia Khera (Masudpur I), Haryana. Indian Journal of Archaeology. 2017;2(1):25–60. [Google Scholar]
- Joshi V.K. CRC Press, Taylor and Francis Group; Boca Raton: 2016. Indigenous Fermented Foods of South Asia. [Google Scholar]
- Kanginakudru S., Metta M., Jakati R.D., Nagaraju J. Genetic evidence from Indian red jungle fowl corroborates multiple domestication of modern-day chicken. BMC Evol. Biol. 2008;8 doi: 10.1186/1471-2148-1188-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap A., Weber S.A. Harappan plant use revealed by starch grains from Farmana, India. Antiquity Project Gallery. 2010;84(326) http://www.antiquity.ac.uk/projgall/kashyap326/Kashyap [Google Scholar]
- Kenoyer J.M. Urban process in the Indus tradition: a preliminary model from Harappa. In: Meadow R.H., editor. Harappa Excavations 1986-1990. Prehistory Press; Madison: 1991. pp. 71–80. [Google Scholar]
- Kenoyer J.M. Early City-States in South Asia: Comparing the Harappan Phase and the Early Historic Period. In: Nichols D.L., Charlton T.H., editors. The Archaeology of City-States, Cross-Cultural Approaches. Smithsonian Institute Press; Washington, D.C.: 1997. pp. 51–70. [Google Scholar]
- Kenoyer J.M. Ancient Cities of the Indus Valley Civilization. Oxford University Press; American Institute of Pakistan Studies; Karachi; Islamabad: 1998. [Google Scholar]
- Kenoyer J.M. Indus urbanism: new perspectives in its origin and character. In: Marcus J., Sabloff J.J.A., editors. The Ancient City: New Perspectives in the Old and New World. 2008. pp. 85–109. Santa Fe, New Mexico: SAR. [Google Scholar]
- Kolattukudy P. Plant waxes. Lipids. 1970;5(2):9–275. doi: 10.1007/BF02532477. [DOI] [Google Scholar]
- Krishnan K. Indus ceramic industries: complexities, challenges and prospects. Indian J. Hist. Sci. 2018;53(3) [Google Scholar]
- Lancelotti C. ‘Not all that burns is wood’. A social perspective on fuel exploitation and use during the Indus urban period (2600-1900 BC) PloS One. 2018;13(3):192364. doi: 10.1371/journal.pone.0192364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law R.W. Occasional Paper: Linguistics, Archaeology and the Human Past. Vol. 11. Research Institute for Humanity and Nature; Kyoto, Japan: 2011. Inter-regional interaction and urbanism in the ancient Indus valley: a geologic provenience study of Harappa’s rock and mineral assemblage. [Google Scholar]
- Lightfoot E., Jones P.J., Joglekar P.P., Tames-Demauras M., Smith E., Muschinski J., Shinde V., Singh R.N., Jones M.K., O’Connell T.C., Petrie C.A. Feeding the herds: stable isotope analysis of animal diet and its implication for understanding social organisation in the Indus Civilisation, Northwest India. Archaeological Research in Asia. 2020;24:100212. doi: 10.1016/j.ara.2020.100212. [DOI] [Google Scholar]
- Liu Y.P., Wu G.S., Yao Y.G., Miao Y.W., Luikart G., Baig M., Beja-Pereira A., Ding Z.L., Palanichamy M.G., Zhang Y.P. Multiple maternal origins of chickens: out of the Asian jungles. Mol. Phylogenet. Evol. 2006;38(1):12–19. doi: 10.1016/j.ympev.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Lucquin A., Gibbs K., Uchiyama J., Saul H., Ajimoto M., Eley Y., Radini A. Ancient lipids document continuity in the use of early hunter–gatherer pottery through 9,000 years of Japanese prehistory. Proc. Natl. Acad. Sci. 2016;113(15):3991–3996. doi: 10.1073/pnas.1522908113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay E.J.H. F. U. Brockhaus; Leipzig: 1938. Die Induskultur. [Google Scholar]
- Madella M. Of crops and food: a social perspective on rice in the Indus civilization. In: Madella M., Lancelotti C., Savard M., editors. Ancient Plants and People: Contemporary Trends in Archaeology. Univeristy of Arizona Press; Tucson: 2014. 218–36. [Google Scholar]
- Madella M., Fuller D.Q. Palaeoecology and the Harappan civilisation of South Asia: a reconsideration. Quat. Sci. Rev. 2006;25(11):1283–1301. doi: 10.1016/j.quascirev.2005.10.012. [DOI] [Google Scholar]
- March R.J., Lucquin A., Joly D., Ferreri J.C., Muhieddine M. Processes of formation and alteration of archaeological fire structures: complexity viewed in the light of experimental approaches. J. Archaeol. Method Theor. 2014;21:1–45. doi: 10.1007/s10816-012-9134-7. [DOI] [Google Scholar]
- Meadow R.H. Early Animal Domestication in South Asia: A First Report of the Faunal Remains from Mehrgarh, Pakistan. In: Hartel H., editor. South Asian Archaeology 1979. Dietrich Reimer Verlag; Berlin: 1981. [Google Scholar]
- Meadow R.H. Continuity and Change in the Agriculture of the Greater Indus Valley: The Palaeoethnobotanical and Zooarchaeological Evidence. In: Kenoyer J.M., editor. Old Problems and New Perspectives in the Archaeology of South Asia. University of Wisconsin; Madison: 1989. pp. 61–74. [Google Scholar]
- Meadow R.H. Animal Domestication in the Middle East: A Revised View from the Eastern Margin. In: Possehl G.L., editor. Harappan Civilization: A Recent Perspective, Oxford and IBH Publishing Co. Pvt. Ltd.; New Delhi: 1993. pp. 295–322. [Google Scholar]
- Meadow R.H. The Origins and Spread of Agriculture and Pastoralism in Northwestern South Asia. In: Harris D., editor. The Origins and Spread of Agriculture and Pastoralism in Eurasia. UCL Press; London: 1996. pp. 390–412. [Google Scholar]
- Miao Y.-W., Peng M.-S., Wu G.-S., Ouyang Y.-N., Yang Z.-Y., Yu N., Liang J.-P. Chicken domestication: an updated perspective based on mitochondrial genomes. Heredity. 2013;110(3):277–282. doi: 10.1038/hdy.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L.J. Secondary products and urbanism in South Asia: the evidence for traction at Harappa. In: Weber S., Belcher W.R., editors. Indus Ethnobiology. Lexington Books; Oxford: 2003. pp. 251–326. [Google Scholar]
- Miller L.J. New York University; New York: 2004. Urban Economies in Early States: the Secondary Products Revolution in the Indus Civilization. Unpublished PhD Thesis. [Google Scholar]
- Mukherjee A.J., Gibson A.M., Evershed R.P. Trends in pig product processing at British Neolithic Grooved Ware sites traced through organic residues in potsherds. J. Archaeol. Sci. 2008;35(7):2059–2073. doi: 10.1016/j.jas.2008.01.010. [DOI] [Google Scholar]
- Murphy C.A., Fuller D.Q. The transition to agricultural production in India: South Asian entanglements of domestication. In: Schug G.R., Walimbe S.R., editors. A Companion to South Asia in the Past. vols. 344–57. John Wiley & Sons, Inc; Hoboken, NJ: 2016. [Google Scholar]
- Nandy A. The changing popular culture of Indian food: preliminary notes. S. Asia Res. 2004;24(1):9–19. doi: 10.1177/0262728004042760. [DOI] [Google Scholar]
- Nath A. Chrono-cultural clue of the rise of the harappans in the Satluj-Yamuna plain. Puratattva. 2018;48:93–129. [Google Scholar]
- Neogi S., French C.A.I., Durcan J., Singh R.N., Petrie C.A. Geoarchaeological insights into the location of Indus settlements on the plains of northwest India. Quat. Res. 2019;(94):137–155. doi: 10.1017/qua.2019.70. [DOI] [Google Scholar]
- Outram A.K., Stear N.A., Bendrey R., Olsen S., Kasparov A., Zaibert V., Thorpe N., Evershed R.P. The earliest horse harnessing and milking. Science. 2009;323(5919):1332–1335. doi: 10.1126/science.1168594. [DOI] [PubMed] [Google Scholar]
- Parikh D., Petrie C.A. ‘We are inheritors of a rural civilisation’: rural complexity and the ceramic economy in the Indus civilisation in northwest India. World Archaeology. 2019:1–21. doi: 10.1080/00438243.2019.1601463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie . South Asia. In: Clark P., editor. Oxford Handbook of Cities in World History. Oxford University Press; Oxford: 2013. pp. 83–104. [Google Scholar]
- Petrie C.A., Bates J. ‘Multi-cropping’, intercropping and adaptation to variable environments in Indus South Asia. J. World PreHistory. 2017;30(2):81–130. doi: 10.1007/s10963-017-9101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie C.A., Singh R.N., Singh A.K. Investigating changing settlement dynamics on the plains: the 2009 survey and excavations at Masudpur (Hissar District, Haryana) Puratattva. 2009;39:38–49. [Google Scholar]
- Petrie C.A., Bates J., Higham T., Singh R.N. Feeding ancient cities in South Asia: dating the adoption of rice, millet and tropical pulses in the Indus civilisation. Antiquity. 2016;90(354):1489–1504. doi: 10.15184/aqy.2016.210. [DOI] [Google Scholar]
- Petrie C.A., Singh R.N., Bates J., Dixit Y., French C.A.I., Hodell D.A., Jones P.J. Adaptation to Variable Environments, Resilience to Climate Change: Investigating Land, Water and Settlement in Indus northwest India. Curr. Anthropol. 2017;58(1):1–30. doi: 10.1086/690112. [DOI] [Google Scholar]
- Ponton C., Giosan L., Eglinton T.I., Fuller D.Q., Johnson J.E., Kumar P., Collett T.S. Holocene aridification of India. Geophys. Res. Lett. 2012;39(3) doi: 10.1029/2011GL050722. [DOI] [Google Scholar]
- Possehl G.L. The end of a state and the continuity of a tradition. In: Fox R., editor. Realm and Region in Traditional India. Carolina Academic Press; Durham: 1977. pp. 235–256. [Google Scholar]
- Possehl G.L. The transformation of the Indus Civilization. J. World Prehistory. 1997;11(4):425–472. doi: 10.1007/BF02220556. [DOI] [Google Scholar]
- Possehl G.L. AltaMira Press; Walnut Creek, Lanham, New York, Oxford: 2002. The Indus Civilization: A Contemporary Perspective. [Google Scholar]
- Prasad B. Animal remains from Harappa. Memoirs of the Archaeological Survey of India. 1936;51:1–62. [Google Scholar]
- Raikes R. The end of the ancient cities of the Indus. Am. Anthropol. 1964;66(2):284–299. doi: 10.1525/aa.1964.66.2.02a00040. [DOI] [Google Scholar]
- Raikes R. Kalibangan: Death from Natural Causes. Antiquity. 1968;42(168):286–291. doi: 10.1017/S0003598X00034505. [DOI] [Google Scholar]
- Raikes R. The Mohenjo-daro floods: the debate continues. In: Taddei M., editor. South Asian Archaeology 1977. Instituto Univeristario Oriental, Seminario de Studi Asiatici; Naples: 1979. 561–66. Series Minor 6. [Google Scholar]
- Reber E.A., Kerr M.T., Whelton H.L., Evershed R.P. Lipid residues from low-fired pottery. Archaeometry. 2019;61(1):131–144. doi: 10.1111/arcm.12403. [DOI] [Google Scholar]
- Regert M. Analytical strategies for discriminating archeological fatty substances from animal origin. Mass Spectrom. Rev. 2011;30(2):177–220. doi: 10.1002/mas.20271. [DOI] [PubMed] [Google Scholar]
- Regert M., Bland H.A., Dudd S.N., van Bergen P.F., Evershed R.P. Free and Bound Fatty Acid Oxidation Products in Archaeological Ceramic Vessels. Proceedings: Biological Sciences. 1998;265(1409):2027–2032. doi: 10.1098/rspb.1998.0536. [DOI] [Google Scholar]
- Roffet-Salque M., Dunne J., Altoft D.T., Casanova E., Cramp L.J.E., Smyth J., Whelton H., Evershed R.P. From the inside out: upscaling organic residue analyses of archaeological ceramics. J. Archaeol. Sci.: Report. 2017;16:627–640. doi: 10.1016/j.jasrep.2016.04.005. [DOI] [Google Scholar]
- Salque M., Bogucki P.I., Pyzel J., Sobkowiak-Tabaka I., Grygiel R., Szmyt M., Evershed R.P. Earliest evidence for cheese making in the sixth millennium BC in northern Europe. Nature. 2013;493(7433):522–525. doi: 10.1038/nature11698. [DOI] [PubMed] [Google Scholar]
- Sarkar S., Prasad S., Wilkes H., Riedel N., Stebich M., Basavaiah N., Sachse D. Monsoon source shifts during the drying Mid-holocene: Biomarker isotope based evidence from the core “Monsoon Zone” (CMZ) of India. Quat. Sci. Rev. 2015;123(September):144–157. [Google Scholar]
- Sarkar A., Deshpande-Mukherjee A., Bera M.K., Das B., Juyal N., Morthekai P., Deshpande R.D., Shinde V.S., Rao L.S. Oxygen isotope in archaeological bioapatites from India: implications to climate change and decline of Bronze Age Harappan civilization. Sci. Rep. 2016;6(1):26555. doi: 10.1038/srep26555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell R.B.S., Guha B.S. Zoological remains. In: Marshall J.H., editor. Mohenjo-Daro and the Indus Civilization. vols. 649–72. Arthur Probstain; London: 1931. [Google Scholar]
- Shinde V.S. Site and its environs. In: Shinde V.S., Osada T., Kumar M., editors. Excavations at Farmana: District Rohtak, Haryana, India, 2006-2008. Research Institute for Humanity and Nature; Kyoto, Japan: 2011. pp. 5–19. [Google Scholar]
- Singh R.N., Petrie C.A., Joglekar P.P., Neogi S., Lancelotti C., Pandey A.K., Pathak A. Recent excavations at Alamgirpur, Meerut district: a preliminary report. Man and Environment. 2013;XXXVIII(1):32–54. [Google Scholar]
- Spangenberg J.E., Jacomet S., Schibler J. Chemical analyses of organic residues in archaeological pottery from Arbon Bleiche 3, Switzerland – evidence for dairying in the late Neolithic. J. Archaeol. Sci. 2006;33(1):1–13. doi: 10.1016/j.jas.2005.05.013. [DOI] [Google Scholar]
- Spiteri C.D., Gillis R.E., Roffet-Salque M., Navarro L.C., Guilane J., Manen C., Muntoni I.M., Segui M.S., Urem-Kotsou D., Whelton H., Craig O.E., Vigne J-D, Evershed R.P. Regional asynchronicity in dairy production and processing in early farming communities of the northern Mediterranean. PNAS. 2016;113(48):13594–13599. doi: 10.1073/pnas.1607810113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staubwasser M., Sirocko F., Grootes P.M., Segl M. Climate change at the 4.2 ka BP termination of the Indus valley civilization and holocene South Asian monsoon variability. Geophys. Res. Lett. 2003;30(8) doi: 10.1029/2002GL016822. [DOI] [Google Scholar]
- Steele V.J., Stern B., Stott A.W. Olive oil or lard?: distinguishing plant oils from animal fats in the archeological record of the eastern mediterranean using gas chromatography/combustion/isotope ratio mass spectrometry. Rapid Commun. Mass Spectrom. 2010;24(23):3478–3484. doi: 10.1002/rcm.4790. [DOI] [PubMed] [Google Scholar]
- Storey A.A., Athens J.S., Bryant D., Carson M., Emery K., deFrance S S. Investigating the global dispersal of chickens in prehistory using ancient mitochondrial DNA signatures. PloS One. 2012;7(7):39171. doi: 10.1371/journal.pone.0039171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P.K. Investigations into the archaeofauna of Harappan sites in western India. In: Korisettar Ravi, Settar Shadakshari., editors. vol. 2. ICHR and Manohar; New Delhi: 2002. pp. 409–420. (Indian Archaeology in Retrospect: Protohistory). [Google Scholar]
- Thomas P.K., Joglekar P.P. Holocene faunal studies. Man and Environment. 1994;XIX:179–204. [Google Scholar]
- Tiwari M., Nagoji S.S., Ganeshram R.S. Multi-centennial scale SST and Indian summer monsoon precipitation variability since the mid-Holocene and its nonlinear response to solar activity. Holocene. 2015;25(9):1415–1424. doi: 10.1177/0959683615585840. [DOI] [Google Scholar]
- Uesugi A. Pottery from the Settlement Area. In: Shinde V.S., Osada T., Kumar M., editors. Excavations at Farmana, Rohtak District, Haryana, India 2006-2008. Research Institute for Humanity and Nature; Kyoto: 2011. pp. 168–368. [Google Scholar]
- Uesugi A. Pottery from the Cemetery. In: Shinde V.S., Osada T., Kumar M., editors. Excavations at Farmana, Rohtak District, Haryana, India 2006-2008. Research Institute for Humanity and Nature; Kyoto: 2011. pp. 674–800. [Google Scholar]
- Uesugi A. A note on the diachronic changes of Harappan pottery - a preliminary analysis. Heritage: Journal of Multidisciplinary Studies in Archaeology. 2013;1:356–371. [Google Scholar]
- Uesugi A. South Asian Archaeology and Art, Volume 1: Man and Environment in Prehistoric and Protohistoric South Asia - New Perspectives. Brepol; Turnhout: 2017. Ceramic sequence in the Ghaggar plains from pre-Indus to post-Indus urban periods. [Google Scholar]
- Vahia M.N., Kumar P., Bhogale A., Kothari D.C., Chopra S., Shinde V.S., Jadhav N., Shastri R. Radiocarbon dating of charcoal samples from Rakhigarhi using AMS. Curr. Sci. 2016;111(1):27–28. [Google Scholar]
- Vidale M. Aspects of palace life at Mohenjo-daro. S. Asian Stud. 2010;26(1):59–76. doi: 10.1080/02666031003737232. [DOI] [Google Scholar]
- Vishnu-Mittre, Savithri R. Food economy of the Harappans. In: Possehl G.L., editor. Harappan Civilisation. Oxford and IBH Publishing Co. Pvt. Ltd; New Delhi: 1982. pp. 205–221. [Google Scholar]
- Wang C., Eley Y., Oakes A., Hren M. Hydrogen isotope and molecular alteration of n-alkanes during heating in open and closed systems. Org. Geochem. 2017;112:47–58. doi: 10.1016/j.orggeochem.2017.07.006. [DOI] [Google Scholar]
- Weber S. Seeds of urbanism: palaeoethnobotany and the Indus Civilization. Antiquity. 1999;73(282):813–826. doi: 10.1017/S0003598X00065558. [DOI] [Google Scholar]
- Weber S. Archaeobotany at Harappa: indications for change. In: Weber S., Belcher W.R., editors. Indus Ethnobiology. Lexington Books; Oxford: 2003. pp. 175–198. [Google Scholar]
- Weber S.A., Kashyap A. The vanishing millets of the Indus civilization. Archaeol Anthropol Sci. 2016;8:9–15. doi: 10.1007/s12520-013-0143-6. [DOI] [Google Scholar]
- Weber S., Barela T., Lehman H. Ecological continuity: an explanation for agricultural diversity in the Indus Civilisation and beyond. Man and Environment. 2010;35(1):62–75. [Google Scholar]
- Weber S., Kashyap A., Mounce L. Archaeobotany at Farmana. In: Shinde V.S., Osada T., Kumar M., editors. Excavations at Farmana, Rohtak District, Haryana, India 2006-2008. Research Institute for Humanity and Nature; Kyoto: 2011. 808–25. [Google Scholar]
- Whelton H.L., Roffet-Salque M., Kotsakis K., Urem-Kotsou D., Evershed R.P. Strong bias towards carcass product processing at Neolithic settlements in northern Greece revealed through absorbed lipid residues of archaeological pottery. Quat. Int. 2018;496(December):127–139. doi: 10.1016/j.quaint.2017.12.018. [DOI] [Google Scholar]
- Wright R.P. Women’s labor and pottery production in prehistory. In: Gero J., Conkey M., editors. Engendering Archaeology: Women and Prehistory. Basil Blackwell; Oxford: 1991. pp. 194–223. [Google Scholar]
- Wright R.P. Cambridge University Press; Cambridge: 2010. The Ancient Indus: Urbanism, Economy and Society. [Google Scholar]
- Zeder M.A. Archaeological approaches to documenting animal domestication. In: Zeder M.A., Bradley D.G., Emshwiller E., Smith B.D., editors. Documenting Domestication: New Genetic and Archaeological Paradigms. University of California Press; Berkeley: 2006. pp. 181–206. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.