Abstract
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2, has posed a serious threat to global public health security. With the increase in the number of confirmed cases globally, the World Health Organization has declared the outbreak of COVID-19 an international public health emergency. Despite atypical pneumonia as the primary symptom, liver dysfunction has also been observed in many clinical cases and is associated with the mortality risk in patients with COVID-19, like severe acute respiratory syndrome and Middle East respiratory syndrome. Here we will provide a schematic overview of the clinical characteristics and the possible mechanisms of liver injury caused by severe acute respiratory syndrome coronavirus 2 infection, which may provide help for optimizing the management of liver injury and reducing mortality in COVID-19 patients.
Keywords: COVID-19, Novel coronavirus, SARS-CoV-2, Liver injury
Core Tip: With the number of confirmed cases increasing worldwide, abnormal liver function has been observed in many patients with coronavirus disease 2019 (COVID-19). COVID-19-associated liver injury refers to any hepatic damage that occurs during disease progression and treatment in COVID-19 patients with or without underlying liver diseases. Underlying mechanisms may be viral infection in liver cells, systemic inflammation induced by cytokine storm, drug induced liver injury or pneumonia-associated hypoxia. A close monitor of liver function is recommended in COVID-19 patients, especially in critical individuals.
INTRODUCTION
Since the 21st century, the outbreak of coronaviruses has brought great harm to human society; the most serious of which are the severe acute respiratory syndrome (SARS) in 2003, the Middle East respiratory syndrome (MERS) in 2012 and the novel coronavirus disease in 2019 (COVID-19). The ongoing outbreak of COVID-19 has become a pandemic. As of December 9, 2020, the total number of diagnosed cases globally exceeded 67530912 with a total of more than 1545140 infection-related deaths, carrying a mortality of approximately 2%[1]. Up to now, no specific antiviral therapies have been identified. Thus, an early monitor of critical complications is vital in preventing disease progression and improving survival.
With the number of confirmed cases increasing worldwide, abnormal liver function has been observed in many patients with COVID-19, making this organ one of the most frequently damaged outside of the respiratory system (summarized in Table 1). COVID-19-associated liver injury refers to any hepatic damage that occurs during disease progression and treatment in COVID-19 patients with or without underlying liver diseases[2]. However, due to the different design and sample size, the incidence and clinical manifestations of liver injury in these studies are not the same. The mechanism of hepatic damage caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is still unclear.
Table 1.
Main characteristics related to liver injury in patients with coronavirus disease 2019 based on a series of case reports
|
Ref.
|
Sample size
|
Liver injury
|
Elevated ALT
|
Elevated AST
|
Elevated TBIL
|
Elevated ALP
|
Elevated GGT
|
Factors related to liver injury
|
|
| [25] | 1099 | NA | 21.3%: Severe 28.1%, Non-severe 19.8% | 22.2%: Severe 39.4%, Non-severe 18.2% | 10.5%: Severe 13.3%, Non-severe 9.9% | NA | NA | NA | |
| [26] | 548 | NA | 23.1%: Severe 24.1%, Non-severe 22.3% | 33.1%: Severe 43.4%, Non-severe 23.3% | 4.4%: Severe 6.4%, Non-severe 2.3% | NA | NA | NA | |
| [27] | 417 | 21.5% | 41.2%: Severe 82.4%, Non-severe 50.2% | 47.2%: Severe 75.3%, Non-severe 36.9% | 64.2%: Severe 75.3%, Non-severe 60.1% | 10.9%: Severe 12.2%, Non-severe 10.5% | 48.5%: Severe 75.3%, Non-severe 39.1% | Older, male, higher BMI, Underlying liver diseases (NAFLD, alcoholic liver disease and chronic hepatitis B), drugs (lopinavir/ritonavir) | |
| [28] | 324 | NA | 15.7% | 10.5% | 6.5% | 1.2% | 0.9% | NA | |
| [29] | 298 | 14.8% | NA | NA | NA | NA | NA | NA | |
| [30] | 274 | NA | 22.0%: Deceased 27.0%, Recovered 19.0% | 31.0%: Deceased 52.0%, Recovered 16.0% | NA | NA | NA | NA | |
| [31] | 148 | 37.2% | 18.2% | 21.6% | 6.1% | 4.1% | 17.6% | Male, higher levels of procalcitonin and CRP. PCT, LDH, received lopinavir / ritonavir | |
| [32] | 85 | 38.8% | 61.2% | NA | NA | NA | NA | Older, lactic acid, myoglobin, neutrophils, critical illness, aCRP, alymphocyte count | |
| [33] | 79 | 36.7% | 31.6% | 35.4% | 5.1% | NA | NA | Male, white blood cell counts, neutrophils, CRP, athe extent of pulmonary alesions on CT | |
| [34] | 40 | 55% | 52.5% | 40% | 25% | NA | NA | Many types of drugs, large amounts of hormones, underlying diseases, lymphocyte count, acritical illness | |
| [35] | 82 | 78% | 30.6% | 61.1% | 30.6% | NA | NA | NA | |
Represents independent risk factors for liver injury in coronavirus disease 2019. ALP: Alkaline phosphatase; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; BMI: Body mass index; COVID-19: Coronavirus disease 2019; CRP: C-reactive protein; CT: Computed tomography; GGT: Gamma-glutamyl transpeptidase; LDH: Lactate dehydrogenase; NA: Not available; NAFLD: Non-alcoholic fatty liver diseases; PCT: Procalcitonin; TBIL: Total bilirubin.
SARS-CoV, Middle East respiratory syndrome coronavirus (MERS-CoV) and SARS-CoV-2 are the causative agents of SARS, MERS and COVID-19, respectively, and they all belong to the highly pathogenic human beta coronaviruses[3]. Genomics analyses have found that SARS-CoV-2 shares 79.5% genome sequence similarity to SARS-CoV and 50% genome sequence homology to MERS-CoV[4]. SARS-CoV-2 uses the same cell entry receptor-angiotensin converting enzyme II (ACE2)-as SARS-CoV[5]. These common points hint that SARS-CoV-2 may partly mimic SARS-CoV and MERS-CoV infection.
In this review, we summarized the characteristics and mechanism of liver injury caused by SARS-CoV-2 infection to provide a reference for further study.
Liver injury in SARS and MERS
Liver injury is not uncommon in patients infected with SARS-CoV and MERS-CoV according to previous studies[6]. In patients with SARS, liver injury mainly manifests as elevated alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) in the early phase of the disease and is associated with the severity of the illness[7-13]. The pathogenesis of hepatic damage caused by SARS-CoV appears to be multifactorial, including a direct injury to the target cells by the virus and an indirect injury mediated by subsequent immune system dysfunction. As the functional receptor for SARS-CoV, ACE2 is abundantly expressed on endothelial cells of the liver, implying that SARS-CoV may directly bind to ACE2 positive cells to dysregulate liver function[5,13]. Liver biopsies in SARS patients demonstrated localization of virus in liver and hepatocyte apoptosis, which further confirmed the direct injury by SARS-CoV[14]. In an analysis of 145 cases of SARS, serum interleukin (IL)-1β, IL-6 and IL-10 were higher in patients with elevated serum levels of ALT than those in ALT normal group, indicating that liver damage was part of the manifestation of system inflammation reactive syndrome induced by SARS-CoV infection[10]. In addition, some studies have also found that hypoxemia and medication are closely related to abnormal liver function[15,16].
Elevated liver enzymes and bilirubin levels, as well as decreased albumin levels were highlighted during the hospital course of MERS-CoV infection in a series of case reports[17-20]. Different from SARS-CoV, MERS-CoV uses dipeptide base peptidase 4 as a cellular receptor to infect cells[21]. In humans, dipeptide base peptidase 4 is expressed constitutively on epithelial cells of liver[22], suggesting a direct hepatic damage caused by MERS-CoV. MERS also involves a mechanism of the upregulation of proinflammatory cytokines, such as interferon-γ, tumor necrosis factor-α, IL-15 and IL-17[23]. However, studies on the relationship between cytokine storm and liver injury are scarce so far.
Clinical characteristics of liver injury in patients with COVID-19
Since Chen et al[24] reported that 43 cases of 99 patients (43.4%) in Wuhan Jinyintan Hospital had different degrees of abnormal liver function, the abnormality of liver function test in patients with COVID-19 has aroused widespread concern among clinicians. As shown in Table 1, the incidence of liver injury ranged from 14.8% to 55.0% in recent case studies reporting clinical features of patients with COVID-19[25-34]. In death cases of COVID-19, the rate reached as high as 78.0%[35]. Liver injury presented in 30 out of 113 deceased patients from our previous report[30]. Abnormal liver function mainly manifests as slightly elevated ALT/AST and bilirubin levels, which usually occurs around the second week of the disease course[33-35]. Rarely, severe acute hepatitis associated with COVID-19 has been reported[36].
Contradictory to other hepatitis-induced liver injury, AST-dominant aminotrans-ferase elevation is common in COVID-19, which may provide a clue about the underlying pathophysiology of the impact of COVID-19 on liver. A retrospective study including 60 patients revealed that median AST was higher than ALT at admission (46 U/L vs 30 U/L) and during the hospital course[37]. In a multicenter retrospective cohort-derived data set of 5771 individuals, the elevation of serum AST level was earlier, more frequent and significant than the increase of ALT in severe patients, and AST levels had the highest correlation with mortality when compared with other indicators reflecting liver injury including elevated ALT, alkaline phosphatase (ALP) and total bilirubin (TBIL) levels in patients with COVID-19[38]. Likewise, an elevated baseline AST level has been shown to correlate with intensive care unit (ICU) admission, intubation and death in another study[39].
To our knowledge, three possible reasons may account for this phenomenon. First, given that AST is also distributed in myocardium and skeletal muscle, the American Association for the Study of Liver Diseases has recommended consideration of myositis or cardiac injury as contributors to the AST elevation[40]. Second, recent data identified ribosomal proteins as important host-dependency factors for SARS-CoV-2[41]. Therefore, the virus may directly cause hepatic mitochondrial injury and subsequent AST elevation. Third, AST-predominant aminotransferase elevations have been reported in alcohol-related liver disease, ischemia and cirrhosis. It is possible that hypoxia as well as metabolic changes such as hepatic steatosis may account for AST elevation in COVID-19 patients[42,43].
It is worth noting that liver dysfunction is closely related to the severity of the disease. On the one hand, severe patients have a higher proportion of liver injury: Guan et al[25] extracted a cohort regarding 1099 patients with laboratory-confirmed COVID-19 from 552 hospitals in mainland China. The results showed more patients with severe disease had elevated AST and ALT than those with non-severe disease. Like the result, Wang et al[44] showed that more patients admitted to the ICU had elevated AST levels. Huang et al[45] showed that patients admitted to the ICU had significantly higher ALT levels. On the other hand, patients with abnormal liver tests had higher risks of progressing to a severe disease course: Bloom et al[37] showed that admission AST, peak AST and peak ALT were higher in intubated patients. Of 417 patients with COVID-19, patients with abnormal liver tests of hepatocellular, cholestatic or mixed type at admission had higher odds of progressing to severe pneumonia[27]. Among 148 confirmed SARS-CoV-2-infected patients, the emerging abnormal liver functions after admission caused a prolonged length of stay[31]. In a large United States COVID-19 cohort of 3381 patients, 2273 patients who tested positive for SARS-CoV-2 had higher initial and peak ALT than those who tested negative[46]. Compared with mild [upper limit of normal (ULN) < ALT < two times ULN] and moderate (two times ULN < ALT < five times ULN) liver injury, patients with severe liver injury (ALT > five times ULN) had a more severe clinical course, including higher rates of ICU admission (69%), intubation (65%), renal replacement therapy (33%) and mortality (42%).
Other hepatic manifestations in COVID-19 patients were hypoproteinemia and changes in coagulation[47,48]. A large cohort study including 2623 patients reported marked hypoalbuminemia in the critically ill and death groups than non-critically ill patients (38.2%, 71.2% and 82.4% on admission and 45.9%, 77.7% and 95.6% during hospitalization, respectively)[49]. Meanwhile, the patients in this study displayed dramatically prolonged activated partial thromboplastin time in critically ill patients reflected coagulopathy.
Further analysis shows that risk factors associated with hepatic damage include older males, a longer time from illness onset to admission, a history of drinking, higher serum levels of C-reactive protein (CRP), white blood cell counts, neutrophils and medication (lopinavir/ritonavir, hormones)[27,31-34,50]. Disease severity (severe/critical), CRP, lymphocyte count and the extent of pulmonary lesions on computed tomography are independent risk factors for liver injury[32-34].
Accumulating data reveals that patients with pre-existing liver diseases are more susceptible to SARS-CoV-2 infection and have poorer prognosis. In our study, viral hepatitis (hepatitis B and hepatitis C) was much more frequent among patients with liver injury than those without[51]. Another study revealed that COVID-19 patients with non-alcoholic fatty liver disease had a significantly higher likelihood of abnormal liver function from admission to discharge when compared to those with non-alcoholic fatty liver disease subjects[52]. Qiu et al[53] first reported a case of acute-on-chronic liver failure due to SARS-COV-2 infection in a patient with decompensated alcoholic cirrhosis. In a multicenter retrospective study, fifty cirrhotic patients with SARS-CoV-2 infection were studied to evaluate the impact of COVID-19 on the clinical outcome[54]. The results showed 30-d mortality rate was higher in cirrhotic patients with COVID-19 than in cirrhotic patients with bacterial infection and in COVID-19 patients without cirrhosis, indicating that COVID-19 was associated with liver function deterioration and elevated mortality in cirrhotic patients. In addition, a study of 2780 COVID-19-positive patients found that those with cirrhosis were at a particularly increased risk for mortality by analyzing a large United States database (risk ratio, 4.6; 95% confidence interval, 2.6-8.3)[55].
Whether the COVID-19 patients with more severe liver injury were positive for hepatotrophis viruses is still controversial. In a retrospective study, the authors analyzed liver function parameters including ALT, AST and TBIL in COVID-19 patients with or without HBV infection and found no significant differences between the two groups[56]. Another study reached a similar conclusion and further proved the longitudinal changes of median values for liver biochemistries were not significantly different between the two groups either[57]. These findings indicated that SARS-CoV-2 will not exacerbate liver injury in patients with HBV co-infection. However, Lin et al[58] drew a completely opposite conclusion. In their cohort, COVID-19 cases with HBV coinfection had higher levels of ALT, AST, TBIL and ALP than the COVID-19 cases without HBV coinfection, showing that inactive HBV carriers with SARS-CoV-2 coinfection are at risk of greater liver injury. Moreover, SARS-CoV-2 was reported to induce HBV reactivation, which may cause severe liver injury in patients with coinfection[57,59]. In addition, a case of COVID-19 with Epstein-Barr virus coinfection was reported recently. On admission, he showed acute liver injury with liver enzymes that were much higher than typically seen solely with COVID-19 infection[60]. Although the evidence is limited, more attention should be paid to COVID-19 patients with other viral coinfections during clinical treatment.
Mechanisms of liver injury in patients with COVID-19
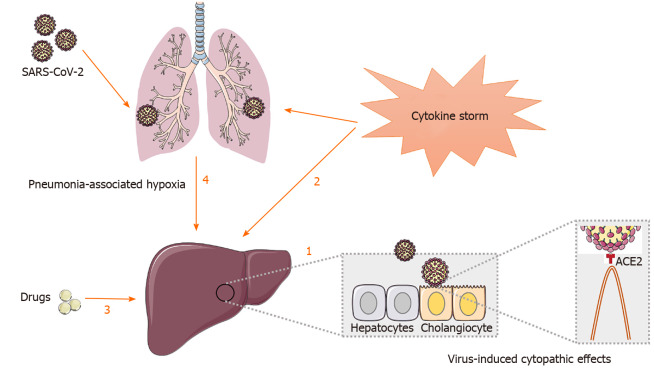
Underlying mechanisms involved in liver injury in patients with COVID-19 are complex and interactive, which might include viral infection in liver cells, systemic inflammation induced by cytokine storm, drug induced liver injury or pneumonia-associated hypoxia[61] (Figure 1).
Figure 1.
Potential mechanisms of liver injury in patients with coronavirus disease 2019. 1: Severe acute respiratory syndrome coronavirus-2 may directly bind to angiotensin converting enzyme II positive cholangiocytes to dysregulate liver function; 2: Inflammatory cytokine storm leads to persistent activation of lymphocytes and macrophages that secrete huge amount of inflammatory cytokine, thus contributing to lung as well as liver damage; 3: Drugs including antipyretics, antiviral medications (lopinavir/ritonavir), antibiotics (macrolides, quinolones) and steroids may have potential hepatotoxicity and lead to abnormal liver function; 4: Hepatic ischemia and hypoxia-reperfusion dysfunction induced by complications such as respiratory failure may cause liver damage, especially in critically ill patients.
Virus-induced cytopathic effects
It is well known that SARS-CoV-2 predominantly enters alveolar epithelial cells through the human ACE2 receptor, thus making the lung the main target organ of SARS-CoV-2 infection[62]. However, previous studies have found that ACE2 receptor is also specifically expressed in bile duct epithelial cells but is rarely expressed in hepatocytes[63,64] and absent of Kupffer cells and hepatic stellate cells[65]. A further study employing single-cell ribonucleic acid-seq suggested that TROP2+ cholangiocytes could be a main target for SARS-CoV-2 infection, leading to impaired liver regeneration and liver function[66]. In a mouse model of acute liver injury, ACE2 was upregulated in liver tissue due to compensatory proliferation of hepatocytes derived from bile duct epithelial cells[64]. During this compensatory process, some newborn hepatocytes still expressed ACE2 receptor and were susceptible to SARS-CoV-2. Recently, Wang et al[67] investigated the patterns of liver impairment by electron microscopy and pathological studies in two COVID-19 cases. In this study, typical coronavirus particles were identified in the cytoplasm of hepatocytes. Histologically, massive hepatic apoptosis and binuclear hepatocytes were observed. Our previous clinical report showed that the bile duct injury related ALP and gamma-glutamyl transpeptidase was elevated in deceased patients[30]. These findings suggest that the liver injury in COVID-19 patients may be due to hepatocyte damage as well as cholangiocyte dysfunction.
Other studies reported conflicting results. For example, Qian et al[28] showed that ALP, gamma-glutamyl transpeptidase and TBIL elevations were rare among 324 cases with SARS-CoV-2 pneumonia. Zhang et al[47] reported that after SARS-CoV-2 infection, the overall ALP level is even lower than that with community-acquired pneumonia patients, implying that the duct epithelium injury by SARS-CoV-2 itself is very slight. Thus, SARS-COV-2 infection may not be the major reason related to liver injury. Given the conflicting results above, the role of virus-induced cytopathic effects in COVID-19-related liver injury warrants further investigation.
Inflammatory cytokine storm
Cell entry of SARS-CoV-2 depends on binding of the viral spike (S) proteins to cellular ACE2 receptor and on spike protein priming by host cell proteases[68]. While the virus enters the cells via fusion with the host membrane, its antigen will be recognized by the antigen presentation cells and then presented to cytotoxic and regulatory T lymphocytes, which initiate an antiviral immune response that includes inflammatory cytokine production and a weak interferon response[69]. In young individuals with an intact immune system, the virus is cleared away during the initial phase, so they show only mild symptoms[45]. However, in the elderly and individuals with underlying chronic diseases, the insufficient viral clearance due to altered immune response will lead to a cytokine storm, which may trigger a violent attack to the body and cause multiple organ failure including the liver[45,70]. Inflammatory cytokine storm is an overactive inflammatory response caused by virus infection, which leads to persistent activation of lymphocytes and macrophages that secrete huge amounts of inflammatory cytokines[71]. For example, SARS-CoV-2 can rapidly activate pathogenic Th1 cells to secrete proinflammatory cytokines, such as granulocyte-macrophage colony-stimulating factor and IL-6[72]. Granulocyte-macrophage colony-stimulating factor further activates CD14+CD16+ inflammatory monocytes to produce large quantities of IL-6, tumor necrosis factor-α and other cytokines. Among these cytokines, IL-6 can bind to sIL-6R to activate STAT3 in nonimmune cells and can bind to membrane-bound IL-6 receptor to lead to pleiotropic effects on acquired and innate immune cells[73]. Meanwhile, sIL-2R may regulate cytotoxic T cells negatively and contribute to lymphopenia through IL-2 signaling inhibition[74].
Accumulating evidence revealed a broad spectrum of proinflammatory cytokines and chemokines dramatically increased in patients with liver dysfunction compared to those with normal liver function[10,32]. Consistent with these results, our data showed the levels of inflammatory markers including high sensitivity CRP, neutrophil-to-lymphocyte ratio, white blood cells, neutrophils, serum ferritin, lactate dehydrogenase, procalcitonin, erythrocyte sedimentation rate and proinflammatory cytokines including IL-2R, IL-6, tumor necrosis factor-α in the liver injury group were significantly higher compared with the group without liver injury[51]. In an analysis of 85 patients with COVID-19, lymphopenia and CRP may even serve as the risk factors related to hepatic injury[32]. Moreover, the postmortem liver biopsy in one patient confirmed that liver injury in COVID-19 is likely immune mediated[43]. These findings indicated that immune-mediated inflammatory response following SARS-CoV-2 infection may cause or contribute to liver damage. In the future, more research is needed to understand the concrete mechanisms involved in cytokine accumulation in COVID-19 and subsequent liver injury.
Drug-induced liver injury
Fever was one of the most common symptoms on admission and during hospitalization in patients with COVID-19[25]. Therefore, antipyretic therapy is very ordinary in infected patients. Acetaminophen, a common ingredient in antipyretic drugs, is proven to cause significant liver damage or induce liver failure according to a dose-dependent mechanism[75]. Recently, a 27-year-old healthy African American female with a positive SARS-CoV-2 test and acute liver failure secondary to acetaminophen overdose was reported[76]. She had a remote history of focal segmental glomerular sclerosis. To manage her pain, she ingested > 50 tablets of acetaminophen over the 3-4 d preceding presentation. Initial blood work revealed the acetaminophen level was 42 µg/mL (upper limit of normal 30 µg/mL) and elevated aminotransferases with alanine transaminase of 2791 U/L and aspartate transaminase of 3202 U/L. Two days after admission, her hepatic synthetic function worsened significantly, and aminotransferases peaked to an AST 9741 U/L and ALT 11322 U/L. Thus, although paracetamol is a safe and effective first line agent in almost all patients regardless of liver disease etiology[77], the clinicians cannot be too careful in the dose.
Although there is currently no specific therapy for COVID-19, many patients especially severe and critical patients, were often treated with multiple drugs, including antiviral medications (lopinavir/ritonavir), antibiotics (macrolides, quinolones) and steroids in clinical practice[78]. These drugs may have potential hepatotoxicity and lead to abnormal liver function. Recent data on liver tests in patients with COVID-19 showed that the use of lopinavir/ritonavir led to increased odds of liver injury[27]. Fan et al[31] reported that among 148 COVID-19 patients, patients receiving treatment with lopinavir/ritonavir were more likely to develop abnormal liver function tests. In another clinical report, liver function injury was more likely to occur in patients who used many types of drugs and large amounts of hormones[34]. Our study also revealed a higher proportion of patients with liver injury had received systemic glucocorticoids (unpublished). The liver biopsy specimens of the patient with COVID-19 showed moderate microvascular steatosis and mild lobular and portal activity, further conforming the possibility of drug-induced hepatic damage[43]. But on the contrary, in a randomized, controlled, open-label trial, Cao et al[79] reported that lopinavir/ritonavir treatment did not significantly increase liver enzymes in patients with serious COVID-19. Due to limited data, we cannot draw a definitive conclusion about whether lopinavir/ritonavir or glucocorticoids increase the risk of developing liver damage.
Pneumonia-associated hypoxia
Hypoxic hepatitis, also known as ischemic hepatitis or shock liver, is commonly seen in patients with hypotension shock or severe hypoxemia caused by severe heart failure, respiratory failure, surgery, trauma and other causes[80]. Its clinical feature is a massive, rapid rise in serum transaminase (which can exceed 20 × ULN) and is often accompanied by an increase in lactate dehydrogenase. In patients with COVID-19, hypoxia and shock caused by respiratory distress syndrome, system inflammation reactive syndrome, multiple organ dysfunction and other complications can lead to hepatic ischemia and hypoxia-reperfusion dysfunction. Experimental data revealed that hepatocyte death and inflammatory cytokines production caused by hypoxia can be seen in both in vivo and in vitro models of hepatic ischemia and hypoxia[81]. Furthermore, liver histological findings on autopsy of patients with COVID-19 revealed the watery degeneration of some hepatocytes, proving the possibility of hepatic ischemia and hypoxia[27]. However, according to the available evidence, the distribution of aminotransferase levels among patients with COVID-19 do not support pneumonia-associated hypoxia being a common cause of liver injury[82]. Whether hypoxia is related to abnormal liver function in COVID-19 patients remains to be further investigated.
Management of COVID-19 patients with liver disease
It was reported that about 2%-11% of patients with COVID-19 had underlying chronic liver disease[83]. Chinese Society of Hepatology, Chinese Medical Association, American Association for the Study of Liver Diseases, Asian Pacific Association for the Study of the Liver and European Association for the Study of the Liver have all issued relevant guidelines to help clinicians manage chronic liver disease and liver transplant patients during the epidemic of COVID-19. Because there is no complete and systematic data at present, most of the recommendations for management of COVID-19 patients with liver disease are based on expert consensus. Among these guidelines, the basic principles are infection control, delay of medical treatment, risk classification and supportive management[84-87]. More details are summarized in Table 2.
Table 2.
Management of coronavirus disease 2019 patients with liver disease
|
|
Management of COVID-19 patients with liver disease |
| Out-patient care | Use telemedicine or visits by phone wherever possible. Consider seeing in person only patients with urgent issues and clinically significant liver disease (e.g., jaundice, elevated ALT or AST > 500 U/L, or recent onset of hepatic decompensation)[40,84,86]. Seeing at the fever clinic[40] |
| Hospital treatment | Separate management from non-COVID-19 patients[40,85]. Monitor liver biochemistries regularly, particularly in patients treated with remdesivir or tocilizumab[40]. Avoid ultrasound or other advanced imaging unless it is likely to change management, for example, clinical suspicion for biliary obstruction or venous thrombosis[40]. Hospitalize COVID-19 patients with advanced liver disease as soon as possible[85] |
| Patients with hepatitis B, hepatitis C | Document discussion with patient regarding CLD diagnosis and management[84]. Delay starting DAA therapy until after their recovery from COVID-19 disease if there is no suspicion of advanced liver disease[87]. Continue treatment and provide 90-d supplies for HBV oral antiviral drugs or a full course of DAA medications to complete HCV treatment[87] |
| Patients with autoimmune liver disease | Continue immunosuppressive therapy in stable patients with AIH[87]. Lower the doses of azathioprine or mycophenolate mofetil when patients develop lymphopenia[87]. Avoid liver biopsy and start empiric therapy in new patients presenting with features of AIH[87]. Avoid high doses of prednisone in AIH patients on corticosteroids[87] |
| Patients with HCC | Continue HCC surveillance schedule for high-risk subjects[40]. Document discussion of risks and benefits of delaying surveillance with patient[40]. Proceed with HCC treatments as appropriate[40]. Postpone elective transplant and resection surgery, withhold immunotherapy[84] |
| Pretransplant and post-transplant patients | Have low threshold for admitting patients on transplant waiting list diagnosed with COVID-19[40,84]. Consider reduction of immunosuppression therapy as appropriate for posttransplant patients with moderate COVID-19[40,84]. Avoid reductions in immunosuppressive therapy in patients with mild COVID-19 disease[40,84] |
AIH: Autoimmune hepatitis; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; CLD: Chronic liver disease; COVID-19: Coronavirus disease 2019; DAA: Direct acting antiviral; HBV: Hepatitis B virus; HCC: Hepatocellular carcinoma; HCV: Hepatitis C virus.
CONCLUSION
Liver injury is a common complication in COVID-19 patients and may result from virus-induced cytopathic effects, immune mediated inflammation, drug toxicity and pneumonia-associated hypoxia. Like SARS and MERS, abnormal liver function mainly manifested as transient elevation of serum aminotransferases. Moreover, patients with abnormal liver tests had higher risks of progressing to severe disease. From a clinical perspective, in addition to actively dealing with the primary disease caused by SARS-CoV-2 infection, a close monitor of liver function is recommended in COVID-19 patients, especially in severe/critical individuals.
Footnotes
Conflict-of-interest statement: There is no conflict of interest.
Manuscript source: Unsolicited manuscript
Peer-review started: September 19, 2020
First decision: November 26, 2020
Article in press: December 23, 2020
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Iorio R, Kharbanda KK S-Editor: Zhang L L-Editor: Filipodia P-Editor: Li X
Contributor Information
Mei-Wen Han, Institute of Infectious Disease, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China.
Ming Wang, Institute of Infectious Disease, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China.
Meng-Ying Xu, Institute of Infectious Disease, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China.
Wei-Peng Qi, Institute of Infectious Disease, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China.
Peng Wang, Institute of Infectious Disease, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China.
Dong Xi, Institute of Infectious Disease, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China. xidong@tjh.tjmu.edu.cn.
References
- 1.Johns Hopkins Coronavirus Resource Center. Cited 9 December 2020. Available from: https://coronavirus.jhu.edu/
- 2.Sun J, Aghemo A, Forner A, Valenti L. COVID-19 and liver disease. Liver Int. 2020;40:1278–1281. doi: 10.1111/liv.14470. [DOI] [PubMed] [Google Scholar]
- 3.Su S, Wong G, Shi W, Liu J, Lai ACK, Zhou J, Liu W, Bi Y, Gao GF. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang HL, Chen KT, Lai SK, Kuo HW, Su IJ, Lin RS, Sung FC. Hematological and biochemical factors predicting SARS fatality in Taiwan. J Formos Med Assoc. 2006;105:439–450. doi: 10.1016/S0929-6646(09)60183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tie ZJ, Zhang TC, Zhang M, Cui ZM, Tian G. Prevention and treatment of liver injury in SARS patients-222 cases analysis. Beijing Yixue Zazhi. 2004;26:318–320. [Google Scholar]
- 9.Zhao LF, Xing HC, Xu LP. [Effect of SARS-associated coronavirus on peripheral blood picture and liver function] Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2004;16:660–663. [PubMed] [Google Scholar]
- 10.Duan ZP, Chen Y, Zhang J, Zhao J, Lang ZW, Meng FK, Bao XL. [Clinical characteristics and mechanism of liver injury in patients with severe acute respiratory syndrome] Zhonghua Gan Zang Bing Za Zhi. 2003;11:493–496. [PubMed] [Google Scholar]
- 11.Wu KL, Lu SN, Changchien CS, Chiu KW, Kuo CH, Chuah SK, Liu JW, Lin MC, Eng HL, Chen SS, Lee CM, Chen CL. Sequential changes of serum aminotransferase levels in patients with severe acute respiratory syndrome. Am J Trop Med Hyg. 2004;71:125–128. [PubMed] [Google Scholar]
- 12.Duan XF, Liu Z, Hao R, Luo L, Zhang YN. [The dynamic change of liver injury in patients with severe acute respiratory syndrome] Zhonghua Gan Zang Bing Za Zhi. 2004;12:439. [PubMed] [Google Scholar]
- 13.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chau TN, Lee KC, Yao H, Tsang TY, Chow TC, Yeung YC, Choi KW, Tso YK, Lau T, Lai ST, Lai CL. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39:302–310. doi: 10.1002/hep.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu SQ, Zhang TC, Zhang M. The relationship between hypoxemia and SARS complicated liver injury and the efficacy of reduced glutathione in treatment of the disease. Zhongguo Xinyao Zazhi . 2004;013:1046–1048. [Google Scholar]
- 16.Liu Z, Guo JZ. Dynamic changes of liver function and myocardial enzyme in 259 patients with severe acute respiratory syndrome. Shiyong Ganzangbing Zazhi . 2003;3:129–131. [Google Scholar]
- 17.Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, Al-Rabiah FA, Al-Hajjar S, Al-Barrak A, Flemban H, Al-Nassir WN, Balkhy HH, Al-Hakeem RF, Makhdoom HQ, Zumla AI, Memish ZA. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Tawfiq JA, Hinedi K, Ghandour J, Khairalla H, Musleh S, Ujayli A, Memish ZA. Middle East respiratory syndrome coronavirus: a case-control study of hospitalized patients. Clin Infect Dis. 2014;59:160–165. doi: 10.1093/cid/ciu226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Assiri A, McGeer A, Perl TM, Price CS, Al Rabeeah AA, Cummings DA, Alabdullatif ZN, Assad M, Almulhim A, Makhdoom H, Madani H, Alhakeem R, Al-Tawfiq JA, Cotten M, Watson SJ, Kellam P, Zumla AI, Memish ZA KSA MERS-CoV Investigation Team. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saad M, Omrani AS, Baig K, Bahloul A, Elzein F, Matin MA, Selim MA, Al Mutairi M, Al Nakhli D, Al Aidaroos AY, Al Sherbeeni N, Al-Khashan HI, Memish ZA, Albarrak AM. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301–306. doi: 10.1016/j.ijid.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang N, Shi X, Jiang L, Zhang S, Wang D, Tong P, Guo D, Fu L, Cui Y, Liu X, Arledge KC, Chen YH, Zhang L, Wang X. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res. 2013;23:986–993. doi: 10.1038/cr.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boonacker E, Van Noorden CJ. The multifunctional or moonlighting protein CD26/DPPIV. Eur J Cell Biol. 2003;82:53–73. doi: 10.1078/0171-9335-00302. [DOI] [PubMed] [Google Scholar]
- 23.Mahallawi WH, Khabour OF, Zhang Q, Makhdoum HM, Suliman BA. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8–13. doi: 10.1016/j.cyto.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, Shi J, Zhou M, Wu B, Yang Z, Zhang C, Yue J, Zhang Z, Renz H, Liu X, Xie J, Xie M, Zhao J. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qian ZP, Mei X, Zhang YY, Zou Y, Zhang ZG, Zhu H, Guo HY, Liu Y, Ling Y, Zhang XY, Wang JF, Lu HZ. [Analysis of baseline liver biochemical parameters in 324 cases with novel coronavirus pneumonia in Shanghai area] Zhonghua Gan Zang Bing Za Zhi. 2020;28:229–233. doi: 10.3760/cma.j.cn501113-20200229-00076. [DOI] [PubMed] [Google Scholar]
- 29.Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, Su Y, Ma Z, Zhang Y, Li Z, He Q, Liu L, Fu Y, Chen J. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742–1752. doi: 10.1111/all.14309. [DOI] [PubMed] [Google Scholar]
- 30.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L, Li S, Xu M, Yu P, Zheng S, Duan Z, Liu J, Chen Y, Li J. Risk factors related to hepatic injury in patients with corona virus disease 2019. medRxiv. 2020 [Google Scholar]
- 33.Xie H, Zhao J, Lian N, Lin S, Xie Q, Zhuo H. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: A retrospective study. Liver Int. 2020;40:1321–1326. doi: 10.1111/liv.14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yao N, Wang SN, Lian JQ, Sun YT, Zhang GF, Kang WZ, Kang W. [Clinical characteristics and influencing factors of patients with novel coronavirus pneumonia combined with liver injury in Shaanxi region] Zhonghua Gan Zang Bing Za Zhi. 2020;28:234–239. doi: 10.3760/cma.j.cn501113-20200226-00070. [DOI] [PubMed] [Google Scholar]
- 35.Zhang B, Zhou X, Qiu Y, Song Y, Feng F, Feng J, Song Q, Jia Q, Wang J. Clinical characteristics of 82 cases of death from COVID-19. PLoS One. 2020;15:e0235458. doi: 10.1371/journal.pone.0235458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wander P, Epstein M, Bernstein D. COVID-19 Presenting as Acute Hepatitis. Am J Gastroenterol. 2020;115:941–942. doi: 10.14309/ajg.0000000000000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bloom PP, Meyerowitz EA, Reinus Z, Daidone M, Gustafson J, Kim AY, Schaefer E, Chung RT. Liver Biochemistries in Hospitalized Patients With COVID-19. Hepatology. 2020 doi: 10.1002/hep.31326. [DOI] [PubMed] [Google Scholar]
- 38.Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, Zhang XJ, Cai J, Lin L, Ouyang S, Wang X, Yang C, Cheng X, Liu W, Li H, Xie J, Wu B, Luo H, Xiao F, Chen J, Tao L, Cheng G, She ZG, Zhou J, Wang H, Lin J, Luo P, Fu S, Zhou J, Ye P, Xiao B, Mao W, Liu L, Yan Y, Liu L, Chen G, Li H, Huang X, Zhang BH, Yuan Y. Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology. 2020;72:389–398. doi: 10.1002/hep.31301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferm S, Fisher C, Pakala T, Tong M, Shah D, Schwarzbaum D, Cooley V, Hussain S, Kim SH. Analysis of Gastrointestinal and Hepatic Manifestations of SARS-CoV-2 Infection in 892 Patients in Queens, NY. Clin Gastroenterol Hepatol 2020; 18: 2378-2379. :e1. doi: 10.1016/j.cgh.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fix OK, Hameed B, Fontana RJ, Kwok RM, McGuire BM, Mulligan DC, Pratt DS, Russo MW, Schilsky ML, Verna EC, Loomba R, Cohen DE, Bezerra JA, Reddy KR, Chung RT. Clinical Best Practice Advice for Hepatology and Liver Transplant Providers During the COVID-19 Pandemic: AASLD Expert Panel Consensus Statement. Hepatology. 2020;72:287–304. doi: 10.1002/hep.31281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, O'Meara MJ, Guo JZ, Swaney DL, Tummino TA, Hüttenhain R, Kaake RM, Richards AL, Tutuncuoglu B, Foussard H, Batra J, Haas K, Modak M, Kim M, Haas P, Polacco BJ, Braberg H, Fabius JM, Eckhardt M, Soucheray M, Bennett MJ, Cakir M, McGregor MJ, Li Q, Naing ZZC, Zhou Y, Peng S, Kirby IT, Melnyk JE, Chorba JS, Lou K, Dai SA, Shen W, Shi Y, Zhang Z, Barrio-Hernandez I, Memon D, Hernandez-Armenta C, Mathy CJP, Perica T, Pilla KB, Ganesan SJ, Saltzberg DJ, Ramachandran R, Liu X, Rosenthal SB, Calviello L, Venkataramanan S, Lin Y, Wankowicz SA, Bohn M, Trenker R, Young JM, Cavero D, Hiatt J, Roth T, Rathore U, Subramanian A, Noack J, Hubert M, Roesch F, Vallet T, Meyer B, White KM, Miorin L, Agard D, Emerman M, Ruggero D, García-Sastre A, Jura N, von Zastrow M, Taunton J, Schwartz O, Vignuzzi M, d'Enfert C, Mukherjee S, Jacobson M, Malik HS, Fujimori DG, Ideker T, Craik CS, Floor S, Fraser JS, Gross J, Sali A, Kortemme T, Beltrao P, Shokat K, Shoichet BK, Krogan NJ. A SARS-CoV-2-Human Protein-Protein Interaction Map Reveals Drug Targets and Potential Drug-Repurposing. bioRxiv. 2020 [Google Scholar]
- 42.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, Fox AN, Zucker J, Verna EC. Acute Liver Injury in COVID-19: Prevalence and Association with Clinical Outcomes in a Large U.S. Cohort. Hepatology. 2020;72:807–817. doi: 10.1002/hep.31404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40:2095–2103. doi: 10.1111/liv.14455. [DOI] [PubMed] [Google Scholar]
- 48.Li LY, Wu W, Chen S, Gu JW, Li XL, Song HJ, Du F, Wang G, Zhong CQ, Wang XY, Chen Y, Shah R, Yang HM, Cai Q. Digestive system involvement of novel coronavirus infection: Prevention and control infection from a gastroenterology perspective. J Dig Dis. 2020;21:199–204. doi: 10.1111/1751-2980.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang W, Li C, Wang Z, Wang H, Zhou N, Jiang J, Ni L, Zhang XA, Wang DW. Decreased serum albumin level indicates poor prognosis of COVID-19 patients: hepatic injury analysis from 2,623 hospitalized cases. Sci China Life Sci. 2020;63:1678–1687. doi: 10.1007/s11427-020-1733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qi X, Liu C, Jiang Z, Gu Y, Zhang G, Shao C, Yue H, Chen Z, Ma B, Liu D, Zhang L, Wang J, Xu D, Lei J, Li X, Huang H, Wang Y, Liu H, Yang J, Pan H, Liu W, Wang W, Li F, Zou S, Zhang H, Dong J. Multicenter analysis of clinical characteristics and outcomes in patients with COVID-19 who develop liver injury. J Hepatol. 2020;73:455–458. doi: 10.1016/j.jhep.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang M, Yan W, Qi W, Wu D, Zhu L, Li W, Wang X, Ma K, Ni M, Xu D, Wang H, Chen G, Yu H, Ding H, Xing M, Han M, Luo X, Chen T, Guo W, Xi D, Ning Q. Clinical characteristics and risk factors of liver injury in COVID-19: a retrospective cohort study from Wuhan, China. Hepatol Int. 2020;14:723–732. doi: 10.1007/s12072-020-10075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Implication of non-alcoholic fatty liver diseases (NAFLD) in patients with COVID-19: a preliminary analysis. J Hepatol. 2020;73:451–453. doi: 10.1016/j.jhep.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qiu H, Wander P, Bernstein D, Satapathy SK. Acute on chronic liver failure from novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Liver Int. 2020;40:1590–1593. doi: 10.1111/liv.14506. [DOI] [PubMed] [Google Scholar]
- 54.Iavarone M, D'Ambrosio R, Soria A, Triolo M, Pugliese N, Del Poggio P, Perricone G, Massironi S, Spinetti A, Buscarini E, Viganò M, Carriero C, Fagiuoli S, Aghemo A, Belli LS, Lucà M, Pedaci M, Rimondi A, Rumi MG, Invernizzi P, Bonfanti P, Lampertico P. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020;73:1063–1071. doi: 10.1016/j.jhep.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh S, Khan A. Clinical Characteristics and Outcomes of Coronavirus Disease 2019 Among Patients With Preexisting Liver Disease in the United States: A Multicenter Research Network Study. Gastroenterology 2020; 159: 768-771. :e3. doi: 10.1053/j.gastro.2020.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen L, Huang S, Yang J, Cheng X, Shang Z, Lu H, Cheng J. Clinical characteristics in patients with SARS-CoV-2/HBV co-infection. J Viral Hepat. 2020;27:1504–1507. doi: 10.1111/jvh.13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu J, Wang T, Cai Q, Sun L, Huang D, Zhou G, He Q, Wang FS, Liu L, Chen J. Longitudinal changes of liver function and hepatitis B reactivation in COVID-19 patients with pre-existing chronic hepatitis B virus infection. Hepatol Res. 2020;50:1211–1221. doi: 10.1111/hepr.13553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin Y, Yuan J, Long Q, Hu J, Deng H, Zhao Z, Chen J, Lu M, Huang A. Patients with SARS-CoV-2 and HBV co-infection are at risk of greater liver injury. Genes Dis. 2020 doi: 10.1016/j.gendis.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aldhaleei WA, Alnuaimi A, Bhagavathula AS. COVID-19 Induced Hepatitis B Virus Reactivation: A Novel Case From the United Arab Emirates. Cureus. 2020;12:e8645. doi: 10.7759/cureus.8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh T, Alameri A, Rampy J, Brady III C, Guerrero J. S2658 Two for One: A Case of COVID-19 and Epstein-Barr Virus-Induced Acute Liver Injury. AJG. 2020 [Google Scholar]
- 61.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, Li T, Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, Zhou J, Shi G, Fang N, Fan J. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoVinfection. bioRxiv. 2020 [Google Scholar]
- 64.Guan GW, Gao L, Wang JW, Wen XJ, Mao TH, Peng SW, Zhang T, Chen XM, Lu FM. [Exploring the mechanism of liver enzyme abnormalities in patients with novel coronavirus-infected pneumonia] Zhonghua Gan Zang Bing Za Zhi. 2020;28:100–106. doi: 10.3760/cma.j.issn.1007-3418.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 65.Nardo AD, Schneeweiss-Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2020 doi: 10.1111/liv.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wen Seow JJ, Pai R, Mishra A, Shepherdson E, Hon Lim TK, Goh BKP, Chan JKY, Chow PKH, Ginhoux F, DasGupta R, Sharma A. scRNA-seq reveals ACE2 and TMPRSS2 expression in TROP2+ Liver Progenitor Cells: Implications in COVID-19 associated Liver Dysfunction. bioRxiv . 2020 doi: 10.3389/fmed.2021.603374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807–816. doi: 10.1016/j.jhep.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020; 181: 271-280. :e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sahin TT, Akbulut S, Yilmaz S. COVID-19 pandemic: Its impact on liver disease and liver transplantation. World J Gastroenterol. 2020;26:2987–2999. doi: 10.3748/wjg.v26.i22.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10:102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi Y, Sun R, Tian Z, Xu X, Wei H. Aberrant pathogenic GM-CSF+ T cells and inflammatory CD14+CD16+ monocytes in severe pulmonary syndrome patients of a new coronavirus. BioRXiv. 2020 [Google Scholar]
- 73.Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol. 2020 doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Y, Wang X, Li X, Xi D, Mao R, Wu X, Cheng S, Sun X, Yi C, Ling Z, Ma L, Ning Q, Fang Y, Sun B, Wu D. Potential contribution of increased soluble IL-2R to lymphopenia in COVID-19 patients. Cell Mol Immunol. 2020;17:878–880. doi: 10.1038/s41423-020-0484-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu PF, Wu Q, Duan ZP, Chen Y. Research advances in the mechanism of drug-induced liver injury due to paracetamol. J Clin Hepatol . 2019;9:2108–2111. [Google Scholar]
- 76.Rouphael C, D'Amico G, Ricci K, Cywinski J, Miranda C, Koval C, Duggal A, Quintini C, Menon KVN, Miller C, Modaresi Esfeh J. Successful orthotopic liver transplantation in a patient with a positive SARS-CoV2 test and acute liver failure secondary to acetaminophen overdose. Am J Transplant. 2020 doi: 10.1111/ajt.16330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hayward KL, Powell EE, Irvine KM, Martin JH. Can paracetamol (acetaminophen) be administered to patients with liver impairment? Br J Clin Pharmacol. 2016;81:210–222. doi: 10.1111/bcp.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Musa S. Hepatic and gastrointestinal involvement in coronavirus disease 2019 (COVID-19): What do we know till now? Arab J Gastroenterol. 2020;21:3–8. doi: 10.1016/j.ajg.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Waseem N, Chen PH. Hypoxic Hepatitis: A Review and Clinical Update. J Clin Transl Hepatol. 2016;4:263–268. doi: 10.14218/JCTH.2016.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang L, Wang W, Wang X, Zhao J, Xiao L, Gui W, Fan H, Xia J, Li Z, Yan J, Alasbahi A, Zhu Q, Hou X. Creg in Hepatocytes Ameliorates Liver Ischemia/Reperfusion Injury in a TAK1-Dependent Manner in Mice. Hepatology. 2019;69:294–313. doi: 10.1002/hep.30203. [DOI] [PubMed] [Google Scholar]
- 82.Bangash MN, Patel J, Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5:529–530. doi: 10.1016/S2468-1253(20)30084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID-19 and the liver. J Hepatol. 2020;73:1231–1240. doi: 10.1016/j.jhep.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.APASL Covid-19 Task Force, Lau G, Sharma M. Clinical practice guidance for hepatology and liver transplant providers during the COVID-19 pandemic: APASL expert panel consensus recommendations. Hepatol Int. 2020;14:415–428. doi: 10.1007/s12072-020-10054-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Boettler T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Cornberg M, Berg T. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2:100113. doi: 10.1016/j.jhepr.2020.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wong GL, Wong VW, Thompson A, Jia J, Hou J, Lesmana CRA, Susilo A, Tanaka Y, Chan WK, Gane E, Ong-Go AK, Lim SG, Ahn SH, Yu ML, Piratvisuth T, Chan HL Asia-Pacific Working Group for Liver Derangement during the COVID-19 Pandemic. Management of patients with liver derangement during the COVID-19 pandemic: an Asia-Pacific position statement. Lancet Gastroenterol Hepatol. 2020;5:776–787. doi: 10.1016/S2468-1253(20)30190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hamid S, Alvares da Silva MR, Burak KW, Chen T, Drenth JPH, Esmat G, Gaspar R, LaBrecque D, Lee A, Macedo G, McMahon B, Ning Q, Reau N, Sonderup M, van Leeuwen DJ, Armstrong D, Yurdaydin C. WGO Guidance for the Care of Patients With COVID-19 and Liver Disease. J Clin Gastroenterol. 2021;55:1–11. doi: 10.1097/MCG.0000000000001459. [DOI] [PMC free article] [PubMed] [Google Scholar]