Abstract
BACKGROUND
Pyogenic ventriculitis caused by extensively drug-resistant Acinetobacter baumannii (A. baumannii) is one of the most severe complications associated with craniotomy. However, limited therapeutic options exist for the treatment of A. baumannii ventriculitis due to the poor penetration rate of most antibiotics through the blood-brain barrier.
CASE SUMMARY
A 68-year-old male patient with severe traumatic brain injury developed pyogenic ventriculitis on postoperative day 24 caused by extensively drug-resistant A. baumannii susceptible to tigecycline only. Successful treatment was accomplished through multi-route administration of tigecycline, including intravenous combined with continuous ventricular irrigation plus intraventricular administration. The pus was cleared on the 3rd day post-irrigation, and cerebrospinal fluid cultures were negative after 12 d.
CONCLUSION
Our findings suggest that multi-route administration of tigecycline can be a therapeutic option against pyogenic ventriculitis caused by extensively drug-resistant A. baumannii.
Keywords: Pyogenic ventriculitis, Acinetobacter baumannii, Extensively drug-resistant, Tigecycline, Ventricular irrigation, Case report
Core Tip: Pyogenic Ventriculitis caused by extensively drug-resistant (XDR) Acinetobacter baumannii (A. baumannii) is one of the most severe complications associated with craniotomy. We present herein a rare case of pyogenic ventriculitis caused by XDR A. baumannii. Successful treatment was accomplished through multi-route administration of tigecycline, including intravenous combined with continuous ventricular irrigation plus intraventricular administration. This case suggests that multi-route administration of tigecycline can be a therapeutic option against pyogenic ventriculitis caused by XDR A. baumannii.
INTRODUCTION
Ventricular infection caused by Acinetobacter baumannii (A. baumannii) accounts for 3.6% to 11.2% of all cases of hospital-acquired ventriculitis[1]. In recent years, strains of extensively drug-resistant (XDR) A. baumannii have emerged due to the overuse of antibiotics[2]. Meningitis and ventriculitis caused by XDR strains are associated with a dismal prognosis, with mortality rates of up to 71%[3-5].
Tigecycline, a new type of glycylcycline, is one of the few newly developed antimicrobial agents that are active against Gram-negative bacteria[2]. In vitro experiments[6-8] have shown that tigecycline is effective against A. baumannii, with strong antibacterial effects on XDR A. baumannii. Previous case reports and case series have provided information on the efficacy and safety of tigecycline in the treatment of ventriculitis or meningitis[5,9-11]. In these cases, tigecycline was commonly administrated via intravenous (IV) with or without intraventricular (IVT) route. Additionally, intraoperative intraventricular lavage for pyogenic ventriculitis has also been reported[12]. However, the efficacy and safety profile of multi-route administration of tigecycline, including IV plus continuous ventricular irrigation (CVI)/IVT, remain relatively uncertain.
Here, we present the first case of successful treatment of pyogenic ventriculitis caused by XDR A. baumannii with multi-route tigecycline.
CASE PRESENTATION
Chief complaints
A 68-year-old male patient was admitted to our hospital due to a severe car accident and was diagnosed as having a severe traumatic brain injury.
History of present illness
The patient was admitted to our hospital due to a severe car accident 3 h ago. The clinical neurological examination revealed a deep coma. The diagnosis was a severe traumatic brain injury.
History of past illness
The patient did not have a specific history of past illness.
Personal and family history
The patient did not have a specific personal and family history.
Physical examination
When admitted to the hospital, the patient’s temperature was 37.2 °C, heart rate was 98 bpm, respiratory rate was 18 breaths per minute, blood pressure was 138/78 mmHg, and oxygen saturation in room air was 98%. The clinical neurological examination revealed a deep coma, with a Glasgow coma scale score of 3/15.
Laboratory examinations
On hospital day 18, laboratory tests showed 105 mg/L of C-reactive protein (CRP), and the white blood cell count (WBC) was 10.37 × 109/L. Additionally, his renal and liver function tests were normal. The cerebral spinal fluid (CSF) obtained through lumbar puncture showed increased WBC, while the glucose level was 0.8 mmol/L, and the total protein was 4.49 g/L. Three sets of sputum, blood, and CSF cultures were obtained, but no bacterial growth was detected in any of the three samples. On hospital day 21, analysis of the sputum and CSF cultures again demonstrated the absence of any bacterial growth. On hospital day 26, the patient's CSF culture tested positive for A. baumannii that was susceptible only to tigecycline, though susceptibility to polymyxin was not tested (Table 1). The same strain of XDR A. baumannii was persistently isolated from the CSF until hospital day 35. The results of blood and CSF analysis are shown in Table 2.
Table 1.
Antimicrobial susceptibility testing for Acinetobacter baumannii in cerebrospinal fluid
|
Antibiotic
|
Susceptibility
|
MIC (mg/L)
|
| Amikacin | R | > 32 |
| Ceftriaxone | I | > 32 |
| Cefotaxime | R | > 32 |
| Ceftazidime | R | > 16 |
| Cefepime | R | > 16 |
| Tetracycline | R | > 8 |
| Levofloxacin | R | > 4 |
| Selectrin | R | > 2 |
| Piperacillin | R | > 64 |
| Tigecycline | S | |
| Gentamicin | R | > 8 |
| Ciprofloxacin | R | > 2 |
| Tobramycin | R | > 8 |
| Meropenem | R | > 8 |
| Ticarcillin | R | > 64 |
R: Resistance; I: Intermediary; S: Sensitivity; MIC: Minimum inhibitory concentration.
Table 2.
Clinical course and cerebrospinal fluid examination
|
Hospital stay
|
Highest body temperature (°C)
|
Blood WBC (× 109/L)
|
Blood CRP (mg/L)
|
CSF glucose (mmol/L)
|
CSF protein (g/L)
|
CSF WBC (× 106/L)
|
Culture of CSF
|
| Day 18 | 39.1 | 10.37 | 104 | 0.8 | 4.49 | 8800 | N |
| Day 24 | 38.5 | 9.85 | 71 | 0.8 | 5.75 | 14000 | MDRAB |
| Day 27 | 37.6 | 6.79 | 65 | 1.8 | 2.58 | 1200 | MDRAB |
| Day 31 | 37.9 | 7.02 | 43 | 2.9 | 2.57 | 590 | MDRAB |
| Day 32 | 37.2 | 7.71 | 2.6 | 2.25 | 160 | MDRAB | |
| Day 33 | 37.1 | 8.08 | 3.2 | 2.22 | 240 | MDRAB | |
| Day 36 | 37.4 | 8.32 | 14 | 3.4 | 3.88 | 40 | N |
| Day 41 | 37.0 | 8.21 | 3.5 | 2.64 | 68 | N | |
| Day 42 | 37.2 | 6.51 | 3 | 2.13 | 90 | N | |
| Day 43 | 37.1 | 5.76 | 6 | 3.6 | 2.32 | 64 | N |
MDRAB: Multi-drug resistant Acinetobacter baumannii; N: Negative; CSF: Cerebrospinal fluid; WBC: White blood cell; CRP: C reactive protein.
Imaging examinations
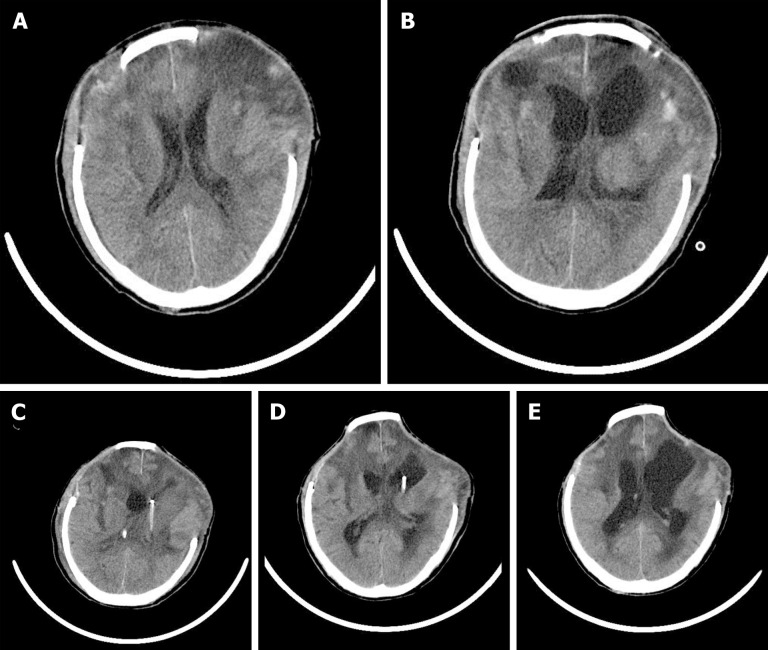
On hospital day 18, head computed tomography (CT) showed normal postoperative changes after the operations (Figure 1A). On hospital day 24, head CT indicated ventricular empyema (Figure 1B). On hospital day 29, no ventricular pus was detected on the head CT.
Figure 1.
Computed tomography images of the patient. A: No ventricle pus was detected by head computed tomography (CT) on day 18; B: Head CT image obtained on day 24 showing ventricle pus; C: Head CT image after bilateral ventricular drainage; D: Head CT image obtained on day 30 showing that the ventricle pus had disappeared; E: Head CT image after removing the left ventricular drainage tube.
FINAL DIAGNOSIS
Pyogenic ventriculitis caused by XDR A. baumannii.
TREATMENT
The patient underwent bilateral decompressive craniectomy and evacuation of cerebellar hematoma simultaneously after admission. Then the patient was admitted to the intensive care unit following the operation. In order to prevent the development of an infection, 2 g of sulperazone was administered every 8 h (q8h) and ceased on hospital day 5.
On hospital day 7, the patient was transferred to the general ward. The body temperature of the patient remained normal until hospital day 18, when it increased to 39.1 ℃. Laboratory tests showed 105 mg/L of CRP, and the WBC was 10.37 × 109/L. Additionally, his renal and liver function tests were normal. The CSF obtained through lumbar puncture showed increased WBC, while the glucose level was 0.8 mmol/L, and the total protein was 4.49 g/L. He was started empirically on 1 g meropenem q8h and 1 g vancomycin q12h. Three sets of sputum, blood, and CSF cultures were obtained, but no bacterial growth was detected in any of the three samples. In the following 6 d, the patient presented with remittent fever (peak at 38.8 °C). On hospital day 21, analysis of the sputum and CSF cultures again demonstrated the absence of any bacterial growth. On hospital day 24, the patient's fever increased to 40.1 ℃, which was associated with meningeal signs and altered mental status. Head CT scans indicated ventricular empyema (Figure 1B). And emergency treatment included lumbar cistern drainage, right frontal external ventricular drainage (EVD), and left occipital EVD. The drainage tube used was VentriClear of Medtronic, which consists of translucent silicone elastomer impregnated with the antimicrobial agents. The pus was copiously irrigated with normal saline (NS) from both the frontal and occipital EVD. Meropenem and vancomycin continued to be administered.
On hospital day 26, the patient's CSF culture tested positive for A. baumannii that was susceptible only to tigecycline, though susceptibility to polymyxin was not tested (Table 1). Therefore, with the permission of the family and written consent, antimicrobial therapy was changed to IV tigecycline (starting at 100 mg and then 50 mg q12h) combined with CVI tigecycline. The CVI tigecycline was performed as follows: A 4 mg dose in 50 mL of NS, which was controlled with a syringe pump, was administered at a rate of 12.5 mL per hour at a frequency of q6h. The CVI tigecycline was administrated via the right frontal EVD, during which the left occipital EVD was drained at a similar rate to balance the intracranial pressure. During continuous ventricular irrigation and EVD handling, to maintain asepsis, we disinfected the incision and drainage tube interface of the patient every other day. The lumbar cistern drainage was removed on hospital day 27 as no CSF was discharged.
On hospital day 29, the CSF drained from the left occipital EVD was clear grossly, and no ventricular pus was detected on the head CT. However, the same strain of A. baumannii was again isolated from the CSF. Then, the right frontal EVD was removed, and the antimicrobial therapy was changed to IV tigecycline (50 mg q12h) combined with IVT tigecycline. The IVT tigecycline was performed as follows: A 2 mg dose in 4 mL of NS was injected through the left occipital EVD in 2 min at a frequency of q8h. After each dose, the left occipital EVD was closed for 2 h to prevent early antibiotic outflow. The same strain of XDR A. baumannii was persistently isolated from the CSF until hospital day 35. The patient still presented with remittent fever, but the peak decreased to 38.4 °C. The results of blood and CSF analysis are shown in Table 2. Antibiotic therapy remained unchanged.
On hospital day 36, the patient's CSF culture tested negative for the first time. The IV plus IVT tigecycline was continued until hospital day 43, on which day the CSF culture was negative for the third time. Then, the occipital EVD was removed, and the patient was transferred to another hospital for rehabilitation treatment.
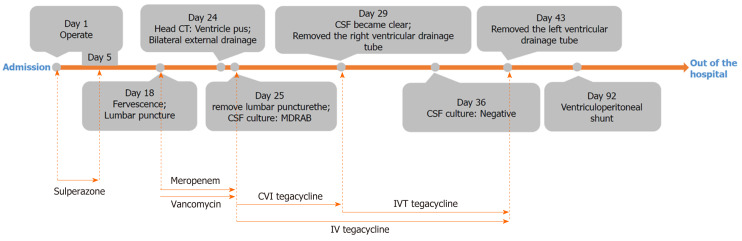
The timeline and antibiotics usage of the patient are shown in Figure 2.
Figure 2.
The timeline and antibiotics usage of the patient. IVT: Intraventricular; CVI: Continuous ventricular irrigation; CSF: Cerebral spinal fluid.
OUTCOME AND FOLLOW-UP
Three months after the car accident, the patient received a ventriculoperitoneal shunt due to hydrocephalus.
DISCUSSION
There are three categories of antimicrobial-resistant drugs, multidrug-resistant (MDR), XDR, and pandrug-resistant. XDR is defined as non-sensitivity to all penicillins and cephalosporins (including inhibitor combinations), fluoroquinolones, aminoglycosides, and carbapenems. We report the first case of pyogenic ventriculitis caused by XDR A. baumannii that was successfully treated by multi-route administration of tigecycline, including IV combined with CVI plus IVT.
A. baumannii is an opportunistic nosocomial pathogen that is listed by the Infectious Disease Society of America as one of the six most common MDR microorganisms in hospitals worldwide[13]. Resistance to A. baumannii has been steadily increasing since the 1970s, when most strains were sensitive to commonly used antibiotics. By 2007, the proportion of MDR isolates was as high as 70%[14]. A. baumannii has developed resistance to the vast majority of available antibiotics due to its ability to adapt rapidly to the environment and employ various resistance mechanisms, including the upregulation of efflux pumps, lactamases, and cell wall channels[4,15-17]. Moreover, few antibiotics are available to treat XDR A. baumannii ventriculitis, as achieving effective concentrations within CSF via traditional administration is challenging[3,18,19]. Hence, the treatment of MDR/XDR A. baumannii ventriculitis, especially pyogenic ventriculitis, remains a challenge for neurosurgeons. It has been reported that the average CSF concentration of tigecycline is equal to 7.9% of the serum concentration[5]. Hence, tigecycline is not commonly recommended as the first-line antibiotic for A. baumannii ventriculitis. In this case, the strain isolated from CSF was found to be sensitive to only tigecycline. Thus, this limited our choice of antibiotics.
Tigecycline was the first clinically available glycylcycline antibiotic that was designed to overcome MDR. The clinical use of IV tigecycline was approved in the United States in 2005, in the European Union in 2006, and in China in 2012. The first successful use of IVT tigecycline for the treatment of MDR A. baumannii meningitis was reported in 2016[5]. In 2017, the first case treated with continuous saline lavage through an intraventricular drainage tube combined with intraventricular meropenem in the prone position was reported[20]. In 2018, a case report and literature review reported the successful treatment of MDR A. baumannii ventriculitis with continuous ventricular lavage of tigecycline. In the case reported by Long et al[21], tigecycline was administered through occipital EVD, and continued drainage was performed through a contralateral occipital EVD. In this case, in order to ensure the effective CSF concentration of tigecycline, we combined IV with CVI and IVT administration. This strategy has not been previously reported in the literature but was necessary due to the life-threatening infection and CT evidence of suppurations in the ventricles. We obtained satisfactory results as the CSF was clear on day 5 of irrigation, and CSF cultures were negative on day 12. The dose of tigecycline in the ventricle was 8 mg/d and then 6 mg/d. Previous studies have shown that the dose of tigecycline in the ventricle was 4-20 mg/d[5,21-23]. These cases have demonstrated the safety and efficacy of this strategy.
Tigecycline is also used to treat other central nervous system infections. Soto-Hernández et al[23] and Wu et al[24] reported a case of intracranial infection with multidrug-resistant Klebsiella treated with tigecycline. And Sahin et al[25] reported a child with enterococcal ventriculitis treated with tigecycline. These cases demonstrate the efficacy and safety of multi-route tigecycline in the treatment of central nervous system infections.
Tigecycline shows low toxicity, particularly for neurological adverse events. As with most other antibiotics, adverse reactions of the digestive tract are common[26]. Additionally, no reports of adverse events following the intrathecal injection of tigecycline during the treatment of ventriculitis were noted. However, adverse reactions still cannot be ignored and can include headache, numbness, muscle spasms, weakness, etc[27,28]. Controlling the rate of intrathecal administration can effectively prevent the occurrence of such complications. In this case, the speed of administration was controlled with a micropump. However, due to the lack of research on such treatment, large scale clinical studies are required to evaluate the safety of the intraventricular lavage and intraventricular tigecycline.
CONCLUSION
In summary, we report the efficacy and safety of multi-route tigecycline for pyogenic ventriculitis caused by XDR A. baumannii. Our findings suggest that multi-route administration of tigecycline can be a therapeutic option against pyogenic ventriculitis caused by XDR A. baumannii. However, there is a lack of research on this topic, and therefore, larger samples and prospective randomized controlled trials are required to evaluate the safety of the intraventricular lavage and intraventricular administration of tigecycline.
ACKNOWLEDGEMENTS
We thank the Department of Neurosurgery, Radiology of The Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University for the diagnosis and treatment of the patient.
Footnotes
Informed consent statement: Informed written consent was obtained from the patient for publication of this report and any accompanying images.
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Unsolicited manuscript
Peer-review started: September 2, 2020
First decision: November 8, 2020
Article in press: January 26, 2021
Specialty type: Neurosciences
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Barik R, Moschovi MA S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Zhang YL
Contributor Information
Wei Li, Department of Neurosurgery, The Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University, Wenzhou 325000, Zhejiang Province, China.
Dan-Dong Li, Department of Neurosurgery, The Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University, Wenzhou 325000, Zhejiang Province, China. andonglmn@163.com.
Bo Yin, Department of Neurosurgery, The Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University, Wenzhou 325000, Zhejiang Province, China.
Dong-Dong Lin, Department of Neurosurgery, The Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University, Wenzhou 325000, Zhejiang Province, China.
Han-Song Sheng, Department of Neurosurgery, The Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University, Wenzhou 325000, Zhejiang Province, China.
Nu Zhang, Department of Neurosurgery, The Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University, Wenzhou 325000, Zhejiang Province, China.
References
- 1.Karaiskos I, Galani L, Baziaka F, Giamarellou H. Intraventricular and intrathecal colistin as the last therapeutic resort for the treatment of multidrug-resistant and extensively drug-resistant Acinetobacter baumannii ventriculitis and meningitis: a literature review. Int J Antimicrob Agents. 2013;41:499–508. doi: 10.1016/j.ijantimicag.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol. 2007;5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- 3.Kim BN, Peleg AY, Lodise TP, Lipman J, Li J, Nation R, Paterson DL. Management of meningitis due to antibiotic-resistant Acinetobacter species. Lancet Infect Dis. 2009;9:245–255. doi: 10.1016/S1473-3099(09)70055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karageorgopoulos DE, Falagas ME. Current control and treatment of multidrug-resistant Acinetobacter baumannii infections. Lancet Infect Dis. 2008;8:751–762. doi: 10.1016/S1473-3099(08)70279-2. [DOI] [PubMed] [Google Scholar]
- 5.Lauretti L, D'Alessandris QG, Fantoni M, D'Inzeo T, Fernandez E, Pallini R, Scoppettuolo G. First reported case of intraventricular tigecycline for meningitis from extremely drug-resistant Acinetobacter baumannii. J Neurosurg. 2017;127:370–373. doi: 10.3171/2016.6.JNS16352. [DOI] [PubMed] [Google Scholar]
- 6.Bradford PA, Weaver-Sands DT, Petersen PJ. In vitro activity of tigecycline against isolates from patients enrolled in phase 3 clinical trials of treatment for complicated skin and skin-structure infections and complicated intra-abdominal infections. Clin Infect Dis. 2005;41 Suppl 5:S315–S332. doi: 10.1086/431673. [DOI] [PubMed] [Google Scholar]
- 7.Sader HS, Farrell DJ, Flamm RK, Jones RN. Variation in potency and spectrum of tigecycline activity against bacterial strains from U.S. medical centers since its approval for clinical use (2006 to 2012) Antimicrob Agents Chemother. 2014;58:2274–2280. doi: 10.1128/AAC.02684-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta S, Aruna C, Nagaraj S, Dias M, Muralidharan S. In vitro activity of tigecycline against multidrug-resistant Gram-negative blood culture isolates from critically ill patients. J Antimicrob Chemother. 2012;67:1293–1295. doi: 10.1093/jac/dkr593. [DOI] [PubMed] [Google Scholar]
- 9.Polat M, Ozkaya-Parlakay A. Tigecycline salvage therapy for ventriculoperitoneal shunt meningitis due to extensively drug-resistant Acinetobacter baumannii. Eur J Pediatr. 2019;178:117–118. doi: 10.1007/s00431-018-3271-2. [DOI] [PubMed] [Google Scholar]
- 10.Pratheep R, Ray S, Mukhopadhyay K, Gautam V, Shafiq N, Dutta S, Saini SS, Bhatia A. First Case Report of Intraventricular Tigecycline in a Neonate with Extensively Drug-resistant Acinetobacter baumannii Ventriculitis. Pediatr Infect Dis J. 2019;38:e172–e174. doi: 10.1097/INF.0000000000002348. [DOI] [PubMed] [Google Scholar]
- 11.Deng ZW, Wang J, Qiu CF, Yang Y, Shi ZH, Zhou JL. A case report of intraventricular and intrathecal tigecycline infusions for an extensively drug-resistant intracranial Acinetobacter baumannii infection. Medicine (Baltimore) 2019;98:e15139. doi: 10.1097/MD.0000000000015139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar A, Agrawal D, Sharma BS. The Role of Endoscopic Lavage in Recalcitrant Multidrug-Resistant Gram-Negative Ventriculitis Among Neurosurgical Patients. World Neurosurg. 2016;93:315–323. doi: 10.1016/j.wneu.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 13.Talbot GH, Bradley J, Edwards JE Jr, Gilbert D, Scheld M, Bartlett JG Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis. 2006;42:657–668. doi: 10.1086/499819. [DOI] [PubMed] [Google Scholar]
- 14.Kempf M, Rolain JM. Emergence of resistance to carbapenems in Acinetobacter baumannii in Europe: clinical impact and therapeutic options. Int J Antimicrob Agents. 2012;39:105–114. doi: 10.1016/j.ijantimicag.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Lima AL, Oliveira PR, Paula AP. Acinetobacter infection. N Engl J Med. 2008;358:2846; author reply 2846–2846; author reply 2847. [PubMed] [Google Scholar]
- 16.Lockhart SR, Abramson MA, Beekmann SE, Gallagher G, Riedel S, Diekema DJ, Quinn JP, Doern GV. Antimicrobial resistance among Gram-negative bacilli causing infections in intensive care unit patients in the United States between 1993 and 2004. J Clin Microbiol. 2007;45:3352–3359. doi: 10.1128/JCM.01284-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howard A, O'Donoghue M, Feeney A, Sleator RD. Acinetobacter baumannii: an emerging opportunistic pathogen. Virulence. 2012;3:243–250. doi: 10.4161/viru.19700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaynes R, Edwards JR National Nosocomial Infections Surveillance System. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis. 2005;41:848–854. doi: 10.1086/432803. [DOI] [PubMed] [Google Scholar]
- 19.Michalopoulos AS, Falagas ME. Colistin: recent data on pharmacodynamics properties and clinical efficacy in critically ill patients. Ann Intensive Care. 2011;1:30. doi: 10.1186/2110-5820-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan AK, Birk HS, Yue JK, Winkler EA, McDermott MW. Bilateral External Ventricular Drain Placement and Intraventricular Irrigation Combined with Concomitant Serial Prone Patient Positioning: A Novel Treatment for Gravity-Dependent Layering in Bacterial Ventriculitis. Cureus. 2017;9:e1175. doi: 10.7759/cureus.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long W, Yuan J, Liu J, Liu J, Wu M, Chen X, Peng G, Wu C, Zhang C, Wang X, Zhao W, Liu Q. Multidrug Resistant Brain Abscess Due to Acinetobacter baumannii Ventriculitis Cleared by Intraventricular and Intravenous Tigecycline Therapy: A Case Report and Review of Literature. Front Neurol. 2018;9:518. doi: 10.3389/fneur.2018.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Pu ZH, Zhao MM. Case Report of Successful Treatment of Extensively Drug-Resistant Acinetobacter baumannii Ventriculitis with Intravenous plus Intraventricular Tigecycline. Antimicrob Agents Chemother . 2018;62:e01625–18. doi: 10.1128/AAC.01625-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soto-Hernández JL, Soto-Ramírez A, Pérez-Neri I, Angeles-Morales V, Cárdenas G, Barradas VA. Multidrug-resistant Klebsiella oxytoca ventriculitis, successfully treated with intraventricular tigecycline: A case report. Clin Neurol Neurosurg. 2020;188:105592. doi: 10.1016/j.clineuro.2019.105592. [DOI] [PubMed] [Google Scholar]
- 24.Wu Y, Chen K, Zhao J, Wang Q, Zhou J. Intraventricular administration of tigecycline for the treatment of multidrug-resistant bacterial meningitis after craniotomy: a case report. J Chemother. 2018;30:49–52. doi: 10.1080/1120009X.2017.1338846. [DOI] [PubMed] [Google Scholar]
- 25.Şahin A, Dalgic N. Intraventricular Plus Intravenous Tigecycline for the Treatment of Daptomycin Nonsusceptible Vancomycin-Resistant Enterococci in an Infant with Ventriculoperitoneal Shunt Infection. World Neurosurg. 2019;130:470–473. doi: 10.1016/j.wneu.2019.07.045. [DOI] [PubMed] [Google Scholar]
- 26.Pankey GA, Steele RW. Tigecycline: a single antibiotic for polymicrobial infections. Pediatr Infect Dis J. 2007;26:77–78. doi: 10.1097/01.inf.0000253038.78188.26. [DOI] [PubMed] [Google Scholar]
- 27.Lucas JT, Ducker TB, Perot PL Jr. Adverse reactions to intrathecal saline injection for control of pain. J Neurosurg. 1975;42:557–561. doi: 10.3171/jns.1975.42.5.0557. [DOI] [PubMed] [Google Scholar]
- 28.Klibanov OM, Filicko JE, DeSimone JA Jr, Tice DS. Sensorineural hearing loss associated with intrathecal vancomycin. Ann Pharmacother. 2003;37:61–65. doi: 10.1345/aph.1C145. [DOI] [PubMed] [Google Scholar]