Abstract
Intracellular growth of pathogenic Legionella in free-living amoebae (FLA) results in the critical concentrations that are problematic in engineered water systems (EWS). However, being amoeba-resistant bacteria (ARB), how Legionella spp. becomes internalized within FLA is still poorly understood. Using fluorescent microscopy, we investigated in real-time the preferential feeding behavior of three water-related FLA species, Willaertia magna, Acanthamoeba polyphaga, and Vermamoeba vermiformis regarding Legionella pneumophila and two Escherichia coli strains. Although all the studied FLA species supported intracellular growth of L. pneumophila, they avoided this bacterium to a certain degree in the presence of E. coli and mostly fed on it when the preferred bacterial food-sources were limited. Moreover, once L. pneumophila were intracellular, it inhibited digestion of co-occurring E. coli within the same trophozoites. Altogether, based on FLA–bacteria interactions and the shifts in microbial population dynamics, we propose that FLA’s feeding preference leads to an initial growth of FLA and depletion of prey bacteria, thus increases the relative abundance of Legionella and creates a “forced-feeding” condition facilitating the internalization of Legionella into FLA to initiate the cycles of intracellular multiplication. These findings imply that monitoring of FLA levels in EWS could be useful in predicting possible imminent high occurrence of Legionella.
Keywords: free-living amoebae, Legionella, engineered water systems, Legionnaires’ disease
1. Introduction
Legionella pneumophila, a Gram-negative bacterium, indigenous to natural and engineered water systems (EWS) [1,2], has become the number one cause of drinking water-related disease outbreaks in developed countries [3,4,5]. EWS including building water systems and cooling towers, are often reported as the source of exposure to pathogenic Legionella [6,7]. Legionella coexists in natural and engineered aquatic environments with other bacteria and microscopic eukaryotes like free-living amoebae (FLA), ciliates, and nematodes [8,9,10,11,12], as bacteria serve as a major source of food for FLA and other microeukaryotes [13,14]. Acanthamoeba, Vermamoeba (Hartmannella), and Naegleria are the most commonly reported amoebae isolated from different EWS [15,16]. Growth of pathogenic Legionella in EWS is considered to occur predominantly via intracellular growth within the susceptible FLA hosts [17,18,19], to very high concentrations considered necessary for causing infections through aerosol exposures [20].
Over millennia, various bacteria that have developed mechanisms against protozoan predation and digestion [21,22,23,24] and even to replicate within predatory host cells [25,26,27] are referred to as amoeba-resistant bacteria (ARB). The increased environmental “fitness” of Legionella has been considered to have resulted from amoeba–bacteria interactions [28,29,30]. About one-third of L. pneumophila’s genome encodes effector proteins that are required to prevent digestion and to grow intracellularly in amoebae and, coincidently, in human macrophages [31,32]. L. pneumophila effectors proteins are functionally redundant (presumably to deal with a wide range of predatory FLA), as elimination of one or many does not affect its overall pathogenic behavior [33,34]. However, the Type IV secretion system called the Icm/Dot system is essential for L. pneumophila pathogenicity, to resist digestion by amoebae and to replicate within various host cells [35,36]. However, the question is how the initial interactions occur between Legionella and FLA, and if FLA avoid phagocytosing pathogenic Legionella in a multispecies aquatic environment [19], what conditions make the FLA phagocytose Legionella to enable them to grow intracellularly.
Protozoa, including FLA appear to have recognition mechanisms to choose particular food. While preferential feeding behavior of some protozoa has been studied [37], there is limited information on these selection processes and feeding preferences of amoeba-resistant bacteria (ARB) in multispecies environments. It is apparent that preferential predation by amoebae would affect the biofilm microbial compositions and play an important role in shaping biofilm bacterial communities [38], but the mechanisms and microbial dynamics are not well understood [10,37,39,40]. Certain bacterial species like Pseudomonas aeruginosa has been reported to promote L. pneumophila uptake by amoeba hosts [41], in contrast, amoeba-symbionts were presumed to prevent intracellular growth of L. pneumophila [23], although the mechanisms is unknown. P. aeruginosa and Klebsiella pneumonia have also been reported to interfere with the growth and persistence of L. pneumophila in biofilms [42,43].
Low-level presence of FLA and Legionella spp. are expected in natural and EWS [44,45] but it is unclear who (prey or predator) plays the primary role in internalizing the Legionella cells in FLA. In addition, how likely Legionella cells are picked-up by the amoeba trophozoites to initiate the intracellular growth is unknown. Given the complex interactions of L. pneumophila with FLA within water-biofilms, we used fluorescent microscopy to observe in situ the interactions of three FLA species with L. pneumophila in the presence of two E. coli K12 strains to explore microbial selection processes through amoeba–bacteria interactions. The two very similar E. coli strains also would help to determine the precision in this selection process. We used bacteria that expressed different fluorescent proteins (different colors) to facilitate locating (intracellular/planktonic) the cells in-situ and in real time. Overall, this work would help us to understand how the predatory preference of FLA species may cause problematic concentrations of L. pneumophila in EWS.
2. Materials and Methods
2.1. L. pneumophila Culture
L. pneumophila Lp02 (ATCC®33152) with a pKB127 plasmid containing green fluorescence protein (GFP) (from Ann Karen Brassinga, University of Manitoba, Canada) [46] was grown on BCYE (Buffered Charcoal Yeast Extract) agar plates without antibiotics at room temperature (RT, 22 ± 1 °C) for 5–7 days (to avoid filamentous growth) [47]. L. pneumophila cell suspension was prepared by following the procedure described previously [48]. When appropriate, heat-killed L. pneumophila (GFP) cells were also used in co-culture experiments. The L. pneumophila (GFP) cell suspension in tap water was heated at 75 °C for 10 min in a heat-block to kill them (confirmed by culturing on BCYE agar plate at 37 °C for 7 days).
2.2. E. coli Culture
E. coli TOP10 (Invitrogen) cells were transformed with pBad-EBFP2 plasmid (provided by Prof. Robert E. Campbell, University of Alberta) to express a blue fluorescent protein. The other E. coli K-12 strain (MG 1655, genotype: F−, λ−, rph-1) contains the plasmid (pTV-mCherry) expressing a red fluorescent protein (provided by Dr. Tracy Raivio, University of Alberta). These two E. coli strains were grown on LB agar plates at 37 °C for 24 h. Cell suspension of each bacterium was prepared in filtered-sterile tap water, and the cell concentration was estimated by checking the optical density at 600 nm and confirmed by culture method.
2.3. Amoebae Culture
W. magna (ATCC®50035) was grown in Serum Casein Glucose Yeast Extract Medium (SCGYEM) at RT for 3 d in 25-cm2 cell-culture flasks to obtain trophozoites. A. polyphaga (ATCC®30461) and V. vermiformis (ATCC®50237) were grown separately at RT in Peptone Yeast Extract Glucose (PYG) medium for 2 days in 25-cm2 cell-culture flasks for trophozoites. The trophozoites of each amoeba species were harvested individually by centrifugation at 400× g for 10 min, washed three times with filtered-sterile, dechlorinated tap water, and re-suspended in the same medium to a concentration of approximately 105 trophozoites mL−1.
2.4. Amoeba–Bacteria Co-Culture
All bacterial strains were mixed together at equal concentrations and added to individual FLA species to make a final ratio of bacteria:trophozoites of 300:1 in filtered-sterile tap water. L. pneumophila cells were also mixed separately with different FLA species trophozoites at a ratio of 100:1 in filtered-sterile tap water. Five milliliters of these bacteria-amoeba suspensions were dispensed in 25-cm2 cell-culture flasks (about 4.0 × 105 trophozoites per flask). The mixed bacterial suspension was also dispensed in 25-cm2 cell-culture flasks and diluted to 5 mL to have a final concentration of 4.0 × 107 cells of individual strains in sterile tap water in each flask (as an amoeba-negative control) to observe whether different bacterial species has any effect on each other. Heat-killed L. pneumophila cells were added with viable E. coli cells in amoeba co-culture in a similar experimental setup. The experiments were undertaken at RT and in triplicate.
2.5. Fluorescent Microscopy and Image Processing
The amoebae–bacteria co-cultures were observed at multiple time points (at 5 and 30 min and 24, 48, 72, and 96 h of incubation) to check the amoebae–bacteria interactions and physical locations (intracellular in amoeba trophozoites and extracellular outside the trophozoites in the medium) of different bacteria using an EVOS Cell Imaging Systems (Thermo Fisher Scientific). When required (for microscopy and before antibiotic treatment), the co-cultures were washed with the filtered-sterile tap water, to reduce the planktonic bacterial cell number by gently changing the water without interrupting the surface-adhered amoeba trophozoites to observe better the intracellular (food vacuoles) location of the bacteria. Random bright field and fluorescent images were taken from each 25-cm2 cell-culture flasks at different time points. The images were further processed using ImageJ software (version 1.52e), if required and the number of trophozoites with and without internalized bacteria (different strains) in each field of view was counted.
2.6. Determining the Intracellular Bacteria
To enumerate the intracellular bacteria, only two bacterial strains (L. pneumophila and E. coli TOP10) were mixed together at equal concentrations and added to W. magna (ATCC®30035) trophozoites to make a final ratio of bacteria:trophozoites of 200:1 in filtered-sterile tap water in 25-cm2 cell-culture flasks and incubated at RT. At different time points (0.5, 24, 48, and 96 h) of the co-culture, the medium was aspirated gently from the flasks (in duplicate) and replaced once with 3 mL of sterile water to reduce the planktonic bacterial cell number (as much as possible without disturbing the adhered trophozoites to the flask bottom). Three milliliters of filtered-sterile tap water containing gentamicin (200 µg/mL) was added to the cell-culture flask containing amoeba trophozoites with mostly internalized bacteria and incubated at RT for 1 h to kill the remaining planktonic bacterial cells. The trophozites were harvested from the flasks after 1 h and re-suspended in 1.5 mL water in 2-mL tubes. The trophozoites were washed three times by centrifuging at 2000× g and resuspending in water. Finally, the trophozoites were lysed by passing through (back and forth) a 23-gauge needle five times to release the internalized bacteria. Appropriate dilutions of these cell suspensions were plated (spread plate technique) on BCYE plate with antibiotics (Polymyxin, Cycloheximide and Vancomycin) and LB agar plate to determine the number of L. pneumophila and E. coli TOP10 cells, respectively. The plates were incubated at 37 °C overnight for E. coli TOP10 and 5 days for L. pneumophila.
2.7. Statistical Analysis
The number of trophozoites were counted from 12 random fields of view photo taken under different fluorescent and bright field channels for visualizing internalized bacteria (studied bacteria produce Green, Red, and Blue fluorescent proteins). Student t-test was carried out to compare the feeding preference of the amoeba trophozoites for different bacterial strains.
3. Results
3.1. Differential Feeding Preference of W. magna
Large number of E. coli cells were found accumulated in the food vacuoles of W. magna trophozoites as early as 5 min of co-culturing them in sterile tap water at RT. Even though W. magna fed on both the E. coli strains, there was a clear preference (visual observation of microscopic images) for E. coli TOP10 cells over E. coli MG1655 (as determined by the apparent number of food vacuoles containing bacteria and the intensity of fluorescence) (Figure 1).
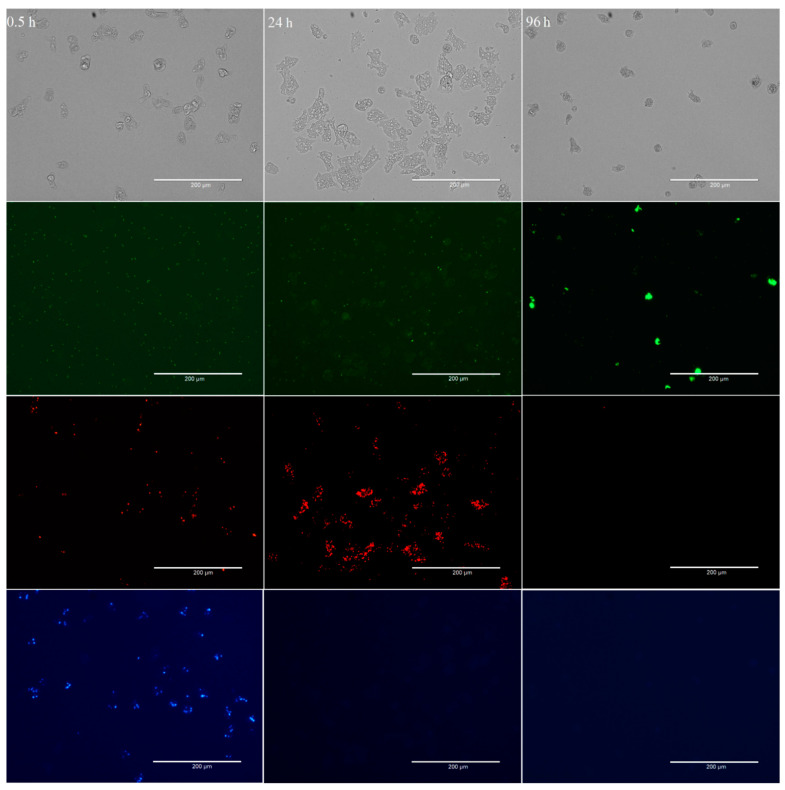
Figure 1.
Preferential feeding on bacteria by W. magna at RT at different time points (0.5, 24, and 96 h) of co-culture. The four images (from top to bottom) represent the same field of view under different fluorescent light channels, Monocolor transmission light channel, Green fluorescent channel to observe GFP-L. pneumophila, Texas-Red channel for mCherry-E. coli MG1655, and DAPI channel for BFP-E. coli TOP10. The clusters of color dots in images indicate the presence of different intracellular bacteria in the food vacuoles of W. magna trophozoites. The scattered color dots (smaller in size) indicate planktonic bacterial cells in the medium.
After 0.5 h of co-incubation, 87.1 ± 9.0% of the total W. magna trophozoites contained E. coli TOP10 cells, 69.7 ± 9.0% E. coli MG1655 and none contained L. pneumophila, despite being present in equally high numbers in close proximity to the amoeba trophozoites (Figure 2). Although both the E. coli strains were present in the same trophozoites, the number of food vacuoles containing E. coli TOP10 cells was much higher than that contained the E. coli MG1655 cells.
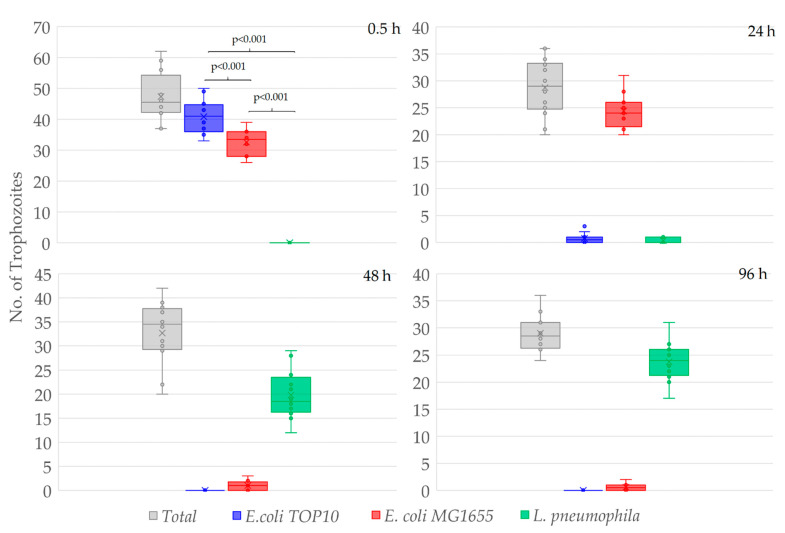
Figure 2.
The number of W. magna trophozoites containing E. coli TOP10, E. coli MG1655, and L. pneumophila cells at different time points of co-culture. The number of trophozoites were counted from four fields of view of three replicates (total 48 images/ time point), the bars represent standard deviations of the mean and the box represent 25–75 percentiles. The t-test indicates significant differences in prey preference (p < 0.001).
After 24 h of co-incubation, no E. coli TOP10 cells were observed in the medium and hardly any in the food vacuoles of the trophozoites (E. coli TOP10 cells were digested), however, E. coli MG1655 cells were numerous within food vacuoles. After 24 h, 2.8 ± 2.8% of the total amoeba trophozoites contained E. coli TOP10 cells, 87.0 ± 10.1% contained E. coli MG1655, and 1.5 ± 1.9% contained L. pneumophila. Hence, E. coli MG1655 appeared to be the second preferred food by W. magna under the study conditions and exhibited some resistance to amoeboid digestion as compared to E. coli TOP10, but both were eventually digested by the amoeba trophozoites within 48–72 h of co-culture. After 48 and 96 h of co-culture no E. coli, TOP10 cells were observed within trophozoites, only a few (3.5 ± 3.1% and 2.1 ± 2.1%, respectively) contained E. coli MG1655 and most (61.0 ± 8.6% and 81.8 ± 6.5%, respectively) contained L. pneumophila. The intracellular concentrations of different bacteria at different time points confirmed the preferential feeding of bacteria by W. magna (Figure S1). W. magna also showed similar preferential feeding behavior with heat-killed L. pneumophila when provided in presence of the E. coli strains (Figure S2).
Thus, W. magna only appeared to phagocytose L. pneumophila when other bacterial strains (E. coli) were unavailable in the co-culture due to prior predation. After 72 h, intracellular growth of L. pneumophila was observed in many trophozoites but not in all that contain L. pneumophila in the food vacuoles. None of the E. coli strains produced any apparent adverse effect on W. magna growth and activity (i.e., all demonstrated regular gliding movement (Supplementary Information Video S3 and Video S4). The gliding movement of the trophozoites with fluorescent food vacuoles also confirmed the intracellular status of the targeted cells. W. magna even avoided heat-killed L. pneumophila when present with the two E. coli strains in the same culture. However, W. magna phagocytosed L. pneumophila within 24 h of co-culture in sterile tap water, when provided as a single species bacterial prey. Interestingly, trophozoites that had recently acquired L. pneumophila also subsequently phagocytosed E. coli strains upon adding them to the culture (Figure 3 and Supplementary Information Video S5), but could not digest them as quickly as they could without having the intracellular L. pneumophila.
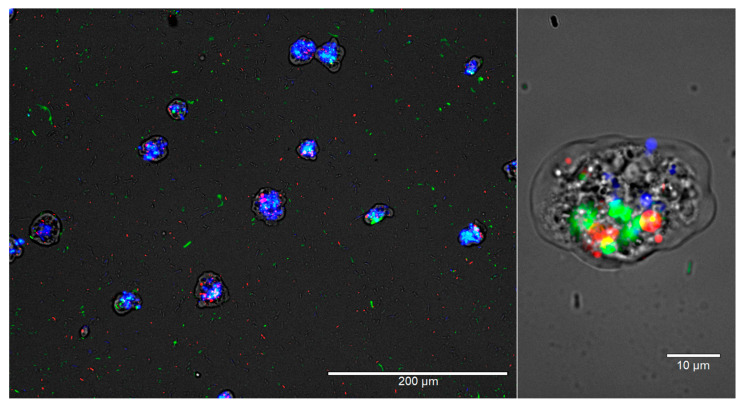
Figure 3.
Intracellular L. pneumophila interferes with W. magna’s digestion process. W. magna and L. pneumophila were co-cultured at RT, and after 24 h, E. coli TOP10 and E. coli MG1655 were added to the culture. The images represent overlaid images of the same field of view under four light channels, Monocolor transmission light channel, Green fluorescent channel, Texas-Red channel, and DAPI channel at 200× and 1000× magnification after 26 h of co-culture. L. pneumophila cells are green, E. coli MG1655 cells are red, and E. coli TOP10 cells are blue in color.
Hence, internalized L. pneumophila seemed to interfere with the overall digestion process of trophozoites and may lead to long-term intracellular persistence of the bacteria without initiating active intracellular growth at RT. All the bacterial species remained fluorescent without losing their number in amoeba-negative culture up to 4 days in sterile tap water at RT. No adverse effect was observed on each other by the studied bacterial species (Figure S6).
3.2. Interactions of A. polyphaga and V. vermiformis with Bacteria
A. polyphaga and V. vermiformis exhibited a higher preference for both E. coli strains when all three bacterial species were present in co-culture in sterile tap water or PAGE’s saline at RT (Figures S7 and S8). However, A. polyphaga did not avoid L. pneumophila as strongly as W. magna and V. vermiformis did. In tap water A. polyphaga tend to form cyst within 48 h and therefore, PAGE’s saline was used. E. coli TOP10 was found to be phagocytosed and mostly digested within 24 h by V. vermiformis, therefore hardly any E. coli TOP10 cells were found in the medium as well as in the food vacuoles of the trophozoites after 24 h of co-culture. However, due to presence of internalized L. pneumophila in A. polyphaga trophozoites, E. coli cells were still observed in the food vacuoles after 24 h. Due to the rapid encystation of A. polyphaga and E. coli MG1655′s moderate resistance to the amoeboid digestion, it stayed in the cysts, most likely in between the two outer layers of the cysts (Figure 4). Releasing of vesicles during encystation was also observed in A. polyphaga, as reported previously for the protozoa Giardia [49,50]. Both the amoebae phagocytosed L. pneumophila when provided as a single culture and supported intracellular growth.
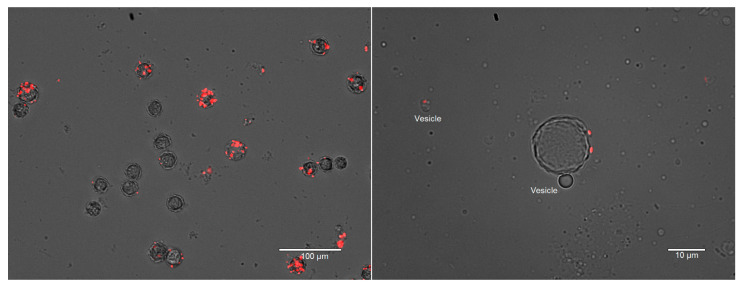
Figure 4.
Intracellular locations of E. coli MG1655 (red) in A. polyphaga cysts at 96 h of co-culture. Many of the cysts did not contain any E. coli MG1655 cells, which indicate that the digestion may depend on the intracellular load of the bacterial cells and encystation time. Releasing of vesicle during encystation and vesicle-containing E. coli MG1655 after 48 h of co-culture (Right image).
3.3. Amoebae–Bacteria Interactions
When all three species of the amoebae (trophozoites) were co-cultured with the studied bacterial species in sterile tap water at RT, the amoebae phagocytosed and digested the E. coli strains first before effectively engulfing the L. pneumophila. Of particular note, co-presence of the amoebae species did not appear to affect their feeding preferences. However, in water A. polyphaga and V. vermiformis underwent encystation earlier than W. magna, resulting in E. coli MG1655 cells being entrapped in cysts (Supplementary Information Video S9 and Video S10), as previously reported for L. pneumophila [51]. W. magna was also observed to phagocytose cysts of the other amoeba species present under the studied conditions (Figure S11).
4. Discussion
It is well known that FLA support the intracellular growth of pathogenic Legionella and other similar opportunistic water-based human pathogens. In fact, the ability to grow within amoebae has led to the evolution of L. pneumophila as a human pathogen [39]. However, we have very limited information on how pathogenic Legionella interacts with FLA in its natural water-biofilm environment. Our previous study suggested that Legionella might not be the preferred prey for W. magna within drinking water-biofilms [19]. This study provided visual evidence to support earlier observation by showing the interactions of different FLA species and L. pneumophila in the presence of other bacteria in real-time in situ. Accumulation of large numbers of E. coli cells and no Legionella in food vacuoles of W. magna trophozoite within 5 min of co-incubation indicated a very fast, effective, and precise recognition mechanisms since the bacteria were present in a homogenous equally high concentration suspension. The sequential feeding order of the two E. coli strains and Legionella by W. magna also indicated that amoeba played the active role in recognizing food through a highly selective manner. Although the two E. coli strains (TOP10 and MG1655) are very similar and originated from a common ancestor (E. coli K12), it was surprising that W. magna distinguished even between these two strains. The higher tolerance of E. coli MG1655 to amoeboid digestion implied that the resistance to digestion was from the bacterial side. W. magna was reluctant to feed on L. pneumophila until other options were limited, therefore, amoebae–bacteria interactions increased the relative abundance of L. pneumophila and created a “forced-feeding” situation to insist the amoebae to phagocytose Legionella [19]. Phagocytosing of L. pneumophila by A. polyphaga and V. vermiformis but not by W. magna in the presence of other bacteria indicated that the recognition systems for food were different among the amoeba species and might not be very stringent in all amoebae, as no apparent effect of non-Legionella bacteria on the uptake of L. pneumophila by A. castellanii and N. lovaniensis was reported earlier [41].
Further research is required to characterize FLA-L. pneumophila interactions in complex natural environments where other organisms and biofilms are present. Nonetheless, it is apparent from the current study that FLA may recognize extracellular chemicals and/or virulence-associated surface markers of L. pneumophila since W. magna was even ‘unwilling’ to phagocytose heat-killed Legionella in the presence of E. coli. Chemotaxis movement of amoeba toward certain bacterial cell lysate supports this observation [37]. Amoebae phagocytosed L. pneumophila when provided as the only option—suggested a “forced-feeding” condition when FLA had no choice but to feed on L. pneumophila, despite being detrimental to them. Larger amoeba trophozoites also phagocytosed other amoeba cysts, which could be another example of “forced-feeding.” Thus, this study strengthens our hypothesis that selection of L. pneumophila through preferential feeding of FLA creates conditions when L. pneumophila becomes the main available food and “forced-feeding” by FLA leads to L. pneumophila’s ultimate rapid growth in water [19].
The prolong presence of undigested E. coli in trophozoites containing L. pneumophila indicates that L. pneumophila actively interferes with the amoeboid digestion process. Hence, low intracellular concentrations of L. pneumophila may render the amoeba cell a reservoir of the bacteria by potentially “intoxicating” the amoeba trophozoites and impairing their digestion process. Similarly, the trapped bacteria within the amoeba cysts also could serve as a source of contamination when the cysts germinate. Moreover, the cysts protect the internal bacteria from the harsh environment and chemical disinfectants [52]. These cysts with L. pneumophila explain the recurrent LD outbreaks within hospital plumbing systems with clonal strains over decades [53,54,55,56]. Vigorous treatment of water systems in case of LD outbreak may remove the planktonic or biofilm-associated cells to some extent but leave behind the cysts with bacteria, which may act as a source for subsequent LD outbreaks [57].
Although the Icm/Dot, the type IV secretion system of L. pneumophila, is essential for replication within the amoebae and kill them [35,36], no adverse effects on E. coli suggested that the Icm/Dot system has no antibacterial activity as reported for type VI secretion system of V. cholerae [58]. The type VI secretion system of V. cholerae is also required to kill the protozoa, Dictyostelium discoideum [59].
Overall, this study supports our previous hypothesis that preferential feeding of FLA might be the driving force for rapid growth of pathogenic Legionella and other ARB in EWS. Although it is well known that the pathogenic L. pneumophila can grow intracellularly and disperse in water as free or vesicle-bound cluster of cells [48,60,61], the current work has described a possible mechanism of attaining critical concentrations of L. pneumophila. Ultimately, this work helped to understand the ecological perspective of L. pneumophila’s growth in EWS where it is usually present at very low concentrations. FLA’s reluctance to graze on L. pneumophila in the presence of non-ARB also suggests that a probiotic approach could work to control pathogenic Legionella’s growth in water systems. Ecological interactions such as competition, antagonism, and obligate parasite–host relationships have been described for potential targets for probiotic control of opportunistic pathogens in EWS [62]. In fact, upstream microbiota has been described to have a profound effect on the downstream biofilm bacterial compositions in water pipes [63,64].
5. Conclusions
Since intracellular multiplication in FLA is the major means for L. pneumophila’s growth, understanding the ecology of L. pneumophila, especially its interactions with FLA are fundamental to better management of water in EWS and to ensure public health safety. This study provided visual evidence of how FLA–bacteria interactions could lead to the problematic growth of pathogenic Legionella in EWS. Hence, the current monitoring of L. pneumophila without any consideration on FLA appears to be a weakness in water quality monitoring for EWS. Since FLA appears to be a major driving force for bacterial community shifts towards selection of opportunistic water-based pathogens, more research on other FLA and opportunistic pathogens like nontuberculous mycobacteria and Pseudomonas spp. is required to develop generalized approaches for monitoring and control of these environmental pathogens.
Acknowledgments
The authors would like to thank Ann Karen Brassinga, University of Manitoba for providing the L. pneumophila Lp02 strain (Harboring GFP plasmid) and Rober E. Campbell and Tracy Raivio, University of Alberta, for providing pBad-EBFP2 plasmid (Blue fluorescent protein) and E. coli MG 1655 (genotype F−, λ−, rph-1) with the plasmid (pTV-mCherry), respectively.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2607/9/1/174/s1.
Author Contributions
Conceptualization, methodology, formal analysis, data curation, writing—original draft preparation, M.S.; review and editing, supervision, project administration, funding acquisition, N.J.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Alberta Innovates, Alberta, Canada (Grant 201300490), the Canadian Fund for Innovation JEFL Grant 34575, Canada, and Natural Science and Engineering Research Council of Canada Discovery Grant GPIN-2017-04004.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. All the image data have not been stored in publicly available domain but additional images and videos are provided in the supplementary information.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Atlas R. Legionella: From environmental habitats to disease pathology, detection and control. Environ. Microbiol. 1999;1:283–293. doi: 10.1046/j.1462-2920.1999.00046.x. [DOI] [PubMed] [Google Scholar]
- 2.Orrison L.H., Cherry W.B., Fliermans C.B., Dees S.B., McDougal L.K., Dodd D.J. Characteristics of environmental isolates of Legionella pneumophila. Appl. Environ. Microbiol. 1981;42:109–115. doi: 10.1128/AEM.42.1.109-115.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beaute J., Zucs P., de Jong B. On behalf of the European Legionnaires’ Disease Surveillance Network (2013) Legionnaires’ disease in Europe, 2009–2010. Euro Surveill. 2013;18:6–12. doi: 10.2807/ese.18.10.20417-en. [DOI] [PubMed] [Google Scholar]
- 4.Beer K.D., Gargano J.W., Roberts V.A., Hill V.R., Garrison L.E., Kutty P.K., Hilborn E.D., Wade T.J., Fullerton K.E., Yoder J.S. Surveillance for Waterborne Disease Outbreaks Associated with Drinking Water—United States, 2011–2012. Morb. Mortal. Wkly. Rep. 2015;64:842–848. doi: 10.15585/mmwr.mm6431a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gargano J., Adam E., Collier S., Fullerton K., Feinman S., Beach M. Mortality from selected diseases that can be transmitted by water–United States, 2003–2009. J. Water Health. 2017;15:438–450. doi: 10.2166/wh.2017.301. [DOI] [PubMed] [Google Scholar]
- 6.Craun G.F., Brunkard J.M., Yoder J.S., Roberts V.A., Carpenter J., Wade T., Calderon R.L., Roberts J.M., Beach M.J., Roy S.L. Causes of outbreaks associated with drinking water in the United States from 1971 to 2006. Clin. Microbiol. Rev. 2010;23:507–528. doi: 10.1128/CMR.00077-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fields B., Benson R.F., Besser R.E. Legionella and Legionnaires’ disease: 25 years of investigation. Clin. Microbiol. Rev. 2002;15:506–526. doi: 10.1128/CMR.15.3.506-526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Declerck P., Behets J., van Hoef V., Ollevier F. Detection of Legionella spp. and some of their amoeba hosts in floating biofilms from anthropogenic and natural aquatic environments. Water Res. 2007;41:3159–3167. doi: 10.1016/j.watres.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Koubar M., Rodier M.H., Garduno R.A., Frere J. Passage through Tetrahymena tropicalis enhances the resistance to stress and the infectivity of Legionella pneumophila. FEMS Microbiol. Lett. 2011;325:10–15. doi: 10.1111/j.1574-6968.2011.02402.x. [DOI] [PubMed] [Google Scholar]
- 10.Taylor M., Ross K., Bentham R. Legionella, protozoa, and biofilms: Interactions within complex microbial systems. Microb. Ecol. 2009;58:538–547. doi: 10.1007/s00248-009-9514-z. [DOI] [PubMed] [Google Scholar]
- 11.van Heijnsbergen E., Schalk J.A., Euser S., Brandsema P.S., den Boer J.W., De Roda Husman A.M. Confirmed and potential sources of Legionella reviewed. Environ. Sci. Technol. 2015 doi: 10.1021/acs.est.5b00142. [DOI] [PubMed] [Google Scholar]
- 12.Rasch J., Krüger S., Fontvieille D., Ünal C.M., Michel R., Labrosse A., Steinert M. Legionella-protozoa-nematode interactions in aquatic biofilms and influence of Mip on Caenorhabditis elegans colonization. Int. J. Med. Microbiol. 2016;306:443–451. doi: 10.1016/j.ijmm.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Parry J.D. Protozoan grazing of freshwater biofilms. Adv. Appl. Microbiol. 2004;54:167–196. doi: 10.1016/S0065-2164(04)54007-8. [DOI] [PubMed] [Google Scholar]
- 14.Sherr B.F., Sherr E.B., Berman T. Grazing, growth, and ammonium excretion rates of a heterotrophic microflagellate fed with four species of bacteria. Appl. Environ. Microbiol. 1983;45:1196–1201. doi: 10.1128/AEM.45.4.1196-1201.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coşkun K.A., Özçelik S., Tutar L., Elaldı N., Tutar Y. Isolation and identification of free-living amoebae from tap water in Sivas, Turkey. Biomed Res. Int. 2013;2013:675145. doi: 10.1155/2013/675145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delafont V., Brouke A., Bouchon D., Moulin L., Hechard Y. Microbiome of free-living amoebae isolated from drinking water. Water Res. 2013;47:6958–6965. doi: 10.1016/j.watres.2013.07.047. [DOI] [PubMed] [Google Scholar]
- 17.Declerck P., Behets J., Margineanu A., van Hoef V., De Keersmaecker B., Ollevier F. Replication of Legionella pneumophila in biofilms of water distribution pipes. Microbiol. Res. 2009;164:593–603. doi: 10.1016/j.micres.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Murga R., Forster T.S., Brown E., Pruckler J.M., Fields B.S., Donlan R.M. Role of biofilms in the survival of Legionella pneumophila in a model potable-water system. Microbiology. 2001;147:3121–3126. doi: 10.1099/00221287-147-11-3121. [DOI] [PubMed] [Google Scholar]
- 19.Shaheen M., Scott C., Ashbolt N.J. Long-term persistence of infectious Legionella with free-living amoebae in drinking water biofilms. Int. J. Hyg. Environ. Health. 2019;222:678–686. doi: 10.1016/j.ijheh.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Schoen M.E., Ashbolt N.J. An in-premise model for Legionella exposure during showering events. Water Res. 2011;45:5826–5836. doi: 10.1016/j.watres.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 21.König L., Siegl A., Penz T., Haider S., Wentrup C., Polzin J., Mann E., Schmitz-Esser S., Domman D., Horn M. Biphasic metabolism and host interaction of a chlamydial symbiont. mSystems. 2017;2 doi: 10.1128/mSystems.00202-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mou Q., Leung P.H. Differential expression of virulence genes in Legionella pneumophila growing in Acanthamoeba and human monocytes. Virulence. 2018;9:185–196. doi: 10.1080/21505594.2017.1373925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okubo T., Matsushita M., Nakamura S., Matsuo J., Nagai H., Yamaguchi H. Acanthamoeba S13WT relies on its bacterial endosymbiont to backpack human pathogenic bacteria and resist Legionella infection on solid media. Environ. Microbiol. Rep. 2018;10:344–354. doi: 10.1111/1758-2229.12645. [DOI] [PubMed] [Google Scholar]
- 24.Schmitz-Esser S., Tischler P., Arnold R., Montanaro J., Wagner M., Rattei T., Horn M. The genome of the amoeba symbiont “Candidatus Amoebophilus asiaticus” reveals common mechanisms for host cell interaction among amoeba-associated bacteria. J. Bacteriol. 2010;192:1045–1057. doi: 10.1128/JB.01379-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matz C., Kjelleberg S. Off the hook–how bacteria survive protozoan grazing. Trends Microbiol. 2005;13:302–307. doi: 10.1016/j.tim.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Jousset A. Ecological and evolutive implications of bacterial defences against predators. Environ. Microbiol. 2012;14:1830–1843. doi: 10.1111/j.1462-2920.2011.02627.x. [DOI] [PubMed] [Google Scholar]
- 27.Erken M., Weitere M., Kjelleberg S., McDougald D. In situ grazing resistance of Vibrio cholerae in the marine environment. FEMS Microbiol. Ecol. 2011;76:504–512. doi: 10.1111/j.1574-6941.2011.01067.x. [DOI] [PubMed] [Google Scholar]
- 28.Cavalier-Smith T. The phagotrophic origin of eukaryotes and phylogenetic classification of Protozoa. Int. J. Syst. Evol. Microbiol. 2002;52:297–354. doi: 10.1099/00207713-52-2-297. [DOI] [PubMed] [Google Scholar]
- 29.Hahn M.W., Höfle M.G. Grazing of protozoa and its effect on populations of aquatic bacteria. FEMS Microbiol. Ecol. 2001;35:113–121. doi: 10.1111/j.1574-6941.2001.tb00794.x. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann C., Harrison C.F., Hilbi H. The natural alternative: Protozoa as cellular models for Legionella infection. Cell. Microbiol. 2014;16:15–26. doi: 10.1111/cmi.12235. [DOI] [PubMed] [Google Scholar]
- 31.Segal G., Shuman H.A. Legionella pneumophila utilizes the same genes to multiply within Acanthamoeba castellanii and human macrophages. Infect. Immun. 1999;67:2117–2124. doi: 10.1128/IAI.67.5.2117-2124.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomez-Valero L., Rusniok C., Carson D., Mondino S., Pérez-Cobas A.E., Rolando M., Pasricha S., Reuter S., Demirtas J., Crumbach J. More than 18,000 effectors in the Legionella genus genome provide multiple, independent combinations for replication in human cells. Proc. Natl. Acad. Sci. USA. 2019;116:2265–2273. doi: 10.1073/pnas.1808016116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Quadan T., Price C.T., Kwaik Y.A. Exploitation of evolutionarily conserved amoeba and mammalian processes by Legionella. Trends Microbiol. 2012;20:299–306. doi: 10.1016/j.tim.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo Z.-Q. Striking a balance: Modulation of host cell death pathways by Legionella pneumophila. Front. Microbiol. 2011;2 doi: 10.3389/fmicb.2011.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Segal G., Purcell M., Shuman H.A. Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila genome. Proc. Natl. Acad. Sci. USA. 1998;95:1669–1674. doi: 10.1073/pnas.95.4.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vogel J.P., Andrews H.L., Wong S.K., Isberg R.R. Conjugative transfer by the virulence system of Legionella pneumophila. Science. 1998;279:873–876. doi: 10.1126/science.279.5352.873. [DOI] [PubMed] [Google Scholar]
- 37.Dopheide A., Lear G., Stott R., Lewis G. Preferential feeding by the ciliates Chilodonella and Tetrahymena spp. and effects of these protozoa on bacterial biofilm structure and composition. Appl. Environ. Microbiol. 2011;77:4564–4572. doi: 10.1128/AEM.02421-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raghupathi P.K., Liu W., Sabbe K., Houf K., Burmølle M., Sørensen S.J. Synergistic Interactions within a Multispecies Biofilm Enhance Individual Species Protection against Grazing by a Pelagic Protozoan. Front. Microbiol. 2018;8:2649. doi: 10.3389/fmicb.2017.02649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amaro F., Wang W., Gilbert J.A., Anderson O.R., Shuman H.A. Diverse protist grazers select for virulence-related traits in Legionella. ISME J. 2015;9:1607. doi: 10.1038/ismej.2014.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huws S.A., McBain A.J., Gilbert P. Protozoan grazing and its impact upon population dynamics in biofilm communities. J. Appl. Microbiol. 2005;98:238–244. doi: 10.1111/j.1365-2672.2004.02449.x. [DOI] [PubMed] [Google Scholar]
- 41.Declerck P., Behets J., Delaedt Y., Margineanu A., Lammertyn E., Ollevier F. Impact of non-Legionella bacteria on the uptake and intracellular replication of Legionella pneumophila in Acanthamoeba castellanii and Naegleria lovaniensis. Microb. Ecol. 2005;50:536–549. doi: 10.1007/s00248-005-0258-0. [DOI] [PubMed] [Google Scholar]
- 42.Stewart C.R., Muthye V., Cianciotto N.P. Legionella pneumophila persists within biofilms formed by Klebsiella pneumoniae, Flavobacterium sp., and Pseudomonas fluorescens under dynamic flow conditions. PLoS ONE. 2012;7:e50560. doi: 10.1371/journal.pone.0050560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kimura S., Tateda K., Ishii Y., Horikawa M., Miyairi S., Gotoh N., Ishiguro M., Yamaguchi K. Pseudomonas aeruginosa Las quorum sensing autoinducer suppresses growth and biofilm production in Legionella species. Microbiology. 2009;155:1934–1939. doi: 10.1099/mic.0.026641-0. [DOI] [PubMed] [Google Scholar]
- 44.Thomas J.M., Ashbolt N.J. Do free-living amoebae in treated drinking water systems present an emerging health risk? Environ. Sci. Technol. 2011;45:860–869. doi: 10.1021/es102876y. [DOI] [PubMed] [Google Scholar]
- 45.Falkinham J.O., 3rd, Hilborn E.D., Arduino M.J., Pruden A., Edwards M.A. Epidemiology and Ecology of Opportunistic Premise Plumbing Pathogens: Legionella pneumophila, Mycobacterium avium, and Pseudomonas aeruginosa. Environ. Health Perspect. 2015 doi: 10.1289/ehp.1408692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morash M.G., Brassinga A.K.C., Warthan M., Gourabathini P., Garduno R.A., Goodman S.D., Hoffman P.S. Reciprocal expression of integration host factor and HU in the developmental cycle and infectivity of Legionella pneumophila. Appl. Environ. Microbiol. 2009;75:1826–1837. doi: 10.1128/AEM.02756-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piao Z., Sze C.C., Barysheva O., Iida K., Yoshida S. Temperature-regulated formation of mycelial mat-like biofilms by Legionella pneumophila. Appl. Environ. Microbiol. 2006;72:1613–1622. doi: 10.1128/AEM.72.2.1613-1622.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shaheen M., Ashbolt N.J. Free-living amoebae supporting intracellular growth may produce vesicle-bound respirable doses of Legionella within drinking water systems. Expos. Health. 2018;10:201–209. doi: 10.1007/s12403-017-0255-9. [DOI] [Google Scholar]
- 49.Benchimol M. The release of secretory vesicle in encysting Giardia lamblia. FEMS Microbiol. Lett. 2004;235:81–87. doi: 10.1111/j.1574-6968.2004.tb09570.x. [DOI] [PubMed] [Google Scholar]
- 50.Marti M., Hehl A.B. Encystation-specific vesicles in Giardia: A primordial Golgi or just another secretory compartment? Trends Parasitol. 2003;19:440–446. doi: 10.1016/S1471-4922(03)00201-0. [DOI] [PubMed] [Google Scholar]
- 51.Greub G., Raoult D. Morphology of Legionella pneumophila according to their location within Hartmanella vermiformis. Res. Microbiol. 2003;154:619–621. doi: 10.1016/j.resmic.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 52.Kilvington S., Price J. Survival of Legionella pneumophila within cysts of Acanthamoeba polyphaga following chlorine exposure. J. Appl. Bacteriol. 1990;68:519–525. doi: 10.1111/j.1365-2672.1990.tb02904.x. [DOI] [PubMed] [Google Scholar]
- 53.Stout J.E., Muder R.R., Mietzner S., Wagener M.M., Perri M.B., DeRoos K., Goodrich D., Arnold W., Williamson T., Ruark O., et al. Role of environmental surveillance in determining the risk of hospital-acquired legionellosis: A national surveillance study with clinical correlations. Infect. Control Hosp. Epidemiol. 2007;28:818–824. doi: 10.1086/518754. [DOI] [PubMed] [Google Scholar]
- 54.Bernander S., Jacobson K., Helbig J.H., Lück P.C., Lundholm M. A hospital-associated outbreak of Legionnaires’ disease caused by Legionella pneumophila serogroup 1 is characterized by stable genetic fingerprinting but variable monoclonal antibody patterns. J. Clin. Microbiol. 2003;41:2503–2508. doi: 10.1128/JCM.41.6.2503-2508.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bernander S., Jacobson K., Lundholm M. A hospital-associated outbreak of Legionnaires′ disease caused by Legionella pneumophila serogroups 4 and 10 with a common genetic fingerprinting pattern. APMIS. 2004;112:210–217. doi: 10.1111/j.1600-0463.2004.apm1120307.x. [DOI] [PubMed] [Google Scholar]
- 56.Oberdorfer K., Müssigbrodt G., Wendt C. Genetic diversity of Legionella pneumophila in hospital water systems. Int. J. Hyg. Environ. Health. 2008;211:172–178. doi: 10.1016/j.ijheh.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 57.Phin N., Parry-Ford F., Harrison T., Stagg H.R., Zhang N., Kumar K., Lortholary O., Zumla A., Abubakar I. Epidemiology and clinical management of Legionnaires’ disease. Lancet. Infect. Dis. 2014;14:1011–1021. doi: 10.1016/S1473-3099(14)70713-3. [DOI] [PubMed] [Google Scholar]
- 58.MacIntyre D.L., Miyata S.T., Kitaoka M., Pukatzki S. The Vibrio cholerae type VI secretion system displays antimicrobial properties. Proc. Natl. Acad. Sci. USA. 2010;107:19520–19524. doi: 10.1073/pnas.1012931107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miyata S.T., Kitaoka M., Brooks T.M., McAuley S.B., Pukatzki S. Vibrio cholerae requires the type VI secretion system virulence factor VasX to kill Dictyostelium discoideum. Infect. Immun. 2011;79:2941–2949. doi: 10.1128/IAI.01266-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dupuy M., Binet M., Bouteleux C., Herbelin P., Soreau S., Hechard Y. Permissiveness of freshly isolated environmental strains of amoebae for growth of Legionella pneumophila. FEMS Microbiol. Lett. 2016;363 doi: 10.1093/femsle/fnw022. [DOI] [PubMed] [Google Scholar]
- 61.Fields B.S., Sanden G.N., Barbaree J.M., Morrill W.E., Wadowsky R.M., White E.H., Feeley J.C. Intracellular multiplication of Legionella pneumophila in amoebae isolated from hospital hot water tanks. Curr. Microbiol. 1989;18:131–137. doi: 10.1007/BF01570838. [DOI] [Google Scholar]
- 62.Wang H., Edwards M.A., Falkinham J.O., III, Pruden A. Probiotic approach to pathogen control in premise plumbing systems? A review. Environ. Sci. Technol. 2013;47:10117–10128. doi: 10.1021/es402455r. [DOI] [PubMed] [Google Scholar]
- 63.Lu J., Buse H., Gomez-Alvarez V., Struewing I., Santo Domingo J., Ashbolt N.J. Impact of drinking water conditions and copper materials on downstream biofilm microbial communities and Legionella pneumophila colonization. J. Appl. Microbiol. 2014;117:905–918. doi: 10.1111/jam.12578. [DOI] [PubMed] [Google Scholar]
- 64.Pinto A.J., Xi C., Raskin L. Bacterial community structure in the drinking water microbiome is governed by filtration processes. Environ. Sci. Technol. 2012;46:8851–8859. doi: 10.1021/es302042t. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author. All the image data have not been stored in publicly available domain but additional images and videos are provided in the supplementary information.