Abstract
Simple Summary
Colistin is a widely used antibiotic against infections caused by extensively drug-resistant Gram-negative bacteria. It is critical to track and monitor the presence of mcr-like genes and colistin resistance to protect a last resort treatment against highly antibiotic-resistant bacteria. In the present study, the colistin resistance gene mcr-1 was investigated, and its in-silico functional analysis in Salmonella isolates was determined. Out of 100 chicken samples (liver and intestine), 82 Salmonella spp. were isolated and characterized. Antimicrobial sensitivity was determined using different antimicrobial agents. The isolates were characterized using PCR targeting genus-specific invA and mcr-1 genes followed by in-silico functional analysis. The majority of isolates (92.68%) was found highly resistant to colistin, which demonstrated the occurrence of the colistin resistance mcr-1 gene in Salmonella isolates of chicken origin in Bangladesh. The study also showed the in-silico functional analysis and phylogenetic relationship of the colistin resistance mcr-1 gene among Salmonella isolates. The findings of the present study highlight the increasing issue of transferable colistin resistance and call for immediate action and measures to review the imprudent use of colistin in poultry production systems in Bangladesh.
Abstract
Colistin (polymyxin E) is widely used in animal and human medicine and is increasingly used as one of the last-resort antibiotics against Gram-negative bacilli. Due to the increased use of colistin in treating infections caused by multidrug-resistant Gram-negative bacteria, resistance to this antibiotic ought to be monitored. The study was undertaken to elucidate the molecular mechanisms, genetic relationships and phenotype correlations of colistin-resistant isolates. Here, we report the detection of the mcr-1 gene in chicken-associated Salmonella isolates in Bangladesh and its in-silico functional analysis. Out of 100 samples, 82 Salmonella spp. were isolated from chicken specimens (liver, intestine). Phenotypic disc diffusion and minimum inhibitory concentration (MIC) assay using different antimicrobial agents were performed. Salmonella isolates were characterized using PCR methods targeting genus-specific invA and mcr-1 genes with validation for the functional analysis. The majority of the tested Salmonella isolates were found resistant to colistin (92.68%), ciprofloxacin (73.17%), tigecycline (62.20%) and trimethoprim/sulfamethoxazole (60.98%). When screened using PCR, five out of ten Salmonella isolates were found to carry the mcr-1 gene. One isolate was confirmed for Salmonella enterica subsp. enterica serovar Enteritidis, and other four isolates were confirmed for Salmonella enterica subsp. enterica serovar Typhimurium. Sequencing and phylogenetic analysis revealed a divergent evolutionary relationship between the catalytic domain of Neisseria meningitidis lipooligosaccharide phosphoethanolamine transferase A (LptA) and MCR proteins, rendering them resistant to colistin. Three-dimensional homology structural analysis of MCR-1 proteins and molecular docking interactions suggested that MCR-1 and LptA share a similar substrate binding cavity, which could be validated for the functional analysis. The comprehensive molecular and in-silico analyses of the colistin resistance mcr-1 gene of Salmonella spp. of chicken origin in the present study highlight the importance of continued monitoring and surveillance for antimicrobial resistance among pathogens in food chain animals.
Keywords: antimicrobial resistance, Enterobacteriaceae, colistin, mcr-1 gene, Salmonella enterica, foodborne, poultry, Bangladesh, chicken, phosphoethanolamine, LptA
1. Introduction
In spite of the significant improvements in the poultry sector in Bangladesh [1], there is a potential risk of infectious diseases due to associated bacterial pathogens, which can result in huge economic losses [2]. Infections due to Salmonella spp. are the most commonly reported bacterial diseases in poultry and may cause foodborne illnesses in human. Salmonellosis remains a persistent threat to both human and animal health [2,3]. Although vaccination and good hygienic practices are among the most effective measures to prevent salmonellosis [4], antibiotics are extensively used either as growth promoters [5,6], prophylactic agents, or therapeutics in the poultry industry in Bangladesh [7]. The widespread misuse and overuse of antimicrobial agents in poultry settings contribute to the emergence and the development of antimicrobial resistance in livestock pathogens such as Salmonella [8].
Antimicrobial-resistant Salmonella in poultry is a potential risk and a common vector for the dissemination of antimicrobial resistance to humans [8,9,10]. Standard microbiological and serological methods are usually employed for isolation and identification of Salmonella species. However, the invasion gene invA, commonly involved in bacterial virulence, is also routinely used for the accurate detection of Salmonella spp. in clinical samples [11]. The nucleotide sequences of the invA gene are distinctive to the genus Salmonella, and the amplification of the invA gene by the polymerase chain reaction (PCR) is a suitable diagnostic method of detection of Salmonella due to its reliability, sensitivity, specificity and detection speed [12]. Of note, poultry is a major source of Salmonella infections that cause mild to severe illnesses in humans. Therefore, the detection of Salmonella species in the poultry production food chain, particularly at the farm level, is of great concern. Furthermore, the escalating antimicrobial resistance of some Salmonella serotypes to multiple antibiotics [8] has led to the study of antimicrobial susceptibility profiles and the underlying resistance genetic determinants of priority [13].
Recently, the colistin resistance mcr-1 gene was firstly reported from animals and human in China [14]. Subsequently, mcr-1 positive Enterobacteriaceae was reported in animals, humans, food, and the environment in more than 25 countries across 4 continents, and the majority of studies reported mcr-1 positive Escherichia (E.) coli [14,15,16]. In comparison to E. coli, very few studies in Europe reported mcr-1 detection in Salmonella in poultry [17]. However, there is no data from Bangladesh in the literature on the molecular characterization and mcr-1 gene detection in Salmonella in poultry. Therefore, the present study was conducted to detect the colistin resistance mcr-1 gene in Salmonella isolates of chicken origin and to study the associated antimicrobial resistance patterns. The study was also conducted to elucidate the molecular mechanisms, genetic relationships and phenotype correlations of colistin-resistant Salmonella isolates. Phylogenetic relationships between the tested local Salmonella isolates and other published data from different parts of the world were also analyzed. Additionally, molecular docking of the phosphatidylethanolamine substrate with MCR-1 and LptA were investigated.
2. Materials and Methods
2.1. Ethical Standards
The experimental protocol was reviewed and approved by the Animal Experimentation Ethics Committee of Sylhet Agricultural University (SAU) (approval number #AUP2018004).
2.2. Study Area and Sampling
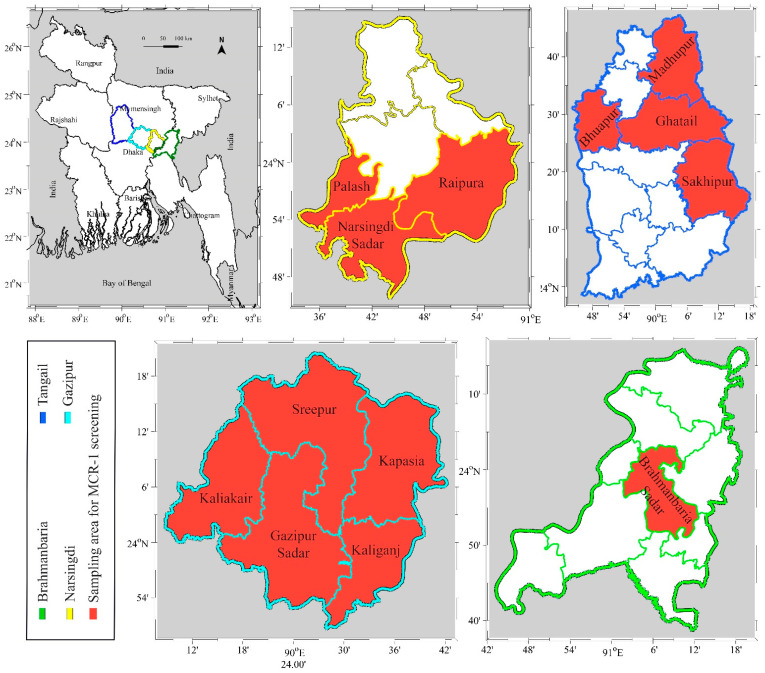
The study was conducted from January 2019 to June 2019 in widely occupied poultry zones of Gazipur, Narsingdi, Tangail and Brahmanbaria in Bangladesh (Figure 1).
Figure 1.
Geographical map of Bangladesh showing the locations of the sampling areas in selected districts in the present study. Areas where the colistin resistance gene mcr-1 was screened are highlighted in red.
Selected dead and sick chicken (Gallus gallus domesticus) were randomly collected and transferred to the laboratory. Postmortem examinations were immediately conducted on receiving the birds, and the anamnesis and clinical information were collected by a local veterinarian in accordance with the standard guidelines. During postmortem examinations, liver and intestinal tissue samples were collected for further analysis. Blood samples were collected from sick live chicken and sera samples were prepared before the postmortem examination.
2.3. Isolation and Biochemical Identification of Bacterial Isolates
Before isolation of Salmonella spp., samples were initially screened using a rapid serum plate agglutination test (Innovative Diagnostics, Grabels, France) followed by clinical and postmortem examinations. Liver and intestinal samples from 100 Salmonella suspected infected chicken were subjected to pre-enrichment step by culturing in 225 mL buffered peptone water medium in a ratio of 1:10 and samples were incubated at 37 °C for 24 h. For Salmonella specific pre-enrichment, cultures were further transferred to modified semi-solid Rappaport-Vassiliadis (MSRV) agar (HiMedia Laboratories Pvt. Ltd., Mumbai, India) and tetrathionate (TT) broth (HiMedia Laboratories Pvt. Ltd., Mumbai, India) consecutively and were incubated at 42 °C for 24 h. Following enrichment, a loopful of enriched broth was initially streaked on Salmonella–Shigella (SS) agar, and pure colonies (single pinkish with black center) were streaked on xylose–lysine–deoxycholate (XLD) agar (HiMedia Laboratories Pvt. Ltd, Mumbai, India) and plates were incubated at 37 °C for 24 h. Colonies were identified as Salmonella based on morphological and biochemical properties using Gram’s stain, catalase test and indole test as was previously described [18]. For further confirmation, 4–6 colonies from each sample were tested biochemically by dilution streaking and stabbed onto triple sugar iron (TSI) agar (Merck, Germany) and tubes were incubated at 37 °C for 16–24 h [19].
2.4. Antimicrobial Susceptibility Testing
Antibiogram profiles were obtained for Salmonella isolates using 19 antimicrobial agents by the Kirby Bauer’s disk diffusion method as previously described [20]. The results were interpreted by measuring the zones of inhibition (in mm), and results were reported as sensitive, intermediate or resistant according to CLSI guidelines [21]. The minimum inhibitory concentrations (MICs) of the tested antimicrobials were determined as described by global CLSI based and natural resistance [21,22]. MIC was performed using a VITEK 2 compact AST card N280. E. coli (ATCC 25922) was used as the quality-control strain. The susceptibility breakpoints of the tested antimicrobials were interpreted based on CLSI-EUCAST plus natural resistance guidelines [21,22]. Isolates that were found resistant to at least 3 different classes of antibiotics were considered multidrug-resistant (MDR) isolates [23].
2.5. Bacterial DNA Extraction
Ten isolates out of the 82 phenotypically identified Salmonella cultures were randomly selected and were subjected to further testing using Salmonella spp. detection kit (AddBio Inc. Ltd., Daejeon, South Korea) using PCR molecular methods for the detection of the invA gene. Bacterial DNA was extracted from the 10 isolates using the boiling method as previously described [24]. Briefly, a loopful of an overnight culture suspension was heated at 100 °C for 8–10 min in a heating block and then immediately cooled on ice for 5 min. Cellular debris was pelleted by centrifugation at 13,000 rpm for 1 min, and the remaining supernatant was used as the DNA template in PCR molecular tests.
2.6. Polymerase Chain Reaction (PCR) and Gel Electrophoresis
Extracted DNA was subjected to PCR for the initial confirmation of Salmonella isolates using specific primers targeting invA gene (forward 3′ TAATGCCAGACGAAAGAGCGT 5′ and reverse 3′ GATA TTGGTGTTTATGGGGTCGTT 5′) as previously described [25] using a Salmonella spp. detection kit (AddBio Inc. Ltd., Daejeon, South Korea) according to the manufacturer guidelines. Lambda primers (forward sequence 3′ CAGATCTCCAGCACGGAACTATTGAGTACGAACG 5′ and reverse sequence 5′ GCATAAAATGCGGGGATTCACTGGCTGC 3′) were used as internal controls, and an expected amplicon size of 100 bp indicated positive Salmonella spp. (AddBio Inc. Ltd., Daejeon, South Korea). The reaction volume was 20 µL and consisted of 10 µL of 2X master mix with uracil–DNA glycosylase (UDG), 5 µL of primer mix and 5 µL of DNA sample. Forward and reverse primers were used at 5 pmole each per reaction. The PCR was performed using a DLAB TC100-G machine (DLAB Scientific Co., Ltd., Beijing, China), and reaction conditions were as follows: UDG reaction for 3 min at 50 °C, initial denaturation for 10 min at 95 °C, 35 cycles denaturation for 30 s at 95 °C, primer annealing and extension for 45 s at 68 °C followed by a final elongation step for 5 min at 72 °C.
For the detection of mcr-1 colistin resistance genes, DNA extracts of the 10 Salmonella isolates were subjected to PCR reaction using designed primers (NeoProbe, Daejeon, South Korea), as shown in Table 1, which cover the reading frame of mcr-1 gene with the allocated two amplicons (1197 bp and 799 bp). For the primer design, mcr-1 gene sequences were retrieved from NCBI and sequences were aligned using the BioEdit version 7.2 (Ibis Biosciences, http://www.mbio.ncsu.edu/bioedit/bioedit.html). Two primers were designed using the Primer3 program available at NCBI, and these two primers yielded two amplicons (1197 bp and 799 bp), which covered the coding sequence of the full-length mcr-1 gene (1626 bp). The reaction volume was 20 µL and consisted of 10 µL of 2X master mix, 5 µL of primer mix and 5 µL of DNA sample. Forward and reverse primers were used at 5 pmole each per reaction. The PCR was performed using a DLAB TC100-G machine (DLAB Scientific Co., Ltd., Beijing, China), and reaction conditions were as follows: initial denaturation for 10 min at 95 °C, 40 cycles denaturation for 30 s at 95 °C, primer annealing and extension for 45 s at 68 °C followed by a final elongation step for 5 min at 72 °C. The amplified products were visualized by gel electrophoresis using 1.5% agarose gel and viewed under UV transillumination in a gel documentation system (Bio-Rad, Hercules, CA, USA).
Table 1.
Primer sequence for mcr-1 gene detection in Salmonella isolates.
| Target Gene | Primer Name | Primer Sequence | Size (bp) |
|---|---|---|---|
| MCR1 | MCR1-P1F MCR1-P1R |
F: CAGTATGGGATTGCGCAATGATT R: TTATCCATCACGCCTTTTGAGTC |
1197 |
| MCR1 | MCR1-P2F MCR1-P2R |
F: TGTCGATACCGCCAAATACCAAG R: GGAGTGTGCGGTGGGTTTG |
799 |
2.7. Sequencing, Multiple Sequence Alignment and Phylogenetic Analysis
Amplicons of mcr-1 gene were purified using a DNA purification kit (AddBio Inc. Ltd., Daejeon, South Korea) and were subjected to Sanger sequencing (SolGent Co., Ltd., Daejeon, South Korea). Sequences were checked using BLAST search tool and annotated to GenBank. In case of invA gene, five representative invA gene amplicons were sequenced from 10 positive invA gene isolates to confirm the results and the identity of invA gene PCR amplicons using the BLAST search tool (Supplementary File S1).
The BLASTp search tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi) was used to retrieve the homologous sequences of the MCR-1 and MCR-1 like proteins containing LptA (formerly named EptA) and others from the NCBI database using five SAUVM MCR-1 proteins translated from the mcr-1 genes of Salmonella spp. (Supplementary Files S2 and S3). Multiple sequence alignment of Salmonella SAUVM MCR-1 proteins and retrieved MCR-1 of Salmonella species was performed using T-Coffee default parameters [26]. The maximum likelihood method of MEGA X [27] was used to construct a phylogenetic tree using aligned sequences of MCR-1 from ClustalW [28]. Results were validated using 500 bootstrap replicates.
2.8. Transmembrane Topology Analysis, Structural Modelling, Refinement and Validation
To predict the transmembrane helices of MCR-1 proteins, the TMHMM server v.2.0 (Department of Health Technology, Lyngby, Denmark; http://www.cbs.dtu.dk/services/TMHMM/) was used with standard parameters. The topology was given as the position of the transmembrane helices differentiated by “I” and “o” when the loop is on the inside and outside, respectively [29]. Three dimensional (3D) modeling of Salmonella SAUVM MCR-1 proteins was performed by I-TASSER, which functions by identifying structure templates from the Protein Data Bank (PDB) library. The confidence of each model was quantitatively measured by C-score [30]. To enhance the accuracy of the predicted structures, refinement was performed using ModRefiner [31] followed by the FG-MD refinement server [32]. Finally, the refined structures were also validated using the Verified 3D, ERRAT and Ramachandran Plot Assessment server (RAMPAGE) [33].
2.9. Molecular Docking of Phosphatidylethanolamine Substrate with mcr-1 and LptA
The chemical structure of phosphatidylethanolamine (ZINC identification number (ID): ZINC32837869) was obtained from the ZINC database [34], while the 3D structure of LptA (PDB ID: 5FGN; Neisseria meningitidis), the best template of MCR-1 of Salmonella SAUVM isolates was retrieved from the RCSB Protein Data Bank server [35]. Binding interactions of the phosphatidylethanolamine to the MCR-1 LptA were investigated by molecular docking using the Autodock Vina algorithm in PyRx software [36]. OpenBabel (version 2.3.1) was used to convert the output PDBQT files into PDB format. PyMol and Discovery Studio software were used to optimize and visualize the protein structures and ligand binding interaction patterns [37].
3. Results
3.1. Confirmation of Salmonella spp.
A total of 82 Salmonella spp. were isolated from 100 samples based on their morphological and biochemical properties. Out of 82 isolates, 10 Salmonella isolates were randomly selected for further confirmation by PCR molecular methods for the presence of the invA virulence gene. It was found that all the 10 Salmonella isolates tested were invA gene positive. One isolate was subjected to 16S rRNA. The obtained sequences of five mcr-1 genes were checked using the BLAST search tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi) and annotated to GenBank. Among the five mcr-1 positive Salmonella isolates, one isolate (GenBank accession numbers of the forward and reverse 16S rRNA partial sequences are MW425840 and MW425841, respectively) was confirmed as Salmonella enterica subsp. enterica serovar Enteritidis using 16S rRNA analysis, and four isolates were confirmed as Salmonella enterica subsp. enterica serovar Typhimurium based on mcr-1 gene similarity to other published mcr-1 sequences of Salmonella enterica serovar Typhimurium.
3.2. Antimicrobial Susceptibility Testing
All Salmonella isolates (n = 82) were subjected to antibiotic resistance testing using 19 antimicrobial agents (Table 2). In general, a considerable percentage of resistance was observed among the tested Salmonella isolates using the disk diffusion test according to CLSI guidelines [21]. Specifically, high percentage of resistance were found against colistin (92.68%), ciprofloxacin (73.17%), tigecycline (62.20%), trimethoprim/sulfamethoxazole (60.98%), amoxicillin/clavulanate (41.46%) and ceftriaxone (40.24%). The susceptibility rates of the tested Salmonella isolates were for ampicillin (85.37%), piperacillin/tazobactam (84.15%), nitrofurantoin (73.17%), imipenem (68.29%) and amikacin (64.63). The multidrug resistance (MDR) patterns were also evaluated by MIC among mcr-1 positive Salmonella spp. isolates.
Table 2.
Antimicrobial susceptibility profile of Salmonella isolates (n = 82) in the present study.
| Antimicrobial Agents | Susceptible (S) | Intermediate (I) | Resistance (R) | I + R | |||
|---|---|---|---|---|---|---|---|
| Number of Isolates | % | Number of Isolates | % | Number of Isolates | % | ||
| Penicillins | |||||||
| Ampicillin (AMP, 10 μg) | 70 | 85.37 | 4 | 4.88 | 8 | 9.76 | 14.63 |
| Amoxycillin/clavulanate (AMC, 20/10 µg) | 45 | 54.88 | 3 | 3.66 | 34 | 41.46 | 45.12 |
| Piperacillin/tazobactam (PTZ, 100/10 μg) | 69 | 84.15 | 6 | 7.32 | 8 | 8.54 | 15.85 |
| Aminoglycosides | |||||||
| Amikacin (AMK, 30 μg) | 53 | 64.63 | 7 | 8.54 | 22 | 26.83 | 35.37 |
| Gentamicin (GEN, 10 μg) | 47 | 57.32 | 8 | 9.76 | 27 | 32.93 | 42.68 |
| Cephalosporins | |||||||
| Cefuroxime (CFX, 30 μg) | 46 | 56.10 | 10 | 12.20 | 26 | 31.71 | 43.90 |
| Cefuroxime axetil (CFA, 30 μg) | 44 | 53.66 | 21 | 25.61 | 17 | 20.73 | 46.34 |
| Ceftriaxone (CTR, 30 μg) | 43 | 52.44 | 6 | 7.32 | 33 | 40.24 | 47.56 |
| Cefoperazone/sulbactam (CFS, 75/30 μg) | 42 | 51.22 | 33 | 40.24 | 7 | 8.54 | 48.78 |
| Cefepime (CFP, 30 μg) | 38 | 46.34 | 29 | 35.37 | 15 | 18.29 | 53.66 |
| Carbapenems | |||||||
| Ertapenem (ETP, 10 μg) | 45 | 54.88 | 32 | 39.02 | 5 | 6.10 | 45.12 |
| Imipenem (IMP, 10 μg) | 56 | 68.29 | 5 | 6.10 | 21 | 25.61 | 31.71 |
| Meropenem (MPM, 10 μg) | 48 | 58.54 | 6 | 7.32 | 28 | 34.15 | 41.46 |
| Tetracyclines | |||||||
| Tigecycline (TIG, 15 μg) | 23 | 28.05 | 8 | 9.76 | 51 | 62.20 | 71.95 |
| Fluoroquinolones | |||||||
| Ciprofloxacin (CIP, 5 μg) | 16 | 19.51 | 6 | 7.32 | 60 | 73.17 | 80.49 |
| Nitrofurans | |||||||
| Nitrofurantoin (NIT, 300 µg) | 60 | 73.17 | 8 | 9.76 | 14 | 17.07 | 26.83 |
| Polymyxins | |||||||
| Colistin (COL, 10 μg) | 0 | 0.00 | 6 | 7.32 | 76 | 92.68 | 100.00 |
| Folate Pathway Inhibitors | |||||||
| Trimethoprim/Sulfamethoxazole (SXT, 1.25/23.75 µg) |
23 | 28.05 | 9 | 10.98 | 50 | 60.98 | 71.95 |
% is the percentage (number of isolates/total number of isolates). CLSI zone diameter (in mm) interpretive criteria.
All the tested mcr-1 positive Salmonella isolates (n = 5) showed a MDR phenotype, where all five isolates were resistant to at least three different classes of antimicrobial agents (at least colistin, ciprofloxacin and trimethoprim/sulfamethoxazole) (Table 3). As shown in Table 3, three out of five tested isolates (60%) were resistant to six antibiotics, and two out of five isolates (40%) were resistant to seven antibiotics.
Table 3.
Multidrug resistance patterns among mcr-1 positive Salmonella spp. isolates in the present study.
| COL | SXT | CIP | TIG | AMC | CTR | GEN * | AMK * | IMP | MPM | NIT | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolate Number | (10 μg) | (25 μg) | (5 μg) | (15 μg) | (30 μg) | (30 μg) | (10 μg) | (30 μg) | (10 μg) | (10 μg) | (300 μg) |
| SAUVM S6 | R | R | R | R | S | S | S | S | R | R | R |
| SAUVM S7 | R | R | R | S | R | R | R | R | S | S | S |
| SAUVM S8 | R | S | R | R | S | R | R | S | S | S | R |
| SAUVM S9 | R | R | R | S | S | S | S | R | R | R | S |
| SAUVM S10 | R | S | R | R | R | S | R | S | S | S | R |
S = susceptible; R = resistance; COL = colistin; SXT = trimethoprim/sulfamethoxazole; CIP = ciprofloxacin; TIG = tigecycline; AMC = amoxicillin/clavulanic acid; CTR = ceftriaxone; GEN = gentamicin; AMK = amikacin; IMP = imipenem; MPM = meropenem; NIT = nitrofurantoin; resistant if, colistin: ≥4; trimethoprim/sulfamethoxazole: ≥80; amoxicillin/clavulanic acid: ≥32; ceftriaxone: ≥4; ciprofloxacin: ≥1; * gentamicin: ≥0.5; * amikacin: ≥0.5; imipenem: ≥4; meropenem: ≥4; nitrofurantoin: ≥128. VITEK 2 systems version: 08.01; control: E. coli (ATCC 25922). MIC interpretation guideline/parameter set: copy of global CLSI based + natural resistance. * AES parameter set: copy of global CLSI based + natural resistance [22].
3.3. Detection of Salmonella Isolates Using PCR and Sequencing of mcr-1 Genes
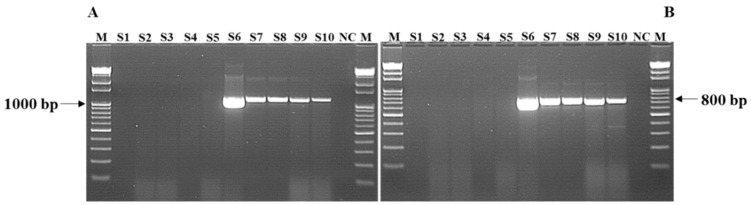
Five out of 10 randomly selected Salmonella isolates obtained from chickens were found positive for mcr-1 using primer-specific PCR (Figure 2). The five mcr-1 carrying Salmonella spp. strains isolated from chicken in the present study were deposited to NCBI under bioproject number PRJNA687398 with accession numbers SAMN17142409, SAMN17142425, SAMN17142450, SAMN17142964, SAMN17145260 for SAUVM S6, SAUVM S7, SAUVM S8, SAUVM S9 and SAUVM S10, respectively.
Figure 2.
PCR amplification of antimicrobial resistance mcr-1 gene of Salmonella isolates. In Salmonella (S6 to S10) isolates, fragments of (A) 1197 bp and (B) 799 bp were detected. Lane M: DNA ladder. Lane NC: negative control. Lanes S1 to S5 represents mcr-1 negative isolates while lanes S6 to S10 represents mcr-1 positive Salmonella isolates SAUVM S6 to SAUVM S10.
The sequences of the mcr-1 genes of Salmonella spp. were deposited into the GenBank under accession numbers MN873694, MN873695, MN873696, MN873697 and MN873698 for SAUVM S6, SAUVM S7, SAUVM S8, SAUVM S9 and SAUVM 10, respectively.
DNA sequencing of the mcr-1 amplicons confirmed them as mcr-1, and the obtained sequences showed 100% similarity in four isolates (Supplementary File S4), while one isolate SAUVM S9 (accession number MN873697) contained a new allele of the MCR-1 family (mcr-1.23 allele) than others retrieved from the NCBI database.
3.4. Sequence Acquisition, Multiple Sequence Alignment and Phylogenetic Analysis
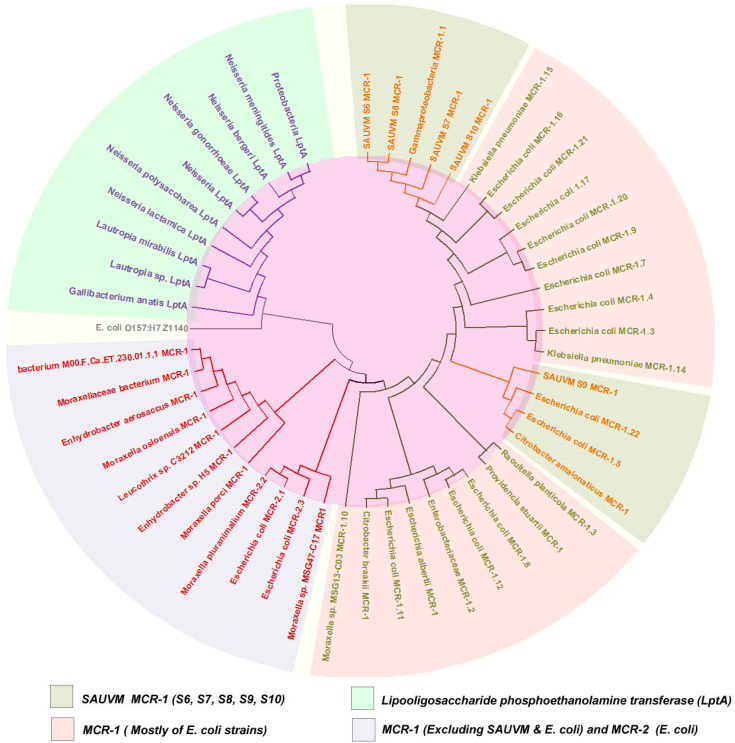
In order to analyze sequence similarities, phylogeny and structural insights of mcr-1 gene products, the respective translated MCR-1 proteins were subjected to different bioinformatics analyses. A total of 52 homologous sequences of the MCR-1 and MCR-1 like proteins were retrieved from the NCBI database, while 44 sequences were subjected to phylogenetic analysis including SAUVM MCR-1 proteins. Again, eight MCR-1 proteins of Salmonella spp. were aligned with SAUVM MCR-1 proteins for further analysis. The evolutionary relation inferred via phylogeny analysis is given in Figure 3. The phylogenetic analysis revealed that all of the MCR-1 and MCR-1 like proteins were distinctly categorized into two major groups, namely chromosomally-encoded LptA and plasmid encoded MCR types, indicating a divergent evolutionary relation between the LptA and MCR proteins.
Figure 3.
Phylogeny analysis showing ancestral origin and diversification of MCR-1 and MCR-1 like proteins. The BLAST search tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi) was used to retrieve the homologous sequences of the MCR-1 and MCR-1 like proteins from the NCBI database using amino acid sequences of five SAUVM MCR-1 proteins. Sequences were categorized into MCR-1 and MCR-1 like proteins of Salmonella, E. coli, strains containing LptA (formerly named EptA) and others. The maximum likelihood method of MEGA X was used to construct a phylogenetic tree using aligned sequences of MCR-1 from CLUSTALW.
3.5. Transmembrane Topology Analysis, Structural Modelling, Refinement and Validation
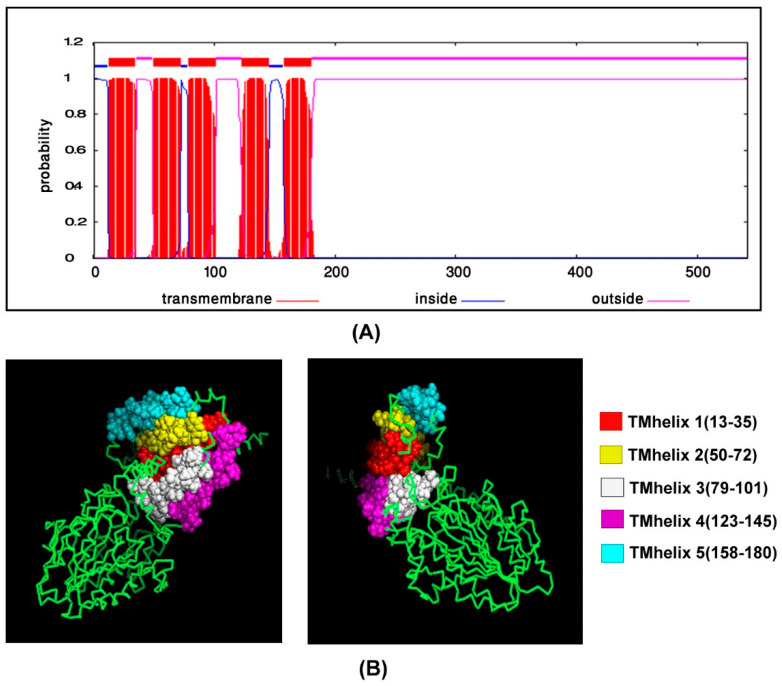
Prediction of transmembrane helices is significant in functional analysis of proteins. Therefore, the TMHMM server was used to predict transmembrane helices in mcr-1 genes of colistin-resistant Salmonella isolates in the present study. TMHMM predicted that there were five transmembrane domains in the Salmonella SAUVM MCR-1 proteins, namely TMhelix1 (13–35), TMhelix 2 (50–72), TMhelix 3 (79–101), TMhelix 4 (123–145) and TMhelix 5 (158–180), which spanned the inner membrane region (Figure 4).
Figure 4.
Transmembrane topology prediction of Salmonella SAUVM S6 MCR-1 protein. TMHMM server v.2.0 (http://www.cbs.dtu.dk/services/TMHMM/) was used to predict the (A) transmembrane helices of MCR-1 proteins and the image showed TMHMM posterior probabilities of SAUVM S6. (B) The topology was shown as the position of the transmembrane helices differentiated by “i” and “o” when the loop is on the inside and outside, respectively.
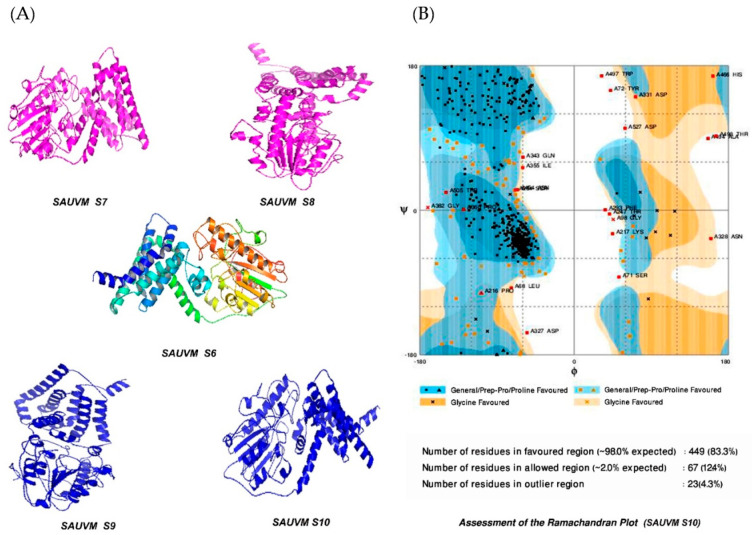
The structure of Salmonella SAUVM MCR-1 protein was modeled using the I-TASSER server, where EptA (PDB ID: 5FGN) of Nisseria meningitidis was used as the structural template. Salmonella SAUVM MCR-1 proteins showed 35.4% (35.6%) identity to EptA, and their modeled structure possessed a coverage score of 96% compared with that of EptA. Refinement was performed to enrich the quality of predicted structures beyond the accuracy. After refinement, Ramachandran plot analysis revealed that 83.3% of residues were in the favored, 12.4% of residues were in the allowed while only 4.3% of residues were in the outlier region (Figure 5). Moreover, ERRAT showed a 94.4% quality factor (Supplementary Figure S1A,B), and Verify3D suggested that 94.74% of the residues showed an average 3D-1D score of ≥0.2 (Supplementary Figure S2).
Figure 5.
Modelled structures of Salmonella SAUVM MCR-1 proteins and validation. (A) Three dimensional (3D) modeling of Salmonella SAUVM MCR-1 proteins (SAUVM S6 to SAUVM S10) were performed using I-TASSER, which functions by identifying structure templates from the PDB library. The confidence of each model was quantitatively measured by C-score. From these models of MCR-1 proteins, the Salmonella SAUVM S10 model was randomly selected, (B) analyzed and structurally validated with the Ramachandran plot assessment server (RAMPAGE).
3.6. Molecular Docking of PE Substrate with MCR-1 and LptA
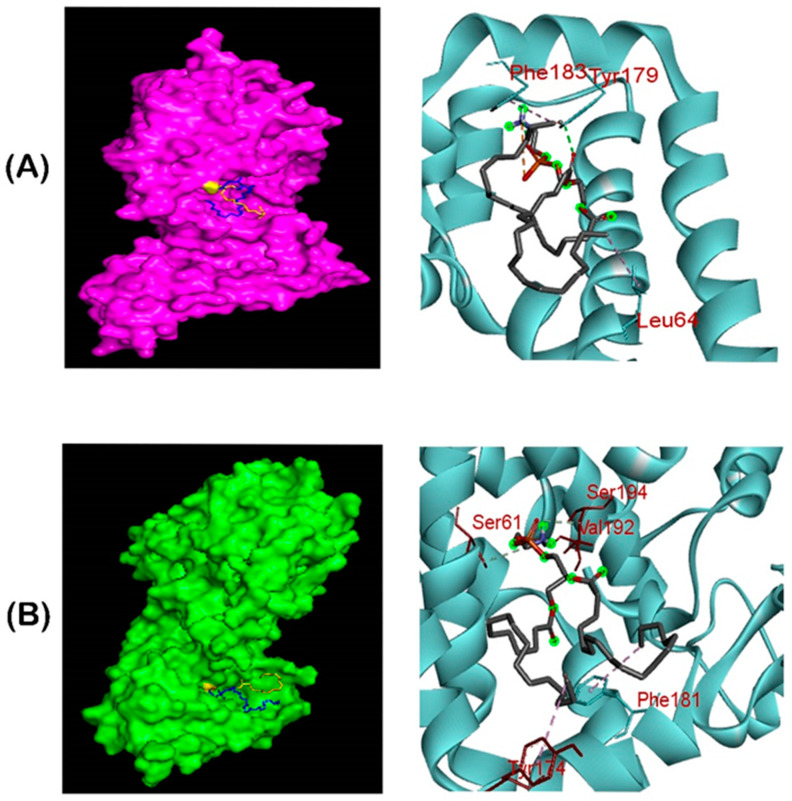
The grid box was set to 82.0138 A° x 82.7041 A° x 82.471 A° (x, y and z) with 1 A° spacing between the grid points, while other parameters were left as default. Molecular docking of the PE substrate to SAUVM MCR-1 and LptA generated five binding conformations for each docking, but the binding pattern with the lowest energy was selected (PE and MCR-1: −3.4kcal/mol and PE and LptA: −3.6 kcal/mol). It was demonstrated that Leu 64, Tyr 179 and Phe 183 were the key interactive molecules in the PE binding cavity of SAUVM MCR-1, whereas Ser 61, Tyr 174, Phe 181, Val 192 and Ser 194 were the key interactive molecules for LptA (Figure 6).
Figure 6.
Ligand–binding interaction pattern of phosphatidylethanolamine substrate with colistin resistance MCR-1 and LptA. The modeled ribbon structure for PE substrate with MCR-1 protein. The ribbon structure was given via PyMol software. In both (A,B) cases, the substrates tend to bind in the groove of MCR-1 and LptA, mostly spanning the 175–195 region, in which Phe, Tyr and Ser residues were abundantly found in the substrate binding region for phosphatidylethanolamine interaction. (B) Ligand–binding interaction revealed that both MCR-1 and LptA proteins exhibited similar localization of phosphatidylethanolamine binding sites (SAUVM MCR-1: Leu 64, Tyr 179, Phe 183; LptA: Ser 61, Tyr 174, Phe 181, val 192, Ser 194).
4. Discussion
In the present study, out of 100 suspected clinical samples obtained from chicken in different poultry zones, 82 samples were confirmed as containing Salmonella spp. using conventional phenotypic methods and molecular methods of PCR and DNA sequencing of the invA gene. The finding of 82% of the tested samples in the present study containing Salmonella is similar to other studies that reported that apparently healthy commercial poultry farms and their surrounding environments may be a potential source of Salmonella spp. [38].
Molecular methods were used for identification of Salmonella isolates and the mcr-1 gene using specific primers followed by confirmation using DNA sequencing and phylogenetic analysis. The pathogenesis of Salmonella is due to a combination of chromosomal and plasmid-mediated factors. A number of genes such as invA, fimA, stn and spv account for salmonellosis, of which the chromosomally located invasion (invA) gene codes for a protein in the inner membrane of the bacterium, which is necessary for invasion of the host epithelial cells [11,12]. In the present study, detection of the invA gene in 10 tested Salmonella isolates indicated their pathogenic nature. Antimicrobial resistance in livestock associated Salmonella spp., including fluoroquionolone and colistin resistant Salmonella spp., is an emerging global public health threat [4]. In the present study, antimicrobial susceptibility results showed that the majority of the tested Salmonella isolates were found resistant to colistin, ciprofloxacin and tigecycline. This high resistance rates in the present study reflects the widespread use of antibiotics in animal feed, as was previously reported [14,39]. Antimicrobial resistance was also detected against amoxicillin and doxycycline suggesting overuse or misuse of these antibiotics [8,40]. Resistance genes, such as mcr-1, associated with these phenotypes are often located on plasmids and may be due to co-selection of other antimicrobials rather than directly due to their misuse [41]. Moreover, higher resistance to ciprofloxacin (73.17%) and trimethoprim/sulfamethoxazole (60.98%) deserves attention, because Salmonella spp. may cause human infections, and fluoroquinolones are the first-line gut active antimicrobial agents used for the treatment of Salmonella infections.
Previous studies have reported that Salmonella isolated from poultry in Bangladesh were sensitive to ciprofloxacin [37,42,43]. However, in the present study, Salmonella isolates were found to be susceptible to ampicillin, piperacillin/tazobactam, nitrofurantoin, imipenem and amikacin, as was previously reported [39]. These antimicrobial agents are commonly used for therapeutic purposes in veterinary practice and not for feed supplement/growth promotion in Bangladesh, which could explain the susceptibility of the isolates in the current study to such antimicrobial agents. All of the five mcr-1 positive Salmonella isolates in the current study were found to be MDR. Similar findings were also reported on the detection of MDR in Salmonella isolates in Bangladesh and different parts of the world [44,45]. The detection of MDR Salmonella isolates including colistin resistance is a serious concern in animal and human health due to the high risk of zoonotic transmission of resistant isolates from animals to humans [46].
Since the early 1980s, colistin (also known as polymyxin E) was reported to be widely used in the agricultural and veterinary practice [14], and its overuse has contributed to the initial emergence and spread of mcr-1 worldwide [14,47]. In this study, an unexpected high rate of phenotypic colistin resistance (92.68% resistant, no isolate was susceptible) was detected in Salmonella spp. isolated from chicken samples. While we only screened ten isolates for the underlying genetic determinant (mcr-1 gene), five isolates were found to be positive for mcr-1 gene. This alarmingly high rate of colistin resistance in the tested Salmonella isolates in chicken suggests that mcr-1 might be escalating or widespread in food animals in Bangladesh. However, the actual data on antimicrobial use in the tested farms where the samples originated were not available. Therefore, the presence of mcr-1 gene may suggest imprudent use of colistin and possibly other antimicrobial agents in the poultry industry and livestock production systems in this region, although a recent study reported that 28% of samples obtained from poultry in China harbored mcr-1 colistin resistance, which is likely to emulate other global antimicrobial resistance [14]. Another study reported an unexpected high prevalence (24.8%) of colistin resistance gene mcr-1 in retail chicken meat samples in the Netherlands [48]. Further investigations have confirmed the presence of mcr-1 in Salmonella isolates recovered from food animals [49,50]. Various species of Enterobacteriaceae carrying mcr-1 plasmid-mediated resistance have been identified from humans, animals and environments in Asia, Europe, Africa, North America and South America [51]. Furthermore, the proportion of mcr-1 carriers among colistin-resistant isolates was higher in food-producing animals investigated in Italy [52], Germany [53], UK [54], Poland [55], Bangladesh [56], India [57], Pakistan [58], and South Korea [59]. Interestingly, the detection of the colistin resistance mcr-1 gene associated with the risk of subsequent transmission to unexposed human populations in southern Vietnam was also reported [60]. Therefore, the high prevalence of the mcr-1 gene in bacterial isolates of poultry origin in Enterobacteriaceae is certainly concerning in a densely populated country such as Bangladesh, where the use of antimicrobial agents in humans and animals may be poorly regulated. Thus, the recent emergence of colistin resistance has triggered an international review and recommendations for restrictions of colistin use in farm animals [47].
As detailed studies on molecular mechanisms of antimicrobial resistance in Salmonella isolates of poultry origin in Bangladesh are lacking, we aimed to assess mcr-1 from Salmonella samples using an integrative approach ranging from nucleotide sequence analysis, bioinformatics and structural modeling of bacterial genetics. The detection of mcr-1-harbouring Salmonella isolates adds new knowledge to the newly emerging issue of colistin resistance and mcr-1 genes regarding homology, structure and validation of this gene in Salmonella isolates. In the present study, multiple sequence alignments for mcr-1 genes in Salmonella spp. isolated from chicken were conducted. In order to avoid hits from very closely related species, retrieved sequences of Salmonella species were excluded from the phylogenetic study and those were only aligned with SAUVM MCR-1 proteins. The multiple sequence alignments of SAUVM MCR-1 proteins clearly indicated that they belong to the mobilized colistin resistance (MCR) protein family with putative conserved sites (Supplementary File S2).
In the present study, the detection of colistin resistance gene mcr-1 in Salmonella isolates was confirmed using primer specific PCR (Figure 2) and also by analyzing the possible convergent evolutionary lineages of these newly identified mcr-1 genes with other previously reported mcr-1 genes. To address this concern, we conducted phylogenetic analysis which showed a divergent evolutionary pattern between the catalytic domain of LptA of Nisseria meningitidis and MCR-1, including MCR-1 like proteins.
The constructed phylogenetic tree provides information about the ancestral origin and diversification of the MCR-1 proteins in different microorganisms which were divided into chromosomally-encoded LptA and plasmid-encoded MCR types, indicating a divergent evolutionary relationship between the LptA and MCR proteins (Figure 3). Further, the MCR protein group was divided into two apparent subgroups; one features MCR-1 proteins, mostly of E. coli strains including SAUVM MCR-1, and the other includes a small subclade of MCR-2 with MCR-1 proteins from diverse microorganisms. All of the SAUVM MCR-1 proteins were closely related to the MCR-1 proteins of E. coli, sharing the position in the same clade. However, SAUVM MCR-1 and LptA fell into two separate subclades within the tree, which indicated low sequence identity was previously reported [61,62]. The MCR-2 proteins of E. coli mostly aligned with MCR-1 proteins of non E. coli groups. The Z1140 locus of E. coli O157:H7, a member of the phosphatidylethanolamine lipid A transferases lacking a role in colistin resistance, apparently formed an individual clade in the phylogeny, which strengthened the findings.
To increase the structural insight, 3D homology modeling of the five SAUVM MCR-1 proteins was performed using LptA of Neisseria meningitidis as a structural template, and five distinct transmembrane helices spanned in the inner membrane region were identified, which was also reported in other studies [47,62]. Molecular interactions between the phosphatidylethanolamine substrate with MCR-1 and LptA were investigated as colistin resistance proteins MCR-1, MCR-2 and LptA were found to share a similar phosphatidylethanolamine lipid substrate-recognizing cavity. Ligand–binding interaction patterns of phosphatidylethanolamine substrate with SAUVM MCR-1 and LptA revealed that both proteins exhibited similar localization of the phosphatidylethanolamine binding sites spanning the 175–195 region in which Phe, Tyr and Ser residues were abundantly found.
5. Conclusions
The results of the current study demonstrated the presence of colistin resistance gene mcr-1 mediated resistance in Salmonella spp. isolated from chicken and highlighted the increasing issue of transferable colistin resistance in Bangladesh. The study also showed the in-silico functional analysis and the phylogenetic relationship of the colistin-resistance mcr-1 genes among Salmonella isolates. The results of the present study call for urgent actions to review the extensive use of colistin in poultry production and to limit the imprudent use of colistin and other antimicrobial agents in poultry production systems in Bangladesh.
Acknowledgments
Authors thank the staff of Kazi Farms Group laboratory, Gazipur, Bangladesh for their technical support. Authors thank the two anonymous reviewers for their insightful comments which significantly improved the manuscript.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2615/11/1/206/s1, Figure S1 (A): Structure validation by ERRAT and (B): Structure validation by ERRAT.; Figure S2: Structure validation by Verify3D server; Supplementary File S1: Amplicons of 100 bp invA gene sequence of Salmonella isolates (SAUVM S6 to SAUVM S10), Supplementary File S2: Retrieved sequences of MCR-1 and MCR-1 like proteins, Supplementary File S3: The multiple sequence alignments of SAUVM MCR-1 proteins with putative conserved sites of other Salmonella MCR-1., Supplementary File S4: Alignment of MCR-1 proteins.
Author Contributions
Conceptualization, S.S.U.A., M.E.E.Z. and M.B.U.; methodology, M.B.U., M.N.A., S.R., R.B., A.H.-A.-R., M.S.R.C., F.E., M.Y.E.C., M.M.H., M.M.R., S.M.B.H. and M.H.; software, M.H. and M.B.U.; validation, M.B.U., S.S.U.A., J.D.J. and M.E.E.Z.; formal analysis, M.B.U., M.E.E.Z., S.M.B.H. and M.H.; investigation, M.B.U., M.H.; resources, M.D., M.B.U.; data curation, S.S.U.A., M.E.E.Z., J.D.J. and M.B.U.; writing—original draft preparation, S.S.U.A., M.E.E.Z., J.D.J. and M.B.U.; writing—review and editing, S.S.U.A., M.E.E.Z., J.D.J. and M.B.U.; visualization, M.B.U. and M.E.E.Z.; supervision, M.E.E.Z.; S.S.U.A. and M.B.U.; project administration, M.B.U., M.E.E.Z., and S.S.U.A.; funding acquisition, J.D.J., S.S.U.A. and M.B.U.; critical manuscript revisions, M.E.E.Z., J.D.J., S.S.U.A. and M.B.U.; critical proof revisions and editing, M.B.U. and M.E.E.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The study was partially funded by the University Grants Commission (UGC) and Sylhet Agricultural University Research System (SAURES) (grant number: SAURES-ID-268) and the Swedish Research Council (Vetenskapsrådet) (grant number: 2016-02606).
Institutional Review Board Statement
The sampling protocol and procedures were performed in accordance with the Bangladesh legislation (Cruelty to Animals Act 1920, Act No. I of 1920 of the Government of the People’s Republic of Bangladesh). The sampling and experimental protocol was reviewed and approved by the Animal Experimentation Ethics Committee (approval number #AUP2018004) of the Sylhet Agricultural University, Bangladesh.
Informed Consent Statement
Consents were obtained from the farm owners.
Data Availability Statement
All data generated and analyzed in the present study were included in the manuscript or available as supplementary files.
Conflicts of Interest
The authors declare no conflict of interest.
Disclaimer
The conclusions, findings and opinions expressed in this scientific manuscript reflect only the view of the authors and not the official position of the funder or the Swedish Research Council.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hamid M.A., Rahman M.A., Ahmed S., Hossain K.M. Status of Poultry Industry in Bangladesh and the Role of Private Sector for its Development. Asian J. Poult. Sci. 2016;11:1–13. doi: 10.3923/ajpsaj.2017.1.13. [DOI] [Google Scholar]
- 2.El-Sharkawy H., Tahoun A., El-Gohary A.E.G.A., El-Abasy M., El-Khayat F., Gillespie T., Kitade Y., Hafez H.M., Neubauer H., El-Adawy H. Epidemiological, molecular characterization and antibiotic resistance of Salmonella enterica serovars isolated from chicken farms in Egypt. Gut Pathog. 2017;9:1–12. doi: 10.1186/s13099-017-0157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cosby D.E., Cox N.A., Harrison M.A., Wilson J.L., Jeff Buhr R., Fedorka-Cray P.J. Salmonella and antimicrobial resistance in broilers: A review. J. Appl. Poult. Res. 2015;24:408–426. doi: 10.3382/japr/pfv038. [DOI] [Google Scholar]
- 4.Hoque M., Mohiuddin R., Khan M., Hannan M., Alam M. Outbreak of Salmonella in Poultry of Bangladesh and possible remedy. J. Adv. Biotechnol. Exp. Ther. 2019;2:87. doi: 10.5455/jabet.2019.d30. [DOI] [Google Scholar]
- 5.Kumar H., Chen B.H., Kuca K., Nepovimova E., Kaushal A., Nagraik R., Bhatia S.K., Dhanjal D.S., Kumar V., Kumar A., et al. Understanding of colistin usage in food animals and available detection techniques: A review. Animals. 2020;10:1892. doi: 10.3390/ani10101892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies M., Walsh T.R. A colistin crisis in India. Lancet. Infect. Dis. 2018;18:256–257. doi: 10.1016/S1473-3099(18)30072-0. [DOI] [PubMed] [Google Scholar]
- 7.Al Masud A., Rousham E.K., Islam M.A., Alam M.U., Rahman M., Al Mamun A., Sarker S., Asaduzzaman M., Unicomb L. Drivers of Antibiotic Use in Poultry Production in Bangladesh: Dependencies and Dynamics of a Patron-Client Relationship. Front. Vet. Sci. 2020;7:1–9. doi: 10.3389/fvets.2020.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antunes P., Mourão J., Campos J., Peixe L. Salmonellosis: The role of poultry meat. Clin. Microbiol. Infect. 2016;22:110–121. doi: 10.1016/j.cmi.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Hoque R., Ahmed S.M., Naher N., Islam M.A., Rousham E.K., Islam B.Z., Hassan S. Tackling antimicrobial resistance in Bangladesh: A scoping review of policy and practice in human, animal and environment sectors. PLoS ONE. 2020;15:1–22. doi: 10.1371/journal.pone.0227947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mund M.D., Khan U.H., Tahir U., Mustafa B.E., Fayyaz A. Antimicrobial drug residues in poultry products and implications on public health: A review. Int. J. Food Prop. 2017;20:1433–1446. doi: 10.1080/10942912.2016.1212874. [DOI] [Google Scholar]
- 11.Kadry M., Nader S.M., Dorgham S.M., Kandil M.M. Molecular diversity of the invA gene obtained from human and egg samples. Vet. World. 2019;12:1033–1038. doi: 10.14202/vetworld.2019.1033-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma I. Detection of invA Gene in Isolated Salmonella from Marketed Poultry Meat by PCR Assay. J. Food Process. Technol. 2016;7:2. doi: 10.4172/2157-7110.1000564. [DOI] [Google Scholar]
- 13.Elkenany R., Elsayed M.M., Zakaria A.I., El-Sayed S.A.E.S., Rizk M.A. Antimicrobial resistance profiles and virulence genotyping of Salmonella enterica serovars recovered from broiler chickens and chicken carcasses in Egypt. BMC Vet. Res. 2019;15:1–9. doi: 10.1186/s12917-019-1867-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y.Y., Wang Y., Walsh T.R., Yi L.X., Zhang R., Spencer J., Doi Y., Tian G., Dong B., Huang X., et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016;16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y., Zhang R., Li J., Wu Z., Yin W., Schwarz S., Tyrrell J.M., Zheng Y., Wang S., Shen Z., et al. Comprehensive resistome analysis reveals the prevalence of NDM and MCR-1 in Chinese poultry production. Nat. Microbiol. 2017;2:16260. doi: 10.1038/nmicrobiol.2016.260. [DOI] [PubMed] [Google Scholar]
- 16.Amin M.B., Sraboni A.S., Hossain M.I., Roy S., Mozmader T.A.U., Unicomb L., Rousham E.K., Islam M.A. Occurrence and genetic characteristics of mcr-1-positive colistin-resistant E. coli from poultry environments in Bangladesh. J. Glob. Antimicrob. Resist. 2020;22:546–552. doi: 10.1016/j.jgar.2020.03.028. [DOI] [PubMed] [Google Scholar]
- 17.El Garch F., de Jong A., Bertrand X., Hocquet D., Sauget M. mcr-1-like detection in commensal Escherichia coli and Salmonella spp. from food-producing animals at slaughter in Europe. Vet. Microbiol. 2018;213:42–46. doi: 10.1016/j.vetmic.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Sobur A., Hasan M., Haque E., Mridul A.I., Noreddin A., El Zowalaty M.E., Rahman T. Molecular detection and antibiotyping of multidrug-resistant Salmonella isolated from houseflies in a fish market. Pathogens. 2019;8:191. doi: 10.3390/pathogens8040191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gad A.H., Abo-Shama U.H., Harclerode K.K., Fakhr M.K. Prevalence, serotyping, molecular typing, and antimicrobial resistance of Salmonella isolated from conventional and organic retail ground poultry. Front. Microbiol. 2018;9:1–10. doi: 10.3389/fmicb.2018.02653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bauer A.W., Kirby W.M., Sherris J.C., Turck M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 2018;45:493–496. doi: 10.1093/ajcp/45.4_ts.493. [DOI] [PubMed] [Google Scholar]
- 21.CLSI . Clinical and Laboratory Standards Institute: Performance Standards for Antimicrobial Susceptibility Testing Supplement M100S. 26th ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2016. [Google Scholar]
- 22.Satlin M.J., Weinstein M.P., Patel J., Romney M., Kahlmeter G., Giske C.G., Turnidge J. Clinical and Laboratory Standards Institute and European Committee on Antimicrobial Susceptibility Testing Position Statements on Polymyxin B and Colistin Clinical Breakpoints. Clin. Infect. Dis. 2020;71:e523–e529. doi: 10.1093/cid/ciaa121. [DOI] [PubMed] [Google Scholar]
- 23.Sweeney M.T., Lubbers B.V., Schwarz S., Watts J.L. Applying definitions for multidrug resistance, extensive drug resistance and pandrug resistance to clinically significant livestock and companion animal bacterial pathogens. J. Antimicrob. Chemother. 2018;73:1460–1463. doi: 10.1093/jac/dky043. [DOI] [PubMed] [Google Scholar]
- 24.Dashti A.A., Jadaon M.M., Abdulsamad A.M., Dashti H.M. Heat treatment of bacteria: A simple method of DNA extraction for molecular techniques. Kuwait Med. J. 2009;41:117–122. [Google Scholar]
- 25.Khan M.N.K., Das M.R., Sabur M.A., Rahman M.M., Uddin M.B., Cho H.S., Hossain M.M., Sciences B. Isolation, identification, molecular detection and sensitivity to antibiotics of Salmonella from cattle faeces. Bulg. J. Vet. Med. 2019 doi: 10.15547/bjvm.2019-0061. [DOI] [Google Scholar]
- 26.Notredame C., Higgins D.G., Heringa J. T-coffee: A novel method for fast and accurate multiple sequence alignment. Microb. Pathog. 2019;130:19–37. doi: 10.1006/jmbi.2000.4042. [DOI] [PubMed] [Google Scholar]
- 27.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larkin M.A., Blackshields G., Brown N.P., Chenna R., Mcgettigan P.A., McWilliam H., Valentin F., Wallace I.M., Wilm A., Lopez R., et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 29.Krogh A., Larsson B., Von Heijne G., Sonnhammer E.L. Predicting transmembrane protein topology with a hidden markov model: Application to complete genomes1. J. Mol. Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinform. 2008;9:1–8. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu D., Zhang Y. Improving the physical realism and structural accuracy of protein models by a two-step atomic-level energy minimization. Biophys. J. 2011;101:2525–2534. doi: 10.1016/j.bpj.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J., Liang Y., Zhang Y. Atomic-level protein structure refinement using fragment-guided molecular dynamics conformation sampling. Structure. 2011;19:1784–1795. doi: 10.1016/j.str.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lovell S.C., Davis I.W., Adrendall W.B., de Bakker P.I.W., Word J.M., Prisant M.G., Richardson J.S., Richardson D.C. Structure validation by Calpha geometry: Phi, psi and Cbeta deviation. Proteins. 2003;50:437–450. doi: 10.1002/prot.10286. [DOI] [PubMed] [Google Scholar]
- 34.Irwin J.J., Shoichet B.K. ZINC--a free database of commercially available compounds for virtual screening. J. Chem. Inf. Model. 2005;45:177–182. doi: 10.1021/ci049714+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deshpande N., Addess K.J., Bluhm W.F., Merino-Ott J.C., Townsend-Merino W., Zhang Q., Knezevich C., Xie L., Chen L., Feng Z., et al. The RCSB Protein Databa Bank: A redesigned query system and relational database based on the mmCIF schema. Nucleic Acids Res. 2005;33:233–237. doi: 10.1093/nar/gki057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dallakyan S., Olson A.J. Chemical Biology. Springer Nature Switzerland AG; Cham, Switzerland: 2014. Small-molecule library screening by docking with PyRx; pp. 243–250. [DOI] [PubMed] [Google Scholar]
- 37.Temml V., Kaserer T., Kutil Z., Landa P., Vanek T., Schuster D. Pharmacophore modeling for COX-1 and-2 inhibitors with LigandScout in comparison to Discovery Studio. Future Med. Chem. 2014;6:1869–1881. doi: 10.4155/fmc.14.114. [DOI] [PubMed] [Google Scholar]
- 38.Parvej M.S., Rahman M., Uddin M.F., Nazir K.N.H., Jowel M.S., Khan M.F.R., Rahman M.B. Isolation and Characterization of Salmonella enterica Serovar Typhimurium Circulating Among Healthy Chickens of Bangladesh. Turkish J. Agric. Food Sci. Technol. 2016;4:519. doi: 10.24925/turjaf.v4i7.519-523.695. [DOI] [Google Scholar]
- 39.Zhang L., Fu Y., Xiong Z., Ma Y., Wei Y., Qu X., Zhang H., Zhang J., Liao M. Highly prevalent multidrug-resistant Salmonella from chicken and pork meat at retail markets in Guangdong, China. Front. Microbiol. 2018;9:1–9. doi: 10.3389/fmicb.2018.02104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clifford K., Desai D., da Costa C.P., Meyer H., Klohe K., Winkler A., Rahman T., Islam T., Zaman M.H. Antimicrobial resistance in livestock and poor quality veterinary medicines. Bull. World Health Organ. 2018;96:662–664. doi: 10.2471/BLT.18.209585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wales A.D., Davies R.H. Co-selection of resistance to antibiotics, biocides and heavy metals, and its relevance to foodborne pathogens. Antibiotics. 2015;4:567–604. doi: 10.3390/antibiotics4040567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faruque M.O., Mahmud S., Munayem M.A., Sultana R., Molla M.T., Ali M.F., Wasim M., Sarker S., Evamoni F.Z. Bacteriological Analysis and Public Health Impact of Broiler Meat: A Study on Nalitabari Paurosova, Sherpur, Bangladesh. Adv. Microbiol. 2019;9:581–601. doi: 10.4236/aim.2019.97036. [DOI] [Google Scholar]
- 43.Mridha D., Uddin M.N., Alam B., Akhter A.H.M.T., Islam S.K.S., Islam M.S., Khan M.S.R., Kabir S.M.L. Identification and characterization of Salmonella spp. From samples of broiler farms in selected districts of Bangladesh. Vet. World. 2020;13:275–283. doi: 10.14202/vetworld.2020.275-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wajid M., Saleemi M.K., Sarwar Y., Ali A. Detection and characterization of multidrug-resistant Salmonella enterica serovar Infantis as an emerging threat in poultry farms of Faisalabad, Pakistan. J. Appl. Microbiol. 2019;127:248–261. doi: 10.1111/jam.14282. [DOI] [PubMed] [Google Scholar]
- 45.Akhi M.A., Das N.C., Banik A., Abony M., Juthi M., Uddin M.E. Detection of Drug-resistant S. aureus from Poultry Samples Collected from Different Areas of Bangladesh. Microbiol. Res. J. Int. 2019;29:1–10. doi: 10.9734/mrji/2019/v29i130154. [DOI] [Google Scholar]
- 46.Marshall B.M., Levy S.B. Food animals and antimicrobials: Impacts on human health. Clin. Microbiol. Rev. 2011;24:718–733. doi: 10.1128/CMR.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang R., Van Dorp L., Shaw L.P., Bradley P., Wang Q., Wang X., Jin L., Zhang Q., Liu Y., Rieux A., et al. The global distribution and spread of the mobilized colistin resistance gene mcr-1. Nat. Commun. 2018;9:1–9. doi: 10.1038/s41467-018-03205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schrauwen E.J.A., Huizinga P., van Spreuwel N., Verhulst C., Kluytmans-van den Bergh M.F.Q., Kluytmans J.A.J.W. High prevalence of the mcr-1 gene in retail chicken meat in the Netherlands in 2015. Antimicrob. Resist. Infect. Control. 2017;6:4–8. doi: 10.1186/s13756-017-0242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lima T., Domingues S., Da Silva G.J. Plasmid-mediated colistin resistance in Salmonella enterica: A review. Microorganisms. 2019;7:55. doi: 10.3390/microorganisms7020055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang X., Yu L., Chen X., Zhi C., Yao X., Liu Y., Wu S., Guo Z., Yi L., Zeng Z., et al. High prevalence of colistin resistance and mcr-1 gene in Escherichia coli isolated from food animals in China. Front. Microbiol. 2017;8:1–5. doi: 10.3389/fmicb.2017.00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skov R.L., Monnet D.L. Plasmid-mediated colistin resistance (mcr-1 gene): Three months later, the story unfolds. Eurosurveillance. 2016;21:1–6. doi: 10.2807/1560-7917.ES.2016.21.9.30155. [DOI] [PubMed] [Google Scholar]
- 52.Carnevali C., Morganti M., Scaltriti E., Bolzoni L., Pongolini S., Casadei G. Occurrence of mcr-1 in colistin-resistant Salmonella enterica isolates recovered from humans and animals in Italy, 2012 to 2015. Antimicrob. Agents Chemother. 2016;60:7532–7534. doi: 10.1128/AAC.01803-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borowiak M., Baumann B., Fischer J., Thomas K., Deneke C., Hammerl J.A., Szabo I., Malorny B. Development of a Novel mcr-6 to mcr-9 Multiplex PCR and Assessment of mcr-1 to mcr-9 Occurrence in Colistin-Resistant Salmonella enterica Isolates From Environment, Feed, Animals and Food (2011–2018) in Germany. Front. Microbiol. 2020;11:1–8. doi: 10.3389/fmicb.2020.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doumith M., Godbole G., Ashton P., Larkin L., Dallman T., Day M., Day M., Muller-Pebody B., Ellington M.J., de Pinna E., et al. Detection of the plasmid-mediated mcr-1 gene conferring colistin resistance in human and food isolates of Salmonella enterica and Escherichia coli in England and Wales. J. Antimicrob. Chemother. 2016;71:2300–2305. doi: 10.1093/jac/dkw093. [DOI] [PubMed] [Google Scholar]
- 55.Zając M., Sztromwasser P., Bortolaia V., Leekitcharoenphon P., Cavaco L.M., Ziȩtek-Barszcz A., Hendriksen R.S., Wasyl D. Occurrence and Characterization of mcr-1-Positive Escherichia coli Isolated From Food-Producing Animals in Poland, 2011–2016. Front. Microbiol. 2019;10:1753. doi: 10.3389/fmicb.2019.01753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahmed S., Das T., Islam M.Z., Herrero-Fresno A., Biswas P.K., Olsen J.E. High prevalence of mcr-1-encoded colistin resistance in commensal Escherichia coli from broiler chicken in Bangladesh. Sci. Rep. 2020;10:18637. doi: 10.1038/s41598-020-75608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ghafur A., Shankar C., GnanaSoundari P., Venkatesan M., Mani D., Thirunarayanan M.A., Veeraraghavan B. Detection of chromosomal and plasmid-mediated mechanisms of colistin resistance in Escherichia coli and Klebsiella pneumoniae from Indian food samples. J. Glob. Antimicrob. Resist. 2019;16:48–52. doi: 10.1016/j.jgar.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 58.Lv J., Mohsin M., Lei S., Srinivas S., Wiqar R.T., Lin J., Feng Y. Discovery of a mcr-1-bearing plasmid in commensal colistin-resistant Escherichia coli from healthy broilers in Faisalabad, Pakistan. Virulence. 2018;9:994–999. doi: 10.1080/21505594.2018.1462060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oh S.S., Song J., Kim J., Shin J. Increasing prevalence of multidrug-resistant mcr-1-positive Escherichia coli isolates from fresh vegetables and healthy food animals in South Korea. Int. J. Infect. Dis. 2020;92:53–55. doi: 10.1016/j.ijid.2019.12.025. [DOI] [PubMed] [Google Scholar]
- 60.Trung N.V., Matamoros S., Carrique-Mas J.J., Nghia N.H., Nhung N.T., Chieu T.T.B., Mai H.H., van Rooijen W., Campbell J., Wagenaar J.A., et al. Zoonotic transmission of mcr-1 colistin resistance gene from small-scale poultry farms, Vietnam. Emerg. Infect. Dis. 2017;23:529–532. doi: 10.3201/eid2303.161553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao R., Hu Y., Li Z., Sun J., Wang Q., Lin J., Ye H., Liu F., Srinivas S., Li D., et al. Dissemination and Mechanism for the MCR-1 Colistin Resistance. PLoS Pathog. 2016;12:1–19. doi: 10.1371/journal.ppat.1005957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu Y., Wei W., Lei S., Lin J., Srinivas S., Feng Y. An evolutionarily conserved mechanism for intrinsic and transferable polymyxin resistance. MBio. 2018;9:1–18. doi: 10.1128/mBio.02317-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated and analyzed in the present study were included in the manuscript or available as supplementary files.