Abstract
V-domain Ig suppressor of T-cell activation (VISTA), a newly discovered negative immune checkpoint, is thought to be related to immunotherapy resistance and may become a new immune therapeutic target. Here, we evaluated the expression of VISTA in a cohort containing 254 patients with untreated triple-negative breast cancer. The relevance of VISTA expression, clinicopathologic parameters, expression of other immune markers, and prognosis were investigated in the whole cohort. Genomic analysis of 139 triple-negative breast cancer (TNBC) patients from the cancer genome atlas (TCGA) was also performed. VISTA was expressed in the immune cells (ICs) and in the tumor cells (TCs) in 87.8% (223/254) and 18.5% (47/254) of the cohort, respectively. VISTA-positive ICs were associated with no lymph node metastasis (p < 0.001), American Joint Committee on Cancer (AJCC) stage I and II (p = 0.001) and basal-like subtype (p < 0.001). VISTA expression in ICs positively correlated with some tumor-infiltrating lymphocytes (TILs) types, particularly with the CD4+TILs, which was consistent with mRNA level analysis from the TCGA database. Survival analysis showed that patients with VISTA-positive ICs had prolonged relapse-free and overall survival compared with the negative ones, especially among T1-2N0 stage patients. Multivariate analysis showed that it independently predicted the prognosis. These data confirmed the regulatory role of VISTA in anti-tumor immunity, changed our perception of VISTA as a negative immune checkpoint, and suggested VISTA as a potential therapeutic target for TNBC.
Keywords: V-domain Ig suppressor of T-cell activation, triple-negative breast cancer, prognosis, tumor microenvironment, immune checkpoint
Introduction
Breast cancer (BC) is a major cause of death in women worldwide, with approximately 2.09 million new diagnoses and 626,679 BC-related deaths in 2018 (1). The incidence of BC among Chinese female from urban and rural areas has been increasing over the past 30 years (2, 3). According to the Chinese epidemiological investigation, 367,900 new BC cases and 97,972 BC-related deaths occurred in 2018 (4). Westernized lifestyles and changes in fertility patterns exacerbate BC development (5, 6), and the lack of early diagnostic tools and effective adjuvant therapies contribute to the higher mortality.
Triple-negative breast cancer (TNBC), defined as not expressing the estrogen receptor (ER), the progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), accounts for nearly one fifth of all BCs (7). It is often diagnosed at a young age, has more aggressive biological features and lacks effective targeting treatment, resulting in a poor prognosis (8, 9). Nearly 20% of TNBC patients carry BRCA gene mutations, especially BRCA1 (10). Cytotoxic chemotherapy is the main systemic treatment. However, patients with metastatic TNBC respond poorly to rescue chemotherapy and have shorter overall survival than other BC subtypes (11). Adverse effects, including pain, nausea, vomiting, and even cardiovascular dysfunction severely affect the treatment of patients (12). Compared with the luminal subtype, TNBC is more immunogenic because it has more tumor-infiltrating lymphocytes (TILs) and higher mutational burden (13).
Recently, numerous clinical trials were performed to assess the effectiveness of immune checkpoint inhibitors. Blocking immune checkpoints improved the treatment response of various cancers. This may become an effective long-term treatment strategy for previously intractable cancers. Last year, the FDA approved atezolizumab (anti-PD-L1 inhibitor) combined with protein-bound paclitaxel for treating locally progressive or metastatic TNBC (14). However, inhibiting T-lymophocyte-associated antigen 4 (CTLA-4), programmed cell death-1 (PD-1) or PD-1-ligand 1 (PD-L1) resulted in lower than 30% response rates (15). Thus, new targets or combination therapy is needed.
V-domain Ig suppressor of T cell activation (VISTA) is a negative checkpoint regulator and type I transmembrane protein that shares an extracellular domain homologous with PD-L1 (16, 17).VISTA is expressed in myeloid cells, inflammatory monocytes, dendritic cells(DC), and lymphocytes including CD4 and CD8 T cells (18, 19). In multiple mouse models, the high expression of VISTA in myeloid cells plays a significant role in anti-tumor immunity (20, 21). A series of in vitro tests suggested that VISTA inhibited CD4+T cell activation by activating as both a co-inhibitory ligand and a receptor (17, 22). It also regulated naïve T cell quiescence and peripheral tolerance (23). VISTA has also been shown to non-redundantly inhibit T cell activation from the PD-1/PD-L1 pathway in murine models (24). Recent studies have found increased VISTA level in prostate cancer and metastatic melanoma after anti-CTLA4 (25) and anti-PD-1 treatment (26). This implied the potential for acquired immunotherapy resistance. These also showed that VISTA may become a new target, or it may be used in combination with other immunotherapy for cancer treatments.
Studies on VISTA in BC have been limited, and the correlation between VISTA and TNBC has not been reported. This study aimed to analyze the expression of VISTA in the TNBC tumor microenvironment (TME) and its relevance in relation to clinicopathological characteristics, other immune markers and clinical outcomes.
Materials and Methods
Patients and Tissue Microarray
Formalin-fixed paraffin-embedded (FFPE) tumor specimens were collected from 254 stage I-III TNBC patients and fabricated into a tumor microarray (TMA). To construct the TMA, we used the core needle to select 1 mm diameter areas containing tumor epithelial cells and tumor stroma from each FFPE section after hematoxylin and eosin (HE)-staining. Patients with de novo stage IV or inflammatory TNBC as well as those who received neoadjuvant chemotherapy were excluded. Patients who had incomplete medical records or adequate tumor and stromal contents for TMA cores were excluded.
All selected patients underwent curative surgery at our hospital from January 2011 to December 2014, and they subsequently received standard adjuvant therapy. After that, they were followed up regularly (median: 68 months; range: 3–103 months).
This study conformed to the Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK criteria) (27) and the principles of the Declaration of Helsinki, approved by the committee on Human Research of Peking Union Medical College Hospital. The ethics committee on Human Research of Peking Union Medical College Hospital approved this study. Consent was obtained from all patients participating in the study.
Immunohistochemistry
The sections of TMA were stained with VISTA, CD3, CD4, CD8, and CD19 antibodies. The rabbit IgG monoclonal antibody (D1L2G; dilution1:200, Cell Signaling Technology, USA) was used to detect the expression of VISTA. Human placental and tonsil tissues obtained from our hospital were used as positive control samples. SignalStain®Antibody Diluent #8112 and SignalStain®Boost (HRP, Rabbit) #8114 were used as diluent and detection reagents, respectively. The details of primary antibodies and immunohistochemistry were described in Supplementary Table 1. All sections were stained using the Leica stainer (Leica Biosystems, Germany) complying with the instructions. Two pathologists (XYR and HWW) independently reviewed the immunohistochemical staining and scored for each sample. The consensus of the two observers was more than 90%. Less than 10% of the sections had inconsistent results which were resolved via the joint evaluation of the particular tumor area.
Assess of Immunostaining
The expression of VISTA was observed in the TME, including tumor cells (TCs) and immune cells (ICs). Tumor-infiltrated ICs were defined as the dendritic cells, myeloid cells, macrophages, and lymphocytes occupied the tumor. VISTA-positive TCs or ICs were those with membrane and/or cytoplasmic staining at any intensity. We dichotomized the VISTA expression in TCs and ICs into “negative” and “positive” groups, according to the median of raw proportion values. The median value of VISTA expression in ICs was 5%, which was consistent with the cutoff values recommended in previous studies (28, 29), while expression in tumor cells was 0%.
The percentages of CD3+TILs, CD4+TILs, CD8+TILs, and CD19+ TILs among the nucleated cells were also investigated as continuous values, and were dichotomized into “low” and “high” groups based on a median of 20, 10, 10, and 1%, respectively. TILs were evaluated according to recommendation of the International TILs Working Group 2014 (30) and were divided into “low” and “high” groups by median value of 5%.
The staining methods of BC related routine immune indexes were described previously (31, 32) and have been used during clinical practice in our hospital. Basal-like was defined as that ER, PR, and HER2 negative cancers that were positive for either EGFR or CK5/6.
Analysis of Public Datasets
mRNA Expression from TCGA Database
Date on the gene-level RNA-seq expression date and clinicopathological information of patients were collected from the Breast Invasive Carcinoma (TCGA, PanCancer Atlas) (http://www.cbioportal.org/) database in January 2020. A total of 139 female patients with ER- and PR-and HER2- referred as TNBC were enrolled. Inclusion criteria included: (1) female patients; (2) immunohistochemical staining (IHC) of ER was negative; (3) IHC of PR was negative; (4) IHC of HER2 and HER2 FISH test were negative; or IHC of HER2 was equivocal or not available but HER2 FISH test was negative; and (5) complete mRNA expression information. The total 139 samples with a next generation sequencing date were assessed for correlation with genes encoding VISTA(VSIR), CD3(CD3G), CD4(CD4), CD8(CD8A), and CD19(CD19). The date ID of the 139 TNBCs from the TCGA was shown in Supplementary Table 2.
Survival Analysis
Kaplan-Meier plotter website (http://kmplot.com/analysis) is a public database which evaluated the predictive effect of 54k genes on survival among 6,234 BC cases, followed up for an average of 69 months. It was used to analyze the relapse-free survival (RFS) and overall survival (OS) of basal-like BCs, based on the mRNA level of the VISTA coding gene C10orf54. Multiple genes incuding C10orf54 for VISTA and CD4 for CD4 were analyzed through the mean expression of proposed immune markers. The Affymetrix probe set IDs of VISTA and CD4 were 225372_at and 203547_at, respectively. The patients were analyzed for prognosis on the basis of gene expression.
Statistical Analysis
SPSS (version 19.0) was used for statistical analysis and GraphPad Prism (version 7.0) was used for plotting. Chi-square test was performed to assess the relevance of VISTA expression in the TME and clinicopathological parameters. The Spearman’s correlation test analyzed the association between the proportion of VISTA+ ICs, CD3+TILs, CD4+TILs, CD8+TILs, CD19+TILs, and total TILs. This was also done for VISTA+ TCs and TILs. Person’s correlation was employed to assess the mRNA expression of the above immune markers according to the TCGA database. The outcomes of this study were RFS and OS, which were plotted via the Kaplan-Meier method and compared using log-rank tests. The Cox regression models were performed to analyze prognostic factors and survival in TNBC. A two-sided p <0.05 was considered statistically significant. All the statistical methods corresponding to tables or figures were summarized in Supplementary Table 3.
Results
VISTA Expression and Clinicopathological Factors
A total of 254 patients were finally enrolled. The mean age was 49 ± 11 years (range 25–79 years). There were 145 (57.1%) patients younger than 50 years old, 120 (47.2%) patients had tumors ≤2 cm, and 120 (47.2%) patients had tumors between 2–5 cm. There were 108 (42.5%) patients with lymph node metastasis. Among them 53 (20.9%) patients had at least 4 involved nodes. According to the American Joint Committee on Cancer (AJCC), there were 116 (45.7%) and 55 (21.6%) patients with stage II and III, respectively.
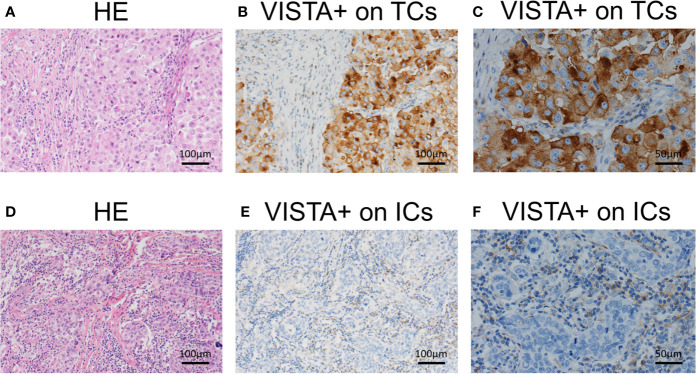
VISTA expression was greater in ICs than in TCs. In both cell types, VISTA expression was exclusively cytoplasmic (Figure 1). VISTA was expressed by ICs in 223 cases (87.8%) with 143 “positive” cases (56.3%) showing high expression. Only 47 cases (18.5%) had VISTA expression in TCs in total. Only 24 cases were both positive for TCs and ICs while 88 cases tested negative for both. 119 cases were positive for ICs only, while 23 cases tested positive for TCs only.
Figure 1.
Representative immunohistochemical staining of V-domain Ig suppressor of T-cell activation (VISTA) on tumor cells and immune cells in triple-negative breast cancer. (A) Hematoxylin and eosin (HE) staining of the tumor microenvironment. (B) VISTA expression on tumor cells (Original magnifications×100). (C) VISTA expression on tumor cells (Original magnifications×200). (D) HE staining of tumor microenvironment. (E) VISTA expression on immune cells (Original magnifications×100). (F) VISTA expression on immune cells (Original magnifications×200).
VISTA-positive ICs were strongly correlated with no lymph node metastasis (p < 0.001), AJCC stage I and II (p = 0.001), and basal-like subtype (p < 0.001). VISTA-positive ICs did not show correlation with age, Ki-67, differentiation, and VISTA expression in TCs (Table 1). No correlation was established between VISTA-positive TCs and all the clinicopathological features (Supplementary Table 4).
Table 1.
VISTA on immune cells and clinicopathological characteristics in TNBC.
| Parameters | VISTA on immune cells | ||||||
|---|---|---|---|---|---|---|---|
| Negative | Positive | p value | |||||
| N | % | N | % | N | % | ||
| Age | 0.501 | ||||||
| < 50 years | 145 | 57.1 | 66 | 59.5 | 79 | 55.2 | |
| ≥ 50 years | 109 | 42.9 | 45 | 40.5 | 64 | 44.8 | |
| Tumor stage | 0.907 | ||||||
| pT1 | 120 | 47.2 | 52 | 46.8 | 68 | 47.6 | |
| pT2 | 120 | 47.2 | 52 | 46.8 | 68 | 47.6 | |
| pT3 | 9 | 3.6 | 4 | 3.6 | 5 | 3.5 | |
| pT4 | 5 | 2 | 3 | 2.8 | 2 | 1.3 | |
| Lymph node | <0.001 | ||||||
| pN0 | 146 | 57.5 | 53 | 47.7 | 93 | 65 | |
| pN1 | 55 | 21.7 | 22 | 19.8 | 33 | 23.1 | |
| pN2 | 23 | 9.1 | 19 | 17.1 | 4 | 2.8 | |
| pN3 | 30 | 11.7 | 17 | 15.4 | 13 | 9.1 | |
| AJCC stage | 0.001 | ||||||
| I | 83 | 32.7 | 30 | 27 | 53 | 37.1 | |
| II | 116 | 45.7 | 45 | 40.5 | 71 | 49.7 | |
| III | 55 | 21.6 | 36 | 32.5 | 19 | 13.2 | |
| Ki 67 | 0.737 | ||||||
| < 14% | 39 | 15.4 | 18 | 16.2 | 21 | 14.7 | |
| ≥ 14% | 215 | 84.6 | 93 | 83.8 | 122 | 85.3 | |
| Differentiation | 0.08 | ||||||
| Well | 2 | 0.8 | 0 | 0 | 2 | 1.4 | |
| Moderate | 67 | 26.4 | 36 | 32.4 | 31 | 21.7 | |
| Poor | 185 | 72.8 | 75 | 67.6 | 110 | 76.9 | |
| Basal like | <0.001 | ||||||
| Yes | 223 | 87.8 | 86 | 77.5 | 137 | 95.8 | |
| No | 31 | 12.2 | 25 | 22.5 | 6 | 4.2 | |
| VISTA on TCs | 0.423 | ||||||
| Positive | 47 | 18.5 | 23 | 20.7 | 24 | 16.8 | |
| Negative | 207 | 81.5 | 88 | 79.3 | 119 | 83.2 | |
VISTA, V-domain Ig suppressor of T-cell activation; TNBC, triple-negative breast cancer; AJCC, American Joint Committee on Cancer; TC, tumor cell.
VISTA Expression and Other Immune Markers in the TME
CD3+TILs, CD4+TILs, CD8+TILs, and CD19+TILs were also detected in the whole TNBC cohort. Representative IHC staining density of these immune marker were shown in Supplementary Figure 1. A high density of CD3+TILs, CD4+TILs, CD8+TILs, and CD19+TILs were found in 50.8% (129/254), 59.1% (150/254), 52.4% (133/254), and 54.7% (139/254) of TNBC tissues, respectively. VISTA expression in ICs showed a strong correlation with total TILs (p < 0.001), CD3+TILs (p = 0.009), CD4+TILs (p < 0.001), CD8+TILs (p = 0.021), and CD19+TILs (p < 0.001) (Table 2). No connection was established between VISTA expression in TCs and the four types of TILs (Supplementary Table 5).
Table 2.
Correlation of VISTA on immune cells and other immune markers in TNBC.
| Markers | VISTA on immune cells | ||||||
|---|---|---|---|---|---|---|---|
| Negative | Positive | p value | |||||
| N | % | N | % | N | % | ||
| TILs | <0.001 | ||||||
| < 5% | 143 | 56.3 | 77 | 69.4 | 66 | 46.2 | |
| ≥ 5% | 111 | 43.7 | 34 | 30.6 | 77 | 53.8 | |
| CD3 | 0.009 | ||||||
| < 20% | 125 | 49.2 | 65 | 58.6 | 60 | 42 | |
| ≥ 20% | 129 | 50.8 | 46 | 41.4 | 83 | 58 | |
| CD4 | <0.001 | ||||||
| < 10% | 104 | 40.9 | 67 | 60.4 | 37 | 25.9 | |
| ≥ 10% | 150 | 59.1 | 44 | 39.6 | 106 | 74.1 | |
| CD8 | 0.021 | ||||||
| < 10% | 121 | 47.6 | 62 | 55.9 | 59 | 41.3 | |
| ≥ 10% | 133 | 52.4 | 49 | 44.1 | 84 | 58.7 | |
| CD19 | <0.001 | ||||||
| < 1% | 115 | 45.3 | 75 | 67.6 | 40 | 28 | |
| ≥ 1% | 139 | 54.7 | 36 | 32.4 | 103 | 72 | |
VISTA, V-domain Ig suppressor of T-cell activation; TNBC, triple-negative breast cancer; TIL, tumor-infiltrating lymphocyte.
In the further Spearman’s correlation analysis, the proportion of VISTA-positive ICs were moderately and positively correlated with the percentage of CD4+TILs (ρ = 0.423, p < 0.001) and CD19+TILs (ρ = 0.465, p < 0.001), respectively. They were weakly related to CD3+TILs (ρ = 0.222, p < 0.001), CD8+TILs (ρ = 0.214, p < 0.001), and total TILs (ρ = 0.266, p < 0.001). Moreover, the percentage of CD4+TILs was strongly and positively related to CD3+TILs (ρ = 0.670, p < 0.001) and CD8+TILs (ρ = 0.632, p < 0.001). CD19+TILs were significantly related to CD3+TILs (ρ = 0.474, p < 0.001), CD4+TILs (ρ = 0.549, p < 0.001) and CD8+TILs (ρ = 0.487, p < 0.001). CD8+TILs and CD3+TILs were strongly correlated (ρ = 0.918, p < 0.001) (Supplementary Table 6).
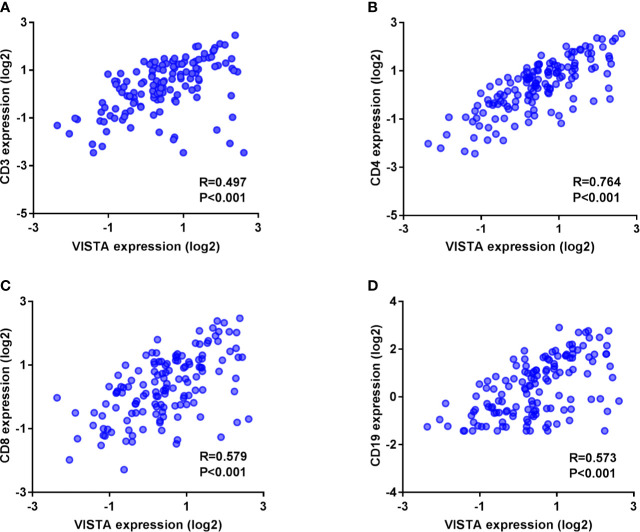
To further evaluate the relevance of VISTA expression and other immune markers in TNBC at the mRNA level, we assessed the correlation between the C10orf54 gene(encoding VISTA), CD3G(encoding CD3), CD4(encoding CD4), CD8A(encoding CD8), and CD19(encoding CD19) according to the mRNA expression of 139 TNBC patients downloaded from the TCGA database. The gene encoding VISTA was strongly and positively related to the gene encoding CD4 (R = 0.764, p < 0.001) (Figure 2B). It was also moderately related to the genes encoding CD8 (R = 0.579, p < 0.001) (Figure 2C), CD19 (R = 0.573, p < 0.001) (Figure 2D) and CD3 (R = 0.497, p < 0.001) (Figure 2A) (Supplementary Table 7).
Figure 2.
V-domain Ig suppressor of T cell activation (VISTA)-encoding co-expressed with genes that encode CD3 (A), CD4 (B), CD8 (C), and CD19 (D) in triple-negative breast cancer samples from The Cancer Genome Atlas public database.
VISTA Expression on ICs Reveals Long-Term Survival in TNBC Patients
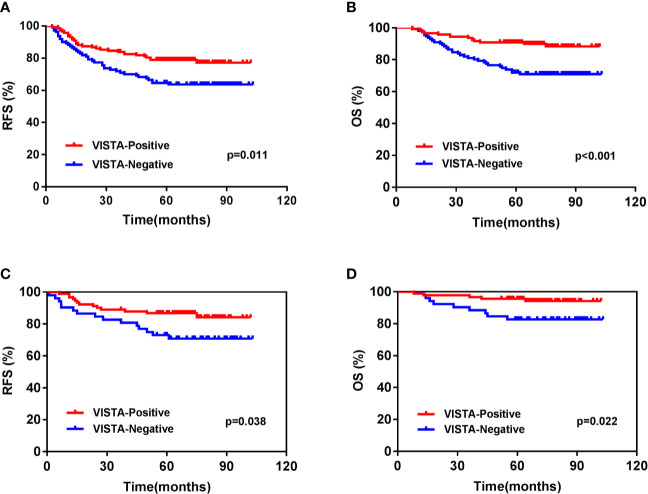
For the entire cohort, the 5-year RFS and OS rates were 72.7 and 82.6%, respectively. The VISTA-positive ICs group had a more favorable 5-years RFS (79.0 vs. 64.6%, p = 0.011) (Figure 3A) and OS (90.0 vs. 72%, p < 0.001) (Figure 3B) than the negative group (Supplementary Table 8). The correlation between mRNA level and survival was further analyzed. The Kaplan-Meier plots showed that basal-like BCs with high level of C10orf54 had a better RFS (HR = 0.64, p = 0.007) (Supplementary Figure 2A), but OS remained the same (HR = 0.95, p = 0.860) (Supplementary Figure 2B). Multivariate Cox regression analysis of the TNBC cohort showed that VISTA-positive ICs (p = 0.012) and AJCC stage (p = 0.001) independently predicted the OS, while age, Ki-67, and tumor differentiation did not (Table 3).
Figure 3.
Kaplan-Meier survival analysis of V-domain Ig suppressor of T cell activation (VISTA) expression on immune cells in triple-negative breast cancer patients. (A) Relapse-free survival (RFS) of the whole cohort. (B) Overall survival (OS) of the whole cohort. (C) RFS of T1-2N0 patients. (D) OS of T1-2N0 patients.
Table 3.
Multivariate analysis for patients with TNBC.
| Relapse-free survival | Overall survival | ||||
|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | ||
| VISTA on ICs | 0.158 | 0.012 | |||
| Negative | 1 | 1 | |||
| Positive | 0.698 (0.423–1.151) | 0.436 (0.228–0.831) | |||
| Age | 0.999 | 0.709 | |||
| < 50 years | 1 | 1 | |||
| ≥ 50 years | 1.000 (0.619–1.615) | 1.119 (0.620–2.019) | |||
| AJCC stage | <0.001 | 0.001 | |||
| I | 1 | 1 | |||
| II | 1.884 (0.959–3.703) | 0.066 | 2.181 (0.863–5.508) | 0.099 | |
| III | 4.182 (2.066–8.466) | <0.001 | 5.217 (2.068–13.164) | <0.001 | |
| Ki 67 | 0.2 | 0.366 | |||
| < 14% | 1 | 1 | |||
| ≥ 14% | 1.683 (0.760–3.727) | 1.549 (0.600–3.996) | |||
| Differentiation | 0.922 | 0.951 | |||
| Moderate+Well | 1 | 1 | |||
| Poor | 1.028 (0.594–1.777) | 1.021 (0.522–1.996) | |||
TNBC, triple-negative breast cancer; VISTA, V-domain Ig suppressor of T-cell activation; IC, immune cell; AJCC, American Joint Committee on Cancer; CI, confidence interval.
On further subgroup analysis, the predictive effect of VISTA expression in ICs was significant for OS in T1 (p = 0.032), T2 (p = 0.005), and N0 (p = 0.020) patients. This effect was also significant for RFS in T2 (p = 0.017) and N0 (p = 0.030) patients (Supplementary Table 8). We further analyzed the T1-2N0 subgroups. The results showed that the VISTA-positive ICs group had a longer 5-years RFS (86.8 vs. 73.1%, p = 0.038) (Figure 3C) and OS (95.6 vs. 82.7%, p = 0.022) (Figure 3D) than the negative groups. Multivariate analysis showed that VISTA-positive ICs was the only prognostic indicators for both RFS (p = 0.044) and OS (p = 0.024) (Table 4) in T1-2N0 TNBC patients.
Table 4.
Multivariate analysis for patients with T1-2N0 TNBC.
| Relapse-free survival | Overall survival | |||||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |||||
| VISTA on ICs | 0.044 | 0.024 | ||||||
| Negative | 1 | 1 | ||||||
| Positive | 0.462 (0.218–0.979) | 0.281 (0.093–0.848) | ||||||
| Age | 0.469 | 0.303 | ||||||
| < 50 years | 1 | 1 | ||||||
| ≥ 50 years | 1.328 (0.617–2.858) | 1.758 (0.601–5.147) | ||||||
| Tumor stage | 0.115 | 0.302 | ||||||
| T1 | 1 | 1 | ||||||
| T2 | 1.851 (0.861–3.982) | 1.764 (0.601–5.179) | ||||||
| Ki 67 | 0.409 | 0.966 | ||||||
| < 14% | 1 | 1 | ||||||
| ≥ 14% | 1.677 (0.492–5.717) | 1.034 (0.220–4.854) | ||||||
| Differentiation | 0.924 | 0.612 | ||||||
| Moderate+Well | 1 | 1 | ||||||
| Poor | 1.043 (0.436–5.717) | 1.399 (0.382–5.115) | ||||||
TNBC, triple-negative breast cancer; VISTA, V-domain Ig suppressor of T-cell activation; IC, immune cell; AJCC, American Joint Committee on Cancer; CI, confidence interval.
Classification of the TME According to VISTA+ ICs and CD4+TILs
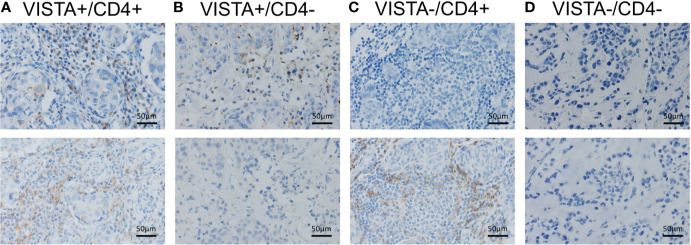
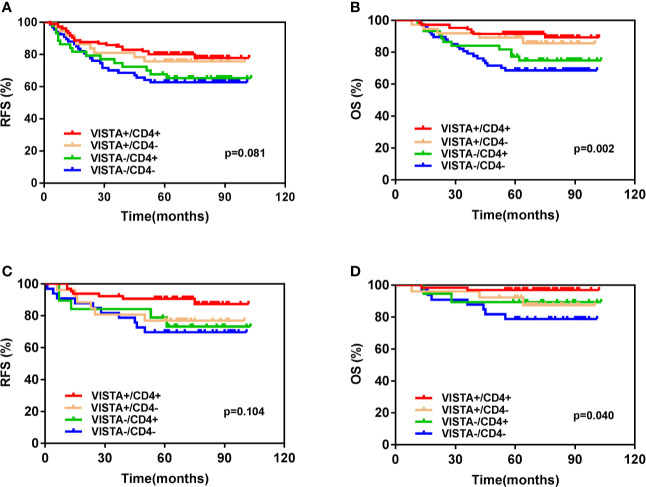
We classified the TNBC immune microenvironment into four subtypes based on the expression of VISTA in ICs and the density of CD4+ TILs: VISTA+/CD4+ (41.7%, 106/254), VISTA+/CD4- (14.6%, 37/254), VISTA-/CD4+ (17.3%, 44/254), and VISTA-/CD4- (26.4%, 67/254) (Figure 4). Kaplan–Meier survival analysis indicated that patients with both VISTA-positive ICs and high CD4+TILs density had a significantly increased 5-year OS rate than others (VISTA+/CD4+ vs. VISTA +/CD4- vs. VISTA-/CD4+ vs. VISTA-/CD4-: 91.5 vs. 89.2 vs. 77.3 vs. 68.7%, p = 0.002) (Figure 5B).A similar trend was observed in 5-years RFS without statistical significance (VISTA+/CD4+ vs. VISTA +/CD4- vs. VISTA-/CD4+ vs. VISTA-/CD4-: 80.1 vs. 75.7 vs. 67.7 vs. 62.7%, p = 0.081) (Figure 5A). In the T1-2N0 subgroups, similar trends and statistical differences were observed in RFS (p = 0.104) (Figure 5C) and OS (p = 0.040) (Figure 5D).
Figure 4.
Classification of the triple-negative breast cancer immune microenvironment according to the expression of V-domain Ig suppressor of T cell activation (VISTA) on immune cells and CD4+ tumor-infiltrating lymphocytes (TILs). (A) VISTA+/CD4+. (B) VISTA+/CD4-. (C) VISTA-/CD4+. (D) VISTA-/CD4-.
Figure 5.
Kaplan-Meier survival analysis based on different immune subtypes according to V-domain Ig suppressor of T cell activation (VISTA) and CD4 of (A) Relapse-free survival (RFS) of the whole cohort. (B) Overall survival (OS) of the whole cohort. (C) RFS of T1-2N0 patients. (D) OS of T1-2N0 patients.
We assessed the prognostic effect of the co-expression of mRNA encoding for VISTA and CD4 in basal-like BC patients via a multigene classifier and survival analysis. Patients with high levels of the co-expression of C10orf54 and CD4 had significantly prolonged RFS(HR = 0.36, p < 0.001) (Supplementary Figure 2C) and OS (HR = 0.49, p = 0.034) (Supplementary Figure 2D).
Discussion
VISTA is a new immune checkpoint that has been studied previously in cell lines, murine models (17), and human cancer cohorts, including breast cancer (29) and other malignances (19, 28, 33–39). However, there are still few reports about VISTA, with no large-scale studies in a TNBC cohort. In this research, we explored the distribution of VISTA in the TME, its correlation with other immune markers, and its prognostic value in a large-scale TNBC cohort. We found that VISTA was expressed in both ICs and TCs. High VISTA expression in ICs was significantly related to survival benefits in the whole TNBC cohort, especially for T1-2N0 patients. In the TME, VISTA expression in ICs was positively related to T lymphocyte markers (CD3, CD4, and CD8) and B lymphocyte marker (CD19). Patients with VISTA-positive ICs and high CD4+TILs density had significantly longer survival than those in other subgroups.
VISTA expression was greater in ICs than in TCs, with membrane and/or cytoplasmic staining. Previous experiments suggested that VISTA functioned as both a ligand on antigen-presenting cells (APCs) and a receptor on T cells (17). In vitro studies on cell lines and mouse models demonstrated that VISTA was principally expressed in leukocytes infiltrating the TME (18). Zong et al. (29) suggested that in BCs, the expression of VISTA in ICs was higher than that in TCs, but the overall positive rate was lower than that of this study. Studies on gastric cancer (34) and non–small cell lung cancer (NSCLC) (19) suggested that VISTA was expressed more in ICs than in TCs. Loeser et al (28). showed that VISTA was rarely present in esophageal adenocarcinoma (1.2%). However, in hepatocellular carcinoma (35) and ovarian cancer (39), the expression rates of VISTA in TCs and ICs were similar. The variable level of expression may be caused by the differences of immunogenicity among different human malignancies.
In the cross-table analysis, VISTA-positive ICs were associated with nodal negative patients (p < 0.001) and early AJCC stage (I/II) (p = 0.001). However, it was not correlated with age, Ki-67 and differentiation. This indicated that VISTA expressed in ICs affected local tumor progression in the TME. VISTA expression may also have been caused by the decrease in tumor immunogenicity to achieve immune evision and disease development according to the theory of cancer immunoediting. This mechanism leads to a decrease in tumor infiltrating ICs and VISTA expression.
Previous studies reported VISTA as a negative immune checkpoint, which potentially restrained T-cells proliferation and activation. However, the relevance of VISTA expression in the TME in survival remains controversial. In this study, TNBC patients with VISTA-positive ICs, especially the T1-2N0 stage patients and basal-like subgroup patients had a significantly favorable prognosis in terms of RFS and OS Recent studies on esophageal adenocarcinoma (28) and breast cancer (29) also confirmed that high levels of VISTA in ICs was related to a better prognosis. Research on NSCLC (19) showed that a high VISTA expression in the tumor area was related to prolonged survival, while in most cases, it was only expressed in the tumor stroma. Meanwhile, studies on hepatocellular carcinoma (35) and high-grade serous ovarian cancer (39) showed that VISTA expression in TCs was significantly related to a favorable survival. However, studies on gastric cancer (34) and oral squamous cell carcinoma (37) showed no relationship between VISTA expression and survival. A study on cutaneous melanoma (38) discovered a negative correlation between VISTA expression in ICs and prognosis. All these findings showed that the mechanism of VISTA expression in TCs and ICs behaved differently, and its relationship with prognosis varied across the different cancers.
A positive connection was also found between VISTA expression and TILs, especially the CD4+TILs. Similar results were obtained at mRNA level. Previous studies demonstrated that VISTA was constitutively expressed in naïve CD4+T cell as a co-inhibitory T cell receptor, limiting CD4+T cell activation and function (22, 40). A recent study also showed that VISTA played a critical role in regulating naïve T cell quiescence and peripheral tolerance. However, this was lost under inflammatory conditions (23). This may explain why the high expression of VISTA in ICs was closely related to CD4 +TILs in TNBC. This was consistent with a study on esophageal adenocarcinoma (28) which showed a strong co-expression between VISTA and CD4. However, it contradicts the study on hepatocellular carcinoma (35) which showed a strong association between VISTA and CD8+TILs rather than CD4+TILs.
Previous studies demonstrated that VISTA in both T cells and APCs contributed to the suppression of immunity.(17.22) However, in this study, we found that TNBC patients with both VISTA-positive ICs and high density of CD4+TILs had a significantly better prognosis than patients with only one or no increased index. The tumor associated inflammatory environment may have relieved naïve T cells suppression by VISTA. A ligand may have also bound to VISTA on the CD4+T cell and stimulated cell differentiation into type 1 T helper cells, which play an important role in anti-tumor immunity.
Recent studies showed that VISTA expression increased after the blockade of PD-1 in metastatic melanoma (26) and of CTLA-4 in prostate cancer (25),respectively. This indicated that VISTA may play a significant role in immunotherapy tolerance. Further studies exploring the potential relationship between VISTA and the PD-1 axis or other inhibitors are under way. There are also some clinical trials about VISTA. NCT02671955 is a phase 1 trial of JNJ-61610588 (a VISTA inhibitor) in human progressed cancers. NCT02812875 is another phase 1 clinical trial of CA-170 (inhibit PD-L1/PD-L2/VISTA) in human advanced solid tumors. We look forward to the results of these experiments, which will help understand the immune mechanism of VISTA in the TME and its synergistic anti-tumor effect with other immune checkpoints.
This research had limitations. First, it was a retrospective study with a limited sample size. Second, owing to intra-tumoral heterogeneity, TMA may not have been able to completely reflect the TME. Third, immunofluorescence may accurately show the co-expression of VISTA and lymphocyte markers (CD3, CD4, CD8, and CD19).
Conclusion
Our study revealed that VISTA expression was greater in ICs than in TCs in the TNBC cohort. It was also associated with prolonged RFS and OS, especially among T1-2N0 stage patients. Expression of VISTA in ICs was closely correlated with tumor-infiltrated ICs. We look forward to more studies on the immunoregulation mechanism of VISTA as this will provide more options for TNBC immunotherapy.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: Breast Invasive Carcinoma (TCGA, PanCancer Atlas) (https://www.cbioportal.org/study/summary?id=brca_tcga_pan_can_atlas_2018).
Ethics Statement
The studies involving human participants were reviewed and approved by Human Research of Peking Union Medical College Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
XC and QS conceived, designed, and supervised the whole study. XC performed experiments, collected and analyzed data; and wrote, edited, and reviewed the manuscript. XR and HW performed the experiments and assessed the immunostaining results. YZ, FM, and YL evaluated and analyzed database from the public website and the whole TNBC cohort. They also guided the statistical methods. All authors contributed to the article and approved the submitted version.
Funding
This study is supported by the Fundamental Research Funds for the Central Universities (3332020001), and the funders are not involved in this study and have no interest connection.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Thanks to all the patients who participated in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.583966/full#supplementary-material
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2018) 68(6):394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Chen WQ, Zheng RS, Zeng HM, Zhang SW, Zhao P, He J. [Trend analysis and projection of cancer incidence in China between 1989 and 2008]. Zhonghua zhong liu za zhi [Chinese J Oncol] (2012) 34(7):517–24. 10.3760/cma.j.issn.0253-3766.2012.07.010 [DOI] [PubMed] [Google Scholar]
- 3. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA: Cancer J Clin (2016) 66(2):115–32. 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 4. Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond Engl) (2019) 39(1):22. 10.1186/s40880-019-0368-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goss PE, Strasser-Weippl K, Lee-Bychkovsky BL, Fan L, Li J, Chavarri-Guerra Y, et al. Challenges to effective cancer control in China, India, and Russia. Lancet Oncol (2014) 15(5):489–538. 10.1016/s1470-2045(14)70029-4 [DOI] [PubMed] [Google Scholar]
- 6. Varghese C, Shin HR. Strengthening cancer control in China. Lancet Oncol (2014) 15(5):484–5. 10.1016/s1470-2045(14)70056-7 [DOI] [PubMed] [Google Scholar]
- 7. Schneider BP, Winer EP, Foulkes WD, Garber J, Perou CM, Richardson A, et al. Triple-negative breast cancer: risk factors to potential targets. Clin Cancer Res (2008) 14(24):8010–8. 10.1158/1078-0432.ccr-08-1208 [DOI] [PubMed] [Google Scholar]
- 8. Gierach GL, Burke A, Anderson WF. Epidemiology of triple negative breast cancers. Breast Dis (2010) 32(1-2):5–24. 10.3233/bd-2010-0319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gadi VK, Davidson NE. Practical Approach to Triple-Negative Breast Cancer. J Oncol Pract (2017) 13(5):293–300. 10.1200/jop.2017.022632 [DOI] [PubMed] [Google Scholar]
- 10. Gonzalez-Angulo AM, Timms KM, Liu S, Chen H, Litton JK, Potter J, et al. Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer. Clin Cancer Res (2011) 17(5):1082–9. 10.1158/1078-0432.ccr-10-2560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. den Brok WD, Speers CH, Gondara L, Baxter E, Tyldesley SK, Lohrisch CA. Survival with metastatic breast cancer based on initial presentation, de novo versus relapsed. Breast Cancer Res Treat (2017) 161(3):549–56. 10.1007/s10549-016-4080-9 [DOI] [PubMed] [Google Scholar]
- 12. Tao JJ, Visvanathan K, Wolff AC. Long term side effects of adjuvant chemotherapy in patients with early breast cancer. Breast (Edinburgh Scotland) (2015) 24 Suppl 2(0 2):S149–53. 10.1016/j.breast.2015.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cimino-Mathews A, Foote JB, Emens LA. Immune targeting in breast cancer. Oncol (Williston Park NY) (2015) 29(5):375–85. [PubMed] [Google Scholar]
- 14. Narayan P, Wahby S, Gao JJ, Amiri-Kordestani L, Ibrahim A, Bloomquist E, et al. FDA Approval Summary: Atezolizumab Plus Paclitaxel Protein-bound for the Treatment of Patients with Advanced or Metastatic TNBC Whose Tumors Express PD-L1. Clin Cancer Res (2020) 26(10):2284–9. 10.1158/1078-0432.ccr-19-3545 [DOI] [PubMed] [Google Scholar]
- 15. Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol (2015) 33(17):1974–82. 10.1200/jco.2014.59.4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ni L, Dong C. New B7 Family Checkpoints in Human Cancers. Mol Cancer Ther (2017) 16(7):1203–11. 10.1158/1535-7163.mct-16-0761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang L, Rubinstein R, Lines JL, Wasiuk A, Ahonen C, Guo Y, et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med (2011) 208(3):577–92. 10.1084/jem.20100619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lines JL, Pantazi E, Mak J, Sempere LF, Wang L, O’Connell S, et al. VISTA is an immune checkpoint molecule for human T cells. Cancer Res (2014) 74(7):1924–32. 10.1158/0008-5472.can-13-1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Villarroel-Espindola F, Yu X, Datar I, Mani N, Sanmamed M, Velcheti V, et al. Spatially Resolved and Quantitative Analysis of VISTA/PD-1H as a Novel Immunotherapy Target in Human Non-Small Cell Lung Cancer. Clin Cancer Res (2018) 24(7):1562–73. 10.1158/1078-0432.ccr-17-2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Le Mercier I, Chen W, Lines JL, Day M, Li J, Sergent P, et al. VISTA Regulates the Development of Protective Antitumor Immunity. Cancer Res (2014) 74(7):1933–44. 10.1158/0008-5472.can-13-1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu W, Dong J, Zheng Y, Zhou J, Yuan Y, Ta HM, et al. Immune-Checkpoint Protein VISTA Regulates Antitumor Immunity by Controlling Myeloid Cell-Mediated Inflammation and Immunosuppression. Cancer Immunol Res (2019) 7(9):1497–510. 10.1158/2326-6066.CIR-18-0489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Flies DB, Wang S, Xu H, Chen L. Cutting edge: A monoclonal antibody specific for the programmed death-1 homolog prevents graft-versus-host disease in mouse models. J Immunol (2011) 187(4):1537–41. 10.4049/jimmunol.1100660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. ElTanbouly MA, Zhao Y, Nowak E, Li J, Schaafsma E, Le Mercier I, et al. VISTA is a checkpoint regulator for naïve T cell quiescence and peripheral tolerance. Science (2020) 367(6475):eaay0524. 10.1126/science.aay0524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu J, Yuan Y, Chen W, Putra J, Suriawinata AA, Schenk AD, et al. Immune-checkpoint proteins VISTA and PD-1 nonredundantly regulate murine T-cell responses. Proc Natl Acad Sci U S A (2015) 112(21):6682–7. 10.1073/pnas.1420370112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gao J, Ward JF, Pettaway CA, Shi LZ, Subudhi SK, Vence LM, et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat Med (2017) 23(5):551–5. 10.1038/nm.4308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kakavand H, Jackett LA, Menzies AM, Gide TN, Carlino MS, Saw RPM, et al. Negative immune checkpoint regulation by VISTA: a mechanism of acquired resistance to anti-PD-1 therapy in metastatic melanoma patients. Modern Pathol (2017) 30(12):1666–76. 10.1038/modpathol.2017.89 [DOI] [PubMed] [Google Scholar]
- 27. Altman DG, McShane LM, Sauerbrei W, Taube SE. Reporting recommendations for tumor marker prognostic studies (REMARK): explanation and elaboration. BMC Med (2012) 10:51. 10.1186/1741-7015-10-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Loeser H, Kraemer M, Gebauer F, Bruns C, Schröder W, Zander T, et al. The expression of the immune checkpoint regulator VISTA correlates with improved overall survival in pT1/2 tumor stages in esophageal adenocarcinoma. Oncoimmunology (2019) 8(5):e1581546. 10.1080/2162402x.2019.1581546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zong L, Mo S, Yu S, Zhou Y, Zhang M, Chen J, et al. Expression of the immune checkpoint VISTA in breast cancer. Cancer Immunol Immunother CII (2020) 69(8):1437–46. 10.1007/s00262-020-02554-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol (2015) 26(2):259–71. 10.1093/annonc/mdu450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu Y, Wu S, Shi X, Luo Y, Pang J, Wang C, et al. HER2 double-equivocal breast cancer in Chinese patients: a high concordance of HER2 status between different blocks from the same tumor. Breast Cancer Res Treat (2019) 178(2):275–81. 10.1007/s10549-019-05387-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ren X, Yuan L, Shen S, Wu H, Lu J, Liang Z. c-Met and ERβ expression differences in basal-like and non-basal-like triple-negative breast cancer. Tumour Biol (2016) 37(8):11385–95. 10.1007/s13277-016-5010-5 [DOI] [PubMed] [Google Scholar]
- 33. Xie S, Huang J, Qiao Q, Zang W, Hong S, Tan H, et al. Expression of the inhibitory B7 family molecule VISTA in human colorectal carcinoma tumors. Cancer Immunol Immunother CII (2018) 67(11):1685–94. 10.1007/s00262-018-2227-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Böger C, Behrens HM, Krüger S, Röcken C. The novel negative checkpoint regulator VISTA is expressed in gastric carcinoma and associated with PD-L1/PD-1: A future perspective for a combined gastric cancer therapy? Oncoimmunology (2017) 6(4):e1293215. 10.1080/2162402x.2017.1293215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang M, Pang HJ, Zhao W, Li YF, Yan LX, Dong ZY, et al. VISTA expression associated with CD8 confers a favorable immune microenvironment and better overall survival in hepatocellular carcinoma. BMC Cancer (2018) 18(1):511. 10.1186/s12885-018-4435-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liao H, Zhu H, Liu S, Wang H. Expression of V-domain immunoglobulin suppressor of T cell activation is associated with the advanced stage and presence of lymph node metastasis in ovarian cancer. Oncol Lett (2018) 16(3):3465–72. 10.3892/ol.2018.9059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu L, Deng WW, Huang CF, Bu LL, Yu GT, Mao L, et al. Expression of VISTA correlated with immunosuppression and synergized with CD8 to predict survival in human oral squamous cell carcinoma. Cancer Immunol Immunother CII (2017) 66(5):627–36. 10.1007/s00262-017-1968-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kuklinski LF, Yan S, Li Z, Fisher JL, Cheng C, Noelle RJ, et al. VISTA expression on tumor-infiltrating inflammatory cells in primary cutaneous melanoma correlates with poor disease-specific survival. Cancer Immunol Immunother CII (2018) 67(7):1113–21. 10.1007/s00262-018-2169-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zong L, Zhou Y, Zhang M, Chen J, Xiang Y. VISTA expression is associated with a favorable prognosis in patients with high-grade serous ovarian cancer. Cancer Immunol Immunother CII (2020) 69(1):33–42. 10.1007/s00262-019-02434-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Flies DB, Han X, Higuchi T, Zheng L, Sun J, Ye JJ, et al. Coinhibitory receptor PD-1H preferentially suppresses CD4+T cell-mediated immunity. J Clin Invest (2014) 124(5):1966–75. 10.1172/JCI74589 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: Breast Invasive Carcinoma (TCGA, PanCancer Atlas) (https://www.cbioportal.org/study/summary?id=brca_tcga_pan_can_atlas_2018).