The activity of individual nuclear receptors is not only determined by specific actions in specific target cells, but also by their regulation in the tissue environment, as exemplified by the role of liver X receptors in regulating T cell development and effector functions.
Abstract
Nuclear receptors control the transcriptional program of target cells and thereby their phenotype and activities. Two complementary studies by Micheals et al. (https://doi.org/10.1084/jem.20201311) and Chan et al. (https://doi.org/10.1084/jem.20200318) published in JEM uncover the cell type–specific expression and role of the nuclear receptors liver X receptors in the regulation of T cell homeostasis and function.
As ligand-dependent transcription factors, nuclear receptors (NRs) critically regulate a variety of metabolic and cellular processes through the modulation of target gene expression. The NRs liver X receptors (LXRs) are sensors of oxysterols and sterol intermediates from the cholesterol biosynthetic pathway and exert metabolic control over lipid and cholesterol homeostasis (Peet et al., 1998; Yang et al., 2006). There are two isoforms with sequence homology: LXRα (NR1H3), expressed in metabolically active tissues and cells including the liver, intestine, adipose, and macrophages; and LXRβ (NR1H2), which is ubiquitously expressed (Peet et al., 1998; Janowski et al., 1999). In addition to their transcriptional integration of metabolism, both isoforms were reported to directly regulate immune responses and inflammation through modulation of pro-inflammatory genes in macrophages and T cells (Bensinger et al., 2008; Cui et al., 2011; Glass and Saijo, 2010). However, the exact role of LXR in T cell development, homeostasis, and effector function remained thus far unclear.

Insights from Truong San Phan and Thomas Brunner.
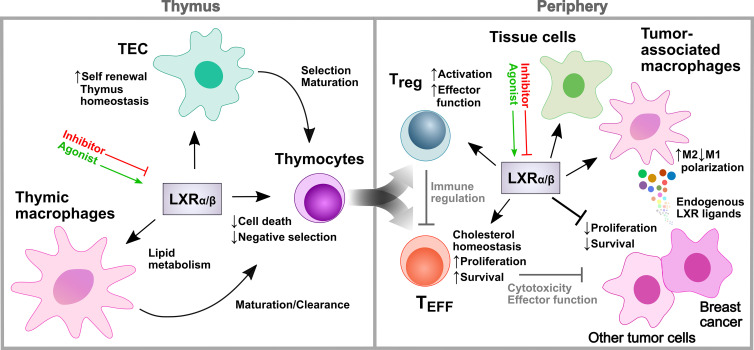
In this issue of JEM, both presented studies address this question using comprehensive genetic models. Chan et al. (2020) demonstrate in their study the cell type–specific role and relevance of LXRαβ for T cell development in different cell lineages within the thymus. While LXRαβ-deficient macrophages were shown to accumulate lipids, thymic epithelial cell (TEC)–specific deletion resulted in increased sensitivity to thymic involution due to reduced proliferation capacity, resulting in insufficient TEC self-renewal and recovery. T cell development, however, was critically impaired when LXRαβ was deleted in thymocytes, resulting in an enhanced Fas/Bim-mediated activation-induced cell death during negative selection and associated reduced sensitivity toward experimental autoimmune encephalomyelitis. With this study, the authors show the distinct and differential roles of LXRαβ in several thymic cell lineages to maintain T cell homeostasis in the thymus and the periphery. Complementarily, the study by Michaels et al. (2020) demonstrates that LXRβ not only regulates thymocyte development, but also effector functions of mature T cells. Specifically, they investigated T cell phenotypes using a CD4-Cre LXRβ knockout mouse model and observed, similar to the study by Chan et al. (2020), T cell lymphopenia, decreased proliferative capacity, and also spontaneous T cell activation. Using elegant experiments with bone marrow chimeric mice harboring mixed wild-type and LXR-deficient T cells, the authors found that LXRβ is cell-intrinsically required for T cell fitness and effector T cell (TEFF) development, and that spontaneous T cell activation may derive from deficient regulatory T (T reg) cell functions. In this regard, they demonstrated that CD4 T cell development was critically compromised in the absence of LXRβ. However, the most pronounced developmental defect was observed in the T reg cell subset. In line with these findings, the authors further show that loss of a single copy of the Nr1h2 gene in T reg cells was sufficient to cause early onset of fatal autoimmune inflammatory diseases. Furthermore, they determined that T reg cell activation requires a higher LXRβ expression compared with conventional CD4 T cells, and that LXRβ deficiency impairs T reg activity and effector functions. Together, both studies reveal multiple and complex roles of LXRs in the regulation of T cell development and function in various cell types.
Cell type–distinct functions of LXRs (LXRα/β) in the regulation of T cell development and function in the thymus and periphery. LXR in thymic macrophages and TECs regulates lipid homeostasis and thymus integrity, while thymocyte LXR controls negative selection and thymocyte development. TEFF-specific LXR is important for their survival and expansion, whereas LXR in T reg cells controls their immunoregulatory activity, and thus indirectly that of TEFF. Enhanced LXR signaling mediates anti-proliferative and tumor-suppressive effects, whereas some breast cancer tumors benefit from LXR signaling through increased polarization of tumor-associated macrophages to tumor-supporting M2 macrophages. LXR activity in various cells can be modulated using pharmacological inventions (agonists/inhibitors), but differ from physiological, cell-distinct LXR activities observed in studies using cell-specific genetic knockout models.
LXRs do not stand alone. Several other (orphan) NRs, such as peroxisome proliferator-activated receptor (PPAR) γ, retinoid acid receptor (RAR), RAR-related orphan receptor (ROR), retinoid X receptor (RXR), Ear2 (NR2F6), liver receptor homologue-1 (LRH-1), NURR1 (NR4A2), or glucocorticoid receptor (GR) have been extensively studied, revealing their critical and pleiotropic control over diverse branches of cellular processes regulating developmental, metabolic to homeostatic functions. Additionally, their ability to heterodimerize with other NRs further augments the versatility and complexity of their actions. Most of these NRs, specifically PPARγ, RXR, and LXR, are also known to exert regulatory and anti-inflammatory activities in epithelial, stromal, and immune cells through repression of pro-inflammatory genes, while a handful (RORα, RORγt, and NURR1) exclusively induce differentiation of pro-inflammatory TH17 cells (Glass and Saijo, 2010). Increasing evidence revealed a nonredundant role of such NRs in cancer and inflammatory disorders, but detailed understanding of their complex mechanisms is so far limited.
The emerging relevance of LXRs in metabolism and inflammation has led to extensive preclinical research and several clinical trials with LXR modulators (Wang and Tontonoz, 2018). However, to date, none of them showed satisfying outcomes, resulting in the termination of their investigation. Their clinical failure likely reflects the complexity and many faces of LXRs in the regulation of different cellular processes, which is still not fully understood, but observed for many other NRs, including the prominent GR. Yet, several attempts have shown that LXR modulation may hold the key to targeting inflammation-related diseases, as the capacity of LXR to suppress pro-inflammatory genes is well documented (Glass and Saijo, 2010). However, recent studies also substantiated its ligand-induced anti-proliferative and tumor-suppressive activity, as well as its capability to reduce myeloid-derived suppressor cells within the tumor microenvironment (Dhiman et al., 2018; Tavazoie et al., 2018). On the other hand, an inverse LXR agonist was shown to reduce tumor-associated myeloid-derived suppressor cells, as well as to enhance cytotoxic CD8 T cell–mediated anti-tumor responses in breast cancer through pharmacological LXR inactivation (Carpenter et al., 2019). Both studies show beneficial effects in cancer therapy based on opposite LXR mechanisms, indicating that LXR manipulation by ligand-dependent activation or inactivation depends on the varying preconditions or composition of different tumor microenvironments. Thus, cellular targets, as well as the overall context, likely strongly impact differential LXR expression and different LXR downstream effector functions.
These recent studies show that pharmacological inventions are facing the challenges of targeting specific cell types but still have to consider the diverse cell type–specific activities of LXR in a heterogenous cellular environment. Additionally, unresolved issues regarding LXR complex formation with other NRs, such as RXR, remain to be investigated in order to explore potential NR crosstalk mechanisms, mutual NR dependencies, and their impact on the target gene expression. Analysis of NR complex formation and downstream pathways may further reveal beneficial effects in clinical settings or even unwanted outcomes accountable for adverse side effects, as observed, for example, in long-term glucocorticoid therapy. Further in-depth understanding of LXR dynamics also necessitates a deeper characterization of endogenous LXR ligands, including their cellular sources and targets, and their local and global role in regulating physiological and pathological processes in conjunction with the underlying metabolic pathways.
The studies presented by Chan et al. (2020) and Michaels et al. (2020) provided detailed mechanistic and metabolic evidence for why LXR in T cells could be an interesting pharmacological target. Cell type–specific and context-dependent abrogation of LXRαβ, LXRβ, or even a single copy loss illustrated diverse LXR activities across several cell lineages, resulting in different and independent phenotypes. Similar findings had been attributed to other NRs, such as PPARγ, LRH-1, and NR2F6, which exert tissue protective functions during inflammatory disorders but also regulate pro-inflammatory T cell and macrophage effector functions (Klepsch et al., 2019; Seitz et al., 2019; Schwaderer et al., 2020). Together, the present studies indicate that cell type–specific differential LXR expression and function are likely directly linked to cell type–distinct responses toward LXR deletion or ligand-induced increased activity. These observations, as described by Chan et al. (2020) and together with the fact that different NRs are able to cooperate, point out that LXR-mediated actions have to be carefully interpreted when effects cannot be related to specific cellular events. This represents another crucial factor that needs to be considered for future pharmacological interventions and drug development. Despite this, clinical application of inverse agonists, for example, may only induce minor adverse side effects on tissue cells (e.g., hepatocytes with high expression of LXR), while lower expression in immune cells may indicate higher sensitivity toward pharmacological inactivation, possibly resulting in more beneficial anti-tumor therapies. Such scenarios might represent a window of opportunity, depending on the overall context and the targeted cells. With the fact that T reg cell activation and effector function are strongly dependent on LXR, as shown by Michaels et al. (2020), LXR in tumor-associated T reg cells may present a novel target, in addition to the anti-inflammatory role of LXR in tumor-associated macrophages.
Taken together, both studies not only provide advanced knowledge on the crucial role of LXR in the regulation of T cell development, homeostasis, and specific effector functions, but also offer a more differentiated point of view suggesting that only deep understanding of the cell-specific processes regulated by LXRαβ in health and disease will allow us to pharmacologically target this complex regulatory network in specific diseases for the net benefit of the patient.
References
- Bensinger, S.J., et al. 2008. Cell. 10.1016/j.cell.2008.04.052 [DOI] [Google Scholar]
- Carpenter, K.J., et al. 2019. Sci. Rep. 10.1038/s41598-019-56038-1 [DOI] [Google Scholar]
- Chan, C.T., et al. 2020. J. Exp. Med. 10.1084/jem.20200318 [DOI] [Google Scholar]
- Cui, G., et al. 2011. J. Clin. Invest. 10.1172/JCI42974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhiman, V.K., et al. 2018. Nat. Rev. Genet. 10.1038/nrg.2017.102 [DOI] [PubMed] [Google Scholar]
- Glass, C.K., and Saijo K.. 2010. Nat. Rev. Immunol. 10.1038/nri2748 [DOI] [PubMed] [Google Scholar]
- Janowski, B.A., et al. 1999. Proc. Natl. Acad. Sci. USA. 10.1073/pnas.96.1.266 [DOI] [Google Scholar]
- Klepsch, V., et al. 2019. Front. Immunol. 10.3389/fimmu.2019.01070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels, A., et al. 2020. J. Exp. Med. 10.1084/jem.20201311 [DOI] [Google Scholar]
- Peet, D.J., et al. 1998. Cell. 10.1016/S0092-8674(00)81432-4 [DOI] [Google Scholar]
- Schwaderer, J., et al. 2020. Cell Death Dis. 10.1038/s41419-020-2348-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz, C., et al. 2019. Sci. Adv. 10.1126/sciadv.aav9732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie, M.F., et al. 2018. Cell. 10.1016/j.cell.2017.12.026 [DOI] [Google Scholar]
- Wang, B., and Tontonoz P.. 2018. Nat. Rev. Endocrinol. 10.1038/s41574-018-0037-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, C., et al. 2006. J. Biol. Chem. 10.1074/jbc.M603781200 [DOI] [Google Scholar]