Abstract
Mycobacterium abscessus (Mab) is an emerging, nontuberculosis mycobacterium (NTM) that infects humans. Mab has two morphotypes, smooth (S) and rough (R), related to the production of glycopeptidolipid (GPL), that differ in pathogenesis. To further understand the pathogenicity of these morphotypes in vivo, the amphibian Xenopus laevis was used as an alternative animal model. Mab infections have been previously modeled in zebrafish embryos and mice, but Mab are cleared early from immunocompetent mice, preventing the study of chronic infection, and the zebrafish model cannot be used to model a pulmonary infection and T cell involvement. Here, we show that X. laevis tadpoles, which have lungs and T cells, can be used as a complementary model for persistent Mab infection and pathogenesis. Intraperitoneal (IP) inoculation of S and R Mab morphotypes disseminated to tadpole tissues including liver and lungs, persisting for up to 40 days without significant mortality. Furthermore, the R morphotype was more persistent, maintaining a higher bacterial load at 40 days postinoculation. In contrast, the intracardiac (IC) inoculation with S Mab induced significantly greater mortality than inoculation with the R Mab form. These data suggest that X. laevis tadpoles can serve as a useful comparative experimental organism to investigate pathogenesis and host resistance to M. abscessus.
Keywords: morphotype, dissemination, microbial persistence, Xenopus, mycobacteria, non-mammalian model, aquatic vertebrates, larval stage
1. Introduction
Mycobacterium abscessus (Mab) is a nontuberculosis mycobacterium (NTM) commonly found in water and soil [1,2,3]. This emerging pathogen can infect multiple human tissues including skin, lungs, and other vital organs [2,4]. Mab has become a particular threat to humans with chronic lung diseases such as bronchiectasis and cystic fibrosis as well as immunocompromised patients [5,6,7]. In fact, Mab is the second most prevalent cause of NTM pulmonary infections in the United States, causing 2.6–13.0% of all mycobacterial pulmonary infections [8]. However, Mab can also cause cutaneous infections in healthy individuals [2,5]. Treatment of infections caused by Mab is challenging due to its multiantibiotic resistance and the ability of this pathogen to evade the host’s immune system, aiding in its persistence [5]. In addition, determining the mechanisms by which Mab causes disease can be used to better understand the pathogenicity of Mycobacterium tuberculosis (Mtb). Both pathogens share multiple physiological features [9]. As such, Mab can be used to better understand the virulence mechanisms that Mtb presents since Mab has a lower Biohazard Safety Level and grows faster than Mtb in simple media [10].
Beyond the plasma membrane, all mycobacteria have a complex mycolyl–arabinogalactan–peptidoglycan cell wall with a second lipid bilayer, the mycomembrane, distal to the wall [11]. Mab has two morphotypes, smooth (S) and rough (R), which are due to the presence of glycopeptidolipid (GPL), a structure found on the outer leaflet of the mycomembrane [12]. The R morphotype has low, or lacks, GPL production, resulting in the formation of dry colonies in vitro on agar plates, while the S morphotype has high production of GPL, resulting in the formation of circular, moist colonies [13]. It has been shown that the R morphotype derives from a S precursor [14]. Multiple genes are responsible for the assembly and transport of GPL across the plasma membrane and cell wall; therefore, a deletion mutation in any one of these genes could hinder or completely arrest the production of GPL. A large fraction of R variant clinical isolates is due to single nucleotide deletions or insertions within two genes: mmpl4 or mps1 [12]. These two genes, located within the GPL locus, play essential roles for GPL synthesis but are not essential for the organism’s survival [15].
Epidemiological surveys have revealed that R Mab is more prominent in patients with severe pulmonary infections [1,3,12]. The S morphotype, which is commonly isolated from nature, may be acquired by susceptible individuals through hospital contamination, drinking water, or soil [12]. However, once an infection with S Mab is acquired, a S to R switch may occur. A spontaneous S to R switch has a frequency of approximately 1 in 105 to 1 in 106 [13]. The preferential selection of the R morphotype has been observed in many clinical isolates with and without cystic fibrosis [16].
GPL impacts many aspects of pathogenicity by changing sliding mobility, bacteria interaction, and biofilm formation [12,17]. High GPL production is associated with increased sliding mobility and biofilm formation as well as tolerance to acidic pH [12,17]. In contrast, low GPL production promotes aggregation and cording [12,13,17]. Cords are irregular filaments that form ridges and extend outward [13]. It is noteworthy that cords produced by R Mab are morphologically similar to those produced by Mtb [13]. S Mab lack the ability to form cords because GPL blocks the interaction of trehalose dimycolate (TDM) molecules, which are involved in the cord formation mechanism [13,17]. The rough morphotype’s cording ability and aggregation properties may increase its pathogenesis in the host environment, leading to a more persistent and invasive infection.
The pathogenicity of R and S morphotypes remains poorly understood. In fact, studies on the host–pathogen interaction remain fragmented and inconsistent. The differential expression of GPL appears to affect interactions with host cells, including phagocytosis and immune reaction. Although the R morphotype is considered hypervirulent, inducing tumor necrosis factor (TNF) production and phagocytosis through toll-like receptor 2 (TLR2) signaling, the immune response of S Mab is not clearly characterized [18]. If GPL is highly immunogenic, inflammation and innate immune cell recruitment caused by GPL may promote the dissemination of the pathogen [12]. During the early stages of infection, GPL continues to induce the production of cytokines and chemokines, promoting the recruitment of macrophages and additional neutrophils [12]. Neutrophils are only able to kill approximately 25% of the Mab by phagocytosis and neutrophil extracellular traps (NETs) [19]. This may select for the survival of R, GPL-deficient, variants and mark the transition from S to R morphotypes during Mab infection, which would drive colonization toward persistence and invasion of host tissues (19). Furthermore, it has been reported that the presence of GPL could mask ligands that bind to macrophage surface receptors, preventing phagocytosis [20]. In contrast, toll-like receptor 2 (TLR-2) has been shown to bind phosphatidyl-myo-inositol mannoside (PIMs), a ligand on the outer surface of R Mab, to signal phagocytosis [21]. Mab is an intracellular pathogen; therefore, macrophage engulfment may be beneficial for replication. In addition, R Mab may be more equipped to survive phagocytosis due to its ability to aggregate and form cords. Aggregation upon phagocytosis leads to the formation of phagocytic cups resulting in phagocyte cell death [22].
For in vivo studies, Mab infections have been modeled using zebrafish embryos and mice [12]. Studies in mice have been hampered by a lack of persistent infection with Mab, as immunocompetent mice clear the pathogen within the first couple of weeks postinfection [12]. Moreover, TNFR−/−, Cybb−/−, and Nos2−/− mice were all able to completely clear Mab in the lungs, spleen, and liver 40 days after intravenous injection of 1 × 106 CFUs [23]. Even though these mice lacked an intact innate immune system, the infection triggered an innate response one day postinfection, resulting in early clearance [23]. Mab clearance was less efficient in MyD88−/− and GKO−/− mice, with 3.5 log10 CFUs Mab at 40 days postinfection [23].
The attractiveness of the zebrafish model includes a convenient visualization of host–pathogen interactions because their embryos are transparent [12]. However, there are some limitations of this model, including the lack of lungs, which are necessary to model pulmonary infections, and the lack of functional adaptive immunity during the early stages of development [12]. Infection of zebrafish embryos with the R morphotype resulted in 65% mortality by 13 days postinfection, whereas less than 10% of the S-infected embryos died by 13 days postinfection [22]. There was no difference in the phagocytosis of the S and R morphotypes [9]. Engulfment did initially limit the pathogen’s growth, but ultimately enhanced the spread of the microbe to tissues beyond the site of infection. The innate mechanisms of macrophages only protected the host from infection with the S morphotype [9]. The R morphotype was more pathogenic because, at the site of infection, the bacteria were immediately engulfed by macrophages. Additional macrophages were recruited to the infection site and caused granuloma formation [9]. Phagocytized bacteria then migrated via the circulatory system to other tissues. Intracellular growth and cord formation triggered macrophage apoptosis, which released the bacteria [9]. Further cord formation prevented repeated phagocytosis, resulting in replication of the bacteria and tissue damage [9]. Altogether, these studies in murine and zebrafish experimental organisms show numerous inconsistencies indicating that the host–pathogen interactions in Mab are complex and would benefit from the development of complementary animal models.
X. laevis tadpoles have lungs that become functional for breathing between four and five days postfertilization, which permits the study of pulmonary infections [24]. Furthermore, like zebrafish embryos, X. laevis tadpoles are transparent, allowing visualization of pathogen dissemination, while their immune system is more similar to that of humans, including the adaptive arm. Previous research has concluded that the mammalian and Xenopus adaptive and innate immune systems are evolutionarily conserved based on extensive similarity [25]. We have previously developed a Mycobacterium marinum infection model with X. laevis and hypothesized that this model could also be used to study Mab pathogenesis [26].
The purpose of this study was to evaluate whether the amphibian X. laevis tadpole could provide a reliable model relevant to humans for studying Mab infection by comparing the survival of the host and the survival and proliferation of S and R Mab. Since R and S Mab present different structural surface ligands, we postulate that different immune responses to these morphotypes should occur resulting in distinct survival rates in infected tadpoles. Our results provide new in vivo evidence of differences in the pathogenesis of S and R Mab variants and establish X. laevis tadpoles as a useful new comparative experimental organism to examine host interaction with Mab.
2. Results
2.1. Clearance of Mab in Mice
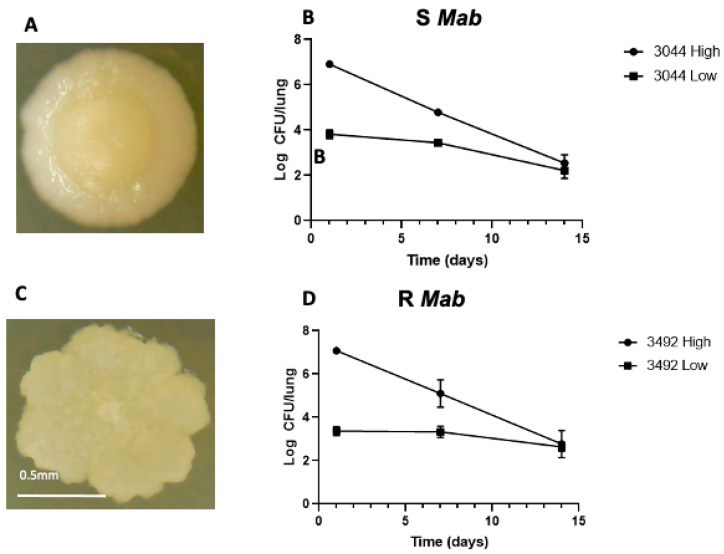
We confirmed that our S and R Mab strains are cleared in the mouse model as expected. After intranasal challenge of C57BL/6 mice with high (107 CFUs) and low (104 CFUs) doses, each morphotype was cleared, with a faster decrease seen in the high dose experiments (Figure 1). Unlike zebrafish, where increasing the dose of the rough morphotype led to increased mortality [22], high (107 CFUs) and low (104 CFUs) doses of S and R Mab resulted in a reduction in concentration in the lungs to less than 103 CFUs 14 days postinfection.
Figure 1.
Colony morphology and bacterial abundance in C57BL/6 mice 1, 7, and 14 dpi. (A) Picture of a smooth (S) Mab colony cultured 5 days at 37 °C on agar plates showing a circular shape and a moist appearance; (C) Rough (R) Mab colony with rugged edges and dry appearance. Mice were infected with high (107 CFUs) and low (104 CFUs) of (B) S (3044) and (D) R (3492) Mab by intranasal infection. The right lung was collected from four mice per timepoint, and lysates were plated on agarose media. Average bacterial load decreased from 1 to 14 days postinfection (dpi) for each morphotype and dose.
2.2. Dissemination and Persistence of S and R Mab in Intraperitoneally Infected X. laevis Tadpoles
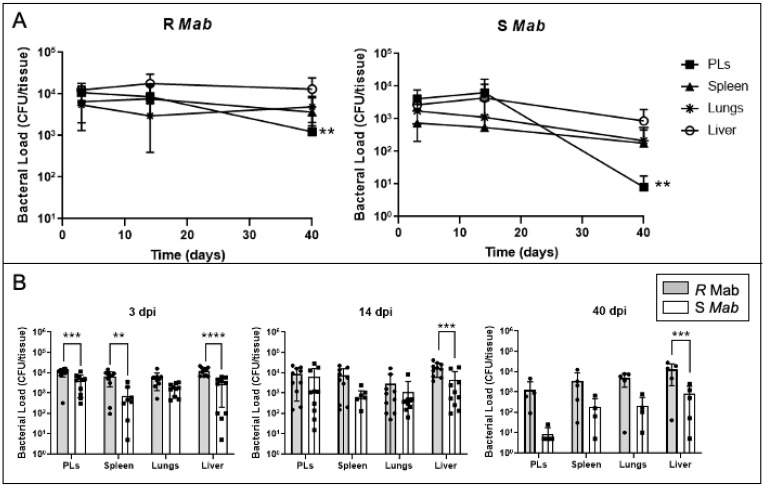
To determine whether S and R Mab strains can infect X. laevis tadpoles, we followed the same intraperitoneal (IP) inoculation procedure that we previously used with M. marinum [26]. Following the injection of 5 × 105 CFUs of Mab, we used bacterial plate counts to determine the abundance of S and R Mab in lysates of different X. laevis tissues 3 days postinfection (dpi), 14 dpi, and 40 dpi (Figure 2A). We observed wide dissemination and persistence of both S and R Mab since significant CFU were recovered from all the tissues tested until 40 dpi. On average, 103 and 104 R Mab CFU/tissue were recovered at each time point. More detailed comparisons between S and R Mab (Figure 2B), indicates that tadpoles infected with S Mab presented fewer CFU in lysates across tissues and time points, between 101 and 103 CFU/tissue, with declining bacterial abundance from 3 to 40 dpi. Notably, significantly less S than R Mab were recovered from the liver at the three time points, and very low CFU counts were obtained for peritoneal leucocytes (PLs) at 40 dpi. In conclusion, a relatively modest dose of either S and R Mab inoculum can infect, disseminate, and persist in tadpoles, while R Mab can persist at higher loads than S Mab within multiple organs.
Figure 2.
Bacterial recovery from tadpoles 3, 14, and 40 days after intraperitoneal (IP) inoculation of either R or S Mab. (A) Timeline and (B) two-by-two comparison. Tadpoles were IP-injected with 5 × 105 CFU in a 10 μL volume. Spleen, liver, peritoneal leukocytes (PLs), and lungs were collected, suspended in 500 µL of aPBS, lysed by bead beating, and plated on agar media. The bacterial abundance was determined by the number of CFUs/tissue. **p < 0.005, ***p < 0.0005, and ****p < 0.0001 (two-way ANOVA followed by post hoc analysis using Tukey’s multiple comparisons test).
2.3. Survival Analysis of Tadpole X. laevis Inoculated with S or R Mab
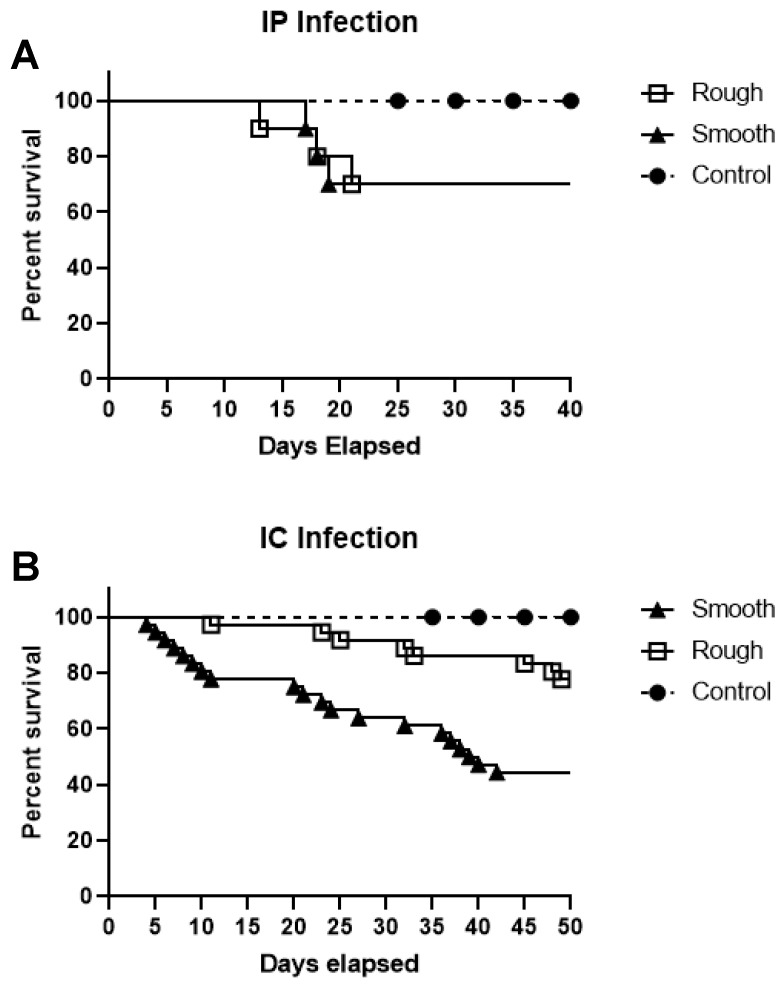
To examine tadpole host resistance to S and R Mab, survival assays were performed using 5 × 105 CFU of Mab by IP inoculation. Over the course of 40 days, only modest mortality (30%) was associated with S and R Mab infection (Figure 3A), and survivorship was not affected by the Mab morphotype (p = 0.4983).
Figure 3.
Tadpole survival following IP (A) or IC (B) inoculation with either R or S Mab. (A) Twenty tadpoles, ten in each group, were inoculated with 5 × 105 CFU of R or S Mab by IP infection and monitored for 40 days. (B) Seventy-two tadpoles were inoculated with 5 × 105 CFU of R or S Mab by IC infection and monitored for 50 days for survival. There was a significant difference in the survival of S and R IC inoculated tadpoles (p < 0.005) based on the log-rank (Mantel–Cox) test.
The apparent tadpole resistance to S and R Mab infection, despite the wide dissemination and persistence of the pathogens, prompted us to determine whether tadpole survival would be affected by another route of inoculation. To be close to an intravenous inoculation resulting in a rapidly spreading infection in the whole organism, we decided to use intracardiac (IC) injection, which is well tolerated in tadpoles. Notably, tadpoles were found to be more susceptible to S Mab infection following IC inoculation compared to IP inoculation of 5 × 105 CFUs of bacteria (Figure 3B). Indeed, tadpoles infected with S Mab started to die at 4 dpi, whereas all control tadpoles injected with aPBS survived the duration of the 50-day study. The median survival of tadpoles infected with S Mab was 39.5 days. In contrast, only 1 tadpole infected with R Mab died at 8 dpi, and less than 10 died over the 50-day period, which suggests that tadpoles are more resistant or tolerant to R Mab.
2.4. Dissemination and Persistence of S and R Mab in IC-Infected X. laevis Tadpoles
Since the intracardiac route of Mab inoculation induced higher mortality in tadpoles infected with the S morphotype, we tested whether this was associated with higher bacterial loads and/or differences in dissemination. We assayed bacterial burden at 3 dpi and 14 dpi and expanded the bacterial recovery to include gills, kidney, and brain, in addition to the liver, spleen, PLs, and lungs (Figure 4A). Overall, CFU recovered at 3 and 14 dpi were comparable between S and R morphotypes, with the exception of the gills that exhibited significantly lower S Mab load at 3 dpi. We also noted an increase in bacterial loads for both morphotypes in the liver for the two time points. When comparing CFUs between 3 and 14 dpi, there was a consistent expansion (1 log on average) of R Mab in most tissues, whereas S Mab CFUs remained stable (Figure 4B).
Figure 4.
Bacterial recovery from tadpoles 3 and 14 days after intracardiac (IC) inoculation of either R or S Mab. (A) Comparison of R and S Mab CFUs for different tissues at 3 and 14 dpi. (B) CPU comparison between 3 and 14 dpi for R and S Mab. Tadpoles were IC-injected with 5 × 105 CFU in a 10 μL volume. Spleen, liver, PLs, and lungs were collected, suspended in 500 µL of aPBS, lysed by bead beating, and plated on agar media. The bacterial abundance was determined by the number of CFUs/tissue. * p < 0.05, ** p < 0.005, and **** p < 0.0001 (two-way ANOVA followed by post hoc analysis using Tukey’s multiple comparisons test).
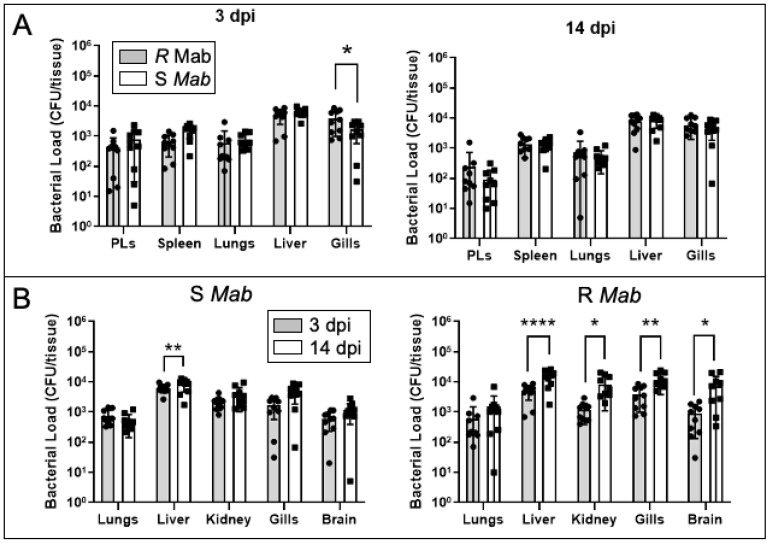
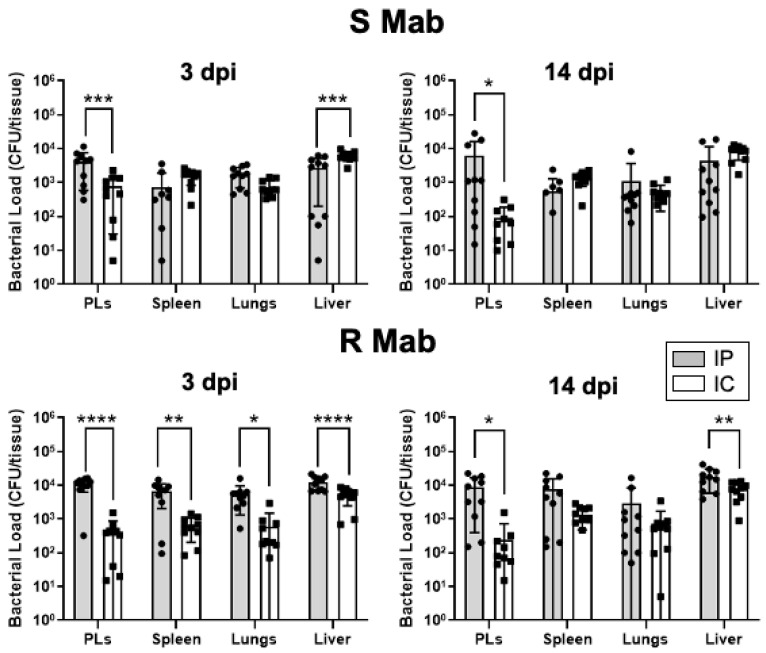
Because of the difference in tadpole survival, we also compared the bacterial loads recovered following IP versus IC inoculation (Figure 5). Early establishment and dissemination of R Mab at 3 dpi by the IP route were significantly higher than the IC route, and this difference persisted at 14 dpi. In contrast, except for PLs where lower S Mab loads were detected at 3 and 14 dpi, comparable dissemination was detected in other organs.
Figure 5.
Comparison of bacterial recovery between IP and IC inoculation at 3 and 14 dpi for S and R Mab. * p < 0.05, ** p < 0.005, *** p < 0.0005, and **** p < 0.0001 (two-way ANOVA followed by post hoc analysis using Tukey’s multiple comparisons test).
3. Discussion
The goal of this study was to evaluate the potential of X. laevis tadpole to serve as a useful comparative experimental organism alternative to mice and zebrafish to investigate Mab pathogenesis and host–pathogen interactions. Our data show that both S and R Mab morphotypes can readily infect tadpoles, disseminate widely in tadpole tissues, and persist up to 50 days. Compared to the mouse infection model, which showed marked and rapid decreases in bacterial abundance from the time of infection within 14 dpi, X. laevis tadpoles did not clear the pathogen as rapidly, indicating it could serve as a reliable animal model to study chronic mycobacterial infections. This provides a convenient time period for future investigation.
In addition, the dissemination, expansion, and persistence of Mab, especially when inoculated by intraperitoneal injection, did not induce sizable mortality. It is noteworthy that the recovery of bacteria in culture is indicative of an active persisting infection rather than typical latency as with Mtb [27]. This is reminiscent of our previous study with M. marinum, where we demonstrated that tadpoles show resistance to M. marinum by promoting an active tolerance to the pathogen [26]. This system will now permit us to investigate the host immune response to determine whether it is characterized by the induction of anti-inflammatory cytokine (e.g., IL-10) as for M. marinum and whether distinct responses are elicited against R and S Mab.
It is noteworthy that IP inoculation did affect the survival of the S morphotype in vivo in tadpoles, especially in PLs. Infection of the peritoneal cavity may have induced a more robust innate immune response, resulting in phagocytosis of this morphotype, whereas aggregation of the R morphotype may have allowed it to escape engulfment. Alternatively, R Mab may have a better capacity to resist host cell microbicidal activity. In fact, the rapid dissemination of R Mab with IP injection in comparison to S Mab suggests that R Mab is more equipped to persist within phagocytes, which may trigger their rapid dissemination through the organism.
Another notable finding is the unanticipated higher tadpole susceptibility to S Mab when inoculated by IC injection. Unlike the zebrafish survival assays, in which mortality is higher in R Mab infected larvae, IC injection of the S morphotype was more lethal than IP injection of S Mab [22]. Relatively low doses of S Mab (5 × 105 CFUs) were used for the IP and IC injection in tadpoles. Our results suggest that S Mab induces an acute infection, resulting in early fatality due to a robust immune response. The R morphotype appears to affect the host during later stages of the infection, supporting its characterization as a persistent pathogen.
In humans, Mab can persist in tissues for years without showing symptoms but can reactivate into a systemic infection [16]. After two to three months, X. laevis tadpoles undergo metamorphosis, which is accompanied by a drastic remodeling of their immune system [28]. It will be interesting to examine the outcome of persisting S and R Mab during this developmental period. Notably, metamorphosis marks the transition between the tadpole adaptive immune system dominated by innate T cells and tolerance to mycobacteria pathogens to an adult adaptive immune system driven by conventional T cells and proinflammatory antimycobacterial host responses [26]. Furthermore, during the metamorphosis transition, T cells are no longer being generated in the thymus and T cell function is suppressed [29]. This suppression of the adaptive immune response, resembling immunocompromised conditions in human patients, may allow the R morphotype to flourish and cause tissue damage. Evaluating the pathogenicity of R and S Mab in X. laevis tadpoles during metamorphosis, between stages 60 and 66 [30], could provide a unique model to examine Mab disease progression in vivo in naturally immunodeficient X. laevis hosts. It will also be interesting to determine whether Mab infection at the tadpole stage will result in tolerance or pathogenesis after metamorphosis.
4. Materials and Methods
4.1. Animal Husbandry
Outbred X. laevis tadpoles were obtained from our X. laevis Research Resources for Immunology at the University of Rochester, New York, USA, (http://www.urmc.rochester.edu/smd/mbi/xenopus/index.htm). All animals during the experiment were carefully handled under the University of Rochester Committee on Animal Resources regulations (approval number 100577/2003-151).
Six-week-old female C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, MN). The mice were maintained in the Syracuse Veterans Affairs Medical Center Veterinary Medical Unit in an animal Biosafety Level 2 facility. Mice were housed in micro-isolator cages with a maximum of 8 mice per cage. There was a one-week acclimation period before mice were manipulated in any way. The mice ingested sterile water and Prolab® RMH 3000 rodent chow (PMI Nutrition International, Brentwood, MO, USA.) and libitum throughout the course of the study. The animal protocols, ACORP# 021, were approved by the Veterans Affairs Medical Center’s IACUC (Subcommittee for Animal Studies (SAS), Syracuse, NY, USA).
4.2. Mycobacterial Strains and Culture Conditions:
M. abscessus S strain PM3044, a smooth colony clone of the type strain ATCC19977, and the R strain PM3492, a ∆mbtH mutant derived from PM3044 via suicide-vector mediated allelic exchange, were used for all comparisons in mouse and X. laevis infections [31]. The R phenotype of PM3492 can be complemented to the wild-type S phenotype by the mbtH+ gene in trans [31]. Mab cultures were grown at 37 °C for 5 days in Middlebrook 7H9 broth supplemented with 0.2% glycerol, 10% albumin–dextrose–saline (ADS) supplement, and 0.05% Tween-80. The cultures were then centrifuged, washed with amphibian phosphate buffer (aPBS) with 0.05% of Tween 80, resuspended in 50% aPBS and 50% glycerol, and frozen at −80 °C. After freezing, the number of viable colony-forming units (CFU/mL) was determined. The working concentration of all bacterial samples had to be at or above 107 CFU/mL to be used for tadpole infection. R Mab samples were gently sonicated prior to plating and infection to disperse clumps.
To avoid contamination by X. laevis microbiota, Middlebrook 7H10 media supplemented with 0.5% glycerol, 10% ADS, 200 μg/mL ampicillin, and 40 μg/mL polymyxin B was used for determination of bacterial burden. Growth of Mab on 7H10 media was not affected by the antibiotics.
4.3. Mab Mouse Infection Model
Mab S strain PM3044 and R strain PM3492 were grown in 7H10 broth with 10% oleic acid–albumin–dextrose–catalase (OADC) supplement and 0.05% Tween 80 on a rotary shaker at 37 °C for 5–7 days. On the day of infection, the organism was diluted to 100 Klett units (corresponding to 5 × 108 CFU/mL) and 0.1 Klett units (5 × 105 CFU/mL). The actual CFU count was determined by diluting the culture to 5 × 102 CFU/mL and plating in triplicate on Cation-adjusted Mueller Hinton agar. The agar plates were incubated in ambient air at 37 ℃ for 5–7 days.
C57BL/6 mice were anesthetized by intramuscular delivery of a telazol (45 mg/kg)/xylazine (7.5 mg/kg) cocktail (Lederle Parenterals, Carolina, Puerto Rico and Bayer Corp., Shawnee Mission, Kansas, respectively) and infected intranasally with approximately 1 × 107 and 1 × 104 viable Mab S (PM3044) or R Mab (PM3492) in a 20 μL volume.
The actual inoculum of PM3044 was 2.4 × 106 CFU (high) and 2.4 × 103 CFU (low). The inoculum for PM3492 was 2.2 × 106 CFU (high) and 2.2 × 103 CFU (low). At 1, 7, and 14 days postinfection, 4 mice from each group were euthanized by CO2 asphyxiation, and their right lung was removed aseptically and homogenized in saline with Tween-80. The homogenates were serially diluted and plated on Mueller Hinton agar to determine CFUs/lung. Four mice were used for each timepoint. Agar plates were incubated in ambient air at 37 °C for 7–14 days.
4.4. Mab Inoculation in Tadpoles
Three-week old tadpoles (development stage 52) [30,32], were infected either by intraperitoneal (IP) injection of R or S Mab (5 × 105 CFU in 10 μL volume) or by intracardiac (IC) injection (5 × 105 CFU in 5 μL volume). Controls were injected with sterile aPBS. For survival analysis following Mab IP inoculation, 20 tadpoles were monitored daily for 40 days. For IC inoculation, 72 tadpoles were monitored for 50 days. Remaining survivors were euthanized, and organs were collected to determine bacterial loads.
4.5. Colony-Forming Assay
Peritoneal leukocytes (PLs), liver, spleen, and lungs were collected and lysed by bead beating in 500 μL aPBS. Undiluted and 10× diluted lysates (100 μL out of 500 μL total lysate) were plated on an antibiotic medium. Colony counts were obtained 5 days after incubating the samples at 37 °C. Organ homogenates not used for plating were saved for DNA isolation at −80 °C.
4.6. Statistical Analysis
Statistical significance of survival data was determined by a log-rank (Mantel–Cox) test using the GraphPad Prism 8 software (GraphPad Software, Inc., La Jolla, CA, USA). Two-way ANOVA statistical analysis and multiple comparisons analysis followed by using Tukey’s multiple comparisons test was performed to the compare CFU/tissue of R and S Mab extracted from organ lysates. A value of p > 0.05 was considered significant.
5. Conclusions
Our data show that while Mab was rapidly cleared in C57BL/6 mice, both S and R Mab morphotypes inoculated by intraperitoneal injection in X. laevis tadpoles widely disseminated in tissues, including liver and lungs, and persisted in active form for up to 40 days without causing significant mortality. Furthermore, the R morphotype persisted at a higher bacterial load. In contrast, intravenous-like intracardiac inoculation with S Mab induced significant mortality compared to that with R Mab. These data provide evidence that tadpoles of the amphibian X. laevis, which have lungs and functional T cells, can serve as a useful experimental organism complementary to the murine model for persistent Mab infection and pathogenesis.
Acknowledgments
We would like to thank Tina Martin for the expert animal husbandry; Francisco DeJesus, Zanah Khadijah Francis, David Barnard, Rhoo and Baglia for technical assistance.
Author Contributions
Conceptualization, A.L., C.S., M.S.P.J. and J.R.; software, A.L., C.S.; validation, A.L., C.S., M.S.P.J., M.C., D.D., M.P. and J.R.; formal analysis, A.L., C.S., M.C., D.D., M.P., M.S.P.J. and J.R.; investigation, A.L., C.S., M.C., D.D., M.P., M.S.P.J. and J.R.; resources, M.C., M.S.P.J. and J.R.; data curation, A.L., C.S., D.D., and M.P.; writing—original draft preparation, A.L., M.S.P.J. and J.R.; writing—review and editing, A.L., C.S., M.C., D.D., M.P., M.S.P.J. and J.R.; visualization, A.L.; supervision, M.C., M.S.P.J. and J.R.; project administration, M.S.P.J. and J.R.; funding acquisition, M.S.P.J. and J.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by R21AI139718, R24AI059830 and R21AI115527 from the National Institute of Allergy and Infectious Diseases (NIH/NIAID) and IOS-1456213 from the National Science Foundation.
Institutional Review Board Statement
All animals during the experiment were carefully handled under the University of Rochester Committee on Animal Resources regulations (approval number 100577/2003-151).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Thomson R., Tolson C., Carter R., Coulter C., Huygens F., Hargreaves M. Isolation of Nontuberculous Mycobacteria (NTM) from Household Water and Shower Aerosols in Patients with Pulmonary Disease Caused by NTM. J. Clin. Microbiol. 2013;51:3006–3011. doi: 10.1128/JCM.00899-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howard S.T., Byrd T.F. The rapidly growing mycobacteria: Saprophytes and parasites. Microbes Infect. 2000;2:1845–1853. doi: 10.1016/S1286-4579(00)01338-1. [DOI] [PubMed] [Google Scholar]
- 3.Brown-Elliott B.A., Wallace R.J. Clinical and Taxonomic Status of Pathogenic Nonpigmented or Late-Pigmenting Rapidly Growing Mycobacteria. Clin. Microbiol. Rev. 2002;15:716–746. doi: 10.1128/CMR.15.4.716-746.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nessar R., Cambau E., Reyrat J.M., Murray A., Gicquel B. Mycobacterium abscessus: A new antibiotic nightmare. J. Antimicrob. Chemother. 2012;67:810–818. doi: 10.1093/jac/dkr578. [DOI] [PubMed] [Google Scholar]
- 5.Catherinot E., Clarissou J., Etienne G., Ripoll F., Emile J.F., Daffe M., Perronne C., Soudais C., Gaillard J.L., Rottman M. Hypervirulence of a rough variant of the Mycobacte-rium abscessus type strain. Infect. Immun. 2007;75:1055–1058. doi: 10.1128/IAI.00835-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piersimoni C., Scarparo C. Pulmonary infections associated with non-tuberculous mycobacteria in immunocompetent pa-tients. Lancet Infect. Dis. 2008;8:323–334. doi: 10.1016/S1473-3099(08)70100-2. [DOI] [PubMed] [Google Scholar]
- 7.Esther C.R., Jr., Esserman D.A., Gilligan P., Kerr A., Noone P.G. Chronic Mycobacterium abscessus infection and lung function decline in cystic fibrosis. J. Cyst. Fibros. 2010;9:117–123. doi: 10.1016/j.jcf.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee M.-R., Sheng W.-H., Hung C.-C., Yu C.-J., Lee L.-N., Hsueh P.-R. Mycobacterium abscessusComplex Infections in Humans. Emerg. Infect. Dis. 2015;21:1638–1646. doi: 10.3201/2109.141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernut A., Herrmann J.-L., Ordway D., Kremer L. The diverse cellular and animal models to decipher the physiopathological traits of Mycobacterium abscessus infection. Front. Cell. Infect. Microbiol. 2017;7:100. doi: 10.3389/fcimb.2017.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medjahed H., Gaillard J.-L., Reyrat J.-M. Mycobacterium abscessus: A new player in the mycobacterial field. Trends Microbiol. 2010;18:117–123. doi: 10.1016/j.tim.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Alderwick L., Harrison J., Lloyd G.S., Birch H.L. The Mycobacterial Cell Wall—Peptidoglycan and Arabinogalactan. Cold Spring Harb. Perspect. Med. 2015;5:a021113. doi: 10.1101/cshperspect.a021113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gutiérrez A.V., Viljoen A., Ghigo E., Herrmann J.-L., Kremer L. Glycopeptidolipids, a Double-Edged Sword of the Mycobacterium abscessus Complex. Front. Microbiol. 2018;9:1145. doi: 10.3389/fmicb.2018.01145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howard S.T., Rhoades E., Recht J., Pang X., Alsup A., Kolter R., Lyons C.R., Byrd T.F. Spontaneous reversion of Mycobacterium abscessus from a smooth to a rough morphotype is associated with reduced expression of glycopeptidolipid and reacquisition of an invasive phenotype. Microbiology. 2006;152:1581–1590. doi: 10.1099/mic.0.28625-0. [DOI] [PubMed] [Google Scholar]
- 14.Byrd T.F., Lyons C.R. Preliminary Characterization of aMycobacterium abscessus Mutant in Human and Murine Models of Infection. Infect. Immun. 1999;67:4700–4707. doi: 10.1128/IAI.67.9.4700-4707.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nessar R., Reyrat J.-M., Davidson L.B., Byrd T.F. Deletion of the mmpL4b gene in the Mycobacterium abscessus glycopep-tidolipid biosynthetic pathway results in loss of surface colonization capability, but enhanced ability to replicate in human macrophages and stimulate their innate immune response. Microbiology. 2011;157:1187–1195. doi: 10.1099/mic.0.046557-0. [DOI] [PubMed] [Google Scholar]
- 16.Park I.K., Hsu A.P., Tettelin H., Shallom S.J., Drake S.K., Ding L., Wu U.-I., Adamo N., Prevots D.R., Olivier K.N., et al. Clonal Diversification and Changes in Lipid Traits and Colony Morphology in Mycobacterium abscessus Clinical Isolates. J. Clin. Microbiol. 2015;53:3438–3447. doi: 10.1128/JCM.02015-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Recht J., Kolter R. Glycopeptidolipid Acetylation Affects Sliding Motility and Biofilm Formation in Mycobacterium smegmatis. J. Bacteriol. 2001;183:5718–5724. doi: 10.1128/jb.183.19.5718-5724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sampaio E.P., Elloumi H.Z., Zelazny A., Ding L., Paulson M.L., Sher A., Bafica A.L., Shea Y.R., Holland S.M. Mycobacterium abscessus and M. avium trigger Toll-like receptor 2 and distinct cytokine response in human cells. Am. J. Respir. Cell Mol. Biol. 2008;39:431–439. doi: 10.1165/rcmb.2007-0413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malcolm K.C., Nichols E.M., Caceres S.M., Kret J.E., Martiniano S.L., Sagel S.D., Chan E.D., Caverly L., Solomon G.M., Reynolds P., et al. Mycobacterium abscessus Induces a Limited Pattern of Neutrophil Activation That Promotes Pathogen Survival. PLoS ONE. 2013;8:e57402. doi: 10.1371/journal.pone.0057402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan E.D., Bai X., Kartalija M., Orme I.M., Ordway D. Host Immune Response to Rapidly Growing Mycobacteria, an Emerging Cause of Chronic Lung Disease. Am. J. Respir. Cell Mol. Biol. 2010;43:387–393. doi: 10.1165/rcmb.2009-0276TR. [DOI] [PubMed] [Google Scholar]
- 21.Ryan K., Byrd T.F. Mycobacterium abscessus: Shapeshifter of the Mycobacterial World. Front. Microbiol. 2018;9:2642. doi: 10.3389/fmicb.2018.02642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernut A., Herrmann J.L., Kissa K., Dubremetz J.F., Gaillard J.L., Lutfalla G., Kremer L. Mycobacterium abscessus cording prevents phagocytosis and promotes abscess formation. Proc. Natl. Acad. Sci. USA. 2014;111:E943–E952. doi: 10.1073/pnas.1321390111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obregón-Henao A., Arnett K.A., Henao-Tamayo M., Massoudi L., Creissen E., Andries K., Lenaerts A.J., Ordway D. Susceptibility of Mycobacterium abscessus to Antimycobacterial Drugs in Preclinical Models. Antimicrob. Agents Chemother. 2015;59:6904–6912. doi: 10.1128/AAC.00459-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rose C.S., James B. Plasticity of lung development in the amphibian, Xenopus laevis. Biol. Open. 2013;2:1324–1335. doi: 10.1242/bio.20133772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robert J., Ohta Y. Comparative and developmental study of the immune system inXenopus. Dev. Dyn. 2009;238:1249–1270. doi: 10.1002/dvdy.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhoo K.H., Edholm E.S., Forzán M.J., Khan A., Waddle A.W., Pavelka M.S., Robert J. Distinct Host–Mycobacterial Pathogen Interac-tions between Resistant Adult and Tolerant Tadpole Life Stages of Xenopus laevis. J. Immunol. 2019;203:2679–2688. doi: 10.4049/jimmunol.1900459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manabe Y.C., Bishai W.R. Latent Mycobacterium tuberculosis–persistence, patience and winning by waiting. Nat. Med. 2000;6:1327–1329. doi: 10.1038/82139. [DOI] [PubMed] [Google Scholar]
- 28.Du Pasquier L., Schwager J., Flajnik M.F. The Immune System of Xenopus. Annu. Rev. Immunol. 1989;7:251–275. doi: 10.1146/annurev.iy.07.040189.001343. [DOI] [PubMed] [Google Scholar]
- 29.Rollins-Smith L.A., Blair P.J., Davis A.T. Thymus Ontogeny in Frogs: T-Cell Renewal at Metamorphosis. Dev. Immunol. 1992;2:207–213. doi: 10.1155/1992/26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nieuwkoop P., Faber J. Normal Table of Xenopus Laevis (Daudin) Garland Publishing; New York, NY, USA: 1994. p. 252. [Google Scholar]
- 31.Gregoire S.A., Byam J., Pavelka M.S., Jr. Galk-based suicide vector mediated allelic exchange in Mycobacterium abscessus. Microbiology. 2017;163:1399. doi: 10.1099/mic.0.000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zahn N., Levin M., Adams D.S. The Zahn drawings: New illustrations of Xenopus embryo and tadpole stages for studies of craniofacial development. Development. 2017;144:2708–2713. doi: 10.1242/dev.151308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.