Abstract
A single dose of psilocybin, a psychedelic and serotonin 2A receptor (5-HT2AR) agonist, may be associated with antidepressant effects. The mechanism behind its antidepressive action is unknown but could be linked to increased synaptogenesis and down-regulation of cerebral 5-HT2AR. Here, we investigate if a single psychedelic dose of psilocybin changes synaptic vesicle protein 2A (SV2A) and 5-HT2AR density in the pig brain. Twenty-four awake pigs received either 0.08 mg/kg psilocybin or saline intravenously. Twelve pigs (n = 6/intervention) were euthanized one day post-injection, while the remaining twelve pigs were euthanized seven days post-injection (n = 6/intervention). We performed autoradiography on hippocampus and prefrontal cortex (PFC) sections with [3H]UCB-J (SV2A), [3H]MDL100907 (5-HT2AR antagonist) and [3H]Cimbi-36 (5-HT2AR agonist). One day post psilocybin injection, we observed 4.42% higher hippocampal SV2A density and lowered hippocampal and PFC 5-HT2AR density (−15.21% to −50.19%). These differences were statistically significant in the hippocampus for all radioligands and in the PFC for [3H]Cimbi-36 only. Seven days post-intervention, there was still significantly higher SV2A density in the hippocampus (+9.24%) and the PFC (+6.10%), whereas there were no longer any differences in 5-HT2AR density. Our findings suggest that psilocybin causes increased persistent synaptogenesis and an acute decrease in 5-HT2AR density, which may play a role in psilocybin’s antidepressive effects.
Keywords: psilocybin, psychedelics, neuroplasticity, SV2A, 5-HT2A, depression, autoradiography, functional-selectivity
1. Introduction
Serotonergic psychedelic drugs have for centuries been extensively used in religious practices and also recreationally [1]. Their neurobiological and behavioral effects in mammals are mediated through stimulation of the serotonin 2A receptor (5-HT2AR) as reviewed by Vollenweider et al. [2,3]. Upon ingestion of psilocybin, a tryptamine psychedelic [1], it quickly dephosphorylates to the active compound psilocin, which has a high affinity to 5-HT2AR, but also to other 5-HT receptors such as 5-HT1AR and 5-HT2CR [1,4,5].
Psychedelic stimulation of 5-HT2AR, a G-protein-coupled receptor (GPCR), has recently shown potential as an anxiolytic and antidepressant therapy. Some clinical studies suggest that a single dose of psilocybin rapidly and effectively relieves symptoms in depression and anxiety, with effects that persist long after the psychedelic experience [6,7,8,9]. Research in rodents suggests that psilocybin, lysergic acid diethylamide (LSD), 2,5-dimethoxy-4-iodoamphetamine (DOI), N,N-dimethyltryptamine (DMT), and alkaloids like harmine, tetrahydroharmine, and harmaline (present in ayahuasca) induce structural neuroplasticity and alter the expression of important proteins like VGLUT1, BDNF, kalirin-7 and MAP2 [10,11,12,13,14]. The mechanism behind these synaptic changes is hypothesized to be exerted via the 5-HT2AR pathway [10].
Changes in synaptic density in brain regions associated with emotional processing, i.e., the hippocampus and prefrontal cortex (PFC), may play a vital role in the pathophysiology of mood disorders, e.g., major depressive disorder. Both post-mortem human brain [15,16] and in vivo [17] studies in depressed individuals have shown a loss of synapses through the down-regulation of synaptic proteins and genes. Hence, upregulation of presynaptic proteins and an increase in synaptic density may be associated with the potential antidepressive effects of psychedelics.
Synaptic vesicle protein 2A (SV2A) is an integral 12-transmembrane domain glycoprotein expressed in synaptic vesicles throughout the brain [18], and SV2A density is thought to reflect presynaptic density [19]. The levetiracetam derivative UCB-J, which binds selectively to SV2A, has in its radiolabeled form been shown to correspond to synaptic density as measured with the well-characterized presynaptic protein synaptophysin [20,21,22].
Classical receptor binding assay studies have demonstrated that 5-HT2AR (and other GPCRs) exist in two affinity states, a high- and a low- affinity state [23,24,25]. The affinity states of the receptors are considered to represent different functional states of the receptor, high-affinity being functionally active (activation of Gαi1-protein pathway) in contrast to the low-affinity state (activation of canonical Gαq/11-protein pathway) [26]. Whereas 5-HT2AR antagonists bind to the total pool of 5-HT2AR, 5-HT2AR agonists bind to the high-affinity state GPCRs [27]. Stimulation of 5-HT2AR leads to rapid receptor internalization [28]. This endosomal internalization may lead to lysosomal degradation and down-regulation of 5-HT2AR, as extensively reviewed by Gray J.A. and Roth B.L [29].
In the present study, we hypothesize that a psychedelic dose of psilocybin increases presynaptic density, as reflected in SV2A protein levels in the pig brain. We also test the hypothesis that the availability of 5-HT2AR is decreased after agonist stimulation with psilocybin.
Using in vitro autoradiography, we measure SV2A and 5-HT2AR protein levels one and seven days post-injection of a single dose of psilocybin, known to induce 5-HT2AR associated behavioral changes corresponding to psychedelic effects, in healthy pigs [30]. We investigate brain effects one day after psilocybin administration because the days following a psychedelic experience may provide a therapeutic window to treat mood disorders [6,7]. A follow-up seven days after a psilocybin intervention was done because this is when depressive scores have been reported to be the lowest [6]. To investigate if potential psilocybin-induced reductions in 5-HT2AR are due to changes in the total receptor pool or confined to functionally active 5-HT2ARs, we used both an antagonist ([3H]MDL100907) and an agonist radioligand ([3H]Cimbi-36) for autoradiography.
2. Results
2.1. SV2A Autoradiography
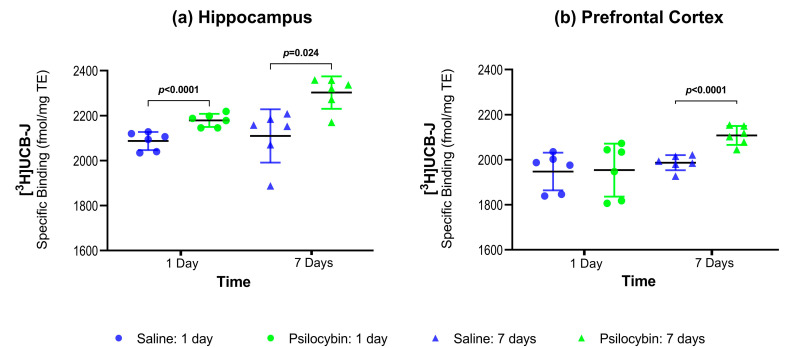
Figure 1a,b show the SV2A protein density as determined by [3H]UCB-J autoradiography in the hippocampus and the PFC. Compared to the saline-treated group, psilocybin treatment was associated with 4.42% higher SV2A in the hippocampus (p < 0.0001) one day after psilocybin injection and 9.24% higher SV2A in the hippocampus (p = 0.024) seven days after psilocybin (Figure 1a and Table 1). One day after psilocybin, there was no difference in PFC SV2A (Figure 2b and Table 1, 0.25%, p = 1), but seven days after psilocybin administration there was 6.10% higher SV2A in the PFC (p < 0.0001).
Figure 1.
Group-wise comparisons of synaptic vesicle protein 2A (SV2A) density (mean ± SD) in the hippocampus (a) and prefrontal cortex (PFC) (b) as measured with [3H]UCB-J autoradiography.
Table 1.
Group-wise summary of statistical tests performed for each radioligand. All tests show the adjusted p-values using the Holm method. NA (not applicable) indicates test was not performed.
| Hippocampus | Prefrontal Cortex | |||
|---|---|---|---|---|
| 1 day | 7 Days | 1 Day | 7 Days | |
| [3H]UCB-J (psilocybin vs. saline) |
+4.42% (p < 0.0001) |
+9.24% (p = 0.024) |
+0.25% (p = 1) |
+6.10% (p < 0.0001) |
| [3H]MDL100907 (psilocybin vs. saline) |
−29.60% (p < 0.0001) |
−3.58% (p = 1) |
−15.21% (p = 0.162) |
+1.32% (p = 1) |
| [3H]Cimbi-36 (psilocybin vs. saline) |
−43.39% (p = 0.013) |
+3.31% (p = 1) |
−50.19% (p < 0.0001) |
+2.23% (p = 1) |
| [3H]MDL100907 vs. [3H]Cimbi-36 | NA | NA | −41.26% (p = 0.033) |
+0.90% (p = 0.921) |
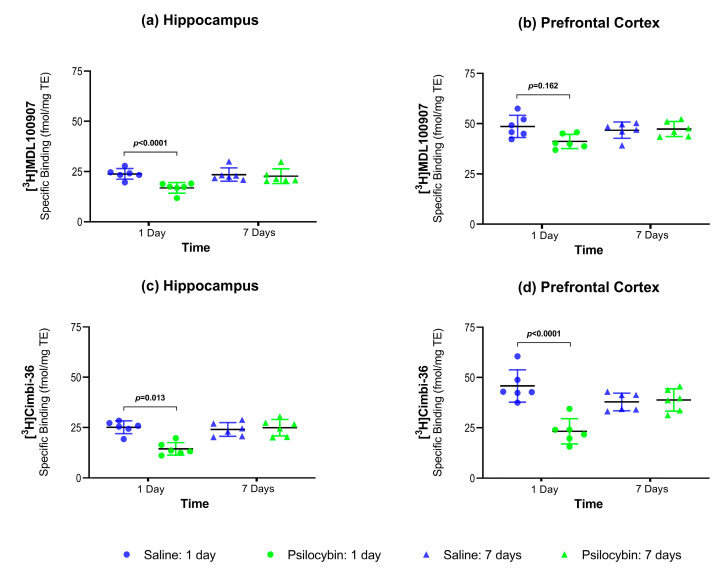
Figure 2.
Group-wise comparison of 5-HT2AR density (mean ± SD) as measured with [3H]MDL100907 and [3H]Cimbi-36 in the hippocampus (a,c) and PFC (b,d) using autoradiography.
2.2. 5-HT2AR Antagonist Autoradiography:
Figure 2a,b show the 5-HT2AR density as measured with the antagonist radioligand [3H]MDL100907 in hippocampus and PFC. One day after the intervention, hippocampal 5-HT2AR density (Figure 2a and Table 1) was 29.60% lower (p < 0.0001) and PFC 5-HT2AR density was similar (−15.21%, p = 0.162, Figure 2b and Table 1) in the psilocybin-treated group than in the saline-treated group. Seven days after the psilocybin interventions, hippocampal and PFC 5-HT2AR density was not significantly different from the saline-treated animals.
2.3. 5-HT2AR Agonist Autoradiography
Figure 2c,d show the 5-HT2AR density as measured with the agonist radioligand [3H]Cimbi-36 in hippocampus and PFC. One day after psilocybin intervention, hippocampal 5-HT2AR/5-HT2CR density (Figure 2c and Table 1) was 43.39% lower (p = 0.013), and PFC 5-HT2AR density (Figure 2d and Table 1) was 50.19% lower (p < 0.0001) in the psilocybin-treated group than in the saline-treated group. With [3H]Cimbi-36, similar to [3H]MDL100907, 5-HT2AR density was not significantly different in the hippocampus and the PFC seven days after the psilocybin intervention compared to saline.
2.4. Antagonist vs. Agonist Radioligand for 5-HT2AR Density
We found a more pronounced reduction of 5-HT2AR density when measured with [3H]Cimbi-36 compared to [3H]MDL100907 one day after psilocybin intervention in the PFC. This difference of 41.26% was statistically significant at p = 0.033 (Table 1). We found no significant difference seven days after psilocybin intervention for either radioligand.
2.5. Plasma Psilocin
Plasma psilocin levels at euthanasia one and seven days after the psilocybin intervention were all below the detection limit.
3. Discussion
To the best of our knowledge, this is the first large-animal study to investigate how a single dose of psilocybin changes the key proteins SV2A and 5-HT2AR in brain regions involved in emotional processing. We find that a single dose of psilocybin increases the presynaptic marker, SV2A already after one day and that it remains higher seven days after. We also show a transient reduction in the hippocampus and PFC 5-HT2AR density; it is reduced one day after intervention but not seven days after.
The increase in synaptic marker SV2A may result from the stimulation of the 5-HT2AR, TrkB and mTOR-signaling pathways [10]. The activation of 5-HT2AR by DOI has been shown to induce a kalirin-7-dependent increase in dendritic spine size that may play a role in regulating structural plasticity in the cortex [14]. To understand the neurobiological basis of neuroplasticity and the implication of these changes, future proteomics studies must reveal which other proteins in these pathways are changed and the temporal evolvement of such changes. Our data support the notion of increased synaptogenesis following psychedelic exposure, which is hypothesized to underlie the antidepressant effects observed in humans: We find higher SV2A density in the hippocampus and the PFC, which are also regions where SV2A is reduced in patients with major depressive disorder [17]. Atypical antidepressants like ketamine are also associated with neuroplastic effects through proteins like cFos, pERK, and BDNF in the PFC and hippocampus in a social defect stress rodent model [31]. We propose that the increase in SV2A represents an increase in presynaptic density through the same pathways. The absence of psilocybin-associated changes in mRNA for cFos, pERK, and BDNF described in Donovan et al. [30] does not, however, exclude these pathways as instrumental mediators of psilocin’s effects on SV2A. This requires separate studies of protein levels or experiments where the pathways were interrupted. Together with other markers of neuroplasticity, increased levels of SV2A after intervention with a psychedelic drug adds to the scientific evidence that psychedelics enhance neuroplasticity, which may explain the mechanism of action of its antidepressant properties [32].
We have previously reported that 5-HT2AR mRNA expression is unaltered in the brains of these pigs [30]. It is, however, well known that brain 5-HT2AR mRNA expression does not correlate with 5-HT2AR protein content [33]. Our finding of a transient decrease in 5-HT2AR density, but not mRNA, one day after psilocybin is in line with results for the psychedelic substance DOI, where a significant difference in 5-HT2AR protein expression was not accompanied by a change in mRNA gene expression [34].
Compared to other protein-measuring techniques such as Western blot and immunohistochemistry, autoradiography provides an added advantage of measuring receptors in the functionally active vs. total receptor pool by the use of agonist or antagonist radioligands, respectively. The use of adjacent brain sections provides the ability to directly compare receptors. We find a statistically significant reduction in 5-HT2AR density in the PFC one day after psilocybin administration when measuring with [3H]Cimbi-36 compared to [3H]MDL100907. The difference between the two radioligands offers circumstantial evidence of the differential binding of antagonists versus agonists, at least when it comes to the PFC. More caution should be exerted when comparing the radioligands in the hippocampus because the hippocampus has high levels of 5-HT2CR, with a density similar to 5-HT2AR [35,36] and [3H]Cimbi-36 also has affinity to 5-HT2CR [37,38]. That is, we cannot exclude the possibility that some of the observed reduction in [3H]Cimbi-36 in the hippocampus could be due to a down-regulation of 5-HT2CR. It could be a concern that the reduction in 5-HT2AR one day after psilocybin was due to partial blocking by residual psilocin. However, plasma psilocin levels at euthanasia one day after psilocybin administration were under the detection limit in all animals.
The fraction of functional 5-HT2AR has to some extent been assessed in vivo in non-human primates [38] and humans [39] using [11C]Cimbi-36 as an agonist and [11C]MDL100907 or [18F]altanserin as antagonist radioligands, but a more precise estimate is difficult in vivo because of missing information about the free fraction of the radioligand and the radioligand affinity. A little unexpectedly, Bmax did not differ substantially between agonist and antagonist radioligands in our study, but it should be kept in mind that uncertainties in the determination of specific activities are reflected in the calculation of Bmax. Functional receptors can also be measured with [35S]guanosine triphosphate (GTP) γS binding stimulation mediated with DOI followed by immunoprecipitation with specific antibodies, while the complex is captured with protein A-polyvinyl toluene scintillation proximity assay. When this approach is made on post-mortem brain tissue from patients with schizophrenia, the canonical Gαq/11-protein pathway of 5-HT2ARs is found to be unaltered in the PFC, whereas the pro-hallucinogenic Gαi1-protein pathway is functionally overactive in the PFC [40,41]. GTPγS binding assay may be more sensitive to the measurement of functional receptors and could generate an outcome that was more straight-forward to interpret.
It is already well-known that a transient 5-HT2AR down-regulation occurs upon agonist stimulation, followed by a return to baseline [42]. We have previously found that the 5-HT2AR binding has normalized seven days after healthy individuals take a single psychedelic dose of psilocybin [43]. To what extent the transient down-regulation of 5-HT2AR is a prerequisite for boosting the formation of new synapses is intriguing and should be examined in future studies.
Some limitations of the study should also be mentioned. We chose to investigate only two time-points and selected a few highly relevant proteins in two relevant brain regions. It would be interesting to investigate whether the synaptic density increases further beyond one week, and for how long it is maintained. Although we cannot be certain that our findings translate to humans that consume a single dose of psilocybin, the SV2A density in the hippocampus and the PFC in the saline treated pigs is in the same range as that reported in post-mortem human and non-human primates by Varnäs et al. [22]. The changes in SV2A and 5-HT2AR were seen in healthy pigs; it might also be relevant to investigate changes in a psychosocial chronic-stress pig model [44]. Further, to ensure that the pigs received a well-defined dose of psilocybin, we chose to administer the drug intravenously rather than perorally. This differs from the typical approach in patients. Despite the faster pharmacokinetics after intravenous administration, the dose and administration route result in the same 5-HT2AR occupancy as in humans that take it perorally [30,43].
4. Materials and Methods
4.1. Animals and Drug Dosage
The brain tissue was retrieved from pigs entering a previously published study [30] where more details are described. Briefly, female Danish slaughter pigs (Yorkshire × Duroc × Landrace) weighing around 20 kg (approximately nine weeks old) were used in the study. The animals were sourced from a local farm and allowed to acclimatize for at least one week before the start of the experiment. The animals were housed in individual pens with an enriched environment on a 12-h light/dark cycle, with free access to water, and weight-adjusted food twice daily. The welfare of the animals was assessed daily. After arriving in the stables, animals were trained for up to a week to allow for handling by humans.
Donovan et al. [30] identified which dose of psilocybin to give to make it comparable to a dose that elicits psychedelic effects in humans. This was based on behavioral response (headshakes, hindlimb scratches, and rubbing against the pen wall) and on Positron emission tomography (PET) studies of the 5-HT2AR occupancy using the agonist radioligand [11C]Cimbi-36; 67% 5-HT2AR occupancy will elicit psychedelic effects in humans [43]. Intravenous injection of psilocybin was given in an ear vein catheter to the awake pigs, and the animals were under no form of external stress during the experiment. At the time of euthanasia, blood was drawn for the measurement of plasma psilocin levels, which were measured by ultra-high-performance liquid chromatography coupled to tandem mass spectrometry as previously described [43].
4.2. Ethical Statement
All animal experiments conformed to the European Commission’s Directive 2010/63/EU and the ARRIVE guidelines. The Danish Council of Animal Ethics had approved all procedures (Journal no. 2016-15-0201-01149).
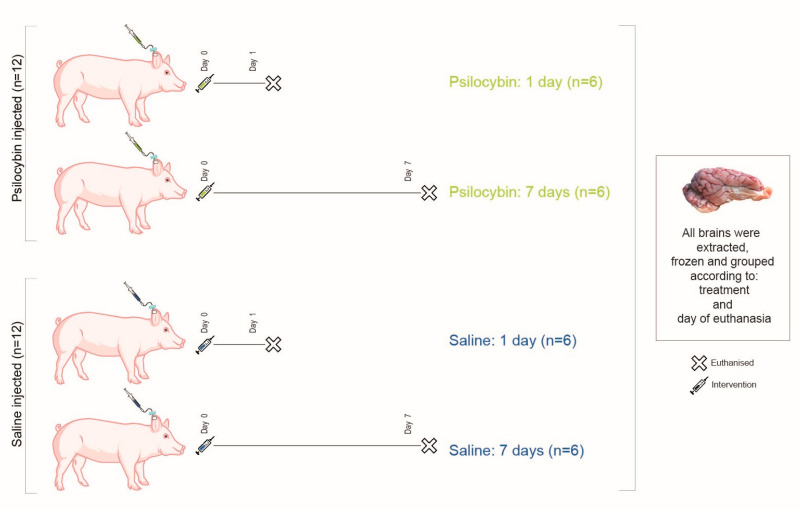
4.3. Study Design
Figure 3 shows the overall design of the study. Twenty-four awake pigs were given an intravenous dose of either 0.08 mg/kg psilocybin (n = 12) or saline (n = 12) through an ear vein catheter. Half of the animals in each group were euthanized one day post-injection (n = 6/intervention). The remaining 12 animals were euthanized seven days post-injection (n = 6/intervention). That is, the animals were divided into four groups: Saline: 1 day, Psilocybin: 1 day, Saline: 7 days and Psilocybin: 7 days (Figure 3). The extracted brains were snap-frozen and stored at −80 °C. From one hemisphere, 20 µm thick frozen sections were sliced on a cryostat (Leica CM1800, Leica Biosystems, Buffalo Grove, IL, USA) from the PFC and the hippocampus and mounted on Superfrost Plus™ adhesion microscope slides. Sections were stored at −20 °C for the remaining period of the study.
Figure 3.
Study design. Twenty-four pigs received an intravenous dose of either 0.08 mg/kg psilocybin or saline. Six pigs from each type of intervention were euthanized one day or seven days post-injection. The pigs were divided into four groups, as depicted in the figure.
4.4. Autoradiography
Radioligands used for autoradiography included SV2A imaging with [3H]UCB-J (UCB pharma, Brussels, Belgium, specific activity 14 Ci/mmol or Pharmaron Ltd., Hoddesdon, UK, specific activity 28 Ci/mmol). [3H]MDL100907 (ViTrax, Placentia, CA, USA, specific activity 56 Ci/mmol) was used as an antagonist radioligand for 5-HT2AR and [3H]Cimbi-36 (kindly provided by Prof. Dr C. Halldin, Department of Neuroscience, Karolinska Institute, Stockholm, Sweden, specific activity 53 Ci/mmol,) as an agonist radioligand for 5-HT2AR/5-HT2cR. Radio-Thin-Layered-Chromatography (R-TLC) was performed for all radioligands to measure the radiochemical purity (RCP) and integrity of the parent compound. The mobile phase for [3H]UCB-J R-TLC was Acetonitrile:Ammonium formate [25:75] (0.1 M, with 0.5% AcOH, pH 4.2). The mobile phase for [3H]MDL100907 R-TLC was Chloroform:Methanol:Ammonia solution [90:9:1]. The mobile phase for [3H]Cimbi-36 R-TLC was Chloroform:Methanol:Triethylamine [94:5:1]. [3H]UCB-J and [3H]MDL100907 had high RCP (96–98%) while [3H]Cimbi-36 had an RCP of 52–57%. Radioactivity was corrected for RCP of [3H]Cimbi-36 after TLC.
Sections were thawed to room temperature for 30–45 min before prewashing twice for 10 min in 50 mM Tris-HCl pre-incubation buffer set to 7.4 pH containing 0.5% bovine serum albumin (BSA) for [3H]UCB-J or 0.01% ascorbic acid, 4 nM CaCl2 and 0.1% BSA for [3H]MDL100907 and [3H]Cimbi-36.
For SV2A, the sections were incubated in assay buffer containing 60 nM [3H]UCB-J in 50 mM Tris-HCl buffer containing 5 mM MgCl2, 2 mM EGTA and 0.5% BSA (pH 7.4) for 1 h. Incubation was terminated by three 5-min washes with 4 °C pre-incubation buffer followed by a rapid rinse in 4 °C deionized H2O (dH2O). For 5-HT2AR, sections were incubated in assay buffer containing 3 nM [3H]MDL100907 or 1 nM [3H]Cimbi-36 in 50 mM Tris-HCl containing 0.01% ascorbic acid, 4 nM CaCl2 and 0.1% BSA (pH 7.4) for 1 h. Incubation was terminated by two 10-min washes in ice-cold pre-incubation buffer followed by a rapid rinse in ice-cold dH2O.
The assay buffer concentration of the respective radioligands was determined using 4–5 × KD values (Appendix A: Figure A1) to determine Bmax values in the section. After washing, the slides were rapidly air-dried and fixated in a paraformaldehyde vapor chamber overnight in cold storage (4 °C). The next day, the samples were moved to an exicator for 45–60 min to remove any excess moisture and then placed in a cassette for autoradiography with tritium sensitive image plates (BAS-IP TR2040, Science Imaging Scandinavia AB, Nacka, Sweden) along with radioactive tritium standards (RPA510, Amersham Bioscience, GE Healthcare, Chicago, IL, USA) (Figure 4). The image plates were exposed for seven days. After the exposure, the image plates were read using a Fujifilm BAS 1000 scanner (Fujifilm Europe, GmbH, Duesseldorf, Germany). Calibration, quantification and data evaluation were done using ImageJ software (NIH Image, Bethesda, MD, USA) [45]. The regions of interest were hand-drawn or drawn using the wand tool and visually inspected after automated delineation, as shown in Figure 4. The four-parameter general curve fit (David Rodbard, NIH) of decay corrected tritium standards was used to convert mean pixel density (grayscale) to nCi/mg tissue equivalent (TE). Total binding was determined in the hippocampal and cortical grey matter while non-specific binding was determined in the white matter on the same slides. Finally, the decay-corrected specific activity of the representative radioligand was used to convert nCi/mg TE to fmol/mg TE. Specific binding was calculated as the difference between total binding and non-specific binding. All experiments were performed in triplicates, and experimenters were blinded.
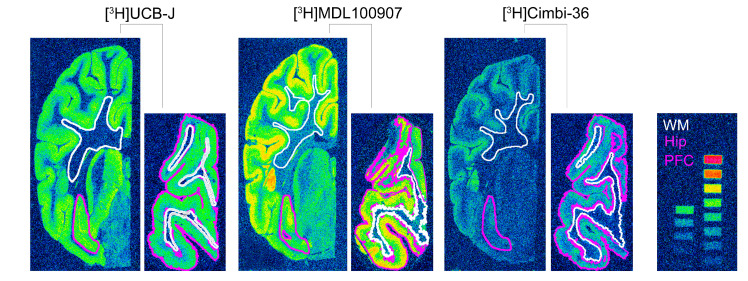
Figure 4.
Representative autoradiograms of the radioligands used in this study. Half hemisphere sections of the hippocampus (larger coronal sections) and PFC (smaller coronal sections) from the same animal belonging to the Saline: 1 day group. The color-coded lines show representations of the region of interest that were either hand-drawn or drawn using the wand tool in ImageJ and visually inspected. The figure also shows the radioactive standards used in the study; approximately 16 nCi/mg to 0.2 nCi/mg decay corrected to the time of experiment and day of exposure.
4.5. Statistical Analyses
The data were analyzed using R (v. 4.0.3; R core team, Vienna, Austria), while GraphPad Prism (v. 9.0.0; GraphPad Software, San Diego, CA, USA) was used for data visualization. Comparisons between group means () for the respective radioligands (Equations (1) and (2)) were done using a permutation test (with 1000 permutations) on log-transformed values and adjusted for multiple comparisons (overtime, radioligand, and brain regions: 12 tests) using the Holm method. Comparison between the 5-HT2AR radioligands, [3H]Cimbi-36 and [3H]MDL100907, was performed for the PFC using a permutation test (with 1000 permutations) on log-transformed values of treatment effect at one day and seven days (Equations (3) and (4)).
| (1) |
| (2) |
| (3) |
| (4) |
Acknowledgments
The psilocybin was kindly supplied by the National Institute of Mental Health, Klecany, Czech Republic and the Forensic Laboratory of Biologically Active Compounds, Department of Chemistry of Natural Compounds, University of Chemistry and Technology Prague, Prague, Czech Republic. We would also like to acknowledge the help of Jesper Langgaard Kristensen for procuring psilocybin. The authors would like further to thank Malthe Scharff for his assistance in the experiments. The authors would also like to thank and show sincere gratitude to the veterinarians and staff at the Department of Experimental Medicine, University of Copenhagen for their continued assistance with animal experiments.
Abbreviations
| 5-HT | 5-Hydroxytryptamine (serotonin) |
| 5-HT1AR | 5-Hydroxytryptamine (serotonin) 1A Receptor |
| 5-HT2AR | 5-Hydroxytryptamine (serotonin) 2A Receptor |
| 5-HT2CR | 5-Hydroxytryptamine (serotonin) 2C Receptor |
| ARRIVE | Animal Research: Reporting of In Vivo Experiments |
| BDNF | Brain-Derived Neurotropic Factor |
| Bmax | Total density (concentration) of the receptor |
| BPND | Binding Potential (Non-displaceable) |
| BSA | Bovine Serum Albumin |
| DMT | N,N-dimethyltryptamine |
| DOI | 2,5-dimethoxy-4-iodoamphetamine |
| GPCR | G-protein coupled receptor |
| KD | Equilibrium dissociation Constant |
| LSD | Lysergic acid diethylamide |
| MAP2 | Microtubule-associated protein 2 |
| mTOR | Mammalian Target of Rapamycin |
| NSB | Non-specific Binding |
| PET | Positron Emission Tomography |
| pERK | Endoplasmic Reticulum Protein Kinase |
| PFC | Prefrontal Cortex |
| RCP | Radiochemical purity |
| R-TLC | Radio-Thin Layer Chromatography |
| SV2A | Synaptic Vesicular Protein 2A |
| TB | Total Binding |
| TE | Tissue Equivalent |
| TrkB | Tropomyosin Receptor Kinase B |
| VGLUT1 | Vesicular Glutamate Transporter 1 |
| Mean |
Appendix A
Appendix A.1. Saturation Assays
Appendix A.1.1. Methods
Two female Danish slaughter pigs’ brains that were not included in this study were used to perform a saturation assay. The extracted brains were snap-frozen and stored at −80 °C. From one hemisphere, 20 µm thick frozen sections were sliced on a cryostat (Leica CM1800, Leica Biosystems, Buffalo Grove, IL, USA) from the prefrontal cortex (PFC) and mounted on Superfrost Plus™ adhesion microscope slides. The sections were stored at −80 °C for the remaining period of the study.
All radioligands used for this autoradiography study were included for a saturation assay. The radioligands used are as follows: [3H]UCB-J for SV2A (UCB pharma, Brussels, Belgium, specific activity 14 Ci/mmol), [3H]MDL100907 as an antagonist radioligand for 5-HT2AR (ViTrax, Placentia, CA, USA, specific activity 56 Ci/mmol) and [3H]Cimbi-36 as an agonist radioligand for 5-HT2AR/5-HT2c R (kindly provided by Prof. Dr C. Halldin, Department of Neuroscience, Karolinska Institute, Stockholm, Sweden, specific activity 53 Ci/mmol) for 5-HT2A/5-HT2c. The saturation assay for [3H]Cimbi-36 was performed in 2012, when the tracer was produced, hence high radiochemical purity was assumed.
Experimental conditions previously described were also used for the saturation assay. Briefly, after the sections were thawed to room temperature, they were prewashed twice for 10 min in 50 mM Tris-HCl pre-incubation buffer set to 7.4 pH containing 0.5% bovine serum albumin (BSA) for [3H]UCB-J or 0.01% ascorbic acid, 4 nM CaCl2 and 0.1% BSA for [3H]MDL100907 and [3H]Cimbi-36.
For SV2A, the sections (n = 4) were incubated in assay buffer (50 mM Tris-HCl buffer containing 5 mM MgCl2, 2 mM EGTA and 0.5% BSA (pH 7.4)) containing varying concentrations (0 to 100 nM) of [3H]UCB-J for total binding (TB) and the same varying concentration of [3H]UCB-J with 10 mM of levetiracetam (Keppra, UCB pharma, Brussels, Belgium) for non-specific binding (NSB). The sections were incubated for 60 min. Incubation was terminated by three 5-min washes with 4 °C pre-incubation buffer followed by a rapid rinse in 4 °C deionized H2O (dH2O).
For 5-HT2AR, sections (n = 4 for [3H]MDL100907 and n = 1 for [3H]Cimbi-36) were incubated in assay buffer (50 mM Tris-HCl containing 0.01% ascorbic acid, 4 nM CaCl2 and 0.1% BSA (pH 7.4)) containing varying concentrations of [3H]MDL100907 or [3H]Cimbi-36 (0 to 4 nM) for TB and the same varying concentration of [3H]MDL100907 or [3H]Cimbi-36 with 10 mM ketanserin (Sigma-Aldrich, Søborg, Denmark) for NSB. The sections were incubated for 60 min. Incubation was terminated by two 10-min washes in ice-cold pre-incubation buffer followed by a rapid rinse in ice-cold dH2O.
After washing, the slides were rapidly air-dried and fixated in a paraformaldehyde vapor chamber overnight in cold storage (4 °C). The next day, the samples were moved to an exicator for 45–60 min to remove any excess moisture and then placed in a cassette for autoradiography with tritium sensitive image plates (BAS-IP TR2040, Science Imaging Scandinavia AB, Nacka, Sweden) along with radioactive tritium standards (ART0123B, American Radiolabelled Chemical, Inc., St. Loui, MO, USA and RPA510, Amersham Bioscience, GE Healthcare, Chicago, IL, USA) (Figure 4). The image plates were exposed for seven days. After the exposure, the image plates were read using a Fujifilm BAS 1000 scanner (Fujifilm Europe, GmbH, Duesseldorf, Germany). Calibration, quantification and data evaluation was done using ImageJ software (NIH Image, Bethesda, MD, USA) [45]. The regions of interest were hand-drawn or drawn using the wand tool and visually inspected after automated delineation, as shown in Figure 4 of the main manuscript. The four-parameter general curve fit (David Rodbard, NIH) of decay corrected tritium standards was used to convert mean pixel density (grayscale) to nCi/mg TE. TB was determined in cortical grey matter from TB slides while NSB was determined in the cortical grey matter of NSB slides, and white matter was defined on the same TB slides. Finally, the decay-corrected specific activity of the representative radioligand was used to convert nCi/mg TE to fmol/mg TE. Specific binding was calculated as the difference between TB and NSB.
The data were analyzed using GraphPad Prism (v. 9.0.0; GraphPad Software, San Diego, CA, USA). Non-linear regression analysis (One site- Fit total and non-specific binding) was used for all radioligands. The curve fitting used for saturation assays was “One site-fit total and non-specific binding”. The fitting method used was the least squared regression with no weighting.
Appendix A.1.2. Results
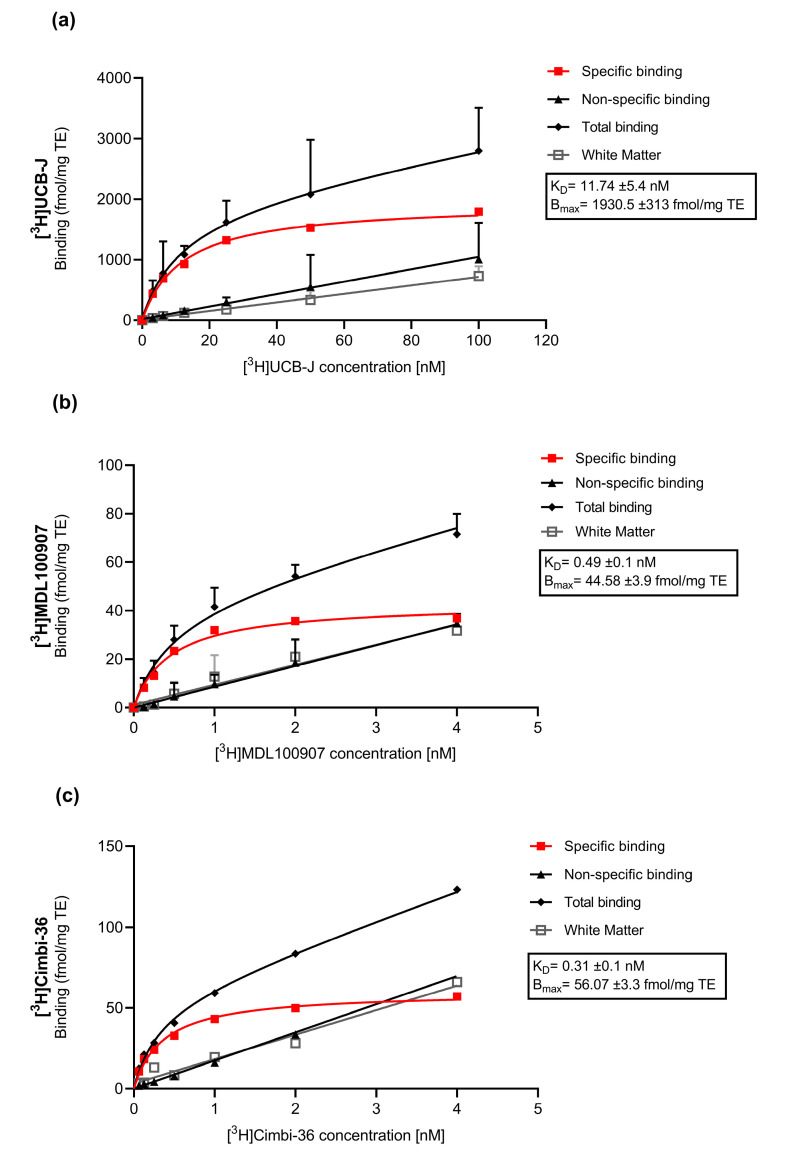
The saturation assay with [3H]UCB-J generated a KD of 11.47 ± 7.8 nM and Bmax of 1090 ± 253 fmol/mg TE. [3H]MDL100907 generated a KD of 0.49 ± 0.1) nM and Bmax of 44.58 ± 3.9 fmol/mg TE while [3H]Cimbi-36 shows a KD of 0.31 ± 0.1 nM and Bmax of 56.07 ± 3.3 fmol/mg TE. [3H]UCB-J white mater binding was lower (slope = 7.0, R2 = 0.99) compared to NSB in the grey matter of levetiracetam blocked slides (slope = 10.0, R2 = 0.99) but with less variation. On the other hand, white matter binding with [3H]MDL100907 (slope = 8.2, R2 = 0.95) and [3H]Cimbi-36 (slope = 15.1, R2 = 0.97) correlate (visually) with NSB in the grey matter of ketanserin blocked slides (slope = 8.8, R2 = 0.99 and 16.4, R2 = 0.99, respectively).
Figure A1.
Saturation assay of [3H]UCB-J (a), [3H]MDL100907 (b) and [3H]Cimbi-36 (c) in the grey matter of the pig brain.
Author Contributions
Conceptualization, G.M.K., H.D.H., N.R.R., L.L.D. and A.J.; methodology, N.R.R., A.J., B.O. and N.F.R.; software, N.R.R., and B.O.; validation, N.R.R., A.J., and N.F.R.; formal analysis, N.R.R., A.J. and B.O.; investigation, N.R.R., N.F.R., A.J.; resources, L.L.D.; data curation, N.R.R., A.J., and B.O.; writing—original draft preparation, N.R.R.; writing—review and editing, N.R.R., A.J., L.L.D., B.O., H.D.H. and G.M.K.; visualization, N.R.R.; supervision, H.D.H. and G.M.K.; funding acquisition, G.M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No 813528. The Lundbeck Foundation supported running costs and a stipend for Lene L. Donovan. The Independent Research Fund Denmark funded Annette Johansen.
Institutional Review Board Statement
All animal experiments conformed to the European Commission’s Directive 2010/63/EU and the ARRIVE guidelines. The Danish Council of Animal Ethics had ap-proved all procedures (Journal no. 2016-15-0201-01149).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to other on-going studies.
Conflicts of Interest
G.M.K.: H. Lundbeck A/S (research collaboration), Novo Nordisk/Novozymes/Chr. Hansen (stockholder), Sage Therapeutics and Sanos (Advisor). G.M.K. is currently the president of the European College of Neuropsychopharmacology. All other authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nichols D.E. Hallucinogens. Pharmacol. Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Vollenweider F.X., Kometer M. The neurobiology of psychedelic drugs: Implications for the treatment of mood disorders. Nat. Rev. Neurosci. 2010;11:642–651. doi: 10.1038/nrn2884. [DOI] [PubMed] [Google Scholar]
- 3.Vollenweider F.X., Preller K.H. Psychedelic drugs: Neurobiology and potential for treatment of psychiatric disorders. Nat. Rev. Neurosci. 2020 doi: 10.1038/s41583-020-0367-2. [DOI] [PubMed] [Google Scholar]
- 4.Rickli A., Moning O.D., Hoener M.C., Liechti M.E. Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. Eur. Neuropsychopharmacol. 2016;26:1327–1337. doi: 10.1016/j.euroneuro.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Blair J.B., Kurrasch-Orbaugh D., Marona-Lewicka D., Gumbay M.G., Watts V.J., Barker E.L., Nichols D.E. Effect of ring fluorination on the pharmacology of hallucinogenic tryptamines. J. Med. Chem. 2000;43:4701–4710. doi: 10.1021/jm000339w. [DOI] [PubMed] [Google Scholar]
- 6.Carhart-Harris R.L., Bolstridge M., Rucker J., Day C.M.J.J., Erritzoe D., Kaelen M., Bloomfield M., Rickard J.A., Forbes B., Feilding A., et al. Psilocybin with psychological support for treatment-resistant depression: An open-label feasibility study. Lancet Psychiatry. 2016;3:619–627. doi: 10.1016/S2215-0366(16)30065-7. [DOI] [PubMed] [Google Scholar]
- 7.Griffiths R.R., Johnson M.W., Carducci M.A., Umbricht A., Richards W.A., Richards B.D., Cosimano M.P., Klinedinst M.A. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J. Psychopharmacol. 2016;30:1181–1197. doi: 10.1177/0269881116675513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross S., Bossis A., Guss J., Agin-Liebes G., Malone T., Cohen B., Mennenga S.E., Belser A., Kalliontzi K., Babb J., et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: A randomized controlled trial. J. Psychopharmacol. 2016;30:1165–1180. doi: 10.1177/0269881116675512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis A.K., Barrett F.S., May D.G., Cosimano M.P., Sepeda N.D., Johnson M.W., Finan P.H., Griffiths R.R. Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry. 2020 doi: 10.1001/jamapsychiatry.2020.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ly C., Greb A.C., Cameron L.P., Wong J.M., Eden V., Wilson P.C., Burbach K.F., Zarandi S.S., Paddy M.R., Duim W.C., et al. Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep. 2018;23:3170–3182. doi: 10.1016/j.celrep.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morales-García J.A., De La Fuente Revenga M., Alonso-Gil S., Rodríguez-Franco M.I., Feilding A., Perez-Castillo A., Riba J. The alkaloids of Banisteriopsis caapi, the plant source of the Amazonian hallucinogen Ayahuasca, stimulate adult neurogenesis in vitro. Sci. Rep. 2017;7:1–13. doi: 10.1038/s41598-017-05407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fortunato J.J., Réus G.Z., Kirsch T.R., Stringari R.B., Fries G.R., Kapczinski F., Hallak J.E., Zuardi A.W., Crippa J.A., Quevedo J. Chronic administration of harmine elicits antidepressant-like effects and increases BDNF levels in rat hippocampus. J. Neural Transm. 2010;117:1131–1137. doi: 10.1007/s00702-010-0451-2. [DOI] [PubMed] [Google Scholar]
- 13.Morales-Garcia J.A., Calleja-Conde J., Lopez-Moreno J.A., Alonso-Gil S., Sanz-SanCristobal M., Riba J., Perez-Castillo A. N,N-dimethyltryptamine compound found in the hallucinogenic tea ayahuasca, regulates adult neurogenesis in vitro and in vivo. Transl. Psychiatry. 2020;10:331. doi: 10.1038/s41398-020-01011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones K.A., Srivastava D.P., Allen J.A., Strachan R.T., Roth B.L., Penzes P. Rapid modulation of spine morphology by the 5-HT2A serotonin receptor through kalirin-7 signaling. Proc. Natl. Acad. Sci. USA. 2009;106:19575–19580. doi: 10.1073/pnas.0905884106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duric V., Banasr M., Stockmeier C.A., Simen A.A., Newton S.S., Overholser J.C., Jurjus G.J., Dieter L., Duman R.S. Altered expression of synapse and glutamate related genes in post-mortem hippocampus of depressed subjects. Int. J. Neuropsychopharmacol. 2013;16:69–82. doi: 10.1017/S1461145712000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang H.J., Voleti B., Hajszan T., Rajkowska G., Stockmeier C.A., Licznerski P., Lepack A., Majik M.S., Jeong L.S., Banasr M., et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat. Med. 2012;18:1413–1417. doi: 10.1038/nm.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmes S.E., Scheinost D., Finnema S.J., Naganawa M., Davis M.T., DellaGioia N., Nabulsi N., Matuskey D., Angarita G.A., Pietrzak R.H., et al. Lower synaptic density is associated with depression severity and network alterations. Nat. Commun. 2019;10 doi: 10.1038/s41467-019-09562-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartholome O., Van den Ackerveken P., Sánchez Gil J., de la Brassinne Bonardeaux O., Leprince P., Franzen R., Rogister B., Surguchov A., Conti F., Haucke V., et al. Puzzling Out Synaptic Vesicle 2 Family Members Functions. Front. Mol. Neurosci. 2017;10:148. doi: 10.3389/fnmol.2017.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heurling K., Ashton N.J., Leuzy A., Zimmer E.R., Blennow K., Zetterberg H., Eriksson J., Lubberink M., Schöll M. Synaptic vesicle protein 2A as a potential biomarker in synaptopathies. Mol. Cell. Neurosci. 2019;97:34–42. doi: 10.1016/j.mcn.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Nowack A., Yao J., Custer K.L., Bajjalieh S.M. SV2 regulates neurotransmitter release via multiple mechanisms. Am. J. Physiol. Cell Physiol. 2010;299:960–967. doi: 10.1152/ajpcell.00259.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finnema S.J., Nabulsi N.B., Eid T., Detyniecki K., Lin S.F., Chen M.K., Dhaher R., Matuskey D., Baum E., Holden D., et al. Imaging synaptic density in the living human brain. Sci. Transl. Med. 2016;8 doi: 10.1126/scitranslmed.aaf6667. [DOI] [PubMed] [Google Scholar]
- 22.Varnäs K., Stepanov V., Halldin C. Autoradiographic mapping of synaptic vesicle glycoprotein 2A in non-human primate and human brain. Synapse. 2020 doi: 10.1002/syn.22157. [DOI] [PubMed] [Google Scholar]
- 23.López-Giménez J.F., Villazón M., Brea J., Loza M.I., Palacios J.M., Mengod G., Vilaró M.T. Multiple conformations of native and recombinant human 5-hydroxytryptamine2A receptors are labeled by agonists and discriminated by antagonists. Mol. Pharmacol. 2001;60:690–699. [PubMed] [Google Scholar]
- 24.Song J., Hanniford D., Doucette C., Graham E., Poole M.F., Ting A., Sherf B., Harrington J., Brunden K., Stricker-Krongrad A. Development of homogenous high-affinity agonist binding assays for 5-HT2 receptor subtypes. Assay Drug Dev. Technol. 2005;3:649–659. doi: 10.1089/adt.2005.3.649. [DOI] [PubMed] [Google Scholar]
- 25.Shalgunov V., van Waarde A., Booij J., Michel M.C., Dierckx R.A.J.O., Elsinga P.H. Hunting for the high-affinity state of G-protein-coupled receptors with agonist tracers: Theoretical and practical considerations for positron emission tomography imaging. Med. Res. Rev. 2019;39:1014–1052. doi: 10.1002/med.21552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kent R.S., De Lean A., Lefkowitz R.J. A quantitative analysis of beta-adrenergic receptor interactions: Resolution of high and low affinity states of the receptor by computer modeling of ligand binding data. Mol. Pharmacol. 1980;17:14–23. [PubMed] [Google Scholar]
- 27.López-Giménez J.F., Vilaró M.T., Palacios J.M., Mengod G. Multiple conformations of 5-HT2A and 5-HT2C receptors in rat brain: An autoradiographic study with [125I](±)DOI. Exp. Brain Res. 2013;230:395–406. doi: 10.1007/s00221-013-3636-8. [DOI] [PubMed] [Google Scholar]
- 28.Berry S.A., Shah M.C., Khan N., Roth B.L. Rapid agonist-induced internalization of the 5-hydroxytryptamine(2A) receptor occurs via the endosome pathway in vitro. Mol. Pharmacol. 1996;50:306–313. [PubMed] [Google Scholar]
- 29.Gray J.A., Roth B.L. Paradoxical trafficking and regulation of 5-HT2A receptors by agonists and antagonists. Brain Res. Bull. 2001;56:441–451. doi: 10.1016/S0361-9230(01)00623-2. [DOI] [PubMed] [Google Scholar]
- 30.Donovan L.L., Johansen J.V., Ros N.F., Jaberi E., Johansen S.S., Ozenne B., Issazadeh-Navikas S., Hansen H.D., Knudsen G.M. Effects of a single dose of psilocybin on behaviour, brain 5-HT2A receptor occupancy and gene expression in the pig. Eur. Neuropsychopharmacol. 2021;42:1–11. doi: 10.1016/j.euroneuro.2020.11.013. [DOI] [PubMed] [Google Scholar]
- 31.Zhang M., Radford K.D., Driscoll M., Purnomo S., Kim J., Choi K.H. Effects of subanesthetic intravenous ketamine infusion on neuroplasticity-related proteins in the prefrontal cortex, amygdala, and hippocampus of Sprague-Dawley rats. IBRO Rep. 2019;6:87–94. doi: 10.1016/j.ibror.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hutten N.R.P.W., Mason N.L., Dolder P.C., Theunissen E.L., Holze F., Liechti M.E., Varghese N., Eckert A., Feilding A., Ramaekers J.G., et al. Low Doses of LSD Acutely Increase BDNF Blood Plasma Levels in Healthy Volunteers. ACS Pharmacol. Trans. Sci. 2020:acsptsci.0c00099. doi: 10.1021/acsptsci.0c00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beliveau V., Ganz M., Feng L., Ozenne B., Højgaard L., Fisher P.M., Svarer C., Greve D.N., Knudsen G.M. A high-resolution in vivo atlas of the human brain’s serotonin system. J. Neurosci. 2017;37:120–128. doi: 10.1523/JNEUROSCI.2830-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anji A., Kumari M., Sullivan Hanley N.R., Bryan G.L., Hensler J.G. Regulation of 5-HT2A receptor mRNA levels and binding sites in rat frontal cortex by the agonist DOI and the antagonist mianserin. Neuropharmacology. 2000;39:1996–2005. doi: 10.1016/S0028-3908(00)00026-5. [DOI] [PubMed] [Google Scholar]
- 35.Pompeiano M., Palacios J.M., Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: Comparison between 5-HT2A and 5-HT2C receptors. Mol. Brain Res. 1994;23:163–178. doi: 10.1016/0169-328X(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 36.Marazziti D., Rossi A., Giannaccini G., Zavaglia K.M., Dell’Osso L., Lucacchini A., Cassano G.B. Distribution and characterization of [ 3 H]mesulergine binding in human brain postmortem. Eur. Neuropsychopharmacol. 1999;10:21–26. doi: 10.1016/S0924-977X(99)00045-0. [DOI] [PubMed] [Google Scholar]
- 37.Ettrup A., Holm S., Hansen M., Wasim M., Santini M.A., Palner M., Madsen J., Svarer C., Kristensen J.L., Knudsen G.M. Preclinical safety assessment of the 5-HT2A receptor agonist PET radioligand [11C]cimbi-36. Mol. Imaging Biol. 2013;15:376–383. doi: 10.1007/s11307-012-0609-4. [DOI] [PubMed] [Google Scholar]
- 38.Finnema S.J., Stepanov V., Ettrup A., Nakao R., Amini N., Svedberg M., Lehmann C., Hansen M., Knudsen G.M., Halldin C. Characterization of [11C]Cimbi-36 as an agonist PET radioligand for the 5-HT2A and 5-HT2C receptors in the nonhuman primate brain. Neuroimage. 2014;84:342–353. doi: 10.1016/j.neuroimage.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 39.Ettrup A., Svarer C., McMahon B., da Cunha-Bang S., Lehel S., Møller K., Dyssegaard A., Ganz M., Beliveau V., Jørgensen L.M., et al. Serotonin 2A receptor agonist binding in the human brain with [11C]Cimbi-36: Test–retest reproducibility and head-to-head comparison with the antagonist [18 F]altanserin. Neuroimage. 2016;130:167–174. doi: 10.1016/j.neuroimage.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 40.García-Bea A., Miranda-Azpiazu P., Muguruza C., Marmolejo-Martinez-Artesero S., Diez-Alarcia R., Gabilondo A.M., Callado L.F., Morentin B., González-Maeso J., Meana J.J. Serotonin 5-HT2A receptor expression and functionality in postmortem frontal cortex of subjects with schizophrenia: Selective biased agonism via Gαi1-proteins. Eur. Neuropsychopharmacol. 2019;29:1453–1463. doi: 10.1016/j.euroneuro.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 41.Miranda-Azpiazu P., Díez-Alarcia R., García-Bea A., González-Maeso J., Morentín B., Meana J.J. P.1.g.022 Hallucinogenic and non-hallucinogenic 5-HT2A receptor agonists induce distinct patterns of G protein coupling in postmortem human brain. Eur. Neuropsychopharmacol. 2013;23:S201. doi: 10.1016/S0924-977X(13)70309-2. [DOI] [Google Scholar]
- 42.Buckholtz N.S., Zhou D., Freedman D.X. Serotonin2 agonist administration down-regulates rat brain serotonin2 receptors. Life Sci. 1988;42:2439–2445. doi: 10.1016/0024-3205(88)90342-6. [DOI] [PubMed] [Google Scholar]
- 43.Madsen M.K., Fisher P.M., Burmester D., Dyssegaard A., Stenbæk D.S., Kristiansen S., Johansen S.S., Lehel S., Linnet K., Svarer C., et al. Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels. Neuropsychopharmacology. 2019;44:1328–1334. doi: 10.1038/s41386-019-0324-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menneson S., Ménicot S., Ferret-Bernard S., Guérin S., Romé V., Le Normand L., Randuineau G., Gambarota G., Noirot V., Etienne P., et al. Validation of a psychosocial chronic stress model in the pig using a multidisciplinary approach at the gut-brain and behavior levels. Front. Behav. Neurosci. 2019;13:161. doi: 10.3389/fnbeh.2019.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 Years of Image Analysis HHS Public Access. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to other on-going studies.