Abstract
BACKGROUND
Colorectal cancer is a common tumor with a quite high-related mortality. Despite the used curative treatments, patients will develop cancer recurrence in up to 50% of the cases and/or other primary neoplasms. Although most of the recurrences are discovered within 3 years from the first treatment, a small percentage is found after 5 years. The early detection of recurrence is crucial to allow further therapies improving patients’ survival. Several follow-up programs have been developed but the optimal one is far from being established.
AIM
To evaluation of potential prognostic factors for timing and patterns of recurrence in order to plan tailored follow-up programs.
METHODS
Perioperative and long-term data of all consecutive patients surgically treated with curative intent, from January 2006 to June 2009, for colorectal adenocar-cinoma, were retrospectively reviewed to find potential prognostic factors associated with: (1) Recurrence incidence; (2) Incidence of an early (within 3 years from surgery) or late recurrence; and (3) Different sites of recurrence. In addition, the incidence of other primary neoplasms has been evaluated in a cohort of patients with a minimum potential follow-up of 10 years.
RESULTS
Our study included 234 patients. The median follow-up period has been 119 ± 46.2 mo. The recurrence rate has been 25.6%. Patients with a higher chance to develop recurrence had also the following characteristics: Higher levels of preoperative glycemia and carcinoembryonic antigen, highest anaesthesiologists Score score, occlusion, received a complex operation performed with an open technique, after a longer hospital stay, and showed advanced tumors. The independent prognostic factors for recurrence were the hospital stay, N stage 2, and M stage 1 (multivariate analysis). Younger ages were significantly associated with an early recurrence onset. Patients that received intermediate colectomies or segmental resections, having an N stage 2 or American Joint Committee on Cancer stage 3 tumors were also associated with a higher risk of liver recurrence, while metastatic diseases at diagnosis were linked with local recurrence. Neoadjuvant treatments showed lung recurrence. Finally, bigger tumors and higher lymph node ratio were associated with peritoneal recurrence (marginally significant). Thirty patients developed a second malignancy during the follow-up time.
CONCLUSION
Several prognostic factors should be considered for tailored follow-up programs, eventually, beyond 5 years from the first treatment.
Keywords: Prognostic factors, Recurrence, Recurrence patterns, Colorectal cancer, Long-term follow-up, Follow-up programs
Core Tip: In this retrospective study, several potential prognostic factors for recurrence, timing, and recurrence sites have been evaluated in patients who received curative colorectal surgery for adenocarcinoma with a potential minimum follow-up of 10 years. The independent prognostic factors for recurrence were the hospital stay, N stage 2, and M stage 1. Of note, younger ages were significantly associated with an early onset of recurrence. Some prognostic factors have been found for each site of recurrence: Liver, local, lung, and peritoneum. Thirty patients developed a second malignancy during the follow-up period. These findings may help in providing a tailored follow-up program.
INTRODUCTION
Colorectal cancer (CRC) is one of the most common malignant neoplasms in the world with an age-standardized worldwide incidence of 19.7 and mortality of 8.9 per 100000 person-year, respectively[1].
Surgical resection is the cornerstone of CRC management. However, according to the clinical and pathological stage, this treatment should be integrated with neoadjuvant and/or adjuvant therapies, where appropriate[2].
Despite the curative-intent of the first treatment, the patients have a considerable risk of developing cancer recurrence and/or other primary tumors[3,4]. Liver is the most frequent site of recurrence and liver metastases are diagnosed in up to 50% of the patients. Lungs are the second site for frequency of recurrence but, unfortunately, only a small percentage of them will develop lungs-only recurrence susceptible to resection[5]. Locoregional and peritoneal recurrences are reported in 4% to 11.5% and 3% to 6%, respectively[5,6]. Finally, the increasing cumulative risk to develop a second colorectal cancer is reported to be 3% every 6 years[7].
Analysis of prognostic factors for recurrence and of the specific recurrence patterns is seldom reported since the great majority of the registries rarely detailed the sites of the development of metastases[8].
About 80% of the recurrences occurs within the first 3 years of primary surgery and about 95% within 5 years with a small percentage of the patients (0.9%-9%) who will suffer from recurrence 5 years after the treatment[9]. The clinical and pathologic characteristics of early or late recurrence after curative surgery have been rarely described. Therefore, it is unclear whether there is a significant difference between these two groups in terms of prognosis[10,11]. However, the early recurrence detection or the diagnosis of other neoplasms is an important factor to allow radical resection, when technically feasible and oncologically appropriate. Although the relation between early detection of recurrence and prognosis is still under debate[12], many follow-up programs have been developed over the years and in different countries. There are significant variations in the length and strength of follow-up strategies in the different centers, in the type and timing of the examination, in the staff conducting and reviewing the tests, and the optimum follow-up schedule is far from being defined[12,13]. The great majority of the surveillance programs ends in 5 years after the primary colorectal resection[3,9,12] while colonoscopy follow-up programs are recommended to be continued beyond 5 years with the timing established on endoscopic findings[7].
Our study aims to evaluate - in a cohort of patients who received curative surgery and who had a potential minimum of 10-years of follow-up - the clinical, operative, and pathological potential prognostic factors that may influence the recurrence development, the timing and site of disease presentation. This evaluation will allow us to fine-tune a tailored follow-up program.
MATERIALS AND METHODS
Study design
All patients submitted to an elective oncological colorectal surgery at the actually renamed Hepatobiliary Surgery Unit of Careggi Teaching Hospital, Florence, Italy from January 2006 to June 2009 and identified for follow-up of at least 10 years were evaluated for inclusion in this retrospective study.
Exclusion criteria were: Final histopathological diagnosis of benign pathology; other concomitant malignancies; histological report different from adenocarcinoma; previous oncological colorectal surgery; palliative-intent surgery, unavailability of data about recurrence status.
Data concerning demographic aspects, primary lesion, operative and postoperative outcomes, histopathological response, and long-term outcomes were prospectively recorded in a specific database. Standard preoperative work-up included triple-phase contrast-enhanced computed tomography (CT) scan and pancolonoscopy. Other radiological tests, including magnetic resonance imaging and positron emission tomography scan, were performed when required. Every decision about patients' treatments was taken after the weekly Multidisciplinary Team evaluation.
Some definitions: “Right colon” has been defined as the tract between the caecum and middle transverse colon while “left colon” has been defined as the tract between the splenic flexure and sigmoid colon.
The surgical technique was chosen according stage disease, patient conditions, and surgeon’s preference and it was reported according to an “intention-to-treat” evaluation. Associated procedures were defined as “minor” including appendi-cectomy, oophorectomy, or cholecystectomy, and “major” including hepatic resections. Histopathological evaluation was performed following the tumor node metastasis (TNM) classification, 6th edition[14]. The lymph node ratio was defined as the number of positive lymph nodes on the total lymph nodes retrieved. Chemotherapy was considered if administered to the patient despite the interruption of the initially scheduled program due to intolerance or any other reasons. Recurrence was considered in case of high radiological suspicion and/or after the biopsy and it was divided into liver, lung, peritoneal, and “locoregional” metastasis (including tumor recurrence on the previous anastomotic line or in the lymph nodes or soft tissue near the previous surgical site). Recurrence was defined as “early” or “late” if it occurred within or beyond 3 years from surgery, respectively. In case of patient presentation with CRC and synchronous liver metastasis treated with two a 2-step surgery, disease-free survival (DFS) was considered as the time between the second intervention and the time of the first available diagnosis of recurrence or death. Recurrences were treated with surgery, chemotherapy, radiation, palliation of the symptoms including jaundice or best supportive care, as appropriated.
Within the subgroup of patients experiencing recurrence, ananalysis of the potential prognostic factors for each site of recurrence was performed to try to find clinicopathological patterns of recurrence.
Follow-up program
Follow-up was conducted according to a standardized program. The complete follow-up program is reported in Table 1. Follow-up included a physical examination, carcinoembrionyc antigen (CEA) determination, and a routine blood examination, endoscopy, chest radiography, abdominal sonography, and/or a CT scan. If recurrence was suspected or patients developed abdominal symptoms, further examinations were performed (i.e. whole-body positron emission tomography or hepatic magnetic resonance imaging). Scheduled tests could have been modified according to the oncologist’s indications.
Table 1.
Complete follow-up program
| Time from surgery |
Tests
|
||||
|
Full blood count, liver function tests, CEA
|
Abdominal US
|
Chest X-Ray
|
Abdominal CT scan
|
Colonoscopy
|
|
| 3 mo | √ | ||||
| 6 mo | √ | √ | √ | ||
| 9 mo | √ | ||||
| 12 mo | √ | √ | √ | √ | |
| 18 mo | √ | ||||
| 2 yr | √ | √ | √ | √ | |
| 3 yr | √ | √ | √ | √ | |
| 4 yr | √ | √ | √ | ||
| 5 yr | √ | √ | √ | √ | |
CEA: Carcinoembrionyc antigen; US: Ultrasonography; CT: Computed tomography.
Retrieval of follow-up data was completed with a revision via available medical records and phone call interviews.
Analysis
Patients’ data were prospectively collected into a database that was retrospectively reviewed. Continuous variables were reported as mean ± SD while categorical variables were reported as frequency and percentage.
To evaluate the association between possible prognostic factors and DFS and overall survival (OS) a Cox model, Kaplan-Meier method, and log-rank test were used.
To estimate possible independent prognostic factors for recurrence a multiple Cox model with a backward selection method was used. To assess the association between each possible prognostic factor and timing to recurrence (< 3 years or ≥ 3 years) a simple logistic regression model was used.
Statistical significance was defined as P value ≤ 0.05.
Data were analyzed using the statistical software SPSS, version 24 (IBM Corp.). The statistical review of the study was performed by the biostatistic Lorenzo Tofani.
RESULTS
During the study period, 360 patients underwent colorectal surgery for neoplasms. According to the exclusion criteria, 234 patients were included in our analysis. Further details are shown in Figure 1.
Figure 1.
Inclusion flow-chart. Patients excluded from the study (white boxes) and included in the study.
Analysis of survival and recurrence
The median follow-up time was 119 ± 46.2 mo. Tumor recurrences occurred in 60 patients (25.6%). The OS rate was 86.7%, 78.1%, and 59.9% at 3, 5, and 10 years, respectively. The DFS rate was 75.7%, 71.2%, and 58.3% at 3, 5, and 10 years, respectively, with a median DFS of 150 mo.
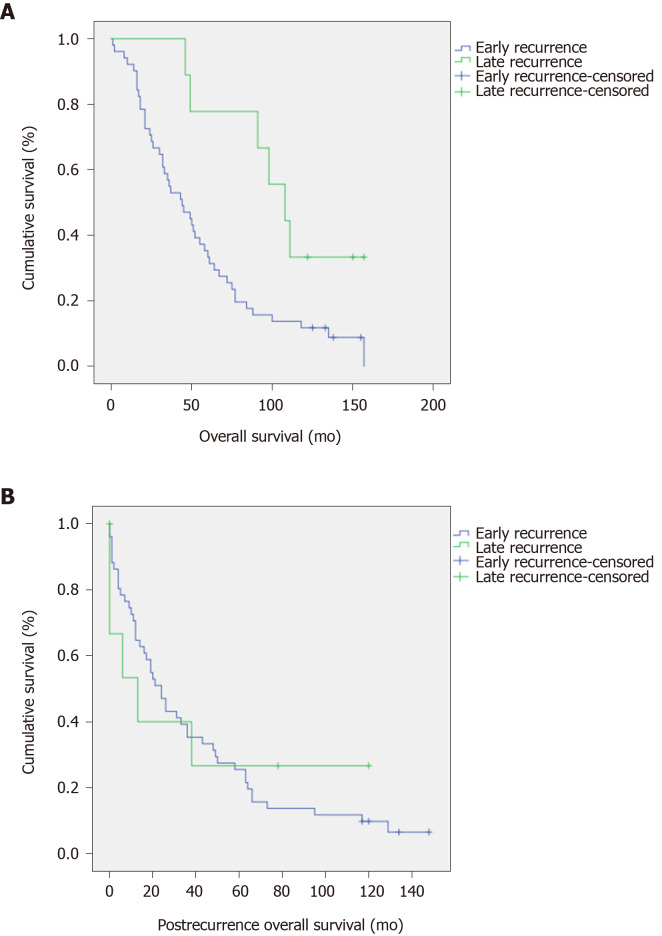
Table 2 includes the significant potential prognostic factors for DFS and OS. The American Joint Committee on Cancer (AJCC) stage and the pathological M stage resulted as strong prognostic factors for both DFS and OS. The recurrence timing resulted in a significant prognostic factor for OS but there were no significant differences in post recurrence OS (P = 0.011 and P = 0.991, respectively, Figure 2).
Table 2.
Potential prognostic factor for disease-free survival and overall survival
|
|
Disease free survival (DFS)
|
P
value
|
Overall survival (OS)
|
P
value
|
||||
|
3 yr (%)
|
5 yr (%)
|
10 y (%)
|
3 yr (%)
|
5 yr (%)
|
10 yr (%)
|
|||
| CEA | 0.095 | 0.045 | ||||||
| < 5 ng/mL | 84.4 | 80.8 | 73.1 | 88.3 | 79.8 | 69.6 | ||
| ≥ 5 ng/mL | 72.2 | 61.1 | 50.0 | 81.0 | 66.7 | 42.9 | ||
| AJCC stage | < 0.0001 | < 0.0001 | ||||||
| 1 | 86.9 | 82.9 | 70.6 | 91.1 | 83.5 | 70.4 | ||
| 2 | 87.7 | 76.2 | 57.9 | 92.9 | 82.1 | 54.3 | ||
| 3 | 59.3 | 57.9 | 50.6 | 72.0 | 61.0 | 48.5 | ||
| 4 | 20.0 | - | - | 63.6 | 45.5 | 18.2 | ||
| Pathologic M stage1 | < 0.0001 | 0.002 | ||||||
| 0 | 77.0 | 71.2 | 58.4 | 85.1 | 74.4 | 57.0 | ||
| 1 | 20.0 | - | - | 63.6 | 45.5 | 18.2 | ||
| Retrieved LN | 0.819 | 0.688 | ||||||
| < 12 | 81.5 | 75.4 | 58.1 | 87.5 | 76.4 | 54.7 | ||
| ≥ 12 | 73.3 | 69.3 | 58.8 | 83.2 | 73.8 | 56.4 | ||
| LN ratio | 0.068 | 0.043 | ||||||
| < 15 | 66.8 | 64.0 | 55.6 | 78.0 | 70.7 | 56.0 | ||
| ≥ 15 | 41.5 | 41.5 | 36.2 | 61.7 | 44.7 | 33.8 | ||
| Timing of recurrence | 0.028 | |||||||
| Early (< 3 yr2) | 54.9 | 33.3 | 11.8 | |||||
| Late (≥ 3 yr2) | 100 | 77.8 | 22.2 | |||||
According to the tumor node metastasis staging system.
Recurrence from the first treatment.
CEA: Carcinoembryonic antigen; AJCC: American Joint Committee on Cancer; LN: Lymph node.
Figure 2.
Kaplan-Meier curves for overall survival. A: Kaplan–Meier curve of overall survival (OS) stratified by Timing of Recurrence. Median OS for patients with recurrence < 3 years was 44 mo (95% Confidential interval 29-59 mo) vs 108 (95% Confidential interval 79-137 mo) for those with recurrence ≥ 3 years (P = 0.011); B: Kaplan Meier curve of post recurrence OS stratified by Timing of Recurrence. Median post recurrence OS for patients with recurrence < 3 years was 24 mo (95% Confidential interval 16-32 mo) vs 13 (95% Confidential interval 0-30 mo) for those with recurrence ≥ 3 years (P = 0.991).
Recurrence characteristics and related treatments are reported in Table 3.
Table 3.
Recurrence characteristics and related treatment
|
|
Total, n = 234
|
%
|
| Recurrence | ||
| No | 174 | 67.7 |
| Yes | 60 | 23.3 |
| Timing of recurrence1 | ||
| < 3 yr | 51 | 19.8 |
| ≥ 3 yr | 9 | 3.5 |
| Liver recurrence | ||
| No | 207 | 80.5 |
| Yes | 25 | 9.7 |
| Missing | 2 | 9.7 |
| Lung recurrence | ||
| No | 216 | 84.0 |
| Yes | 16 | 6.2 |
| Missing | 2 | 9.7 |
| Local recurrence | ||
| No | 215 | 83.7 |
| Yes | 17 | 6.6 |
| Missing | 2 | 9.7 |
| Peritoneal seeding | ||
| No | 225 | 87.5 |
| Yes | 7 | 2.7 |
| Missing | 2 | 9.7 |
| Treatment of the first recurrence | ||
| Surgery | 25 | 41.7 |
| Chemotherapy | 20 | 33.3 |
| Best supportive care | 8 | 13.3 |
| Palliation | 2 | 3.3 |
| Missing | 5 | 8.3 |
Recurrence from the first treatment.
Fifty-one patients (85% of those experiencing recurrence) recurred within 3 years from the first intervention while 9 (15%) of them recurred beyond 3 years. The recurrence rate after 5 years was 1.7% within the entire cohort and 6.7% within the recurrence group. Consequently, 15.3 patients had to be observed between 5 and 10 years from the first treatment in order to detect 1 recurrence beyond 5 years.
Twenty-two out of the 24 patients who received a second surgical curative intervention, with or without subsequent chemotherapy protocols, achieved a status of disease-free. Four of them resulted in disease-free and alive 10 years after surgery while twelve of them developed further recurrence within a mean time from the first intervention of 44.4 mo (11-85 mo). Additional analysis involving recurrence treatments will be no object of the present study.
Fifty patients (83.3% of those experiencing recurrence) presented with the persistence of tumoral disease at death or at the time of the last follow-up.
Actual 10-years survivors were 111 (47.4%), 6 of them had developed a recurrence during the follow-up time and had received a second treatment.
Moreover, during the follow-up period, 30 patients developed a second malignancy: Colorectal (n = 6), breast (n = 4), prostate (n = 2), other urologic cancer (n = 3), intracranial cancer (n = 4), pancreas (n = 4) and other (n = 7). The mean time of second malignancies development was 80 mo (range 8-153). Amongst these patients, 18 developed a second neoplasm beyond 5 years and 6 died because of second neoplasms.
Analysis of the prognostic factors for recurrence and comparison between early and late recurrence
Demographic and patient-related potential prognostic factors for recurrence and evaluation of early vs late recurrence are shown in Table 4.
Table 4.
Demographic and patient-related preoperative potential prognostic factors for recurrence and evaluation of early vs late recurrence (univariate analysis)
|
Recurrence, n = 234
|
Timing of recurrence, n = 60
|
||||||||
|
All
|
No
|
Yes
|
HR (95%CI)
|
P
value
|
Early recurrence (< 3 yr)
|
Late recurrence (≥ 3 yr)
|
OR (95%CI)
|
P
value
|
|
|
n
(%)
|
n
= 174 (67.7%)
|
n
= 60 (23.3%)
|
n
= 51 (85.0%)
|
n
= 9 (15.0%)
|
|||||
| Age, yr; mean ± SD | 68 ± 12 | 68 ± 11 | 69 ± 12 | 1.00 (0.98-1.02) | 0.868 | 67 ± 12 | 77 ± 8 | 1.10 (1.00-1.21) | 0.050 |
| Gender, n (%) | 0.179 | 0.421 | |||||||
| Male | 128 (54.7) | 99 (77.3) | 29 (22.6) | Reference | 25 (86.2) | 4 (13.8) | Reference | ||
| Female | 106 (45.3) | 75 (70.7) | 31 (29.2) | 1.42 (0.85-2.37) | 26 (83.9) | 5 (16.1) | 1.85 (0.41-8.21) | ||
| Preop Hb, g/dL, mean ± SD | 12.3 ± 2.1 | 12.6 ± 2.1 | 11.6 ± 2.3 | 0.84 (0.71-1.01) | 0.058 | 11.9 ± 2.2 | 9.67 ± 2.3 | 0.60 (0.31-1.17) | 0.136 |
| Preop glycemia, g/dL, mean ± SD | 0.99 ± 0.26 | 0.96 ± 0.24 | 1.08 ± 0.31 | 3.44 (1.05-11.24) | 0.040 | 1.10 ± 0.34 | 0.99 ± 0.19 | 0.25 (0.00-16.06) | 0.518 |
| Preop total proteins, g/dL, mean ± SD | 6.8 ± 0.7 | 6.8 ± 0.6 | 6.8 ± 1 | 1.07 (0.54-2.11) | 0.839 | 6.7 ± 1.0 | 7.3 ± 0.2 | 2.43 (0.47-12.54) | 0.288 |
| Preop CEA, n (%) | 0.029 | 0.440 | |||||||
| < 5 ng/mL | 85 (82.5) | 71 (83.5) | 14 (16.5) | 0.36 (0.15-0.90) | 12 (85.7) | 2 (14.3) | Reference | ||
| ≥ 5 ng/mL | 18 (17.5) | 11 (61.1) | 7 (38.9) | Reference | 5 (71.4) | 2 (28.6) | 2.40 (0.26-22.10) | ||
| Missing | 131 | 131 | 39 | ||||||
| BMI, mean ± SD | 26 ± 4 | 25 ± 4 | 27 ± 5 | 1.06 (0.99-1.14) | 0.085 | 27 ± 5 | 27 ± 5 | 0.98 (0.81-1.19) | 0.866 |
| ASA, n (%) | |||||||||
| 1 | 31 (14.5) | 26 (83.9) | 5 (16.1) | Reference | 5 (100) | 0 (0) | Reference | ||
| 2 | 80 (37.4) | 61 (76.2) | 19 (23.8) | 1.50 (0.56-4.02) | 0.421 | 16 (84.2) | 3 (15.8) | - | 0.951 |
| 3 | 94 (43.9) | 72 (76.6) | 22 (23.4) | 1.51 (0.57-3.40) | 0.404 | 16 (72.7) | 6 (27.3) | - | 0.948 |
| 4 | 9 (4.2) | 5 (55.6) | 4 (44.4) | 3.85 (1.03-14.41) | 0.045 | 4 (100) | 0 (0) | - | 1.000 |
| Missing | 20 | 20 | 10 | ||||||
| Presentation with occlusion, n (%) | 0.021 | 0.366 | |||||||
| No | 42 (59.2) | 33 (78.6) | 9 (21.4) | Reference | 7 (77.8) | 2 (22.2) | Reference | ||
| Yes | 29 (40.8) | 15 (51.7) | 14 (48.3) | 2.60 (1.15-5.87) | 13 (92.9) | 1 (7.1) | 0.31 (0.02-3.97) | ||
| Missing | 163 | 163 | 37 | ||||||
| Tumor site, n (%) | |||||||||
| Right colon | 70 (29.9) | 53 (75.7) | 17 (24.3) | Reference | 13 (76.5) | 4 (23.5) | Reference | ||
| Left colon | 103 (44) | 76 (73.8) | 27 (26.2) | 0.93 (0.50-1.70) | 0.807 | 24 (88.9) | 3 (11.1) | 0.41 (0.08-2.10) | 0.282 |
| Rectum | 61 (26.1) | 45 (73.8) | 16 (26.2) | 0.94 (0.47-1.85) | 0.851 | 14 (87.5) | 2 (12.5) | 0.46 (0.07-2.98) | 0.418 |
HR: Hazard ratio; OR: Odds ratio; CI: Confidential intervals; Preop: Preoperative value; Hb: Haemoglobin; CEA: Carcinoembryonic antigen; BMI: Body mass index; ASA: American Society of Anaesthesiologists Score.
Preoperative glycemia and abnormal CEA values were significantly higher in the recurrence group.
Anaesthesiologists Score (ASA) score grade 4 has a more than 3-fold higher recurrence risk compared to the American Society of ASA grade 1 (P = 0.045). Patients presenting with bowel obstruction were 32. Four of them received a transverse loop colostomy before curative surgery. The tumor appearance with occlusive symptoms was higher in the recurrence group (P = 0.021).
Younger ages resulted significantly associated with early recurrence (P = 0.050).
Table 5 reports the treatment-related potential prognostic factors for recurrence and evaluation of early vs late recurrence.
Table 5.
Treatment-related potential prognostic factors for recurrence and evaluation of early versus late recurrence (univariate analysis)
|
Recurrence, n = 234
|
Timing of recurrence, n = 60
|
||||||||
|
All
|
No
|
Yes
|
HR (95%CI)
|
P
value
|
Early recurrence (< 3 yr)
|
Late recurrence (≥ 3 yr)
|
OR (95%CI)
|
P
value
|
|
|
n
, %
|
n
= 174 (67.7%)
|
n
= 60 (23.3%)
|
n
= 51 (85.0%)
|
n
= 9 (15.0%)
|
|||||
| Neoadjuvant therapy, n (%) | 0.921 | 0.949 | |||||||
| No | 97 (93.3) | 68 (70) | 29 (30) | Reference | 25 (86.2) | 4 (13.8) | Reference | ||
| Yes | 7 (6.7) | 5 (71) | 2 (29) | 1.07 (0.26-4.51) | 2 (100) | 0 (0) | 1.13 (0.02-53.57) | ||
| Missing | 130 | 130 | 29 | ||||||
| Surgery, n (%) | |||||||||
| Right emicolectomy | 55 (23.5) | 42 (76.4) | 13 (23.6) | Reference | 10 (76.9) | 3 (23.1) | Reference | ||
| Extended right emicolectomy | 15 (6.4) | 12 (80) | 3 (20) | 1.16 (0.37-3.59) | 0.801 | 2 (66.7) | 1 (33.3) | 4.20 (0.37-47.64) | 0.247 |
| Intermediate colectomy | 10 (4.3) | 6 (60) | 4 (40) | 2.16 (0.70-6.71) | 0.182 | 3 (75) | 1 (25) | 1.80 (0.14-23.6) | 0.655 |
| AR/Hartmann | 106 (45.3) | 80 (75.5) | 26 (24.5) | 1.09 (0.55-2.17) | 0.798 | 24 (92.3) | 2 (7.7) | 0.43 (0.06-3.02) | 0.395 |
| LAR/Miles | 38 (16.2) | 28 (73.7) | 10 (26.3) | 1.16 (0.50-2.68) | 0.731 | 8 (80) | 2 (20) | 1.23 (0.16-9.69) | 0.841 |
| Total/Sub-total Colectomy | 6 (2.6) | 3 (50) | 3 (50) | 2.69 (0.76-9.53) | 0.126 | 3 (100) | 0 (0.0) | 0.60 (0.01-24.50) | 0.787 |
| Segmental resection | 4 (1.7) | 3 (75) | 1 (25) | 1.12 (0.14-8.68) | 0.916 | 1 (100) | 0 (0.0) | 1.7 (0.02-162.35) | 0.819 |
| Associated procedure, n (%) | |||||||||
| No | 52 (50.5) | 39 (75) | 13 (25) | Reference | 11 (84.6) | 2 (15.4) | Reference | ||
| Minor | 40 (38.8) | 27 (67.5) | 13 (32.5) | 1.30 (0.60-2.80) | 0.505 | 11 (84.6) | 2 (15.4) | 1.00 (0.13-7.44) | 1.00 |
| Major | 11 (10.7) | 4 (36.4) | 7 (63.6) | 3.56 (1.41-8.96) | 0.007 | 7 (100) | 0 (0) | 0.31 (0.01-8.95) | 0.492 |
| Missing | 131 | 131 | 27 | ||||||
| Operative technique, n (%) | < 0.001 | 0.061 | |||||||
| Open | 99 (42.7) | 61 (61.6) | 38 (38.4) | Reference | 35 (92.1) | 3 (7.9) | Reference | ||
| Laparoscopy | 133 (57.3) | 111 (83.5) | 22 (16.5) | 0.37 (0.22-0.62) | 16 (72.7) | 6 (27.3) | 4.00 (0.94-17.01) | ||
| Missing | 2 | 2 | - | ||||||
| Duration of surgery, min – mean ± SD | 232 ± 67 | 230 ± 61 | 239 ± 83 | 1.00 (0.99-1.01) | 0.496 | 238 ± 78 | 242 ± 112 | 1.00 (0.99-1.01) | 0.808 |
| Postop blood transfusion, n (%) | 0.123 | 0.119 | |||||||
| No | 174 (75.7) | 134 (77) | 40 (23) | Reference | 36 (90) | 4 (10) | Reference | ||
| Yes | 56 (24.3) | 37 (66.1) | 19 (33.9) | 1.54 (0.89-2.66) | 14 (73.7) | 5 (26.3) | 3.08 (0.75-12.64) | ||
| Missing | 4 | 4 | 1 | ||||||
| Reoperation, n (%) | 0.262 | 0.559 | |||||||
| No | 82 (91.1) | 60 (73.2) | 22 (26.8) | Reference | 17 (77.3) | 5 (22.7) | Reference | ||
| Yes | 8 (8.9) | 5 (62.5) | 3 (37.5) | 1.99 (0.60-6.61) | 2 (66.7) | 1 (33.3) | 2.13 (0.17-26.68) | ||
| Missing | 144 | 144 | 35 | ||||||
| Hospital stay, d, mean ± SD | 9.2 ± 6 | 8.7 ± 3.7 | 10.9 ± 9.1 | 1.05 (1.02-1.07) | 0.001 | 10.9 ± 9.8 | 10.6 ± 4.0 | 1.01 (0.95-1.08) | 0.737 |
| Adjuvant therapy, n (%) | 0.064 | 0.057 | |||||||
| No | 127 (56.4) | 103 (81.1) | 24 (18.9) | Reference | 18 (75) | 6 (25) | Reference | ||
| Yes | 98 (43.5) | 67 (68.4) | 31 (31.6) | 1.65 (0.97-2.81) | 28 (90.3) | 3 (9.7) | 0.22 (0.04-1.04) | ||
| Missing | 9 | 9 | 5 | ||||||
| Start of adj CHT, n (%) | 0.268 | 0.401 | |||||||
| < 6 wk | 26 (48.1) | 13 (50) | 13 (50) | Reference | 12 (92.3) | 1 (7.7) | Reference | ||
| ≥ 6 wk | 28 (51.9) | 19 (67.9) | 9 (32.1) | 1.63 (0.69-3.95) | 8 (88.9) | 1 (11.1) | 4.4 (0.14-141.01) | ||
| Missing | 180 | 180 | 38 | ||||||
HR: Hazard ratio; OR: Odds ratio; CI: Confidential intervals; AR: Anterior resection of the rectum; LAR: Low anterior resection of the rectum; Adj CHT: Adjuvant chemotherapy.
Ten percent of the patients with a rectal cancer location received neoadjuvant chemoradiation and administration of neoadjuvant therapy did not result in a significant prognostic factor although the high rate of missing data has to be taken into account. Patients treated with an associated major procedure have a 3.5-fold higher risk of recurrence when compared to the patients receiving only the colorectal resection (P = 0.007). Patients treated with open surgery have a high risk of recurrence (P < 0.001) and the open technique showed a marginally significant association (P = 0.061) with “early recurrence”.
During the hospital stay, 10 patients required reoperations due to complications: Anastomotic leak (n = 5), hemoperitoneum (n = 2), wound dehiscence (n = 1), rectal bleeding not amenable to endoscopic treatments (n = 1), acute pancreatitis (n = 1). However, reoperation did not result associated with higher recurrence risk. A longer hospital stay resulted significantly associated with recurrence (P = 0.001).
Chemotherapy mainly consisted of 5-fluorouracil and folinic acid. Some patients were also treated with capecitabine, irinotecan, and oxaliplatin-based chemotherapy. Administration of adjuvant chemotherapy showed a marginally significant association with recurrence rate and with the early onset of the disease (P = 0.064 and P = 0.057, respectively).
Pathological-related potential prognostic factors for recurrence and evaluation of early vs late recurrence are presented in Table 6.
Table 6.
Pathological-related potential prognostic factors for recurrence and evaluation of early versus late recurrence (univariate analysis)
|
|
Recurrence, n = 234
|
Timing of recurrence, n = 60
|
|||||||
|
All
|
No
|
Yes
|
HR (95%CI)
|
P
value
|
Early recurrence (< 3 yr)
|
Late recurrence (≥ 3 yr)
|
OR (95%CI)
|
P
value
|
|
|
n
(%)
|
n
= 174 (67.7%)
|
n
= 60 (23.3%)
|
n
= 51 (85.0%)
|
n
= 9 (15.0%)
|
|||||
| Major tumor diameter, mm, mean ± SD | 43 ± 23 | 41.6 ± 23 | 46.8 ± 24.5 | 1.01 (0.99-1.02) | 0.130 | 47.6 ± 23.5 | 42.8 ± 29.8 | 0.99 (0.96-1.02) | 0.694 |
| Pathological T stage1, n (%) | |||||||||
| 1 | 46 (19.7) | 44 (95.7) | 2 (4.3) | Reference | 2 (100) | 0 (0) | Reference | ||
| 2 | 40 (17.2) | 32 (80) | 8 (20) | 3.44 (0.91-12.99) | 0.068 | 6 (75) | 2 (25) | 0.64 (0.04-10.57) | 0.756 |
| 3 | 141 (60.5) | 94 (66.7) | 47 (33.3) | 6.14 (1.91-19.77) | 0.002 | 40 (85.1) | 7 (14.9) | 0.27 (0.02-3.20) | 0.298 |
| 4 | 6 (2.6) | 4 (66.7) | 2 (33.3) | 6.19 (1.03-37.96) | 0.046 | 2 (100) | 0 (0) | 0.33 (0.00-24.94) | 0.624 |
| Missing | 1 | 1 | 1 | ||||||
| Pathological N stage1, n (%) | |||||||||
| 0 | 155 (66.5) | 129 (83.2) | 26 (16.8) | Reference | 18 (69.2) | 8 (30.8) | Reference | ||
| 1 | 51 (21.9) | 36 (70.6) | 15 (29.4) | 1.95 (1.03-3.69) | 0.039 | 15 (100) | 0 (0) | 0.07 (0.00-1.44) | 0.085 |
| 2 | 27 (11.6) | 9 (33.3) | 18 (66.7) | 6.35 (3.50-11.64) | < 0.001 | 17 (94.4) | 1 (5.6) | 0.19 (0.03-1.25) | 0.084 |
| Missing | 1 | 1 | 1 | ||||||
| Pathological M stage1, n (%) | < 0.001 | 0.408 | |||||||
| 0 | 223 (95.6) | 171 (76.7) | 52 (23.3) | Reference | 43 (82.7) | 9 (17.3) | Reference | ||
| 1 | 10 (4.3) | 2 (20) | 8 (80) | 5.31 (2.50-11.30) | 8 (100) | 0 (0) | 0.27 (0.01-6.02) | ||
| Missing | 1 | 1 | - | ||||||
| AJCC stage, n (%) | |||||||||
| 1 | 77 (32.9) | 68 (88.3) | 9 (11.7) | Reference | 7 (77.8) | 2 (22.2) | Reference | ||
| 2 | 75 (32) | 59 (78.7) | 16 (21.3) | 1.65 (0.74-3.67) | 0.220 | 10 (62.5) | 6 (37.5) | 1.12 (0.20-6.17) | 0.894 |
| 3 | 72 (30.8) | 45 (62.5) | 27 (37.5) | 3.59 (1.73-7.42) | < 0.001 | 26 (96.3) | 1 (3.7) | 0.12 (0.01-1.03) | 0.053 |
| 4 | 10 (4.3) | 2 (20) | 8 (80) | 11.1 (4.35-28.52) | < 0.001 | 8 (100) | 0 (0) | 0.13 (0.00-3.43) | 0.220 |
| Retrieved LN, n (%) | 0.535 | 0.741 | |||||||
| < 12 | 65 (29.3) | 53 (81.5) | 12 (18.5) | Reference | 10 (83.3) | 2 (16.7) | Reference | ||
| ≥ 12 | 157 (70.7) | 112 (71.3) | 45 (28.7) | 0.84 (0.47-1.47) | 38 (84.4) | 7 (15.6) | 0.78 (0.18-3.40) | ||
| Missing | 12 | 12 | 3 | ||||||
| LN ratio, mean ± SD | 20 ± 16 | 16 ± 13 | 25 ± 19 | 25.8 (8.22-80.93) | < 0.001 | 25 ± 19 | 22 | 0.00 (0.00-2.20) | 0.073 |
| Colloid component, n (%) | 0.168 | 0.607 | |||||||
| No | 63 (60) | 49 (77.8) | 14 (22.2) | Reference | 12 (85.7) | 2 (14.3) | Reference | ||
| Yes | 42 (40) | 27 (64.3) | 15 (35.7) | 1.68 (0.80-3.53) | 13 (86.7) | 2 (13.3) | 0.55 (0.06-5.22) | ||
| Missing | 129 | 129 | 31 | ||||||
According to the tumor node metastasis staging system.
AJCC: American Joint Committee on Cancer; LN: Lymph nodes; HR: Hazard ratio; OR: Odds ratio; CI: Confidential intervals.
Each parameter of the TNM classification and the AJCC stage resulted strongly associated with recurrence and the group patients of the AJCC stage 3 showed a marginally significant higher chance to develop an early recurrence when compared to the AJCC stage 1 (P = 0.053).
The mean number of lymph nodes retrieved was 19.4 (range 3-133). An incorrect disease stadiation following the retrieval of fewer than 12 nodes did not significantly influence the recurrence (P = 0.535). The lymph node ratio was significantly higher in the recurrence group (P < 0.001).
At the multivariate analysis, the only independent prognostic factors for recurrence were the hospital stay, N stage 2, and M stage 1 (Table 7). None of the prognostic factors analyzed remained significant at the multivariate analysis of the comparison between early or late recurrence.
Table 7.
Multivariate analysis of potential prognostic factors for recurrence
|
|
HR
|
95%CI
|
P
value
|
| Hospital stay, d | 1.053 | 1.023-1.084 | < 0.001 |
| Pathological N stage1 | |||
| 0 | Reference | ||
| 1 | 1.608 | 0.773-3.343 | 0.204 |
| 2 | 6.129 | 3.070-12.236 | < 0.001 |
| Pathological M stage1 | |||
| 0 | Reference | ||
| 1 | 5.521 | 2.113-14.425 | < 0.001 |
According to the tumor node metastasis staging system.
HR: Hazard ratio; CI: Confidential intervals.
Pattern of recurrence
Two patients were excluded from this analysis because there were no available data about the recurrence site. The most frequent site of recurrence was the liver (41.7%), followed by the locoregional recurrence (28.3%), the lung (26.7%), and the peritoneum (11.7%).
Tables 8-10 reported the results of the univariate analysis of the previously reported potential prognostic factors for liver and lung recurrence.
Table 8.
Demographic and patient-related preoperative potential prognostic factors for liver and lung recurrence
|
|
Recurrence, n = 58
|
|||||||
|
Liver
|
Lung
|
|||||||
|
No, n = 33 (55.0%)
|
Yes, n = 25 (41.7%)
|
HR (95%CI)
|
P
value
|
No, n = 42 (70.0%)
|
Yes, n = 16 (26.7%)
|
HR (95%CI)
|
P
value
|
|
| Age, yr, mean ± SD | 67.4 ± 12.3 | 68.0 ± 12.3 | 1.00 (0.97-1.03) | 0.945 | 67.3 ± 12.7 | 68.7 ± 11.0 | 1.00 (0.96-1.04) | 0.837 |
| Gender, n (%) | 0.702 | 0.185 | ||||||
| Male | 16 (55.2) | 13 (44.8) | Reference | 19 (65.5) | 10 (34.5) | Reference | ||
| Female | 17 (58.6) | 12 (41.4) | 0.86 (0.39-1.88) | 23 (79.3) | 6 (20.7) | 0.50 (0.18-1.39) | ||
| Preop Hb, g/dL, mean ± SD | 11.28 | 11.93 | 1.16 (0.88-1.53) | 0.296 | 11.4 ± 2.3 | 12.0 ± 2.5 | 1.14 (0.81-1.61) | 0.446 |
| Preop glycemia, g/dL, mean ± SD | 1.08 ± 0.36 | 1.08 ± 0.31 | 1.72 (0.14-10.00) | 0.884 | 1.06 ± 0.33 | 1.14 ± 0.35 | 1.47 (0.12-18.20) | 0.764 |
| Preop total proteins, g/dL, mean ± SD | 6.7 ± 0.7 | 7.1 ± 0.8 | 1.33 (0.56-3.17) | 0.514 | 6.9 ± 0.9 | 6.9 ± 0.5 | 0.87 (0.19-3.93) | 0.861 |
| Preop CEA, n (%) | 0.868 | 0.891 | ||||||
| < 5 ng/mL | 6 (46.2) | 7 (53.8) | Reference | 10 (76.9) | 3 (23.1) | Reference | ||
| ≥ 5 ng/mL | 4 (57.1) | 3 (42.9) | 0.89 (0.23-3.46) | 5 (71.4) | 2 (28.6) | 1.14 (0.18-7.14) | ||
| Missing | 23 | 15 | 27 | 11 | ||||
| BMI, mean ± SD | 26.3 ± 4.9 | 27.8 ± 4.7 | 1.04 (0.94-1.15) | 0.465 | 27.6 ± 4.8 | 25.7 ± 4.6 | 0.96 (0.83-1.13) | 0.646 |
| ASA, n (%) | ||||||||
| 1 | 3 (60) | 2 (40) | Reference | 4 (80) | 1 (20) | Reference | ||
| 2 | 8 (42.1) | 11 (57.9) | 0.64 (0.15-2.75) | 0.544 | 14 (73.7) | 5 (26.3) | 0.46 (0.06-3.38) | 0.447 |
| 3 | 13 (61.9) | 8 (38.1) | 0.34 (0.07-1.55) | 0.163 | 15 (71.4) | 6 (28.6) | 0.31 (0.04-2.35) | 0.258 |
| 4 | 3 (100) | 0 (0.0) | 0.30 (0.01-7.69) | 0.471 | 2 (66.7) | 1 (33.3) | 2.09 (0.16-27.56) | 0.573 |
| Missing | 6 | 4 | 7 | 3 | ||||
| Presentation with occlusion, n (%) | 0.465 | 0.470 | ||||||
| No | 5 (62.5) | 3 (37.5) | Reference | 6 (75) | 2 (25) | Reference | ||
| Yes | 6 (46.2) | 7 (53.8) | 1.58 (0.46-5.44) | 9 (69.2) | 4 (30.8) | 1.89 (0.34-10.61) | ||
| Missing | 23 | 15 | 27 | 11 | ||||
| Tumour site, n (%) | ||||||||
| Right colon | 11 (68.7) | 5 (31.3) | Reference | 13 (81.3) | 3 (18.7) | Reference | ||
| Left colon | 11 (42.3) | 15 (57.7) | 2.48 (0.89-6.88) | 0.081 | 20 (76.9) | 6 (23.1) | 1.73 (0.42-7.04) | 0.445 |
| Rectum | 11 (68.7) | 5 (31.3) | 0.98 (0.28-3.41) | 0.972 | 9 (56.3) | 7 (43.7) | 2.34 (0.59-9.32) | 0.226 |
HR: Hazard ratio; OR: Odds ratio; CI: Confidential intervals; Preop: Preoperative value; Hb: Haemoglobin; CEA: Carcinoembryonic antigen; BMI: Body mass index; ASA: American Society of Anaesthesiologists Score.
Table 10.
Pathological-related potential prognostic factors for liver and lung recurrence
|
|
Recurrence, n = 58
|
|||||||
|
Liver
|
Lung
|
|||||||
|
No, n = 33 (55.0%)
|
Yes, n = 25 (41.7%)
|
HR (95%CI)
|
P
value
|
No, n = 42 (70.0%)
|
Yes, n = 16 (26.7%)
|
HR (95%CI)
|
P
value
|
|
| Major tumor diameter, mm, mean ± SD | 55.71 | 42.74 | 0.99 (0.98-1.01) | 0.368 | 29.09 | 30.01 | 0.99 (0.97-1.01) | 0.326 |
| Pathological T stage1, n (%) | ||||||||
| 1 | 1 (50) | 1 (50) | Reference | 1 (50) | 1 (50) | Reference | ||
| 2 | 6 (75) | 2 (25) | 0.68 (0.06-7.50) | 0.752 | 6 (75) | 2 (25) | 0.77 (0.07-8.58) | 0.835 |
| 3 | 25 (55.6) | 20 (44.4) | 1.66 (0.22-12.44) | 0.623 | 33 (73.3) | 12 (26.7) | 1.52 (0.19-12.45) | 0.695 |
| 4 | 1 (50) | 1 (50) | 2.18 (0.13-35.75) | 0.585 | 1 (50) | 1 (50) | 4.28 (0.24-76.14) | 0.981 |
| Missing | - | - | 1 | - | ||||
| Pathological N stage1, n (%) | ||||||||
| 0 | 17 (65.4) | 9 (34.6) | Reference | 18 (69.2) | 8 (30.8) | Reference | ||
| 1 | 9 (64.3) | 5 (35.7) | 1.75 (0.55-5.53) | 0.341 | 10 (71.4) | 4 (28.6) | 2.31 (0.60-8.88) | 0.222 |
| 2 | 7 (41.2) | 10 (58.8) | 3.48 (1.31-9.27) | 0.013 | 13 (76.5) | 4 (23.5) | 2.17 (0.57-8.51) | 0.264 |
| Missing | - | 1 | 1 | - | ||||
| Pathological M stage1, n (%) | 0.321 | 0.092 | ||||||
| 0 | 29 (58) | 21 (42) | Reference | 37 (74) | 13 (26) | Reference | ||
| 1 | 4 (50) | 4 (50) | 1.74 (0.58-5.22) | 5 (62.5) | 3 (37.5) | 3.13 (0.83-11.79) | ||
| AJCC stage, n (%) | ||||||||
| 1 | 7 (77.8) | 2 (22.2) | Reference | 6 (66.7) | 3 (33.3) | Reference | ||
| 2 | 10 (62.5) | 6 (37.5) | 2.12 (0.43-10.51) | 0.359 | 11 (68.7) | 5 (31.3) | 1.45 (0.33-6.33) | 0.623 |
| 3 | 12 (48) | 13 (52) | 5.09 (1.10-23.52) | 0.037 | 20 (80) | 5 (20) | 2.11 (0.42-10.47) | 0.361 |
| 4 | 4 (50) | 4 (50) | 5.57 (0.96-32.24) | 0.055 | 5 (62.5) | 3 (37.5) | 3.06 (0.85-30.09) | 0.075 |
| Retrieved LN, n (%) | 0.803 | 0.961 | ||||||
| < 12 | 6 (54.5) | 5 (45.5) | Reference | 8 (72.7) | 3 (27.3) | Reference | ||
| ≥ 12 | 26 (59.1) | 18 (40.9) | 0.90 (0.38-2.12) | 31 (70.5) | 13 (29.5) | 0.97 (0.34-2.82) | ||
| Missing | 1 | 2 | 3 | - | ||||
| LN ratio, mean ± SD | 22 ± 18 | 28 ± 20 | 4.21 (0.79-22.42) | 0.092 | 27 ± 21 | 18 ± 8 | 1.07 (0.96-18.11) | 0.963 |
| Colloid component, n (%) | 0.367 | 0.200 | ||||||
| No | 6 (46.2) | 7 (53.8) | Reference | 8 (61.5) | 5 (38.5) | Reference | ||
| Yes | 11 (73.3) | 4 (26.7) | 0.56 (0.16-1.98) | 14 (93.3) | 1 (6.7) | 0.24 (0.03-2.12) | ||
| Missing | 16 | 14 | 20 | 10 | ||||
According to the tumor node metastasis staging system. HR: Hazard ratio; CI: Confidential intervals; AJCC: American Joint Committee on Cancer; LN: Lymph nodes.
Table 9.
Treatment-related potential prognostic factors for liver and lung recurrence
|
|
Recurrence, n = 58
|
|||||||
|
Liver
|
Lung
|
|||||||
|
No, n = 33 (55.0%)
|
Yes, n = 25 (41.7%)
|
HR (95%CI)
|
P
value
|
No, n = 42 (70.0%)
|
Yes, n = 16 (26.7%)
|
HR (95%CI)
|
P
value
|
|
| Neoadjuvant therapy, n (%) | 0.952 | 0.010 | ||||||
| No | 13 (46.4) | 15 (53.6) | Reference | 21 (75) | 7 (25) | Reference | ||
| Yes | 2 (100) | 0 (0) | 0.91 (0.04-18.42) | 0 (0) | 2 (100) | 13.21 (1.86-93.92) | ||
| Missing | 18 | 10 | 21 | 7 | ||||
| Surgery, n (%) | ||||||||
| Right emicolectomy | 10 (83.3) | 2 (16.7) | Reference | 10 (83.3) | 2 (16.7) | Reference | ||
| Extended right emicolectomy | 1 (33.3) | 2 (66.7) | 2.02 (0.31-13.21) | 0.461 | 2 (66.7) | 1 (33.3) | 0.81 (0.09-7.66) | 0.857 |
| Intermediate colectomy | 0 (0) | 4 (100) | 7.05 (1.34-36.94) | 0.021 | 4 (100) | 0 (0) | 0.75 (0.03-21.49) | 0.864 |
| AR/Hartmann’s procedure | 11 (44) | 14 (56) | 2.44 (0.59-10.18) | 0.220 | 19 (76) | 6 (24) | 1.07 (0.21-5.39) | 0.936 |
| Low anterior resection/Miles | 9 (90) | 1 (10) | 0.54 (0.06-4.67) | 0.579 | 3 (30) | 7 (70) | 2.20 (0.45-10.78) | 0.332 |
| Total/Sub-total colectomy | 2 (66.7) | 1 (33.3) | 2.37 (0.27-20.60) | 0.433 | 3 (100) | 0 (0) | 0.84 (0.03-24.19) | 0.920 |
| Segmental resection | 0 (0) | 1 (100) | 110 (5.3-2304.7) | 0.002 | 1 (100) | 0 (0) | - | - |
| Associated procedure, n (%) | ||||||||
| No | 4 (33.3) | 8 (66.7) | Reference | 10 (83.3) | 2 (16.7) | Reference | ||
| Minor | 9 (69.2) | 4 (30.8) | 0.37 (0.11-1.23) | 0.106 | 10 (76.9) | 3 (23.1) | 0.92 (0.16-5.43) | 0.928 |
| Major | 2 (28.6) | 5 (71.4) | 1.65 (0.53-5.47) | 0.377 | 4 (57.1) | 3 (42.9) | 3.37 (0.55-20.65) | 0.188 |
| Missing | 18 | 8 | 18 | 8 | ||||
| Operative technique, n (%) | 0.253 | 0.658 | ||||||
| Open | 19 (51.4) | 18 (48.6) | Reference | 27 (73) | 10 (27) | Reference | ||
| Laparoscopy | 14 (66.7) | 7 (33.3) | 0.60 (0.25-1.44) | 15 (71.4) | 6 (28.6) | 0.79 (0.28-2.24) | ||
| Duration of surgery, min, mean ± SD | 250 ± 90 | 227 ± 75 | 1.00 (0.99-1.00) | 0.579 | 231 ± 83 | 264 ± 86 | 1.00 (1.00-1.01) | 0.221 |
| Hospital stay, d, mean ± SD | 10.0 ± 3.9 | 9.5 ± 2.1 | 0.97 (0.84-1.11) | 0.637 | 9.8 ± 2.8 | 9.9 ± 4.3 | 1.00 (0.86-1.17) | 0.980 |
| Postoperative blood transfusion, n (%) | 0.067 | 0.992 | ||||||
| No | 20 (50) | 20 (50) | Reference | 30 (75) | 10 (25) | Reference | ||
| Yes | 13 (76.5) | 4 (23.5) | 0.37 (0.12-1.07) | 11 (64.7) | 6 (35.3) | 0.99 (0.36-2.75) | ||
| Missing | - | 1 | 1 | - | ||||
| Reoperation due to complications, n (%) | 0.723 | 0.705 | ||||||
| No | 12 (57.1) | 9 (42.9) | Reference | 18 (85.7) | 3 (14.3) | Reference | ||
| Yes | 2 (100) | 0 (0) | 0.58 (0.03-12.07) | 1 (50) | 1 (50) | 1.53 (0.17-13.57) | ||
| Missing | 19 | 16 | 23 | 12 | ||||
| Adjuvant therapy, n (%) | 0.055 | 0.703 | ||||||
| No | 16 (72.7) | 6 (27.2) | Reference | 15 (68.2) | 7 (31.8) | Reference | ||
| Yes | 14 (45.2) | 17 (54.8) | 2.52 (0.98-6.48) | 23 (74.2) | 8 (25.8) | 1.23 (0.42-3.62) | ||
| Missing | 3 | 2 | 4 | 1 | ||||
| Start of adj CHT, n (%) | 0.913 | 0.344 | ||||||
| < 6 wk from surgery | 6 (46.2) | 7 (53.8) | Reference | 8 (61.5) | 5 (38.6) | Reference | ||
| ≥ 6 wk from surgery | 4 (44.4) | 5 (55.6) | 1.07 (0.33-3.43) | 8 (88.9) | 1 (11.1) | 0.35 (0.04-1.04) | ||
| Missing | 23 | 13 | 26 | 10 | ||||
HR: Hazard ratio; OR: Odds ratio; CI: Confidential intervals; Adj CHT: Adjuvant chemotherapy.
Patients receiving intermediate colectomies or segmental resections, having an N stage 2 or AJCC stage 3 tumor showed a higher risk of developing a liver recurrence. Patients receiving postoperative blood transfusions and adjuvant chemotherapy had a marginally significant higher chance of suffering from liver recurrence (P = 0.067 and P = 0.055).
Patients receiving neoadjuvant treatments had a higher rate of lung recurrence (P = 0.010).
In Tables 11-13, we have summarized the results of the analysis of the previously reported potential prognostic factors for locoregional and peritoneal recurrence.
Table 11.
Demographic and patient-related preoperative potential prognostic factors for local and peritoneal recurrence
|
|
Recurrence, n = 58
|
|||||||
|
Peritoneal
|
Local
|
|||||||
|
No, n = 51 (87.9%)
|
Yes, n = 7 (12.1%)
|
HR (95%CI)
|
P
value
|
No, n = 41 (70.7%)
|
Yes, n = 17 (29.3%)
|
HR (95%CI)
|
P
value
|
|
| Age, yr, mean ± SD | 67.6 ± 11.9 | 66.3 ± 14.9 | 0.98 (0.92-1.04) | 0.489 | 68.6 ± 11.3 | 67.5 ± 14.2 | 0.98 (0.95-1.02) | 0.345 |
| Gender, n (%) | 0.434 | 0.862 | ||||||
| Male | 27 (93.1) | 2 (6.9) | Reference | 21 (72.4) | 8 (27.6) | Reference | ||
| Female | 24 (82.8) | 5 (17.2) | 1.97 (0.36-10.77) | 20 (69) | 9 (31) | 0.91 (0.32-2.61) | ||
| Preop Hb, g/dL, mean ± SD | 11.7 ± 2.3 | 11.1 ± 2.7 | 0.91 (0.55-1.50) | 0.712 | 11.9 ± 2.2 | 9.9 ± 1.9 | 0.71 (0.38-1.31) | 0.273 |
| Preop glycemia, g/dL, mean ± SD | 1.08 ± 0.32 | 1.08 ± 0.41 | 2.42 (0.05-123.6) | 0.659 | 1.12 ± 0.33 | 0.82 ± 0.16 | - | 0.210 |
| Preop total proteins, g/dL, mean ± SD | 7.0 ± 0.7 | 6.5 ± 1.1 | 0.17 (0.02-1.23) | 0.079 | 6.9 ± 0.9 | 7.0 ± 0.4 | 0.60 (0.02-14.06) | 0.750 |
| Preop CEA, n (%) | 0.727 | 0.860 | ||||||
| < 5 ng/mL | 11 (84.6) | 2 (15.4) | Reference | 12 (92.3) | 1 (7.7) | Reference | ||
| ≥ 5 ng/mL | 6 (85.7) | 1 (14.3) | 0.52 (0.01-21.28) | 5 (71.4) | 2 (28.6) | 1.32 (0.06-29.55) | ||
| Missing | 34 | 4 | 24 | 14 | ||||
| BMI, mean ± SD | 26.7 ± 4.5 | 30.7 ± 7.2 | 1.15 (0.91-1.44) | 0.238 | 27.4 ± 5.1 | 26.0 ± 3.6 | 0.95 (0.81-1.11) | 0.510 |
| ASA, n (%) | ||||||||
| 1 | 4 (80) | 1 (20) | Reference | 4 (80) | 1 (20) | Reference | ||
| 2 | 17 (89.5) | 2 (10.5) | 0.25 (0.02-2.83) | 0.264 | 15 (78.9) | 4 (21.1) | 0.42 (0.06-3.18) | 0.401 |
| 3 | 18 (85.7) | 3 (14.3) | 0.19 (0.02-2.14) | 0.178 | 14 (66.7) | 7 (33.3) | 0.35 (0.05-2.61) | 0.307 |
| 4 | 3 (100) | 0 (0) | 0.47 (0.01-23.31) | 0.707 | 1 (33.3) | 2 (66.7) | 2.32 (0.24-21.92) | 0.464 |
| Missing | 9 | 1 | 7 | 3 | ||||
| Presentation with occlusion, n (%) | 0.674 | 0.962 | ||||||
| No | 7 (87.5) | 1 (12.5) | Reference | 6 (75) | 2 (25) | Reference | ||
| Yes | 11 (84.6) | 2 (15.4) | 1.67 (0.15-18.56) | 12 (92.3) | 1 (7.7) | 0.93 (0.06-15.21) | ||
| Missing | 33 | 4 | 23 | 14 | ||||
| Tumour site, n (%) | ||||||||
| Right colon | 14 (87.5) | 2 (12.5) | Reference | 9 (56.3) | 7 (43.7) | Reference | ||
| Left colon | 24 (92.3) | 2 (7.7) | 0.80 (0.11-5.70) | 0.825 | 19 (73.1) | 7 (26.9) | 0.79 (0.27-2.30) | 0.671 |
| Rectum | 13 (81.2) | 3 (18.8) | 1.36 (0.23-8.17) | 0.735 | 13 (81.3) | 3 (18.7) | 0.44 (0.11-1.69) | 0.232 |
HR: Hazard ratio; OR: Odds ratio; CI: Confidential intervals; Preop: Preoperative value; Hb: Haemoglobin; CEA: Carcinoembryonic antigen; BMI: Body mass index; ASA: American Society of Anaesthesiologists Score.
Table 13.
Pathological-related potential prognostic factors for local and peritoneal recurrence
|
|
Recurrence, n = 58
|
|||||||
|
Peritoneal
|
Local
|
|||||||
|
No, n = 51 (87.9)
|
Yes, n = 7 (12.1)
|
HR (95%CI)
|
P
value
|
No, n = 41 (70.7)
|
Yes, n = 17 (29.3)
|
HR (95%CI)
|
P
value
|
|
| Major tumor diameter, mm, mean ± SD | 46.2 ± 22.4 | 64.0 ± 35.1 | 1.02 (0.99-1.05) | 0.062 | 44.2 ± 19 | 55.8 ± 31.9 | 1.01 (0.99-1.03) | 0.107 |
| Pathological T stage1, n (%) | ||||||||
| 1 | 2 (100) | 0 (0) | Reference | 2 (100) | 0 (0) | Reference | ||
| 2 | 7 (87.5) | 1 (12.5) | 1.41 (0.03-62.92) | 0.858 | 4 (50) | 4 (50) | 4.91 (0.19-124) | 0.335 |
| 3 | 39 (86.7) | 6 (13.3) | 3.8 (0.05-270.74) | 0.538 | 33 (73.3) | 12 (23.7) | 6.29 (0.22-177) | 0.281 |
| 4 | 2 (100) | 0 (0) | 7.03 (0.04-1358) | 0.468 | 1 (50) | 1 (50) | 21.47 (0.51-897) | 0.107 |
| Missing | 1 | - | 1 | - | ||||
| Pathological N stage1, n (%) | 0.196 | |||||||
| 0 | 25 (96.2) | 1 (3.8) | Reference | 17 (65.4) | 9 (34.6) | Reference | ||
| 1 | 11 (78.6) | 3 (21.4) | 14.5 (0.52-405.2) | 0.115 | 8 (57.1) | 6 (42.9) | 2.41 (0.76-7.65) | 0.135 |
| 2 | 14 (82.4) | 3 (17.6) | 15.4 (0.55-428) | 0.107 | 15 (88.2) | 2 (11.8) | 0.88 (0.19-4.11) | 0.874 |
| Missing | 1 | - | 1 | - | ||||
| Pathological M stage1, n (%) | 0.546 | 0.032 | ||||||
| 0 | 44 (88) | 6 (12) | Reference | 37 (74) | 13 (26) | Reference | ||
| 1 | 7 (87.5) | 1 (12.5) | 1.84 (0.25-13.22) | 4 (50) | 4 (50) | 3.57 (1.12-11.40) | ||
| AJCC Stage, n (%) | ||||||||
| 1 | 8 (88.9) | 1 (11.1) | Reference | 5 (55.6) | 4 (44.4) | Reference | ||
| 2 | 16 (100) | 0 (0) | 0.78 (0.01-73.61) | 0.916 | 12 (75) | 4 (25) | 1.45 (0.28-7.51) | 0.659 |
| 3 | 20 (80) | 5 (20) | 6.16 (0.21-178) | 0.290 | 20 (80) | 5 (20) | 1.62 (0.31-8.37) | 0.567 |
| 4 | 7 (87.5) | 1 (12.5) | 5.16 (0.12-215) | 0.389 | 4 (50) | 4 (50) | 4.64 (0.84-25.69) | 0.079 |
| Retrieved LN, n (%) | 1.000 | 0.477 | ||||||
| < 12 | 10 (90.9) | 1 (11.1) | Reference | 8 (72.7) | 3 (27.3) | Reference | ||
| ≥ 12 | 38 (86.4) | 6 (13.6) | 31 (70.5) | 13 (29.5) | 1.56 (0.46-5.32) | |||
| Missing | 3 | - | ||||||
| LN Ratio, mean ± SD) | 24 ± 17 | 27 ± 29 | 12.13 (0.84-174) | 0.066 | 29 ± 20 | 15 ± 16 | 0.64 (0.04-11.17) | 0.757 |
| Colloid component, n (%) | 0.106 | 0.396 | ||||||
| No | 13 (100) | 0 (0) | Reference | 9 (69.2) | 4 (30.8) | Reference | ||
| Yes | 10 (66.7) | 5 (33.3) | 13.77 (0.57-330) | 9 (60) | 6 (40) | 1.77 (0.47-6.62) | ||
| Missing | 28 | 2 | 23 | 7 | ||||
According to the tumor node metastasis staging system.
HR: Hazard ratio; CI: Confidential intervals; AJCC: American Joint Committee on Cancer; LN: Lymph node.
Table 12.
Treatment-related potential prognostic factors for local and peritoneal recurrence
|
|
Recurrence, n = 58
|
|||||||
|
Peritoneal
|
Local
|
|||||||
|
No, n = 51 (87.9%)
|
Yes, n = 7 (12.1%)
|
HR (95%CI)
|
P
value
|
No, n = 41 (70.7)
|
Yes, n = 17 (29.3)
|
HR (95%CI)
|
P
value
|
|
| Neoadjuvant therapy, n (%) | 0.569 | 0.475 | ||||||
| No | 23 (82.1) | 5 (17.9) | Reference | 23 (81.1) | 5 (17.9) | Reference | ||
| Yes | 2 (100) | 0 (0) | 2.71 (0.09-84.27) | 2 (100) | 0 (0) | 3.88 (0.09-160.22) | ||
| Missing | 26 | 2 | 16 | 12 | ||||
| Surgery, n (%) | ||||||||
| Right emicolectomy | 10 (83.3) | 2 (16.7) | Reference | 6 (50) | 6 (50) | Reference | ||
| Extended right emicolectomy | 3 (100) | 0 (0) | 0.23 (0.01-11.90) | 0.468 | 2 (66.7) | 1 (33.3) | 0.20 (0.02-1.68) | 0.140 |
| Intermediate colectomy | 4 (100) | 0 (0) | 1.20 (0.03-47.50) | 0.923 | 4 (100) | 0 (0) | 0.35 (0.01-8.18) | 0.511 |
| AR/Hartmann’s procedure | 23 (92) | 2 (8) | 0.36 (0.04-3.04) | 0.350 | 17 (68) | 8 (32) | 0.41 (0.13-1.29) | 0.127 |
| Low Anterior resection/Miles | 8 (80) | 2 (20) | 0.68 (0.07-6.27) | 0.731 | 9 (90) | 1 (10) | 0.15 (0.02-1.07) | 0.059 |
| Total/Sub-total colectomy | 2 (66.7) | 1 (33.3) | 2.99 (0.25-35.10) | 0.383 | 2 (66.7) | 1 (33.3) | 0.98 (0.14-6.95) | 0.981 |
| Segmental resection | 1 (100) | 0 (0) | - | - | 1 (100) | 0 (0) | - | - |
| Associated procedure, n (%) | ||||||||
| No | 11 (91.7) | 1 (8.3) | Reference | 11 (91.7) | 1 (8.3) | Reference | ||
| Minor | 11 (84.6) | 2 (15.4) | 1.16 (0.10-14.11) | 0.905 | 7 (53.8) | 6 (46.2) | 2.41 (0.33-17.37) | 0.384 |
| Major | 7 (100) | 0 (0) | 0.61 (0.01-31.26) | 0.808 | 5 (71.4) | 2 (28.6) | 3.26 (0.36-29.71) | 0.295 |
| Missing | 22 | 4 | 18 | 8 | ||||
| Operative technique, n (%) | 0.128 | 0.368 | ||||||
| Open | 35 (94.6) | 2 (5.4) | Reference | 25 (67.6) | 12 (32.4) | Reference | ||
| Laparoscopy | 16 (76.2) | 5 (23.8) | 3.64 (0.69-19.14) | 16 (76.2) | 5 (23.8) | 0.61 (0.21-1.77) | ||
| Duration of surgery, min, mean ± SD | 239 ± 85 | 249 ± 87 | 1.00 (0.99-1.01) | 0.869 | 244 ± 81 | 232 ± 93 | 1.00 (0.99-1.01) | 0.934 |
| Hospital stay, d, mean ± SD | 9.8 ± 3.2 | 10.0 ± 4.1 | 0.98 (0.76-1.27) | 0.907 | 9.4 ± 3.3 | 10.7 ± 3.1 | 1.09 (0.96-1.23) | 0.181 |
| Postoperative blood transfusion, n (%) | 0.272 | 0.518 | ||||||
| No | 37 (92.5) | 3 (7.5) | Reference | 30 (75) | 10 (25) | Reference | ||
| Yes | 13 (76.5) | 4 (23.5) | 2.32 (0.52-10.38) | 10 (58.8) | 7 (41.2) | 1.39 (0.51-3.74) | ||
| Missing | 1 | - | 1 | - | ||||
| Reoperation due to complications, n (%) | 0.344 | 0.182 | ||||||
| No | 17 (81) | 4 (19) | Reference | 18 (75) | 6 (25) | Reference | ||
| Yes | 1 (50) | 1 (50) | 3.00 (0.31-29.24) | 1 (50) | 1 (50) | 4.35 (0.50-37.59) | ||
| Missing | 33 | 2 | 22 | 10 | ||||
| Adjuvant therapy, n (%) | 0.481 | 0.852 | ||||||
| No | 20 (90.9) | 2 (9.1) | Reference | 14 (63.6) | 8 (36.4) | Reference | ||
| Yes | 28 (90.3) | 3 (9.7) | 2.26 (0.23-21.77) | 23 (74.2) | 8 (25.8) | 1.11 (0.38-3.21) | ||
| Missing | 3 | 2 | 4 | 1 | ||||
| Start of adj chemotherapy, n (%) | 0.648 | 0.953 | ||||||
| < 6 wk from surgery | 12 (92.3) | 1 (7.7) | Reference | 9 (69.2) | 4 (30.8) | Reference | ||
| ≥ 6 wk from surgery | 8 (88.8) | 1 (11.1) | 1.91 (0.12-30.85) | 7 (77.8) | 2 (22.2) | 0.95 (0.18-5.13) | ||
| Missing | 31 | 5 | 25 | 1 | ||||
HR: Hazard ratio; OR: Odds ratio; CI: Confidential intervals; Adj: Adjuvant.
Patients with bigger tumors and higher lymph node ratio had a marginally significant probability to develop a peritoneal recurrence (P = 0.062 and P = 0.066, respectively).
Patients having metastatic disease at diagnosis had a significantly higher probability to experience a local recurrence while patients receiving a low anterior resection of the rectum or a Miles intervention had a marginally significant higher chance to develop a local recurrence (P = 0.059).
Due to the paucity of significant prognostic factors found for each subgroup, multivariate analysis was not performed.
DISCUSSION
Many known and unknown factors, including patient and tumor characteristics together with surgical technical aspects, take part in the recurrence after curative treatments for colorectal cancer. Therefore, it is rather difficult to investigate every single variable, especially in a cohort with a very long follow-up period.
In agreement with previous reports[3,4,9], we documented that the recurrence rate was 25.6% with 85% during the 3 years from surgery.
In this study, a higher value of preoperative glycemia and abnormal (> 5 ng/mL) preoperative CEA levels resulted in prognostic recurrence factors. Although there are conflicting results[15,16], a relationship between metabolic syndrome and a higher risk of recurrence has been reported in a large cohort study including more than 1000 patients[17]. The discrepancies between these studies may be explained with the use of non-uniform definitions, however, the insulin role in stimulating cell proliferation via mitogen-activated protein kinase pathway is well documented[18]. The role of preoperative CEA as an independent risk factor for both DFS and OS has been already reported, especially in AJCC stage I-III, with the optimal cut off value ranging from 3 ng/mL[19] to 5 ng/mL if associated with positive lymph nodes or 10 ng/mL if in presence of negative lymph nodes[20].
Patients with ASA score grade 4 had a more than 3-fold higher risk of recurrence compared to ASA score grade 1 in our study. Association between ASA score and recurrence has been seldom reported[21,22]. It is reasonable to think that patients with more comorbidity may show a less efficient anti-tumoral response. Similarly, few data are available regarding the relationship between clinical presentation modalities and prognosis after elective surgery. A presentation with perforation, but not with obstruction, was found to be associated with very late (more than 5 years) onset of recurrence[23]. A possible explanation may be related to the presence of an undetectable micro-perforation and/or bacterial translocation with an impairment of the immune response, but it was not demonstrated yet.
Administration of neoadjuvant therapies, especially the combination of radio and chemotherapy for rectal cancer, has been progressively increased during the last decade but in the study period, only a few patients underwent these treatments. Therefore, it was impossible to evaluate this parameter in the present study.
The finding of higher recurrence risk in patients receiving additional major procedures, treated with the open technique, and receiving adjuvant chemotherapy (marginally significant) is easily explainable with more advanced tumor stages diagnosed in each subgroup. Similarly, the interpretation of the length of hospital stay as an independent prognostic factor should take into account that this parameter is actually the result of many other variables including patient’s conditions, kind of received operation, and postoperative course.
Our data confirmed the prognostic value of the TNM staging system. Furthermore, the N stage 2 and M stage 1 resulted to be independent prognostic factors for recurrence. Patients with a higher lymph node ratio had significantly higher to develop recurrence. Interestingly, a potential incorrect disease stadiation following the retrieval of fewer than 12 nodes did not significantly influence the recurrence.
Lymph nodes ratio is considered as an effective parameter for stratification, especially because it seems to be independent of the resection length[24], and as a strong predictor for tumor recurrence[25,26]. However, the more recent paper of Jakob et al[27] reported the bigger impact of pN than lymph node ratio on recurrence suggesting that the latter parameter could be more helpful in presence of a relatively low number of harvested lymph nodes[27].
Tumor size did not confer a higher risk of recurrence in this analysis. However, previous studies reported a direct correlation between diameter and TNM parameter or, on the contrary, a poorer prognosis of small tumors when associated with advanced T stage[28,29]. These findings could suggest that tumor biology may have a bigger impact on prognosis than tumor size.
Administration of adjuvant chemotherapy and an earlier start of it (within 6 wk from surgery) did not affect the recurrence risk while previously published papers reported opposite results[13]. Administration of chemotherapy in more advanced cancer stages, together with the possible impact of missing data of the present analysis may explain these different findings.
Several factors affecting the timing of recurrence have been proposed and they may differ in subgroups’ analysis of different CRC stages[13].
We found that younger ages resulted significantly associated with early recurrence while AJCC stage 3 and administration of adjuvant chemotherapy showed a trend toward higher probability to develop an early recurrence without reaching statistical significance. These findings may be related to more aggressive tumor biology. Advanced T and N stages have been reported to be related to the early onset of recurrence[9,10,13]. It has been reported that adjuvant chemotherapy may or not influence the rate of recurrence[9,13]. These conflicting results may be explained with the use of different chemotherapy protocols and, again, by different aggressiveness of the disease. Although the early or late recurrence is a debate prognostic factor for the OS[13], we observed that the early recurrence onset resulted significantly associated with a shorter OS rate but not with post recurrence overall survival. Similarly, Lan et al[13] reported that there was no difference in terms of post-recurrence survival between early or late recurrence[13].
Regarding the recurrence site, we documented that liver was the most frequent site (41.7%) followed by locoregional recurrence, lung, and peritoneal recurrence. In detail, patients receiving intermediate colectomies or segmental resections, having an N stage 2 or AJCC stage 3 tumor had a higher risk of developing a liver recurrence. Patients receiving postoperative blood transfusions and adjuvant chemotherapy had a marginally significant higher chance of suffering from recurrence.
Patients receiving neoadjuvant treatments had a higher rate of lung recurrence suggesting the probable association with the presence of advanced primary tumors located in the lower rectum as a prognostic factor.
Finally, in the present study, patients with bigger tumors and higher lymph node ratio had a marginally significant probability to develop a peritoneal recurrence. Previously reported risk factors for peritoneal carcinomatosis included also mucinous and signet ring adenocarcinomas[8].
Patients having metastatic disease at diagnosis had a significantly higher probability to experience a local recurrence while patients receiving a low anterior resection of the rectum or a Miles intervention had a marginally significant higher chance to develop a local recurrence. These findings may be related to complex technical surgical aspects and to pathological aspects including the circumferential radial margin, which was recently introduced in routine practice as a parameter of correct dissection. While in this analysis anastomotic leak did not result in a higher risk of local recurrence, this association has been previously reported[30,31]. Notably, as we previously reported, the research of a relationship between gut microbiota, anastomotic leak, and local recurrence is a promising field under evaluation[32].
Kind of tests, frequency, and duration of the follow-up is still debated[33,34].
Data from a review of several moderate-high quality randomized controlled trials showed better OS and a higher resectability rate of the recurrence but no differences in cancer-specific mortality rate with a more intensive follow-up program[12].
Most of the follow-up programs end in five years after primary surgery. Only routine surveillance is recommended beyond this period but recurrence continues to occur after 5 years. Our data showed a rate of very late first recurrence (> 5 years) of 1.7%, similar to other previously published studies[3,9]. Moreover, a mean time of more than 60 mo for the development of the second recurrence in the patients with lung metastases was observed and the presentation of the second liver recurrence ranged between 24 and 76 mo. Finally, as a collateral finding, 15 patients developed a second neoplasm beyond 5 years from primary colorectal surgery. Perhaps, in these patients, a longer surveillance program could have allowed an earlier diagnosis. Similarly, Chauvenet et al[35] estimated the achievement of cure at 9.3 years[35] while Bouvier et al[23] reported a recurrence rate of 6.7% between 5 and 10 years after the first treatments, independently from cancer stage[23]. These data could suggest the necessity to extend the actual follow up programs up to 10 years, especially for selected patients. On the other hand, patients may suffer from test-related anxiety and the tests may rarely provoke adverse reactions[12]. No relevant reports about the economic aspect have been found[12]. Consequently, a tailored approach for each patient seems advisable.
Our study has several drawbacks. This has been a retrospective study with an inherent selection bias. Missing data, mostly due to the chosen long follow-up period, may also cause bias throughout the analysis. Patients lost during follow-up and patients who did not undergo radiological imaging after the 5th years of follow-up could have a silent recurrence causing a recurrences’ underestimation. On the contrary, not all the patients with a radiological suspicion of metastasis underwent a histological assessment causing a potential overestimation of the recurrence rate. Data concerning chemotherapy are quite simplified and those about molecular biology were almost completely missing, therefore further statistical analysis could not be performed. Similarly, data regarding mesorectal excision were still not available in the histological reports making impossible the analysis of its adequacy. Finally, an accurate cost/benefit evaluation should be considered. Therefore, further analyses, possibly in a larger sample, are needed and could offer stronger evidence.
CONCLUSION
In conclusion, several prognostic factors for recurrence and some specific factors for each site of recurrence have been found and should be taken into account in scheduling a tailored follow-up program for each patient. Since recurrence or other primary tumors may occur even 5 years from the first treatment, an extension of the recommended 5-years follow-up program should be evaluated according to the presence of potential prognostic factors.
ARTICLE HIGHLIGHTS
Research background
Colorectal cancer is a common malignancy with a quite high recurrence rate in spite of the curative treatments utilized. Although most of the recurrences occur within the first three years, a percentage of them appear beyond five years after surgery. Early detection of these recurrences is of paramount importance to allow further curative treatments and improve patient prognosis. However, several different follow-up programs have been proposed over the year, mostly ending in 5 years after surgery.
Research motivation
Prognostic factors for recurrence, patterns of recurrence, and different prognostic factors for early or late recurrence are rarely reported in the literature, especially in cohorts of patients with a long follow-up period. Identifications of these parameters may allow a correct allocation of the patients in specific and tailored follow-up programs to improve patient prognosis and to reduce the costs.
Research objectives
The objectives of this study are the research of prognostic factors for overall recurrence, for early or late recurrence, and the analysis of the potential patterns of recurrence for the most frequent sites of recurrence evaluating patients with a potential minimum follow-up period of 10 years. Clinical, operative, and pathological potential prognostic factors were evaluated and significant results were found for each one of the prospected objectives. These results may help clinicians in predicting patient prognosis and in choosing more cost-effective patient surveillance strategies.
Research methods
All the consecutive patients curatively treated for colorectal adenocarcinoma from January 2006 to June 2009 were prospectively included in a database that was retrospectively reviewed. A standardized follow-up program was applied to all the patients. Several prognostic factors about the patient, the treatment used, and the pathological response were evaluated. To evaluate the association between possible prognostic factors and disease-free survival and overall survival a Cox model, Kaplan-Meier method, and log-rank test were used. To estimate possible independent prognostic factors for recurrence a multiple Cox model with a backward selection method was used. To assess the association between each possible prognostic factor and timing to recurrence (< 3 years or ≥ 3 years) a simple logistic regression model was used.
Research results
Patients with higher levels of preoperative glycemia and carcinoembrionyc antigen, highest anaesthesiologists score score, presenting with occlusion, receiving a complex operation performed with an open technique, after a longer hospital stay, and showing advanced tumors had a higher chance to develop recurrence. At the multivariate analysis, the independent prognostic factors for recurrence were the hospital stay, N stage 2, and M stage 1. Younger ages were significantly associated with an early recurrence onset. Receiving intermediate colectomies or segmental resections, having an N stage 2 or American Joint Committee on Cancer stage 3 tumors was associated with a higher risk of liver recurrence; metastatic disease at diagnosis with local recurrence; receiving neoadjuvant treatments with lung recurrence; bigger tumors and higher lymph node ratio with peritoneal recurrence (marginally significant). However, these results and, in particular, those about the early vs late recurrence and the pattern of recurrence should be verified in larger series.
Research conclusions
Several prognostic factors for recurrence and some specific factors for each site of recurrence have been found and should be taken into account to perform a correct allocation of the patient within tailored cost-effective follow-up programs, eventually extended beyond five years after surgery.
Research perspectives
Further studies are needed to confirm these results, possibly prospective studies. The use of the learning machine may offer interesting opportunities in this area. Finally, the analysis of the second malignancies developed during the follow-up, which is marginally mentioned in this study, may represent another potential field of research.
ACKNOWLEDGEMENTS
We thank Mascha Stroobant for the language revision.
Footnotes
Institutional review board statement: This study is under revision and approval by the Ethics Committee named Comitato Etico Area Vasta Centro.
Conflict-of-interest statement: All the authors have no financial relationships or any other conflicting interests to disclose.
Manuscript source: Invited manuscript
Peer-review started: October 27, 2020
First decision: November 30, 2020
Article in press: December 17, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bordonaro M S-Editor: Zhang L L-Editor: A P-Editor: Li JH
Contributor Information
Filippo Melli, Department of Clinical and Experimental Medicine, University of Florence, Azienda Ospedaliero-Universitaria Careggi, Florence 50134, Italy.
Ilenia Bartolini, Department of Clinical and Experimental Medicine, University of Florence, Azienda Ospedaliero-Universitaria Careggi, Florence 50134, Italy.
Matteo Risaliti, Department of Clinical and Experimental Medicine, University of Florence, Azienda Ospedaliero-Universitaria Careggi, Florence 50134, Italy.
Rosaria Tucci, Department of Clinical and Experimental Medicine, University of Florence, Azienda Ospedaliero-Universitaria Careggi, Florence 50134, Italy.
Maria Novella Ringressi, Department of Clinical and Experimental Medicine, University of Florence, Azienda Ospedaliero-Universitaria Careggi, Florence 50134, Italy.
Paolo Muiesan, Department of Clinical and Experimental Medicine, University of Florence, Azienda Ospedaliero-Universitaria Careggi, Florence 50134, Italy.
Antonio Taddei, Department of Clinical and Experimental Medicine, University of Florence, Azienda Ospedaliero-Universitaria Careggi, Florence 50134, Italy.
Amedeo Amedei, Department of Clinical and Experimental Medicine, University of Florence, Azienda Ospedaliero-Universitaria Careggi, Florence 50134, Italy. aamedei@unifi.it.
Data sharing statement
Technical appendix, statistical code, and dataset available from the corresponding author at aamedei@unifi.it.
References
- 1. Cancer Today [accessed on 16 October 2020]. Available from: https://www.cancertodaymag.org/
- 2.Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, Saito Y, Hamaguchi T, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kawano H, Kinugasa Y, Kokudo N, Murofushi K, Nakajima T, Oka S, Sakai Y, Tsuji A, Uehara K, Ueno H, Yamazaki K, Yoshida M, Yoshino T, Boku N, Fujimori T, Itabashi M, Koinuma N, Morita T, Nishimura G, Sakata Y, Shimada Y, Takahashi K, Tanaka S, Tsuruta O, Yamaguchi T, Yamaguchi N, Tanaka T, Kotake K, Sugihara K Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2018;23:1–34. doi: 10.1007/s10147-017-1101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seo SI, Lim SB, Yoon YS, Kim CW, Yu CS, Kim TW, Kim JH, Kim JC. Comparison of recurrence patterns between ≤5 years and >5 years after curative operations in colorectal cancer patients. J Surg Oncol. 2013;108:9–13. doi: 10.1002/jso.23349. [DOI] [PubMed] [Google Scholar]
- 4.Tjandra JJ, Chan MK. Follow-up after curative resection of colorectal cancer: a meta-analysis. Dis Colon Rectum. 2007;50:1783–1799. doi: 10.1007/s10350-007-9030-5. [DOI] [PubMed] [Google Scholar]
- 5.Brown KGM, Koh CE. Surgical management of recurrent colon cancer. J Gastrointest Oncol. 2020;11:513–525. doi: 10.21037/jgo-2019-ccm-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liska D, Stocchi L, Karagkounis G, Elagili F, Dietz DW, Kalady MF, Kessler H, Remzi FH, Church J. Incidence, Patterns, and Predictors of Locoregional Recurrence in Colon Cancer. Ann Surg Oncol. 2017;24:1093–1099. doi: 10.1245/s10434-016-5643-z. [DOI] [PubMed] [Google Scholar]
- 7.Renehan AG, Egger M, Saunders MP, O'Dwyer ST. Impact on survival of intensive follow up after curative resection for colorectal cancer: systematic review and meta-analysis of randomised trials. BMJ. 2002;324:813. doi: 10.1136/bmj.324.7341.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riihimäki M, Hemminki A, Sundquist J, Hemminki K. Patterns of metastasis in colon and rectal cancer. Sci Rep. 2016;6:29765. doi: 10.1038/srep29765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho YB, Chun HK, Yun HR, Lee WS, Yun SH, Lee WY. Clinical and pathologic evaluation of patients with recurrence of colorectal cancer five or more years after curative resection. Dis Colon Rectum. 2007;50:1204–1210. doi: 10.1007/s10350-007-0247-0. [DOI] [PubMed] [Google Scholar]
- 10.Aghili M, Izadi S, Madani H, Mortazavi H. Clinical and pathological evaluation of patients with early and late recurrence of colorectal cancer. Asia Pac J Clin Oncol. 2010;6:35–41. doi: 10.1111/j.1743-7563.2010.01275.x. [DOI] [PubMed] [Google Scholar]
- 11.Sargent D, Sobrero A, Grothey A, O'Connell MJ, Buyse M, Andre T, Zheng Y, Green E, Labianca R, O'Callaghan C, Seitz JF, Francini G, Haller D, Yothers G, Goldberg R, de Gramont A. Evidence for cure by adjuvant therapy in colon cancer: observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2009;27:872–877. doi: 10.1200/JCO.2008.19.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Guideline Alliance part of the Royal College of Obstetricians and Gynaecologists (UK) Follow-up to detect recurrence after treatment for non-metastatic colorectal cancer: Colorectal cancer (update): Evidence review E1. London: National Institute for Health and Care Excellence (UK); 2020. [PubMed] [Google Scholar]
- 13.Lan YT, Chang SC, Yang SH, Lin CC, Wang HS, Jiang JK, Chen WS, Lin TC, Chiou SH, Lin JK. Comparison of clinicopathological characteristics and prognosis between early and late recurrence after curative surgery for colorectal cancer. Am J Surg. 2014;207:922–930. doi: 10.1016/j.amjsurg.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 14.Greene FL, Page DL, Fleming ID, Fritz AG. AJCC Cancer Staging Manual. 6th Ed. Chicago: Springer-Verlag; 2002. [Google Scholar]
- 15.Yang Y, Mauldin PD, Ebeling M, Hulsey TC, Liu B, Thomas MB, Camp ER, Esnaola NF. Effect of metabolic syndrome and its components on recurrence and survival in colon cancer patients. Cancer. 2013;119:1512–1520. doi: 10.1002/cncr.27923. [DOI] [PubMed] [Google Scholar]
- 16.Colangelo LA, Gapstur SM, Gann PH, Dyer AR, Liu K. Colorectal cancer mortality and factors related to the insulin resistance syndrome. Cancer Epidemiol Biomarkers Prev. 2002;11:385–391. [PubMed] [Google Scholar]
- 17.You J, Liu WY, Zhu GQ, Wang OC, Ma RM, Huang GQ, Shi KQ, Guo GL, Braddock M, Zheng MH. Metabolic syndrome contributes to an increased recurrence risk of non-metastatic colorectal cancer. Oncotarget. 2015;6:19880–19890. doi: 10.18632/oncotarget.4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Rohan TE, Manson JE, Howard BV, Wylie-Rosett J, Anderson GL, Ho GY, Kaplan RC, Li J, Xue X, Harris TG, Burk RD, Strickler HD. Insulin, insulin-like growth factor-I, endogenous estradiol, and risk of colorectal cancer in postmenopausal women. Cancer Res. 2008;68:329–337. doi: 10.1158/0008-5472.CAN-07-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim CG, Ahn JB, Jung M, Beom SH, Heo SJ, Kim JH, Kim YJ, Kim NK, Min BS, Koom WS, Kim H, Roh YH, Ma BG, Shin SJ. Preoperative Serum Carcinoembryonic Antigen Level as a Prognostic Factor for Recurrence and Survival After Curative Resection Followed by Adjuvant Chemotherapy in Stage III Colon Cancer. Ann Surg Oncol. 2017;24:227–235. doi: 10.1245/s10434-016-5613-5. [DOI] [PubMed] [Google Scholar]
- 20.Huang SH, Tsai WS, You JF, Hung HY, Yeh CY, Hsieh PS, Chiang SF, Lai CC, Chiang JM, Tang R, Chen JS. Preoperative Carcinoembryonic Antigen as a Poor Prognostic Factor in Stage I-III Colorectal Cancer After Curative-Intent Resection: A Propensity Score Matching Analysis. Ann Surg Oncol. 2019;26:1685–1694. doi: 10.1245/s10434-019-07184-3. [DOI] [PubMed] [Google Scholar]
- 21.Manceau G, Voron T, Mege D, Bridoux V, Lakkis Z, Venara A, Beyer-Berjot L, Abdalla S, Sielezneff I, Lefèvre JH, Karoui M AFC (French Surgical Association) Working Group. Prognostic factors and patterns of recurrence after emergency management for obstructing colon cancer: multivariate analysis from a series of 2120 patients. Langenbecks Arch Surg. 2019;404:717–729. doi: 10.1007/s00423-019-01819-5. [DOI] [PubMed] [Google Scholar]
- 22.Bartolini I, Ringressi MN, Melli F, Risaliti M, Brugia M, Mini E, Batignani G, Bechi P, Boni L, Taddei A. Analysis of Prognostic Factors for Resected Synchronous and Metachronous Liver Metastases from Colorectal Cancer. Gastroenterol Res Pract. 2018;2018:5353727. doi: 10.1155/2018/5353727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouvier AM, Launoy G, Bouvier V, Rollot F, Manfredi S, Faivre J, Cottet V, Jooste V. Incidence and patterns of late recurrences in colon cancer patients. Int J Cancer. 2015;137:2133–2138. doi: 10.1002/ijc.29578. [DOI] [PubMed] [Google Scholar]
- 24.Amri R, Klos CL, Bordeianou L, Berger DL. The prognostic value of lymph node ratio in colon cancer is independent of resection length. Am J Surg. 2016;212:251–257. doi: 10.1016/j.amjsurg.2015.10.037. [DOI] [PubMed] [Google Scholar]
- 25.Dedavid e Silva TL, Damin DC. Lymph node ratio predicts tumor recurrence in stage III colon cancer. Rev Col Bras Cir. 2013;40:463–470. doi: 10.1590/s0100-69912013000600008. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg R, Engel J, Bruns C, Heitland W, Hermes N, Jauch KW, Kopp R, Pütterich E, Ruppert R, Schuster T, Friess H, Hölzel D. The prognostic value of lymph node ratio in a population-based collective of colorectal cancer patients. Ann Surg. 2010;251:1070–1078. doi: 10.1097/SLA.0b013e3181d7789d. [DOI] [PubMed] [Google Scholar]
- 27.Jakob MO, Guller U, Ochsner A, Oertli D, Zuber M, Viehl CT. Lymph node ratio is inferior to pN-stage in predicting outcome in colon cancer patients with high numbers of analyzed lymph nodes. BMC Surg. 2018;18:81. doi: 10.1186/s12893-018-0417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang B, Feng Y, Mo SB, Cai SJ, Huang LY. Smaller tumor size is associated with poor survival in T4b colon cancer. World J Gastroenterol. 2016;22:6726–6735. doi: 10.3748/wjg.v22.i29.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saha S, Shaik M, Johnston G, Saha SK, Berbiglia L, Hicks M, Gernand J, Grewal S, Arora M, Wiese D. Tumor size predicts long-term survival in colon cancer: an analysis of the National Cancer Data Base. Am J Surg. 2015;209:570–574. doi: 10.1016/j.amjsurg.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Ramphal W, Boeding JRE, Gobardhan PD, Rutten HJT, de Winter LJMB, Crolla RMPH, Schreinemakers JMJ. Oncologic outcome and recurrence rate following anastomotic leakage after curative resection for colorectal cancer. Surg Oncol. 2018;27:730–736. doi: 10.1016/j.suronc.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Ha GW, Kim JH, Lee MR. Oncologic Impact of Anastomotic Leakage Following Colorectal Cancer Surgery: A Systematic Review and Meta-Analysis. Ann Surg Oncol. 2017;24:3289–3299. doi: 10.1245/s10434-017-5881-8. [DOI] [PubMed] [Google Scholar]
- 32.Bartolini I, Risaliti M, Ringressi MN, Melli F, Nannini G, Amedei A, Muiesan P, Taddei A. Role of gut microbiota-immunity axis in patients undergoing surgery for colorectal cancer: Focus on short and long-term outcomes. World J Gastroenterol. 2020;26:2498–2513. doi: 10.3748/wjg.v26.i20.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Y, Yi C, Zhang Y, Fang F, Faramand A. Intensive follow-up strategies after radical surgery for nonmetastatic colorectal cancer: A systematic review and meta-analysis of randomized controlled trials. PLoS One. 2019;14:e0220533. doi: 10.1371/journal.pone.0220533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pita-Fernández S, Alhayek-Aí M, González-Martín C, López-Calviño B, Seoane-Pillado T, Pértega-Díaz S. Intensive follow-up strategies improve outcomes in nonmetastatic colorectal cancer patients after curative surgery: a systematic review and meta-analysis. Ann Oncol. 2015;26:644–656. doi: 10.1093/annonc/mdu543. [DOI] [PubMed] [Google Scholar]
- 35.Chauvenet M, Lepage C, Jooste V, Cottet V, Faivre J, Bouvier AM. Prevalence of patients with colorectal cancer requiring follow-up or active treatment. Eur J Cancer. 2009;45:1460–1465. doi: 10.1016/j.ejca.2009.01.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Technical appendix, statistical code, and dataset available from the corresponding author at aamedei@unifi.it.