Abstract
Neonatal hypoxic-ischemic (HI) brain injury is one of the major drawbacks of mortality and causes significant short/long-term neurological dysfunction in newborn infants worldwide. To date, due to multifunctional complex mechanisms of brain injury, there is no well-established effective strategy to completely provide neuroprotection. Although therapeutic hypothermia is the proven treatment for hypoxic-ischemic encephalopathy (HIE), it does not completely chang outcomes in severe forms of HIE. Therefore, there is a critical need for reviewing the effective therapeutic strategies to explore the protective agents and methods. In recent years, it is widely believed that there are neuroprotective possibilities of natural compounds extracted from plants against HIE. These natural agents with the anti-inflammatory, anti-oxidative, anti-apoptotic, and neurofunctional regulatory properties exhibit preventive or therapeutic effects against experimental neonatal HI brain damage. In this study, it was aimed to review the literature in scientific databases that investigate the neuroprotective effects of plant extracts/plant-derived compounds in experimental animal models of neonatal HI brain damage and their possible underlying molecular mechanisms of action.
Keywords: hypoxic-ischemic encephalopathy, neonatal hypoxic-ischemic brain damage, birth asphyxia, perinatal hypoxia-ischemia, neonates, newborns, neuroprotective, plant extracts, natural compounds
1. Introduction
Hypoxia-ischemia (HI) is regarded as one of the major drawbacks of brain damage in the neonates and infants. Neonatal hypoxic-ischemic brain damage (HIBD) (synonymous with hypoxic-ischemic encephalopathy (HIE)) is the main drawback of newborn deaths and irretrievable and long-lasting neurodevelopmental disabilities in the sufferers [1]. HIE complications estimate 23% of infant deaths all over the world, and have impacted 0.7–1.2 million newborns per annum [2]. Cerebral palsy, epilepsy, mental retardation, motor and cognitive deficits, learning and behavioral disabilities, and other severe neurological disorders were regularly observed in the sufferers based on brain injury grade [3,4]. In infants with mild HIE, the death probability is 10% and the risk of neurodevelopmental disorders is 30%, while 60% of infants with severe HIE are at risk of death and all sufferers risk exposure to remarkable disabilities.
The neonatal HI (NHI) incidence is near 1–8 of every one thousand live full-term births in developed countries, and nearly 25 in one thousand live full-term births in developing countries [5,6]. Lately, advanced neonatology has evidenced a great improvement in survival of seriously premature newborns. The rate of incidence in the very low birth weight infants is 60% of all live births.
NHI commonly occurs as a result of prenatal or birth asphyxia near the time of birth [7,8]. The ordinary birth process that is correlated with asphyxia and severe asphyxia may affect a pre-existing normal brain, prompting acute encephalopathy. Severe asphyxia can arise for numerous causes, including abnormal uterine contractions, abruption of the placenta, umbilical cord compression, or failure of the neonate to initiate breathing successfully. Infection, growth retardation, and fever can enhance the brain sensitivity to nonpathogenic asphyxia. On the other hand, the prematurity subject is even more complicated which is frequently concomitant with impaired fetal growth and infection. Also, the general handling of the severe premature infants causes bases of postnatal insults. The most common form of perinatal injury is systemic asphyxia, which impacts the neonate/fetus cardiovascular system and results in a hypoperfusion by hypoxemic blood. Consequently, such injury is discussed to be reperfusion/hypoperfusion damage [9].
On the cellular status, HI actuates the activation of complex biochemical reactions, consisting of switching from oxidative to anaerobic metabolism, cellular energy failure, excitotoxicity, dysfunction of mitochondria, overload of intracellular calcium, production of free radicals, and inflammatory responses, which results in significant neuronal impairment and ultimately contributes to change in degrees of brain infarction, BBB (blood–brain barrier) dysfunction, and brain edema, finally resulting in irreversible brain injury.
The neurologic disorders extremely dwindle the quality of life in HIBD kids and escalate the socioeconomic burden on caregivers, families, and society. Currently, hypothermia is the only standard treatment for neonatal HIBD for full-term infants with moderate to severe HIE [10]. By this treatment, infants with the most severe type of HIE might not be saved, and this strategy involves the risk of severe disability or death, as nearly 50% of treated cases still die [11], and as many as 1/3 to 1/2 of sufferers demonstrate low Intelligence Quotient (IQ) at 6 to 7 years of age or constant neurologic abnormalities [12,13]. Therefore, more effective neuroprotective strategies are required. In this respect, several agents have been investigated to save the brain from irreversible damage or to postpone the pathological process, such as Erythropoietin, Melatonin, Metformin, stem cell therapy, etc., but only a few agents have been used in clinical studies [14]. Therefore, a search to explore better neuroprotective agents is under way.
In recent times, focus on plant research has increased globally and significant attention has been paid to medicinal plants due to their safety, cost-effectiveness, availability, long-term use, and limited side-effects. So far, the latest investigations have been looking at the potential of natural compounds isolated from plants, vegetables, fruits, and beverages to treat neurological disorders. Among them, the majority of naturally derived neuroprotective agents are polyphenolic compounds such as flavonoids which show protective effects in several animal models of experimental neurological disorders [15,16]. These naturally derived compounds have been reported as neuro-functional regulatory, anti-apoptotic, anti-inflammatory, and anti-oxidative agents [17,18,19]. In these topics, several plant extracts and plant-derived compounds, such as grape seed extract, resveratrol, and cannabinol, have been scientifically identified to be useful in preventing or treating animal models of neonatal HIBD.
Although in previous review articles, natural products were studied as neuroprotective agents in various neurological and neurodegenerative diseases, none of them have focused on neonatal HIBD. In addition to introducing neonatal HI brain damage and its pathophysiology, in this study, we aimed to review the literature in scientific databases that investigate the protective effects of plant extracts or their constituents in experimental animal models of neonatal HI brain damage and their possible underlying mechanisms of action. For this purpose, articles indexed in PubMed, Science direct, and Scopus databases were evaluated, using the following keywords in English: (Neonatal [Title]) OR (Perinatal [Title]) OR (Neonate [Title]) OR (Newborn [Title]) AND (hypoxic-ischemic [Title]) OR (hypoxia-ischemia [Title]) OR (hypoxic ischemic encephalopathy [Title]) AND (brain [Title]) AND (natural [Title/Abstract]) OR (plant [Title/Abstract]) OR (extract [Title/Abstract]) OR (herb [Title/Abstract]). In addition, the reference listing of the reclaimed essays and further resources comprising Google Scholar was screened.
2. Pathophysiology of Neonatal Hypoxic-Ischemic Brain Damage
2.1. Excitatory
In fact, HIE is caused by a restriction in blood supply (ischemia) and deprivation of adequate oxygen supply (hypoxia), which causes a switch to anaerobic metabolism. Anaerobic metabolism leads to depletion of adenosine triphosphate (ATP) and accumulation of lactic acid. This condition is known as primary energy failure, and occurs immediately after initial HI insult. At the cellular level, energy failure and ATP reduction results in failure of the cell membrane ion in pumping and consequently in flow of sodium and calcium ions accompanied by water flow into the cell. Following the process, the cytotoxic edema is eventuated due to the water flow which might lead to cellular swelling and cell lysis [9]. The following membrane depolarization unrolls voltage-sensitive calcium channels and leads to excessive calcium influx. Increase in intracellular Ca2+ leads to production and release of excitatory amino acids, particularly glutamate. In the following stage, excess glutamate activates receptors such as α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartate (NMDA) receptors [20]. Activation of these receptors additionally promotes influx of Ca2+ into the affected cells. Finally, the rise in intracellular Ca2+ promotes production of reactive oxygen species (ROS), release of pro-radicals (such as free ions), and synthesis of excessive nitric oxide (NO) by neuronal NO synthase (nNOS), which is modulated by the NMDA receptors [20]. Furthermore, activation of lipases, proteases, and endonucleases are increased, which causes a release of fatty acids and membrane damage, and triggers a cascade reaction series which leads to cell death [20].
2.2. Free Radical Toxicity
Reoxygenation and reperfusion oxygen availability lead to the initial ischemic tissue and more ROS generation by mitochondria (via the electron transport chain) and damage integrity of membrane and organelle’s function. These activities lead to mitochondrial dysfunction which in turn can trigger ROS-induced ROS generation. Furthermore, hypoxanthine and pro-radicals such as iron cause large amounts of ROS formation. Mitochondrial impairment is one of the main factors involved in the apoptotic pathway of cell death. The second phase of injury is known as reperfusion injury and occurs hours later [20].
2.3. Inflammation
In addition to the described mechanisms, damaged neurons and activated endothelium produce various cytokines, including interleukins (IL-1, IL-6, IL-8, IL-10) and tumor necrosis factor (TNF)-α, which activate inflammatory response and trigger biochemical pathways that lead to secondary energy failure and a delay in neuronal cell death [20]. Secondary energy failure generally eventuates 6 to 48 h after initial HI insult and delays necrotic and apoptotic processes, that might continue up to days or weeks after birth [20]. Also, a delay in brain damage is associated with a decrease in production of neurotrophic growth factors, including epidermal growth factor (EGF), nerve growth factor (NGF), insulin-like growth factor (IGF), GDNF (glial cell-derived neurotrophic factor), BDNF (brain-derived neurotrophic factor), and VEGF (vascular endothelial growth factor), which apparently inhibit apoptosis and prompt cell proliferation and differentiation of the developing brain. On the other side, transcription factors comprising JNK (c-Jun N-terminal kinase) and NF-kβ (nuclear factor kappa-beta) also demonstrate an important function in this point [20]. The damage progression proceeds and enters into the tertiary phase by progress in inflammation, alteration in neurogenesis, and impaired synaptogenesis and axonal growth [9].
2.4. Neuronal Death
Neuronal cell death induced by neonatal HI occurs either by necrosis or apoptosis. Necrotic cell death occurs immediately after the insult and is the prevailing pathway of cell death subsequent to irreversible or severe damage [9]. Apoptosis or programmed cell death planned apoptosis is a significant part of ultimate cell death. Apoptosis can last for days and even weeks after the initial insult. It was proposed that, in infants, apoptosis is possibly more critical in provoking cell death compared with necrosis [20].
3. Potential Mechanisms for Neuroprotective Strategies
As mentioned above, HIE consists of complex pathophysiologic mechanisms occurring in two energy failure stages. The first stage eventuates immediately after HI and the secondary stage eventuates 6–15 h after the initial insult. This delay offers a temporary therapeutic window for neuroprotective strategies, during which pharmacological interventions can be applied. To date, a broad range of propitious neuroprotective structures have been evaluated on experimental animal models of neonatal HIBD, but only a few agents have been used in clinical studies [14,21].
The pathophysiology of HIE enables multiple targets at different time points of the disease process. As an example, in the first stage, treatments are mostly concentrated on reduction of oxidative, apoptotic, and excitotoxic mediators of injury, whereas in the later stages, reduction of neurotrophic-evoked excitation features and inflammatory cytokines in the immature brain are targeted to contribute to regeneration of oligodendrocyte and neurons [22,23].
3.1. Reduction of Cellular Apoptosis and Oxidative Stress in HI
Apoptosis is categorized into two pathways, one is caspase-dependent and the second is caspase-independent, and both play an important task in the neonatal pathogenic process in HIBD. Therefore, apoptotic pathways are the main target for therapeutic interventions. Apaf-1, caspase, and Bcl-2 gene families demonstrate the major role in neuronal apoptosis determination. The Bcl-2 gene family includes pro-apoptotic (Bok, Bak, and Bax) and anti-apoptotic (Bcl-XL and Bcl-2) members that regulate cytochrome c translocation from mitochondria to cytoplasm, which is the initiator phase of the caspase-independent apoptosis. The mechanisms of release of pro-apoptotic Bcl-2 from the membrane of mitochondria might include the rupture of membrane of mitochondria, and anti-apoptotic members prevent this process. Caspases are intracellular proteases that function as initiators, and effectors of apoptosis function to activate other caspases’ cleavage [24]. Numerous agents have been evaluated to function on anti-apoptotic pathways, for example the proteins of Bcl-2 family become a prosperous object for drug targeting. Caspases are also one of the intriguing classes of proteins as a pharmacological target since caspase inhibitors are able to block apoptosis, for example, casp-3 inhibitors demonstrated neuroprotective activities in animal models of neonatal HIBD [25,26,27]. Furthermore, it has been recently demonstrated that autophagy (programmed cell death) reveals a significant efficacy in HIBD and neuroprotection, which can be obtained by regulating autophagy.
As discussed above, a large amount of free radical species, mainly ROS and RNS (reactive nitrogen species), comprising hydroxyl radical, superoxide, peroxynitrite, and hydrogen peroxide, accumulate rapidly in the brain after HI and cause oxidative stress that is able to initiate protein oxidation, lipid peroxidation, nucleic acid, and cell membrane damage, and eventually, lead to cell death. Due to the high level of polyunsaturated fatty acids, low concentration of endogenous antioxidant enzymes: SOD (superoxide dismutase), CAT (catalase), GPx (gluthathione peroxidase), GST (glutathione S-transferase), GSH-Px (gluthathione peroxidase), and high oxygen consumption, neonatal encephalon is more sensitive to oxidative damage than adults. Oxidative stress has a critical role in neuroinflammation and apoptosis, especially in neonatal HIBD. In addition, ROS are in charge of the numerous downstream factors which directly change the blood–brain barrier (BBB) integrity, and also effect the tight junction (TJ) proteins’ modification and the matrix metalloproteinases’ (MMPs) activation. Thus, the activation of MMPs with regards to oxidative stress is deemed as an inappropriate factor to segregate the integrity of the BBB. According to the evidence, it has been demonstrated that the changes in permeability of BBB and oxidative stress are generally involved in the neonatal pathogenesis of encephalon injury following HI insult. Many agents with anti-oxidative properties, including free radical scavengers (FRS) or free radical production inhibitors, such as melatonin or erythropoietin, have been reported to provide a protective effect in animal models of neonatal HIE [28,29]. Furthermore, stabilization of the BBB after HIBD in neonates is a fundamental target in order to keep the microenvironment of cerebral homeostatic and the normal function of the neurons unchanged.
3.2. Reduction of Neurotrophic-Evoked Excitation and Inflammatory Cytokines in HI
Neuroinflammation subsequently occurs following HI, and anti-inflammatory treatments have been shown to heal brain injuries in neonates. For example, agents with anti-inflammatory effects such as allopurinol, deferoxamine, N-acetylcysteine, melatonin, and minocycline have demonstrated beneficial effects in animal models. Moreover, NF-kB inhibitors have also shown sustainable neuroprotection [30,31,32].
Supplementation of insufficient growth factors seems to be a promising therapeutic strategy, and administration of BDNF, IGF-1, and Erythropoietin ameliorate functional recovery and improve neurogenesis after neonatal HI injury. More recently, promoting neuronal regeneration with endogenous stem cells, such as mesenchymal progenitor/stem cells, has also been represented to promote functional outcomes in neonates [33,34].
3.3. Animal Model of HI
Animal models are normally the primary step in identifying mechanisms underlying illness and disease, to discover effective therapeutic treatments. Neonatal brain damage requires gestation for some months, and also the fetal and newborn maturation of the brain. Many animal models have reported information concerning the brain damage pathophysiology in term infants [35]. A combination of proper animal models to direct these investigations will help us to comprehend human neonatal damage and the approach of therapeutic interventions. In this review, the bulk of the studies reviewed three kinds of animal models, mice, rat, and piglet, in HI. Neonatal HI mice studies were performed as outlined in the following procedure: At postnatal day 7, pups were anesthetized, and the left common carotid artery was subjected and Bovie cauterized. Subjecting to hypoxia was carried out in sealed chambers immersed in a water bath [36]. In the rat model, usually, the neonatal rats (7 days old) were exposed to HIBD by the RiceVannucci modeling method. After anesthetizing rats, a longitudinal incision was made in the middle of their neck. The left common carotid artery was separated and was ligated on both sides. After cutting the blood vessel, the wound was quickly sutured [37]. In the piglet model, 1- to 2-day-old male piglets were anesthetized. Then, the two common carotid arteries were subjected, and they were tightened by elastic bands. After the surgery, HI brain injury was induced in the piglets by total interruption of the carotid blood flow by tightening the elastic bands around the arteries [38].
4. Pharmacological Evidence of Natural Plant Products in Neonatal Hypoxic-Ischemic Brain Damage
4.1. Plant Extracts
4.1.1. Grape Seed Extract
Grape is one of the fruits that is a rich source of phenolic compounds and 60–70% of grape phenolic compounds are discovered in the seeds of the fruit. Grape seed extract (GSE) comprises several phenolic compounds, mainly procyanidins and proanthocyanodins monomers, polymers, and their gallate ester and resveratrol, which is the major compound that is extracted from the skin and seeds of grape [39,40,41]. GSE is a powerful Free Radical Scavenger (FRS) of oxygen and has anti-lipid peroxidation activity [32,33,34,35,36,37,38,39,40,41,42,43,44,45]. GSE revealed anti-oxidant efficacy more than vitamins C and E [46]. GSE also displayed anti-inflammatory and anti-apoptotic actions [47,48,49,50]. In addition, GSE can reduce brain injury in forebrain ischemia in a gerbil model in adults [51].
Zheng and coworkers, in 2005, investigated the neuroprotective effect of GSE against neonatal HI brain injury [52]. In this study, rat pups at postnatal day 7 (P7) were subjected to HI insult and received GSE (50 mg/kg by intra peritoneal (i.p.)) 5 min before hypoxia and 4 h after reoxygenation (twice daily for 1 day). Brain weight loss was reduced from 20.0% in vehicle rats to 3.1% in treated rats, as well as improvement in the histopathologic brain score in hippocampus, thalamus, and cortex after GSE pretreatment. Additionally, lipid peroxidation markers 8-isoPGF2α (8-isoprostaglandin F2α) and TBARS (thiobarbituric acid reactive substances) levels significantly reduced. In another study [53], GSE was injected at 5 min to 5 h after reoxygenation and it was found that GSE post-treatment reduces neurofunctional abnormalities and brain weight loss even when administered 3 h after injury. In addition, the reduction of lipid peroxidation marker 8-isoPGF2α and pro-apoptotic protein c-jun in the rat brain cortex suggested that GSE exhibits neuroprotection through free radical inhibition and anti-apoptotic effects [53].
4.1.2. Grape Seed Proanthocyanidin Extract
Grape seed proanthocyanidin extract (GSPE) is a combination of biologically active flavanol compounds ranging from monomers such as gallic acid, epicatechin, catechins, and their gallate forms, to oligomeric proanthocyanidins. GSPE is known as one of the more potent natural antioxidants and its antioxidant capacity is significantly higher than vitamins E and C [54]. GSPE has been identified in various plants, for instance, cinnamon bark, pine bark, lotus, and apple, and also is widely distributed in red and white grape seeds. Many studies have reported a variety of pharmacological activities for GSPE including antioxidative [55,56], anti-inflammatory, anti-apoptosis [43], and anticancer properties. Recent studies have also been reported that suggest that GSPE possesses neuroprotective effects [56,57,58], for example against an adult rat model of ischemia-reperfusion injury [59].
Tu et al. demonstrated that GSPE significantly decreased brain infarct volume (approximately 50%) and improved neurobehavioral recovery in HIE mice models when pups were pretreated with GSPE (30 mg/kg, intraperitoneal (i.p.) injection) 20 min before HI [60]. In addition, the number of apoptotic cells and pro-apoptotic proteins’ expression (cleaved caspase-3 and bax) was significantly reduced, while expression of anti-apoptosis protein bcl-2 and the bcl2/bax ratio increased in the pretreated group, demonstrating the antiapoptotic role of GSPE [60].
4.1.3. Pomegranate Juice and Pomegranate Polyphenol Extract
The scientific name for pomegranate is Punica granatum Linn. Different parts of pomegranate (peel, seeds, and arils) are an important source of polyphenols, including tannins, mainly ellagitannins and gallotanins, flavonoids, mainly luteolin, quercetin, anthocyanidins, catechin, and epicatechin, alkaloids, etc. Moreover, pomegranate contains hydroxybenzoic acids such as gallagic acid, ellagic acid, and ellagic acid glycosides [61,62,63,64]. Many studies have demonstrated that pomegranate has been linked with the prevention and treatment of a wide range of disorders, such as cardiovascular disease [65], hypertension [66], cancer (skin, breast, prostate, colon, lung, pancreatic) [67,68], arthritis [69,70], hyperlipidemia [71], obesity [72], diabetes [73], and wound healing activities [74]. The therapeutic potential of pomegranate fractions are correlated with its antioxidative [75,76,77], anti-inflammatory [78,79], anti-infective [80], anti-atherogenic, anti-hyperglycemic, immunomodulation [81,82], and anti-proliferative [83,84] properties. In recent decades, a number of in vivo and in vitro experimental researches have indicated the neuroprotective properties of pomegranate [85,86,87] against neurodegenerative diseases, particularly Alzheimer’s and depression-related models in several animal models [88,89,90]. In addition, other studies have demonstrated the effect of pomegranate and pomegranate seed extract on improvement of memory deficit in rat models of cerebral ischemia [91,92,93] and ischemia-induced anxiety in male rats [94]. In another study, pomegranate has been shown to improve cognitive and functional recovery after ischemic stroke [95].
In order to investigate the neuroprotective effect of pomegranate juice against neonatal HI brain insult, in 2005, Loren et al. [36] conducted a study on mature C57BL6 mice. In this study, pregnant mice received pomegranate juice in their drinking water at three dose levels (1:60, 1:80, and 1:320 dilutions of pomegranate juice concentrate). The treatment time for total groups was, in most cases, 15 days, which included 7 days in utero and 8 days ex utero for mice that sustained caspase-3 analysis, and 21 days, which included 7 days in utero and 14 days ex utero for mice that sustained histologic analysis. Seven-day-old neonatal mice were subjected to hypoxic-ischemic insult. They found significantly (>60%) decreased brain tissue loss as well as a decrease in activation of caspase-3 (84% in the hippocampus tissue and 64% in the cortex tissue) in the treated group as compared to control pups. The results demonstrated the effectiveness of pomegranate juice against neonatal HIBD through maternal dietary supplementation. The neuroprotective effects were observed. In a follow-up study, using the same neonatal HI mouse model, Loren et al. showed that maternal supplementation with pomegranate polyphenol extract (PPE) resulted in a significant decrease of HI-induced caspase-3 activation. The authors suggested that phenolic compounds of the juice are responsible for its neuroprotective effect [96]. Recent studies demonstrated that among polyphenol-containing foods, pomegranate juice had the highest concentration of measurable polyphenol due to the fact that the pomegranate juice antioxidant capacity was higher than green tea or red wine. Anthocyanins are the largest and most important group of flavonoids which exist in pomegranate juice [61,62,97,98].
4.1.4. Dendrobium officinale Extract
Dendrobium officinale (DO), that is used in traditional Chinese herbal medicine, represents various beneficial therapeutic activities, like chronic inflammation reduction. The neuroprotective activity of DO extract in ischemia-reperfusion-induced arrhythmia rat models has also been reported [99,100,101].
In 2019, Li found that DO extract (DOE) exerted neuroprotection against neonatal HIBD when 7-day-old rats were intra-gastrically administered different doses of DOE ((75–150–300) mg/kg/day, continuously for 14 consecutive days) after HI [37]. Results showed that after treatment with DOE, the levels of MDA (malondialdehyde), NO (nitric oxide), and NOS diminished and SOD activity increased in a dose-dependent manner, which indicated the enhancement of the antioxidant capacity in HIBD rats. In addition, the behavioral ability of rats significantly improved after DOE treatment in a dose-dependent manner. Besides, DOE led to downregulation of Bax and caspase-3 and upregulation of Bcl-2 (demonstrating reduced neuronal apoptosis). Moreover, CI area percentage was remarkably diminished after treatment. They also examined the protein expression related to brain injury (KCC2 and HDAC1) and it was reported that after treatment with DOE, the expression of HDAC1 was diminished and KCC2 expression was elevated in the hippocampus and cortex. Meanwhile, determining the neurotrophic factors’ expression: HIF-1α (hypoxia-inducible factor 1- alpha), bFGF (basic fibroblast growth factor), CNTF (ciliary neurotrophic factor), and BDNF, after treatment with DOE, indicated that HIF-1α expression was remarkably diminished, and the bFGF, CNTF, and BDNF expression were enhanced. Altogether, this study demonstrated that the DOE is able to increase the neurotrophic factors’ expression and leads to neuronal apoptosis suppression and stimulates antioxidant capacity.
4.2. Phytochemicals
4.2.1. Verbascoside
Phenylethanoid glycosides are a kind of water-soluble natural phenolic compound that have exhibited neuroprotective activity [102]. Verbascoside (VB) (the chemical structure is shown in Figure 1a) is a typical phenylethanoid glycoside from lemon verbena [103]. VB is found in more than 150 types of plants [104]. There is numerous evidence that VB has various biological activities, including antioxidant [105,106], anti-inflammatory [107,108,109], immunoregulatory [110], anticancer [111], and antimicrobial. In addition, several research studies have shown that VB also has neuroprotective properties against various neurological disorders in in vitro and in vivo experiments [112,113,114].
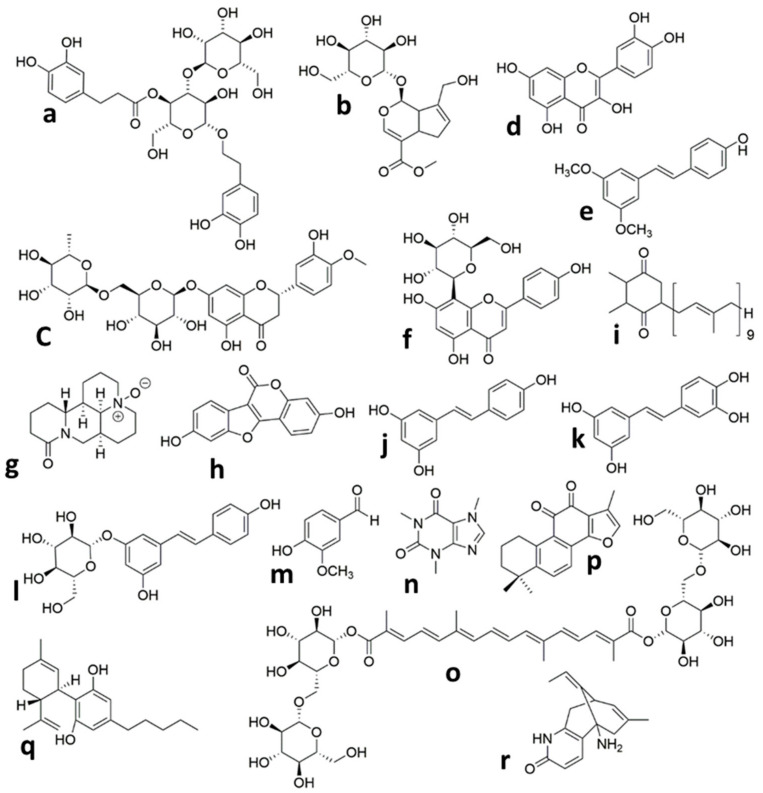
Figure 1.
Chemical structures of the isolated compounds from plants in animal models of neonatal Hypoxic Ischemic brain injury. (a) Verbascoside, (b) Geniposide, (c) Hesperidin, (d) Quercetin, (e) Pterostilbene, (f) Vitexin, (g) Oxymatrine, (h) Coumestrol, (i) Plastoquinone, (j) Resveratrol, (k) Piceatannol, (l) Polydatin, (m) Vanillin, (n) Caffeine, (o) Crocin, (p) Tanshinone IIA, (q) Cannabidiol, (r) Huperzine A.
Wei et al. [115] investigated the neuroprotective effects of VB on a HIBD model in rat neonates. In this study, animals were subjected to HIBD at p7 and VB groups (60, 120, and 240 mg/kg) were administered through i.p. injection every 12 h after HI for two consecutive days. VB post-treatment (240 mg/kg) significantly reduced prolonged reflex latencies and brain infarct volume dose-dependently (120 and 240 mg/kg). The VB-treated group (240 mg/kg) also remarkably decreased the degree of degeneration, morphological damage, and necrosis in the cortex and hippocampus CA3 region compared with the HI group. Furthermore, autophagosome formation and the autophagy-related proteins’ expression (P62, Beclin-1, and LC3-II/I ratio) reduced after VB post-treatment.
4.2.2. Geniposide
Iridoid glycosides are phytochemicals that naturally occur in many plants [116,117]. Geniposide (Figure 1b) is one of the major iridoid glycoside compounds that are purified from the Chinese herb Gardenia jasminoides [118]. Nearly 40 plants have been identified that contain geniposide [119]. Geniposide possesses diverse pharmacological activities, including anti-inflammatory [120,121], antidiabetic [122], anti-oxidative, and hepatoprotective [123]. In recent years, geniposide demonstrated excellent neuroprotective activities in experimental neurological dysfunctions, such as Parkinson’s disease [124], Alzheimer’s disease [125,126], and cerebral ischemia [127,128,129].
Liu et al., in 2019, studied the therapeutic effects and underlying mechanisms of geniposide against HI brain damage in neonatal mice. In this study, mice pups were subjected to HI insult at P10 and geniposide was administered (20 mg/kg) daily, intra-gastrically after HI. Findings showed that geniposide treatment significantly reduced the number of apoptotic neurons and also decreased the leakage of serum Immunoglobulin type G (IgG) into brain tissue. In addition, mRNA expression levels of pericyte markers, adherens, and tight junction proteins were upregulated after treatment, which meant that geniposide decreased the disruption of the BBB, which was induced by HI. Moreover, geniposide post-treatment attenuated astrogliosis and microgliosis. Microglia and astrocytes are two of the major neuroinflammation mediators [130]. To further elucidate the underlying molecular mechanism of geniposide-induced neuroprotection, it was found that Phosphoinositide 3-kinases/Protein kinase B (PI3K/Akt) expression signaling pathway-related protein was increased after treatment, which showed that geniposide probably exploits its neuroprotection activity via PI3K/Akt signaling pathway activation.
4.2.3. Hesperidin
Hesperidin (Figure 1c) belongs to the flavanone glycoside class of compounds, which was originally discovered in citrus fruits [131] but has been reported to occur in many plants other than citrus [132]. Several biological activities have been reported from Hesperidin, for example, antioxidative [133,134], anti-inflammatory [135], anticarcinogenic, and antiallergic properties. Hesperidin also has the ability to pass through the BBB [136]. In recent studies, both the in vivo and in vitro neuroprotective properties of hesperidin have been shown [137], which are attributed to its antioxidant and anti-inflammatory activities. This compound also had neuroprotective effects on amyloid β [138] against 3-nitropropionic acid-induced [139,140] and H2O2-induced [141] neurotoxicity. It also possesses antidepressant activities [142]. Another study reported that hesperidin enhances learning and memory in a rat model of cerebral ischemic-reperfusion injury [143].
Rong et al. demonstrated that hesperidin pretreatment in HIBD rat neonates increased the surviving brain volume from 49.8% to 72.9% and improved long-term behavioral development in oral administration of hesperidin (50 mg/kg/day) during 3 days in animals. Hesperidin pretreatment also markedly decreased Fluoro-Jade B stain-positive neurons in the cortex of the brain at post-HI [144]. In the culture media, pretreatment with hesperidin (1.6 μM) reduced the release of LDH (lactate dehydrogenase) and enhanced the levels of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), which indicated that hesperidin promotes neuronal survival. Moreover, levels of ROS and MDA decreased with hesperidin pretreatment in both in vivo and in vitro experimental models. Results also showed that hesperidin pretreatment suppressed FoxO3 phosphorylation, which indicated that possible molecular mechanisms of hesperidin neuroprotection are likely the result of activation of the PI3K/Akt survival signaling pathway and free radical reduction.
4.2.4. Quercetin
Quercetin (Que) is a flavonoid (3,3′,4′,5,6-Pentahydroxyflavone) (Figure 1d) which is distributed all over the world in fruits, vegetables, and many other dietary sources, including green tea, red wine, berries, and onions [145]. Quercetin is generally known as a strong antioxidant and free radical scavenger [146,147,148]. It also possesses anti-inflammatory and neuroprotective effects in brain injuries, such as traumatic [149] and ischemic/reperfusion [150]. Many recent studies have been conducted to examine the neuroprotective effects of quercetin on ischemic brain damage [151] in vitro [152,153] and in various types of in vivo models, such as focal cerebral ischemia/reperfusion [154,155] and global cerebral ischemia [156]. Moreover, in some studies, delivery systems such as liposomal [157] and the nano-encapsulation approach have been used to deliver quercetin into the rat’s brain [158].
Qu, in 2014, conducted a study on postnatal 3-day-old rats and found that administration of Que (intra-gastrically once a day, 20 or 40 mg/kg from 2 h after HI to the day rats were sacrificed) significantly improved HI-induced myelin damage through strengthening survival of oligodendrocytes [159]. In 2019, Wu used a 7-day-old neonatal rat model of HIBD and administered Que (40 mg/kg/day) intra-gastrically once a day for 7 days. Results showed that Que treatment enhanced anti-apoptotic (Bcl-2) and reduced pro-apoptotic (Bax) proteins in cortical cells, as well as significantly attenuated the DNA-strand break induced by HI, which demonstrated the antiapoptotic effect of Que. Data also showed that Que treatment significantly decreased astrogliosis and microgliosis and downregulated the inflammatory factors’ expression (TNF-α, IL-1β, and IL-6) in rat cortex, which showed that Que hads an anti-inflammatory effect and could protect brain cortex tissue from subsequent damage [160]. To study the mechanism of Que, authors examined the expressions of the TLR4/NF-κB signaling pathway. Based on the results, the phosphorylation of TLR4 (Toll-like receptor 4) reduced significantly and signals of p-p65 and p-p-IκBα were downregulated. These data confirmed that Que probably exerts its neuroprotection activity via suppression of the TLR4-mediated NF-κB pathway.
4.2.5. Pterostilbene
Pterostilbene (PTE) (3,5-dimethoxy-4-hydroxystilbene) (Figure 1e) is one of the resveratrol derivatives and was predominantly discovered in numerous varieties of grapes and blueberries [161,162]. PTE belongs to the phytoalexins family, and biosynthesizes in plants in response to biotic and abiotic stress [163]. The following evidence demonstrates that PTE possesses a protective effect against oxidative stress, inflammation, apoptosis, and shows anticancer and analgesic activities [164,165,166]. Recent studies suggest that pterostilbene also has neuroprotective effects in nervous system disorders such as lipopolysaccharide-induced learning and memory deficit [167], Alzheimer’s disease [168], anxiety [169], and ischemia-reperfusion brain damage [170,171].
In 2016, Li and coworkers reported that PTE (single dose—50 mg/kg) had neuroprotective effects against HI brain damage in rats when injected i.p. 30 min before insult. Brain infarct volume and brain edema markedly decreased (nearly 50%) and animals’ survival enhanced (from 78% to 95%). Additionally, PTE pretreatment significantly decreased neurological score and improved working memory impairment and motor deficit. Moreover, pro-inflammatory cytokines’ expression (IL-1, IL-6, and TNFα,) and transcription factors (p-65 and NF-κβ) decreased in hippocampus CA1 and represented an anti-inflammatory effect of PTE. The results also showed inhibition of programmed cell death. Moreover, the level of TBARS and ROS were decreased and SOD activity was increased, which indicated the antiapoptotic and antioxidative role of PTE [172].
PTE pretreatment significantly prevented mRNA and protein expression of HO-1 (hemeoxygenase-1). On the other hand, HO-1 inhibitor, ZnPP, can inhibit the neuroprotective effect of PTE. Authors suggested that HO-1 is a possible target of PTE in neuroprotection for oxidative, apoptotic, and inflammatory damage induced by HI [172].
4.2.6. Vitexin
Vitexin (5, 7, 4-trihydroxyflavone-8-glucoside) is an apigenin flavone glycoside (Figure 1f) that has been discovered in several plant species, including Vitex negundo seed [173], Passion flower [174], mimosa [175], wheat leaves [176], and hawthorn [177]. Earlier studies revealed that it possesses potent pharmacological actions such as antioxidant, anticancer [178], antiviral [179], anti-inflammatory [180], antihypertensive [181], and anti-depressant-like actions [182]. In addition, there are few reports on the neuroprotective efficacy of vitexin [183,184,185] such as its protective effect against cerebral I/R (ischemia/reperfusion) [186]. Vitexin is an HIF-1α inhibitor [187] and former researches have represented that the early suppression of HIF-1α after HI injury produced neuroprotection.
A study by Min et al. [188] revealed that administration of vitexin (single-dose: 30–60 mg/kg i.p.) at 5 min or 3 h after HI can significantly decrease brain infarct volume and improve long-term behavioral development as well as reduce neuronal cell death and brain tissue loss in neonatal rats dose-dependently. In addition, vitexin significantly inhibited upregulation of HIF-1α and VEGF protein levels. Vitexin also reduced the BBB disruption following brain edema. Throughout hypoxia, HIF-1α showed a significant function in VEGF expression. VEGF had a destructive effect on the surrounding vasculature that resulted in BBB disruption and subsequently, brain edema. Previous reports have shown that the VEGF expression inhibition throughout the primary cerebral ischemia stage or hypoxia led to the BBB protection. These data suggest that the primary HIF- 1α inhibition along with vitexin decreased VEGF levels and led to protection of the BBB and brain edema reduction. Additionally, vitexin (45 mg/kg) was the most efficacious dose at 5 min after HI but lost its effects when administered 3 h after HI. These results indicated that the neuroprotection activity of vitexin is related to primary inhibition of HIF-1α and VEGF expression.
4.2.7. Oxymatrine
Oxymatrine (OMT) (Figure 1g) is one of the main alkaloids isolated from traditional Chinese herbal medicine Sophora japonica [189]. Oxymatrine has indicated anti-inflammatory [190,191], antiviral [192], antioxidant [193], anti-hepatic fibrosis [194], anti-apoptosis [195,196], and anticancer [197,198] effects. In recent years, in vitro [199] and in vivo investigations have represented that OMT possesses a neuroprotective effect against experimental brain injury models such as traumatic injury [200] and ischemia/reperfusion damage [201,202].
Liu et al., in 2015 [203], reported that OMT could ameliorate brain damage in HI rat neonates. In order to identify OMT’s specific effect on HI rat neonates and underlying mechanism, another in vivo and in vitro experimental study was designed on a model of neonatal HIBD [204]. In this study, 7-day-old rat pups were administered oxymatrine (120 mg/kg, i.p. injection) after HI every 12 h for 2 days. OMT treatment attenuated neuronal damage and necrotic cell loss and degeneration in cerebral hippocampus CA3 and cortex. In addition, the reflex latencies were reduced in treated pups.
The results of cultural hippocampal neurons obviously indicated that OMT improved cell viability, reduced LDH leakage, and attenuated intracellular Ca2+ and loss of MMP, and decreased the rate of cell apoptosis in neurons exposed to OGD/RP. In addition, OMT adjusted the expression of the mRNA and protein of the PI3K/Akt/GSK3β pathway, inhibited the expression of NR2B protein, and regulated apoptosis mediators (reduced caspase-3 and enhanced Bcl-2 and MCL-1 levels). These results demonstrated that OMT exerts neuroprotection against neonatal HIBD via regulating apoptosis, NR2B downregulation, and activation of the PI3K/Akt/GSK3β signaling pathway [203,204].
4.2.8. Coumestrol
Neuroprotective effects of estrogens against ischemic brain damage have been demonstrated previously [205]. Phytoestrogens are naturally occurring estrogen-like molecules found in many dietary plant sources and are structurally similar to estradiol [206,207,208]. Isoflavonoids are a subgroup of phytoestrogens with estrogen agonist ability and mimic estrogen’s neuroprotective properties [209,210]. Coumestrol (Figure 1h) is an isoflavonoid-like natural compound present in soybean, alfalfa, and red clover [211,212,213], and various studies have proven its anti-inflammatory [214], anti-adipogenesis [215], anti-aging [216], antioxidant, and anticancer [217,218,219] activities. In addition, the literature also indicates that coumestrol exerts neuroprotection against Alzheimer’s disease [220,221], multiple sclerosis, and brain global ischemia [222,223].
Recently, in one study, effects of coumestrol administration on neonatal HIBD have been investigated in male rats [224]. In this study, coumestrol (20 mg/kg) was injected i.p. immediately before HI, as a preventive, or 3 h post-hypoxia as a therapeutic intervention. Results showed that coumestrol pretreatment prevents HI-induced mitochondrial swelling. Furthermore, pre- and post-treatment with coumestrol protected the reference and the working memory from impairments as well as partially prevented late cognitive deficits in both the reference and working spatial memory tasks that were evaluated at P60. In addition, pre- or post-hypoxia treatment reduced tissue death in both ipsilateral brain hemisphere (20% recovery) and hippocampus (25% recovery). Finally, Coumestrol pre- and post-treatment attenuated late GFAP (glial fibrillary acidic protein, a marker of late HI-induced reactive astrogliosis) overexpression in the hippocampus and also demonstrated that this compound can provide long-term protective activity for astroglia against cell damage. Based on these data, the authors suggested that coumestrol neuroprotection mechanisms involve prevention of mitochondrial dysfunction and long-term cognitive deficits.
4.2.9. Plastoquinone
During the photosynthesis process, a large amount of ROS is generated in plants by the sunlight. So, chloroplasts are kept by a potent antioxidative system. Plastoquinone (Figure 1i) is an important component of the photosynthetic electron transport chains in chloroplasts and the reduced form of plastoquinone (plastoquinol) is a powerful antioxidant which diminishes generated ROS during photosynthetic reactions [225,226,227,228].
As mentioned above, HI causes mitochondrial dysfunction which actuates the pathological ROS production. Thus, antioxidants’ targeted delivery to the mitochondria is a productive therapeutic strategy to treat the detrimental impacts of HIBD. Previous data have indicated that SkQR1 (10-(6′-plastoquinonyl) decylrhodamine 19) (mitochondria-targeted antioxidant), which is a plastoquinone molecule linked to rhodamine derivative [229], has a protective effect on ROS-induced pathological conditions in a number of tissues, such as heart [230], kidney [231,232], eye [233], and brain [230,231,232,234,235]. SkQR1 also showed a neuroprotective effect in experimental models of brain trauma [232] and brain ischemia [236].
The neuroprotective effect of SkQR1 in neonatal HIBD was explored by Silachev et al. in 2019 [237]. They found that pretreatment with SkQR1 in neonatal HI rats at a dose of 2 µmol/kg (i.p. injection) markedly reduced infarct volume and diminished oxidative stress in the brain, as well as reduced nervous tissue loss. SkQR1 pretreatment also led to a significant decrease of long-term neurological deficits. Finally, SkQR1 improved short- and long-term behavioral deficiency as a consequence of HI-induced and remarkably enhanced sensorimotor performance. Administration of SkQR1 before or immediately after (10 min) HI exerts neuroprotection via diminishing brain deterioration and ameliorating long-term neurological tasks. It seems that the rationale of neuroprotective action of SkQR1 can reduce the ROS generation with mitochondrial origin.
4.2.10. Resveratrol
Resveratrol (3,5,4′-trans-trihydroxystilbene) (RES) is a natural phenolic compound with a stilbene structure (Figure 1j). Resveratrol is found naturally in various plant species; however, its dietary sources are limited. Some dietary sources of RES are peanuts, pistachio, berries, plums, pomegranates, grapes, and red wine (grapevines). Of all of these, grapes and red wine present the highest content [238,239,240]. RES has received extensive attention for its diverse pharmacological activities [241,242,243,244,245,246]. Besides, accumulating evidence indicated that RES treatment exerted neuroprotection against various brain disorders [247,248,249], including neurodegenerative diseases [250,251,252] such as Parkinson’s, Alzheimer’s, and Huntington’s diseases [253,254]. In addition, resveratrol also has neuroprotective effects in animal models of stroke [255], traumatic brain injury [256], and various kinds of ischemic brain injury ischemia [257,258] such as focal cerebral ischemia [259,260,261], global cerebral ischemia [262,263], and ischemia/reperfusion [264,265,266,267]. The RES mechanisms of action in neuroprotection might comprise antioxidation [268,269], anti-inflammation [270,271], and anti-apoptosis [272] effects.
Few reports have exhibited potential neuroprotective effects of RES in the pilot model of neonatal HI encephalopathy using both in vitro and in vivo approaches. West et al., in 2007, investigated the resveratrol effects on HI brain injury in both neonatal rats and mice [96]. In this study, the pups subjected to HI at postnatal day 7 and different concentrations of RES (20 mg/kg, 200 μg/kg, and 2 μg/kg) at several different points in time (24 h before hypoxia, 10 min before hypoxia, and 3 h after hypoxia) were administered via i.p. injection. Results indicated that RES at doses of 200 μg/kg or greater diminished caspase-3 and calpain activation at 24 h after the injury time dose-dependently, but only when its use before the injury showed both anti-apoptotic and necrotic cell death activity. RES also protected the brain from tissue loss 7 days after the injury. In addition to its protective effect in mouse neonates, RES also protects neonatal rats against HI, interestingly, when it is used after the injury. In another study in 2008, rat pups received RES (30 mg/kg) i.p. 30 min before or 30 min after HI. Results displayed an anti-apoptotic mechanism of RES via a remarkable decrease in expression level of Bax, the ratio of Bax/Bcl-2, and caspase-3, upon administration of RES at 30 min before HI but not when it was used 30 min after the injury [273]. Most of the surveys highlighted the short-term immunohistochemical and histological measurement of a set of biological markers and generally downgrade the long-term adverse effects and consequences of the treatment. In 2011, Karalis et al. investigated the delayed consequence of RES early administration through analysis of behavior and late neuropathological examination. Shortly, rats at P7 experienced HI and were treated instantly after hypoxia, receiving the 90 mg/kg of RES via i.p. injection. The remarkable difference was reported among the groups in righting water maze, rotarod, and reflex tests. According to the neuropathology study, a remarkable decrease in preservation of myelination and infarct eventuated after RES treatment. These results demonstrate that RES long-term neuroprotective activity on neonatal HI-induced damage on the gray and white tissue of the brain may be connected with the protection of behavioral functions [274].
Arteaga et al. conducted a study aiming to determine the RES activity as a preventive (before HI injury) or therapeutic agent (immediately after HI injury) on cellular and morphological damage and impairments in behavior in rat neonates. They found that pretreatment with RES reduced volume of infarction, preserved myelination, and minimized the astroglial reactive response. Moreover, long-term cognitive impairment significantly improved in adulthood. It was noticeable that none of these protective characteristics were found when RES was administered following HI [275]. All of these reported studies demonstrated the protective property of RES only when administered before HI (pre-treatment). However, Pan et al. reported that post-treatment with RES (100 mg/kg, i.p. injection three times at 0, 8, and 18 h respectively, after HI) remarkably decreased brain damage. They found that RES also decreased the expression of important inflammatory factors at the protein and mRNA levels, and at least partially through inhibition of microglia activation. However, RES demonstrated an anti-apoptotic activity, and changed the apoptosis-related genes’ expression, such as caspase3, Bax, and Bcl-2. These data indicate that inhibition of inflammation and apoptosis is a prosperous therapeutic strategy of RES post-treatment. The post-insult administration of RES has higher clinical relevance than pre-insult administration, though its specific molecular mechanism in the treatment of neuroinflammation is unclear [276]. Therefore, recently, Le and coworkers investigated the potential anti-inflammatory mechanism of RES on immature brains. They used the HIBI model in neonatal mice for in vivo and microglial cells for in vitro study. They found that RES significantly reduced the levels mRNA and protein, which were related to cytokines (TNF-α, IL-1β, and IL-6) in brain tissues following HI. As a result, RES remarkably ameliorated brain damage and neurobehavioral deficits by reducing neuroinflammatory responses. Additionally, RES reversed the reduction in the levels of SIRT1, like raising the levels of TLR4, MyD88, and NF-κB, whereas the administration of SIRT1-specific inhibitor prior to RES significantly reversed this effect, which demonstrated that RES exerted anti-inflammatory activity via inhibition of the TLR4/MyD88/NF-κB signaling pathway in vivo [277].
4.2.11. Piceatannol
Piceatannol (PIC) (Figure 1k) is a polyphenolic stilbene phytochemical that is found in large amounts in passion fruit seeds, berries, and grapes [278,279]. PIC is a hydroxylated analog of RES but has stronger free-radical-scavenging activity as well as greater bioavailability than RES [280,281]. Previous studies have reported that PIC has antioxidant, anti-inflammatory, and anticancer activity [282,283]. In addition, it exerts cardioprotective and neuroprotective properties [284,285,286].
Dumont et al. [287] recently showed that nutritional supplementation of pregnant and breastfeeding female rats with PIC (0.15 mg/kg/day via drinking water) could provide neuroprotection for HI neonates. In behavioral terms, maternal supplementation of PIC restored sensorimotor functions and recovered cognitive functions. Also, the number of pups with brain lesions, brain lesion size, and severity of edema inside brain lesions decreased in the treated group. Moreover, the spatial distribution of white matter fiber bundles significantly improved. These results were observed in pups as early as 3 h post-injury, which indicated the short-term effect of PIC. In addition, the hippocampal-dependent memory of pups was completely restored at P30, which indicated the long-term neuroprotective effect of maternal PIC consumption. The PIC-induced neuroprotection could be attributed to its antioxidant effect or modulation of the brain metabolism.
4.2.12. Polydatin
Polydatin (Figure 1l) is the main component of Polygonum cuspidatum (a traditional Chinese herb) but is also found in many daily diets, such as grape, peanut, hop cones, red wines, and chocolate. Polydatin is the glycoside of RES and the most abundant derivative of RES in nature [288]. Numerous studies have reported that polydatin has various pharmacological activities, comprising cardiovascular protection, anti-inflammation, antioxidation, anticancer, etc. [289]. Protective effects of polydatin against brain damage in animal models of ischemia-reperfusion have also been reported [290,291].
In one study by Sun et al., rat neonates were subjected to HIBI at P7 and polydatin (10 mg/kg) was administrated through i.p. injection once a day for 10 consecutive days. The results of a behavioral test showed that polydatin treatment led to enhancement of long-term learning and memory. In addition, BDNF expression was increased significantly in brains of treated pups. These results demonstrated that the protective effect of polydatin was possibly mediated via upregulation of BDNF, which showed that polydatin may have an important role in promoting neuronal survival and neuronal recovery following injury [292].
4.2.13. Vanillin
Vanillin (Figure 1m), the major component of vanilla orchids, is a natural aromatic compound. Vanillin is one of the best flavor ingredient in cosmetic, food, and drug products [293]. It has been reported that vanillin exhibits various pharmacological activities [294]. Recent reports revealed that vanillin could pass through the BBB and showed neuroprotective effects. For instance, vanillin reduced ischemia-induced neuronal damage in gerbils [295] or it showed a protective role against Parkinson’s disease [296].
In one study, the authors examined neuroprotective effects of Vanillin post-treatment on HIBD in rat neonates at postnatal day 7. Evaluation of brain damage revealed that vanillin (80 mg/kg, i.p. injection) significantly improved HI-induced neurobehavioral deficits and reduced brain infract volume as well as brain edema in a dose-dependent manner. In addition, neuronal degeneration and necrotic cell death were reduced in the hippocampal CA1 and CA3 and cortex regions in the brains of the treated group. Furthermore, vanillin post-treatment decreased IgG leakage into the brain tissue, reduced the levels of MMPs, and upregulated the TJ-related proteins’ expression, which indicated that vanillin could improve BBB ultrastructure damage. Besides, endogenous antioxidant enzymes activities (SOD, GSH-Px, and CAT) and T-AOC (total antioxidant capacity) remarkably increased, and lipid peroxidation (reduction in the level of MDA content) was decreased. These results demonstrated that neuroprotection of vanillin might be attributed to its efficacy in oxidative stress inhibition and preserving BBB integrity, and reducing subsequent brain edema [297].
4.2.14. Caffeine
Caffeine (Figure 1n) belongs to methylxanthine alkaloid, which is found naturally in many plants. Coffee and tea are the main sources of caffeine. The main action of the methylxanthines is to stimulate the central nervous system. Caffeine is a non-selective adenosine antagonist and shows a wide range of actions on the brain [298,299]. Caffeine has been widely used to treat apnea of prematurity for the past 30 years as well as to facilitate the extubation in very low birth weight infants. It is available in the form of caffeine citrate and its active substance is caffeine base [300,301,302].
In one study by Back et al. [303], neonatal mice were subjected to hypoxia and administered caffeine. It was found that hypomyelination and ventriculomegaly were diminished by caffeine treatment. Results also led to a more normally arranged myelinated axon orientation in the caffeine-treated group. Additionally, the proportion of immature oligodendrocytes was enhanced. In order to determine the underlying mechanism of caffeine’s neuroprotective effect, in another study, rat pups were subjected to HI at postnatal day 7 and received caffeine citrate (20 mg/kg) immediately before and at 0, 24, 48, and 72 h after HI [304]. Results demonstrated that administration of caffeine citrate led to a significant decrease in the number of apoptotic cells in the parietal cortex and hippocampus of the treated rat’s brain, which showed that the antiapoptotic effect of caffeine was possibly responsible for its protective effects against neonatal HI brain injury.
4.2.15. Crocin
Crocus sativus L. stigma, generally known as saffron, is widely used to treat various diseases. Crocin (Figure 1o) is a water-soluble carotenoid and one of the main pharmacological active substances isolated from saffron. Previous studies have reported that crocin has high antioxidant capacity along with anti-inflammatory, anti-atherosclerotic, anticancer, and hypolipidemic effects [305]. Beside, neuroprotective properties of crocin have also been reported in experimental models of various brain disease, like cerebral ischemia-reperfusion [306,307], memory impairment [308], traumatic brain injury [309], and depression-related models [310].
Hypothermia therapy is one of the standard treatments for HIE in infants, although the efficacy is limited. Combination treatments are considered to increase the efficacy of hypothermia. Increasing the neuroprotective activity of hypothermia, combination treatments are deemed effective, and numerous therapeutic treatments have been examined as potential strategy in combination with hypothermia, comprising stem cells, erythropoietin, etc.
In one study [311], Huang and Jia investigated the protective effect of crocin and its combination with hypothermia against HIE in mouse neonates. Pups were subjected to HI at P7 followed by treatment with crocin (10 mg/kg) and hypothermia in combination. Levels of NO, ROS, and MDA were significantly reduced as well as mRNA expression of COX-2 (Cyclooxygenase-2) and iNOS (inducible nitric oxide synthase), which demonstrated the enhancement of the anti-oxidative effect of hypothermia after combined treatment. Furthermore, levels of IL-1β and TNF-α were markedly reduced, which indicated the enhancement of the anti-inflammatory effect of single hypothermia by combined treatment. Finally, combined treatment led to a significant reduction of neurological severity score, that showed the improvement of neurological function. These results demonstrated that crocin could increase the neuroprotective effect of hypothermia and can be used as a combined therapy with hypothermia.
4.2.16. Tanshinone IIA
Danshen, isolated from the Salvia miltiorrhizae Bunge, is a popular traditional Chinese herbal medicine which has been used for the treatment of cardiovascular and cerebrovascular disease. Tanshinone IIA (Tan IIA) (Figure 1q) is the most abundant pharmacologically active component of Danshen [312,313,314]. Tan IIA possesses anti-oxidative, anti-inflammatory, anti-apoptosis, antiplatelet aggregation, and anticancer activities [315,316,317], and exerts protective effects against cerebral I/R injury [318]. Tan IIA can be defined as an AchE (acetylcholinesterase) inhibitor that has the ability to penetrate the BBB [319].
Xia et al. found that the HIBI induced in the P7 neonatal rat model with administration of tanshinone IIA (i.p. injection at 10 mg/kg/d from 2 days before HI for 9 or 16 days) improved neuropathology and sensorimotor functions. Also, Tan IIA remarkably enhanced the antioxidant capacity in rat plasma and protected the C17.2 progenitor cells from AAPH-induced death. Furthermore, Tan IIA exhibited a protective activity on mitochondrial membrane potential [320].
4.2.17. Cannabidiol
Cannabinoids are a family of lipid mediators which modulate the synaptic transmission. Cannabinoids have emerged as promising neuroprotective agents in various brain disorders such as seizures, epilepsy, and Multiple Sclerosis (MS) [321,322,323]. In recent years, in vitro and in vivo investigations have reported that the cannabinoid receptor (CB1–CB2) agonist WIN55212–2 provided neuroprotection in animal models of neonatal HIE [324]. However, the production of psychoactive effects limits their therapeutic value. The phytocannabinoid, cannabidiol (CBD), is the major non-psychoactive component of Cannabis sativa plant (marijuana) [325]. CBD is well-known as a potent anti-inflammatory and antioxidant substance which shows neuroprotection in several neurodegenerative disorders using experimental animal models [326,327,328].
The neuroprotective effect of CBD against neonatal HIE was first demonstrated by Alvarez et al. [329]. It was reported that administration of CBD to newborn piglets at a dose of 0.1 mg/kg after HI insult leads to a reduction of brain edema and prevention of brain seizures. Also, CBD reduced cell loss and the number of degenerated neurons and showed additional cardio-protective effects. These effects can be attributed to a CBD-induced modulation of cerebral hemodynamic impairment and improvement of brain metabolic activity. Also, CBD did not show any significant side effects in treated piglets. Castillo et al. examined the effect of CBD (100 µM) in an in vitro model of neonatal HIE by exposing forebrain slices from newborn mice to oxygen-glucose deprivation [330]. The prevention of necrotic (reduction of LDH efflux to the medium) and apoptotic (reduction of caspase-9 concentration in tissue) cell death by CBD was observed. CBD also modulates inflammation (reduced IL-6 and TNF-α production and COX-2 expression) as well as excitotoxicity modulation (decreased glutamate release). Furthermore, iNOS expression was decreased. The results demonstrated that CB2 and adenosine—mainly A2A-receptors—participated in CBD-induced neuroprotection and led to activation of A2A, which was a principal mechanism of CBD neuroprotection. However, these reports demonstrated short-term neuroprotective activities of CBD in the immature brain after HI. In one study, Pazos et al. demonstrated long-term neuroprotective effects of CBD in newborn rats that received 1 mg/kg CBD after HI [331]. In this study, CBD reduced brain infarct volume and modulated brain excitotoxicity, inflammation, and oxidative stress 7 days following HI. It was reported that CBD provided long-lasting neuroprotection and treated animals’ neurobehavioral performance improved one month after HI. Also, they did not observe any noticeable side effects with CBD administration. In one study, Lafuente studied long-term (72 h after HI) effects of CBD. They found that post-HI i.v. injection of 0.1 mg/kg CBD in newborn piglets protects neurons, reduces cell death, and preserves astrocytes, which is related to its anti-inflammatory effect. Also, CBD improved neurobehavioral performance, brain activity, and stabilized metabolic activity [331]. In a fallow-up study by Pazos et al. [332], they investigated the underlying mechanisms in in vivo CBD-induced neuroprotection using the 1–2-day-old piglets treated with 1 mg/kg 30 min after HI. Findings indicated that CBD modulated excitotoxicity, inflammation, and oxidative stress, and both CB2 and 5HT1A receptors were involved in CBD-induced neuroprotection. In a recent study [333], the authors combined CBD with hypothermia in newborn piglets (receiving 30 min after the insult) and observed that the protective effect of combined therapy on excitotoxicity (glutamate/Nacetyl-aspartate ratio), inflammation (TNFα), oxidative stress (oxidized protein levels), and on cell damage was greater than either hypothermia or CBD alone, which indicated that CBD and hypothermia act complementarily if used shortly following the insult. Similarly, Barata et al. [38] observed the additive neuroprotective effects for combining hypothermia and CBD in newborn pigs (received 1 mg/kg) in a longer follow-up study that resulted in more complete neuroprotection than CBD or hypothermia alone. According to the results, CBD can be applied as a promising and novel therapy for neonates with HIE.
4.2.18. Huperzine A
Huperzine A (HupA), a naturally occurring alkaloid which was firstly separated from the Chinese herb Huperzia serrata, is a potent AChE inhibitor [334]. It has been widely demonstrated that this agent represented learning- and memory-enhancing efficacy in animal and human studies. It also reversed or attenuated cognitive deficits in Alzheimer’s disease and other forms of dementia in animal experiments [335,336]. Besides, HupA also exerts multiple neuroprotective activities, for example it demonstrated neuroprotective properties, against hydrogen peroxide-, β-amyloid-, and glutamate-induced neurotoxicity and oxygen–glucose deprivation [337,338,339]. Furthermore, HupA has also been revealed to have neuroprotective activities against cerebral ischemic injury [340].
The protective activity of huperzine A against HIBD in neonatal rats was investigated by Wang et al. [341]. In this study, HI resulted in working memory impairments in pups after 5 weeks. Pups received huperzine A (0.1 mg/kg i.p.) once a day for 5 weeks after HI. They observed significant behavioral and histopathological protection against HI injury in rats treated with HupA. Furthermore, administration of HupA also decreased neuronal damage in ipsilateral hemisphere and provided remarkable protection in the hippocampal CA1 region. HupA showed its pharmacological activities by enhancing synaptic ACh levels via inhibition of AChE.
5. Conclusions
Despite the wide range of studies concerning neuroprotective drugs, currently, the only clinically relevant strategy to treat neonates with HI brain injury is hypothermia, but it is not able to reduce severe disabilities and is not completely successful, as approximately half of the affected neonates do not respond to it. Furthermore, hypothermia is expensive, and its therapeutic window is narrow. Thus, development of alternative and more effective therapeutic strategies is urgently needed.
The evidence presented in this review demonstrates the potential of natural plant products, especially polyphenols, in both prevention (agents administered before HI insult or through maternal consumption) and treatment (those administered after HI event) of neonatal HI brain damage, however regulated clinical trials have not been performed.
These compounds target both the early phase as well as later phase of injury via the following mechanisms: antioxidative, such as GSE, GSPE, and vanillin; anti-apoptosis, such as quercetin, oxymatrine, and caffeine; ameliorating blood barrier dysfunction, such as vanillin and vitexin; preventing microgliosis and inflammatory response, such as pterostilbene, etc. Moreover, some of these agents also regulate cell survival or cell death signaling pathways (Figure 2). For example, geniposide activates the PI3K/Akt cell survival signaling pathway or the neuroprotective effect of vitexin is related to early inhibition of HIF-1α. Many of these agents act not only on direct targets, but also on signaling pathways (see Table 1 and Figure 2). Since HIBD involves a series of complex progressive pathophysiologic processes, therefore, drugs acting on multiple targets or combinations of single target agents may be more effective in treating perinatal HIE.
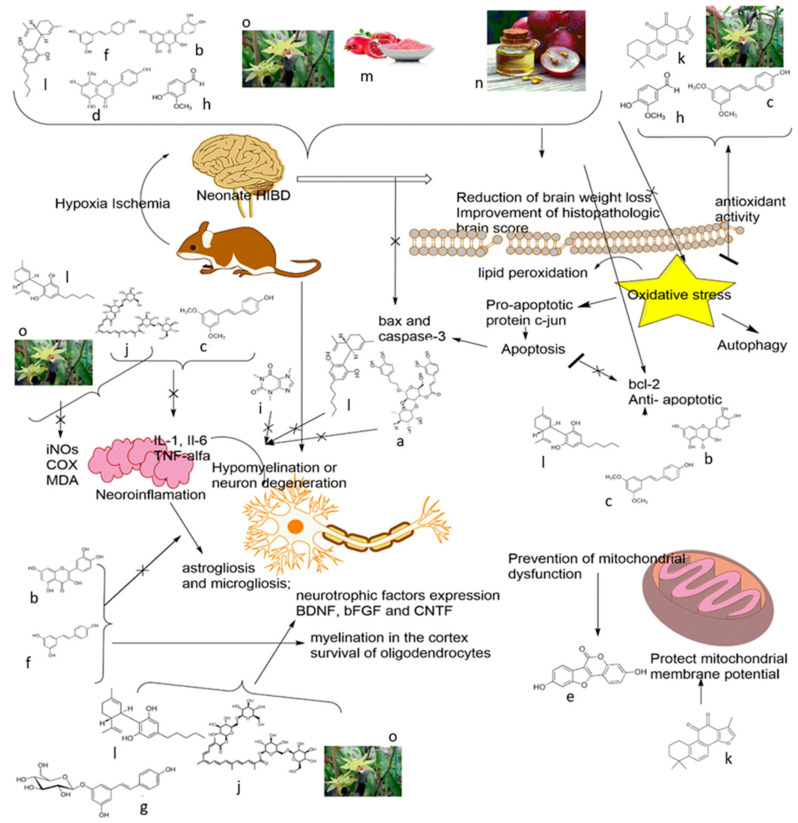
Figure 2.
The excitatory, free radical toxicity, inflammation, and neuronal cell death mechanism of plant extracts/plant-derived compounds in animal models of neonatal hypoxic-ischemic brain injury. (a) Verbascoside, (b) Quercetin, (c) Pterostilbene, (d) Vitexin, (e) Coumestrol, (f) Resveratrol, (g) Polydatin, (h) Vanillin, (i) Caffeine, (j) Crocin, (k) Tanshinone IIA, (l) Cannabidiol, (m) Pomegranate juice and Pomegranate polyphenol extract, (n) Grape seed extract and Grape seed proanthocyanidin extract, (o) Dendribium officinale.
Table 1.
The studied plant extracts/plant-derived compounds in animal models of neonatal hypoxic-ischemic brain injury.
| Plant Extract/Compound |
Administration Regimen | Dose | Species | Synergistic/Complementary Mechanism with/without Hypothermia | Effects/Molecular Mechanism | Reference |
|---|---|---|---|---|---|---|
| Grape seed extract | Pretreatment 5 min before HI Post-treatment 4 h after reoxygenation |
50 mg/kg intra peritoneal (i.p.) injection twice daily for 1 day |
P7 neonatal rat pups |
Pretreatment: Reduction of brain weight loss Improvement of histopathologic brain score Antioxidant effect: Reduction of lipid peroxidation (reduced 8-iso-PGF2α and TBARS level) Post-treatment: No protection |
[52] | |
| Post-treatment 5 min to 5 h after reoxygenation |
50 mg/kg i.p. injection |
P7 neonatal rat pups | Reduction of brain weight loss Antioxidant effect: Reduction of lipid peroxidation (reduced 8-iso-PGF2α level) Anti-apoptotic effect: Reduction of pro-apoptotic protein c-jun |
[53] | ||
| Grape seed proanthocyanidin extract | Pretreatment 20 min before HI |
30 mg/kg i.p. injection |
P7neonatal mouse pups | Reduction of infarct volume Improvement of neurobehavioral recovery Anti-apoptotic effect: Reduction of apoptotic cells number Reduction of pro-apoptotic proteins expression(bax and cleaved-caspase-3) Enhancement of anti-apoptotic (bcl-2) proteins expression and increased ratio of bcl2-to-bax |
[60] | |
| Pomegranate juice | Maternal dietary supplementation | 1:60 or 1:80 or 1:320 dilutions of pj concentrate, during last third of pregnancy and litter suckling | P7 neonatal mouse pups | Reduction of brain tissue loss Anti-apoptotic effect: Reduction of caspase-3 activation |
[36] | |
| Pomegranate polyphenol extract | Maternal dietary supplementation | 4.8 mg/day during pregnancy and litter suckling | P7 neonatal mouse pups | Anti-apoptotic effect Reduction of caspase-3 activation |
[96] | |
| Dendribium officinale extract | Post-treatment | (75 or 150 or 300) mg/kg, intragastric administration, Daily for 14 consecutive days | P7 neonatal rat pups | Improvement of behavioral ability Reduction of CI area percentage Regulation of brain injury-related proteins’ expression (diminished HDAC1 and elevated KCC2 expression) Antioxidant effect: Diminished levels of NOS, NO, and MDA Enhancement of antioxidant capacity (increased SOD activity) Anti-apoptotic effect Downregulated caspase-3 and Bax expressionUpregulated Bcl-2 expression Anti-inflammatory effect: Regulation of neurotrophic factors expression (increased expression of BDNF, bFGF, and CNTF) Mechanism: Inhibition of HIF-1α |
[37] | |
| Caffeine | Pretreatment immediately before HI, post-treatment 0, 24, 48, and 72 h after HI |
20 mg/kg | P7 neonatal rat pups | Decreased ventriculomegaly and hypomyelination More normally arranged myelinated axon orientation Enhanced proportion of immature oligodendrocytes Anti-apoptotic effectDecreased number of apoptotic cells |
[303] | |
| Pterostilbene | Pretreatment 30 min before HI |
50 mg/kg i.p. injection |
P7 neonatal rat pups | Enhancement of animal survival Reduction of neurological score Improvement in motor coordination, motor deficit, and working memory deficit Reduction of brain infarct volume Reduction of brain edema Anti-inflammation: (decreased TNFα, IL-1, and IL-6 expression; decreased p-65 and NF-κβ expression) Antioxidant: (decreased TBARS and ROS level; increased SOD activity) Antiapoptotic: (inhibition of programmed cell death) Mechanism: HO-1 signaling pathway (Prevention of HO-1 mRNA and protein expression) |
[172] | |
| Quercetin | Pretreatment | 40 mg/kg/day Intra-gastrically injection once a day for 7 days, last administration was 2 h after HI |
P7 neonatal rat pups | Anti-apoptosis: (downregulated Bax, upregulated Bcl-2, attenuated DNA-strands breaks) Anti-inflammation: (attenuated astrogliosis and microgliosis; downregulated IL-6, IL-1β, and TNF-α) Mechanism: Suppression of TLR4-mediated NF-κB signaling pathway (decreased TLR4 phosphorylation and downstream signals of p65 and p-IκBα) |
[159] | |
| Post-treatment 2 h after HI to the day of rats’ scarification |
20 or 40 mg/kg Intra-gastrically injection Once a day |
P3 neonatal rat pups | Improvement of myelination in the cortex through strengthening survival of oligodendrocytes | [160] | ||
| Resveratrol | Pretreatment 24 h/10 min before HI Post-treatment3 h after HI |
20 mg/kg or 200 μg/kg or 2 μg/kg i.p. injection |
P7 Neonatal rat and mouse pups |
Pretreatment: Protecting brain from tissue loss Preventing apoptosis and necrosis (diminished caspase-3 and calpain activation) Post-treatment: No protection |
[96] | |
| Pretreatment 30 min before HI Post-treatment 30 min after HI |
30 mg/kg i.p. injection |
P7 neonatal rat pups | Pretreatment: anti-apoptotic: (reduced expression level of Bax, caspase-3, and the ratio of Bax/Bcl-2) Post-treatment Not protection |
[273] | ||
| Post-treatment Immediately after HI |
90 mg/kg i.p. injection |
P7 neonatal rat pups | Reduced infarct volume Preserved myelination Behavioral tests (P8–P66): Improved early reflexes and sensorimotor functions Improved learning/memory function |
[274] | ||
| Pretreatment 10 min before HI Post-treatment Immediately after HI |
20 mg/kg i.p. injection |
P7 neonatal rat pups |
Pretreatment: Reduced infarct volume Preserved myelination Minimized astroglial reactive response Improved long-term cognitive impairment Post-treatment: Not protection |
[275] | ||
| Post-treatment 0 h, 8 h and 18 h after HI |
100 mg/kg i.p. injection |
P7 neonatal rat pups |
Anti- inflammation:(reduction of inflammatory factors expression; inhibition of microglia activation) anti-apoptosis: (regulation of Bax, Bcl-2 and caspase3 expression) |
[276] | ||
| Post-treatment immediately after HI and 12 h later |
100 mg/mL i.p. injection |
P7 neonatal mouse pups |
Anti- inflammation: (reduced expression levels of IL-1β, IL-6, and TNF-α) Enhancement of SIRT1 levelMechanism: Inhibition of TLR4/MyD88/NF-κB signaling pathway (reduced TLR4, MyD88, NF-κB levels) |
[277] | ||
| Tanshinone IIA | From 2 days before HI for 9 or 16 days | 10 mg/kg/day i.p. injection |
P7 neonatal rat pups | Improved neuropathology and sensorimotor functions Improved antioxidant capacity Protect mitochondrial membrane potential |
[320] | |
| Vanillin | Post- treatment Immediately after HI | 80 mg/kg, i.p. injection | P7 neonatal rat pups | Improved neurobehavioral deficits Reduced brain infract volume Reduced brain edema Reduced neuronal degeneration and necrotic cell death Preserving BBB integrity (decreased IgG leakage, reduced levels of MMPs, upregulated expression of TJ-related proteins) Inhibiting oxidative stress (increased SOD, GSH-Px, and CAT; increased T-AOC; decreased lipid peroxidation (MDA content)) |
[297] | |
| Verbascoside | Post-treatment | (60 or 120 or 240) mg/kg, i.p. injection, every 12 h for two consecutive days | P7 neonatal rat pups | Reduction of prolonged reflex latencies Reduction of brain infarct volume Reduction of necrosis Reduction of degeneration and morphological damage of neurons Reduction of autophagosome formation Reverse expression level of autophagy-related proteins (Beclin-1, LC3-II/I ratio, and P62) |
[115] | |
| Vitexin | Post-treatment 5 min or 3 h after HI |
(30 or 45 or 60) mg/kg i.p. injection single dose |
P7 neonatal rat pups | 5 min after HI:Reduction of brain infarct volume Reduction of brain edema Improvement of long-term behavioral development Attenuation of neuronal cell death and brain tissue loss Attenuation of BBB disruption: (reduction of IgG staining and brain water content) Mechanism: inhibition of HIF-1α/VEGF(decreased HIF-1α and VEGF protein levels) 3 h after HI: vitexin lost its neuroprotection |
[188] | |
| Cannabidiol | Post-treatment 15 and 240 min after HI |
0.1 mg/kg i.v. injection |
P3–P5 newborn piglets | Reduction of brain edema Prevention of brain seizures Reduced cell lossReduced number of degenerating neurons Cardio-protective effects |
[329] | |
| Post-treatment | 100 µM | Forebrain slices from P7–P10 neonatal mouse pups | Prevention of necrotic cell death (reduced LDH efflux to the medium) Anti-apoptotic: (reduced caspase-9 level) Anti-inflammatory: (reduced IL-6 and TNF-α level; reduced COX-2 expression level) Excitotoxicity modulation: (decreased glutamate release) Antioxidant: (decreased iNOS expression) Mechanism: Activation of A2A and CBD receptors |
[330] | ||
| Post-treatment 15 and 240 min after HI |
0.1 mg/kg i.v. injection |
P1–P3 newborn piglets | Long follow-up study (72 h after HI): Improved neurobehavioral performance Improved brain activity Stabilized metabolic activity Reduced cell death Anti-inflammatory effect (protects astrocytes) |
[331] | ||
| Post-treatment 10 min after HI |
1 mg/kg i.v. injection |
P7–P10 neonatal rat pups | Long follow-up study (7 days or one month after HI): Reduced infarct volume Modulated brain excitotoxicity, oxidative stress, and inflammation 7 days after HI Improved neurobehavioral performance one month after HI |
[332] | ||
| Post-treatment 30 min after HI |
1 mg/kg i.v. injection |
P1–P2 newborn piglets | Modulation of excitotoxicity, oxidative stress and inflammationMechanism Involvement of CB2 and 5HT1A receptors | [333] | ||
| Post-treatment 30 min after HT Combined with hypothermia |
1 mg/kg i.v. injection |
P1–P2 newborn piglets | Short follow-up study: Modulation of excitotoxicity, inflammation, oxidative stress, and cell damage was greater than either hypothermia or CBD alone |
Cannabidiol treatments reduced the oxidized protein and TNFα levels | [38] | |
| Post-treatment 30 min after HT Combined with hypothermia |
1 mg/kg i.v. injection repeated 24 and 48 h after HI |
P1 newborn piglets | Reverse the induction of increases in lactate/N-acetyl-aspartate, glutamate/Nacetyl-aspartate, TNFα, or apoptosis Modulate the brain excitotoxicity and inflammation More complete neuroprotection than CBD or hypothermia alone |
Long follow-up study: Improved brain activity Decreased microglial activation |
[334] | |
| Coumestrol | Pretreatment Immediately pre-hypoxia Post-treatment 3 h after hypoxia |
20 mg/kg i.p. injection |
P7 male neonatal rat pups |
Pre- and post-treatment: Preventing reference and working memory impairments Preventing late cognitive deficits in reference and working memory at P60 Reduction of brain tissue loss Reduction of late GFAP overexpression (index of late reactive astrogliosis) Pretreatment: Prevention of mitochondrial swelling Mechanism: Prevention of mitochondrial dysfunction and long-term cognitive deficits |
[224] | |
| Crocin | Post-treatment Immediately or 2 h after HI |
10 mg/kg combined with hypothermia | P7 neonatal mouse pups |
Reduced neurological severity score Anti-oxidative effect: (reduced levels of MDA, ROS, and NO; reduced mRNA expression of iNOS and COX-2) Anti-inflammatory effect: (reduced IL-1β and TNF-α levels) |
[311] |
When a HI event occurs, a substantial damage has taken place immediately after HI in the brain. Therefore, treatments have to start as early as possible in order to reduce further injury and promote regeneration. However, the preventive strategies will likely be the most effective therapeutic approaches. In this context, those compounds or extracts that act through maternal dietary supplementation, such as pomegranate juice and PIC, would be very interesting because nutritional approaches can easily be adopted as a preventive strategy in humans.
In conclusion, development of neuroprotective drugs from natural plant products is a prosperous approach for prevention and treatment of HI brain injury in neonates. Results of the presented studies may be useful for this purpose. In the future, more attention should be paid to further investigate the neuroprotective mechanisms, bioavailability, and metabolism of these agents, especially in humans.
Abbreviations
AChE: acetylcholinesterase; Akt: protein kinase B; AMPA: N-methyl-d-aspartate; ATP: adenosine triphosphate; BBB: blood-brain barrier; BDNF: Brain-derived neurotrophic factor; bFGF: basic fibroblast growth factor; i.p.: intraperitoneal; CAT: Catalase; CB1: cannabinoid receptor; CBD: Cannabidiol; CNTF: Ciliary neurotrophic factor; COX-2: Cyclooxygenase-2; DOE: Dendrobium officinale extract; EGF: epidermal growth factor; GDNF: Glial cell-derived neurotrophic factor; GFAP: Glial fibrillary acidic protein; GPx: Glutathione peroxidase; GSE: grape seed extract; GSH-Px: Glutathione peroxidase; GSPE: grape seed proanthocyanidin extract; HI: hypoxia-ischemia; HIBD: hypoxic-ischemic brain damage; HIE: hypoxic-ischemic encephalopathy; HIF-1α: Hypoxia-inducible factor 1-alpha; HO-1: Heme oxygenase-1; HupA: Huperzine A; IGF: insulin-like growth factor; IgG: Immunoglobulin G; IL: interleukin; iNOS: inducible nitric oxide synthase; I/R: ischemia/reperfusion; IQ: intelligence quotient; 8-isoPGF2α: 8-isoprostaglandinF2α; JNK: c-Jun N-terminal kinase; LDH: Lactate dehydrogenase; MDA: Malondialdehyde; MMPs: matrix metalloproteinases; MS: Multiple sclerosis; MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide; NF-kβ: nuclear factor kappa-beta; NGF: nerve growth factor; NMDA: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; NO: nitric oxide; nNOS: neuronal nitric oxide synthase; OMT: Oxymatrine; P7: postnatal day 7; PI3K: phosphatidylinositol 3-kinase; PIC: Piceatannol; PPE: pomegranate polyphenol extract; PTE: Pterostilbene; Que: Quercetin; RNS: reactive nitrogen species; RES: Resveratrol; ROS: reactive oxygen species; SIRT1; SkQR1: 10-(6ʹ-plastoquinonyl) decylrhodamine 19; SOD: Superoxide dismutase; Tan IIA: Tanshinone IIA; T-AOC: total antioxidant capacity; TBARS: Thiobarbituric acid reactive substances; TJ: tight junction; TLR4: Toll-like receptor 4; TNF: tumor necrosis factor; VB: Verbascoside; VEGF: Vascular endothelial growth factor.
Author Contributions
Conceptualization, M.H.F., M.P. and A.P.; methodology, H.M.; investigation, H.M. and A.B.; writing—original draft preparation, H.M. and A.B.; writing—review and editing, P.M.P., M.H.F., M.P. and A.P.; supervision, M.H.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kruszewski S.P. Neonatal Brain Injury. N. Engl. J. Med. 2005;352:1985–1995. doi: 10.1056/NEJM200502243520822. [DOI] [PubMed] [Google Scholar]
- 2.Lawn J.E., Cousens S., Zupan J. 4 Million Neonatal Deaths: When? Where? Why? Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 3.Dilenge M.E., Majnemer A., Shevell M.I. Long-Term Developmental Outcome of Asphyxiated Term Neonates. J. Child Neurol. 2001;16:781–792. doi: 10.1177/08830738010160110201. [DOI] [PubMed] [Google Scholar]
- 4.Graham E.M., Ruis K.A., Hartman A.L., Northington F.J., Fox H.E. A Systematic Review of the Role of Intrapartum Hypoxia-Ischemia in the Causation of Neonatal Encephalopathy. Am. J. Obstet. Gynecol. 2008;199:587–595. doi: 10.1016/j.ajog.2008.06.094. [DOI] [PubMed] [Google Scholar]
- 5.Kurinczuk J.J., White-Koning M., Badawi N. Epidemiology of Neonatal Encephalopathy and Hypoxic-Ischaemic Encephalopathy. Early Hum. Dev. 2010;86:329–338. doi: 10.1016/j.earlhumdev.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Airede A.I. Birth Asphyxia and Hypoxic-Ischaemic Encephalopathy: Incidence and Severity. Ann. Trop. Paediatr. 1991;11:331–335. doi: 10.1080/02724936.1991.11747524. [DOI] [PubMed] [Google Scholar]
- 7.De Haan M., Wyatt J.S., Roth S., Vargha-Khadem F., Gadian D., Mishkin M. Brain and Cognitive-Behavioural Development after Asphyxia at Term Birth. Dev. Sci. 2006;9:350–358. doi: 10.1111/j.1467-7687.2006.00499.x. [DOI] [PubMed] [Google Scholar]
- 8.Roth S.C., Edwards A.D., Cady E.B., Delpy D.T., Wyatt J.S., Azzopardi D., Baudin J., Townsend J., Stewart A.L., Reynolds E.O. Relation between cerebral oxidative metabolism following birth asphyxia, and neurodevelopmental outcome and brain growth at one year. Dev. Med. Child Neurol. 1992;34:285–295. doi: 10.1111/j.1469-8749.1992.tb11432.x. [DOI] [PubMed] [Google Scholar]
- 9.Gluckman P.D., Pinal C.S., Gunn A.J. Hypoxic-Ischemic Brain Injury in the Newborn: Pathophysiology and Potential Strategies for Intervention. Semin. Neonatol. 2001;6:109–120. doi: 10.1053/siny.2001.0042. [DOI] [PubMed] [Google Scholar]
- 10.Bunney P.E., Zink A.N., Holm A.A., Billington C.J., Kotz C.M. Orexin activation counteracts decreases in nonexercise activity thermogenesis (NEAT) caused by high-fat diet. Physiol. Behav. 2017;176:139–148. doi: 10.1016/j.physbeh.2017.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gluckman P.D., Wyatt J.S., Azzopardi D., Ballard R., Edwards A.D., Ferriero D.M., Polin R.A., Robertson C.M., Thoresen M., Whitelaw A., et al. Selective Head Cooling with Mild Systemic Hypothermia after Neonatal Encephalopathy: Multicentre Randomised Trial. Lancet. 2005;365:663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 12.Azzopardi D., Strohm B., Marlow N., Brocklehurst P., Deierl A., Eddama O., Goodwin J., Halliday H.L., Juszczak E., Kapellou O., et al. Effects of Hypothermia for Perinatal Asphyxia on Childhood Outcomes. N. Engl. J. Med. 2014;371:140–149. doi: 10.1056/NEJMoa1315788. [DOI] [PubMed] [Google Scholar]
- 13.Shankaran S., Pappas A., McDonald S.A., Vohr B.R., Hintz S.R., Yolton K., Gustafson K.E., Leach T.M., Green C., Bara R., et al. Childhood Outcomes after Hypothermia for Neonatal Encephalopathy. Obstet. Gynecol. Surv. 2012;67:617–619. doi: 10.1097/01.ogx.0000419766.08585.32. [DOI] [Google Scholar]
- 14.Ournal T.H.E.J., Ediatrics O.F.P. Neuroprotective Strategies in Neonatal Brain Injury. J. Pediatr. 2017;192:22–32. doi: 10.1016/j.jpeds.2017.08.031. [DOI] [PubMed] [Google Scholar]
- 15.Simonyi A., Wang Q., Miller R.L., Yusof M., Shelat P.B., Sun A.Y., Sun G.Y. Polyphenols in Cerebral Ischemia: Novel Targets for Neuroprotection. Mol. Neurobiol. 2005;31:135–147. doi: 10.1385/MN:31:1-3:135. [DOI] [PubMed] [Google Scholar]
- 16.Ahmad N., Khan Z., Hasan N., Basit A., Zohrameena S. Neuroprotective Agents, Natural Plant Herbs & Drugs in Ischemic Stroke: A Review. PharmaTutor. 2017;5:29–36. [Google Scholar]
- 17.Costa S.L., Silva V.D.A., dos Santos Souza C., Santos C.C., Paris I., Muñoz P., Segura-Aguilar J. Impact of Plant-Derived Flavonoids on Neurodegenerative Diseases. Neurotox. Res. 2016 doi: 10.1007/s12640-016-9600-1. [DOI] [PubMed] [Google Scholar]
- 18.Su S., Hsieh C. Anti-Inflammatory Effects of Chinese Medicinal Herbs on Cerebral Ischemia. Chin. Med. 2011;6:1–9. doi: 10.1186/1749-8546-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pohl F., Kong P., Lin T. The Potential Use of Plant Natural Products and Plant Extracts with Antioxidant Properties for the Prevention/Treatment of Neurodegenerative Diseases: In Vitro, In Vivo and Clinical Trials. Molecules. 2018;23:3283. doi: 10.3390/molecules23123283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albrecht M., Zitta K., Groenendaal F., Van Bel F., Peeters-Scholte C. Neuroprotective Strategies Following Perinatal Hypoxia-Ischemia: Taking Aim at NOS. Free Radic. Biol. Med. 2019;142:123–131. doi: 10.1016/j.freeradbiomed.2019.02.025. [DOI] [PubMed] [Google Scholar]
- 21.Nair J., Kumar V.H.S. Current and Emerging Therapies in the Management of Hypoxic Ischemic Encephalopathy in Neonates. Children. 2018;5:99. doi: 10.3390/children5070099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Q., Chen W., Sinha B., Tu Y., Manning S., Thomas N., Zhou S., Jiang H., Ma H., Kroessler D.A., et al. Neuroprotective Agents for Neonatal Hypoxic-Ischemic Brain Injury. Drug Discov. Today. 2015;20:1372–1381. doi: 10.1016/j.drudis.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Dixon B.J., Reis C., Ho W.M., Tang J., Zhang J.H. Neuroprotective strategies after neonatal hypoxic ischemic encephalopathy. Int. J. Mol. Sci. 2015;16:22368–22401. doi: 10.3390/ijms160922368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grow J., Barks J.D.E. Pathogenesis of Hypoxic-Ischemic Cerebral Injury in the Term Infant: Current Concepts. Clin. Perinatol. 2002;29:585–602. doi: 10.1016/S0095-5108(02)00059-3. [DOI] [PubMed] [Google Scholar]
- 25.Northington F.J., Graham E.M., Martin L.J. Apoptosis in perinatal hypoxic-ischemic brain injury: How important is it and should it be inhibited? Brain Res. Rev. 2005;50:244–257. doi: 10.1016/j.brainresrev.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Yin W., Cao G., Johnnides M.J., Signore A.P., Luo Y., Hickey R.W., Chen J. TAT-Mediated Delivery of Bcl-XL Protein Is Neuroprotective against Neonatal Hypoxic-Ischemic Brain Injury via Inhibition of Caspases and AIF. Neurobiol. Dis. 2006;21:358–371. doi: 10.1016/j.nbd.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Han B.H., Xu D., Choi J., Han Y., Xanthoudakis S., Roy S., Tam J., Vaillancourt J., Colucci J., Siman R., et al. Selective, reversible caspase-3 inhibitor is neuroprotective and reveals distinct pathways of cell death after neonatal hypoxic-ischemic brain injury. J. Biol. Chem. 2002;277:30128–30136. doi: 10.1074/jbc.M202931200. [DOI] [PubMed] [Google Scholar]
- 28.Alonso-Alconada D., Álvarez A., Arteaga O., Martínez-Ibargüen A., Hilario E. Neuroprotective Effect of Melatonin: A Novel Therapy against Perinatal Hypoxia-Ischemia. Int. J. Mol. Sci. 2013;14:9379–9395. doi: 10.3390/ijms14059379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arteaga O., Álvarez A., Revuelta M., Santaolalla F., Urtasun A., Hilario E. Role of Antioxidants in Neonatal Hypoxic–Ischemic Brain Injury: New Therapeutic Approaches. Int. J. Mol. Sci. 2017;18:265. doi: 10.3390/ijms18020265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen H., Hu X., Liu C., Wang S., Zhang W., Gao H., Stetler R.A., Gao Y., Chen J. Ethyl Pyruvate Protects against Hypoxic-Ischemic Brain Injury via Anti-Cell Death and Anti-Inflammatory Mechanisms. Neurobiol. Dis. 2010;37:711–722. doi: 10.1016/j.nbd.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang D., Sun Y.Y., Lin X., Baumann J.M., Dunn R.S., Lindquist D.M., Kuan C.Y. Intranasal Delivery of Cell-Penetrating Anti-NF-κB Peptides (Tat-NBD) Alleviates Infection-Sensitized Hypoxic-Ischemic Brain Injury. Exp. Neurol. 2013;247:447–455. doi: 10.1016/j.expneurol.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yıldız E.P., Ekici B., Tatlı B. Neonatal Hypoxic Ischemic Encephalopathy: An Update on Disease Pathogenesis and Treatment. Expert Rev. Neurother. 2017;17:449–459. doi: 10.1080/14737175.2017.1259567. [DOI] [PubMed] [Google Scholar]
- 33.Donega V., van Velthoven C.T., Nijboer C.H., Kavelaars A., Heijnen C.J. The endogenous regenerative capacity of the damaged newborn brain: Boosting neurogenesis with mesenchymal stem cell treatment. J. Cereb. Blood Flow Metab. 2013;33:625–634. doi: 10.1038/jcbfm.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen A.I., Xiong L.-J., Tong Y.U., Mao M. The Neuroprotective Roles of BDNF in Hypoxic Ischemic Brain Injury. Biomed. Rep. 2013;1:167–176. doi: 10.3892/br.2012.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yager J.Y., Ashwal S. Animal models of perinatal hypoxic-ischemic brain damage. Pediatr. Neurol. 2009;40:156–167. doi: 10.1016/j.pediatrneurol.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 36.Loren D.J., Seeram N.P., Schulman R.N., Holtzman D.M. Maternal Dietary Supplementation with Pomegranate Juice Is Neuroprotective in an Animal Model of Neonatal Hypoxic-Ischemic Brain Injury. Pediatr. Res. 2005;57:858–864. doi: 10.1203/01.PDR.0000157722.07810.15. [DOI] [PubMed] [Google Scholar]
- 37.Li X.-L., Hong M. Aqueous Extract of Dendrobium Officinale Confers Neuroprotection against Hypoxic-Ischemic Brain Damage in Neonatal Rats. Kaohsiung J. Med. Sci. 2020;36:43–53. doi: 10.1002/kjm2.12139. [DOI] [PubMed] [Google Scholar]
- 38.Lafuente H., Pazos M.R., Alvarez A., Mohammed N., Santos M., Arizti M., Alvarez F.J., Martinez-Orgado J.A., Davidson J. Effects of Cannabidiol and Hypothermia on Short-Term Brain Damage in New-Born Piglets after Acute Hypoxia-Ischemia. Front. Neurosci. 2016;10:1–11. doi: 10.3389/fnins.2016.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yadav M., Jain S., Bhardwaj A., Nagpal R., Puniya M., Tomar R., Singh V., Parkash O., Prasad G.B.K.S., Marotta F., et al. Biological and Medicinal Properties of Grapes and Their Bioactive Constituents: An Update. J. Med. Food. 2009;12:473–484. doi: 10.1089/jmf.2008.0096. [DOI] [PubMed] [Google Scholar]
- 40.Jang M.H., Piao X.L., Kim J.M., Kwon S.W., Park J.H. Inhibition of Cholinesterase and Amyloid-β Aggregation by Resveratrol Oligomers from Vitis Amurensis. Phyther. Res. 2008;22:544–549. doi: 10.1002/ptr.2406. [DOI] [PubMed] [Google Scholar]
- 41.Fine A.M. Oligomeric Proanthocyanidin Complexes: Applications Oligomeric Proanthocyanidins. Altern. Med. Rev. 2000;5:144–151. [PubMed] [Google Scholar]
- 42.Bian J.T., Bhargava H.N. Protective Effects of Grape Seed Proanthocyanidins and Selected Antioxidants against TPA-Induced Hepatic and Brain Lipid Peroxidation and DNA Fragmentation, and Peritoneal Macrophage Activation in Mice. Gen. Pharmacol. 1998;30:771–776. doi: 10.1016/S0306-3623(97)00332-7. [DOI] [PubMed] [Google Scholar]
- 43.Bagchi D., Sen C.K., Ray S.D., Das D.K., Bagchi M., Preuss H.G., Vinson J.A. Molecular Mechanisms of Cardioprotection by a Novel Grape Seed Proanthocyanidin Extract. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2003;523–524:87–97. doi: 10.1016/S0027-5107(02)00324-X. [DOI] [PubMed] [Google Scholar]
- 44.Shafiee M., Carbonneau M.A., Urban N., Descomps B., Leger C.L. Grape and Grape Seed Extract Capacities at Protecting LDL against Oxidation Generated by Cu2+, AAPH or SIN-1 and at Decreasing Superoxide THP-1 Cell Production. A Comparison to Other Extracts or Compounds. Free Radic. Res. 2003;37:573–584. doi: 10.1080/1071576031000083152. [DOI] [PubMed] [Google Scholar]
- 45.Balu M., Sangeetha P., Murali G., Panneerselvam C. Modulatory Role of Grape Seed Extract on Age-Related Oxidative DNA Damage in Central Nervous System of Rats. Brain Res. Bull. 2006;68:469–473. doi: 10.1016/j.brainresbull.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 46.Ariga T. The Antioxidative Function, Preventive Action on Disease and Utilization of Proanthocyanidins. BioFactors. 2004;21:197–201. doi: 10.1002/biof.552210140. [DOI] [PubMed] [Google Scholar]
- 47.Sato M., Bagchi D., Tosaki A., Das D.K. Grape Seed Proanthocyanidin Reduces Cardiomyocyte Apoptosis by Inhibiting Ischemia/Reperfusion-Induced Activation of JNK-1 and C-JUN. Free Radic. Biol. Med. 2001;31:729–737. doi: 10.1016/S0891-5849(01)00626-8. [DOI] [PubMed] [Google Scholar]
- 48.Ray S.D., Kumar M.A., Bagchi D. A novel proanthocyanidin IH636 grape seed extract increases in vivo Bcl-XL expression and prevents acetaminophen-induced programmed and unprogrammed cell death in mouse liver. Arch. Biochem. Biophys. 1999;369:42–58. doi: 10.1006/abbi.1999.1333. [DOI] [PubMed] [Google Scholar]
- 49.Sugisawa A., Inoue S., Umegaki K. Grape Seed Extract Prevents H2O2-Induced Chromosomal Damage in Human Lymphoblastoid Cells. Biol. Pharm. Bull. 2004;27:1459–1461. doi: 10.1248/bpb.27.1459. [DOI] [PubMed] [Google Scholar]
- 50.Baines C.P., Molkentin J.D. STRESS signaling pathways that modulate cardiac myocyte apoptosis. J. Mol. Cell. Cardiol. 2005;38:47–62. doi: 10.1016/j.yjmcc.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 51.Hwang I.K., Yoo K.Y., Kim D.S., Jeong Y.K., Kim J.D., Shin H.K., Lim S.S., Yoo I.D., Kang T.C., Kim D.W., et al. Neuroprotective Effects of Grape Seed Extract on Neuronal Injury by Inhibiting DNA Damage in the Gerbil Hippocampus after Transient Forebrain Ischemia. Life Sci. 2004;75:1989–2001. doi: 10.1016/j.lfs.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 52.Feng Y., Liu Y.M., Fratkins J.D., LeBlanc M.H. Grape Seed Extract Suppresses Lipid Peroxidation and Reduces Hypoxic Ischemic Brain Injury in Neonatal Rats. Brain Res. Bull. 2005;66:120–127. doi: 10.1016/j.brainresbull.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 53.Feng Y., Liu Y.M., Leblanc M.H., Bhatt A.J., Rhodes P.G. Grape Seed Extract given Three Hours after Injury Suppresses Lipid Peroxidation and Reduces Hypoxic-Ischemic Brain Injury in Neonatal Rats. Pediatr. Res. 2007;61:295–300. doi: 10.1203/pdr.0b013e318030c92d. [DOI] [PubMed] [Google Scholar]
- 54.Fracassetti D., Costa C., Moulay L., Tomás-barberán F.A. Ellagic Acid Derivatives, Ellagitannins, Proanthocyanidins and Other Phenolics, Vitamin C and Antioxidant Capacity of Two Powder Products from Camu-Camu Fruit (Myrciaria Dubia) Food Chem. 2013;139:578–588. doi: 10.1016/j.foodchem.2013.01.121. [DOI] [PubMed] [Google Scholar]
- 55.Margalef M., Guerrero L., Pons Z., Isabel F., Arola L., Muguerza B., Arola-arnal A. A dose—Response study of the bioavailability of grape seed proanthocyanidin in rat and lipid-lowering effects of generated metabolites in HepG2 cells. Food Res. Int. 2014;64:500–507. doi: 10.1016/j.foodres.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 56.Devi S.A., Jolitha A.B., Ishii N. Grape seed proanthocyanidin extract (GSPE) and antioxidant defense in the brain of adult rats. Med Sci. Monit. 2006;12:BR124–BR129. [PubMed] [Google Scholar]
- 57.Ferruzzi M.G., Lobo J.K., Janle E.M., Cooper B., Simon J.E., Wu Q., Welch C., Ho L., Weaver C., Pasinetti G.M. Bioavailability of gallic acid and catechins from grape seed polyphenol extract is improved by repeated dosing in rats: Implications for treatment in alzheimer’s disease. J. Alzheimers Dis. 2009;18:113–124. doi: 10.3233/JAD-2009-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu L., Zhang Q.L., Zhang X.Y., Lv C., Li J., Yuan Y., Yin F.X. Pharmacokinetics and Blood-Brain Barrier Penetration of (+)-Catechin and (-)-Epicatechin in Rats by Microdialysis Sampling Coupled to High-Performance Liquid Chromatography with Chemiluminescence Detection. J. Agric. Food Chem. 2012;60:9377–9383. doi: 10.1021/jf301787f. [DOI] [PubMed] [Google Scholar]
- 59.Kong X., Guan J., Gong S., Wang R. Neuroprotective Effects of Grape Seed Procyanidin Extract on Ischemia-Reperfusion Brain Injury. Chin. Med. Sci. J. 2017;32:92–99. doi: 10.24920/J1001-9294.2017.020. [DOI] [PubMed] [Google Scholar]
- 60.Tu X., Wang M., Liu Y., Zhao W., Ren X., Li Y., Liu H., Gu Z., Jia H., Liu J., et al. Pretreatment of Grape Seed Proanthocyanidin Extract Exerts Neuroprotective Effect in Murine Model of Neonatal Hypoxic-Ischemic Brain Injury by Its Antiapoptotic Property. Cell. Mol. Neurobiol. 2019;39:953–961. doi: 10.1007/s10571-019-00691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kalaycıoğlu Z., Erim F.B. Total Phenolic Contents, Antioxidant Activities, and Bioactive Ingredients of Juices from Pomegranate Cultivars Worldwide. Food Chem. 2017;221:496–507. doi: 10.1016/j.foodchem.2016.10.084. [DOI] [PubMed] [Google Scholar]
- 62.Venkata C., Prakash S., Prakash I. Bioactive Chemical Constituents from Pomegranate (Punica Granatum) Juice, Seed and Peel-A Review. Int. J. Res. Chem. Environ. 2011;1:1–18. [Google Scholar]
- 63.Akhtar S., Ismail T., Fraternale D., Sestili P. Pomegranate Peel and Peel Extracts: Chemistry and Food Features. Food Chem. 2015;174:417–425. doi: 10.1016/j.foodchem.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 64.Landete J.M. Ellagitannins, Ellagic Acid and Their Derived Metabolites: A Review about Source, Metabolism, Functions and Health. Food Res. Int. 2011;44:1150–1160. doi: 10.1016/j.foodres.2011.04.027. [DOI] [Google Scholar]
- 65.Wu P., Fitschen P.J., Kistler B.M., Jeong J.H., Chung H.R., Aviram M., Phillips S.A., Fernhall B., Wilund K.R. Effects of pomegranate extract supplementation on cardiovascular risk factors and physical function in hemodialysis patients. J. Med. Food. 2015;18:1–9. doi: 10.1089/jmf.2014.0103. [DOI] [PubMed] [Google Scholar]
- 66.Sahebkar A., Ferri C., Giorgini P., Bo S., Nachtigal P., Grassi D. Effects of Pomegranate Juice on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pharmacol. Res. 2017;115:149–161. doi: 10.1016/j.phrs.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 67.Panth N., Manandhar B., Paudel K.R. Anticancer Activity of Punica Granatum (Pomegranate): A Review. Phyther. Res. 2017;31:568–578. doi: 10.1002/ptr.5784. [DOI] [PubMed] [Google Scholar]
- 68.Article R., Review G. Anti-cancer activity of pomegranate and its biophenols; general review. EC Nutr. 2016;1:28–52. [Google Scholar]
- 69.Shukla M., Gupta K., Rasheed Z., Khan K.A., Haqqi T.M. Consumption of hydrolyzable tannins-rich pomegranate extract suppresses inflammation and joint damage in rheumatoid arthritis. Nutrition. 2008;24:733–743. doi: 10.1016/j.nut.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghavipour M., Sotoudeh G., Tavakoli E., Mowla K., Hasanzadeh J., Mazloom Z. Pomegranate extract alleviates disease activity and some blood biomarkers of in fl ammation and oxidative stress in Rheumatoid Arthritis patients. Eur. J. Clin. Nutr. 2016;71:92–96. doi: 10.1038/ejcn.2016.151. [DOI] [PubMed] [Google Scholar]
- 71.Sahebkar A., Simental-Mendía L.E., Giorgini P., Ferri C., Grassi D. Lipid Profile Changes after Pomegranate Consumption: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Phytomedicine. 2016 doi: 10.1016/j.phymed.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 72.Al-Muammar M.N., Khan F. Obesity: The Preventive Role of the Pomegranate (Punica Granatum) Nutrition. 2012;28:595–604. doi: 10.1016/j.nut.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 73.Banihani S., Swedan S., Alguraan Z. Pomegranate and Type 2 Diabetes. Nutr. Res. 2013;33:341–348. doi: 10.1016/j.nutres.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 74.Asadi M.S., Mirghazanfari S.M., Dadpay M. Evaluation of wound healing activities of pomegranate (Punica granatum-Lythraceae) Peel and Pulp. JRMDS. 2018;6:230–236. doi: 10.24896/jrmds.20186336. [DOI] [Google Scholar]
- 75.Morvaridzadeh M., Sepidarkish M., Daneshzad E., Akbari A., Mobini G.R., Heshmati J. The Effect of Pomegranate on Oxidative Stress Parameters: A Systematic Review and Meta-Analysis. Complement. Ther. Med. 2020;48:102252. doi: 10.1016/j.ctim.2019.102252. [DOI] [PubMed] [Google Scholar]
- 76.Li Y., Guo C., Yang J., Wei J., Xu J., Cheng S. Evaluation of Antioxidant Properties of Pomegranate Peel Extract in Comparison with Pomegranate Pulp Extract. Food Chem. 2006;96:254–260. doi: 10.1016/j.foodchem.2005.02.033. [DOI] [Google Scholar]
- 77.Acker L.E.P. Antioxidant Activities of Pomegranate Fruit Extract and Its Anthocyanidins: Delphinidin, Cyanidin, and Pelargonidin. J. Agric. Food Chem. 2002;50:166–171. doi: 10.1021/jf0108765. [DOI] [PubMed] [Google Scholar]
- 78.Ismail T., Sestili P., Akhtar S. Pomegranate Peel and Fruit Extracts: A Review of Potential Anti-Inflammatory and Anti-Infective Effects. J. Ethnopharmacol. 2012;143:397–405. doi: 10.1016/j.jep.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 79.Lansky E.P., Newman R.A. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J. Ethnopharmacol. 2007;109:177–206. doi: 10.1016/j.jep.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 80.Singh B., Singh J.P. Antimicrobial Potential of Pomegranate Peel: A Review. Int. J. Food Sci. Technol. 2018:1–7. doi: 10.1111/ijfs.13964. [DOI] [Google Scholar]
- 81.Wu Y., Zhu C., Zhang Y., Li Y., Sun J. Immunomodulatory and Antioxidant Effects of Pomegranate Peel Polysaccharides on Immunosuppressed Mice. Int. J. Biol. Macromol. 2019;137:504–511. doi: 10.1016/j.ijbiomac.2019.06.139. [DOI] [PubMed] [Google Scholar]
- 82.Thilakarathna S.H., Rupasinghe H.P.V. Anti-Atherosclerotic Effects of Fruit Bioactive Compounds: A Review of Current Scientific Evidence. Can. J. Plant Sci. 2012 doi: 10.4141/cjps2011-090. [DOI] [Google Scholar]
- 83.Zhou B., Yi H., Tan J., Wu Y., Liu G., Qiu Z. Anti-proliferative effects of polyphenols from pomegranate rind (Punica Granatum L.) on EJ bladder cancer cells via regulation of p53/miR-34a axis. Phytother. Res. 2015;29:415–422. doi: 10.1002/ptr.5267. [DOI] [PubMed] [Google Scholar]
- 84.Orgil O., Spector L., Holland D., Mahajna J. The anti-proliferative and anti-androgenic activity of different pomegranate accessions. J. Funct. Foods. 2016;26:517–528. doi: 10.1016/j.jff.2016.08.024. [DOI] [Google Scholar]
- 85.Braidy N., Selvaraju S., Essa M.M., Vaishnav R., Al-Adawi S., Al-Asmi A., Al-Senawi H., Abd A., Alobaidy A., Lakhtakia R., et al. Neuroprotective Effects of a Variety of Pomegranate Juice Extracts against MPTP-Induced Cytotoxicity and Oxidative Stress in Human Primary Neurons. Oxid. Med. Cell. Longev. 2013;2013:1–12. doi: 10.1155/2013/685909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Al Damen L., Stockton A., Al-Dujaili E.A. Effects on Cognition of Berry, Pomegranate, Grape and Biophenols: A General Review. JPAD. 2018;5:1–8. [Google Scholar]
- 87.Amri Z., Ghorbel A., Turki M., Akrout F.M., Ayadi F., Elfeki A. Effect of pomegranate extracts on brain antioxidant markers and cholinesterase activity in high fat-high fructose diet induced obesity in rat model. BMC Complement. Altern. Med. 2017:1–9. doi: 10.1186/s12906-017-1842-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morzelle M.C., Salgado J.M., Telles M., Mourelle D., Bachiega P., Buck H.S., Viel T.A. Neuroprotective effects of pomegranate peel extract after chronic infusion with amyloid-β peptide in mice. PLoS ONE. 2016;11:e0141332. doi: 10.1371/journal.pone.0166123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yuan T., Ma H., Liu W., Niesen D.B., Shah N., Crews R., Rose K.N., Vattem D.A., Seeram N.P. Pomegranate’s neuroprotective effects against Alzheimer’s disease are mediated by urolithins, its ellagitannin-gut microbial derived metabolites. ACS Chem. Neurosci. 2015;7:26–33. doi: 10.1021/acschemneuro.5b00260. [DOI] [PubMed] [Google Scholar]
- 90.Subash S., Essa M.M., Asmi A., Al-Adawi S., Vaishnav R., Braidy N., Manivasagam T., Guillemin G.J. Pomegranate from Oman Alleviates the Brain Oxidative Damage in Transgenic Mouse Model of Alzheimer’s Disease. J. Tradit. Complement. Med. 2014;4:232–238. doi: 10.4103/2225-4110.139107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ahmed M.A.E., El Morsy E.M., Ahmed A.A. Pomegranate extract protects against cerebral ischemia/reperfusion injury and preserves brain DNA integrity in rats. Life Sci. 2014 doi: 10.1016/j.lfs.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 92.Sarkaki A., Farbood Y., Hashemi S., Rad M.R. Pomegranate seed hydroalcoholic extract improves memory deficits in ovariectomized rats with permanent cerebral hypoperfusion/ischemia. Avicenna J. Phytomed. 2015;5:43–55. [PMC free article] [PubMed] [Google Scholar]
- 93.Alireza S., Moslem R. Improving active and passive avoidance memories deficits due to permanent cerebral ischemia by pomegranate seed extract in female rats. Malays. J. Med Sci. 2013;20:25–34. [PMC free article] [PubMed] [Google Scholar]
- 94.Rafieirad M., Abbaszadeh H. Pomegranate Seed Extract Reduces Ischemia Induced Anxiety in Male Rats. J. Herbmed Pharmacol. 2017;6:85–89. [Google Scholar]
- 95.Bellone J.A., Murray J.R., Jorge P., Fogel T.G., Kim M., Wallace D.R., Hartman R.E., Bellone J.A., Murray J.R., Jorge P., et al. Pomegranate Supplementation Improves Cognitive and Functional Recovery Following Ischemic Stroke: A Randomized Trial. Nutr. Neurosci. 2019;22:738–743. doi: 10.1080/1028415X.2018.1436413. [DOI] [PubMed] [Google Scholar]
- 96.West T., Atzeva M., Holtzman D.M. Pomegranate polyphenols and resveratrol protect the neonatal brain against hypoxic-ischemic injury. Dev. Neurosci. 2007;29:363–372. doi: 10.1159/000105477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Basu A., Penugonda K. Pomegranate Juice: A Heart-Healthy Fruit Juice. Nutr. Rev. 2009;67:49–56. doi: 10.1111/j.1753-4887.2008.00133.x. [DOI] [PubMed] [Google Scholar]
- 98.Gil I., Toma F.A., Hess-pierce B., Holcroft D.M., Kader A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000;48:4581–4589. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- 99.Teixeira da Silva J.A., Ng T.B. The Medicinal and Pharmaceutical Importance of Dendrobium Species. Appl. Microbiol. Biotechnol. 2017;101:2227–2239. doi: 10.1007/s00253-017-8169-9. [DOI] [PubMed] [Google Scholar]
- 100.Ng T.B., Liu J., Wong J.H., Ye X., Wing Sze S.C., Tong Y., Zhang K.Y. Review of Research on Dendrobium, a Prized Folk Medicine. Appl. Microbiol. Biotechnol. 2012;93:1795–1803. doi: 10.1007/s00253-011-3829-7. [DOI] [PubMed] [Google Scholar]
- 101.Tang H., Zhao T., Sheng Y., Zheng T., Fu L., Zhang Y. Dendrobium Officinale Kimura et Migo: A Review on Its Ethnopharmacology, Phytochemistry, Pharmacology, and Industrialization. Evid. Based Complement. Altern. Med. 2017;2017:7436259. doi: 10.1155/2017/7436259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Koo K.A., Sung S.H., Park J.H., Kim S.H., Lee K.Y., Kim Y.C. In Vitro Neuroprotective Activities of Phenylethanoid Glycosides from Callicarpa Dichotoma. Planta Med. 2005;71:778–780. doi: 10.1055/s-2005-871213. [DOI] [PubMed] [Google Scholar]
- 103.Alipieva K., Korkina L., Erdogan I., Georgiev M.I. Verbascoside—A review of its occurrence, (bio) synthesis and pharmacological significance. Biotechnol. Adv. 2014;32:1065–1076. doi: 10.1016/j.biotechadv.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 104.Taylor P., He J., Hu X., Zeng Y., Li Y., Wu H., Qiu R., Ma W., Li T., Li C., et al. Advanced Research on Acteoside for Chemistry and Bioactivities. J. Asian Nat. Prod. Res. 2011:37–41. doi: 10.1080/10286020.2011.568940. [DOI] [PubMed] [Google Scholar]
- 105.Imperio M.D., Cardinali A., Antuono I.D., Linsalata V., Minervini F., Redan B.W., Ferruzzi M.G. Stability—Activity of Verbascoside, a Known Antioxidant Compound, at Different PH Conditions. Food Res. Int. 2014;66:373–378. doi: 10.1016/j.foodres.2014.09.037. [DOI] [Google Scholar]
- 106.Frum Y., Viljoen A.M., Van Heerden F.R. Verbascoside and luteolin-5-o-β-d-glucoside isolated from Halleria lucida L. exhibit antagonistic anti-oxidant properties in vitro. S. Afr. J. Bot. 2007;73:583–587. doi: 10.1016/j.sajb.2007.05.006. [DOI] [Google Scholar]
- 107.Lee J.H., Lee J.Y., Kang H.S., Jeong C.H., Moon H., Whang W.K., Kim C.J., Sim S.S. The Effect of Acteoside on Histamine Release and Arachidonic Acid Release in RBL-2H3 Mast Cells. Arch. Pharmacal Res. 2006;29:508–513. doi: 10.1007/BF02969425. [DOI] [PubMed] [Google Scholar]
- 108.Speranza L., Franceschelli S., Pesce M., Reale M., Menghini L., Vinciguerra I., De Lutiis M.A., Felaco M., Grilli A. Antiinfl Ammatory Effects in THP-1 Cells Treated with Verbascoside. Phytother. Res. 2010;1404:1398–1404. doi: 10.1002/ptr.3173. [DOI] [PubMed] [Google Scholar]
- 109.Pesce M., Franceschelli S., Ferrone A., Anna M., Lutiis D., Patruno A., Grilli A., Felaco M., Speranza L. Verbascoside Down-Regulates Some pro-Inflammatory Signal Transduction Pathways by Increasing the Activity of Tyrosine Phosphatase SHP-1 in the U937 Cell Line. J. Cell. Mol. Med. 2015;19:1548–1556. doi: 10.1111/jcmm.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hno T.O., Noue M.I., Gihara Y.O., Aracoglu I.S. Antimetastatic Activity of Acteoside, a Phenylethanoid Glycoside. Biol. Pharm. Bull. 2002;25:666–668. doi: 10.1248/bpb.25.666. [DOI] [PubMed] [Google Scholar]
- 111.Zhou L., Feng Y., Jin Y., Liu X., Sui H., Chai N., Chen X., Liu N., Ji Q., Wang Y., et al. Verbascoside Promotes Apoptosis by Regulating HIPK2-P53 Signaling in Human Colorectal Cancer. BMC Cancer. 2014;14:1–11. doi: 10.1186/1471-2407-14-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li N., Wang J., Ma J., Gu Z., Jiang C., Yu L., Fu X. Neuroprotective Effects of Cistanches Herba Therapy on Patients with Moderate Alzheimer ’ s Disease. Evid. Based Complement. Altern. Med. 2015;2015:103985. doi: 10.1155/2015/103985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu J., Huang F.X., Wang J., Shi C.C., Fang G.Y. Protective Effect of Liquiritin on Corticosterone-Induced Neurotoxicity in Pc12 Cells. Trop. J. Pharm. Res. 2018;17:2013–2017. doi: 10.4314/tjpr.v17i10.17. [DOI] [Google Scholar]
- 114.Yuan J., Ren J., Wang Y., He X., Zhao Y. Acteoside Binds to Caspase-3 and Exerts Neuroprotection in the Rotenone Rat Model of Parkinson’s Disease. PLoS ONE. 2016;11:e0162696. doi: 10.1371/journal.pone.0162696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wei W., Lu M., Lan X., Liu N., Wang H., Du J., Sun T., Li Y., Yu J. Neuroprotective Effect of Verbascoside on Hypoxic-Ischemic Brain Damage in Neonatal Rat. Neurosci. Lett. 2019;711:134415. doi: 10.1016/j.neulet.2019.134415. [DOI] [PubMed] [Google Scholar]
- 116.Inda B.D., Ebnath S.D., Anik R.B. Naturally Occurring Iridoids and Secoiridoids. An Updated Review, Part 4. Chem. Pharm. Bull. 2011;59:803–833. doi: 10.1248/cpb.59.803. [DOI] [PubMed] [Google Scholar]
- 117.El-Naggar L.J., Beal J.L. Iridoids. A review. J. Nat. Prod. 1980;43:649–707. doi: 10.1021/np50012a001. [DOI] [PubMed] [Google Scholar]
- 118.Chou G., Wang Z., Medica C.M., Park Z.H. Iridoid glycosides from Gardenia jasminoides Ellis. Helv. Chim. Acta. 2008;91:4–10. [Google Scholar]
- 119.Shan M., Yu S., Yan H., Guo S., Xiao W., Wang Z., Zhang L., Ding A., Wu Q., Fong S., et al. A review on the phytochemistry, pharmacology, pharmacokinetics and toxicology of geniposide, a natural product. Molecules. 2017;22:1689. doi: 10.3390/molecules22101689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li F., Li W., Li X., Li F., Zhang L., Wang B., Huang G., Guo X., Wan L., Liu Y., et al. Geniposide Attenuates Inflammatory Response by Suppressing P2Y14 Receptor and Downstream ERK1/2 Signaling Pathway in Oxygen and Glucose Deprivation-Induced Brain Microvascular Endothelial Cells. J. Ethnopharmacol. 2016;185:77–86. doi: 10.1016/j.jep.2016.03.025. [DOI] [PubMed] [Google Scholar]
- 121.Wang J., Hou J., Zhang P. Geniposide Reduces Inflammatory Responses of Oxygen-Glucose Deprived Rat Microglial Cells via Inhibition of the TLR4 Signaling Pathway. Neurochem. Res. 2012;2:2235–22480. doi: 10.1007/s11064-012-0852-8. [DOI] [PubMed] [Google Scholar]
- 122.Guo L., Xia Z., Gao X., Yin F., Liu J. Glucagon-like Peptide 1 Receptor Plays a Critical Role in Geniposide-Regulated Insulin Secretion in INS-1 Cells. Acta Pharmacol. Sin. 2011;33:237–241. doi: 10.1038/aps.2011.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu J., Yin F., Zheng X., Jing J., Hu Y. Geniposide, a Novel Agonist for GLP-1 Receptor, Prevents PC12 Cells from Oxidative Damage via MAP Kinase Pathway. Neurochem. Int. 2007;51:361–369. doi: 10.1016/j.neuint.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 124.Chen Y., Zhang Y., Li L., Hölscher C. Neuroprotective Effects of Geniposide in the MPTP Mouse Model of Parkinson’s Disease. Eur. J. Pharmacol. 2015;768:21–27. doi: 10.1016/j.ejphar.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 125.Zhang H., Zhao C., Lv C., Liu X., Du S., Li Z., Wang Y., Zhang W. Geniposide Alleviates Amyloid-Induced Synaptic Injury by Protecting Axonal Mitochondrial Trafficking. Front. Cell. Neurosci. 2017;10:1–13. doi: 10.3389/fncel.2016.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lv C., Wang L., Liu X., Yan S., Yan S.S., Wang Y., Zhang W. Multi-Faced Neuroprotective Effects of Geniposide Depending on the RAGE-Mediated Signaling in an Alzheimer Mouse Model. Neuropharmacology. 2015;89:175–184. doi: 10.1016/j.neuropharm.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 127.Wang J., Li D., Hou J., Lei H. Protective Effects of Geniposide and Ginsenoside Rg1 Combination Treatment on Rats Following Cerebral Ischemia Are Mediated via Microglial MicroRNA-155-5p Inhibition. Mol. Med. Rep. 2018;17:3186–3193. doi: 10.3892/mmr.2017.8221. [DOI] [PubMed] [Google Scholar]
- 128.Pan L., Wang W., Shi F., Zhou J., Zhang M., Zhu H., Zeng M. Exploratory Pharmacokinetics of Geniposide in Rat Model of Cerebral Ischemia Orally Administered with or without Baicalin and/or Berberine. Evid. Based Complement. Altern. Med. 2013;2013:349531. doi: 10.1155/2013/349531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huang B., Chen P., Huang L., Li S., Zhu R., Sheng T., Yu W., Chen Z., Wang T. Geniposide Attenuates Post-Ischaemic Neurovascular Damage via GluN2A/AKT/ ERK-Dependent Mechanism. Cell. Physiol. Biochem. 2017;43:705–716. doi: 10.1159/000480657. [DOI] [PubMed] [Google Scholar]
- 130.Liu F., Wang Y., Yao W., Xue Y., Zhou J., Liu Z. Geniposide Attenuates Neonatal Mouse Brain Injury after Hypoxic-Ischemia Involving the Activation of PI3K/Akt Signaling Pathway. J. Chem. Neuroanat. 2019;102:54. doi: 10.1016/j.jchemneu.2019.101687. [DOI] [PubMed] [Google Scholar]
- 131.Wu G.A., Terol J., Ibanez V., López-García A., Pérez-Román E., Borredá C., Domingo C., Tadeo F.R., Carbonell-caballero J., Alonso R., et al. Genomics of the Origin and Evolution of Citrus. Nature. 2018 doi: 10.1038/nature25447. [DOI] [PubMed] [Google Scholar]
- 132.Garg A., Garg S., Zaneveld L.J.D., Singla A.K. Chemistry and Pharmacology of the Citrus Bioflavonoid Hesperidin. Phyther. Res. 2001;15:655–669. doi: 10.1002/ptr.1074. [DOI] [PubMed] [Google Scholar]
- 133.Elavarasan J., Velusamy P., Ganesan T., Ramakrishnan S.K., Rajasekaran D., Periandavan K. Hesperidin-Mediated Expression of Nrf2 and Upregulation of Antioxidant Status in Senescent Rat Heart. J. Pharm. Pharmacol. 2012;64:1472–1482. doi: 10.1111/j.2042-7158.2012.01512.x. [DOI] [PubMed] [Google Scholar]
- 134.Cho J. Antioxidant and Neuroprotective Effects of Hesperidin and Its Aglycone Hesperetin. Arch. Pharmacal Res. 2006;29:699–706. doi: 10.1007/BF02968255. [DOI] [PubMed] [Google Scholar]
- 135.Lorzadeh E., Ramezani-Jolfaie N., Mohammadi M. The Effect of Hesperidin Supplementation on Inflammatory Markers in Human Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. Chem. Biol. Interact. 2019;307:8–15. doi: 10.1016/j.cbi.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 136.Dimpfel W. Different Anticonvulsive Effects of Hesperidin and Its Aglycone Hesperetin on Electrical Activity in the Rat Hippocampus In-Vitro. J. Pharm. Pharmacol. 2006;58:375–379. doi: 10.1211/jpp.58.3.0012. [DOI] [PubMed] [Google Scholar]
- 137.Rainey-smith S., Schroetke L., Bahia P., Fahmi A., Skilton R., Spencer J.P.E., Rice-evans C., Rattray M., Williams R.J. Neuroprotective effects of hesperetin in mouse primary neurones are independent of CREB activation. Neurosci. Lett. 2008;438:29–33. doi: 10.1016/j.neulet.2008.04.056. [DOI] [PubMed] [Google Scholar]
- 138.Huang S., Tsai S., Lin J., Wu C., Yen G. Cytoprotective Effects of Hesperetin and Hesperidin against Amyloid β-Induced Impairment of Glucose Transport through Downregulation of Neuronal Autophagy. Mol. Nutr. Food Res. 2012:601–609. doi: 10.1002/mnfr.201100682. [DOI] [PubMed] [Google Scholar]
- 139.Menze E.T., Tadros M.G., Abdel-tawab A.M., Khalifa A.E. Potential Neuroprotective Effects of Hesperidin on 3-Nitropropionic Acid-Induced Neurotoxicity in Rats. Neurotoxicology. 2012;33:1265–1275. doi: 10.1016/j.neuro.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 140.Kumar P., Kumar A. Protective Effect of Hesperidin and Naringin against 3-Nitropropionic Acid Induced Huntington’s like Symptoms in Rats: Possible Role of Nitric Oxide. Behav. Brain Res. 2010;206:38–46. doi: 10.1016/j.bbr.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 141.Choi E.J., Ahn W.S. Neuroprotective Effects of Chronic Hesperetin Administration in Mice. Arch. Pharmacal Res. 2008;31:1457–1462. doi: 10.1007/s12272-001-2130-1. [DOI] [PubMed] [Google Scholar]
- 142.Donato F., de Gomes M.G., Goes A.T.R., Borges Filho C., Del Fabbro L., Antunes M.S., Souza L.C., Boeira S.P., Jesseb C.R. Hesperidin Exerts Antidepressant-like Effects in Acute and Chronic Treatments in Mice: Possible Role of l-Arginine-NO-CGMP Pathway and BDNF Levels. Brain Res. Bull. 2014;104:19–26. doi: 10.1016/j.brainresbull.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 143.Gaur V., Kumar A. Hesperidin Pre-Treatment Attenuates NO-Mediated Cerebral Ischemic Reperfusion Injury and Memory Dysfunction. Pharmacol. Rep. 2010;62:635–648. doi: 10.1016/S1734-1140(10)70321-2. [DOI] [PubMed] [Google Scholar]
- 144.Rong Z., Pan R., Xu Y., Zhang C., Cao Y., Liu D. Hesperidin pretreatment protects hypoxia—Ischemic brain injury in neonatal rat. Neuroscience. 2013;255:292–299. doi: 10.1016/j.neuroscience.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 145.Lakhanpal P., Rai D.K. Quercetin: A versatile flavonoid. Internet J. Med. Update. 2007;2:22–37. doi: 10.4314/ijmu.v2i2.39851. [DOI] [Google Scholar]
- 146.Erden Inal M., Kahraman A. The Protective Effect of Flavonol Quercetin against Ultraviolet a Induced Oxidative Stress in Rats. Toxicology. 2000;154:21–29. doi: 10.1016/S0300-483X(00)00268-7. [DOI] [PubMed] [Google Scholar]
- 147.Shutenko Z., Henry Y., Pinard E., Seylaz J., Potier P., Berthet F., Girard P., Sercombe R. Influence of the Antioxidant Quercetin in Vivo on the Level of Nitric Oxide Determined by Electron Paramagnetic Resonance in Rat Brain during Global Ischemia and Reperfusion. Biochem. Pharmacol. 1999;57:199–208. doi: 10.1016/S0006-2952(98)00296-2. [DOI] [PubMed] [Google Scholar]
- 148.Huk I., Brovkovych V., Nanobash Vili J., Weigel G., Neumayer C., Partyka L., Patton S., Malinski T. Bioflavonoid Quercetin Scavenges Superoxide and Increases Nitric Oxide Concentration in Ischaemia-Reperfusion Injury: An Experimental Study. Br. J. Surg. 1998;85:1080–1085. doi: 10.1046/j.1365-2168.1998.00787.x. [DOI] [PubMed] [Google Scholar]
- 149.Du G., Zhao Z., Chen Y., Li Z., Tian Y., Liu Z., Liu B., Song J. Quercetin Attenuates Neuronal Autophagy and Apoptosis in Rat Traumatic Brain Injury Model via Activation of PI3K/Akt Signaling Pathway. Neurol. Res. 2016;38:1012–1019. doi: 10.1080/01616412.2016.1240393. [DOI] [PubMed] [Google Scholar]
- 150.Lei X., Chao H., Zhang Z., Lv J., Li S., Wei H., Xue R., Li F., Li Z. Neuroprotective Effects of Quercetin in a Mouse Model of Brain Ischemic/Reperfusion Injury via Anti-Apoptotic Mechanisms Based on the Akt Pathway. Mol. Med. Rep. 2015;12:3688–3696. doi: 10.3892/mmr.2015.3857. [DOI] [PubMed] [Google Scholar]
- 151.Hwang I.K., Lee C.H., Yoo K.Y., Choi J.H., Park O.K., Lim S.S., Kang I.J., Kwon D.Y., Park J., Yi J.S., et al. Neuroprotective Effects of Onion Extract and Quercetin against Ischemic Neuronal Damage in the Gerbil Hippocampus. J. Med. Food. 2009;12:990–995. doi: 10.1089/jmf.2008.1400. [DOI] [PubMed] [Google Scholar]
- 152.Wang X.Q., Yao R.Q., Liu X., Huang J.J., Qi D.S., Yang L.H. Quercetin Protects Oligodendrocyte Precursor Cells from Oxygen/Glucose Deprivation Injury in Vitro via the Activation of the PI3K/Akt Signaling Pathway. Brain Res. Bull. 2011;86:277–284. doi: 10.1016/j.brainresbull.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 153.Kang C.H., Choi Y.H., Moon S.K., Kim W.J., Kim G.Y. Quercetin Inhibits Lipopolysaccharide-Induced Nitric Oxide Production in BV2 Microglial Cells by Suppressing the NF-κB Pathway and Activating the Nrf2-Dependent HO-1 Pathway. Int. Immunopharmacol. 2013;17:808–813. doi: 10.1016/j.intimp.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 154.Yao R.Q., Qi D.S., Yu H.L., Liu J., Yang L.H., Wu X.X. Quercetin Attenuates Cell Apoptosis in Focal Cerebral Ischemia Rat Brain via Activation of BDNF-TrkB-PI3K/Akt Signaling Pathway. Neurochem. Res. 2012;37:2777–2786. doi: 10.1007/s11064-012-0871-5. [DOI] [PubMed] [Google Scholar]
- 155.Ahmad A., Khan M.M., Hoda M.N., Raza S.S., Khan M.B., Javed H., Ishrat T., Ashafaq M., Ahmad M.E., Safhi M.M., et al. Quercetin Protects against Oxidative Stress Associated Damages in a Rat Model of Transient Focal Cerebral Ischemia and Reperfusion. Neurochem. Res. 2011;36:1360–1371. doi: 10.1007/s11064-011-0458-6. [DOI] [PubMed] [Google Scholar]
- 156.Cho J.Y., Kim I.S., Jang Y.H., Kim A.R., Lee S.R. Protective Effect of Quercetin, a Natural Flavonoid against Neuronal Damage after Transient Global Cerebral Ischemia. Neurosci. Lett. 2006;404:330–335. doi: 10.1016/j.neulet.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 157.Rivera F., Costa G., Abin A., Urbanavicius J., Arruti C., Casanova G., Dajas F. Reduction of Ischemic Brain Damage and Increase of Glutathione by a Liposomal Preparation of Quercetin in Permanent Focal Ischemia in Rats. Neurotox. Res. 2008;13:105–114. doi: 10.1007/BF03033562. [DOI] [PubMed] [Google Scholar]
- 158.Ghosh A., Sarkar S., Mandal A.K., Das N. Neuroprotective Role of Nanoencapsulated Quercetin in Combating Ischemia-Reperfusion Induced Neuronal Damage in Young and Aged Rats. PLoS ONE. 2013;8:e57735. doi: 10.1371/journal.pone.0057735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Qu X., Qi D., Dong F., Wang B., Guo R., Luo M., Yao R. Quercetin Improves Hypoxia-Ischemia Induced Cognitive Deficits via Promoting Remyelination in Neonatal Rat. Brain Res. 2014;1553:31–40. doi: 10.1016/j.brainres.2014.01.035. [DOI] [PubMed] [Google Scholar]
- 160.Wu M., Liu F., Guo Q. Quercetin Attenuates Hypoxia-Ischemia Induced Brain Injury in Neonatal Rats by Inhibiting TLR4/NF-κB Signaling Pathway. Int. Immunopharmacol. 2019;74:105704. doi: 10.1016/j.intimp.2019.105704. [DOI] [PubMed] [Google Scholar]
- 161.Adrian M., Jeandet P., Douillet-Breuil A.C., Tesson L., Bessis R. Stilbene Content of Mature Vitis Vinifera Berries in Response to UV-C Elicitation. J. Agric. Food Chem. 2000;48:6103–6105. doi: 10.1021/jf0009910. [DOI] [PubMed] [Google Scholar]
- 162.Roupe K.A., Remsberg C.M., Yáñez J.A., Davies N.M. Pharmacometrics of Stilbenes: Seguing Towards the Clinic. Curr. Clin. Pharmacol. 2006;1:81–101. doi: 10.2174/157488406775268246. [DOI] [PubMed] [Google Scholar]
- 163.Jeandet P., Clément C., Courot E., Cordelier S. Modulation of Phytoalexin Biosynthesis in Engineered Plants for Disease Resistance. Int. J. Mol. Sci. 2013;14:14136–14170. doi: 10.3390/ijms140714136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liu J., Fan C., Yu L., Yang Y., Jiang S., Ma Z., Hu W., Li T., Yang Z., Tian T., et al. Pterostilbene Exerts an Anti-Inflammatory Effect via Regulating Endoplasmic Reticulum Stress in Endothelial Cells. Cytokine. 2016;77:88–97. doi: 10.1016/j.cyto.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 165.McCormack D., McFadden D. A Review of Pterostilbene Antioxidant Activity and Disease Modification. Oxid. Med. Cell. Longev. 2013;2013 doi: 10.1155/2013/575482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Remsberg C.M., Yáñez J.A., Ohgami Y., Vega-villa K.R., Rimando A.M., Davies N.M. Pharmacometrics of Pterostilbene: Preclinical Pharmacokinetics and Metabolism, Anticancer, Antiinflammatory, Antioxidant and Analgesic Activity. Phytotherapy Res. 2008;179:169–179. doi: 10.1002/ptr.2277. [DOI] [PubMed] [Google Scholar]
- 167.Hou Y., Xie G., Miao F., Ding L., Mou Y., Wang L., Su G., Chen G., Yang J., Wu C. Pterostilbene Attenuates Lipopolysaccharide-Induced Learning and Memory Impairment Possibly via Inhibiting Microglia Activation and Protecting Neuronal Injury in Mice. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2014 doi: 10.1016/j.pnpbp.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 168.Chang J., Rimando A., Pallas M., Camins A., Porquet D., Reeves J., Shukitt-hale B., Smith M.A., Joseph J.A., Casadesus G. Low-Dose Pterostilbene, but Not Resveratrol, Is a Potent Neuromodulator in Aging and Alzheimer’s Disease. NBA. 2012;33:2062–2071. doi: 10.1016/j.neurobiolaging.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 169.Papers O. Anxiolytic Action of Pterostilbene: Involvement of Hippocampal ERK Phosphorylation. Planta Med. 2013;79:723–730. doi: 10.1055/s-0032-1328553. [DOI] [PubMed] [Google Scholar]
- 170.Yang Y., Wang J., Li Y., Fan C., Jiang S., Zhao L., Di S., Xin Z., Wang B., Wu G., et al. HO-1 Signaling Activation by Pterostilbene Treatment Attenuates Mitochondrial Oxidative Damage Induced by Cerebral Ischemia Reperfusion Injury. Mol. Neurobiol. 2015 doi: 10.1007/s12035-015-9194-2. [DOI] [PubMed] [Google Scholar]
- 171.Zhou Y., Zhang X., Ma A., Zhang Y., Chen Y., Zhou H., Li W., Jin X. Orally Administrated Pterostilbene Attenuates Acute Cerebral Ischemia—Reperfusion Injury in a Dose- and Time-Dependent Manner in Mice. Pharmacol. Biochem. Behav. 2015;135:199–209. doi: 10.1016/j.pbb.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 172.Li D., Song T., Yang L., Wang X., Yang C., Jiang Y. Neuroprotective Actions of Pterostilbene on Hypoxic-Ischemic Brain Damage in Neonatal Rats through Upregulation of Heme Oxygenase-1. Int. J. Dev. Neurosci. 2016;54:22–31. doi: 10.1016/j.ijdevneu.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 173.Zhou Y., Liu Y.E., Cao J., Zeng G., Shen C., Zhou M., Chen Y., Pu W., Potters L., Shi E.Y. Vitexins, nature-derived lignan compounds, induce apoptosis and suppress tumor growth. Clin. Cancer. 2010;15:5161–5169. doi: 10.1158/1078-0432.CCR-09-0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zucolotto S.M., Fagundes C., Reginatto F.H., Ramos F.A., Castellanos L., Duque C., Schenkel E.P. Analysis of C-Glycosyl Flavonoids from South American Passiflora Species by HPLC-DAD and HPLC-MS. Phytochem. Anal. 2012;23:232–239. doi: 10.1002/pca.1348. [DOI] [PubMed] [Google Scholar]
- 175.Zhang J., Yuan K., Zhou W.L., Zhou J., Yang P. Studies on the Active Components and Antioxidant Activities of the Extracts of Mimosa Pudica Linn. from Southern China. Pharmacogn. Mag. 2011;7:35–39. doi: 10.4103/0973-1296.75899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Moheb A., Ibrahim R.K., Roy R., Sarhan F. Changes in Wheat Leaf Phenolome in Response to Cold Acclimation. Phytochemistry. 2011;72:2294–2307. doi: 10.1016/j.phytochem.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 177.Ma L.Y., Liu R.H., Xu X.D., Yu M.Q., Zhang Q., Liu H.L. The Pharmacokinetics of C-Glycosyl Flavones of Hawthorn Leaf Flavonoids in Rat after Single Dose Oral Administration. Phytomedicine. 2010;17:640–645. doi: 10.1016/j.phymed.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 178.Zhou J., Hu H., Long J., Wan F., Li L., Zhang S., Shi Y.E., Chen Y. Vitexin 6, a Novel Lignan, Induces Autophagy and Apoptosis by Activating the Jun N-Terminal Kinase Pathway. Anticancer Drugs. 2013;24:928–936. doi: 10.1097/CAD.0b013e328364e8d3. [DOI] [PubMed] [Google Scholar]
- 179.Li Y.L., Ma S.C., Yang Y.T., Ye S.M., But P.P.H. Antiviral Activities of Flavonoids and Organic Acid from Trollius Chinensis Bunge. J. Ethnopharmacol. 2002;79:365–368. doi: 10.1016/S0378-8741(01)00410-X. [DOI] [PubMed] [Google Scholar]
- 180.Dong L., Fan Y., Shao X., Chen Z. Vitexin Protects against Myocardial Ischemia/Reperfusion Injury in Langendorff-Perfused Rat Hearts by Attenuating Inflammatory Response and Apoptosis. Food Chem. Toxicol. 2011;49:3211–3216. doi: 10.1016/j.fct.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 181.Dong L.Y., Li S., Zhen Y.L., Wang Y.N., Shao X., Luo Z.G. Cardioprotection of Vitexin on Myocardial Ischemia/Reperfusion Injury in Rat via Regulating Inflammatory Cytokines and MAPK Pathway. Am. J. Chin. Med. 2013;41:1251–1266. doi: 10.1142/S0192415X13500845. [DOI] [PubMed] [Google Scholar]
- 182.Can Ö.D., Demir Özkay Ü., Üçel U.I. Anti-Depressant-like Effect of Vitexin in BALB/c Mice and Evidence for the Involvement of Monoaminergic Mechanisms. Eur. J. Pharmacol. 2013;699:250–257. doi: 10.1016/j.ejphar.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 183.Abbasi E., Nassiri-Asl M., Shafeei M., Sheikhi M. Neuroprotective Effects of Vitexin, a Flavonoid, on Pentylenetetrazole-Induced Seizure in Rats. Chem. Biol. Drug Des. 2012;80:274–278. doi: 10.1111/j.1747-0285.2012.01400.x. [DOI] [PubMed] [Google Scholar]
- 184.Abbasi E., Nassiri-Asl M., Sheikhi M., Shafiee M. Effects of Vitexin on Scopolamine-Induced Memory Impairment in Rats. Chin. J. Physiol. 2013;56:184–189. doi: 10.4077/CJP.2013.BAB123. [DOI] [PubMed] [Google Scholar]
- 185.Yang L., Yang Z.M., Zhang N., Tian Z., Liu S.B., Zhao M.G. Neuroprotective Effects of Vitexin by Inhibition of NMDA Receptors in Primary Cultures of Mouse Cerebral Cortical Neurons. Mol. Cell. Biochem. 2014;386:251–258. doi: 10.1007/s11010-013-1862-9. [DOI] [PubMed] [Google Scholar]
- 186.Wang Y., Zhen Y., Wu X., Jiang Q., Li X., Chen Z., Zhang G., Dong L. Vitexin Protects Brain against Ischemia/Reperfusion Injury via Modulating Mitogen-Activated Protein Kinase and Apoptosis Signaling in Mice. Phytomedicine. 2015;22:379–384. doi: 10.1016/j.phymed.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 187.Choi H.J., Eun J.S., Kim B.G., Kim S.Y., Jeon H., Soh Y. Vitexin, an HIF-1α Inhibitor, Has Anti-Metastatic Potential in PC12 cells. Mol. Cells. 2006;22:291–299. [PubMed] [Google Scholar]
- 188.Min J.W., Hu J.J., He M., Sanchez R.M., Huang W.X., Liu Y.Q., Bsoul N.B., Han S., Yin J., Liu W.H., et al. Vitexin Reduces Hypoxia-Ischemia Neonatal Brain Injury by the Inhibition of HIF-1alpha in a Rat Pup Model. Neuropharmacology. 2015;99:38–50. doi: 10.1016/j.neuropharm.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 189.Funaya N., Haginaka J. Matrine- and Oxymatrine-Imprinted Monodisperse Polymers Prepared by Precipitation Polymerization and Their Applications for the Selective Extraction of Matrine-Type Alkaloids from Sophora Flavescens Aiton. J. Chromatogr. A. 2012;1248:18–23. doi: 10.1016/j.chroma.2012.05.081. [DOI] [PubMed] [Google Scholar]
- 190.Guzman J.R., Koo J.S., Goldsmith J.R., Mühlbauer M., Narula A., Jobin C. Oxymatrine Prevents NF-ΚB Nuclear Translocation and Ameliorates Acute Intestinal Inflammation. Sci. Rep. 2013;3:1–9. doi: 10.1038/srep01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang S., Jia J. Oxymatrine Attenuates Diabetes-Associated Cognitive Deficits in Rats. Acta Pharmacol. Sin. 2014;35:331–338. doi: 10.1038/aps.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ma Z.J., Li Q., Wang J.B., Zhao Y.L., Zhong Y.W., Bai Y.F., Wang R.L., Li J.Y., Yang H.Y., Zeng L.N., et al. Combining Oxymatrine or Matrine with Lamivudine Increased Its Antireplication Effect against the Hepatitis B Virus in Vitro. Evid. Based Complement. Altern. Med. 2013;2013:186573. doi: 10.1155/2013/186573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Papers O. Protective Effects of Oxymatrine on Experimental Diabetic Nephropathy. Planta Med. 2014;80:269–276. doi: 10.1055/s-0033-1360369. [DOI] [PubMed] [Google Scholar]
- 194.Chai N.L., Fu Q., Shi H., Cai C.H., Wan J., Xu S.P., Wu B.Y. Oxymatrine Liposome Attenuates Hepatic Fibrosis via Targeting Hepatic Stellate Cells. World J. Gastroenterol. 2012;18:4199–4206. doi: 10.3748/wjg.v18.i31.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Xiao T., Wang Y., Zhang Y., Bai C., Shen X. Similar to Spironolactone, Oxymatrine Is Protective in Aldosterone-Induced Cardiomyocyte Injury via Inhibition of Calpain and Apoptosis-Inducing Factor Signaling. PLoS ONE. 2014;9:e88856. doi: 10.1371/journal.pone.0088856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhang X., Jiang W., Zhou A.L., Zhao M., Jiang D.R. Inhibitory Effect of Oxymatrine on Hepatocyte Apoptosis via TLR4/PI3K/Akt/GSK-3β Signaling Pathway. World J. Gastroenterol. 2017;23:3839–3849. doi: 10.3748/wjg.v23.i21.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Liu Y., Xu Y., Ji W., Li X., Sun B., Gao Q., Su C. Anti-Tumor Activities of Matrine and Oxymatrine: Literature Review. Tumor Biol. 2014 doi: 10.1007/s13277-014-1680-z. [DOI] [PubMed] [Google Scholar]
- 198.Ying X.J., Jin B., Chen X.W., Xie J., Xu H.M., Dong P. Oxymatrine Downregulates HPV16E7 Expression and Inhibits Cell Proliferation in Laryngeal Squamous Cell Carcinoma Hep-2 Cells in Vitro. Biomed Res. Int. 2015;2015:150390. doi: 10.1155/2015/150390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Dong X., Du Q., Yu W., Zhang Z., Zhu Q., Che Z., Chen F., Wang H., Chen J. Anti-inflammatory effects of oxymatrine through inhibition of nuclear factor–kappa b and mitogen-activated protein kinase activation in lipopolysaccharide-induced BV2 microglia cells. Iran. J. Pharm. Res. 2013;12:165–174. [PMC free article] [PubMed] [Google Scholar]
- 200.Dong X.-Q., Yu W.-H., Hu Y.-Y., Zhang Z.-Y., Huang M. Oxymatrine Reduces Neuronal Cell Apoptosis by Inhibiting Toll-like Receptor 4/Nuclear Factor Kappa-B-Dependent Inflammatory Responses in Traumatic Rat Brain Injury. Inflamm. Res. 2011:533–539. doi: 10.1007/s00011-010-0300-7. [DOI] [PubMed] [Google Scholar]
- 201.Cui L., Zhang X., Yang R., Wang L., Liu L., Li M., Du W. Neuroprotection and Underlying Mechanisms of Oxymatrine in Cerebral Ischemia of Rats. Neurol. Res. 2011:319–324. doi: 10.1179/016164110X12759951866876. [DOI] [PubMed] [Google Scholar]
- 202.Ge X.H., Shao L., Zhu G.J. Oxymatrine Attenuates Brain Hypoxic-Ischemic Injury from Apoptosis and Oxidative Stress: Role of p-Akt/GSK3β/HO-1/Nrf-2 Signaling Pathway. Metab. Brain Dis. 2018;33:1869–1875. doi: 10.1007/s11011-018-0293-4. [DOI] [PubMed] [Google Scholar]
- 203.Zhao P., Zhou R., Li H.N., Yao W.X., Qiao H.Q., Wang S.J., Niu Y., Sun T., Li Y.X., Yu J.Q. Oxymatrine Attenuated Hypoxic-Ischemic Brain Damage in Neonatal Rats via Improving Antioxidant Enzyme Activities and Inhibiting Cell Death. Neurochem. Int. 2015;89:17–27. doi: 10.1016/j.neuint.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 204.Liu Y., Wang H., Liu N., Du J., Lan X., Qi X., Zhuang C., Sun T., Li Y., Yu J. Oxymatrine Protects Neonatal Rat against Hypoxic-Ischemic Brain Damage via PI3K/Akt/GSK3β Pathway. Life Sci. 2020;254 doi: 10.1016/j.lfs.2019.04.070. [DOI] [PubMed] [Google Scholar]
- 205.Inagaki T., Etgen A.M. Neuroprotective Action of Acute Estrogens: Animal Models of Brain Ischemia and Clinical Implications. Steroids. 2013;78:597–606. doi: 10.1016/j.steroids.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ibarreta D., Daxenberger A., Meyer H.H. Possible health impact of phytoestrogens and xenoestrogens in food. Apmis. 2001;109:S402–S425. doi: 10.1111/j.1600-0463.2001.tb05792.x. [DOI] [PubMed] [Google Scholar]
- 207.Cornwell T., Cohick W., Raskin I. Dietary phytoestrogens and health. Phytochemistry. 2004;65:995–1016. doi: 10.1016/j.phytochem.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 208.Stahl S., Chun T., Gray W.G. Phytoestrogens Act as Estrogen Agonists in an Estrogen-Responsive Pituitary Cell Line. Toxicol. Appl. Pharmacol. 1998;48:41–48. doi: 10.1006/taap.1998.8500. [DOI] [PubMed] [Google Scholar]
- 209.Zhao L., Chen Q., Brinton R.D. Neuroprotective and Neurotrophic Efficacy of Phytoestrogens in Cultured Hippocampal Neurons. Exp. Biol. Med. 2002;227:509–519. doi: 10.1177/153537020222700716. [DOI] [PubMed] [Google Scholar]
- 210.Chandsawangbhuwana C., Baker M.E. 3D Models of Human ER α and ER β Complexed with Coumestrol. Steroids. 2014;80:37–43. doi: 10.1016/j.steroids.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 211.Jung E., Zuly L., Kwang J., Seo H., Baeg G., Young N., Kang G., Ryong T. Mass Production of Coumestrol from Soybean (Glycine Max) Adventitious Roots through Bioreactor: Effect on Collagen Production. Plant Biotechnol. Rep. 2019 doi: 10.1007/s11816-019-00589-2. [DOI] [Google Scholar]
- 212.Stopper H., Schmitt E., Kobras K. Genotoxicity of Phytoestrogens. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2005;574:139–155. doi: 10.1016/j.mrfmmm.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 213.Yuk H.J., Lee J.H., Curtis-Long M.J., Lee J.W., Kim Y.S., Ryu H.W., Park C.G., Jeong T.S., Park K.H. The Most Abundant Polyphenol of Soy Leaves, Coumestrol, Displays Potent $α$-Glucosidase Inhibitory Activity. Food Chem. 2011;126:1057–1063. doi: 10.1016/j.foodchem.2010.11.125. [DOI] [Google Scholar]
- 214.You J.S., Cho I.A., Kang K.R., Oh J.S., Yu S.J., Lee G.J., Seo Y.S., Kim S.G., Kim C.S., Kim D.K., et al. Coumestrol Counteracts Interleukin-1β-Induced Catabolic Effects by Suppressing Inflammation in Primary Rat Chondrocytes. Inflammation. 2017;40:79–91. doi: 10.1007/s10753-016-0455-7. [DOI] [PubMed] [Google Scholar]
- 215.Jang Y.J., Son H.J., Ahn J., Jung C.H., Ha T. Coumestrol Modulates Akt and Wnt/β-Catenin Signaling during the Attenuation of Adipogenesis. Food Funct. 2016;7:4984–4991. doi: 10.1039/C6FO01127F. [DOI] [PubMed] [Google Scholar]
- 216.Park G., Baek S., Kim J.E., Lim T.G., Lee C.C., Yang H., Kang Y.G., Park J.S., Augustin M., Mrosek M., et al. Flt3 Is a Target of Coumestrol in Protecting against UVB-Induced Skin Photoaging. Biochem. Pharmacol. 2015;98:473–483. doi: 10.1016/j.bcp.2015.08.104. [DOI] [PubMed] [Google Scholar]
- 217.Lim W., Jeong M., Bazer F.W., Song G. Coumestrol Inhibits Proliferation and Migration of Prostate Cancer Cells by Regulating AKT, ERK1/2, and JNK MAPK Cell Signaling Cascades. J. Cell. Physiol. 2017;232:862–871. doi: 10.1002/jcp.25494. [DOI] [PubMed] [Google Scholar]
- 218.Zafar A., Singh S., Naseem I. Cu(II)-Coumestrol Interaction Leads to ROS-Mediated DNA Damage and Cell Death: A Putative Mechanism for Anticancer Activity. J. Nutr. Biochem. 2016;33:15–27. doi: 10.1016/j.jnutbio.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 219.Lee Y.H., Yuk H.J., Park K.H., Bae Y.S. Coumestrol Induces Senescence through Protein Kinase CKII Inhibition-Mediated Reactive Oxygen Species Production in Human Breast Cancer and Colon Cancer Cells. Food Chem. 2013;141:381–388. doi: 10.1016/j.foodchem.2013.03.053. [DOI] [PubMed] [Google Scholar]
- 220.Koirala P., Seong S.H., Jung H.A., Choi J.S. Comparative Evaluation of the Antioxidant and Anti-Alzheimer’s Disease Potential of Coumestrol and Puerarol Isolated from Pueraria Lobata Using Molecular Modeling Studies. Molecules. 2018;23:785. doi: 10.3390/molecules23040785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Liu M., Tsuang F., Sheu S., Sun J. The Protective Effects of Coumestrol against Lipopolysaccharide-Induced Toxicity on Mice Astrocytes. Neurol. Res. 2011:663–672. doi: 10.1179/1743132810Y.0000000029. [DOI] [PubMed] [Google Scholar]
- 222.Canal Castro C., Pagnussat A.S., Orlandi L., Worm P., Moura N., Etgen A.M., Alexandre Netto C. Coumestrol Has Neuroprotective Effects before and after Global Cerebral Ischemia in Female Rats. Brain Res. 2012;1474:82–90. doi: 10.1016/j.brainres.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 223.Castro C.C., Pagnussat A.S., Moura N., da Cunha M.J., Machado F.R., Wyse A.T.S., Netto C.A. Coumestrol Treatment Prevents Na+, K+-ATPase Inhibition and Affords Histological Neuroprotection to Male Rats Receiving Cerebral Global Ischemia. Neurol. Res. 2014;36:198–206. doi: 10.1179/1743132813Y.0000000286. [DOI] [PubMed] [Google Scholar]
- 224.Beatriz J., Anastacio R., Farias E., Nicola F., Odorcyk F., Bandeira R., Alexandre C., Biochemistry P.P., Federal U., Ufrgs S., et al. Phytoestrogen Coumestrol Attenuates Brain Mitochondrial Dysfunction and Long-Term Cognitive Deficits Following Neonatal Hypoxia—Ischemia. Int. J. Dev. Neurosci. 2019;79:86–95. doi: 10.1016/j.ijdevneu.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 225.Mubarakshina M.M., Ivanov B.N. The Production and Scavenging of Reactive Oxygen Species in the Plastoquinone Pool of Chloroplast Thylakoid Membranes. Physiol. Plant. 2010;140:103–110. doi: 10.1111/j.1399-3054.2010.01391.x. [DOI] [PubMed] [Google Scholar]
- 226.Borisova-Mubarakshina M.M., Vetoshkina D.V., Ivanov B.N. Antioxidant and Signaling Functions of the Plastoquinone Pool in Higher Plants. Physiol. Plant. 2019;166:181–198. doi: 10.1111/ppl.12936. [DOI] [PubMed] [Google Scholar]
- 227.Amesz J. The function of plastoquinone in photosynthetic electron transport. Biochim. Biophys. Acta. 1973;301:35–51. doi: 10.1016/0304-4173(73)90011-6. [DOI] [PubMed] [Google Scholar]
- 228.Ivanov B., Khorobrykh S. Participation of photosynthetic electron transport in production and scavenging of reactive oxygen species. Antioxid. Redox Signal. 2003;5:43–53. doi: 10.1089/152308603321223531. [DOI] [PubMed] [Google Scholar]
- 229.Skulachev V.P. A biochemical approach to the problem of aging: “megaproject” on membrane-penetrating ions. The first results and prospects. Biochemistry. 2007;72:1385–1396. doi: 10.1134/S0006297907120139. [DOI] [PubMed] [Google Scholar]
- 230.Bakeeva L.E., Barskov I.V., Egorov M.V., Isaev N.K., Kapelko V.I., Kazachenko A.V., Kirpatovsky V.I., Kozlovsky S.V., Lakomkin V.L., Levina S.B., et al. Mitochondria Targeted Plastoquinone Derivatives as Tools to Interrupt Execution of the Aging Program. 2. Treatment of Some ROS and Age Related Diseases (Heart Arrhythmia, Heart Infarctions, Kidney Ischemia, and Stroke) Biochemistry. 2008;73:1288–1299. doi: 10.1134/S000629790812002X. [DOI] [PubMed] [Google Scholar]
- 231.Jankauskas S.S., Andrianova N.V., Alieva I.B., Prusov A.N., Matsievsky D.D., Zorova L.D., Pevzner I.B., Savchenko E.S., Pirogov Y.A., Silachev D.N., et al. Dysfunction of Kidney Endothelium after Ischemia / Reperfusion and Its Prevention by Mitochondria Targeted Antioxidant. Biochemistry. 2016;81:1538–1548. doi: 10.1134/S0006297916120154. [DOI] [PubMed] [Google Scholar]
- 232.Plotnikov E.Y., Chupyrkina A.A., Jankauskas S.S., Pevzner I.B., Silachev D.N., Skulachev V.P., Zorov D.B. Biochimica et Biophysica Acta Mechanisms of Nephroprotective Effect of Mitochondria-Targeted Antioxidants under Rhabdomyolysis and Ischemia/Reperfusion. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2011;1812:77–86. doi: 10.1016/j.bbadis.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 233.Neroev V.V., Archipova M.M., Bakeeva L.E., Fursova A.Z., Grigorian E.N., Grishanova A.Y., Iomdina E.N., Ivashchenko Z.N., Katargina L.A., Maslova I.P.K., et al. Mitochondria Targeted Plastoquinone Derivatives as Tools to Interrupt Execution of the Aging Program. 4. Age Related Eye Disease. SkQ1 Returns Vision to Blind Animals. Biochemistry. 2008;73:1317–1328. doi: 10.1134/S0006297908120043. [DOI] [PubMed] [Google Scholar]
- 234.Isaev N.K., Novikova S.V., Stelmashook E.V., Barskov I.V., Silachev D.N., Khaspekov L.G., Skulachev V.P., Zorov D.B. Mitochondria Targeted Plastoquinone Antioxidant SkQR1 Decreases Trauma Induced Neurological Deficit in Rat. Biochemistry. 2012;77:996–999. doi: 10.1134/S0006297912090052. [DOI] [PubMed] [Google Scholar]
- 235.Silachev D.N., Isaev N.K., Pevzner I.B., Zorova L.D. The Mitochondria-Targeted Antioxidants and Remote Kidney Preconditioning Ameliorate Brain Damage through Kidney-to-Brain Cross-Talk. PLoS ONE. 2012;7:e51553. doi: 10.1371/journal.pone.0051553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Plotnikov E.Y., Silachev D.N., Chupyrkina A.A., Danshina M.I., Jankauskas S.S., Morosanova M.A., Stelmashook E.V., Vasileva A.K., Goryacheva E.S., Pirogov Y.A., et al. New Generation Skulachev Ions Exhibiting Nephroprotective and Neuroprotective Properties. Biochemistry. 2010;75:145–150. doi: 10.1134/S0006297910020045. [DOI] [PubMed] [Google Scholar]
- 237.Ischemic H., Injury B., Silachev D.N., Plotnikov E.Y., Pevzner I.B., Zorova L.D., Balakireva A.V., Id M.V.G., Pirogov Y.A., Skulachev V.P., et al. Neuroprotective effects of mitochondria-targeted plastoquinone and thymoquinone in a rat model of brain ischemia/reperfusion injury. Molecules. 2015;20:14487–14503. doi: 10.3390/molecules23081871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Akinwumi B.C., Bordun K.M., Anderson H.D. Biological Activities of Stilbenoids. Int. J. Mol. Sci. 2018;19:792. doi: 10.3390/ijms19030792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Fernández-Mar M.I., Mateos R., García-parrilla M.C., Puertas B., Cantos-Villar E. Bioactive Compounds in Wine: Resveratrol, Hydroxytyrosol and Melatonin: A Review. Food Chem. 2012;130:797–813. doi: 10.1016/j.foodchem.2011.08.023. [DOI] [Google Scholar]
- 240.Sovak M. Grape Extract, Resveratrol, and Its Analogs: A Review. J. Med. Food. 2001;4:93–105. doi: 10.1089/109662001300341752. [DOI] [PubMed] [Google Scholar]
- 241.Smoliga J.M., Baur J.A., Hausenblas H.A. Resveratrol and Health—A Comprehensive Review of Human Clinical Trials. Mol. Nutr. Food Res. 2011:1129–1141. doi: 10.1002/mnfr.201100143. [DOI] [PubMed] [Google Scholar]
- 242.Cottart C.H., Nivet-Antoine V., Beaudeux J.L. Review of Recent Data on the Metabolism, Biological Effects, and Toxicity of Resveratrol in Humans. Mol. Nutr. Food Res. 2014;58:7–21. doi: 10.1002/mnfr.201200589. [DOI] [PubMed] [Google Scholar]
- 243.Rauf A., Imran M., Sulera H.A.R., Ahmad B., Peters D.G., Mubarak M.S. A Comprehensive Review of the Health Perspectives of Resveratrol. Food Funct. 2017;8:4284–4305. doi: 10.1039/C7FO01300K. [DOI] [PubMed] [Google Scholar]
- 244.Savouret J.F., Quesne M. Resveratrol and Cancer: A Review. Biomed. Pharmacother. 2002;56:84–87. doi: 10.1016/S0753-3322(01)00158-5. [DOI] [PubMed] [Google Scholar]
- 245.Meng T., Xiao D., Muhammed A., Deng J., Chen L., He J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules. 2021;26:229. doi: 10.3390/molecules26010229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Neves A.R., Lucio M., LC Lima J., Reis S. Resveratrol in Medicinal Chemistry: A Critical Review of Its Pharmacokinetics, Drug-Delivery, and Membrane Interactions. Curr. Med. Chem. 2012;19:1663–1681. doi: 10.2174/092986712799945085. [DOI] [PubMed] [Google Scholar]
- 247.Albani D., Polito L., Signorini A., Forloni G. Neuroprotective Properties of Resveratrol in Different Neurodegenerative Disorders. BioFactors. 2010;36:370–376. doi: 10.1002/biof.118. [DOI] [PubMed] [Google Scholar]
- 248.Bastianetto S., Ménard C., Quirion R. Neuroprotective Action of Resveratrol. Biochim. Biophys. BBA Acta Mol. Basis Dis. 2015;1852:1195–1201. doi: 10.1016/j.bbadis.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 249.Richard T., Victor U., Bordeau S., Waffo-téguo P., Richard T., Pawlus A.D., Igl M., Pedrot E., Waffo-teguo P. Neuroprotective Properties of Resveratrol and Derivatives Neuroprotective Properties of Resveratrol and Derivatives. Ann. N.Y. Acad. Sci. 2011 doi: 10.1111/j.1749-6632.2010.05865.x. [DOI] [PubMed] [Google Scholar]
- 250.Tellone E., Galtieri A., Russo A., Giardina B., Ficarra S. Resveratrol: A Focus on Several Neurodegenerative Diseases. Oxid. Med. Cell. Longev. 2015;2015:392169. doi: 10.1155/2015/392169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Pallàs M., Casadesús G., Smith M.A., Coto-montes A., Pelegri C., Vilaplana J., Camins A. Resveratrol and Neurodegenerative Diseases: Activation of SIRT1 as the Potential Pathway towards Neuroprotection. Curr. Neurovasc. Res. 2009;6:70–81. doi: 10.2174/156720209787466019. [DOI] [PubMed] [Google Scholar]
- 252.Granados-Soto V. Resveratrol: A Natural Compound with Pharmacological Potential in Neurodegenerative Diseases. CNS Neurosci. Ther. 2008;14:14–234. doi: 10.1111/j.1755-5949.2008.00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Rege S.D., Geetha T., Griffin G.D., Broderick T.L., Babu J.R. Neuroprotective Effects of Resveratrol in Alzheimer Disease Pathology. Front. Aging Neurosci. 2014;6:1–27. doi: 10.3389/fnagi.2014.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Gomes Q., Alexandre B., Paulo J., Silva B., Fernanda C., Romeiro R., Monteiro S., Rodrigues C.A., Gonçalves P.R., Sakai J.T., et al. Neuroprotective Mechanisms of Resveratrol in Alzheimer’s Disease: Role of SIRT1. Oxid. Med. Cell. Longev. 2018;2018:8152373. doi: 10.1155/2018/8152373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Singh N., Agrawal M., Dore S. Neuroprotective Properties and Mechanisms of Resveratrol in in Vitro and in Vivo Experimental Cerebral Stroke Models. ACS Chem. Neurosci. 2013;4:1151–1162. doi: 10.1021/cn400094w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Lopez M.S., Dempsey R.J., Vemuganti R. Resveratrol Neuroprotection in Stroke and Traumatic CNS Injury. Neurochem. Int. 2015 doi: 10.1016/j.neuint.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Dave K.R., Fazio R.A.D.E., Bao Y.C., Raval A.P. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1—Uncoupling protein 2 pathway. Neuroscience. 2009;159:993–1002. doi: 10.1016/j.neuroscience.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Dong W., Li N., Gao D., Zhen H. Resveratrol Attenuates Ischemic Brain Damage in the Delayed Phase after Stroke and Induces Messenger RNA and Protein Express for Angiogenic Factors. J. Vasc. Surg. 2008;48:709–714. doi: 10.1016/j.jvs.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 259.Lei J.R., Tu X.K., Wang Y., Tu D.W., Shi S.S. Resveratrol Downregulates the TLR4 Signaling Pathway to Reduce Brain Damage in a Rat Model of Focal Cerebral Ischemia. Exp. Ther. Med. 2019:3215–3221. doi: 10.3892/etm.2019.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Suo S., Cheng M., Lien C. Resveratrol Reduction of Infarct Size in Long-Evans Rats Subjected to Focal Cerebral Ischemia. Life Sci. 2001;69:1057–1065. doi: 10.1016/s0024-3205(01)01195-x. [DOI] [PubMed] [Google Scholar]
- 261.Li Z., Pang L., Fang F., Zhang G., Zhang J., Xie M., Wang L. Resveratrol Attenuates Brain Damage in a Rat Model of Focal Cerebral Ischemia via Up-Regulation of Hippocampal Bcl-2. Brain Res. 2012;1450:116–124. doi: 10.1016/j.brainres.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 262.Hong J., Lee H., Lee S. Protective effect of resveratrol against neuronal damage following transient global cerebral ischemia in mice. J. Nutr. Biochem. 2015 doi: 10.1016/j.jnutbio.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 263.Wang Q., Xu J., Rottinghaus G.E., Simonyi A., Lubahn D., Sun G.Y., Sun A.Y. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res. 2002;958:439–447. doi: 10.1016/S0006-8993(02)03543-6. [DOI] [PubMed] [Google Scholar]
- 264.Gao D., Zhang X., Jiang X., Peng Y., Huang W., Cheng G., Song L. Resveratrol Reduces the Elevated Level of MMP-9 Induced by Cerebral Ischemia—Reperfusion in Mice. Life Sci. 2006;78:2564–2570. doi: 10.1016/j.lfs.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 265.He Q., Li Z., Wang Y., Hou Y., Li L., Zhao J. Resveratrol Alleviates Cerebral Ischemia/Reperfusion Injury in Rats by Inhibiting NLRP3 Inflammasome Activation through Sirt1-Dependent Autophagy Induction. Int. Immunopharmacol. 2017;50:208–215. doi: 10.1016/j.intimp.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 266.Kizmazoglu C., Aydin H.E., Sevin I.E., Kalemci O., Yuceer N., Atasoy M.A. Neuroprotective Effect of Resveratrol on Acute Brain Ischemia Reperfusion Injury by Measuring Annexin V, P53, Bcl-2 Levels in Rats. J. Korean Neurosurg. Soc. 2015;58:508–512. doi: 10.3340/jkns.2015.58.6.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Abdel-Aleem G.A., Khaleel E.F., Mostafa D.G., Elberier L.K. Neuroprotective effect of resveratrol against brain ischemia reperfusion injury in rats entails reduction of DJ-1 protein expression and activation of PI3K/Akt/GSK3b survival pathway. Arch. Physiol. Biochem. 2016;3455 doi: 10.1080/13813455.2016.1182190. [DOI] [PubMed] [Google Scholar]
- 268.Kovacic P., Somanathan R. Multifaceted Approach to Resveratrol Bioactivity Focus on Antioxidant Action, Cell Signaling and Safety. Oxid. Med. Cell. Longev. 2010;3:86–100. doi: 10.4161/oxim.3.2.11147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Science F. Neuroprotective Effects of Resveratrol on Cerebral Ischemia-Induced Neuron Loss Mediated by Free Radical Scavenging and Cerebral Blood Flow Elevation. J. Agric. Food Chem. 2006;54:3126–3131. doi: 10.1021/jf053011q. [DOI] [PubMed] [Google Scholar]
- 270.Poulsen M.M., Fjeldborg K., Ornstrup M.J., Kjær T.N., Nøhr M.K., Pedersen S.B. Resveratrol and Inflammation: Challenges in Translating Pre-Clinical Fi Ndings to Improved Patient Outcomes. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2015 doi: 10.1016/j.bbadis.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 271.Zhang F., Liu J., Shi J. Anti-Inflammatory Activities of Resveratrol in the Brain: Role of Resveratrol in Microglial Activation. Eur. J. Pharmacol. 2010;636:1–7. doi: 10.1016/j.ejphar.2010.03.043. [DOI] [PubMed] [Google Scholar]
- 272.Fulda S., Debatin K.M. Resveratrol Modulation of Signal Transduction in Apoptosis and Cell Survival: A Mini-Review. Cancer Detect. Prev. 2006;30:217–223. doi: 10.1016/j.cdp.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 273.Shin J.Y., Seo M.A., Choi E.J., Kim J.K., Seo E.S., Lee J.H., Chung H.L., Kim W.T. Neuroprotective effects of resveratrol via anti-apoptosis on hypoxic-ischemic brain injury in neonatal rats. Korean J. Pediatr. 2008;51:1102–1111. doi: 10.3345/kjp.2008.51.10.1102. [DOI] [Google Scholar]
- 274.Karalis F., Soubasi V., Georgiou T., Nakas C.T., Simeonidou C., Guiba-Tziampiri O., Spandou E. Resveratrol Ameliorates Hypoxia/Ischemia-Induced Behavioral Deficits and Brain Injury in the Neonatal Rat Brain. Brain Res. 2011;1425:98–110. doi: 10.1016/j.brainres.2011.09.044. [DOI] [PubMed] [Google Scholar]
- 275.Arteaga O., Revuelta M., Urigüen L., Álvarez A., Montalvo H., Hilario E. Pretreatment with Resveratrol Prevents Neuronal Injury and Cognitive Deficits Induced by Perinatal Hypoxia-Ischemia in Rats. PLoS ONE. 2015;10:e0142424. doi: 10.1371/journal.pone.0142424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Pan S., Li S., Hu Y., Zhang H., Liu Y., Jiang H., Fang M., Li Z., Xu K., Zhang H., et al. Resveratrol Post-Treatment Protects against Neonatal Brain Injury after Hypoxia-Ischemia. Oncotarget. 2016;7:79247–79261. doi: 10.18632/oncotarget.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Le K., Chibaatar Daliv E., Wu S., Qian F., Ali A.I., Yu D., Guo Y. SIRT1-Regulated HMGB1 Release Is Partially Involved in TLR4 Signal Transduction: A Possible Anti-Neuroinflammatory Mechanism of Resveratrol in Neonatal Hypoxic-Ischemic Brain Injury. Int. Immunopharmacol. 2019;75:105779. doi: 10.1016/j.intimp.2019.105779. [DOI] [PubMed] [Google Scholar]
- 278.Matsui Y., Sugiyama K., Kamei M., Takahashi T., Suzuki T., Katagata Y., Ito T. Extract of Passion Fruit (Passiflora Edulis) Seed Containing High Amounts of Piceatannol Inhibits Melanogenesis and Promotes Collagen Synthesis. J. Agric. Food Chem. 2010;58:11112–11118. doi: 10.1021/jf102650d. [DOI] [PubMed] [Google Scholar]
- 279.Rodríguez-Cabo T., Rodríguez I., López P., Ramil M., Cela R. Investigation of Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry Performance for Identification and Determination of Hydroxylated Stilbene Antioxidants in Wine. J. Chromatogr. A. 2014;1337:162–170. doi: 10.1016/j.chroma.2014.02.058. [DOI] [PubMed] [Google Scholar]
- 280.Rossi M., Caruso F., Opazo C., Salciccioli J. Crystal and Molecular Structure of Piceatannol; Scavenging Features of Resveratrol and Piceatannol on Hydroxyl and Peroxyl Radicals and Docking with Transthyretin. J. Agric. Food Chem. 2008;56:10557–10566. doi: 10.1021/jf801923j. [DOI] [PubMed] [Google Scholar]
- 281.Murias M., Jäger W., Handler N., Erker T., Horvath Z., Szekeres T., Nohl H., Gille L. Antioxidant, Prooxidant and Cytotoxic Activity of Hydroxylated Resveratrol Analogues: Structure-Activity Relationship. Biochem. Pharmacol. 2005;69:903–912. doi: 10.1016/j.bcp.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 282.Banik K., Ranaware A.M., Harsha C., Nitesh T., Girisa S., Deshpande V., Fan L., Nalawade S.P., Sethi G., Kunnumakkara A.B. Piceatannol: A Natural Stilbene for the Prevention and Treatment of Cancer. Pharmacol. Res. 2020;153:104635. doi: 10.1016/j.phrs.2020.104635. [DOI] [PubMed] [Google Scholar]
- 283.Lee H.H., Park S.A., Almazari I., Kim E.H., Na H.K., Surh Y.J. Piceatannol Induces Heme Oxygenase-1 Expression in Human Mammary Epithelial Cells through Activation of ARE-Driven Nrf2 Signaling. Arch. Biochem. Biophys. 2010;501:142–150. doi: 10.1016/j.abb.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 284.Gerszon J., Walczak A., Rodacka A. Attenuation of H2O2-Induced Neuronal Cell Damage by Piceatannol. J. Funct. Foods. 2017;35:540–548. doi: 10.1016/j.jff.2017.06.025. [DOI] [Google Scholar]
- 285.Zhang Y., Zhang L.H., Chen X., Zhang N., Li G. Piceatannol Attenuates Behavioral Disorder and Neurological Deficits in Aging Mice: Via Activating the Nrf2 Pathway. Food Funct. 2018;9:371–378. doi: 10.1039/C7FO01511A. [DOI] [PubMed] [Google Scholar]
- 286.Gupta S.C., Prasad S., Aggarwal B.B. Anti-Inflammatory Nutraceuticals and Chronic Diseases. Volume 928. Springer; New York, NY, USA: 2016. pp. 267–289. [DOI] [Google Scholar]
- 287.Dumont U., Sanchez S., Olivier B., Chateil J.F., Pellerin L., Beauvieux M.C., Bouzier-Sore A.K., Roumes H. Maternal consumption of piceatannol: A nutritional neuroprotective strategy against hypoxia-ischemia in rat neonates. Brain Res. 2019;1717:86–94. doi: 10.1016/j.brainres.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 288.Zhang D., Li X., Hao D., Li G., Xu B., Ma G., Su Z., Zhang D., Li X., Hao D., et al. Systematic purification of polydatin, resveratrol and anthraglycoside B from Polygonum cuspidatum Sieb. et Zucc. Sep. Purif. Technol. 2009;66:329–339. doi: 10.1016/j.seppur.2008.12.013. [DOI] [Google Scholar]
- 289.Du Q., Peng C., Zhang H. Polydatin: A Review of Pharmacology and Pharmacokinetics. Pharm. Biol. 2013;0209 doi: 10.3109/13880209.2013.792849. [DOI] [PubMed] [Google Scholar]
- 290.Cheng Y., Zhang H., Sun L., Guo S., Ouyang S., Zhang Y., Xu J. Involvement of Cell Adhesion Molecules in Polydatin Protection of Brain Tissues from Ischemia—Reperfusion Injury. Brain Res. 2006;1110:193–200. doi: 10.1016/j.brainres.2006.06.065. [DOI] [PubMed] [Google Scholar]
- 291.Gao Y., Chen T., Lei X., Li Y., Dai X., Cao Y., Ding Q., Lei X., Li T., Lin X. Neuroprotective Effects of Polydatin against Mitochondrial-Dependent Apoptosis in the Rat Cerebral Cortex Following Ischemia/Reperfusion Injury. Mol. Med. Rep. 2016:5481–5488. doi: 10.3892/mmr.2016.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Sun J., Qu Y., He H., Fan X., Qin Y., Mao W., Xu L. Protective Effect of Polydatin on Learning and Memory Impairments in Neonatal Rats with Hypoxic-ischemic Brain Injury by Up-regulating Brain-derived Neurotrophic Factor. Mol. Med. Rep. 2014;10:3047–3051. doi: 10.3892/mmr.2014.2577. [DOI] [PubMed] [Google Scholar]
- 293.Rao S.R., Ravishankar G.A. Review Vanilla Flavour: Production by Conventional and Biotechnological Routes. J. Sci. Food Agric. 2000;304:289–304. [Google Scholar]
- 294.Anand A., Khurana R., Wahal N., Mahajan S., Mehta M., Satija S., Sharma N., Vyas M., Khurana N. Vanillin: A comprehensive review of pharmacological activities. Plant Arch. 2019;19:1000–1004. [Google Scholar]
- 295.Kim H.J., Hwang I.K., Won M.H. Vanillin, 4-hydroxybenzyl aldehyde and 4-hydroxybenzyl alcohol prevent hippocampal CA1 cell death following global ischemia. Brain Res. 2007;1181 doi: 10.1016/j.brainres.2007.08.066. [DOI] [PubMed] [Google Scholar]
- 296.Dhanalakshmi C., Janakiraman U., Essa M.M., Kalandar A. Vanillin Attenuated Behavioural Impairments, Neurochemical Deficts, Oxidative Stress and Apoptosis Against Rotenone Induced Rat Model of Parkinson’s Disease. Neurochem. Res. 2016;41:1899–1910. doi: 10.1007/s11064-016-1901-5. [DOI] [PubMed] [Google Scholar]
- 297.Lan X., Wang Q., Yang J., Ma L., Zhang W., Zheng P., Sun T. Neuroprotective e effectof Vanillin on hypoxic-ischemic brain damage in neonatal rats. Biomed. Pharmacother. 2019;118:109196. doi: 10.1016/j.biopha.2019.109196. [DOI] [PubMed] [Google Scholar]
- 298.Nawrot P., Jordan S., Eastwood J., Rotstein J., Hugenholtz A., Feeley M., Jordan S., Eastwood J., Rotstein J., Hugenholtz A., et al. Effects of caffeine on human health. Food Addit. Contam. 2017 doi: 10.1080/0265203021000007840. [DOI] [PubMed] [Google Scholar]
- 299.Deslandes A.C., Veiga H., Cagy M., Piedade R., Pompeu F., Ribeiro P. Effects of Caffeine on the Electrophysiological, Cognitive and Motor Responses of the Central Nervous System. Braz. J. Med Biol. Res. 2005;38:1077–1086. doi: 10.1590/S0100-879X2005000700011. [DOI] [PubMed] [Google Scholar]
- 300.Comer A.M., Perry C.M., Figgitt D.P., Bhatia J., Neonatology S., College M. Caffeine Citrate: A Review of Its Use in Apnoea of Prematurity. Paediatr Drugs. 2001;3:61–79. doi: 10.2165/00128072-200103010-00005. [DOI] [PubMed] [Google Scholar]
- 301.Schmidt B., Roberts R.S., Davis P., Doyle L.W., Barrington K.J., Ohlsson A., Solimano A., Tin W. Caffeine Therapy for Apnea of Prematurity. N. Engl. J. Med. 2006:2112–2121. doi: 10.1056/NEJMoa054065. [DOI] [PubMed] [Google Scholar]
- 302.Picone S., Bedetta M., Paolillo P. Caffeine Citrate: When and for How Long. A Literature Review. J. Matern. Neonatal Med. 2012;25:11–14. doi: 10.3109/14767058.2012.712305. [DOI] [PubMed] [Google Scholar]
- 303.Back S.A., Craig A., Luo N.L., Ren J., Akundi R.S., Ribeiro I., Rivkees S.A. Protective Effects of Caffeine on Chronic Hypoxia-Induced Perinatal White Matter Injury. Ann. Neurol. 2006;3:696–705. doi: 10.1002/ana.21008. [DOI] [PubMed] [Google Scholar]
- 304.Kilicdag H., Daglioglu Y.K., Erdogan S., Zorludemir S. Effects of Caffeine on Neuronal Apoptosis in Neonatal Hypoxic-Ischemic Brain Injury. Ann. Neurol. 2014;7058:1470–1475. doi: 10.3109/14767058.2013.878694. [DOI] [PubMed] [Google Scholar]
- 305.Hoda S., Hosseinzadeh H. Bioactivity Assessment and Toxicity of Crocin: A Comprehensive Review. Food Chem. Toxicol. 2014;64:65–80. doi: 10.1016/j.fct.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 306.Abdel-Rahman R.F., El Awdan S.A., Hegazy R.R., Mansour D.F., Ogaly H.A., Abdelbaset M. Neuroprotective Effect of Crocus Sativus against Cerebral Ischemia in Rats. Metab. Brain Dis. 2020:427–439. doi: 10.1007/s11011-019-00505-1. [DOI] [PubMed] [Google Scholar]
- 307.Sarshoori J.R., Asadi M.H., Mohammadi M.T. Neuroprotective Effects of Crocin on the Histopathological Alterations Following Brain Ischemia-Reperfusion Injury in Rat. Iran. J. Basic Med. Sci. 2014;17:895. [PMC free article] [PubMed] [Google Scholar]
- 308.Pitsikas N., Sakellaridis N. Crocus sativus L. extracts antagonize memory impairments in different behavioural tasks in the rat. Behav. Brain Res. 2006;173:112–115. doi: 10.1016/j.bbr.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 309.Wang K., Zhang L., Rao W., Su N., Hui H., Wang L., Peng C., Tu Y., Zhang S., Fei Z. Neuroprotective Effects of Crocin against Traumatic Brain Injury in Mice: Involvement of Notch Signaling Pathway. Neurosci. Lett. 2015;591:53–58. doi: 10.1016/j.neulet.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 310.Hassani F.V., Naseri V., Razavi B.M., Mehri S., Abnous K. Antidepressant Effects of Crocin and Its Effects on Transcript and Protein Levels of CREB, BDNF, and VGF in Rat Hippocampus. DARU J. Pharm. Sci. 2014;22:1–9. doi: 10.1186/2008-2231-22-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Huang A., Jia L. Crocin Enhances Hypothermia Therapy in Hypoxic Ischemia-Induced Brain Injury in Mice. Acta Neurol. Belg. 2019 doi: 10.1007/s13760-019-01198-0. [DOI] [PubMed] [Google Scholar]
- 312.Xu S., Liu P. Tanshinone II-A: New Perspectives for Old Remedies. Expert Opin. Ther. Pat. 2013;23:149–153. doi: 10.1517/13543776.2013.743995. [DOI] [PubMed] [Google Scholar]
- 313.Gao S., Liu Z., Li H., Little P.J., Liu P., Xu S. Cardiovascular Actions and Therapeutic Potential of Tanshinone IIA. Atherosclerosis. 2012;220:3–10. doi: 10.1016/j.atherosclerosis.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 314.Shang Q., Xu H., Huang L. Tanshinone IIA: A Promising Natural Cardioprotective Agent. Evid. Based Complement. Altern. Med. 2012;2012:716459. doi: 10.1155/2012/716459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Zhang Y., Jiang P., Ye M., Kim S.H., Jiang C., Lü J. Tanshinones: Sources, Pharmacokinetics and Anti-Cancer Activities. Int. J. Mol. Sci. 2012;13:13621–13666. doi: 10.3390/ijms131013621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Niu X.L., Ichimori K., Yang X., Hirota Y., Hoshiai K., Li M., Nakazawa H. Tanshinone II-A Inhibits Low Density Lipoprotein Oxidation in Vitro. Free Radic. Res. 2000;33:305–312. doi: 10.1080/10715760000301471. [DOI] [PubMed] [Google Scholar]
- 317.Zhou Z.Y., Zhao W.R., Zhang J., Chen X.L., Tang J.Y. Sodium Tanshinone IIA Sulfonate: A Review of Pharmacological Activity and Pharmacokinetics. Biomed. Pharmacother. 2019;118:109362. doi: 10.1016/j.biopha.2019.109362. [DOI] [PubMed] [Google Scholar]
- 318.Chen Y., Wu X., Yu S., Lin X., Wu J., Li L., Zhao J., Zhao Y. Neuroprotection of Tanshinone IIA against Cerebral Ischemia/Reperfusion Injury through Inhibition of Macrophage Migration Inhibitory Factor in Rats. PLoS ONE. 2012;7:e40165. doi: 10.1371/journal.pone.0040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Ren Y., Houghton P.J., Hider R.C., Howes M.J.R. Novel Diterpenoid Acetylcholinesterase Inhibitors from Salvia Miltiorhiza. Planta Med. 2004;70:201–204. doi: 10.1055/s-2004-815535. [DOI] [PubMed] [Google Scholar]
- 320.Xia W.J., Yang M., Fok T.F., Li K., Chan W.Y., Ng P.-C., Ng H.K., Chik K.W., Wang C.C., Gu G.J.S., et al. Partial Neuroprotective Effect of Pretreatment with Tanshinone IIA on Neonatal Hypoxia-Ischemia Brain Damage. Pediatr. Res. 2005;58:784–790. doi: 10.1203/01.PDR.0000180550.99162.BC. [DOI] [PubMed] [Google Scholar]
- 321.Fowler C.J. Plant-derived, synthetic and endogenous cannabinoids as neuroprotective agents: Non-psychoactive cannabinoids, ‘entourage’ compounds and inhibitors of N-acyl ethanolamine breakdown as therapeutic strategies to avoid pyschotropic effects. Brain Res. Rev. 2003;41:26–43. doi: 10.1016/S0165-0173(02)00218-7. [DOI] [PubMed] [Google Scholar]
- 322.Rosenberg E.C., Patra P.H., Whalley B.J. Therapeutic Effects of Cannabinoids in Animal Models of Seizures, Epilepsy, Epileptogenesis, and Epilepsy-Related Neuroprotection. Epilepsy Behav. 2016:1–9. doi: 10.1016/j.yebeh.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Sánchez A.J., Garcia-Merino A. Neuroprotective Agents: Cannabinoids. Clin. Immunol. 2012;142:57–67. doi: 10.1016/j.clim.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 324.Fernández-López D., Pazos M.R., Tolon R.M., Moro M.A., Romero J., Lizasoain I., Martinez-Orgado J. The cannabinoid agonist WIN55212 reduces brain damage in an in vivo model of hypoxic-ischemic encephalopathy in newborn rats. Pediatric Res. 2007;62:255–260. doi: 10.1203/PDR.0b013e318123fbb8. [DOI] [PubMed] [Google Scholar]
- 325.Zuardi A.W., Crippa J.A.S., Hallak J.E.C., Bhattacharyya S., Martín-Santos R., Mcguire P.K., Guimarães F.S. A Critical Review of the Antipsychotic Effects of Cannabidiol 30 Years of a Trans- Lational Investigation. Curr. Pharm. Des. 2012;15:5131–5140. doi: 10.2174/138161212802884681. [DOI] [PubMed] [Google Scholar]
- 326.Scuderi C., De Filippis D., Iuvone T., Blasio A., Steardo A., Esposito G. Cannabidiol in Medicine: A Review of Its Therapeutic Potential in CNS Disorders. Phytotherapy Res. 2009;602:597–602. doi: 10.1002/ptr.2625. [DOI] [PubMed] [Google Scholar]
- 327.Millar S.A., Stone N.L., Yates A.S., Sullivan S.E.O. A Systematic Review on the Pharmacokinetics of Cannabidiol in Humans. Front. Pharmacol. 2018;9 doi: 10.3389/fphar.2018.01365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Burstein S. CBD Receptor Binding Signaling Events Downstream Events. Bioorg. Med. Chem. 2015 doi: 10.1016/j.bmc.2015.01.059. [DOI] [Google Scholar]
- 329.Alvarez F.J., Lafuente H., Rey-santano M.C., Mielgo V.E., Gastiasoro E., Rueda M., Pertwee R.G., Castillo A.I., Romero J., Martínez-Orgado J. Neuroprotective Effects of the Nonpsychoactive Cannabinoid Cannabidiol in Hypoxic-Ischemic Newborn Piglets. Pediatr. Res. 2008;64:653–658. doi: 10.1203/PDR.0b013e318186e5dd. [DOI] [PubMed] [Google Scholar]
- 330.Castillo A., Tolón M.R., Fernández-Ruiz J., Romero J., Martinez-Orgado J. Neurobiology of Disease The Neuroprotective Effect of Cannabidiol in an in Vitro Model of Newborn Hypoxic—Ischemic Brain Damage in Mice Is Mediated by CB2 and Adenosine Receptors. Neurobiol. Dis. 2010;37:434–440. doi: 10.1016/j.nbd.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 331.Lafuente H., Alvarez F.J., Pazos M.R., Alvarez A., Rey-Santano M.C., Mielgo V., Murgia-Esteve X., Hilario E., Martinez-Orgado J. Cannabidiol Reduces Brain Damage and Improves Functional Recovery after Acute Hypoxia-Ischemia in Newborn Pigs. Pediatr. Res. 2011;70:272–277. doi: 10.1203/PDR.0b013e3182276b11. [DOI] [PubMed] [Google Scholar]
- 332.Pazos M.R., Cinquina V., Gómez A., Layunta R., Santos M., Fernández-ruiz J., Martínez-orgado J. Cannabidiol administration after hypoxia e ischemia to newborn rats reduces long-term brain injury and restores neurobehavioral function. Neuropharmacology. 2012;63:776–783. doi: 10.1016/j.neuropharm.2012.05.034. [DOI] [PubMed] [Google Scholar]
- 333.Pazos M.R., Mohammed N., Lafuente H., Santos M., Martínez-pinilla E., Moreno E., Valdizan E., Romero J., Pazos A., Franco R., et al. Mechanisms of Cannabidiol Neuroprotection in Hypoxice Ischemic Newborn Pigs: Role of 5HT1A and CB2 Receptors. Neuropharmacology. 2013;71:282–291. doi: 10.1016/j.neuropharm.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 334.Barata L., Arruza L., Rodríguez M., Aleo E., Vierge E., Criado E., Sobrino E., Vargas C., Ceprián M., Gutiérrez-Rodríguez A., et al. Neuroprotection by Cannabidiol and Hypothermia in a Piglet Model of Newborn Hypoxic-Ischemic Brain Damage. Neuropharmacology. 2019;146:1–11. doi: 10.1016/j.neuropharm.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 335.Ferreira A., Rodrigues M., Fortuna A., Falcão A., Alves G. Huperzine A from Huperzia Serrata: A Review of Its Sources, Chemistry, Pharmacology and Toxicology. Phytochem. Rev. 2016;15:51–85. doi: 10.1007/s11101-014-9384-y. [DOI] [Google Scholar]
- 336.Desilets A.R., Gickas J.J., Dunican K.C. Role of Huperzine A in the Treatment of Alzheimer’s Disease. Ann. Pharmacother. 2009;43 doi: 10.1345/aph.1L402. [DOI] [PubMed] [Google Scholar]
- 337.Li J., Hm W., Zhou R.L., Liu G.J., Dong B.R. Huperzine A for Alzheimer’s Disease. Cochr. Database Syst. Rev. 2009;2 doi: 10.1002/14651858.CD005592.pub2. [DOI] [PubMed] [Google Scholar]
- 338.Wang R., Can X. Neuroprotective Effects of Huperzine A. Neurosignals. 2005;14:71–82. doi: 10.1159/000085387. [DOI] [PubMed] [Google Scholar]
- 339.Damar U., Gersner R., Johnstone J.T., Schachter S., Rotenberg A., Gersner R., Johnstone J.T., Schachter S., Rotenberg A. Expert Review of Neurotherapeutics Huperzine A as a Neuroprotective and Antiepileptic Drug: A Review of Preclinical Research. Expert Rev. Neurother. 2016;16:671–680. doi: 10.1080/14737175.2016.1175303. [DOI] [PubMed] [Google Scholar]
- 340.Park S. State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Zhangjiang Hi-Tech Park, Shanghai, P.R. China. J. Neurochem. 2008:1594–1603. doi: 10.1111/j.1471-4159.2008.05504.x. [DOI] [PubMed] [Google Scholar]
- 341.Shuan L., Zhou J., Mei X., Can X. Huperzine A Attenuates Cognitive Deficits and Brain Injury in Neonatal Rats after Hypoxia—Ischemia. Brain Res. 2002;949:162–170. doi: 10.1016/s0006-8993(02)02977-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.