Abstract
Myelodysplastic syndromes (MDSs) are a group of heterogeneous hematopoietic stem/progenitor cells clonal diseases, characteristic features with myeloid dysplasia, leading to abnormality of neutrophils. Recent studied have showed that neutrophils act not only as professional killers, but also as regulators of innate and adaptive immune in infection and inflammatory condition. The CD54highCD181low neutrophils are a kind of reverse-transmigrated neutrophils characterized proinflammatory phenotype. We investigated the frequency and functional properties of circulating CD54highCD181low neutrophils in patients with untreated MDS. Frequency of CD54highCD181low neutrophils was significantly increased in MDS patients and related to the severity of the disease. Furthermore, CD54highCD181low neutrophils suppressed CD8+ T cells functions in vitro. CD54highCD181low neutrophils lead to upregulation of PD1 on CD8+ T cells. Higher CD54highCD181low neutrophils were related to poor prognosis and more infections. The frequency of CD54highCD181low neutrophils decreased in high risk MDS patients who had response after treatment with decitabine. Overall, we identified CD54highCD181low neutrophils expanded in MDS. The exact mechanisms of increased CD54highCD181low neutrophils and its effect on immune function remain to be elucidated.
Keywords: myelodysplastic syndromes, neutrophils, immunity, CD54/ICAM-1, CD181
Introduction
Myelodysplastic syndrome (MDS) is a malignant clonal hematopoietic stem cell disease that is heterogeneous (1). It is characterized by malignant clonal hematopoietic stem cells, abnormal development of progenitor cells, ineffective hematopoiesis, and genetic instability, which makes it easy to transform into acute myeloid leukemia (AML) (2, 3).
Over the past decade, there has been increasing evidence of phenotype heterogeneity and functional diversity of neutrophils. In addition to their antibacterial functions, neutrophils appear as decision makers in innate and acquired immune responses. These findings open the door to understanding the role of neutrophils in homeostatic and pathogenic immune processes (4, 5). The differentiation of neutrophils into different functional subpopulations requires these cells to respond to environmental signals, synthesize and release a series of new proteins. Neutrophils resynthesize and release proteins that affected by epigenetics and regulatory genes (6).
Studies have shown that neutrophils have the ability to migrate out of the blood vessel and then return to the blood vessel, challenging the traditional concept of unidirectional neutrophil migration. The phenotype of neutrophils in the circulating pool is CD54lowCD181high, and the phenotype in tissues is CD54lowCD181low in vitro. The phenotype of reverse-transmigrated neutrophils is CD54highCD181low, which is different from that in tissues and blood. These cells have a longer lifespan and more reactive oxygen species (ROS) production than circulating neutrophils (4). Reverse-transmigrated neutrophils exhibited a proinflammatory phenotype characterized by a high CD54 expression, and cannot transmigrate again into the tissue (6).
In the present study, we investigated the level of circulating CD54highCD181low neutrophils in patients with MDS and evaluated the association between the neutrophils and T cells in MDS.
Methods
Patient Characteristics
From September 2016 to June 2019, a total of 37 newly diagnosed MDS patients in the Hematology Department of the General Hospital of Tianjin Medical University were enrolled in the study. The study included 22 males and 15 females with a median age of 61 years (range 27–79 years) (details in Table 1). The patients were divided into two groups based on the revised International Prognostic Scoring System (IPSS-R), the relative low risk group (IPSS-R score less than or equal to 3.5, n=18) and the relative high risk group (IPSS-R score more than 3.5, n=19). The low risk MDS patients were treated with Recombinant Human Erythropoietin (Sansheng, China) and lemalidomide (BeiGene, China) (only for 5q- patient). The high risk MDS patients were treated with decitabine (Janssen, China).
Table 1.
The characteristics of myelodysplastic syndrome (MDS) patients.
| case | sex/age | diagnosis | cytogenetics | IPSS-R |
|---|---|---|---|---|
| 1 | Male/63 | MLD | 46,XY | Very Low |
| 2 | Male/34 | RS | 46,XY | Very Low |
| 3 | Male/67 | EB2 | 46,XY | Very high |
| 4 | Female/62 | 5q- | 5q- | Low |
| 5 | Male/42 | RS | 46,XY,del20q11 | Low |
| 6 | Female/47 | RS | 46,XX | Low |
| 7 | Male/62 | SLD | 46,XY,13q+ | Low |
| 8 | Female/49 | RS | 46,XX | low |
| 9 | Male/50 | EB1 | 46,XX | Int |
| 10 | Male/50 | MLD | 47,XY,+8/46,XY | Int |
| 11 | Male/65 | MLD | 46,XY,del17q31 | Int |
| 12 | Female/61 | MLD | 46,XX | Int |
| 13 | Male/46 | MLD | 46,XY,-2,-12,+mar,19+,9P+ | Int |
| 14 | Male/70 | EB1 | 46,XY | Int |
| 15 | Female/67 | MLD | 46,XX | Int |
| 16 | Male/61 | MLD | 45-46,XY,21p+ | Int |
| 17 | Male/71 | MLD | 46,XY | Int |
| 18 | Female/56 | MLD | 17P+, +8 | Int |
| 19 | Male/58 | MLD | 46,XY | Int |
| 20 | Male/48 | MLD | 46,XY | Int |
| 21 | Male/58 | EB2 | 46,XY | High |
| 22 | Female/73 | EB2 | 46,XX | High |
| 23 | Male/61 | EB2 | 46,XY | High |
| 24 | Female/64 | EB2 | 46,XX | High |
| 25 | Female/59 | EB1 | 46,XY,13q+ | High |
| 26 | Male/62 | EB2 | 46,XY | High |
| 27 | Male/38 | EB2 | 46,XY | High |
| 28 | Female/70 | EB2 | 46,XX | High |
| 29 | Female/69 | EB2 | 46,XX | high |
| 30 | Male/30 | EB2 | 47,XY,+8/46,XY | High |
| 31 | Female/79 | EB2 | 45,XX,-7 | Very High |
| 32 | Female/29 | EB2 | 20q-,5q-,7q- | Very High |
| 33 | Male/68 | EB2 | 46,XY,+8/45,XY+8,-6,-7 | Very High |
| 34 | Female/76 | EB2 | del5q33,del5q31,del7q311,del7q3 | Very High |
| 35 | Female/77 | EB2 | 45,XX,-5,-2,45,XX,+mar,-5,3P- | Very High |
| 36 | Male/27 | EB2 | 3p+,-18,+mar | Very High |
| 37 | Male/60 | EB2 | 45,XY,-7 | Very High |
Twenty-three healthy people were selected as controls in this study, including 13 men and 10 women with a median age of 52 (range 24–74 years).
The study was approved by the Ethics Committee of the General Hospital of Tianjin Medical University. Informed written consents have been obtained from all patients and control groups or their guardians according to the Helsinki Declaration.
CD54highCD181low Neutrophils With Flow Cytometric Analysis
Heparin anticoagulant sterile tube were used to collect peripheral blood samples 5 ml from MDS patients and healthy controls. We used cells’ SSC/FSC to divide peripheral blood mononuclear cells into three subgroups, namely, lymphocytes, monocytes, and granulocytes. The CD 33 positive and CD 11b positive cells were defined neutrophils. The number of CD54highCD181low neutrophils were measured by FCM assay. FITC-CD181, APC-CD11b, PE-CD54, and Cy7-CD33 monoclonal antibodies were purchased from BD Biosciences, USA. Data acquisition and analysis were performed using a FACS-Calibur flow cytometer (BD Biosciences, USA) and Cell Quest software (Becton Dickinson, version 3.1).
CD54highCD181low Neutrophils and CD8+ Cells Isolation
CD8 positive T cells were purified using CD8 MicroBeads isolation kit for human (No: 130-045-201, Miltenyl Biotec; Bergisch Gladbach, Germany) according manufacturer’s operating instructions. CD54highCD181low neutrophils were sorted using FITC-CD181, APC-CD11b, PE-CD54, and Cy7-CD33 monoclonal antibodies by FACS-Aria (BD Biosciences, USA)(purity >95%).
Coculture Experiments
CD54highCD181low neutrophils and CD8+ T cells were coculture activating anti-CD2, -CD3, and -CD28 bead-coupled antibodies (Miltenyi Biotec, Bergisch Gladbach, Germany). The concentration of CD54highCD181low neutrophils is 0,1×105/ml and 2×105/ml. The concentration of CD8+ T cells is 5×104/ml.
T-Cell Proliferation Assay
Proliferation of CD8+ T cells was assessed by Cell counting KIT-8 assay (CCK-8, Beyotime Biotechnology, China) and compared with stimulated T cells alone (set as 100% T-cell proliferation). CD8+ T cells were seeded on a 96-well plate at a density of 1 × 104 with DMEM supplemented and 10% FBS. CD54highCD181low neutrophils were added in experimental groups, and not in control group. After 3 days, 10 µl CCK-8 solution was added for an hour and its absorbance was measured with a microplate reader (Biotek Instruments Inc, Winooski, USA) at a wavelength of 450 nm.
Lactate Dehydrogenase Measure
After coculture, the supernatants were collected and the levels of lactate dehydrogenase (LDH) in supernatant were detected by Roche Biochemistry Analyzer (Roche, Switzerland).
Perforin and Granzyme With Flow Cytometric Analysis
Perforin and granzyme secreted by CD8+ T cells were measured using perforin-PE and granzyme-APC monoantibody (BD Biosciences, USA) by FACS-Calibur flow cytometer (BD Biosciences, USA). Briefly, after coculture, the cells were collected and labeled with CD8-FITC (BD Biosciences, USA). After cell fixation and permeabilization, perforin-PE and granzyme-B-APC were added.
PD1 With Flow Cytometric Analysis
PD1 on CD8+ T cells were measured using PD1-APC monoantibody (BD Biosciences, USA) by FACS-Calibur flow cytometer (BD Biosciences, USA). Briefly, after coculture, the cells were collected and labeled with CD8-FITC (BD Biosciences, USA) and PD1-APC monoantibody.
Statistical Analysis
Result analysis was performed with the GraphPad Prism 8.0 program (GraphPad Software, Inc. San Diego, CA). Data obeyed normal distribution were presented as means ± SD and multiple group comparisons were performed by using one-way analysis of variance (ANOVA). The analysis of correlation was performed by linear regression. The survival analysis was performed by Log-Rank test. The ratio of transformation to AML and infection were performed by Fisher’s exact test. The index changes before and after treatment were performed by paired t test. A P value of <0.05 was considered statistically significant.
Results
Circulating CD54highCD181low Neutrophils Increased in Untreated MDS and Correlated With High Risk According to IPSS-R
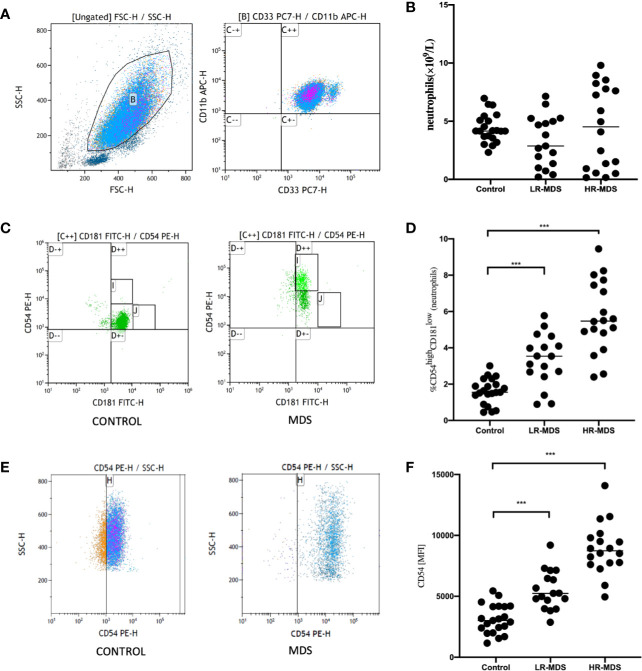
Frequency of CD54highCD181low neutrophils among peripheral blood were significantly increased in MDS patients (3.33 ± 1.42% in LR-MDS and 5.71 ± 1.97% in HR-MDS) as compared with healthy controls (1.61 ± 0.70%) (Figures 1C, D). The mean fluorescence index (MFI) levels of CD54 on the CD33+CD11b+ neutrophils were significantly increased in MDS patients (5510 ± 1590 in LR-MDS and 8906 ± 2103 in HR-MDS) as compared with healthy controls (3182 ± 1187) (Figures 1E, F). But the quantity of neutrophils among peripheral blood were not significantly different among MDS patients and healthy controls (4.49 ± 3.55×109/L, 3.29 ± 2.19×109/L and 4.42 ± 1.25×109/L, respectively) (Figures 1A, B). Therefore, the increase of CD54highCD181low neutrophils is not due to granulocytosis.
Figure 1.
Increased frequency of CD54highCD181low neutrophils cells in the peripheral blood of new diagnosis myelodysplastic syndromes (MDS) patients. (A, B) Frequency of peripheral blood neutrophils were compared between 23 healthy controls (HC) and 37 new diagnosis MDS patients. FSC and SSC were used to gate neutrophils. CD33 positive and CD11b positive were used to confirm neutrophils (C++). (C, D) Representative dot plots from flow cytometric (FACS) analyses showing the CD54highCD181low cell frequency among peripheral blood neutrophils obtained from HC (n = 23) and MDS patients (n = 37) (I represents CD54highCD181low neutrophils). (E, F) Mean fluorescence index (MFI) levels of CD54 on the CD33 and CD11b neutrophils was compared between HC (n = 23) and MDS patients (n = 37). The bars represent the standard error of the mean. ***P < 0.001.
CD54highCD181low Neutrophils From MDS Patients Suppress T Cells Functions
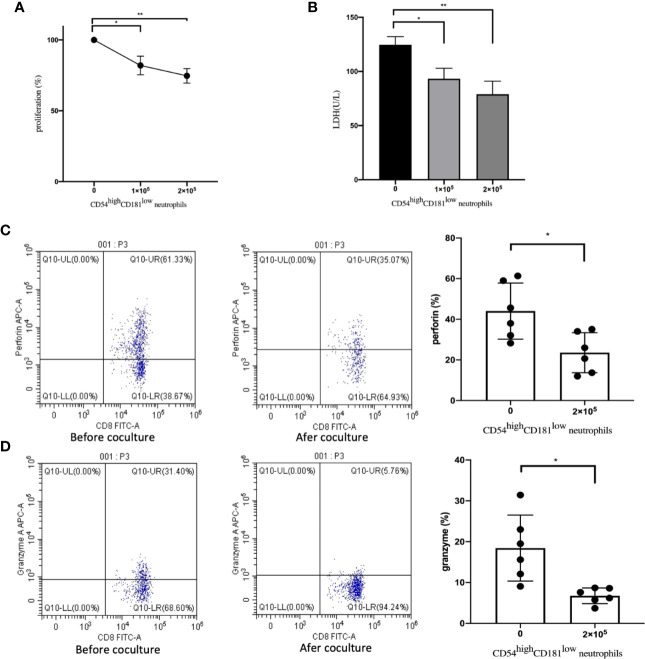
In the following experiment, we sorted CD54highCD181low neutrophils and CD8+ T cells from MDS patients using FACS and CD8 MicroBeads. In order to prove the in vitro suppressive capacity of CD54highCD181low neutrophils on T cell, proliferation of sorted T cells was measured after bead stimulated. It was found that CD54highCD181low neutrophils cells suppressed T cell proliferation significantly with in a dose-dependent manner (Figure 2A). The levels of lactate dehydrogenase (LDH) in supernatant of coculture experiments with CD3+ T cells and effector cells decreased significantly when CD54highCD181low neutrophils was added (Figure 2B). The level of perforin and granzyme secreted by T cells were decreased significantly while co-culturing with CD54highCD181low neutrophils (44.04 ± 5.64 vs. 23.58 ± 4.03 and 18.45 ± 3.30 vs. 6.76 ± 0.79) (Figures 2C, D).
Figure 2.
Impact of CD54highCD181low neutrophils from myelodysplastic syndromes (MDS) patients on T cells. (A) The dose-dependent suppressive activity of FACS-sorted CD54highCD181low neutrophils from MDS patients (n=6) was evaluated in coculture experiments with autologous T cells activated by means of anti-CD2, -CD3, and -CD28 microbeads. Proliferation of T cells was assessed after 5 days by CCK-8 assay and compared with stimulated T cells alone (set as 100% T-cell proliferation). (B) The levels of lactate dehydrogenase (LDH) in supernatant of coculture experiments with CD54highCD181low neutrophils, CD3+ T cells and effector cells. (C) The perforin of CD3+CD8+ T cells before and after coculture with CD54highCD181low neutrophils from the peripheral blood of MDS patients. (D) The granzyme B of CD3+CD8+ T cells before and after coculture with CD54highCD181low neutrophils from the peripheral blood of MDS patients. The bars represent the standard error of the mean. *P < 0.05; **P < 0.01.
CD54highCD181low Neutrophils Lead to Upregulation of PD1 Expression on CD8+ T Cells
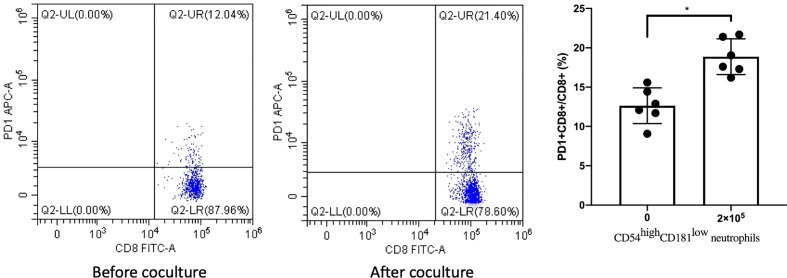
Next, we measured the levels of PD1 on CD8+T cells using FACS before and after co-culturing with CD54highCD181low neutrophils. We found that the expression of PD1 on CD8+T cells from MDS patients was increased significantly after co-culturing with CD54highCD181low neutrophils (12.63 ± 2.28 vs. 18.87 ± 2.31) (Figure 3).
Figure 3.
CD54highCD181low neutrophils from myelodysplastic syndromes (MDS) patients (n = 6) lead to upregulation of PD1 expression on CD8+ T cells. The bars represent the standard error of the mean. *P < 0.05.
Higher CD54highCD181low Neutrophils Related With Poor Prognosis and More Infection
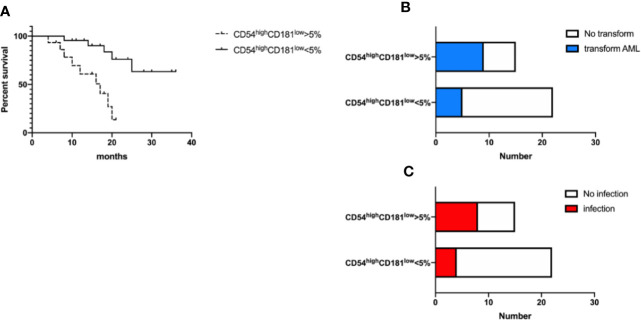
In order to verify the impact of CD54highCD181low neutrophils on survival, acute myeloid leukemia transformation and infection, we divided MDS patients into two groups based on whether CD54highCD181low neutrophils were greater than 5%, a high-proportion group (n=15) and a low-proportion group (n=22). The median follow-up time was 17 months (range 4–36 months). The median overall survival of MDS patients with more than 5 percent CD54highCD181low neutrophils was 17 months. The median overall survival of MDS patients with less than 5 percent CD54highCD181low neutrophils was not reach during the follow-up time. The survival of higher CD54highCD181low neutrophils MDS patients was shorter than that of lower CD54highCD181low neutrophils patients (P<0.05) (Figure 4A).
Figure 4.
CD54highCD181low neutrophils are related to the prognosis and infection of myelodysplastic syndromes (MDS) patients. (A) The frequency of CD54highCD181low neutrophils is related with overall survival of MDS patients. P < 0.05 (B) The ratio of acute myeloid leukemia transformation was higher in higher frequency of CD54highCD181low neutrophils group. P<0.05 (C) The incidence of infection was higher in higher frequency of CD54highCD181low neutrophils group at diagnosis time. P < 0.05.
Nine patients (60%) transformed to acute myeloid leukemia in higher CD54highCD181low neutrophils MDS group during following-up period, while only five patients (22.7%) transformed in lower group (P<0.05) (Figure 4B). Transformed acute myeloid leukemia included five acute monocytic leukemia (M5) cases, three acute myelomonocytic leukemia (M4) cases and one pure erythroid leukemia (M6) case in higher group, and three M5 cases and two M4 cases in lower group.
Eight patients (53.3%) had infection at the time of new diagnosis in higher CD54highCD181low neutrophils MDS group, but only four patients (18.2%) had infection in lower group (Figure 4C). The most common sites of infection are the lungs (6 cases), upper respiratory tract (two cases), skin (one case), mouth (one case), perianal (one case), and blood (one case).
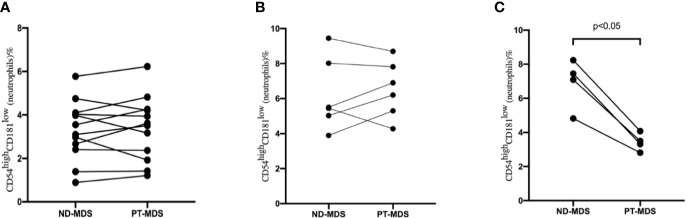
The Frequency of CD54highCD181low Neutrophils Decreased in High Risk MDS Patients Who Had Response
Among the 37 MDS patients, 22 patients had the data of CD54highCD181low neutrophils at new diagnosis and after treatment, including 12 in the low-risk group and 10 in the high-risk group. The median interval time is 3 months (range 1–6 months). The frequency of CD54highCD181low neutrophils in the relatively low-risk group did not change significantly before and after treatment (P>0.05) (Figure 5A). But the frequency of CD54highCD181low neutrophils in the high-risk group who had good responses (complete response or partial response) after treatment was decreased significantly (P<0.05) (Figure 5B). However, the frequency of CD54highCD181low neutrophils did not change significantly in patients with high-risk MDS who did not respond to treatment (P>0.05) (Figure 5C).
Figure 5.
The change of CD54highCD181low neutrophils from myelodysplastic syndromes (MDS) patients at new diagnosis (ND) and post-therapy (PT). (A) Low risk MDS patient (n = 12). P > 0.05. (B) high risk MDS patients without good response (CR+PR) (N = 6). P > 0.05. (C) high risk MDS patients with good response (CR+PR) (N = 4). P < 0.05.
Discussion
MDS is a clonal hematopoietic abnormality disease. Most MDS patients have aberrant neutrophil development. Aberrantly developed neutrophils not only have abnormal quantity, but can also lead to abnormal quality. The abnormality of neutrophils not only leads to an increased chance of infection, but may also affect the immune function of patients. In the past decade, MDS clone leading to abnormal pro-inflammatory signaling and innate immune activation in bone marrow microenvironment were identified as key pathogenic driver factors (7, 8).
Recent evidences indicate that tumors regulate neutrophil function to support tumor growth and development. Tumor associated neutrophils are an important part of the tumor microenvironment and play an active role in tumor occurrence, progression and metastasis (9).
Here, we found that circulating CD54highCD181low neutrophils increased in untreated MDS and correlated with high risk according to IPSS-R. CD54, also known as ICAM -1, is a transmembrane glycoprotein of the immunoglobulin superfamily, involved in tumor cell immune regulation, angiogenesis, invasion and distant metastasis. Some studies have proved that CD54 is abnormally highly expressed in a variety of malignant tumors, which promotes the occurrence and development of tumors and affects their prognosis. Several studies have shown that CD54 is highly expressed in CD34+ cells and CD8+ lymphocytes in MDS patients, and the soluble CD54 level in plasma of MDS is also significantly increased, which is an important indicator of cytopenia, dysplasia, and disease progression to AML (10–14).
CD181, also known as CXCR1, is mainly expressed on the surface of neutrophils in the normal body, when foreign pathogens enter invasion, causing inflammation in the tissues, the inflammatory tissue releases IL-8 and other chemokines, and binds to CD181 on the surface of neutrophils through blood circulation, thereby migrating neutrophils to the site of inflammation to engulf and kill pathogens (15). Fuhler et al. have demonstrate decreased CD181-induced neutrophil chemotaxis in MDS patients (16).
We found that circulating CD54highCD181low neutrophils from MDS patients suppress T cells functions. Our previous study has shown that myeloid-derived suppressor cells (MDSCs) were increased in MDS patients, and related poor clinical outcome. MDSCs could suppress T-cell mediated immune functions (17, 18). MDSCs are a group of immature myeloid cells derived from bone marrow, including monocytic and granulocytic MDSCs. Recently, a few studies have demonstrated that mature neutrophils could exert MDSC activity suppressing T cells functions (19, 20). Our research indicated that not only immature myeloid cells can inhibit T lymphocytes, but mature granulocytes CD54highCD181low neutrophils can also inhibit T lymphocytes. Only activated neutrophils with ROS can suppress T-cell functions. CD54highCD181low neutrophils can produce more ROS and have a longer lifespan. So CD54highCD181low neutrophils could exert MDSC suppressive activity about T cells. Silzle et al. (21) reported that lymphopenia was associated with increased mortality in patients with MDS, and confirmed that innate and adaptive immune system changed in MDS patients. The mechanism of impaired lymphatic homeostasis balance included MDS clonal cell, bone marrow microenvironments and genetic mutations.
Our results showed that CD54highCD181low neutrophils lead to upregulation of PD1 expression on CD8+ T cells. PD1, the full name is programmed cell death protein 1, is majorly expressed on active T lymphocytes, and plays an essential role in balancing immune tolerance, protective immunity and homeostasis. In patients with chronic infection or tumors, PD1 over-express can inhibit T cell effector functions, promote T cell exhaustion and contribute to adaptive resistance. Tumor patients often have physiological stress, for instance, hypoxia and nutrient deprivation. Cancer cells can hijack immune suppression (22). We speculate, in patients with MDS, due to the persistent presence of hypoxia and an inflammatory state, CD54highCD181low neutrophils promote over-expression of PD1 on CD8+ T cells and inhibit anti-tumor immunity of CD8+ T cells.
Does elevated CD54highCD181low neutrophils affect overall survival and acute leukemia transformation? We found that the overall survival time of patients with higher CD54highCD181low neutrophils group was significantly shorter than that of patients with lower proportion. This shows that a high proportion of CD54highCD181low neutrophils is a factor of poor prognosis. The rate of transformation to acute leukemia was also significantly higher in the high proportion of CD54highCD181low neutrophils group than in the low-proportion group. Of course, most patients in the high proportion of CD54highCD181low neutrophils group are patients in the relatively high-risk group, and this part of the patients themselves has a poor prognosis. But generally speaking, the high proportion of neutrophils indicates that patients have a higher chance of acute leukemia transformation and a shorter expected survival. AML transformation, infection and bleeding are the main causes of death in MDS patients. The degree of neutropenia was significantly correlated with mortality (23). The relationship between different phenotypes of neutrophils and prognosis needs to be further studied (24, 25). We also found that the risk of infection in the group with a high proportion of CD54highCD181low neutrophils was higher at the new diagnosis time. Infection is the leading cause of death in MDS patients, which mainly attributed to decreased counts and qualitative defects of neutrophil (26–28).
In order to study the effect of CD54highCD181low neutrophils on the therapeutic effect, we examined the changes of neutrophils before and after treatment. We found that for patients in the low proportion group, the frequency of CD54highCD181low neutrophils did not change significantly before and after treatment. This may be related to the fact that the main clinical manifestation of patients in the relatively low-risk group is anemia. Blood transfusion and rhEPO therapy were used had little effect on neutrophils. The frequency of CD54highCD181low neutrophils decreased in patients who had achieved good response after decitabine treatment. But the frequency of CD54highCD181low neutrophils did not change significantly in patients who had no good response. This also indirectly proves that high CD54highCD181low neutrophils are related to poor prognosis.
In conclusion, we found that CD54highCD181low neutrophils increased in peripheral blood of MDS patients, and CD54highCD181low neutrophils could suppress CD8+ T cells functions and upregulate the PD1 expression on CD8+ T cells. CD54highCD181low neutrophils affect the prognosis and the chance of infection. We conclude CD54highCD181low neutrophils may be involved in MDS pathogenesis and targeting strategies offer potential therapy for MDS.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by The ethics committee of General Hospital Tianjin Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LY, HL, and YL performed research and analyzed the data. HW design studies, ensure the correct analysis of the data and drafted the manuscript. XX, HZ, HN, LX, and ZS assisted in design research, oversaw data collection, and contributed to the writing of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This project is partly supported by The National Natural Science Foundation of China (No. 81170472), Key Technology Research and Development Program of Tianjin China (18ZXDBSY00140), Application Bases and Advanced Technology Research Program of Tianjin China (No. 14JCYBJC27200, 09JCYBJC11200).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.585216/full#supplementary-material
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1. Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood (2016) 127:2391–405. 10.1182/blood-2016-03-643544 [DOI] [PubMed] [Google Scholar]
- 2. Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med (2009) 361:1872–85. 10.1056/NEJMra0902908 [DOI] [PubMed] [Google Scholar]
- 3. Adès L, Itzykson R, Fenaux P. Myelodysplastic syndromes. Lancet (2014) 383:2239–52. 10.1016/S0140-6736(13)61901-7 [DOI] [PubMed] [Google Scholar]
- 4. Silvestre-Roig C, Hidalgo A, Soehnlein O. Neutrophil heterogeneity: implications for homeostasis and pathogenesis. Blood (2016) 127:2173–81. 10.1182/blood-2016-01-688887 [DOI] [PubMed] [Google Scholar]
- 5. Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol (2012) 30:459–89. 10.1146/annurev-immunol-020711-074942 [DOI] [PubMed] [Google Scholar]
- 6. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol (2013) 13:159–75. 10.1038/nri3399 [DOI] [PubMed] [Google Scholar]
- 7. Basiorka AA, McGraw KL, Eksioglu EA, Chen X, Johnson J, Zhang L, et al. The NLRP3 inflammasome functions as a driver of the myelodysplastic syndrome phenotype. Blood (2016) 128:2960–75. 10.1182/blood-2016-07-730556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sallman DA, List A. The central role of inflammatory signaling in the pathogenesis of myelodysplastic syndromes. Blood (2019) 133:1039–48. 10.1182/blood-2018-10-844654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keeley T, Costanzo-Garvey DL, Cook LM. Unmasking the Many Faces of Tumor-Associated Neutrophils and Macrophages: Considerations for Targeting Innate Immune Cells in Cancer. Trends Cancer (2019) 5:789–98. 10.1016/j.trecan.2019.10.013 [DOI] [PubMed] [Google Scholar]
- 10. Buccisano F, Maurillo L, Tamburini A, Del Poeta G, Del Principe MI, Ammatuna E, et al. Evaluation of the prognostic relevance of L-selectin and ICAM1 expression in myelodysplastic syndromes. Eur J Haematol (2008) 80:107–14. 10.1111/j.1600-0609.2007.00986.x [DOI] [PubMed] [Google Scholar]
- 11. Passam FH, Tsirakis G, Boula A, Fragou A, Consolas I, Alegakis A, et al. Levels of soluble forms of ICAM and VCAM in patients with myelodysplastic syndromes and their prognostic significance. Clin Lab Haematol (2004) 26:391–5. 10.1111/j.1365-2257.2004.00640.x [DOI] [PubMed] [Google Scholar]
- 12. Alfinito F, Sica M, Luciano L, Della Pepa R, Palladino C, Ferrara I, et al. Immune dysregulation and dyserythropoiesis in the myelodysplastic syndromes. Br J Haematol (2010) 148:90–8. 10.1111/j.1365-2141.2009.07921.x [DOI] [PubMed] [Google Scholar]
- 13. Maio M, Pinto A, Carbone A, Zagonel V, Gloghini A, Marotta G, et al. Differential expression of CD54/intercellular adhesion molecule-1 in myeloid leukemias and in lymphoproliferative disorders. Blood (1990) 76:783–90. 10.1182/blood.V76.4.783.783 [DOI] [PubMed] [Google Scholar]
- 14. Südhoff T, Germing U, Aul C. Levels of circulating endothelial adhesion molecules in patients with myelodysplastic syndromes. Int J Oncol (2002) 20:167–72. 10.3892/ijo.20.1.167 [DOI] [PubMed] [Google Scholar]
- 15. Teijeira Á, Garasa S, Gato M, Alfaro C, Migueliz I, Cirella A, et al. CXCR1 and CXCR2 Chemokine Receptor Agonists Produced by Tumors Induce Neutrophil Extracellular Traps that Interfere with Immune Cytotoxicity. Immunity (2020) 52:856–71.e8. 10.1016/j.immuni.2020.03.001 [DOI] [PubMed] [Google Scholar]
- 16. Fuhler GM, Knol GJ, Drayer AL, Vellenga E. Impaired interleukin-8- and GROalpha-induced phosphorylation of extracellular signal-regulated kinase result in decreased migration of neutrophils from patients with myelodysplasia. J Leukoc Biol (2005) 77:257–66. 10.1189/jlb.0504306 [DOI] [PubMed] [Google Scholar]
- 17. Jiang H, Fu R, Wang H, Li L, Qu W, Liang Y, et al. Increased circulating of myeloid-derived suppressor cells in myelodysplastic syndrome. Chin Med J (Engl) (2013) 126:2582–4. 10.3760/cma.j.issn.0366-6999.20121794 [DOI] [PubMed] [Google Scholar]
- 18. Chen X, Eksioglu EA, Zhou J, Zhang L, Djeu J, Fortenbery N, et al. Induction of myelodysplasia by myeloid-derived suppressor cells. J Clin Invest (2013) 123:4595–611. 10.1172/JCI67580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aarts CEM, Hiemstra IH, Béguin EP, Hoogendijk AJ, Bouchmal S, van Houdt M, et al. Activated neutrophils exert myeloid-derived suppressor cell activity damaging T cells beyond repair. Blood Adv (2019) 3:3562–74. 10.1182/bloodadvances.2019031609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aarts CEM, Hiemstra IH, Tool ATJ, van den Berg TK, Mul E, van Bruggen R, et al. Neutrophils as Suppressors of T Cell Proliferation: Does Age Matter? Front Immunol (2019) 10:2144. 10.3389/fimmu.2019.02144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Silzle T, Blum S, Schuler E, Kaivers J, Rudelius M, Hildebrandt B, et al. Lymphopenia at diagnosis is highly prevalent in myelodysplastic syndromes and has an independent negative prognostic value in IPSS-R-low-risk patients. Blood Cancer J (2019) 9:63. 10.1038/s41408-019-0223-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol (2018) 18:153–67. 10.1038/nri.2017.108 [DOI] [PubMed] [Google Scholar]
- 23. Nachtkamp K, Stark R, Strupp C, Kündgen A, Giagounidis A, Aul C, et al. Causes of death in 2877 patients with myelodysplastic syndromes. Ann Hematol (2016) 95:937–44. 10.1007/s00277-016-2649-3 [DOI] [PubMed] [Google Scholar]
- 24. Schuster M, Moeller M, Bornemann L, Bessen C, Sobczak C, Schmitz S, et al. Surveillance of Myelodysplastic Syndrome via Migration Analyses of Blood Neutrophils: A Potential Prognostic Tool. J Immunol Baltim Md 1950 (2018) 201:3546–57. 10.4049/jimmunol.1801071 [DOI] [PubMed] [Google Scholar]
- 25. Schmidt CS, Aranda Lopez P, Dopheide JF, Schmidt F, Theobald M, Schild H, et al. Phenotypic and functional characterization of neutrophils and monocytes from patients with myelodysplastic syndrome by flow cytometry. Cell Immunol (2016) 308:19–26. 10.1016/j.cellimm.2016.07.005 [DOI] [PubMed] [Google Scholar]
- 26. Toma A, Fenaux P, Dreyfus F, Cordonnier C. Infections in myelodysplastic syndromes. Haematologica (2012) 97:1459–70. 10.3324/haematol.2012.063420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dayyani F, Conley AP, Strom SS, Stevenson W, Cortes JE, Borthakur G, et al. Cause of death in patients with lower-risk myelodysplastic syndrome. Cancer (2010) 116:2174–9. 10.1002/cncr.24984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goldberg SL, Chen E, Corral M, Guo A, Mody-Patel N, Pecora AL, et al. Incidence and clinical complications of myelodysplastic syndromes among United States Medicare beneficiaries. J Clin Oncol (2010) 28:2847–52. 10.1200/JCO.2009.25.2395 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.