Abstract
Background
We aimed to describe changes in characteristics and treatment strategies of hospitalised patients with COVID-19 and detail the mortality trend over time.
Methods
Observational cohort study of all consecutive patients admitted ≥ 48 h to Hospital Clinic of Barcelona for COVID-19 (1 March–30 September 2020).
Findings
A total of 1645 consecutive patients with COVID-19 were assessed over a 7-month period. Overall mortality (≤30 days) was 9.7% (159 patients), 7.7% in patients hospitalised in regular wards and 16.7 % in patients requiring ICU admission. Overall mortality decreased from 11.6% in the first month to 1.4% in the last month, reflecting a progressive, significant downward trend (p for trend <0.001). Patients’ age changed over time, peaking in June. Most changes in the use of antivirals and anti-inflammatory treatments were documented. Age (OR 1.1, CI 1.1–1.12), chronic heart disease, (OR 1.7, CI 1.1–2.9), D-dimer>700 ng/mL (OR 2.3, CI 1.3–4.1), ferritin>489 ng/mL (OR 1.9; CI 1.5–3.2), C-RP>7 mg/dL (OR 2.6; CI 1.5–4.6), and shorter duration from symptom onset to hospital admission (OR 1.11; CI 1.04–1.17) were factors associated with 30-day mortality at hospital admission. Conversely, hospital admission in the last months (OR 0.80; CI 0.65–0.98) was significantly associated with lower mortality.
Interpretation
In-hospital mortality has decreased in patients with COVID-19 over the last, few months, even though main patient characteristics remain similar. Several changes made when managing patients may explain this decreasing trend. Our study provides current data on mortality of patients hospitalised with COVID-19 that might be useful in establishing quality of standard of care.
Funding
EIT Health, European Union´s Horizon 2020 Research and Innovation Programme), EDRD. PPA [CM18/00132], NGP [FI19/00133], and CGV [FIS PI18/01061], have received grants from Ministerio de Sanidad y Consumo, ISCIII.
Keywords: COVID-19, ICU admission, Outcomes, Mortality
Abstract
Contexto
Nuestro objetivo es describir los cambios en las características y las estrategias de tratamiento de los pacientes hospitalizados por COVID-19, y detallar la tendencia de la mortalidad en el tiempo.
Métodos
Estudio observacional de cohortes de todos los pacientes consecutivos, ingresados por COVID-19 durante más de 48 horas, en el Hospital Clínic de Barcelona (del 1 de marzo al 30 de septiembre de 2020).
Resultados
Un total de 1645 pacientes consecutivos fueron evaluados durante un período de 7 meses. La mortalidad global (≤30 días) fue del 9.7% (159 pacientes): 7.7% en pacientes hospitalizados en salas convencionales, y 16.7% en pacientes que requirieron ingreso en UCI. La mortalidad global disminuyó del 11.6% en el primer mes al 1.4% en el último mes evaluado, reflejando una progresiva y significativa tendencia a la baja (p para la tendencia <0.001). La edad de los pacientes ha cambiado con el tiempo, habiendo alcanzado su pico en junio. La mayoría de cambios en el uso de antivirales y antiinflamatorios se han documentado. La edad (OR 1.1; CI 1.1–1.12), cardiopatía crónica (OR 1.7; CI 1.1–2.9), dímero-D>700 ng/mL (OR 2.3; CI 1.3–4.1), ferritina>489 ng/mL (OR 1.9; CI 1.5–3.2), PCR>7 mg/dL (OR 2.6; CI 1.5–4.6), y una menor duración desde el inicio de síntomas a la hospitalización (OR 1.11; CI 1.04–1.17) fueron factores asociados a la mortalidad intrahospitalaria a 30 días. Por el contrario, el ingreso hospitalario previo en los últimos meses (OR 0.80; CI 0.65–0.98) se asoció significativamente a una menor mortalidad.
Discusión
La mortalidad intrahospitalaria ha disminuido en los pacientes con COVID-19 durante los últimos meses, incluso siendo similares las características de los pacientes. Algunos cambios realizados en el manejo de estos pacientes podrían explicar esta tendencia decreciente. Nuestro estudio aporta datos actualizados en la mortalidad de los pacientes hospitalizados con COVID-19, que podrían ser útiles de cara a establecer unos cuidados estándar de calidad.
Financiación
EIT Health, European Union´s Horizon 2020 Research and Innovation Programme, EDRD. PPA [CM18/00132], NGP [FI19/00133] y CGV [FIS PI18/01061], han recibido becas del Ministerio de Sanidad y Consumo, ISCIII.
Research in context.
Evidence before this study
We searched PubMed for articles that documented risk factors for ICU admission and mortality in patients with COVID-19, as well as treatment options. We used the search terms (“SARS-CoV-2” OR “COVID-19”) AND (“death” OR “mortality” OR “ICU” OR “treatment” OR “management”), with no language or time restrictions. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become the primary cause of global mortality due to infectious diseases in the world. Overall mortality in cohorts of patients hospitalised with coronavirus disease 2019 (COVID-19) ranged from 28% to 39%. However, there is a lack of clinical studies describing changes over months in COVID-19 management and their impact on mortality trends in real-life patients with COVID-19.
Added value of this study
This is the first study to describe how in-hospital mortality in patients with COVID-19 has decreased over months, even though main patient characteristics remain similar.
Implications of all the available evidence
We detail several changes made when managing patients with COVID-19 that may explain the decreasing in-hospital mortality trend. Our study provides current data on mortality for patients hospitalised with COVID-19 that might prove useful in establishing quality of standard of care.
Alt-text: Unlabelled box
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has emerged as a leading cause of death due to infectious diseases. In December 2019, an outbreak of coronavirus disease 2019 (COVID-19) occurred, reaching Spain by the end of February. This infection has been a major challenge for both health care professionals and health systems, with high morbidity and mortality [[1], [2], [3], [4], [5]].
However, our understanding of COVID-19 has rapidly improved. Antiviral treatment options have been better defined and the use of anti-inflammatory therapies and personalised approaches has shown to improve outcomes [6], [7], [8]. Yet, there is a lack of clinical studies describing changes over months in COVID-19 patients’ characteristics and management and their impact on mortality trends in real-life. Such descriptions of mortality rates in current patients with COVID-19 are mandatory, should we aim to place into perspective results obtained from different studies, including trials, that have been carried out during different moments of the pandemic. Further, knowledge of current mortality rates and patient characteristics may serve as references for establishing quality of care.
Our study aimed to provide current data on updated mortality for patients hospitalised with COVID-19. The study also aimed to describe changes over time in characteristics and treatment strategies pertaining to patients with COVID-19 and define risk factors for mortality at hospital admission.
2. Methods
2.1. Study design and patients
This observational cohort study was performed at Hospital Clinic in Barcelona (Spain), a 700-bed university centre that provides broad and specialised medical, surgical, and intensive care for an urban population of 500,000 adults (>18 years old). All patients admitted for COVID-19 for ≥48 h between 1 March and 30 September 2020, were included. Clinical outcomes were monitored until 1 November 2020. All patients had a COVID-19 diagnosis confirmed by real-time reverse transcription polymerase chain reaction (RT-PCR) testing performed on nasopharyngeal throat swab specimens, and/or by fulfilling clinical diagnostic criteria provided during the pandemic peak for SARS-CoV-2 (March 2020 to May 2020). These criteria comprised the presence of any of the following respiratory symptoms, including sore throat, congestion, cough, dyspnoea, new loss of taste and/or smell, as well as uni- or bilateral interstitial infiltrates in chest X-rays. Early mortality was defined as death within the first 5 days of admission. The Institutional Ethics Committee of Hospital Clinic of Barcelona approved the study and due to the nature of the retrospective data review, waived the need for informed consent from individual patients (HCB/2020/0273).
2.2. Data collection and outcomes
For all patients hospitalised with COVID-19, data concerning demographics (age, gender), epidemiology, comorbidities, laboratory tests, microbiological results, treatment, and outcomes were collected directly from electronic health records (EHR). An intelligent system was used to retrieve the high-quality data from EHRs (SILDv1.0 system, S34M@) as described elsewhere [9].
2.3. Statistical analysis
Data are presented as percentages and numbers, means with SDs, medians and interquartile ranges (IQRs), or proportions and 95% CIs. Accordingly, the chi-squared test for equal proportion, t test, and Mann–Whitney U test were used to examine differences. To reduce the variability and noise of random in day-by-day data, we divided the study duration into month-defined timespans, setting March 2020 as the reference period.
To assess factors related with 30-day mortality at hospital admission, a multivariate regression model (step-forward procedure) was constructed using all variables significantly associated with mortality in univariate analyses. These variables included age, month of hospital admission, chronic heart disease, diabetes mellitus, haematological diseases, chronic kidney disease, hypertension, solid cancer, chronic lung disease, a respiratory rate higher than 20, oxygen saturation < 94%, D-dimer levels higher than 700 ng/mL, a lymphocyte count lower than 0.7 (109/L), LDH levels higher than 330 U/L, ferritin levels higher than 489 ng/mL, C-RP higher than 7 mg/dL, and days from symptom onset. Cut-off values were selected after analysing medians for each variable in patients who died, compared with those who survived. Laboratory markers were obtained at COVID-19 diagnosis. A second multivariate analysis was also performed with analytics as a continuous value. Adequacy of the models were assessed with the Hosmer–Lemeshow goodness-of-fit test and the area under the receiver operating characteristic curve was used to measure the predictive ability of the model. Potential confounders were investigated. Significance was set at a p-value of <0.05. Statistical analyses were performed with Microsoft SPSS-PC+, version 23.0 (SPSS, Chicago, IL, USA).
2.4. Role of the funding source
No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
3. Results
3.1. Mortality trends
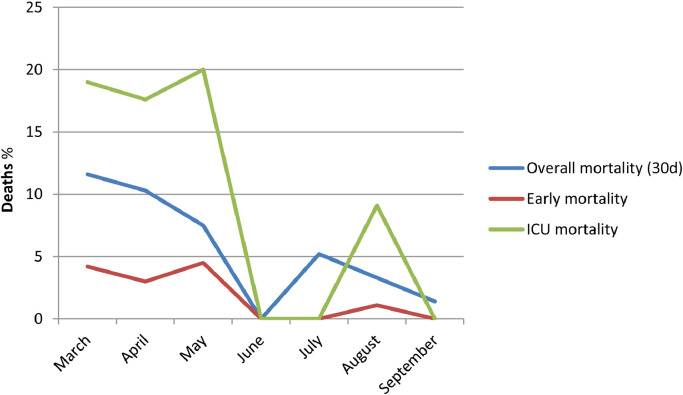
We assessed 1645 consecutive adults, 88.4% Caucasians, with COVID-19 at our hospital during the study period. Overall mortality (≤30 days) was 9.7% (159 patients), 7.7% in patients hospitalised in regular wards and 16.7 % in patients requiring ICU admission. Early mortality (5 days from admission) was 3.2% (53 patients). Furthermore, 60- and 90-day mortality were 10.8% (178 patients) and 11.4% (187 patients), respectively. Fig. 1 details the unadjusted overall mortality rates, with a reported 11.6% in the first month and 1.4% in the last month, and reflecting a progressive, significant downward trend (p for trend <0.001). Trends for ICU mortality (19.1% in the first month and 0% in the last month; p for trend 0.021) and early mortality (4.2% in the first month and 0% in the last month; p for trend 0.004) were also in decline. Table 1 details mortality by 10-year age intervals throughout the study period. Supplementary Tables 1 and 2 describe 60-day and 90-day mortality by age intervals.
Fig. 1.
Overall mortality trends for patients admitted with COVID-19 (distribution by months).
Table 1.
30-day mortality by 10-year age intervals throughout the study periods.
| 30-day mortality (%) | Period 1 March (n=810) | Period 2 April (n=504) | Period 3 May (n=67) | Period 4 June (n=22) | Period 5 July (n=77) | Period 6 August (n=91) | Period 7 September (n=74) | p |
|---|---|---|---|---|---|---|---|---|
| <40 y | 1/90 (1%) | 0/55 (0%) | 0/9 (0%) | 0/6 (0%) | 0/14 (0%) | 0/13 (0%) | 0/7 (0%) | 0•465 |
| 40–49 y | 1/105 (1%) | 0/62 (0%) | 0/7 (0%) | 0/0 (0%) | 0/18 (0%) | 0/12 (0%) | 0/17 (0%) | 0•474 |
| 50–59 y | 6/155 (4%) | 4/86 (5%) | 0/7(0%) | 0/4 (0%) | 0/17(0%) | 0/12(0%) | 0/19(0%) | 0•173 |
| 60–69 y | 9/180 (5%) | 8/90 (9%) | 0/14 (0%) | 0/2 (0%) | 0/8 (0%) | 0/25 (0%) | 1/11 (9%) | 0•482 |
| 70–79 y | 38/186 (20%) | 9/84 (11%) | 2/10 (20%) | 0/6 (0%) | 1/11 (9%) | 1/15 (7%) | 0/11 (0%) | 0•012 |
| 80–89 y | 31/80 (39%) | 22/94 (24%) | 2/11 (18%) | 0/3 (0%) | 1/5 (20%) | 2/12 (17%) | 0/8 (0%) | 0•005 |
| >90 y | 8/14 (57%) | 9/33 (27%) | 1/9 (11%) | 0/1 (0%) | 2/4 (50%) | 0/2 (0%) | 0/1 (0%) | 0•133 |
| All patients | 94/810 (11•6%) | 52/504 (10•3%) | 5/67 (7•5%) | 0/22 (0%) | 4/77 (5•2%) | 3/91 (3•3%) | 1/74 (1•4%) | <0•001 |
3.2. Changes over time in patient characteristics and COVID-19 management
Table 2 details the main changes in epidemiologic characteristics throughout the study period. Mean age of patients changed significantly, peaking in June. Sex and comorbidity remained stable, as well as days from symptom onset to hospital admission. With respect to laboratory tests, ferritin and LDH levels gathered at admission were significantly higher in the final months whilst D-dimer levels were significantly higher within the first, few months of the pandemic. Further, lymphocyte count also significantly varied throughout the months, with the highest values recorded in June and July. Highest values registered for C-RP during hospital admission were in March and April. Regarding vital signs, variations in temperature were documented over time and no clear trend was observed. Oxygen saturation levels were at their poorest in the last, few months of the study.
Table 2.
Changes in clinical characteristics of 1645 consecutive adults with COVID-19, divided by study periods.
| Period 1 March (n=810) | Period 2 April (n=504) | Period 3 May (n=67) | Period 4 June (n=22) | Period 5 July (n=77) | Period 6 August (n=91) | Period 7 September (n=74) | p | |
|---|---|---|---|---|---|---|---|---|
| Patient characteristics | ||||||||
| Age-Median (IQR), in years | 63 (51–74) | 65 (53–81) | 67 (53–84) | 68 (33–79) | 55 (45–72) | 64•5 (50–72) | 59 (45–72) | 0•003 |
| Age > 65 years (%) | 48•5 | 52 | 55 | 57 | 32•5 | 50 | 32 | 0•018 |
| Sex male, n (%) | 60 | 54 | 49 | 48 | 58 | 53 | 61 | 0•404 |
| Comorbidities (%) | ||||||||
| Hypertension | 47 | 50 | 63 | 38 | 44 | 45 | 36•5 | 0•189 |
| Diabetes mellitus | 20 | 21 | 25 | 19 | 23 | 19 | 19 | 0•940 |
| Chronic heart disease | 24 | 3 | 46 | 33 | 18 | 26 | 19 | 0•775 |
| Chronic lung disease | 22 | 24 | 22 | 43 | 27 | 23 | 18 | 0•957 |
| Chronic liver disease | 6 | 5 | 6 | 0 | 5 | 3 | 1 | 0•076 |
| Chronic kidney disease | 12 | 14 | 24 | 9•5 | 8 | 12 | 7 | 0•340 |
| Solid cancer | 14 | 17 | 19 | 14 | 9 | 21 | 16 | 0•348 |
| Haematological diseases | 7 | 8 | 7•5 | 5 | 8 | 12 | 4 | 0•739 |
| Solid organ transplantation | 3 | 3 | 4•5 | 0 | 1 | 3 | 1 | 0•463 |
| HIV | 2 | 2 | 3 | 0 | 4 | 1 | 1 | 0•908 |
| Symptom onset to hospital admission – Median (IQR), in days | 7 (4–9) | 7 (7–10) | 7 (4•5–11) | 7 (6–14) | 6 (4–9) | 5•5 (4–9) | 7 (6–10) | 0•502 |
| Vital signs at admission; Median (IQR) | ||||||||
| Temperature – Median (°C) | 37•3 (36•8–38•2) | 36•9 (36•3–37•6) | 36•8 (36•2–37•45) | 37•2 (36•9–37,85) | 37•2 (36•475–38•125) | 37•35 (36•8–38•275) | 36•75 (36•425–37•975) | <0•001 |
| Respiratory rate – Median (rpm) | 20 (18–24) | 20 (18–24) | 20 (18–26•5) | 22 (18–31) | 20•5 (18–26) | 20 (19–25•5) | 23 (20–27•5) | 0•112 |
| Respiratory rate > 20 (%) | 55 | 59 | 43 | 37 | 62 | 61 | 66 | 0•106 |
| Oxygen saturationa – Median | 95 (93–96) | 95 (94–97) | 95 (93–98) | 95 (92–95•5) | 95 (94–96) | 94 (92–96) | 94•5 (92•25–96•75) | 0•028 |
| Oxygen saturationa < 94% (%) | 43 | 32 | 30 | 32 | 27 | 40 | 39 | 0•102 |
| Laboratory values at admission; Median (IQR) | ||||||||
| Ferritin (ng/mL) | 643 (286–1279) | 447 (246–1103) | 184 (123–482) | 296 (192•50–354) | 474•50 320–1044) | 808 (420•5–1361) | 704 (289–1356•50) | <0•001 |
| C-RP (mg/dL) | 8,22 (3•92–14•10) | 8•17 (4•02–15•45) | 4•69 (2•29–12•44) | 7•53 (4•48–13•41) | 6•16 (4•00–12•86) | 9•51 (5•965–16•875) | 8•355 (3•375–13•820) | 0•148 |
| D-dimer (ng/mL) | 800 (400–1300) | 900 (500–1750) | 1300 (650–4450) | 600 (500–1000) | 450 (375–925) | 500 (300–1750) | 600 (400–900) | <0•001 |
| LDH (U/L) | 327 (251•50–411•50) | 297 (239–384) | 255 (202–315) | 246 (209–292) | 323 (261–376) | 322•5 (268–379) | 295•5 (234–373•5) | <0•001 |
| Procalcitonin (ng/mL) | 0•12 (0•06–0•23) | 0•11 (0•05–0•24) | 0•17 (0•05–0•59) | 0•03 (0•015–0•075) | 0•085 (0•05–0•15) | 0•135 (0•055–0•23) | 0•08 (0•06–0•18) | 0•156 |
| Lymphocyte count | 0•8 (0•6–1•2) | 0•8 (0•6–1•1) | 0•9 (0•7–1•6) | 1•3 (0•95–1•5) | 1•1 (0•8–1•4) | 0•9 (0•5–1•3) | 0•9 (0•7–1•1) | <0•001 |
| C-RP at its highest during hospitalisation ≥ 15 (%) | 43 | 37 | 31 | 42 | 33 | 34 | 33 | 0•008 |
| C-RP at its highest during hospitalisation; median (IQR) | 13 (7–20) | 11 (6–19) | 11 (4–17) | 9•5 (3–20) | 10 (5–19) | 10 (6–17) | 10 (5–18) | 0•042 |
These measurements were taken on room air at time of admission.
Additionally, Table 3 details the main changes in treatments administered to patients throughout the study period. Antiviral approaches initially included hydroxychloroquine and lopinavir/ritonavir. However, use of such drugs decreased throughout the months and use of remdesivir became more prevalent. Time from symptom onset to remdesivir use decreased throughout the months. With respect to immunomodulatory approaches, some changes in corticosteroid use were documented, with dexamethasone progressively replacing methylprednisolone. The use of tocilizumab varied throughout the months, and the use of antibiotics significantly decreased. Supplementary table 3 details differences in treatments between ward and ICU patients.
Table 3.
Changes in treatments and outcomes of 1645 consecutive adults with COVID-19, divided by study periods.
| Period 1 March (n=810) | Period 2 April (n=504) | Period 3 May (n=67) | Period 4 June (n=22) | Period 5 July (n=77) | Period 6 August (n=91) | Period 7 September (n=74) | p | |
|---|---|---|---|---|---|---|---|---|
| Treatment | ||||||||
| Antiviral effect | ||||||||
| Remdesivir (%) | 7 | 3 | 3 | 19 | 62 | 53 | 36•5 | <0•001 |
| Symptom onset to initiating treatment with remdesivir (median, IQR) | 10 (7–12) | 10 (6•5–12) | 15 (8–19) | 6•5 (4–9) | 6 (4–8•5) | 8 (7•5–9) | <0•001 | |
| Lopinavir/ritonavir (%) | 92 | 69 | 42 | 9•5 | 1 | 0 | 0 | <0•001 |
| Hydroxychloroquine (%) | 96 | 85 | 51 | 0 | 0 | 1 | 0 | <0•001 |
| Anti-inflammatory effect | ||||||||
| Any anti-inflammatory treatment (%) | 56 | 57 | 43 | 33 | 57 | 48 | 50 | 0•069 |
| Tocilizumab (%) | 28•5 | 34 | 7•5 | 5 | 30 | 10 | 7 | <0•001 |
| Symptom onset to initiating treatment with tocilizumab (median, IQR) | 10 (7–12) | 9 (7–12) | 13 (11–13) | – | 9 (6–10) | 8 (6–11) | 13 (1–14) | 0•127 |
| C-RP median (IQR) at initiation of tocilizumab treatment | 13 (8–20) | 14 (9–20) | 13 (7–20) | – | 13 (8–18) | 14 (8–20) | 23 (15–27) | 0•762 |
| Any anti_IL-6*(%) | 32 | 34 | 7•5 | 5 | 30 | 10 | 7 | <0•001 |
| Anakinra (%) | 6 | 13•5 | 3 | 5 | 6•5 | 3 | 0 | 0•036 |
| Methylprednisolone (%) | 36 | 31 | 27 | 29 | 13 | 20 | 9•5 | <0•001 |
| Symptom onset to initiating treatment with methylprednisolone (median, IQR) | 10 (7–14) | 10 (6–13) | 8 (4–12) | 7 (7–7) | 14 (7–18) | 16 (5–24) | 10 (4–19) | 0•363 |
| C-RP median (IQR) at initiation of methylprednisolone treatment | 12 (7–19) | 11 (6–18) | 11 (5–14) | 10 (0–22) | 3 (1–5) | 3 (1–6) | 8 (4–12) | 0•002 |
| Dexamethasone (%) | 6 | 5 | 3 | 5 | 38 | 31 | 31 | <0•001 |
| Symptom onset to initiating treatment with dexamethasone (median, IQR) | 9 (6–13) | 11•5 (7•5–13) | – | – | 8 (5–10) | 8 (5–10) | 10 (8–13) | 0•009 |
| C-RP median (IQR) at initiation of dexamethasone treatment | 14 (9–22) | 13 (6–25) | – | – | 11 (5–17) | 14 (6–19) | 12 (2–23) | 0•521 |
| Prednisone (%) | 22 | 24 | 28 | 29 | 17 | 24 | 19 | 0•834 |
| Oxygen therapy | ||||||||
| Any oxygen support (%) | 28 (229/810) | 42 (211/504) | 42 (28/67) | 36 (8/22) | 40 (31/77) | 37 (34/91) | 32 (24/74) | 0•027 |
| Need of high flow oxygen (%) | 3 (26/810) | 2 (9/504) | 0 (0/67) | 9 (2/22) | 3 (2/77) | 7 (6/91) | 3 (2/74) | 0•370 |
| Antibiotic treatment | ||||||||
| Ceftriaxone (%) | 73 | 55 | 49 | 48 | 32•5 | 42 | 45 | <0•001 |
| Ceftaroline (%) | 6 | 2 | 3 | 0 | 9 | 8 | 8 | 0•223 |
| Outcomes | ||||||||
| Length of hospital stay; Median (IQR) | 8 (5–14) | 9 (6–14) | 8 (5–12) | 8 (4–10•5) | 7 (5–10) | 7 (5–11) | 8 (5–12) | 0•345 |
| ICU admission (%) | 24 | 20 | 22 | 24 | 12 | 24 | 15 | 0•048 |
| Need of VM (%) | 12 | 8 | 3 | 5 | 1 | 8 | 4 | <0•001 |
| ICU mortality (%) | 19 | 18 | 20 | 0 | 0 | 9 | 0 | 0•023 |
| Early mortality (%) | 4 | 3 | 4•5 | 0 | 0 | 1 | 0 | 0•004 |
| 30-day mortality (%) | 11•6 | 10•3 | 7•5 | 0 | 5•2 | 3•3 | 1•4 | <0•001 |
A significant, declining trend was observed in patients either requiring ICU admission or who underwent invasive mechanical ventilation. ICU mortality, early mortality, and 30-day mortality significantly declined over time.
3.3. Factors associated with mortality at hospital admission
Independent factors associated with 30-day mortality at hospital admission were age (OR 1.1, CI 1.1–1.12), the presence of chronic heart disease (OR 1.7, CI 1.1–2.9), D-dimer levels higher than 700 ng/mL (OR 2.3, CI 1.3–4.1), ferritin levels higher than 489 ng/mL (OR 1.9; CI 1.5–3.2), C-RP higher than 7 mg/dL (OR 2.6; CI 1.5–4.6) and shorter duration from symptom onset to hospital admission (OR 1.11; CI 1.04–1.17). Conversely, hospital admission within the last, few months was significantly associated with lower mortality (OR 0.80; CI 0.65–0.98). The goodness-of-fit of the model was assessed with the Hosmer–Lemeshow test (p=0.791). The discriminatory power of the model, as evaluated by the area under the receiver operating characteristic curve, was 0.886 (95% CI, 0.86–0.92), demonstrating an excellent ability to predict 30-day mortality at hospital admission.
A second multivariate analysis including analytics as a continuous variable showed similar results. Independent risk factors associated with 30-day mortality at hospital admission were age (OR 1.1, CI 1.1–1.12); the presence of chronic kidney disease (OR 2.1, CI 1.2–3.7); LDH values (OR 1.01; CI 1.0–1.2); C-RP values (OR 1.1; CI 1.04–1.1) and shorter duration from symptom onset to hospital admission (OR 1.2; CI 1.1–1.2). Conversely, hospital admission within the last, few months was significantly associated with lower mortality (OR 0.81; CI 0.66–0.99). The goodness-of-fit of the model was assessed with the Hosmer–Lemeshow test (p=0.734). The discriminatory power of the model had an area under the receiver operating characteristic curve of 0.867 (95% CI, 0.838–0.896), demonstrating an excellent ability to predict 30-day mortality at hospital admission as well.
4. Discussion
This study is the first of its kind to include all consecutive patients with COVID-19 hospitalised for more than 48 hours and details a marked and decreased trend in 30-day mortality. Despite the fact that most countries have reported a decrease in mortality of patients with COVID, perhaps due to massive screening tests implemented to identify large numbers of patients with asymptomatic or mild infections and stop the pandemic [10], data concerning mortality rates in hospitalised patients are scarce. Three studies have reported a decreasing trend in mortality in patients with COVID-19. The first explored hospital mortality in 5121 patients from three academic hospitals in New York City, observing a decrease in mortality from 25.6% in March to 7.6% in August [11]. The second study reported a decreasing mortality amongst critically ill adults with COVID-19 from Emory Hospital in Atlanta, from 34.3% in March to 26.9% in July [12]. Finally, in a third study reporting mortality amongst 21,082 patients admitted to a high-dependency unit or ICU from March to June, rates decreased from 28.4% to 7.3% and 42% to 19.6%, respectively [13]. Our mortality rates significantly declined from the first wave period (March to May) to the second one. No significant differences were detected in the population aged <70 years, as the mortality rate in this group was low in both periods. In contrast, a significant reduction was observed in the subgroup of patients aged between 70–79 years and 80–89 years. In May 2020, Richardson et al. [14] reported the following mortality rates by age group: more than 63% in patients aged ≥ 90 years; 60% in patients aged 80–89 years; and 36% in patients aged 70–79 years. Our current mortality rates extremely differ. Between June and September 2020, only 7 of 79 (8.8%) patients older than 70 years who were admitted to our hospital died. However, it is important to consider differences in patient admission per month.
Potential explanations for the mortality declining in our institution include: 1) overall improvements in medical skills within these last several months; 2) better health care organisation and, as a result, avoidance of a system overload; 3) a change in patient characteristics; 4) the presence of viral variants with less pathogenicity; and/or 5) changes in treatment strategies.
The overload of patients and ICU capacity during the first wave of the pandemic could explain the high mortality rates reported worldwide; however, our institution was able to double the ICU capacity, which could partially explain the low mortality rate in our centre yet not its progressive, declining trend entirely. Similarly, no significant differences were observed in patient comorbidities, whilst the mean age of patients oscillated during the study period, peaking in June and slowly decreasing thereon. As age is one of the biggest driver of mortality in COVID-19 [14], this could be an explanation for the overall mortality rate. Yet, when we examined mortality by age groups, the reduction was observed in all strata. D-dimer and C-RP levels at onset were higher during the first, few months of the pandemic; however, several other factors with negative prognostic influence have been more frequently documented in recent admissions (e.g. higher levels of ferritin and/or LDH, and more cases of hypoxia at admission). Accordingly, it is difficult to confirm whether patient severity has changed during the study period. The spread of viral variants with less pathogenicity was described in Singapore [15]; however, since February 2020, the dominant virus variant in Europe has the G614 form of the Spike protein [16]. No data about the potential variation in the virulence of this variant has been described, although G614-bearing viruses have shown significantly higher infectious titres in vitro than D614 counterparts [16]. This phenomenon is, therefore, unlikely to explain variations in mortality.
We did observe major changes in treatment strategies that may explain the better outcomes. Our experience documented that the use of remdesivir substantially increased over time, and time from symptom onset to initial doses of remdesivir shortened. Other antivirals such as lopinavir/ritonavir or hydroxychloroquine have disappeared. Although some adverse events have been related with these drugs, namely cardiac events with hydroxychloroquine, we reviewed our experience and did not observe an increased mortality in this population (data not published).
Remdesivir has shown a reduction in mortality rate in the subgroup of patients with pneumonia and low-flow oxygen [7]. Along the same line, the impact of remdesivir on reducing viral shedding has been reported in macaques [17]; however, studies powered to assess the impact of such a finding on infection transmissibility and/or severe complications, such as coagulopathy or hyperinflammation, in infected humans are lacking. We observed a significant increase in the use of remdesivir during the second period. We cannot directly attribute changes in mortality rate to the increase in remdesivir use, as this is not a randomised study; however, mortality rate in our hospitalised patients was <5% during the second period, in line with that reported in similar patients included in the ACTT-1 study [7].
Anti-inflammatory therapy has demonstrated a reduction in the mortality rate [8,18]. We therefore cannot attribute such reduction to a change in anti-inflammatory therapies; more details concerning the type of drug and moment of administration could be important, though. In an open-label, randomised trial comparing dexamethasone use vs. routine care in 2104 patients with a mean age of 67 years and 4321 patients, respectively, dexamethasone use resulted in lower 28-day mortality in those individuals receiving oxygen therapy. Most patients undergoing routine care did not receive any anti-inflammatory treatment. In the dexamethasone group, 28-day mortality was 22.9%, which is quite high when compared to our current rates. Trials assessing the utility of tocilizumab in patients with COVID-19 have not reported declining mortality [19], [20], [21], although some significant benefits have been observed. These trials are difficult to analyse due to factors such as the low number of patients included, especially when the routine care arm includes a high number of patients treated with other anti-inflammatory therapies; differences in baseline patient characteristics across groups, namely age; and a high number of patients who had been rescued with tocilizumab in the non-tocilizumab arm. It is worth mentioning that our study showed differences in ICU and non-ICU patients receiving anti-inflammatory and remdesivir treatment.
Improvements in general management of our cohort of patients hospitalised with COVID-19, including antiviral and anti-inflammatory therapies, are evident. The highest serum concentration of C-RP achieved during hospital admission and the need for ICU admission have also significantly decreased throughout the study period.
Risk factors for mortality at hospital admission have been previously described [3,14]. Our study is in agreement with results from these reports and provides two additional, important variables related with mortality: the impact of hospital admission during the first, few months of the pandemic and duration of symptom onset. In our study, those patients admitted with shorter duration of symptom onset independently had higher mortality. This fact may be related with a higher viral load in this population. Unfortunately, this variable was not available for the present analysis. Further studies evaluating the impact of this finding are warranted.
The strengths of this study include the high number of consecutive patients and compressive data collection. However, there are several limitations. First, the study was conducted in a single centre from Spain, where the public health system attends to all patients equally. This fact may make generalisation of our results difficult. Second, our data were collected directly from EHRs. Nonetheless, it is important to note that our hospital used an intelligent system to retrieve data from EHRs (SILDv1.0 system, S34M@). The data review process, which includes nine quality steps, ensures high quality of our data. Finally, since the end of March, we have implemented a programme that, under the supervision of infectious disease experts, comprises a computer-control centre for patients with COVID-19 that uses real-time data from EHRs to support attending physicians with different skill sets to provide quick, personalised medicine to our patients. The impact of this measure is difficult to assess, but as we have reported, has been related with better outcomes [6].
In conclusion, mortality in hospitalised patients with COVID-19 has decreased throughout these last several months, even though main patient characteristics remain similar except for age oscillating during the study period. Several changes made in patient management had been detailed, impacting a decreasing trend in 30-day mortality over various months, especially in elderly patients. Our study provides current data on mortality for patients hospitalised with COVID-19 that may be useful in establishing quality of standard of care.
Contributors
Carolina Garcia-Vidal: Literature search, study design, data collection, data analysis, data interpretation, writing, figures.
Alberto Cózar-Llistó: Literature search, study design, data collection, data analysis, data interpretation, writing, figures.
Fernanda Meira: Literature search, study design, data collection, data analysis, data interpretation, figures.
Gerard Dueñas: Literature search, study design, data collection, data analysis, data interpretation, figures.
Pedro Puerta-Alcalde: Literature search, study design, data collection, data analysis, data interpretation.
Catia Cilloniz: Literature search, study design, data collection, data analysis, data interpretation.
Nicole Garcia-Pouton: Literature search, study design, data collection, data analysis, data interpretation.
Mariana Chumbita: Literature search, study design, data collection, data analysis, data interpretation.
Celia Cardozo: Literature search, study design, data collection, data analysis, data interpretation.
Marta Hernandez: Literature search, study design, data collection, data analysis, data interpretation.
Veronica Rico: Literature search, study design, data collection, data analysis, data interpretation.
Marta Bodro: Literature search, study design, data collection, data analysis, data interpretation.
Laura Morata: Literature search, study design, data collection, data analysis, data interpretation.
Pedro Castro: Study design, data collection, data analysis, data interpretation.
Alex Almuedo-Riera: Study design, data collection, data analysis, data interpretation.
Felipe García: Study design, data collection, data analysis, data interpretation.
Josep Mensa: Literature search, study design, data analysis, data interpretation.
José Antonio Martínez: Study design, data collection, data analysis, data interpretation.
Gemma Sanjuan: Literature search, study design, data collection, data analysis, data interpretation.
Antoni Torres: Study design, data collection, data analysis, data interpretation.
JM Nicolas: Study design, data collection, data analysis, data interpretation.
Alex Soriano: Literature search, study design, data analysis, data interpretation, writing, figures.
Declaration of Interests
This research is part of an activity that has received funding from EIT Health. EIT Health is supported by the European Institute of Innovation and Technology (EIT), a body of the European Union that receives support from the European Union´s Horizon 2020 Research and Innovation Programme. This study has been co-funded by the European Regional Development Fund (EDRD). All authors report grants from EIT Health and the European Regional Development Fund (for themselves or their institution), during the conduct of the study. PPA [CM18/00132], NGP [FI19/00133], and CGV [FIS PI18/01061] have received research grants from the Ministerio de Sanidad y Consumo and Instituto de Salud Carlos III. No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
CGV has received honoraria for talks on behalf of Gilead Science, MSD, Novartis, Pfizer, Janssen, and Lilly, as well as a grant from Gilead Science and MSD. PPA has received honoraria for talks on behalf of Gilead Science and MSD. JM has received honoraria for talks on behalf of Merck Sharp and Dohme, Pfizer, Novartis, and Angellini. AS has received honoraria for talks on behalf of Merck Sharp and Dohme, Pfizer, Novartis, and Angellini, as well as grant support from Pfizer. Other authors do not declare conflict of interest outside the submitted work.
Hospital Clinic of Barcelona COVID-19 Researchers:
Infectious Diseases’ Research Group:
Albiach L, Agüero D, Ambrosioni J, Blanco JL, De la Mora L, González-Cordón A, Inciarte A, Jordan C, Laguno M, Leal L, Lopera C, Macaya I, Mallolas J, Martínez E, Martínez M, Miró JM, Moreno A, Rojas J, Solá M, Torres B, Torres M, and all the staff members.
Medical Intensive Care Unit:
Adrian Téllez, Sara Fernández, and all the staff members.
Department of International Health:
Daniel Camprubi Ferrer, Maria Teresa de Alba, Marc Fernandez, Elisabet Ferrer, Berta
Grau, Helena Marti, Magdalena Muelas, Maria Jesus Pinazo, Natalia Rodríguez,
Montserrat Roldan, Carme Subira, Isabel Vera, Nana Williams, Jose Muñoz, and all the staff members.
Department of Internal Medicine:
Aldea A, Camafort M, Calvo J, Capdevila A, Cardellach F, Carbonell I, Coloma E, Foncillas A, Estruch R, Feliu M, Fernández-Solá J, Fuertes I, Gabara C, Grafia I, Ladino A, López-Alfaro R, López-Soto A, Masanés F, Matas A, Navarro M, Marco-Hernández J, Miguel L, Milisenda J, Moreno P, Naval J, Nicolás D, Oberoi H, Padrosa J, Prieto-González S, Pellicé M, Ribot J, Rodríguez-Núnez O, Sacanella E, Seguí F, Sierra C, Tomé A, Ugarte A, Ventosa H, Zamora-Martínez C, and all the staff members.
Department of Microbiology:
M. Almela, M. Alvarez, J. Bosch, C. Casals, J. Costa, G. Cuesta, M. Fernandez, B. Fidalgo, J. Gonzàlez, J.C. Hurtado, F. Marco, M.A. Marcos, M. Martínez, M. Mosquera, S. Narvaez, C. Pitart, E. Rubio, A. Vergara, M.E.Valls, J. Vila, Y. Zboromyrska and all the staff members.
Department of Farmacy:
E. López, D. Soy, M. Tuset and all the staff members.
Data sharing statement: All data will be available under request after manuscript acceptance.
Acknowledgements
We would like to thank Anthony Armenta for his medical editing assistance.
Footnotes
This translation in Spanish was submitted by the authors and we reproduce it as supplied. It has not been peer reviewed. Our editorial processes have only been applied to the original abstract in English, which should serve as reference for this manuscript.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanepe.2021.100041.
Appendix. Supplementary materials
References
- 1.Cummings MJ, Baldwin MR, Abrams D. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berenguer J, Ryan P, Rodríguez-Baño J. Characteristics and predictors of death among 4035 consecutively hospitalized patients with COVID-19 in Spain. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wendel Garcia PD, Fumeaux T, Guerci P. Prognostic factors associated with mortality risk and disease progression in 639 critically ill patients with COVID-19 in Europe: initial report of the international RISC-19-ICU prospective observational cohort. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang X, Yu Y, Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Vidal C, Moreno-García E, Hernández-Meneses M. Personalized therapy approach for hospitalized patients with COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa964. published online July. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the treatment of Covid-19 – final report. N Engl J Med. 2020 doi: 10.1056/NEJMc2022236. published online Oct 8. DOI:10.1056/NEJMoa2007764. [DOI] [PubMed] [Google Scholar]
- 8.RECOVERY Collaborative Group. Horby P, Lim WS. Dexamethasone in hospitalized patients with Covid-19 – preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. published online July 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Vidal C, Sanjuan G, Puerta-Alcalde P, Moreno-García E, Soriano A. Artificial intelligence to support clinical decision-making processes. EBioMedicine. 2019;46:27–29. doi: 10.1016/j.ebiom.2019.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Vinh Chau N, Lam VT, Dung NT. The natural history and transmission potential of asymptomatic severe acute respiratory syndrome coronavirus 2 infection. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa711. published online June 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horwitz LI, Jones SA, Cerfolio RJ. Trends in COVID-19 risk-adjusted mortality rates. J Hosp Med. 2020 doi: 10.12788/jhm.3552. published online Oct 23. [DOI] [PubMed] [Google Scholar]
- 12.Auld SC, Caridi-Scheible M, Robichaux C, Coopersmith CM, Murphy DJ. Declines in mortality over time for critically ill adults with Coronavirus disease 2019. Crit Care Med. 2020 doi: 10.1097/CCM.0000000000004687. Publish Ah. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dennis JM, McGovern AP, Vollmer SJ, Mateen BA. Improving survival of critical care patients with coronavirus disease 2019 in England. Crit Care Med. 2020 doi: 10.1097/CCM.0000000000004747. Publish Ah. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richardson S, Hirsch JS, Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA – J Am Med Assoc. 2020;323:E1–E8. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young BE, Fong S-W, Chan Y-H. Effects of a major deletion in the SARS-CoV-2 genome on the severity of infection and the inflammatory response: an observational cohort study. Lancet. 2020;396:603–611. doi: 10.1016/S0140-6736(20)31757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korber B, Fischer WM, Gnanakaran S. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182:812–827. doi: 10.1016/j.cell.2020.06.043. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williamson EJ, Walker AJ, Bhaskaran K. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta S, Wang W, Hayek SS. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.6252. published online Oct 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stone JH, Frigault MJ, Serling-Boyd NJ. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermine O, Mariette X, Tharaux P-L. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.6820. published online Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salvarani C, Dolci G, Massari M. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.6615. published online Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.