Abstract
Background
SARS-CoV-2 infection is associated with a wide spectrum of neurological complications, including encephalitis. Most cases showed features consistent with a central nervous system (CNS) cytokine-mediated damage. However, few cases arguing for an autoimmune mechanism have been described, mainly as single reports or sparse in large case series involving other CNS manifestations. In this paper, we described a case of definite autoimmune limbic encephalitis (LE) COVID-19 related and reviewed the existing literature on other reported cases.
Case report
Two weeks after the onset of COVID-19 infection, a 74-year-old woman presented with subacute confusion and focal motor seizures with impaired awareness, starting from left temporal region. Cerebrospinal fluid analysis revealed hyperproteinorrachia. Brain MRI showed bilateral T2/FLAIR hyperintensities in both hippocampi and total body PET/TC scan revealed hypermetabolism in basal ganglia bilaterally. A diagnosis of autoimmune LE was made. Thus, high dose corticosteroids and antiseizure medications were started, with a marked improvement of neurological conditions.
Literature review
We systematically reviewed the literature to identify all well-documented cases of definite autoimmune LE (according to Graus criteria) in patients with COVID-19 infection, identifying other five cases exhibiting a good response to immunomodulating therapy.
Conclusion
A very limited number of autoimmune LE have been described until now. It is important to monitor neurological symptoms in COVID-19 patients and to consider the possibility of an autoimmune LE, in particular when altered mental status and seizures appear late in the disease course. This allows to promptly start the appropriate treatments and avoid unnecessary delays.
Keywords: COVID-19, SARS-CoV-2, Autoimmune limbic encephalitis, Post-infectious encephalitis, Seizures, Memory deficits
Highlights
-
•
A limited number of autoimmune LE covid-19 related have been described until now.
-
•
A specific focus on autoimmune limbic encephalitis covid-19-related is lacking.
-
•
It is important to consider the possibility of covid-19 related limbic encephalitis to promptly start appropriate treatments.
1. Introduction
Neurological complications due to the infection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been increasingly observed (Mao et al., 2020) during the outbreak of coronavirus disease 2019 (COVID-19).
Concerning central nervous system (CNS) involvement, a wide spectrum of encephalitis secondary to a direct viral damage (Zubair et al., 2020) or to a hyperinflammation syndrome (Mehta et al., 2020; Perrin et al., 2021) have been described. Conversely, only few cases of encephalitis due to an antibody-mediated mechanism have been reported (Pilotto et al., 2020; Paterson et al., 2020) and even fewer of autoimmune limbic encephalitis (LE). These cases have been mostly described within case series involving other types of encephalitis or encephalopathies, without receiving a specific attention.
In this paper, we presented the case of a patient with SARS-CoV-2 pneumonia, who developed an autoimmune LE, and we reviewed the existing literature on other reported cases, in order to highlight the possibility of an autoimmune process also in the setting of COVID-19 infection.
2. Case report
On November 2nd, a 74 years-old woman was admitted to the Emergency Department due to persistent fever and worsening dyspnea; symptoms started 8 days before the admission. Her past medical history only revealed a mild hypothyroidism, treated with hormone replacement therapy.
Arterial blood gas analysis demonstrated type 1 respiratory failure and blood tests documented slightly increased C-reactive protein (5.8 mg/dl) and fibrinogen (630 mg/dl), normal white blood cells count, D-dimer, and procalcitonin. A computed tomography (CT) scan of the lungs revealed bilateral diffuse areas of ground-glass pattern with subpleural parenchymal consolidations. Oropharyngeal and nasopharyngeal swabs were positive for SARS-CoV-2 genome. Therefore, the patient was admitted to the COVID-19 Unit and treated with remdesevir (200 mg on first day followed by 100 mg once daily for 4 days) and dexamethasone (6 mg for 10 days). She also needed continuous positive airway pressure (CPAP) therapy for three days, with good response. Fever rapidly resolved and laboratory inflammation indexes reduced to normal values. Five days after the admission, she presented mild confusion and a brief episode of non-responsivity with staring. A CT scan of the head was negative. The subsequent day she had a generalized tonic-clonic seizure, treated with valproic acid at the dose of 1500 mg/die. CT scan was repeated and did not show any acute lesions. On day 7 she had a cluster of focal seizures with impaired awareness and oral automatisms documented with EEG monitoring (Fig. 1, box 2); levetiracetam was added until 1000 mg/die.
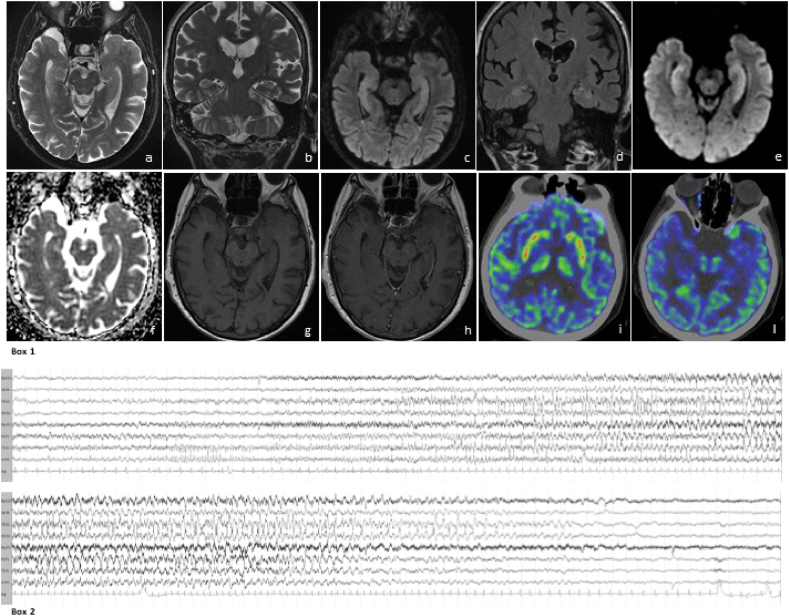
Fig. 1.
Box 1. Brain MRI features: axial (a) and coronal (b) T2 weighted images and axial (c) and coronal (d) Flair images show hyperintensity and mild expansion of both medial temporal lobes. The study of medial temporal lobes with diffusion weighted images demonstrates high signal in DWI b1000 (e) and no diffusivity restriction in ADC maps (f), denoting the presence of vasogenic edema. T1 weighted images acquired before (g) and after (h) contrast administration demonstrate no contrast enhancement. FDG-PET of the brain shows hypermetabolic foci in basal ganglia (i), while no metabolic alteration on both hippocampi (l) is detectable.
Box 2. Ictal EEG: a theta rhythmic activity starts on the left temporal region, then spreads bilaterally and evolves to a delta activity mixed with sharp waves, persisting predominantly on bi-temporal region for a total length of 63 s. Clinically, the patient presents behavioral arrest, right head and eyes deviation, chewing automatisms.
On day 8 a lumbar puncture was performed, showing normal glucose level (67 mg/dl), normal cells (1 cell/mm3), increased protein level (104 mg/dl) and negative oligoclonal bands (OCB). Polymerase chain reactions (PCR) for SARS-CoV-2, neurotropic viruses (Herpes Simplex Virus-1 and 2, Herpes Human Virus-6, Cytomegalovirus, Varicella-Zoster Virus, Epstein Barr Virus), bacteria (E. coli K1, Haemophilus influenzae, Listeria monocytogenes, Neisseria meningitidis, Streptococcus agalactiae, Streptococcus pneumoniae), and Cryptococcus neoformans/gattii were negative. The autoimmune panel for encephalitis - including antibodies anti LGI1, Caspr-2, NMDA, GABA-B, and anti Hu, Yo, Ri - were negative in both serum and cerebrospinal fluid (CSF).
During the following days, patient remained alert, but with marked ideo-motor slowing, confusion, diffuse hyposthenia and prostration.
On day 23, when respiratory and general conditions had improved, a brain MRI showed a bilateral symmetric mesial temporal lobes hyperintensity in FLAIR, T2 and DWI b1000 sequences with mild hippocampal thickening, consistent with LE (Fig. 1, box 1, a-h).
Therefore, patient was treated with steroids (intravenous methylprednisolone 500 mg/daily for 5 days, 250 mg/die for 3 days, 125 mg/die for 3 days, followed by oral prednisolone gradually reduced).
On day 27, after two negative nasopharyngeal swabs, she was transferred to our Neurological Unit; in the following days, her global status and neurological conditions further improved, she was gradually mobilized and was able to walk. She was oriented to person, place and time, with only slight ideo-motor slowing. Mini Mental State Examination was normal with score 30/30, while Montreal Cognitive Assessment Test revealed slight impairment in verbal fluency and delayed verbal recalling (total score 24/30). A total body FDG-PET/TC scan on day 33 showed hypermetabolism in basal ganglia bilaterally (Fig. 1, box 1, i-l), as frequently described in autoimmune non-paraneoplastic encephalitis (Tripathi et al., 2018). No other areas of abnormal metabolism were detected in the rest of the body. On day 35 she was discharged home.
3. Literature review: methods
We performed a systematic review of the literature to identify all well-documented cases of autoimmune LE COVID-19 related. A search was conducted on December 15th, 2020, by using Medline assessed from PubMed; the following search term were used: “SARS-CoV-2” OR “COVID-19” AND “encephalitis”, limited to publications in English. We screened the titles and abstracts of all the obtained results. Potentially relevant studies were reviewed in full text to recognize other cases of definite autoimmune LE according to Graus criteria (Graus et al., 2016): 1) subacute onset of working memory deficits, seizures, or psychiatric symptoms suggesting involvement of the limbic system; 2) bilateral brain abnormalities on T2-weighted fluid-attenuated inversion recovery MRI highly restricted to the medial temporal lobes; 3) at least one of the following: i) CSF pleocytosis, ii) EEG with epileptic or slow wave activity involving the temporal lobes; 4) reasonable exclusion of alternative causes. Diagnosis was made when all four criteria had been met or with the detection of specific antibodies associated with LE.
4. Literature review: results
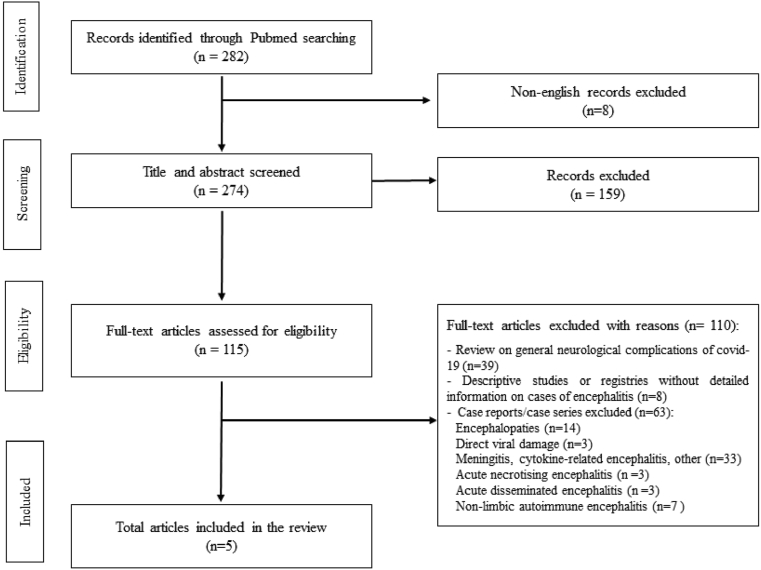
The electronic literature search initially yielded 282 articles, of which 8 articles were excluded because they were published in language other than English and 159 were excluded by title and abstract evaluation (Fig. 2). The remaining articles were assessed for eligibility according to the criteria outlined above. Among the 115 papers included, 39 were excluded because they represented general reviews on neurological complication of COVID-19, 8 because they were registries or descriptive studies involving cases of encephalitis, but without any detailed information. Sixty-three excluded papers were case reports or case series describing encephalopathies COVID-19 related (n = 14), encephalitis with other etiologies than autoimmune (3 direct viral damage, 33 cytokines related or other, 3 acute necrotizing encephalitis, 3 acute disseminated encephalitis) or non-limbic autoimmune encephalitis, the majority of which anti NMDAr (n = 7).
Fig. 2.
Flowchart showing our literature search.
A total of 5 cases met the inclusion criteria of definite autoimmune LE COVID-19 related. One case was a single report while four were extracted from case series (Table 1).
Table 1.
Summary of cases of autoimmune LE covid-19-related.
| Case no | Reference | Age, sex | Respiratory symptoms§ | Neurological symptoms | Days from pneumonia to neurological symptoms | EEG features | MRI findings | CSF cells/mm3; proteins (mg/dl) | CSF, SARS-CoV-2 | Screening performed | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Zambreanu et al. (Zambreanu et al., 2020) (also cited in Paterson et al. (Paterson et al., 2020)) | 66, F | Mild | Confusion, memory deficits, seizure | Not applicable | Not available | T2 hyperintensities in limbic lobes, upper pons and medial thalami, without gadolinium enhancement | 3 cells/mm3; 100 mg/dl |
Negative | CSF PCR negative for HSV1, HSV2, HHV6, enterovirus, parechovirus, CMV, VZV, Cryptococcus, Streptococci, Haemophilus, Listeria, E.Coli. Negative OCB. Autoimmune panel negative on CSF and blood for anti LGI1, CASPR2, NMDAR, Hu, Yo, Ri, Ma1, Ma2, CV2, amphiphysin, Zic4, Sox 1, Tr, GAD, Aquaporin 4, MOG, DPPX. |
CS, IVIg | Incomplete recovery; ongoing cognitive impairment |
| 2 | Pilotto et al. (Pilotto et al., 2020) | 68, M | Severe | Altered mental status, SE, dysarthria | 15 | Abnormal, SE (details not available) | Consistent with LE (details not available) | 1 cell/mm3; 77 mg/dl |
Negative | CSF PCR negative for HSV1, HSV2, CMV, EBV, HHV6, HHV7, VZV, enterovirus, adenovirus. Autoimmune panel negative on CSF and serum for anti LGI1, CASPR2, NMDAR, GABAB1R, GABAB2R, AMPAR1, AMPAR2, Hu, Yo, Ri, CV2, amphiphysin | Spontaneous recovery | Complete recovery |
| 3 | Pilotto et al. (Pilotto et al., 2020) | 76, F | Severe | Altered mental status, aphasia | 13 | Abnormal (details not available) | Consistent with LE (details not available) | 15 cells/mm3; 36 mg/dl |
Negative | CSF PCR negative for HSV1, HSV2, CMV, EBV, HHV6, HHV7, VZV, enterovirus, adenovirus. Autoimmune panel negative on CSF and serum for anti LGI1, CASPR2, NMDAR, GABAB1R, GABAB2R, AMPAR1, AMPAR2, Hu, Yo, Ri, CV2, amphiphysin | CS | Slight disability, unable to perform all previous activities |
| 4 | Guilmot et al. (Guilmot et al., 2020) | 80, M | Mild | Short term memory disturbances, seizure, visual hallucinations, anxiety | Not applicable | Generalized slowing | Normal | 9 cells/mm3; 46 mg/dl |
Negative | CSF PCR for viral agents negative. Positive OCB. CASPR2 positivity on CSF and blood. |
CS, PLEX | Good seizure control, other details Not Available |
| 5 | Hosseini et al. (Hosseini et al., 2020) | 79, F | Mild | Altered mental status, dysphasia, impaired memory, seizures | Not applicable | Not available | T2 and FLAIR hyperintensities in the limbic system, bilaterally but predominantly on the left | 0 cells/mm3; 34 mg/dl |
Negative | CSF PCR negative for HSV1, HSV2, VZV, enterovirus. OCB negative. Autoimmune panel negative on blood and CSF for anti LGI1, CASPR2, NMDAR, GABAb, AMPA, paraneoplastic anti-neuronal antibodies. |
ASM | Impaired verbal fluency, repetition and delayed recall memory |
| 6 | Pizzanelli et al. | 74, F | Severe | Confusion, seizures | 13 | Focal seizure with onset in left temporal lobe | DWI hyperintensity and swelling of both hippocampi, without gadolinium enhancement | 2 cells/mm3; 104 mg/dl |
Negative | CSF PCR negative for HSV1, HSV2, HHV6, CMV, VZV, EBV, E.Coli, H.Influenzae, L. Monocytogenes, N. Meningitidis, Streptococci, Cryptococci. Negative OCB. Autoimmune panel negative on blood and CSF for anti LIG1, CASPR2, NMDAR, GABAb, Hu, Yo, Ri. |
CS | Almost complete recovery with only slight verbal deficits |
Abbreviation: ASM: antiseizure medications; CMV: Cytomegalovirus; CS: corticosteroids; CSF: cerebrospinal fluid; DWI: diffusion weighted imaging; EBV: Epstein-Barr Virus; EEG: electroencephalogram; FLAIR: Fluid Attenuated Inversion Recovery; HHV: Herpes Human Virus; HSV: Herpes Simplex Virus; IVIg: intravenous immunoglobulins; LE: limbic encephalitis; MRI, magnetic resonance imaging; no: number; OCB: oligoclonal bands; PCR: polymerase chain reaction; PLEX: plasma-exchange; SE: status epilepticus; VZV: Varicella-Zoster Virus; §Respiratory symptoms were defined “mild” if patients were asymptomatic or if they presented with hyposmia, dysgeusia or cough without signs of pneumonia; and “severe” if they had documented pneumonia.
Gender was reported in all studies: 3 subjects were women and 2 were men, while patients’ age varied from 66 to 80 years, with a mean of 73.8 years.
Respiratory symptoms were mild in three patients (cases #1, #4 and #5, Table 1) who were either asymptomatic or presented with hyposmia, dysgeusia, or cough without signs of pneumonia, and severe in two subjects (cases #2 and #3, Table 1), who had documented pneumonia.
All patients showed altered mental status (AMS) at the onset of neurological symptoms. Case #1 was admitted to the hospital because of a few-hour history of confusion and amnesia, and later developed a generalized tonic-clonic seizure. Case #2 showed AMS with status epilepticus and dysarthria, while case #3 exhibited AMS and aphasia. Case #4 presented a 3-week history of neuropsychiatric symptoms, including visual hallucinations, short-term memory disturbance and anxiety, and had a tonic-clonic seizure. Case #5 was admitted to the hospital because of a seizure, with confusion and verbal communication difficulties started few hours earlier.
When available, EEG showed abnormal findings, not reported in detail; only one subject (case #2) presented with status epilepticus.
MRI findings were consistent with LE with T2 and FLAIR hyperintensities in the limbic system bilaterally, without gadolinium enhancement in cases #1, #2, #3, #5. Case #4 had normal brain MRI.
CSF examination was abnormal in all subject except case #5, with alterations ranging from hyperproteinorrachia (cases #1, #2 and #4) to pleocytosis (cases #3 and #4). In all patients, CSF PCR for common neurotropic viruses, including SARS-CoV-2, and bacteria was tested and resulted negative. Moreover, an extended autoimmune panel including autoantibodies against intracellular, synaptic and surface antigens associated to LE (Table 1) was performed, resulting negative in all subjects except case #4, who showed CASPR2 positivity, both on CSF and on blood.
All patients except two (cases #2 and #5) were treated with immunomodulating therapy: high dose of corticosteroids and intravenous immunoglobulins or plasma-exchange were used with the aim of counteracting an autoimmune mechanism.
Concerning outcome, case #2 showed complete recovery, while an incomplete recovery was observed in case #1, with ongoing cognitive impairment; case #3 had a slight residual disability while case #5 showed persistent impairment in verbal fluency, repetition, and delayed recall memory. Details on outcome are not available for case #4.
5. Discussion
We described a case of definite autoimmune LE according to Graus criteria (Graus et al., 2016), COVID-19 related, with a good response to corticosteroids and a favorable outcome.
Several cases of encephalitis related to COVID-19 have been recently described, the majority of which seem to be related to a secondary hyperinflammation syndrome (Mehta et al., 2020; Perrin et al., 2021), with a massive release of cytokines and chemokines.
Conversely, a smaller number of cases of encephalitis have been attributed to an autoimmune mechanism through antibodies against neuronal cell-surface or synaptic proteins triggered by the virus, i.e. a para/post-infectious disease associated with molecular mimicry-related mechanisms.
Data that support this hypothesis are: i) the prolonged time interval between respiratory - when present - and neurological symptoms, instead of a concomitant involvement, as occurs during cytokine related syndrome; ii) the good response to immunomodulating therapy with steroids, immunoglobulins or plasma-exchange.
Our patient and the other cases of autoimmune LE who showed severe COVID-19 respiratory symptoms (cases #2, #3 and #6), presented a delayed onset of neurological symptoms compared to respiratory involvement, with a median of two weeks (range 13–15 days, Table 1). In patients asymptomatic or pauci-symptomatic for respiratory involvement (cases #1, #4 and #5), exhibiting autoimmune LE as apparently first manifestation of COVID-19 disease, we hypothesize a previous, though asymptomatic, onset of SARS-CoV2 infection, as a trigger for a subsequent autoimmune process within the CNS. As expected in autoimmune LE, we did not observe severe alterations of blood-brain barrier in any case, with mild pleocytosis in two cases and mild hyperproteinorrachia in four.
Concerning immunomodulating therapy, all treated patients showed a significant improvement, in two cases with almost complete recovery (cases #3 and #6).
In conclusion, although a very limited number of autoimmune LE have been described until now, it is important to consider the possibility of an autoimmune process also in the setting of COVID-19 infection, in particular if neurological impairment appears late, when respiratory symptoms are resolving. In these cases, managing an early diagnostic set-up with CSF analysis, EEG, and brain imaging, represents the best diagnostic strategy, in order to avoid unnecessary delays and promptly start the appropriate treatments.
Future studies oriented towards biomarkers identification may help to correctly identify the pathophysiological mechanisms underlying the wide spectrum of encephalitis and better clarify the pathological process of LE encephalitis in the setting of COVID-19.
Moreover, further data focusing on long-term outcome of autoimmune LE COVID-19 related are needed, with particular regard to long-standing cognitive deficits.
Declaration of competing interest
The Authors have no conflicts of interest to disclose.
References
- Graus F., Titulaer M.J., Balu R. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016 doi: 10.1016/S1474-4422(15)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilmot A., Maldonado Slootjes S., Sellimi A. Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. J Neurol Published Online First. 2020 doi: 10.1007/s00415-020-10108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini A.A., Shetty A.K., Sprigg N. Delirium as a presenting feature in COVID-19: neuroinvasive infection or autoimmune encephalopathy? Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in wuhan, China. JAMA Neurol Published Online First. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020 doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R.W., Brown R.L., Benjamin L. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain Published Online First. 2020 doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin P., Collongues N., Baloglu S. Cytokine release syndrome-associated encephalopathy in patients with COVID-19. Eur J Neurol Published Online First. 2021 doi: 10.1111/ene.14491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilotto A., Masciocchi S., Volonghi I. Clinical presentation and outcomes of severe acute respiratory syndrome coronavirus 2–related encephalitis: the ENCOVID multicenter study. J Infect Dis Published Online First. 2020 doi: 10.1093/infdis/jiaa609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi M., Tripathi M., Roy S.G. Metabolic topography of autoimmune non-paraneoplastic encephalitis. Neuroradiology Published Online First. 2018 doi: 10.1007/s00234-017-1956-2. [DOI] [PubMed] [Google Scholar]
- Zambreanu L., Lightbody S., Bhandari M. A case of limbic encephalitis associated with asymptomatic COVID-19 infection. J. Neurol. Neurosurg. Psychiatry. 2020 doi: 10.1136/jnnp-2020-323839. [DOI] [PubMed] [Google Scholar]
- Zubair A.S., McAlpine L.S., Gardin T. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]