Abstract
Simple Summary
Cellular growth, particularly muscle hypertrophy, requires substantial energetics. In animals, proper substrate utilization is essential. Maximal growth is dependent on both carbohydrates and lipids being oxidized to provide as much energy as possible for protein syntheses, while amino acids must be spared from oxidation to provide an ample supply of proteogenic building blocks. However, maximal cellular growth is not a default metabolic state and is easily overridden by environmental factors such as heat stress (HS). HS has previously been hypothesized to increase metabolic rate, as is typical of a general stress response. Newer evidence, however, suggest that HS may limit energy production by inhibiting the use of lipids as a fuel source. HS metabolism instead depends on carbohydrates and even amino acids as energetic substrate, potentially limiting energy production. This study demonstrates that, contrary to current recommendations, HS reduces metabolic rate. Findings demonstrated a 38% reduction in relative energy expenditure (kcal/day/kg) due to HS. HS also caused a 33% increase in amino acid oxidation. A combination of decreased energy production and increased amino acid oxidation creates a metabolic state with severely limited growth potential which cannot be solved by simply increasing feed.
Abstract
Heat stress (HS) diminishes animal production, reducing muscle growth and increasing adiposity, especially in swine. Excess heat creates a metabolic phenotype with limited lipid oxidation that relies on aerobic and anaerobic glycolysis as a predominant means of energy production, potentially reducing metabolic rate. To evaluate the effects of HS on substrate utilization and energy expenditure, crossbred barrows (15.2 ± 2.4 kg) were acclimatized for 5 days (22 °C), then treated with 5 days of TN (thermal neutral, 22 °C, n = 8) or HS (35 °C, n = 8). Pigs were fed ad libitum and monitored for respiratory rate (RR) and rectal temperature. Daily energy expenditure (DEE) and respiratory exchange ratio (RER, CO2:O2) were evaluated fasted in an enclosed chamber through indirect calorimetry. Muscle biopsies were obtained from the longissimus dorsi pre/post. HS increased temperature (39.2 ± 0.1 vs. 39.6 ± 0.1 °C, p < 0.01) and RER (0.91 ± 0.02 vs. 1.02 ± 0.02 VCO2:VO2, p < 0.01), but decreased DEE/BW (68.8 ± 1.7 vs. 49.7 ± 4.8 kcal/day/kg, p < 0.01) relative to TN. Weight gain (p = 0.80) and feed intake (p = 0.84) did not differ between HS and TN groups. HS decreased muscle metabolic flexibility (~33%, p = 0.01), but increased leucine oxidation (~35%, p = 0.02) compared to baseline values. These data demonstrate that HS disrupts substrate regulation and energy expenditure in growing pigs.
Keywords: heat stress, lipid oxidation, energy expenditure, respiratory exchange ratio
1. Introduction
Heat stress (HS) presents a complex and comprehensive challenge to an organism’s metabolism. Almost all tissue types undergo significant alterations in substrate metabolism in response to HS (brain [1], muscle [2], liver [2], intestine [3], etc.). There are a variety of hormonal adaptations to HS, particularly those associated with a general stress response (i.e., cortisol, nor/epinephrine, thyroid hormone, etc. [4]). However, increases in temperature can directly influence substrate preference in muscle tissue independent of hormonal changes. Unlike typical stress responses that are associated with increased energy expenditure and decreased respiratory quotient [5,6], HS acting independent of a hormonal response has the potential to dramatically limit energy production through reductions in lipolysis and lipid oxidation [2,3,4].
As with neuronal [7] and immune cells [8], muscle fibers express heat-sensing TRPV1 receptors. However, muscle TRPV1 receptors are housed internally on the sarcolemma and, rather than peripherally signaling the sensation of heat, locally coordinate substrate selection through the activation of heat shock factors (HSFs) and subsequent expression of heat shock proteins (HSPs). As a whole, the cellular response to heat suppresses lipolysis [9] and prioritizes lipid storage [10] while increasing cellular glucose uptake [11,12] and glycolysis [13]. The net result of these changes is a metabolic phenotype with limited lipid oxidation that relies on aerobic and anaerobic glycolysis as the predominant source of energy production. Though changes in HSP expression are not typically detected in in vivo HS models, the metabolic effects persist [2,14].
Efficient lean tissue accretion is reliant on an animal devoting a large portion of energy towards developing lean mass [15,16] however, excess accumulated heat promotes a shift in metabolic substrate preference, limiting energy production by blunting lipid oxidation and increasing lipogenesis [2]. If energy production is limited, then hypertrophy will be blunted [15], and substrates will instead be diverted towards storage as is observed in HS animals [17]. The slowed growth and reduced intake during HS have led to the assumption that animals must be in a caloric deficit [18,19]. However, due to HS-induced mechanistic adaptations to metabolism, excess caloric intake may exacerbate metabolic dysfunction. By limiting fat oxidation, even in a caloric surplus, HS could still diminish lean tissue growth by instead diverting fat towards storage, as is observed in metabolic syndrome [20,21].
While the impact of HS on numerous tissues is emerging, the effects of HS on total energy expenditure and basal metabolic rate are largely unknown. During HS, endogenous heat production becomes detrimental and priorities are shifted to promote lowering of core temperature [22,23] via evaporative cooling and behavioral adaptations including food avoidance and decreased physical activity [24]. Therefore, the purpose of this study was (1) to determine to what extent HS can influence shifts in total body substrate oxidation (RER) and (2) to determine if changes in substrate oxidation (RER) coincide with reduced overall metabolic rate.
2. Materials and Methods
2.1. Experimental Design
All procedures involving animals were approved by the Virginia Tech Institutional Animal Care and Use Committee (IACUC protocol number 19-114). Sixteen crossbred barrows were selected based on weight (range: 12–20 kg, mean 15.2 kg), individually housed in metabolic crates and fed commercial feed ab libitum throughout the entire experiment. Pigs were acclimated under thermoneutral conditions (14 h light/10 h dark, 21.6 ± 0.8 °C, with humidity 46–64%) for five days prior to treatment. Pigs were then randomly assigned (n = 8 per group) to thermal neutral (TN, 22.0 ± 0.4 °C, with humidity 43–63%) or heat stress (HS, 33.6 ± 0.5 °C, with humidity 22–40%) treatment groups for five days. Feed intake is reported as a daily average during the acclimation period and during the treatment period. Access to feed was temporarily restricted at 0000 h prior to indirect calorimetry and muscle biopsies and resumed immediately following these procedures. Rectal temperatures and respiratory rates were taken twice daily (0800, 2000 h) during acclimation and thrice daily (0800, 1400, 2000 h) during treatment. Rectal temperatures are reported as an average of the acclimation period (pre) and the treatment period (post).
2.2. Indirect Calorimetry
Pigs were placed in a controlled air plexiglass cage with a rubberized floor mat. Dimensions of the cage allowed 0.5 m2 of floor space for the animals to move if desired and measured approximately 1.0 m in depth by 0.5 m in width by 0.5 m in height for a volume of 0.25 m3. Pigs were evaluated after an 8-h overnight fast two days prior to the start of treatment and on the fifth day of treatment. Indirect calorimetry was performed between 0800 and 1200 h in treatment rooms under TN or HS conditions. Animals remained awake and were permitted to move. After a 60-min habituation period in the chamber, oxygen consumption and carbon dioxide production were measured each minute for 60 min using a ventilated chamber system (Delattre Metabolic Monitor, Sensor Medics Corp., Yorba Linda, CA, USA). Respiratory exchange ratio (RER, CO2expired:O2consumed) and daily energy expenditure (DEE) were calculated over the 60 min assessment using the Weir formula [25]. Values are reported as DEE rather than resting energy expenditure (RER), as the animals were not restricted from movement and allowed enrichment (rubberized bone) per institutional IACUC requirements.
2.3. Skeletal Muscle Metabolic Measures
Prior to the start of environmental treatments, muscle biopsies were taken from pigs while anesthetized under isoflurane after an overnight fast as previously described [2]. Biopsy sites were shaved and sterilized. Muscle tissue was removed from the longissimus dorsi through an incision made at about the first lumbar vertebra. A 10-gauge × 9 cm long Vacora Bard Biopsy Instrument (Bard, Murray Hill, NJ, USA) was used to harvest tissue from the biopsy sites. Approximately 90 mg of skeletal muscle tissue was collected and placed in SET buffer (0.25 M sucrose, 1 mM EDTA, 0.01 M Tris·HCl, and 2 mM ATP) for metabolic measures. After environmental treatment, biopsies were taken from the same approximate location on the contralateral side.
For fatty acid oxidation (FAO) (palmitate ([1–14C] palmitic acid) and pyruvate oxidation ([1–14C] pyruvate)) and metabolic flexibility measures, muscle tissue was homogenized as previously described [2,26]. Palmitate oxidation (FAO-CO2) was evaluated through 14CO2 production and 14C-labeled acid-soluble metabolites (FAO-ASM) from the oxidation of [1–14C] palmitic acid, the combination of palmitate oxidation and acid-soluble metabolites was referred to as the total fat uptake (total-FAO). Pyruvate oxidation was assessed by measuring 14CO2 production from the oxidation of [1–14C] pyruvate. Metabolic flexibility was evaluated by comparing [1–14C] pyruvate oxidation in the presence and absence of 100 μM palmitic acid. The ability of free fatty acid (FFA) to suppress glucose oxidation was used as a representative of metabolic flexibility with a greater reduction in pyruvate oxidation in the presence of palmitate indicating greater metabolic flexibility [26].
2.4. Statistical Analysis
Statistical analyses were performed using PRISM software (Prism version 8.0.0 for Windows, GraphPad Software, La Jolla, CA, USA). Correlations were performed using simple liner regression with an F-test used to determine statistical significance. Pre/post treatment measures (weight, RER, DEE, and metabolic measures) vs. treatment (TN/HS) were analyzed through repeated measures two-way ANOVA. Periodical measurements (feed intake, rectal temperatures, and respiratory rates) were analyzed using a restricted maximum likelihood (REML) mixed-effect analysis with repeated measures, where values are reported as the average within acclimation or treatment periods. Tukey and Sidak’s post hoc analyses determined environmental effects within timepoints and time effects within treatments, respectively. Pig variance was included as a random variable. The model included the treatment (TN and HS) and treatment-by-time point interaction. Data are reported as mean ± SEM and were considered significant at p < 0.05 and defined as a trend if >0.05 but <0.10.
3. Results
3.1. Growth and Thermoregulatory Response
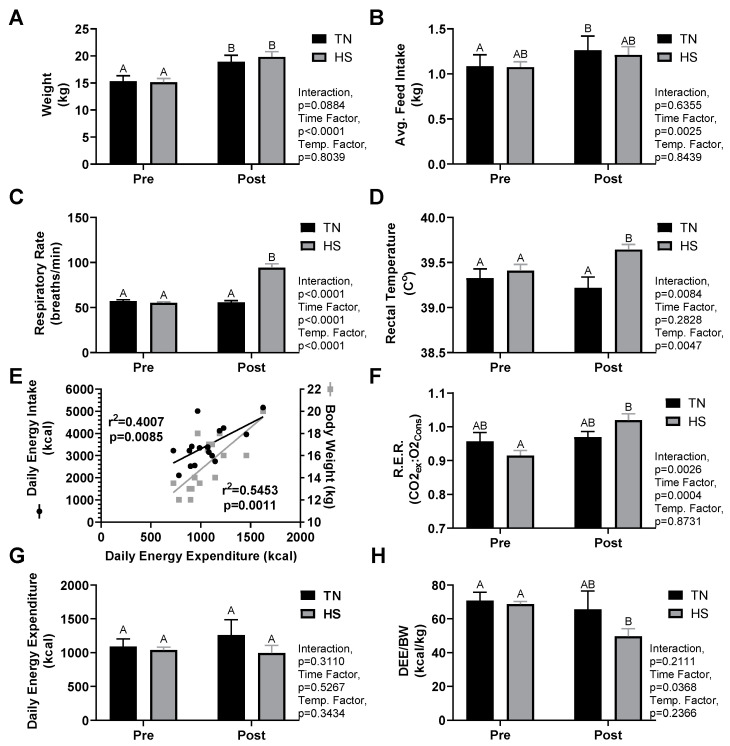
Growth and thermoregulatory parameters are summarized in Table 1 and Figure 1A–D. Pigs in both the TN and HS groups experienced similar weight gain during the study (p < 0.01 time; p = 0.80 treatment). There was no difference in feed intake prior to treatment (p > 0.99) nor was there an effect of treatment on feed intake (p = 0.84). A time effect was detected for feed intake (p < 0.01) with further analysis revealing the TN group did experience a significant increase in feed intake (p = 0.02) form pre to post while the HS group only trended on a change (p = 0.07). Respiratory rates (BPM) increased due to HS treatment (55 ± 1 vs. 94 ± 4, p < 0.01) and were higher than TN controls (p < 0.01). Rectal temperatures of HS animals increased due to treatment (pre 39.4 ± 0.1 °C vs. post 39.6 ± 0.1 °C, p = 0.02) and were higher than those of TN pigs post treatment (39.2 ± 0.1 °C, p = 0.02).
Table 1.
Summary of growth, thermoregulatory, and indirect calorimetry measures.
| Measure | Thermal Neutral | Heat Stress | Interaction | Time Effect | Temperature Effect | ||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||||
| Weight (kg) |
15.3 ± 1.1 | 18.9 ± 1.3 * | 15.1 ± 0.7 | 19.8 ± 1.0 * | 0.0884 | <0.0001 | 0.8039 |
| Rectal Temp. (°C) |
39.3 ± 0.1 | 39.2 ± 0.1 | 39.4 ± 0.1 | 39.6 ± 0.1 *t | 0.0084 | 0.2828 | 0.0470 |
| Respiratory Rate (B.P.M.) |
57 ± 2 | 56 ± 2 | 55 ± 1 | 94 ± 4 *t | <0.0001 | <0.0001 | <0.0001 |
| Feed Intake (kg/day) |
1.1 ± 0.1 | 1.3 ± 0.2 * | 1.1 ± 0.1 | 1.2 ± 0.1 | 0.6355 | 0.0025 | 0.8439 |
| RER (CO2expired:O2consumed) |
0.95 ± 0.03 | 0.97 ± 0.01 | 0.91 ± 0.02 | 1.02 ± 0.02 * | 0.0026 | 0.0004 | 0.8731 |
| DEE (kcal) |
1090 ± 120 | 1252 ± 238 | 1038 ± 48 | 992 ± 123 | 0.3110 | 0.5267 | 0.3434 |
| DEE/BW (kcal/kg) |
70.9 ± 5.1 | 67.5 ± 11.6 | 68.8 ± 1.7 | 49.7 ± 4.8 * | 0.2111 | 0.0368 | 0.2366 |
Pigs were acclimated under thermoneutral conditions (14 h light/10 h dark, 21.6 ± 0.8 °C, with humidity 46–64%) for five days prior to treatment (Pre). Pigs were then randomly assigned (n = 8 per group) to thermal neutral (TN, 22.0 ± 0.4 °C, with humidity 43–63%) or heat stress (HS, 33.6 ± 0.5 °C, with humidity 22–40%) treatment groups for five days (Post). DEE (daily energy expenditure), RER (respiratory exchange ratio). p-values were generated with two-way repeated measures ANOVA. (*) indicates time effect within treatment (p < 0.05). (t) indicates a temperature effect within time point (p < 0.05).
Figure 1.
Effect of environmental conditions on growing pig weight (A), feed intake (B), respiratory rate (C), rectal temperature (D). Correlation of daily energy expenditure (DEE) to animal body weight and energy intake (E). Indirect calorimetry measures on growing pigs of respiratory exchange ratio (RER) (F), DEE (G), and DEE per body weight (BW) (H). Pigs were acclimated under thermoneutral conditions (14 h light/10 h dark, 21.6 ± 0.8 °C, with humidity 46–64%) for five days prior to treatment (Pre). Pigs were then randomly assigned (n = 8 per group) to thermal neutral (TN, 22.0 ± 0.4 °C, with humidity 43–63%) or heat stress (HS, 33.6 ± 0.5 °C, with humidity 22–40%) treatment groups for five days (Post). Means without a shared letter are significantly different, p < 0.05.
3.2. Indirect Calorimetry
Indirect calorimetry parameters are summarized in Figure 1E–H. For both groups, pretreatment measures of DEE correlated with pig body weight (r2 = 0.55, p < 0.01) and average daily feed intake (r2 = 0.40, p < 0.01). RER was unchanged in TN animals (p = 0.74) but increased in the HS group (pre-HS 0.91 ± 0.02 vs. post-HS 1.02 ± 0.02, p < 0.01). DEE did not differ between TN (pre 1090 ± 120 kcal vs. post 1252 ± 238 kcal) and HS (pre 1038 ± 48 kcal vs. post 992 ± 123 kcal) groups prior to treatment nor was there an effect of treatment (p = 0.34). Relative energy expenditure did not exhibit a group or interaction effect, but a significant time effect was detected from pre to post treatment (p = 0.04). Though both TN and HS groups experienced a decrease in relative energy expenditure, a Sidak’s post hoc analysis revealed that this drop was only significant in the HS group (pre-HS 68.8 ± 1.7 kcal/kg vs. post-HS 49.7 ± 4.8 kcal/kg, p < 0.01).
3.3. Substrate Metabolism
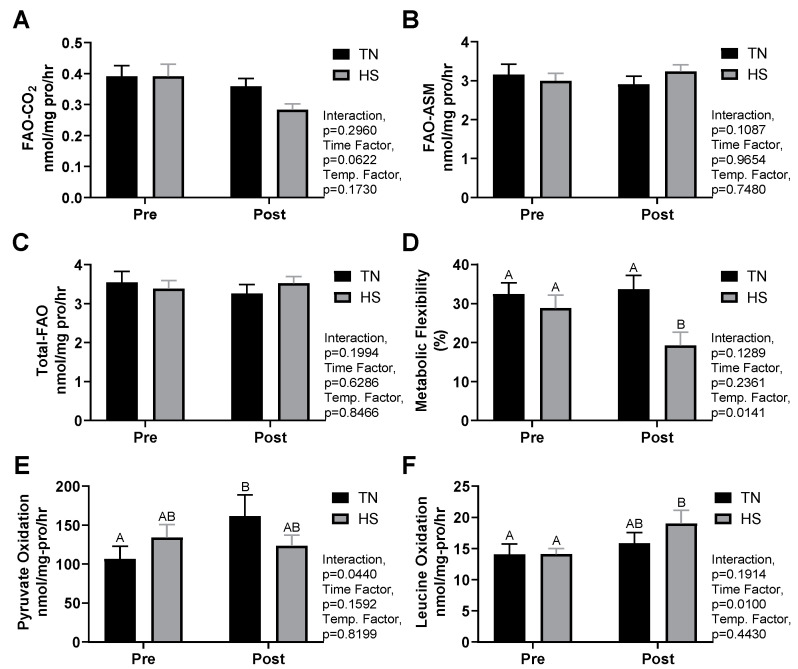
Muscle oxidative measures are summarized in Figure 2. There were no differences between groups for any of the muscle homogenate measures prior to treatment. Measures of FAO (FAO-CO2, FAO-ASM, and total-FAO) did not differ due to treatment, although complete FAO-CO2 did tend to drop in the HS group (p = 0.06) (Figure 2A–C). Metabolic flexibility was reduced in the HS group post-treatment compared to TN (19.2 ± 3.7% vs. 33.7 ± 3.8%, p < 0.01, Figure 2D). Pyruvate oxidation rates increased in the TN group after the treatment period (62%, p = 0.04), though no change was observed in HS pigs (Figure 2E). A time effect was observed for leucine oxidation rates (p = 0.01). A further Sidak’s analysis demonstrated that leucine oxidation was elevated after HS treatment (38 ± 18%, p = 0.02), but not significantly affected by TN (p = 0.48) (Figure 2F). Post metabolic flexibility measures were correlated to change in RER (r2 = 0.40, p < 0.01) and RR (r2 = 0.36, p = 0.01), and tended to be correlated with change in rectal temperature (r2 = 0.24, p = 0.06). Pyruvate oxidation was negatively correlated to changes in rectal temperature (r2 = 0.28, p = 0.04) (graphs not shown).
Figure 2.
Effect of environmental conditions on growing pigs for measures of complete fatty acid oxidation (FAO-CO2) (A), acid soluble metabolites (incomplete oxidation) (FAO-ASM) (B), total-FAO (FAO-CO2 + FAO-ASM) (C), metabolic flexibility (pyruvate oxidation:pyruvate oxidation +100 µM palmitic acid) (D), pyruvate oxidation (E), and leucine oxidation (F). Pigs were acclimated under thermoneutral conditions (14 h light/10 h dark, 21.6 ± 0.8 °C, with humidity 46–64%) for five days prior to treatment (Pre). Pigs were then randomly assigned (n = 8 per group) to thermal neutral (TN, 22.0 ± 0.4 °C, with humidity 43–63%) or heat stress (HS, 33.6 ± 0.5 °C, with humidity 22–40%) treatment groups for five days (Post). Means without a shared letter are significantly different, p < 0.05.
3.4. HS Metabolism and Performance
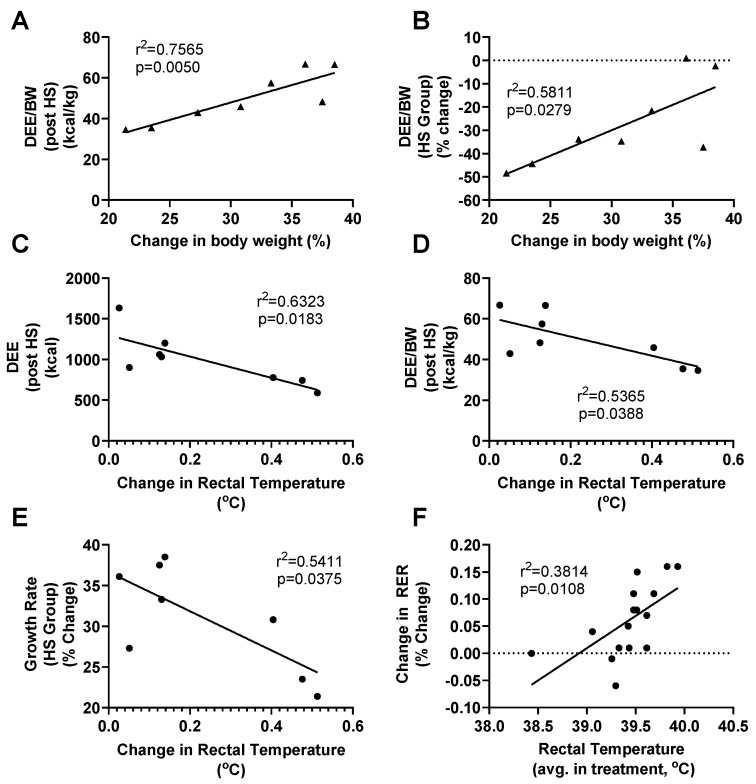
Relative energy expenditure post HS was correlated to growth (r2 = 0.77, p < 0.01, Figure 3A). Reductions in energy expenditure per body weight due to HS were also correlated to growth (r2 = 0.58, p = 0.03, Figure 3B), with the pigs who experienced the greatest decrease in energy expenditure per body weight also experiencing the smallest growth. DEE (r2 = 0.63, p = 0.02, Figure 3C) and DEE/BW (r2 = 0.54, p = 0.04, Figure 3D) of HS animals were negatively correlated with change in rectal temperature where pigs with the greatest increases in rectal temperature due to HS presented with lower rates of exergy expenditure post HS. Changes in rectal temperature were also negatively correlated with growth rates (r2 = 0.54, p = 0.04, Figure 3E), such that pigs with the greatest increases in core temperature exhibited the slowest rates of growth. A positive relationship between change in RER and change in rectal temperature is seen when both TN and HS groups were analyzed together (r2 = 0.38, p = 0.01, Figure 3F).
Figure 3.
Relationship of daily energy expenditure (DEE) per bodyweight (BW) (post treatment, (A), and effect of treatment (B) compared to animal growth rates after 5 days of heat stress (HS)). Relationship of daily energy expenditure (C), daily energy expenditure per bodyweight (D) and growth rate (E) compared to change in rectal temperature during days after HS. Changes in RER after 5 days of HS or thermal neutral environmental treatments compared to average rectal temperature during treatment (F). Pigs were acclimated under thermoneutral conditions (14 h light/10 h dark, 21.6 ± 0.8 °C, with humidity 46–64%) for five days prior to treatment. Pigs were then randomly assigned (n = 8 per group) to thermal neutral (TN, 22.0 ± 0.4 °C, with humidity 43–63%) or heat stress (HS, 33.6 ± 0.5 °C, with humidity 22–40%) treatment groups for five days.
4. Discussion
The major findings of this study are that (1) HS caused an increase in RER and (2) HS decreased metabolic rate. These findings are in opposition of typical stress responses which show increased metabolic rate and FAO [5,6]. This paper is the first to demonstrate a reduction in relative energy expenditure due to HS. Increases in RER are consistent with findings from both acute and chronic HS studies, which highlight increased reliance on glucose as a fuel substrate [12,27,28] paired with suppressed lipid oxidation [2,14]. The extent to which HS increased RER in this study is, however, notable, as fasting values above 1.00 indicate minimal contributions of lipid oxidation to metabolism and either anaerobic energy production or de novo lipogenesis [29,30].
The differences in relative energy expenditure between the TN and HS groups post treatment driven by an increased DEE in TN that was not observed in HS. The increase in TN DEE (~17%) can be attributed to the weight gain (~24%) of TN animals, as weight is highly correlated to energy expenditure [31]. Lower oxidative capacity overall may explain the differences in DEE, as the TN group observed an increase in pyruvate oxidation that was not detected in HS, and HS trended on a reduction in complete FAO that was not detected in the TN group. It should be noted that while feed intake was not different between TN and HS during acclimation or treatment, TN animals did exhibit a statistically significant increase in feed intake from pre to post (p = 0.02) while the HS group trended towards an increase (p = 0.07).
While the effects of HS on metabolic rate have not been extensively evaluated, NRC recommendations [32] state that HS increases energy demand and feed energy should be increased to compensate. These recommendations are based on acute studies of environmental temperature on metabolic rate which indicate that temperature stress (hot or cold), creates a J-shaped response in metabolic rate [33,34,35,36], such that metabolic rates increase proportionally with environmental extremes. The degree of HS for this study was relatively mild and reductions in weight gain and feed intake were not observed, yet the reductions in energy expenditure persisted. This would indicate the pigs were in a positive energy balance with excess energy most likely stored as fat. Following current recommendations [32] of increasing energy intake during HS could result in overfeeding, which would exacerbate the metabolic disruptions associated with HS. Diet-induced obesity (DIO)/diabetes and HS share many parallels in their mechanistic disruption of substrate metabolism. Both DIO/diabetes and HS persist with impaired lipid metabolism, decreased metabolic flexibility, increased reliance on carbohydrates as a fuel source, and ultimately increased adiposity [2,14,37].
The data herein support a new association between HS and decreased energy expenditure highlighting a potential role for HS as a causal factor in the development of obesity, as increased core temperatures have been reported in obese populations [38,39]. That is, obesity drives metabolic dysregulation, this dysregulation may be exacerbated or complicated by increased core temperatures, which also appears to drive independent metabolic dysregulation. This may be particularly relevant to obese populations living in hot environments like southern United States or in areas that trap heat such as urban centers [40]. These data also raise the possibility that treatments designed to ameliorate the metabolic effects of obesity may also be viable as treatments for HS.
RER is a whole-body measure of substrate metabolism. However, due to the lean composition of the production pig and the large contribution of skeletal muscle to overall body mass, skeletal muscle metabolism has major influence over RER values in this species. This study as well as our previous reports [2,41] have demonstrated that HS causes decreases in in situ muscle metabolic flexibility. As measured, a reduction in metabolic flexibility is an indication that pyruvate oxidation is the preferred means of energy production in muscle tissue regardless of lipid availability. Consistent with this, the current study demonstrates a rise in RER due to HS, where post-HS values of 1.02 ± 0.02 VCO2/VO2 indicate that carbohydrates are the primary substrate of oxidation while capacity for FAO is limited. Reduced capacity for FAO can limit muscle hypertrophy [15,42] and can lead to fat accumulation [43] and even myosteatosis [17]. RER values above 1.00 (in a sedentary state) are suggestive of de novo lipogenesis [29,30]. These findings indicate that HS pigs rarely catabolize lipid stores and that nearly all substrate used for energy production is coming directly from dietary intake of carbohydrates or liver gluconeogenesis possibly fueled by increased proteolysis [29,44,45].
Increased proteolysis also appears to provide amino acids (AAs) as alternate and direct fuel sources to muscle tissue, as leucine oxidation increased 35% following HS. Oxidation of branch chain amino acids typically only becomes a significant energy source during prolonged exercise or extended fasting, generally stimulated by rising cortisol levels and elevated proteolysis [46]. Other studies have reported increased proteolysis due to HS [47,48], which was previously thought to provide substrate for gluconeogenesis [45,49]. However, data from this study suggest muscle tissue may mobilize and oxidize significant levels of AAs during HS along with increased glucose oxidation, possibly to compensate for the blunted lipid metabolism. It should be noted that measures from this study looked only at leucine oxidation and it is not known how the oxidation rates of other amino acids are influenced by HS.
5. Conclusions
Data from the current study indicate HS suppresses metabolic rate mostly due to HS-induced suppression of lipid oxidation as indicated by elevated RER levels and reduced muscle fatty acid oxidation. Heat stress also caused increased leucine oxidation in muscle, possibly as a mechanism to compensate for the loss of lipid oxidation. The net effect of HS is a metabolic phenotype that closely resembles overfeeding, possibly leading to an adiposity phenotype shared by HS and overfeeding conditions.
Acknowledgments
The authors would like to thank Kevin P. Davy for providing use of the metabolic cart during this study.
Author Contributions
Conceptualization, D.W.F. and R.P.R.; methodology, R.P.M., M.W.H., and R.P.R.; software, D.W.F., and R.P.R.; validation, D.W.F. and R.P.R.; formal analysis, D.W.F. and R.P.R.; investigation, D.W.F., K.A.K., L.S.M., and R.P.R.; resources, M.W.H., and R.P.R.; data curation, D.W.F. and R.P.R.; writing—original draft preparation, D.W.F.; writing—review and editing, D.W.F., K.A.K., L.S.M., L.H.B., J.T.S., and R.P.R.; visualization, D.W.F., K.A.K., and R.P.R.; supervision, D.W.F., K.A.K., L.S.M., R.P.R.; project administration, D.W.F., K.A.K., and R.P.R.; funding acquisition, L.H.B., J.T.S., and R.P.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by USDA AFRI grants 2017-05931, 2014-67015-21627 and 2011-6700330007.
Institutional Review Board Statement
All procedures involving animals were approved by the Virginia Tech Institutional Animal Care and Use Committee (IACUC protocol number 19-114).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sun H., Jiang R., Xu S., Zhang Z., Xu G., Zheng J., Qu L. Transcriptome responses to heat stress in hypothalamus of a meat-type chicken. J. Anim. Sci. Biotechnol. 2015;6:6. doi: 10.1186/s40104-015-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao L., McMillan R.P., Xie G., Giridhar S., Baumgard L.H., El-Kadi S., Selsby J., Ross J., Gabler N., Hulver M.W., et al. Heat stress decreases metabolic flexibility in skeletal muscle of growing pigs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018;315:R1096–R1106. doi: 10.1152/ajpregu.00404.2017. [DOI] [PubMed] [Google Scholar]
- 3.He J., He Y., Pan D., Cao J., Sun Y., Zeng X. Associations of Gut Microbiota with Heat Stress-Induced Changes of Growth, Fat Deposition, Intestinal Morphology, and Antioxidant Capacity in Ducks. Front. Microbiol. 2019;10:903. doi: 10.3389/fmicb.2019.00903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernabucci U., Lacetera N., Baumgard L.H., Rhoads R.P., Ronchi B., Nardone A. Metabolic and hormonal acclimation to heat stress in domesticated ruminants. Animal. 2010;4:1167–1183. doi: 10.1017/S175173111000090X. [DOI] [PubMed] [Google Scholar]
- 5.Lkhagvasuren B., Nakamura Y., Oka T., Sudo N., Nakamura K. Social defeat stress induces hyperthermia through activation of thermoregulatory sympathetic premotor neurons in the medullary raphe region. Eur. J. Neurosci. 2011;34:1442–1452. doi: 10.1111/j.1460-9568.2011.07863.x. [DOI] [PubMed] [Google Scholar]
- 6.García-Díaz D.F., Campion J., Milagro F.I., Lomba A., Marzo F., Martínez J.A. Chronic mild stress induces variations in locomotive behavior and metabolic rates in high fat fed rats. J. Physiol. Biochem. 2007;63:337–346. doi: 10.1007/BF03165765. [DOI] [PubMed] [Google Scholar]
- 7.Funakoshi K., Nakano M., Atobe Y., Goris R.C., Kadota T., Yazama F. Differential development of TRPV1-expressing sensory nerves in peripheral organs. Cell Tissue Res. 2006;323:27–41. doi: 10.1007/s00441-005-0013-3. [DOI] [PubMed] [Google Scholar]
- 8.Bertin S., Aoki-Nonaka Y., de Jong P.R., Nohara L.L., Xu H., Stanwood S.R., Srikanth S., Lee J., To K., Abramson L., et al. The ion channel TRPV1 regulates the activation and proinflammatory properties of CD4⁺ T cells. Nat. Immunol. 2014;15:1055–1063. doi: 10.1038/ni.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L., Schumann U., Liu Y., Prokopchuk O., Steinacker J.M. Heat shock protein 70 (Hsp70) inhibits oxidative phosphorylation and compensates ATP balance through enhanced glycolytic activity. J. Appl. Physiol. 2012;113:1669–1676. doi: 10.1152/japplphysiol.00658.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuan Y.C., Hashidume T., Shibata T., Uchida K., Shimizu M., Inoue J., Sato R. Heat Shock Protein 90 Modulates Lipid Homeostasis by Regulating the Stability and Function of Sterol Regulatory Element-binding Protein (SREBP) and SREBP Cleavage-activating Protein. J. Biol. Chem. 2017;292:3016–3028. doi: 10.1074/jbc.M116.767277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marshall J.P.S., Estevez E., Kammoun H.L., King E.J., Bruce C.R., Drew B.G., Qian H., Iliades P., Gregorevic P., Febbraio M.A., et al. Skeletal muscle-specific overexpression of heat shock protein 72 improves skeletal muscle insulin-stimulated glucose uptake but does not alter whole body metabolism. Diabetes Obes. Metab. 2018;20:1928–1936. doi: 10.1111/dom.13319. [DOI] [PubMed] [Google Scholar]
- 12.Goto A., Egawa T., Sakon I., Oshima R., Ito K., Serizawa Y., Sekine K., Tsuda S., Goto K., Hayashi T. Heat stress acutely activates insulin-independent glucose transport and 5’-AMP-activated protein kinase prior to an increase in HSP72 protein in rat skeletal muscle. Physiol. Rep. 2015;3:e12601. doi: 10.14814/phy2.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Q., Tu J., Dou C., Zhang J., Yang L., Liu X., Lei K., Liu Z., Wang Y., Li L., et al. HSP90 promotes cell glycolysis, proliferation and inhibits apoptosis by regulating PKM2 abundance via Thr-328 phosphorylation in hepatocellular carcinoma. Mol. Cancer. 2017;16:178. doi: 10.1186/s12943-017-0748-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Victoria Sanz Fernandez M., Johnson J.S., Abuajamieh M., Stoakes S.K., Seibert J.T., Cox L., Kahl S., Elsasser T.H., Ross J.W., Isom S.C., et al. Effects of heat stress on carbohydrate and lipid metabolism in growing pigs. Physiol. Rep. 2015;3:e12315. doi: 10.14814/phy2.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasiakos S.M., Vislocky L.M., Carbone J.W., Altieri N., Konopelski K., Freake H.C., Anderson J.M., Ferrando A.A., Wolfe R.R., Rodriguez N.R. Acute Energy Deprivation Affects Skeletal Muscle Protein Synthesis and Associated Intracellular Signaling Proteins in Physically Active Adults. J. Nutr. 2010;140:745–751. doi: 10.3945/jn.109.118372. [DOI] [PubMed] [Google Scholar]
- 16.Areta J.L., Burke L.M., Camera D.M., West D.W.D., Crawshay S., Moore D.R., Stellingwerff T., Phillips S.M., Hawley J.A., Coffey V.G. Reduced resting skeletal muscle protein synthesis is rescued by resistance exercise and protein ingestion following short-term energy deficit. Am. J. Physiol. Endocrinol. Metab. 2014;306:E989–E997. doi: 10.1152/ajpendo.00590.2013. [DOI] [PubMed] [Google Scholar]
- 17.Gumucio J.P., Qasawa A.H., Ferrara P.J., Malik A.N., Funai K., McDonagh B., Mendias C.L. Reduced mitochondrial lipid oxidation leads to fat accumulation in myosteatosis. bioRxiv. 2019;33:7863–7881. doi: 10.1096/fj.201802457RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearce S.C., Lonergan S.M., Huff-Lonergan E., Baumgard L.H., Gabler N.K. Acute Heat Stress and Reduced Nutrient Intake Alter Intestinal Proteomic Profile and Gene Expression in Pigs. PLoS ONE. 2015;10:e0143099. doi: 10.1371/journal.pone.0143099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baumgard L.H., Rhoads R.P., Jr. Effects of Heat Stress on Postabsorptive Metabolism and Energetics. Annu. Rev. Anim. Biosci. 2013;1:311–337. doi: 10.1146/annurev-animal-031412-103644. [DOI] [PubMed] [Google Scholar]
- 20.Horton T.J., Drougas H., Brachey A., Reed G.W., Peters J.C., Hill J.O. Fat and carbohydrate overfeeding in humans: Different effects on energy storage. Am. J. Clin. Nutr. 1995;62:19–29. doi: 10.1093/ajcn/62.1.19. [DOI] [PubMed] [Google Scholar]
- 21.Joosen A.M.C.P., Westerterp K.R. Energy expenditure during overfeeding. Nutr. Metab. 2006;3:25. doi: 10.1186/1743-7075-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cramer M.N., Jay O. Biophysical aspects of human thermoregulation during heat stress. Auton. Neurosci. 2016;196:3–13. doi: 10.1016/j.autneu.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Seibert J.T., Graves K.L., Hale B.J., Keating A.F., Baumgard L.H., Ross J.W. Characterizing the acute heat stress response in gilts: I. Thermoregulatory and production variables. J. Anim. Sci. 2018;96:941–949. doi: 10.1093/jas/skx036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghassemi Nejad J., Sung K.-I. Behavioral and physiological changes during heat stress in Corriedale ewes exposed to water deprivation. J. Anim. Sci. Technol. 2017;59:13. doi: 10.1186/s40781-017-0140-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weir J.B.d.V. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tarpey M.D., Davy K.P., McMillan R.P., Bowser S.M., Halliday T.M., Boutagy N.E., Davy B.M., Frisard M.I., Hulver M.W. Skeletal muscle autophagy and mitophagy in endurance-trained runners before and after a high-fat meal. Mol. Metab. 2017;6:1597–1609. doi: 10.1016/j.molmet.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanz Fernandez M.V., Stoakes S.K., Abuajamieh M., Seibert J.T., Johnson J.S., Horst E.A., Rhoads R.P., Baumgard L.H. Heat stress increases insulin sensitivity in pigs. Physiol. Rep. 2015;3:e12478. doi: 10.14814/phy2.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimball A.L., McCue P.M., Petrie M.A., Shields R.K. Whole body heat exposure modulates acute glucose metabolism. Int. J. Hyperth. 2018;35:644–651. doi: 10.1080/02656736.2018.1516303. [DOI] [PubMed] [Google Scholar]
- 29.Aarsland A., Chinkes D., Wolfe R.R. Hepatic and whole-body fat synthesis in humans during carbohydrate overfeeding. Am. J. Clin. Nutr. 1997;65:1774–1782. doi: 10.1093/ajcn/65.6.1774. [DOI] [PubMed] [Google Scholar]
- 30.Minehira K., Vega N., Vidal H., Acheson K., Tappy L. Effect of carbohydrate overfeeding on whole body macronutrient metabolism and expression of lipogenic enzymes in adipose tissue of lean and overweight humans. Int. J. Obes. 2004;28:1291–1298. doi: 10.1038/sj.ijo.0802760. [DOI] [PubMed] [Google Scholar]
- 31.Dériaz O., Fournier G., Tremblay A., Després J.P., Bouchard C. Lean-body-mass composition and resting energy expenditure before and after long-term overfeeding. Am. J. Clin. Nutr. 1992;56:840–847. doi: 10.1093/ajcn/56.5.840. [DOI] [PubMed] [Google Scholar]
- 32.National Research Council (US) Subcommittee on Environmental Stress . Effect of Environment on Nutrient Requirements of Domestic Animals. National Academies Press (US); Washington, DC, USA: 1981. [PubMed] [Google Scholar]
- 33.Mount L.E. The metabolic rate of the new-born pig in relation to environmental temperature and to age. J. Physiol. 1959;147:333–345. doi: 10.1113/jphysiol.1959.sp006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuller M.F., Boyne A.W. The effects of environmental temperature on the growth and metabolism of pigs given different amounts of food: 2. Energy metabolism. Br. J. Nutr. 1972;28:373–384. doi: 10.1079/BJN19720046. [DOI] [PubMed] [Google Scholar]
- 35.Saxton C. Effects of severe heat stress on respiration and metabolic rate in resting man. Aviat. Space Environ. Med. 1981;52:281–286. [PubMed] [Google Scholar]
- 36.Mayorga E.J., Renaudeau D., Ramirez B.C., Ross J.W., Baumgard L.H. Heat stress adaptations in pigs. Anim. Front. 2018;9:54–61. doi: 10.1093/af/vfy035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Begaye B., Vinales K.L., Hollstein T., Ando T., Walter M., Bogardus C., Krakoff J., Piaggi P. Impaired Metabolic Flexibility to High-Fat Overfeeding Predicts Future Weight Gain in Healthy Adults. Diabetes. 2020;69:181–192. doi: 10.2337/db19-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bastardot F., Marques-Vidal P., Vollenweider P. Association of body temperature with obesity. The CoLaus study. Int. J. Obes. 2019;43:1026–1033. doi: 10.1038/s41366-018-0218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heikens M.J., Gorbach A.M., Eden H.S., Savastano D.M., Chen K.Y., Skarulis M.C., Yanovski J.A. Core body temperature in obesity. Am. J. Clin. Nutr. 2011;93:963–967. doi: 10.3945/ajcn.110.006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hibbard K.A., Hoffman F.M., Huntzinger D., West T.O. Changes in land cover and terrestrial biogeochemistry. In: Wuebbles D.J., Fahey D.W., Hibbard K.A., Dokken D.J., Stewart B.C., Maycock T.K., editors. Climate Science Special Report: Fourth National Climate Assessment. Volume I. U.S. Global Change Research Program; Washington, DC, USA: 2017. pp. 277–302. [DOI] [Google Scholar]
- 41.Seelenbinder K.M., Zhao L.D., Hanigan M.D., Hulver M.W., McMillan R.P., Baumgard L.H., Selsby J.T., Ross J.W., Gabler N.K., Rhoads R.P. Effects of heat stress during porcine reproductive and respiratory syndrome virus infection on metabolic responses in growing pigs. J. Anim. Sci. 2018;96:1375–1387. doi: 10.1093/jas/sky057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gueugneau M., Coudy-Gandilhon C., Théron L., Meunier B., Barboiron C., Combaret L., Taillandier D., Polge C., Attaix D., Picard B., et al. Skeletal Muscle Lipid Content and Oxidative Activity in Relation to Muscle Fiber Type in Aging and Metabolic Syndrome. J. Gerontol. Ser. A. 2014;70:566–576. doi: 10.1093/gerona/glu086. [DOI] [PubMed] [Google Scholar]
- 43.Zurlo F., Lillioja S., Puente A.E.-D., Nyomba B.L., Raz I., Saad M.F., Swinburn B.A., Knowler W.C., Bogardus C., Ravussin E. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: Study of 24-h RQ. Am. J. Physiol. Endocrinol. Metab. 1990;259:E650–E657. doi: 10.1152/ajpendo.1990.259.5.E650. [DOI] [PubMed] [Google Scholar]
- 44.Colditz P., Kellaway R. The effect of diet and heat stress on feed intake, growth, and nitrogen metabolism in Friesian, and Brahman heifers. Aust. J. Agric. Res. 1972;23:717–725. doi: 10.1071/AR9720717. [DOI] [Google Scholar]
- 45.Gao S.T., Guo J., Quan S.Y., Nan X.M., Fernandez M.V.S., Baumgard L.H., Bu D.P. The effects of heat stress on protein metabolism in lactating Holstein cows. J. Dairy Sci. 2017;100:5040–5049. doi: 10.3168/jds.2016-11913. [DOI] [PubMed] [Google Scholar]
- 46.Simmons P.S., Miles J.M., Gerich J.E., Haymond M.W. Increased proteolysis. An effect of increases in plasma cortisol within the physiologic range. J. Clin. Investig. 1984;73:412–420. doi: 10.1172/JCI111227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chung N., Jenkins G., Hannun Y.A., Heitman J., Obeid L.M. Sphingolipids Signal Heat Stress-induced Ubiquitin-dependent Proteolysis. J. Biol. Chem. 2000;275:17229–17232. doi: 10.1074/jbc.C000229200. [DOI] [PubMed] [Google Scholar]
- 48.Cruzen S.M., Baumgard L.H., Gabler N.K., Pearce S.C., Lonergan S.M. Temporal proteomic response to acute heat stress in the porcine muscle sarcoplasm1. J. Anim. Sci. 2017;95:3961–3971. doi: 10.2527/jas.2017.1375. [DOI] [PubMed] [Google Scholar]
- 49.Koch F., Lamp O., Eslamizad M., Weitzel J., Kuhla B. Metabolic Response to Heat Stress in Late-Pregnant and Early Lactation Dairy Cows: Implications to Liver-Muscle Crosstalk. PLoS ONE. 2016;11:e0160912. doi: 10.1371/journal.pone.0160912. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.