To the editor:
The dynamics of immune response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in kidney transplant recipients (KTRs) remains largely unknown. KTRs have been reported to develop serological responses to SARS-CoV-2.1 , 2 However, information about the duration and significance of antibody response in this immunocompromised population is still critically lacking. We herein report anti–SARS-CoV-2 IgG trajectory in a cohort of KTRs followed at Necker Hospital (Paris, France) between 2 and 6 months after symptomatic coronavirus disease 2019 (COVID-19) infection.
Forty-two patients (22 men [52.4%]; median age of 57.7 years; interquartile range [IQR]: 47.2–67.0), who developed COVID-19 infection between March 14 and May 2, 2020, were included. COVID-19 was defined by typical clinical symptoms associated to a positive SARS-CoV-2 polymerase chain reaction test on nasopharyngeal swab. Sera were tested for the presence of anti-nucleocapsid protein IgG by a chemiluminescent microparticle immunoassay (SARS-CoV-2 IgG assay, Abbott, Abbott Park, IL) at 2 and 6 months after COVID-19 onset. According to the manufacturer’s instructions, an IgG index >1.4 indicates a positive serology while an IgG index between 0.4 and 1.4 is considered to be an equivocal result and IgG index <0.4 to be a negative result. Sera were available for all patients at month 2 and for 33 of 42 patients at month 6.
COVID-19 occurred at a median time of 6.3 years (IQR: 3.1–12.7) after transplantation. In our cohort, 32 patients (76.2%) required hospitalization, including 7 (21.9%) in an intensive care unit (ICU), none of whom died.
At first serological testing (month 2), all patients had recovered and SARS-CoV-2 polymerase chain reaction was negative in 40 patients (95.2%). At month 6, SARS-CoV-2 polymerase chain reaction was negative in all except 1 patient.
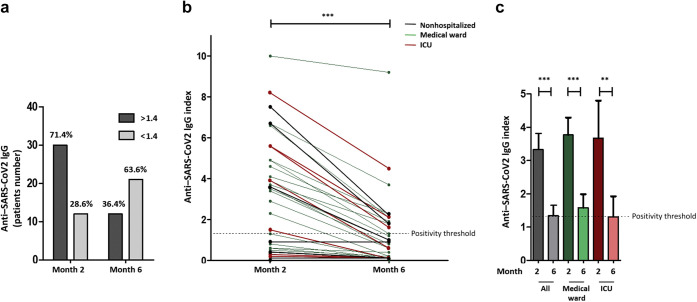
Of the 42 patients, 30 (71.4%) were seropositive (IgG> 1.4) at month 2 (Figure 1 a). Among the 21 of 33 patients (63.6%) who were IgG-positive at month 2 and who had available sera at month 6, 12 (57.1%) remained positive (index ≥1.4) at month 6, while 9 (42.9%) had negative or equivocal results. Overall, 21 of 33 patients (63.6%) had an IgG index <1.4 at month 6 (Figure 1a), including 14 of 24 patients (58.3%) and 4 of 7 (57.1%) who, respectively, required hospitalization and ICU stay at the time of the COVID-19 episode.
Figure 1.
Anti–severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) IgG evolution between 2 and 6 months after coronavirus disease 2019 onset in kidney transplant recipients. (a) A total of 71.4% of patients have a positive IgG response at month 2 while 63.6% of them have a negative or equivocal IgG serology at month 6. (b) Anti–SARS-CoV-2 IgG index decreases in all patients between months 2 and 6. (c) Median anti–SARS-CoV-2 IgG index significantly decreases between months 2 and 6 in all patients, including patients requiring hospitalization or treatment in the intensive care unit (ICU).
IgG index decreased between months 2 and 6 in all patients including in patients requiring hospitalization or ICU stay. Median IgG index fell from 3.6 (IQR: 1.3–5.1) at month 2 to 0.7 (IQR: 0.1–2.0) at month 6 (Figure 1b and c). Median decrease was 80.3% (IQR: 60.8%–83.3%; P < 0.0001). No patient relapsed from COVID-19 infection.
At month 6, there was no correlation between IgG index and initial disease severity (P = 0.65), post-transplantation delay (P = 0.99), or induction therapy by anti-thymocyte globulins (P = 0.77).
In conclusion, this is the first study assessing the anti–SARS-CoV2-IgG trajectory over a period of 6 months after disease onset in KTRs. Our results confirm that most KTRs develop specific antibodies against SARS-CoV-2.1 However, antibody levels rapidly decrease in all patients and more than 60% had negative or equivocal IgG results at month 6. Interestingly, antibodies turned also negative or equivocal in patients with severe forms. Data about anti–SARS-CoV-2 antibodies’ duration in the general population are controversial.3 , 4 However, antibodies’ decline had been mainly described in mild disease forms.4 , 5 Further studies are needed to assess long-term antibody response in KTRs and its potential correlation with COVID-19 reinfections or relapses, as well as the efficacy of the vaccine in this population.
References
- 1.Prendecki M., Clarke C., Gleeson S., et al. Detection of SARS-CoV-2 antibodies in kidney transplant recipients. J Am Soc Nephrol. 2020;31:2753–2756. doi: 10.1681/ASN.2020081152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi M., Bachmann F., Naik M.G., et al. Low seroprevalence of SARS-CoV-2 antibodies during systematic antibody screening and serum responses in patients after COVID-19 in a German transplant center. J Clin Med. 2020;9:3401. doi: 10.3390/jcm9113401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dan J.M., Mateus J., Kato Y., et al. Immunological memory to SARS-CoV-2 assessed for greater than six months after infection. http://biorxiv.org/lookup/doi/10.1101/2020.11.15.383323 Available at: Accessed December 23, 2020. [DOI] [PMC free article] [PubMed]
- 4.Bölke E., Matuschek C., Fischer J.C. Loss of anti–SARS-CoV-2 antibodies in mild Covid-19. N Engl J Med. 2020;383:1694–1698. doi: 10.1056/NEJMc2027051. [DOI] [PubMed] [Google Scholar]
- 5.Patel M.M., Thornburg N.J., Stubblefield W.B., et al. Change in antibodies to SARS-CoV-2 over 60 days among health care personnel in Nashville, Tennessee. JAMA. 2020;324:1781–1782. doi: 10.1001/jama.2020.18796. [DOI] [PMC free article] [PubMed] [Google Scholar]