Abstract
Simple Summary
Chemotherapy ahead of surgery is standard of care for locally advanced stomach cancer or cancer at the junction between esophagus and stomach in Europe. However, response to chemotherapy may depend on microscopic features of the tumor. Three types were defined before: intestinal, diffuse and mixed types. The authors aimed to investigate if these characteristics influence survival after end of treatment (chemotherapy+surgery) in a large cohort treated in a University hospital. It was found that intestinal type patients demonstrate longer survival after chemotherapy+surgery than those with diffuse types. In the mixed type group no clear conclusion regarding the effect of chemotherapy ahead of surgery may be taken. Conclusively, patients with diffuse type tumors do not benefit from chemotherapy ahead of surgery.
Abstract
Background: the purpose of this analysis was to analyze the outcomes of multimodal treatment that are related to Lauren histotypes in gastro-esophageal cancer (GEC). Methods: patients with GEC between 1986 and 2013 were analyzed. Uni- and multivariate regression analysis were performed to identify predictors for overall survival. Lauren histotype stratified overall survival (OS)-rates were analyzed by the Kaplan–Meier method. Further, propensity score matching (PSM) was performed to balance for confounders. Results: 1290 patients were analyzed. After PSM, the median survival was 32 months for patients undergoing primary surgery (PS) and 43 months for patients undergoing neoadjuvant chemotherapy (nCTx) ahead of surgery. For intestinal types, median survival time was 34 months (PS) vs. 52 months (nCTx+surgery) p = 0.07, 36 months (PS) vs. (31) months (nCTx+surgery) in diffuse types (p = 0.44) and 31 months (PS) vs. 62 months (nCTx+surgery) for mixed types (p = 0.28). Five-/Ten-year survival rates for intestinal, diffuse, and mixed types were 44/29%, 36/17%, and 43/33%, respectively. After PSM, Kaplan–Meier showed a survival benefit for patients undergoing nCTx+surgery in intestinal and mixed types. Conclusion: the Lauren histotype might be predictive for survival outcome in GEC-patients after neoadjuvant/perioperative chemotherapy.
Keywords: gastric/gastroesophageal cancer, perioperative chemotherapy, Lauren histotype
1. Introduction
Gastric cancer belongs to the most common malignant diseases worldwide with the highest incidence in Eastern Asia [1]. Despite decreasing incidence in the West, it remains a therapeutic challenge. In the Western hemisphere gastric malignancy is often diagnosed at an advanced stage and, in contrast to Eastern Asia, it is preferably located in the proximal third of the stomach or the gastro-esophageal junction (GEJ) [2]. Hence, multimodal treatment concepts have been introduced after demonstrating outcome benefits in randomized controlled trials [3,4,5]. Nevertheless, not all patients are benefitting from neoadjuvant or perioperative chemotherapy, depending on localization, regimen, and also on the histological subtype. In the past, a signet ring cell, like gastric cancer, was identified to be non-responsive to neoadjuvant chemotherapy [6,7]. However, the data published so far have been difficult to interpret, as there were numerous definitions on the histology of signet ring cell or signet ring cell, like gastric cancer [8]. A pragmatic and feasible sub-classification was only recently published [9]. However, none of the prospective trials investigating the value of neoadjuvant chemotherapy applied this classification system before. Therefore, it is of special interest if already established histopathological classifications may stratify and identify patients to benefit from neoadjuvant/perioperative chemotherapy. This may be accomplished by the widely accepted Lauren classification, because all of the relevant histopathological subtypes (signet ring cell type, poorly-cohesive signet ring cell type, poorly cohesive non-signet-ring cell type, mucinous, papillary, and tubular) are summarized here [10]. Therefore, it was hypothesized that the Lauren histotype dependent histopathologic response may influence survival outcomes after neoadjuvant/perioperative chemotherapy and the aim of this analysis was to evaluate the oncologic outcomes of perioperative/neoadjuvant chemotherapy in a large German single center cohort, depending on the Lauren histotype.
2. Results
For this retrospective analysis, the institutional database for gastric cancer patients was screened and identified 2782 patients having been treated by either surgery or chemotherapy followed by surgery. After removing all cases not fulfilling the defined inclusion criteria (n = 1573), 1209 patients were included in this analysis. 730 patients underwent primary surgery and 479 underwent neoadjuvant/perioperative chemotherapy ahead of surgery. Overall, 663 were diagnosed with Lauren intestinal (398 surgery, 265 nCTx), 359 Lauren diffuse (216 surgery, 143 nCTx), and 187 Lauren mixed type (116 surgery, 71 nCTx). In the entire patient cohort, 247 patients received PLF (20.4%), 41 patients PLF+Taxol (3.4%), 53 (4.4%) OLF, 47 (3.9%) MAGIC, 17 (1.4%) FLOT, and 63 patients received modified regimens (5.2%). The analysis of the baseline characteristics showed significant differences between the primary surgery and neoadjuvant/perioperative chemotherapy group regarding gender distribution (more female patients for intestinal and diffuse. but not mixed Laurentype), older age for patients undergoing primary surgery (all Lauren subtypes), higher proportion of distal cancer locations in primary surgery patients (all groups, especially intestinal type), less advanced cT-stages in the surgery only group (cT2 vs. cT3/4 over all Lauren subtypes), earlier clinical stages, higher proportion of patients requiring extension to the distal esophagus in the chemotherapy group (all Lauren subtypes), higher D2 rates and higher median number of dissected lymph-nodes (LN) in patients undergoing direct surgery (especially in intestinal and mixed Lauren histotype, not so in diffuse type), more pT4a cancers in the primary resection group for all of the Lauren subtypes, earlier UICC stages in those patients undergoing neoadjuvant/perioperative chemotherapy. The proportion of Lauren subtypes, histiopathologic grading, R0-status, and complication rates were balanced between the groups. The histopathologic response rates (Becker Ia/Ib) were 22% in Lauren intestinal type, 9% in Lauren diffuse type, and 21% in Lauren mixed type tumors. Table 1, Table 2 and Table 3 depict the complete baseline characteristics.
Table 1.
Baseline characteristics for patients with intestinal Lauren subtype before and after propensity score matching (PSM).
| Intestinal Subtype (n = 663), Unmatched | Intestinal Subtype (n = 340) PS-Matched | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Surgery Only (n = 398) |
CTX + Surgery (n = 265) |
p-Value | Surgery Only (n = 170) |
CTX + Surgery (n = 170) |
p-Value | |||||
| n | % | n | % | n | % | n | % | |||
| Gender | <0.001 | 0.14 | ||||||||
| Female | 132 | 33.17 | 42 | 15.85 | 30 | 17.65 | 42 | 24.71 | ||
| Male | 266 | 66.83 | 223 | 84.15 | 140 | 82.35 | 128 | 75.29 | ||
| Age | 68.7 ± 10.8 | 60.1 ± 10.5 | <0.001 | 65.8 ±10.3 | 61.6 ±11.1 | <0.001 | ||||
| <70 | 188 | 47.24 | 206 | 77.74 | <0.001 | 112 | 65.88 | 114 | 67.06 | 0.91 |
| >70 | 210 | 52.76 | 59 | 22.26 | 58 | 34.12 | 56 | 32.94 | ||
| Localization | <0.001 | 0.24 | ||||||||
| Proximal | 238 | 59.80 | 219 | 82.64 | 129 | 75.88 | 134 | 78.82 | ||
| Middle | 63 | 15.83 | 21 | 7.92 | 15 | 8.82 | 16 | 9.41 | ||
| Distal | 90 | 22.61 | 25 | 9.43 | 22 | 12.94 | 20 | 11.76 | ||
| Total | 7 | 1.76 | 0 | 0.00 | 4 | 2.35 | 0 | 0.00 | ||
| Clinical Staging | <0.001 | 0.21 | ||||||||
| cT2 cN+/cNx | 161 | 40.45 | 31 | 11.70 | <0.001 | 30 | 17.65 | 31 | 18.24 | 0.99 |
| cT3/cT4 cN0 | 23 | 5.78 | 26 | 9.81 | 15 | 8.82 | 15 | 8.82 | ||
| cT3/cT4 cN+/cNx | 213 | 53.52 | 208 | 78.49 | 125 | 73.53 | 124 | 72.94 | ||
| Dissected LN (Median) | 33 (1–105) | 29 (5–71) | 0.002 | 33 (7–105) | 30 (12–71) | 0.10 | ||||
| ≤25 | 101 | 25.38 | 97 | 36.60 | 44 | 25.88 | 51 | 30.00 | 0.47 | |
| >25 | 297 | 74.62 | 168 | 63.40 | 126 | 74.12 | 119 | 70.00 | ||
| Complications | 0.25 | 0.58 | ||||||||
| None | 298 | 74.87 | 187 | 70.57 | 123 | 72.35 | 125 | 73.53 | ||
| CD I/II | 67 | 16.83 | 46 | 17.36 | 30 | 17.65 | 24 | 14.12 | ||
| CD III-V | 33 | 8.29 | 32 | 12.08 | 17 | 10.00 | 21 | 12.35 | ||
| pT | <0.001 | 0.11 | ||||||||
| pT0/is | 0 | 0.00 | 14 | 5.28 | 0 | 0.00 | 6 | 3.53 | ||
| pT1a | 10 | 2.51 | 5 | 1.89 | 1 | 0.59 | 2 | 1.18 | ||
| pT1b | 35 | 8.79 | 21 | 7.92 | 5 | 2.94 | 7 | 4.12 | ||
| pT2 | 66 | 16.58 | 42 | 15.85 | 24 | 14.12 | 24 | 14.12 | ||
| pT3 | 183 | 45.98 | 139 | 52.5 | 92 | 54.12 | 99 | 58.24 | ||
| pT4a | 84.00 | 21.11 | 38 | 14.3 | 40.00 | 23.53 | 28 | 16.47 | ||
| pT4b | 14 | 3.52 | 6 | 2.26 | 8 | 4.71 | 4 | 2.35 | ||
| pN | 0.65 | 0.27 | ||||||||
| pN0 | 141 | 35.43 | 104 | 39.25 | 55 | 32.35 | 53 | 31.18 | ||
| pN1 | 85 | 21.36 | 54 | 20.4 | 34 | 20.00 | 36 | 21.18 | ||
| pN2 | 74 | 18.59 | 51 | 19.2 | 33 | 19.41 | 38 | 22.35 | ||
| pN3a | 70 | 17.59 | 44 | 16.6 | 28 | 16.47 | 34 | 20.00 | ||
| pN3b | 28 | 7.04 | 12 | 4.53 | 20 | 11.76 | 9 | 5.29 | ||
| UICC | 0.001 | 0.19 | ||||||||
| UICC 0 | 0 | 0.00 | 14 | 5.28 | 0 | 0.00 | 6 | 3.53 | ||
| UICC IA | 34 | 8.54 | 18 | 6.79 | 5 | 2.94 | 4 | 2.35 | ||
| UICC IB | 37 | 9.30 | 32 | 12.1 | 15 | 8.82 | 13 | 7.65 | ||
| UICC IIA | 77 | 19.35 | 44 | 16.6 | 33 | 19.41 | 33 | 19.41 | ||
| UICC IIB | 71 | 17.84 | 49 | 18.5 | 28 | 16.47 | 32 | 18.82 | ||
| UICC IIIA | 83 | 20.85 | 50 | 18.9 | 38 | 22.35 | 38 | 22.35 | ||
| UICC IIIB | 68 | 17.09 | 45 | 17 | 31 | 18.24 | 34 | 20.00 | ||
| UICC IIIC | 28 | 7.04 | 13 | 4.91 | 20 | 11.76 | 10 | 5.88 | ||
| Grading | 0.17 | 0.66 | ||||||||
| G1/G2 | 164 | 41.21 | 124 | 46.79 | 72 | 42.35 | 77 | 45.29 | ||
| G3/G4 | 234 | 58.79 | 141 | 53.21 | 98 | 57.65 | 93 | 54.71 | ||
| R | 0.08 | 0.86 | ||||||||
| R0 | 373 | 93.72 | 238 | 89.81 | 152 | 89.41 | 154 | 90.59 | ||
| R1 | 25 | 6.28 | 27 | 10.19 | 18 | 10.59 | 16 | 9.41 | ||
| Tumor regression grade | ||||||||||
| Becker Ia/Ib | 70 | 26.42 | 37 | 21.76 | ||||||
| Becker II | 66 | 24.91 | 43 | 25.29 | ||||||
| Becker III | 129 | 48.68 | 90 | 52.94 | ||||||
Legend: cT1 = Mucosa/Submucosa; cT2 = Muscularis propria; cT3 = Serosa; cT4 = Adjacent organs; cN0 = no lymph nodemetastasis detected during staging, cN+ = locoregional lymph node metastasis evident during staging; CD = Clavien Dindo Classification; Staging according to UICC 8th edition; p-values printed in bold are considered statistically significant.
Table 2.
Baseline characteristics for patients with diffuse Lauren subtype before and after PSM.
| Diffuse Subtype (n = 359), Unmatched | Diffuse Subtype (n = 210) PS-Matched | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Surgery Only (n = 216) |
CTX + Surgery (n = 143) |
p-Value | Surgery Only (n = 105) |
CTX + Surgery (n = 105) |
p-Value | |||||
| n | % | n | % | n | % | n | % | |||
| Gender | 0.004 | 1.00 | ||||||||
| Female | 114 | 52.78 | 53 | 37.06 | 41 | 39.05 | 42 | 40.00 | ||
| Male | 102 | 47.22 | 90 | 62.94 | 64 | 60.95 | 63 | 60.00 | ||
| Age | 63.9 ±12.3 | 56.2 ±11.9 | <0.001 | 60.8 ±12.1 | 57.1 ±12.4 | 0.03 | ||||
| <70 | 138 | 63.89 | 123 | 86.01 | <0.001 | 84 | 80.00 | 85 | 80.95 | 1.00 |
| >70 | 78 | 36.11 | 20 | 13.99 | 21 | 20.00 | 20 | 19.05 | ||
| Localization | 0.03 | 0.14 | ||||||||
| Proximal | 74 | 34.26 | 63 | 44.06 | 40 | 38.10 | 49 | 46.67 | ||
| Middle | 63 | 29.17 | 35 | 24.48 | 30 | 28.57 | 24 | 22.86 | ||
| Distal | 63 | 29.17 | 27 | 18.88 | 30 | 28.57 | 21 | 20.00 | ||
| Total | 16 | 7.41 | 18 | 12.59 | 5 | 4.76 | 11 | 10.48 | ||
| Clinical Staging | <0.001 | 0.40 | ||||||||
| cT2 cN+/cNx | 75 | 34.72 | 14 | 9.79 | <0.001 | 15 | 14.29 | 14 | 13.33 | |
| cT3/cT4 cN0 | 19 | 8.80 | 21 | 14.69 | 12 | 11.43 | 12 | 11.43 | ||
| cT3/cT4 cN+/cNx | 122 | 56.48 | 108 | 75.52 | 78 | 74.29 | 79 | 75.24 | ||
| Dissected LN (Median) | 34 (1–104) | 30 (9–89) | 0.01 | 35 (1–102) | 31 (9–70) | 0.13 | ||||
| ≤25 | 55 | 25.46 | 36 | 25.17 | 1.00 | 26 | 24.76 | 23 | 21.90 | 0.74 |
| >25 | 161 | 74.54 | 107 | 74.83 | 79 | 75.24 | 82 | 78.10 | ||
| Complications | 0.77 | 0.4 | ||||||||
| None | 160 | 74.07 | 107 | 74.83 | 81 | 77.14 | 76 | 72.38 | ||
| CD I/II | 30 | 13.89 | 22 | 15.38 | 13 | 12.38 | 20 | 19.05 | ||
| CD III-V | 26 | 12.04 | 14 | 9.79 | 11 | 10.48 | 9 | 8.57 | ||
| pT | <0.001 | 0.002 | ||||||||
| pT0/is | 0 | 0.00 | 3 | 2.10 | 0 | 0.00 | 1 | 0.95 | ||
| pT1a | 18 | 8.33 | 1 | 0.70 | 6 | 5.71 | 0 | 0.00 | ||
| pT1b | 18 | 8.33 | 6 | 4.20 | 8 | 7.62 | 2 | 1.90 | ||
| pT2 | 16 | 7.41 | 8 | 5.59 | 4 | 3.81 | 4 | 3.81 | ||
| pT3 | 48 | 22.22 | 65 | 45.5 | 22 | 20.95 | 46 | 43.81 | ||
| pT4a | 104.00 | 48.15 | 54 | 37.8 | 57 | 54.29 | 46 | 43.81 | ||
| pT4b | 12 | 5.56 | 6 | 4.20 | 8 | 7.62 | 6 | 5.71 | ||
| pN | 0.05 | 35 | 33.33 | 29 | 27.62 | 0.03 | ||||
| pN0 | 76 | 35.19 | 61 | 42.66 | 8 | 7.62 | 17 | 16.19 | ||
| pN1 | 23 | 10.65 | 21 | 14.7 | 23 | 21.90 | 20 | 19.05 | ||
| pN2 | 42 | 19.44 | 22 | 15.4 | 18 | 17.14 | 29 | 27.62 | ||
| pN3a | 39 | 18.06 | 29 | 20.3 | 21 | 20.00 | 10 | 9.52 | ||
| pN3b | 36 | 16.67 | 10 | 6.99 | ||||||
| UICC | <0.001 | 0.006 | ||||||||
| UICC 0 | 0 | 0.00 | 3 | 2.1 | 0 | 0.00 | 1 | 0.95 | ||
| UICC IA | 29 | 13.43 | 6 | 4.2 | 12 | 11.43 | 1 | 0.95 | ||
| UICC IB | 11 | 5.09 | 4 | 2.8 | 3 | 2.86 | 1 | 0.95 | ||
| UICC IIA | 22 | 10.19 | 33 | 23.1 | 9 | 8.57 | 17 | 16.19 | ||
| UICC IIB | 30 | 13.89 | 26 | 18.2 | 14 | 13.33 | 15 | 14.29 | ||
| UICC IIIA | 48 | 22.22 | 33 | 23.1 | 28 | 26.67 | 32 | 30.48 | ||
| UICC IIIB | 39 | 18.06 | 26 | 18.2 | 16 | 15.24 | 26 | 24.76 | ||
| UICC IIIC | 37 | 17.13 | 12 | 8.39 | 23 | 21.90 | 12 | 11.43 | ||
| Grading | 0.65 | 1.00 | ||||||||
| G1/G2 | 4 | 1.85 | 1 | 0.70 | 2 | 1.90 | 1 | 0.95 | ||
| G3/G4 | 212 | 98.15 | 142 | 99.30 | 103 | 98.10 | 104 | 99.05 | ||
| R | 0.59 | 1.00 | ||||||||
| R0 | 173 | 80.09 | 118 | 82.52 | 81 | 77.14 | 82 | 78.10 | ||
| R1 | 43 | 19.91 | 25 | 17.48 | 24 | 22.86 | 23 | 21.90 | ||
| Tumor regression grade | ||||||||||
| Becker Ia/Ib | 22 | 15.38 | 9 | 8.57 | ||||||
| Becker II | 37 | 25.87 | 25 | 23.81 | ||||||
| Becker III | 84 | 58.74 | 71 | 67.62 | ||||||
Legend: cT1 = Mucosa/Submucosa; cT2 = Muscularis propria; cT3 = Serosa; cT4 = Adjacent organs; cN0 = no lymph nodemetastasis detected during staging, cN+ = locoregional lymph node metastasis evident during staging; CD = Clavien Dindo Classification; Staging according to UICC 8th edition; p-values printed in bold are considered statistically significant.
Table 3.
Baseline characteristics for patients with mixed Lauren subtype before and after PSM.
| Mixed Subtype (n = 187), Unmatched | Mixed Subtype (n = 112) PS-Matched | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Surgery Only (n = 116) |
CTX + Surgery (n = 71) |
p-Value | Surgery Only (n = 56) |
CTX + Surgery (n = 56) |
p-Value | |||||
| n | % | n | % | n | % | n | % | |||
| Gender | 0.87 | 0.84 | ||||||||
| Female | 40 | 34.48 | 23 | 32.39 | 19 | 33.93 | 17 | 30.36 | ||
| Male | 76 | 65.52 | 48 | 67.61 | 37 | 66.07 | 39 | 69.64 | ||
| Age | <0.0001 | 0.79 | ||||||||
| <70 | 69 | 59.48 | 62 | 87.32 | 49 | 87.50 | 47 | 83.93 | ||
| >70 | 47 | 40.52 | 9 | 12.68 | 7 | 12.50 | 9 | 16.07 | ||
| Localization | 0.04 | 0.21 | ||||||||
| Proximal | 55 | 47.41 | 40 | 56.34 | 28 | 50.00 | 26 | 46.43 | ||
| Middle | 23 | 19.83 | 20 | 28.17 | 12 | 21.43 | 20 | 35.71 | ||
| Distal | 36 | 31.03 | 9 | 12.68 | 15 | 26.79 | 8 | 14.29 | ||
| Total | 2 | 1.72 | 2 | 2.82 | 1 | 1.79 | 2 | 3.57 | ||
| Clinical Staging | <0.0001 | 0.93 | ||||||||
| cT2 cN+/cNx | 39 | 33.62 | 10 | 14.08 | 11 | 19.64 | 10 | 17.86 | ||
| cT3/cT4 cN0 | 10 | 8.62 | 8 | 11.27 | 5 | 8.93 | 6 | 10.71 | ||
| cT3/cT4 cN+/cNx | 67 | 57.76 | 53 | 74.65 | 40 | 71.43 | 40 | 71.43 | ||
| Dissected LN (Median) | 34 (7–83) | 30 (11–68) | 0.02 | 33 (7–83) | 30 (11–60) | 0.19 | ||||
| ≤25 | 24 | 20.69 | 24 | 33.80 | 0.06 | 13 | 23.21 | 20 | 35.71 | 0.21 |
| >25 | 92 | 79.31 | 47 | 66.20 | 43 | 76.79 | 36 | 64.29 | ||
| Complications | 0.89 | 0.81 | ||||||||
| None | 84 | 72.41 | 51 | 71.83 | 43 | 76.79 | 40 | 71.43 | ||
| CD I/II | 17 | 14.66 | 12 | 16.90 | 8 | 14.29 | 10 | 17.86 | ||
| CD III-V | 15 | 12.93 | 8 | 11.27 | 5 | 8.93 | 6 | 10.71 | ||
| pT | 0.17 | 0.41 | ||||||||
| pT0/is | 0 | 0.00 | 3 | 4.23 | 0 | 0.00 | 3 | 5.36 | ||
| pT1a | 2 | 1.72 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | ||
| pT1b | 11 | 9.48 | 7 | 9.86 | 5 | 8.93 | 6 | 10.71 | ||
| pT2 | 15 | 12.93 | 12 | 16.90 | 6 | 10.71 | 9 | 16.07 | ||
| pT3 | 43 | 37.07 | 30 | 42.25 | 24 | 42.86 | 24 | 42.86 | ||
| pT4a | 40.00 | 34.48 | 16 | 22.54 | 18 | 32.14 | 12 | 21.43 | ||
| pT4b | 5 | 4.31 | 3 | 4.23 | 3 | 5.36 | 2 | 3.57 | ||
| pN | 0.02 | 0.03 | ||||||||
| pN0 | 27 | 23.28 | 29 | 40.85 | 10 | 17.86 | 23 | 41.07 | ||
| pN1 | 16 | 13.79 | 14 | 19.72 | 10 | 17.86 | 13 | 23.21 | ||
| pN2 | 23 | 19.83 | 5 | 7.04 | 11 | 19.64 | 5 | 8.93 | ||
| pN3a | 35 | 30.17 | 17 | 23.94 | 17 | 30.36 | 12 | 21.43 | ||
| pN3b | 15 | 12.93 | 6 | 8.45 | 8 | 14.29 | 3 | 5.36 | ||
| UICC | 0.15 | 0.19 | ||||||||
| UICC 0 | 0 | 0.00 | 3 | 4.23 | 0 | 0.00 | 3 | 5.36 | ||
| UICC IA | 8 | 6.90 | 7 | 9.86 | 2 | 3.57 | 6 | 10.71 | ||
| UICC IB | 8 | 6.90 | 7 | 9.86 | 2 | 3.57 | 5 | 8.93 | ||
| UICC IIA | 9 | 7.76 | 10 | 14.08 | 6 | 10.71 | 8 | 14.29 | ||
| UICC IIB | 16 | 13.79 | 12 | 16.90 | 9 | 16.07 | 10 | 17.86 | ||
| UICC IIIA | 26 | 22.41 | 11 | 15.49 | 13 | 23.21 | 10 | 17.86 | ||
| UICC IIIB | 34 | 29.31 | 15 | 21.13 | 17 | 30.36 | 11 | 19.64 | ||
| UICC IIIC | 15 | 12.93 | 6 | 8.45 | 7 | 12.50 | 3 | 5.36 | ||
| Grading | 0.32 | 1 | ||||||||
| G1/G2 | 8 | 6.90 | 2 | 2.82 | 3 | 5.36 | 2 | 3.57 | ||
| G3/G4 | 108 | 93.10 | 69 | 97.18 | 53 | 94.64 | 54 | 96.43 | ||
| R | 0.78 | |||||||||
| R0 | 100 | 86.21 | 61 | 85.92 | 1.00 | 48 | 85.71 | 50 | 89.29 | |
| R1 | 16 | 13.79 | 10 | 14.08 | 8 | 14.29 | 6 | 10.71 | ||
| Tumor regression grade | ||||||||||
| Becker Ia/Ib | 14 | 19.72 | 12 | 21.43 | ||||||
| Becker II | 21 | 29.58 | 18 | 32.14 | ||||||
| Becker III | 36 | 50.70 | 26 | 46.43 | ||||||
Legend: cT1 = Mucosa/Submucosa; cT2 = Muscularis propria; cT3 = Serosa; cT4 = Adjacent organs; cN0 = no lymph nodemetastasis detected during staging, cN+ = locoregional lymph node metastasis evident during staging; CD = Clavien Dindo Classification; Staging according to UICC 8th edition; p-values printed in bold are considered statistically significant.
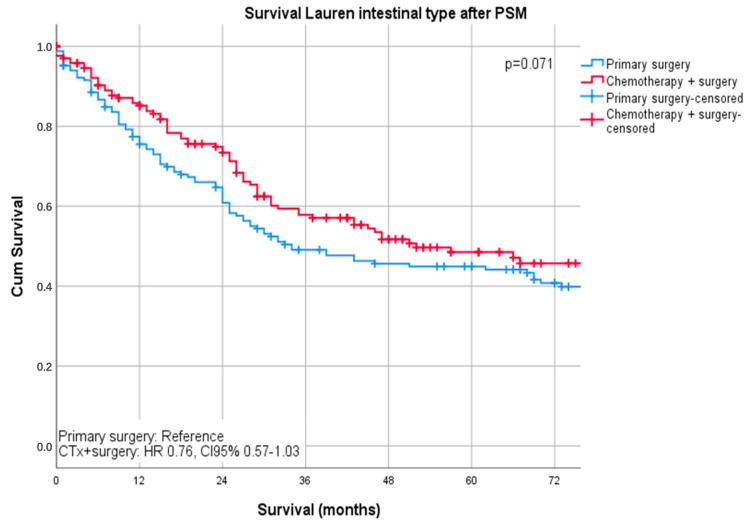
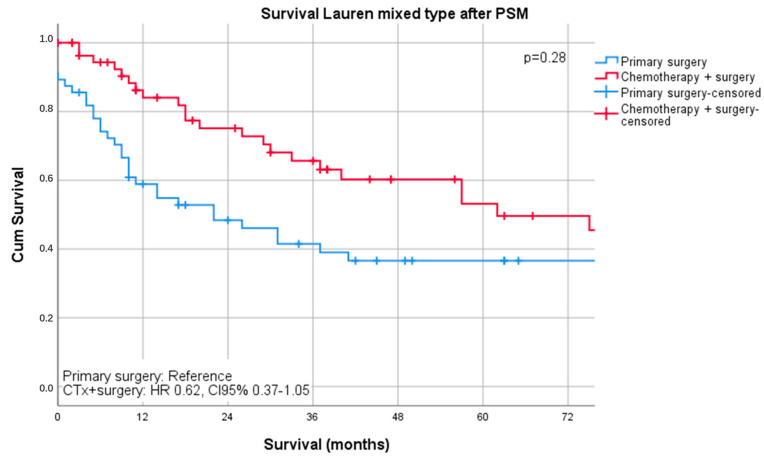
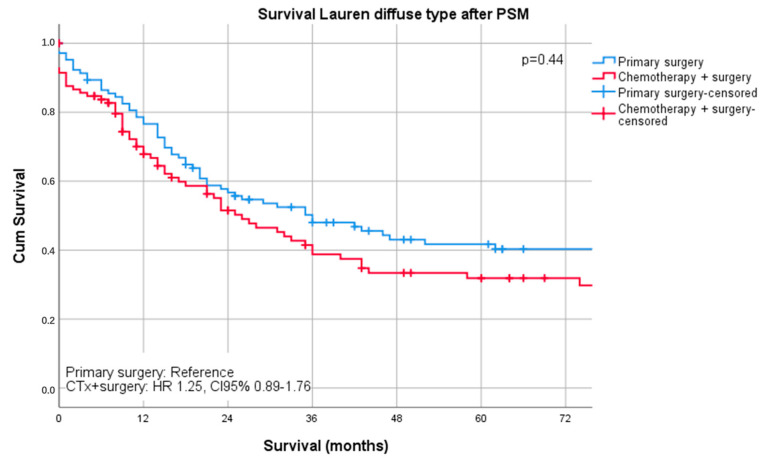
The median follow-up was 30 months (range 1–242 months), comprising of 61 months (range 1–242 months) for survivors and 19 months (range 1–183) months for deceased patients. During the follow-up period 658 patients (54.4%) died, the five-year survival rate was 42%, the ten-year survival rate was 32%. Median survival was 38 months for patients undergoing primary surgery and 46 months for patients undergoing chemotherapy ahead of surgery (p = 0.06). Five-year survival rates (FYSR)/ten-year survival rates (TYSR) after primary surgery and after neoadjuvant/perioperative chemotherapy followed by surgery were identical (44/33%). The UICC stage dependent analysis revealed that this effect was only reproducible in UICC stage III (19 vs. 24 months median survival, p = 0.03), but not in the other UICC stages (UICC I: median survival not met, p = 0.58; UICC II: median survival 72 (surgery) vs. 57 (nCTx+surgery) months, p = 0.7). In patients with Lauren intestinal subtype, the median survival time was 51 months (45 months for primary surgery vs. 57 months for neoadjuvant/perioperative chemotherapy + surgery, p = 0.025), in the diffuse type group 33 months (35 months for primary surgery vs. 28 months for neoadjuvant/perioperative chemotherapy + surgery, p = 0.16) and 40 months (26 months for primary surgery vs. 62 months for neoadjuvant/perioperative chemotherapy + surgery, p = 0.05) in the Lauren mixed type group. FYSR and TYSR for patients with Lauren intestinal, diffuse, and mixed subtype were 48/35%, 39/31%, and 42/32%, respectively.
The following variables were included in the cox regression analysis: age, gender, localization, neoadjuvant/perioperative chemotherapy, UICC-stage, Lauren subtype, number of dissected LN, R-stage, grading, and postoperative complications, because these are the most relevant factors in predicting survival. The pT- and pN-stages were not included, as these factors are summarized in the UICC-stage. All of the factors were entered in the multivariate model without selection. Univariate regression analysis revealed age, tumor location (all locations), all UICC-stages, Lauren intestinal and diffuse subtypes, R-status, grading, and the occurrence of postoperative complications to be significantly associated with post-therapeutic survival (Table 4). The multivariate analysis demonstrated that age, localization (proximal, middle, distal), application of neoadjuvant/chemotherapy, all UICC-stages, all Lauren subtypes, R-stage, and occurrence of postoperative complications were significantly and independently related to postoperative survival. Because of the imbalanced baseline characteristics, propensity score matching (PSM) was performed, and further analysis was performed on the PS-matched cohorts.
Table 4.
Univariate and Multivariate regression analysis for overall survival (OS).
| Univariate | HR | CI 95% | p | Multivariate | HR | CI 95% | p |
|---|---|---|---|---|---|---|---|
| Age > 70 | 1.36 | 1.16–1.60 | <0.001 | Age > 70 | 1.46 | 1.24–1.73 | <0.001 |
| Gender ! | 1.06 | 0.90–1.25 | 0.48 | Gender ! | 1.06 | 0.89–1.26 | 0.50 |
| Proximal $ | 1.00 | <0.001 | Proximal $ | 1.00 | <0.001 | ||
| Middle $ | 0.59 | 0.47–0.74 | <0.001 | Middle $ | 0.56 | 0.44–0.72 | <0.001 |
| Distal $ | 0.67 | 0.54–0.82 | <0.001 | Distal $ | 0.79 | 0.63–0.98 | 0.03 |
| Whole $ | 1.59 | 1.11–2.27 | 0.01 | Whole $ | 1.03 | 0.72–1.48 | 0.88 |
| nCTx | 0.88 | 0.75–1.03 | 0.11 | nCTx | 0.84 | 0.71–1.00 | 0.05 |
| UICC I $ | 1.00 | <0.001 | UICC I $ | 1.00 | <0.001 | ||
| UICC II $ | 2.39 | 1.77–3.23 | <0.001 | UICC II $ | 2.26 | 1.67–3.07 | <0.001 |
| UICC III $ | 5.23 | 3.93–6.92 | <0.001 | UICC III $ | 4.82 | 3.59–6.47 | <0.001 |
| Lauren intestinal $ | 1.00 | 0.016 | Lauren intestinal | 1.00 | <0.001 | ||
| Lauren diffuse $ | 1.27 | 1.08–1.51 | 0.005 | Lauren diffuse $ | 1.40 | 1.15–1.72 | <0.001 |
| Lauren mixed $ | 1.19 | 0.94–1.49 | 0.143 | Lauren mixed $ | 1.29 | 1.01–1.65 | 0.04 |
| Number of LN dissected | 1.06 | 0.89–1.27 | 0.51 | Number of LN dissected | 0.91 | 0.76–1.09 | 0.31 |
| pR0 | 1.00 | ||||||
| pR1 | 2.49 | 2.01–3.09 | <0.001 | pR | 1.55 | 1.23–1.94 | <0.001 |
| Grading (G1/2 vs. G3/4) | 1.23 | 1.03–1.47 | 0.03 | Grading (G1/2 vs. G3/4) | 0.98 | 0.80–1.21 | 0.85 |
| Clavien Dindo 0 $ | 1.00 | <0.001 | Clavien Dindo 0 $ | 1.00 | <0.001 | ||
| Grade I/II $ | 1.29 | 1.04–1.59 | 0.02 | Clavien Dindo I/II $ | 1.27 | 1.03–1.56 | 0.03 |
| Grade III/IV $ | 1.66 | 1.32–2.09 | <0.001 | Clavien Dindo III/IV $ | 1.47 | 1.16–1.86 | <0.001 |
Legend: HR = Hazard Ratio, CI95% lower: 95% Confidence Interval lower boundary, CI95% upper: 95% Confidence Interval upper boundary, p = p-value, ! male vs. female; $ categorical variable, first value is reference (=1.00): Localization, UICC-stage, Lauren subtype, Clavien Dindo grade; p-values printed in bold are considered statistically significant.
Results after PSM
Those variables demonstrating clinically meaningful baseline differences within the respective Lauren subgroups were matched through PSM (age, gender, location, clinical stage, UICC stage) in order to balance possible confounders (Supplementary Figure S1). The matching algorithm matched 170 patients each (surgery/nCTx+surgery) in the Lauren intestinal, 105 patients each in the Lauren diffuse, and 56 patients each in the Lauren mixed subtype groups. Analysis of the baseline characteristics demonstrated that the following variables were then well balanced in all of the groups: Gender, age distribution, tumor localization, clinical stages, D2 dissection rate, number of dissected LN, postop complications, pT-stages (in the Lauren intestinal and mixed, not in the diffuse subtype), UICC (intestinal and mixed subtypes, not diffuse), and grading and R0 status. Table 1, Table 2 and Table 3 show the results.
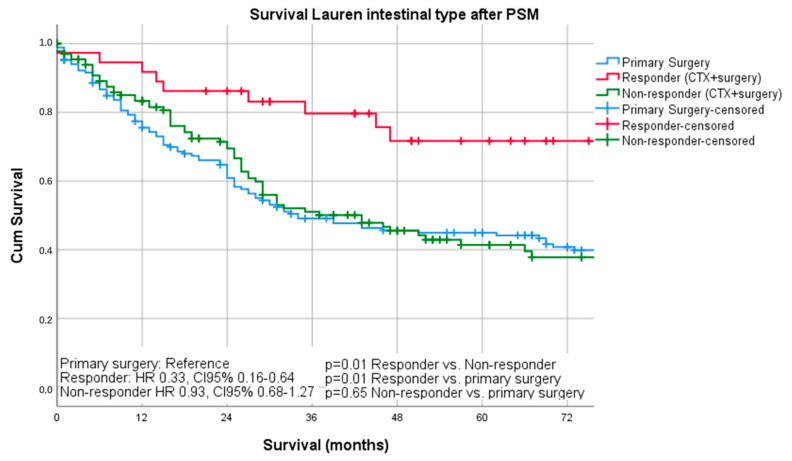
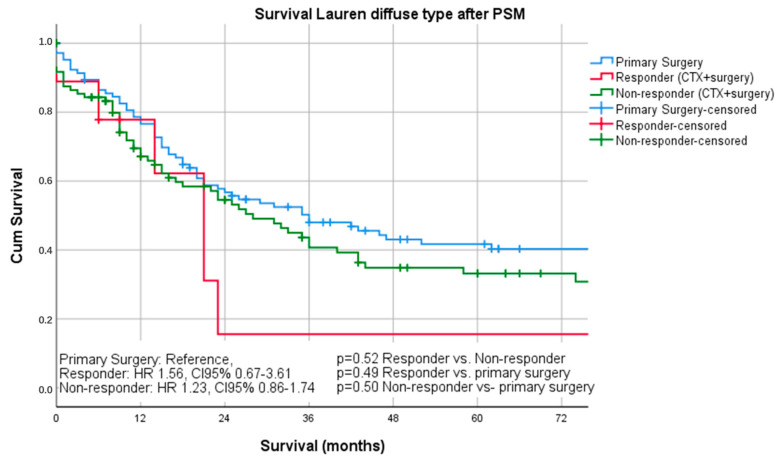
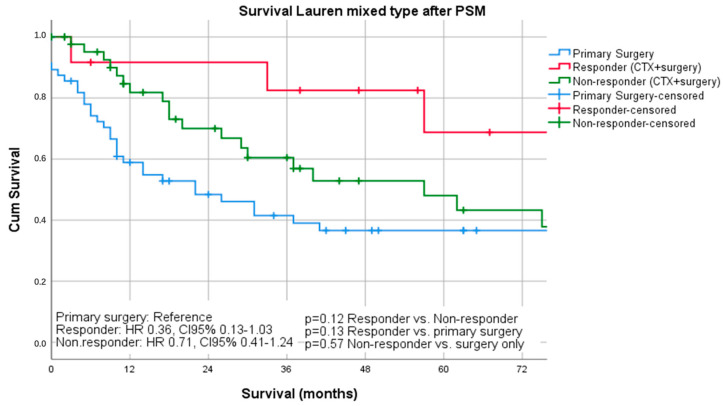
Median follow-up was 26 months (range 1–242 months), comprising of 55 months (range 1–242 months for survivors and 16 months (range 1–144) months for deceased patients. During the follow-up period, 367 patients (55.4%) died, the five-year survival rate was 41%, and the ten-year survival rate was 25%. The median survival was 32 months for patients undergoing primary surgery and 43 months for patients undergoing chemotherapy ahead of surgery (p = 0.16). FYSR/TYSR after primary surgery and after neoadjuvant/perioperative chemotherapy, followed by surgery were 42/31% and 44/32%. The UICC stage dependent analysis revealed no significant survival differences for UICC stages I and II (UICC I: median survival not met, p = 0.33; UICC II: median survival 91 (surgery) vs. 80 (nCTx+surgery) months, p = 0.72). In UICC III, there was a statistically significant survival difference in favor of those patients undergoing neoadjuvant/perioperative chemotherapy (median survival 18 (surgery) vs. 26 (nCTx+surgery) months (p = 0.02), Figures S2–S4. In patients with Lauren intestinal subtype, the median survival time was 46 months (34 months for primary surgery vs. 52 months for neoadjuvant/perioperative chemotherapy + surgery, p = 0.07), in patients with diffuse subtype group 35 months (36 months for primary surgery vs. 31 months for neoadjuvant/perioperative chemotherapy + surgery, p = 0.44) and 57 months (31 months for primary surgery vs. 62 months for neoadjuvant/perioperative chemotherapy + surgery, p = 0.28) in patients with the Lauren mixed subtype. FYSR/TYSR for Lauren intestinal, diffuse, and mixed subtypes were 44/29%, 36/17%, and 43/33%, respectively. Kaplan–Meier analysis revealed that survival benefit for those patients undergoing neoadjuvant/perioperative chemotherapy was detectable for Lauren intestinal (p = 0.07) and mixed types (0.28) without statistical significance (Figure 1 and Figure 2). The overall survival was (statistically not significantly) worse for Lauren diffuse type gastric cancer patients when undergoing neoadjuvant/perioperative chemotherapy (p = 0.44), (Figure 3). A survival benefit was detectable for Lauren intestinal type patients revealing histopathologic response (HPR) (median survival unmet vs. 43 months in non-responders and 34 months in patients undergoing primary surgery, p = 0.01) (Figure 4). There was no significant survival difference between patients undergoing primary surgery and non-responders to nCTx (p = 0.65) (Figure 4). This was not reproducible in Lauren diffuse type patients: The median survival was 21 months in responders vs. 33 months in non-responders (p = 0.52) and 36 months in patients undergoing primary surgery (p = 0.49). There was no survival difference in patients undergoing primary surgery and non-responders to nCTx (p = 0.5) (Figure 5). In the Lauren mixed type patients, there was a trend towards improved survival for responders without statistical significance: the median survival was 103 months in responders vs. 57 months in non-responders (p = 0.12) and 31 months in patients undergoing primary surgery (p = 0.13). There was no survival difference in patients undergoing primary surgery and non-responders to nCTx (p = 0.5) (Figure 6).
Figure 1.
Survival curves for Lauren intestinal subtype after PSM stratified by surgery only vs. chemotherapy plus surgery.
Figure 2.
Survival curves for Lauren mixed subtype after PSM stratified by surgery only vs. chemotherapy plus surgery.
Figure 3.
Survival curves for Lauren diffuse subtype after PSM stratified by surgery only vs. chemotherapy plus surgery.
Figure 4.
Survival curves for intestinal subtype after PSM differentiated by responders, non-responders, and surgery only.
Figure 5.
Survival curves for diffuse subtype after PSM differentiated by responders, non- responders and surgery only.
Figure 6.
Survival curves for mixed subtype after PSM differentiated by responders, non-responders and surgery only.
Recurrence rates and disease free survival were analyzed for the PS-matched groups. In the intestinal type subgroup, the recurrence rates were 79/170 (46.5%) in the surgery only group as compared to 89/170 (52.4%) in the chemotherapy + surgery group (p = 0.33). Disease free median survival was 30 months (1–176) in the primary surgery group and 29.5 months (1–242) in the chemotherapy + surgery group (HR 1.12; CI95% 0.83–1.12; p = 0.45). In the diffuse type subgroup, the recurrence rates were 62/105 (59%) in the surgery only group compared to 67/105 (63.8%) in the chemotherapy + surgery group (p = 0.57). Disease free median survival was 24 months (1–176) in the primary surgery group and 17 months (1–204) in the chemotherapy+surgery group (HR 1.28; CI95% 0.91–1.81; p = 0.16). In the mixed type subgroup, the recurrence rates were 35/56 (62.5%) in the surgery only group when compared to 25/56 (44.6%) in the chemotherapy + surgery group (p = 0.09). The disease free median survival was 15.5 months (1–171) in the primary surgery group and 38 months (3–202) in the chemotherapy+surgery group (HR 0.52; CI95% 0.31–0.87; p = 0.01).
3. Discussion
This analysis of a large single center cohort, including 1209 patients, demonstrates an association between the benefit of neoadjuvant chemotherapy and the Lauren subtype. Based on the presented data, only those patients demonstrating the intestinal subtype benefit from the application of neoadjuvant chemotherapy for locally advanced gastric cancer. However, this only holds true for those patients developing histopathologic regression. In contrast, there was no benefit for those patients with diffuse subtype. In the diffuse type group, those patients undergoing neoadjuvant chemotherapy even demonstrated a deterioration of survival when compared to patients who had primary surgery. Patients with Lauren mixed type features revealed a potential benefit, especially those responding to chemotherapy; however, this was not statistically significant. Neoadjuvant/perioperative chemotherapy has become standard of care in Europe and it has become manifest in most of the guidelines for locally advanced gastric and gastroesophageal junction cancers [3,11,12,13]. However, in recent years, it has become increasingly clear that chemotherapy may not be effective for all patients in the same manner. The overall survival rates still range between 20–40% after five years [7,11,14,15]. This analysis surprisingly demonstrates that patients having undergone surgery only revealed survival rates around 40%, and this was improved to over 70% when intestinal subtype tumors demonstrated good histopathologic response. In many studies, the histological subtype was described as an independent factor of survival [15,16,17,18], but it also determines the effectiveness of the chemotherapy administered. However, the histological subtypes are so far not sufficiently respected in regard of therapeutic decisions, for which the clinical tumor stage is still the only tool to be applied when multimodal therapies are recommended. This is underlined by the present analysis, in which patients were subjected to neoadjuvant/multimodal chemotherapy without respect to the Lauren subtype. Taking into account that only 49 of 331 patients (15%) demonstrated real benefit from preoperative chemotherapy, it has to be stated that 85% of the patients were treated ineffectively and may even have been harmed by (ineffective) cytotoxic drugs (Figure S5). The Spanish AGAMENON research group already published a correlation between the response to chemotherapy and the Lauren subtype in 2017. They also pointed out that there were no subgroup analyses in the large therapy trials for locally advanced stomach cancer, although there were indications of a link [7]. Further, the AGAMENON study incorporated almost only patients undergoing treatment for metastatic disease, which is not comparable to the present analysis. Another important analysis was the multicentric retrospective FREGAT study, which analyzed a similar cohort [19]. However, the French analysis was related to the impact of signet ring cell differentiation on oncologic outcomes and not exactly to Lauren diffuse types. In the present analysis it was found that there was not a single Lauren diffuse type cancer without signet ring cells. None the less, Lauren diffuse types should not be equalized to signet ring cell differentiation. An international European group proposed a new definition for signet ring cell containing gastric cancer, as this is still a matter of debate between surgeons, oncologists, and pathologists [9]. However, this new consensus is neither ratified nor prospectively analyzed nor evaluated in patients undergoing neoadjuvant/multimodal chemotherapy. Biological and prognostic differences for gastric cancer are difficult to evaluate in studies due to the fact that there are different histological classifications for gastric carcinoma histological phenotypes and there is no uniform classification. This is considerably relevant when new chemotherapeutic regimens (FLOT) are propagated as effective in signet ring cell gastric cancer. The prospective FREGAT study (PRODIGE-19-FFCD1103-ADCI002) is currently the only trial that is going to elucidate whether direct surgery is a potential option for signet ring cell type gastric cancer [20]. Another factor that does not allow for direct comparisons is the issue that there is no broad consensus on which tumor regression classification to use (Becker, Mandard, Cologne, etc.). Certainly, this analysis should be replicated by different centers, applying different tumor regression systems in order to determine whether the Lauren diffuse subtype is a non-responding entity. Therefore, the aim of future studies should be to unify the different histological classifications for gastric cancer in order to further investigate the influence of histology on survival and prognosis.
The limitations of this analysis are certainly the monocentric character of the study, the long observation period during which both surgical and perioperative regimens have changed, different chemotherapy regimens having been used, and the fact that FLOT, as the current standard of care, is still underrepresented in this analysis. Although potential biases inherent to the Lauren type subgroups were possibly corrected by PSM, this method cannot compensate for unconscious and biological biases or those factors not having been determined. More than that, it is critical that the PSM resulted in a relatively small number of patients per group. Therefore, no exact statements can be made about unmatched patients. Another limitation is that the PS-matching did not balance adequately for the UICC stages in the Lauren diffuse type subgroup, so the balance is skewed towards more advanced cases in the nCTx+surgery group, which might limit further conclusions regarding survival prognosis. Further, the generalizability of the present results is certainly restricted, as the practice of neoadjuvant/multimodal chemotherapy is only evident in the Western (mostly European) world and the findings are not transferable to Asian patients, due to ethnicity and, more importantly, due to the fact that preoperative chemotherapy is not a standard of care in countries, such as Korea, Japan, and China.
Molecular markers, including microsatellite instability (MSI-H) and the Cancer Genome Atlas (TCGA) molecular subtypes [21], which could have influenced the results of our study were not assessed and is a limitation of our study. A recently published meta-analysis demonstrated that MSI-H could predict outcomes to neoadjuvant chemotherapy [22]. However, this same meta-analysis revealed that MSI-H comprised a very small proportion (2.4%) of non-intestinal type gastric cancers. In the present analysis the number of patients not responding to chemotherapy in the diffuse type group was markedly higher (>80% for non-intestinal type cancer), which does not explain the influence of MSI-status only. Beyond molecular factors, the amount of chemotherapy administered may have also been a confounding factor. Although most of the patients received neoadjuvant chemotherapy only (94.6%), relatively few patients (5.4%) received the perioperative FLOT/MAGIC regimens (i.e., pre-operative + post-operative). We are unable to determine the influence of post-operative chemotherapy on the outcomes in our study due to incomplete data available about the administration of post-operative chemotherapy. Nevertheless, despite these limitations, the results of our study raise questions regarding the benefit of neoadjuvant/perioperative chemotherapy in diffuse-type gastric cancer. Complete surgical resection remains the only curative option for gastric cancer patients, even if overall survival is markedly shorter for patients with diffuse-type histology as compared to intestinal or mixed type histologies. To the authors’ knowledge, except for possibly MSI status, which represents a small proportion of patients, there is no existing biomarker in clinical practice that can adequately predict clinical and histopathologic response to neoadjuvant chemotherapy, and future research on identifying other molecular markers are needed.
The clinical implications of this analysis would be to carefully evaluate the application of neoadjuvant/perioperative chemotherapy in diffuse type patients. It remains speculative as to whether a multimodal treatment concept would be harmful for those patients, but it was demonstrated here that it is not beneficial either. Certainly, R0-surgery remains the only option of curation in these patients, even if the overall survival is markedly shorter than in intestinal or mixed type patients.
4. Materials and Methods
4.1. Patients
Data from patients who underwent curative surgery for gastroesophageal cancer at the Surgical Department of TUM School of Medicine from 1987 to 2017 were extracted from a prospectively documented database. The data were obtained from the medical records and then transferred to the institutional databases as soon as the patients were discharged from inpatient hospital care. The inclusion criteria for this analysis were: histologically proven gastroesophageal cancer (Siewert type II/III, all non-cardia cancers) staged cT2-cT4cNany undergoing neoadjuvant/perioperative chemotherapy after multidisciplinary team review, the Lauren histotype confirmed by expert pathologist (intestinal, diffuse or mixed type). The exclusion criteria were: Siewert type I, metastatic disease, hospital mortality within 30 days, loss of follow-up within a 60 months period, macroscopic residual cancer after surgery (R2), and indeterminate Lauren histotypes. Neoadjuvant/perioperative treatment consisted of either preoperative two cycles cisplatin or oxaliplatin/leucovorin/5-FU (PLF/OLF) only or perioperative three cycles of ECX/ECF (MAGIC) or perioperative four cycles FLOT [12,23]. All of the surgical procedures were performed according to the Japanese guidelines for GC treatment, including standardized D2-lymphnode dissection [24]. In the case of GE junction cancer (Siewert type II and III), the surgical procedure was extended to the distal esophagus. All of the patients received intraoperative frozen sections for the oral resection margin in order to confirm R0 resection. If the resection margin was positive, the surgical procedure was extended to the distal esophagus and esophagectomy was carried out whenever necessary. Circumferential and aboral resection margins were not determined intraoperatively on a routine basis. All of the resected specimens were examined by one or two specialized pathologists, being classified according to the TNM-classification and staged according to UICC-recommendations (8th edition) [25]. The histopathologic response was graded according to the Becker classification. Patients with 0–10% remnant viable tumor cells within the tumor area were graded as histopathologic responders (Becker Ia/Ib), whereas all other patients (Becker II (10–50% remnant viable tumor cells) and Becker III (>50% remnant viable tumor cells)) were graded as histopathologic non-responders [26]. Except for patients receiving FLOT or MAGIC regimens (n = 64, 5.4%, adjuvant chemotherapy was not considered on a routine basis. Following oncologic surgery, all of the patients were followed up every six to twelve months in an outpatient department (Roman Herzog Comprehensive Cancer Center) over the next five years by EGD and CT scans according to the institutional protocol.
Only deceased or surviving patients with a complete follow-up of at least 60 months were included in this analysis. Survival was computed from the day of surgery. The dataset consisted of patients’ gender, age, location (upper, middle, lower third), clinical stages (cT2N0, cT1/cT2cN+, cT3/cT4cN0, cT3/cT4N+), number of dissected lymph nodes, postoperative complications (none, Clavien–Dindo Grade I/II and III/IV), pT- (pT1/pT2/pT3/pT4), pN-(pN0/pN1/pN2/pN3), and UICC-stages (UICC-I/-II/-III), grading (G1/2, G3/4), R-status (R0/R1), Lauren subtype (intestinal, diffuse, mixed), and follow-up period with survival status. Institutional Review Board (IRB)-approval for this study was obtained according to local guidelines (IRB Registration: 364/20 S).
4.2. Statistical Analysis
Descriptive statistics on demographic and clinical tumor characteristics were calculated as the mean ± standard deviation (continuous variables) and frequencies (categorical variables). The survival time was calculated from the day of surgery to death or last follow up date (at least 60 months after surgery for survivors). The Kaplan–Meier method was used in order to estimate the survival probabilities stratified by the application of neoadjuvant/perioperative chemotherapy. The log-rank test was used to compare the estimated survival. Survival prognosticators were analyzed by uni- and multivariate cox regression analyses. The variables that entered into the model were age, tumor location (all locations), all UICC-stages, Lauren intestinal and diffuse subtypes, R-status, grading, and occurrence of postoperative complications. After univariate analysis, all of the variables were entered in the multivariate model. Statistical analyses were performed while using SPSS version 25 (IBM Inc., Ehningen, Germany). PSM was performed with R and the MatchIt Plugin (Version 3.01, Vienna, Austria, URL http://www.R-project.org/). p-values of less than 0.05 were considered to be statistically significant. This retrospective analysis was approved by the local IRB (No.364/20s; Ethikkommission der Fakultät für Medizin, TUM School of Medicine).
5. Conclusions
In conclusion, the present findings demonstrate that the Lauren subtype might be a relevant prognostic factor in relation to overall survival after neoadjuvant/perioperative chemotherapy for locally advanced gastric or gastroesophageal cancer. Data from this analysis suggest that patients with a diffuse subtype may not benefit from neoadjuvant chemotherapy, but further exploration of other factors (e.g., molecular markers, MSI status, EBV-status, etc.), validation in prospective studies, and evaluation of other novel treatments (e.g., immune checkpoint inhibitors) are urgently required.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/13/2/290/s1, Figure S1: Love plot for propensity-score matching of confounding variables within the respective Lauren type subgroups, Figure S2. Kaplan Meier curve for OS in UICC stage I of PSM-cohort, Figure S3. Kaplan Meier curve for OS in UICC stage II of PSM-cohort, Figure S4. Kaplan Meier curve for OS in UICC stage III of PSM-cohort, Figure S5. From 331 patients only 49 (15%) revealed histopathologic response and thus survival benefit.
Author Contributions
Conceptualization: A.N. and D.R.; Methodology: D.R.; Software: D.R.; Validation, R.S., D.R., A.N., J.S.-H., C.O. and H.F.; formal analysis: D.R. and R:S.; investigation: C.O., R.S. and D.R.; Data curation: C.O., R.S. and D.R., writing—original draft preparation: R.S. and D.R., writing—review and editing: R.S., A.N., C.O., J.S.-H., H.F., D.R.; supervision: A.N. and H.F.; project administration D.R.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Institutional Review Board Statement
Institutional Review Board (IRB)-approval for this study was obtained according to local guidelines (IRB Registration: 364/20 S).
Informed Consent Statement
Informed consent was not required due to the retrospective nature of this study according to §27 Bayerisches Krankenhausgesetz.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to European data protection regulation.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guggenheim D.E., Shah M.A. Gastric cancer epidemiology and risk factors. J. Surg. Oncol. 2013;107:230–236. doi: 10.1002/jso.23262. [DOI] [PubMed] [Google Scholar]
- 2.Lagarde S.M., ten Kate F.W., Reitsma J.B., Busch O.R.C., van Lanschot J.J.B. Prognostic factors in adenocarcinoma of the esophagus or gastroesophageal junction. J. Clin. Oncol. 2006;24:4347–4355. doi: 10.1200/JCO.2005.04.9445. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham D., Allum W.H., Stenning S.P., Thompson J.N., Van de Velde C.J.H., Nicolson M., Scarffe J.H., Lofts F.J., Falk S.J., Iveson T.J., et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 4.Ychou M., Boige V., Pignon J.-P., Conroy T., Bouche O., Lebreton G., Ducourtieux M., Bedenne L., Fabre J.-M., Saint-Aubert B., et al. Perioperative Chemotherapy Compared With Surgery Alone for Resectable Gastroesophageal Adenocarcinoma: An FNCLCC and FFCD Multicenter Phase III Trial. J. Clin. Oncol. 2011;29:1715–1721. doi: 10.1200/JCO.2010.33.0597. [DOI] [PubMed] [Google Scholar]
- 5.Van Hagen P., Hulshof M.C., van Lanschot J.J., Steyerberg E.W., van Berge Henegouwen M.I., Wijnhoven B.P., Richel D.J., Nieuwenhuijzen G.A., Hospers G.A., Bonenkamp J.J., et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 6.Hass H.G., Smith U., Jäger C., Schäffer M., Wellhäuber U., Hehr T., Markmann H.U., Nehls O., Denzlinger C. Signet ring cell carcinoma of the stomach is significantly associated with poor prognosis and diffuse gastric cancer (Lauren’s): Single-center experience of 160 cases. Onkologie. 2011;34:682–686. doi: 10.1159/000334545. [DOI] [PubMed] [Google Scholar]
- 7.Jiménez Fonseca P., Carmona-Bayonas A., Hernández R., Custodio A., Cano J.M., Lacalle A., Echavarria I., Macias I., Mangas M., Visa L., et al. Lauren subtypes of advanced gastric cancer influence survival and response to chemotherapy: Real-world data from the AGAMENON National Cancer Registry. Br. J. Cancer. 2017;117:775–782. doi: 10.1038/bjc.2017.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piessen G., Messager M., Robb W.B., Bonnetain F., Mariette C. Gastric signet ring cell carcinoma: How to investigate its impact on survival. J. Clin. Oncol. 2013;31:2059–2060. doi: 10.1200/JCO.2012.47.4338. [DOI] [PubMed] [Google Scholar]
- 9.Mariette C., Carneiro F., Grabsch H.I., van der Post R.S., Allum W., de Manzoni G., European Chapter of International Gastric Cancer A. Consensus on the pathological definition and classification of poorly cohesive gastric carcinoma. Gastric Cancer. 2019;22:1–9. doi: 10.1007/s10120-018-0868-0. [DOI] [PubMed] [Google Scholar]
- 10.LAURÉN P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. Acta Pathol. Microbiol. Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 11.Ronellenfitsch U., Schwarzbach M., Hofheinz R., Kienle P., Kieser M., Slanger T.E., Burmeister B., Kelsen D., Niedzwiecki D., Schuhmacher C., et al. Preoperative chemo(radio)therapy versus primary surgery for gastroesophageal adenocarcinoma: Systematic review with meta-analysis combining individual patient and aggregate data. Eur. J. Cancer. 2013;49:3149–3158. doi: 10.1016/j.ejca.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 12.Schuhmacher C., Gretschel S., Lordick F., Reichardt P., Hohenberger W., Eisenberger C.F., Haag C., Mauer M.E., Hasan B., Welch J., et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J. Clin. Oncol. 2010;28:5210–5218. doi: 10.1200/JCO.2009.26.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moehler M., Al-Batran S.E., Andus T., Anthuber M., Arends J., Arnold D., Aust D., Baier P., Baretton G., Bernhardt J., et al. German S3-guideline “Diagnosis and treatment of esophagogastric cancer”. Z Gastroenterol. 2011;49:461–531. doi: 10.1055/s-0031-1273201. [DOI] [PubMed] [Google Scholar]
- 14.Petrelli F., Berenato R., Turati L., Mennitto A., Steccanella F., Caporale M., Dallera P., de Braud F., Pezzica E., Di Bartolomeo M., et al. Prognostic value of diffuse versus intestinal histotype in patients with gastric cancer: A systematic review and meta-analysis. J. Gastrointest Oncol. 2017;8:148–163. doi: 10.21037/jgo.2017.01.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J.H., Chang K.K., Yoon C., Tang L.H., Strong V.E., Yoon S.S. Lauren Histologic Type Is the Most Important Factor Associated With Pattern of Recurrence Following Resection of Gastric Adenocarcinoma. Ann. Surg. 2018;267:105–113. doi: 10.1097/SLA.0000000000002040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pattison S., Mitchell C., Lade S., Leong T., Busuttil R.A., Boussioutas A. Early relapses after adjuvant chemotherapy suggests primary chemoresistance in diffuse gastric cancer. PLoS ONE. 2017;12:e0183891. doi: 10.1371/journal.pone.0183891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang S.C., Ng K.F., Yeh T.S., Cheng C.T., Lin J.S., Liu Y.J., Chuang H.C., Chen T.C. Subtraction of Epstein-Barr virus and microsatellite instability genotypes from the Lauren histotypes: Combined molecular and histologic subtyping with clinicopathological and prognostic significance validated in a cohort of 1248 cases. Int. J. Cancer. 2019;145:3218–3230. doi: 10.1002/ijc.32215. [DOI] [PubMed] [Google Scholar]
- 18.Van der Kaaij R.T., Snaebjornsson P., Voncken F.E., van Dieren J.M., Jansen E.P., Sikorska K., Cats A., van Sandick J.W. The prognostic and potentially predictive value of the Lauren classification in oesophageal adenocarcinoma. Eur. J. Cancer. 2017;76:27–35. doi: 10.1016/j.ejca.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 19.Messager M., Lefevre J.H., Pichot-Delahaye V., Souadka A., Piessen G., Mariette C. The impact of perioperative chemotherapy on survival in patients with gastric signet ring cell adenocarcinoma: A multicenter comparative study. Ann. Surg. 2011;254:684–693. doi: 10.1097/SLA.0b013e3182352647. [DOI] [PubMed] [Google Scholar]
- 20.Piessen G., Messager M., Le Malicot K., Robb W.B., Di Fiore F., Guilbert M., Moreau M., Christophe V., Adenis A., Mariette C. Phase II/III multicentre randomised controlled trial evaluating a strategy of primary surgery and adjuvant chemotherapy versus peri-operative chemotherapy for resectable gastric signet ring cell adenocarcinomas—PRODIGE 19—FFCD1103—ADCI002. BMC Cancer. 2013;13:281. doi: 10.1186/1471-2407-13-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pietrantonio F., Miceli R., Raimondi A., Kim Y.W., Kang W.K., Langley R.E., Choi Y.Y., Kim K.M., Nankivell M.G., Morano F., et al. Individual Patient Data Meta-Analysis of the Value of Microsatellite Instability As a Biomarker in Gastric Cancer. J. Clin. Oncol. 2019;37:3392–3400. doi: 10.1200/JCO.19.01124. [DOI] [PubMed] [Google Scholar]
- 23.Al-Batran S.E., Homann N., Pauligk C., Goetze T.O., Meiler J., Kasper S., Kopp H.G., Mayer F., Haag G.M., Luley K., et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet. 2019;393:1948–1957. doi: 10.1016/S0140-6736(18)32557-1. [DOI] [PubMed] [Google Scholar]
- 24.Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2014 (ver. 4) Gastric Cancer. 2017;20:1–19. doi: 10.1007/s10120-016-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sano T., Coit D.G., Kim H.H., Roviello F., Kassab P., Wittekind C., Yamamoto Y., Ohashi Y. Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging project. Gastric Cancer. 2017;20:217–225. doi: 10.1007/s10120-016-0601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becker K., Mueller J.D., Schulmacher C., Ott K., Fink U., Busch R., Böttcher K., Siewert J.R., Höfler H. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer. 2003;98:1521–1530. doi: 10.1002/cncr.11660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to European data protection regulation.