Abstract
Radiotherapy (RT) is a primary treatment modality for a number of cancers, offering potentially curative outcomes. Despite its success, tumour cells can become resistant to RT, leading to disease recurrence. Components of the tumour microenvironment (TME) likely play an integral role in managing RT success or failure including infiltrating immune cells, the tumour vasculature and stroma. Furthermore, genomic profiling of the TME could identify predictive biomarkers or gene signatures indicative of RT response. In this review, we will discuss proposed mechanisms of radioresistance within the TME, biomarkers that may predict RT outcomes, and future perspectives on radiation treatment in the era of personalised medicine.
Keywords: biomarkers, immune infiltrate, radiotherapy, stroma, tumour microenvironment
1. Introduction
Radiotherapy (RT) is a primary treatment modality for a number of cancers, offering potentially curative outcomes [1]. Radiation treatment modalities have significantly improved over the last two decades with the introduction of advanced techniques including stereotactic radiotherapy (SRT) and enhanced imaging methodologies to improve the precision of RT delivery, thus limiting damage to healthy tissue. However, despite these advancements, resistance to radiotherapy still occurs, resulting in disease recurrence. Characterisation of radioresistance has traditionally focused on the effects of RT on tumour cells, overlooking the impact on supporting stromal and immune cells that make up the tumour microenvironment (TME) [2]. Although components of the TME have been shown to regulate angiogenesis [3] and promote malignant progression and metastasis [4], their role in the response to RT and their contribution to radioresistance is less well characterised [5]. As such, a greater understanding of the TME response could identify predictive biomarkers indicative of RT success or failure.
Predictive biomarkers offer an approach for stratifying patients who will respond favourably to a particular treatment, in turn sparing those for whom the modality may be less effective. While radiotherapy is intrinsically a precision treatment, directed to the specific architecture of the patient’s tumour, it has so far lacked a personalised approach, taking into consideration patient-specific genomic alterations or TME composition, factors that could predict the outcome of radiotherapy [6,7]. In this review, we summarise some of the recent advances in understanding the TME response to ionising radiation. In particular, we discuss the effect of radiotherapy on the tumour stroma and immune response, and how this may contribute to radioresistance. This review will also consider the biomarkers or gene expression signatures that have been developed to predict radiation outcomes. Lastly, we conclude by exploring how these approaches could be used to develop personalised radiotherapy treatment plans to improve patient outcomes.
2. Radiation Response in the Tumour Microenvironment
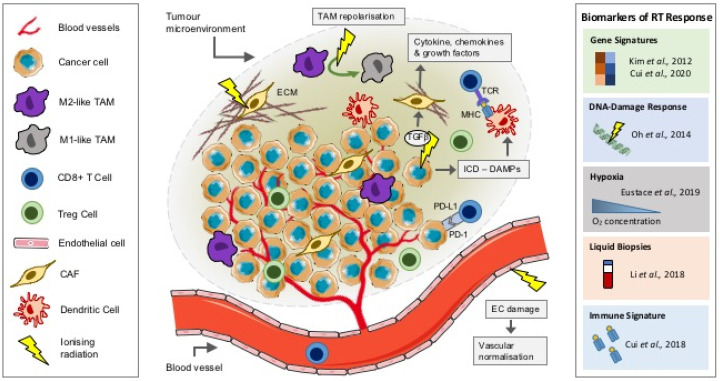
RT can be a cure for many; however, for some patients, the treatment fails or resistance occurs. Though ionizing radiation can induce DNA damage in tumour cells, a potential barrier to the success of RT may be its effects on the other components of the local TME, including the vasculature, stroma and the immune infiltrate (Figure 1). These components can influence tumour progression and response to treatment. Understanding how they are influenced by RT may be critical in predicting disease outcomes. Extracellular vesicles (EVs) including exosomes have also been shown to play a role in cancer progression, immunomodulation and importantly, in modifying the response to radiation; key examples of which are below. However, recent detailed articles focusing on the role of EV-modulated radiation response exist; as such, EVs will not form a primary focus of this review [8,9].
Figure 1.
The effect of radiation on the TME. Schematic showing the role of ionizing radiation on components of the TME and predictive biomarkers of radiation response. DAMPs, damage-associated molecular patterns; EC, endothelial cell; ECM, extracellular matrix; ICD, immunogenic cell death; MHC, major histocompatibility complex; PD-1, programmed cell death protein 1; PD-L1, programmed death ligand-1; RT, radiotherapy; TAM, tumour-associated macrophage; TCR, T-cell receptor; TGFβ, transforming growth factor beta; TME, tumour microenvironment.
2.1. Tumour Immune Microenvironment
Immune evasion, the process by which tumour cells can avoid immune recognition and destruction, has become one of the hallmarks of cancer [10]. Subsequently, more recent therapeutic developments have focused on shifting the TME from an immunosuppressive environment to an immune-activated one through the use of immunotherapeutics: treatments that can effectively remove the brakes on immune signals mounting an anti-tumour response. RT has been shown to have contradictory immunomodulatory effects, influencing both proinflammatory and immunosuppressive responses, which likely influence response to treatment [5]. The inflammatory milieu of the TME, or the tumour immune microenvironment (TIME), is composed of T cells, natural killer (NK) cells, dendritic cells (DCs) and tumour-infiltrating myeloid cells (TIMs) including tumour-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs) and dendritic cells (DCs), all of which are recruited into the TME through altered chemokine and cytokine signalling [11]. The extent and relative proportion of immune infiltration can also influence the response to treatment and progression. Tumours can be broadly separated into two categories based on their TIME: those that are immune “hot”, being infiltrated with T lymphocytes; and those that are immune “cold”, with poor infiltration [12]. In immune “hot” tumours, regulatory T cells (Tregs) and TAMs cooperate to support the immunosuppressive TME and may be more susceptible to the immunomodulatory effects of radiotherapy [13]. Furthermore, these immune-inflamed tumours, including non-small cell lung cancer and melanoma, are more likely to respond favourably to immune checkpoint inhibitors in comparison to immune “cold” tumours, including pancreatic and prostate tumours [14]. Lack of tumour antigens, defects in antigen presentation and poor T-cell homing to the TME by the stroma may all contribute to a “cold” tumour immune phenotype; mechanisms to modulate immune infiltration and turn these tumours “hot” could improve response to therapy [14,15,16].
The ability of radiotherapy to modulate systemic immune responses may contribute towards the observations of tumour regression at non-irradiated sites, an effect described as an abscopal response. Abscopal effects are particularly relevant when RT is combined with immune checkpoint blockade. In preclinical syngeneic models of prostate cancer, a combination of radiotherapy (20 Gy in two fractions) with antibodies against programmed death-1 (anti-PD-1) or programmed death ligand-1 (anti-PD-L1) (iRT) significantly increased median survival (70–130%) in comparison to anti-PD-1 monotherapy, contributing to an abscopal response in which the unirradiated tumours responded similarly to the irradiated tumours. Importantly, this effect was shown to be mediated through antitumour CD8+ (cytotoxic) T cells [17]. Clinical observations of the abscopal effect have been rare in radiation oncology; however, with the development and advancement of immunotherapeutics, these observations are becoming more frequent across a variety of tumour types [18]. Clinically, in patients with unresectable melanoma combining anti-PD-1 therapy with hypofractionated RT (typically 26 Gy in 3–5 fractions) resulted in abscopal treatment responses in 36% of patients [19,20]. Targeting of another immune checkpoint, cytotoxic T-lymphocyte antigen 4 (CTLA-4), with the monoclonal antibody ipilimumab in combination with RT has also been shown to result in abscopal responses both preclinically in models of breast cancer and clinically in melanoma and lung cancer patients [21,22,23,24]. Interestingly, EVs isolated from irradiated tumour cells (H22 cells and 4T1 cells; 8 Gy) in vitro were shown to have immunomodulatory effects when mice were inoculated in vivo, enhancing CD8+ and CD4+ T-cell infiltration in lung metastasis in comparison to nonirradiated EVs [25]. Dose and fractionation are likely to play a critical role in the immunological responses to RT; however, the molecular and cellular mechanisms underpinning this immune-priming effect are still poorly understood [26].
RT-induced cell death is typically thought to occur through DNA damage, particularly in the form of double-strand breaks (DSB). Subsequently, the tumour cell response to radiation-induced DNA damage (RIDD) is dependent on its DNA damage response (DDR), which can activate downstream signalling to repair damage, thus contributing to radioresistance [27]. While the immune cell compartment, including lymphocyte and myeloid populations, may be more resistant to RIDD, RT can modulate immune signalling within the TME, promoting immune cell recruitment and activation and triggering immunogenic cell death [28]. RT-induced immunogenic cell death results in a cascade of events, starting with the release of damage-associated molecular patterns (DAMPs) (Figure 1) [29]. These “danger” signals released by tumour cells include high-mobility group box 1 (HMGB1) and ATP, triggering innate and adaptive immune responses through the expression of major histocompatibility complex (MHC) class I and MHC-II molecules. These antigen-presenting cells (APCs) can in turn can prime CD8+ T cells to induce an antitumour response [28]. In fact, RT has been shown to upregulate MHC-I expression preclinically in tumour cell lines in vivo, an observation that has been recapitulated in ex vivo-irradiated tumour biopsies [30]. Cytosolic double-stranded DNA (dsDNA) released as a result of RIDD can also promote dendritic cell activation through guanosine monophosphate–adenosine monophosphate synthase (cGAS)/stimulator of IFN genes (STING)/interferon (IFN) signalling, leading to CD8+ T-cell activation [31].
TIM populations, including TAMs, form another important component of the TIME and although they have a complex plasticity, they are usually organised as classically activated (M1) or alternatively activated (M2) cells. Numerous stimuli including chemokines can influence TAM polarisation from a proinflammatory (antitumour) M1 to an anti-inflammatory (protumour) M2 phenotype, which promotes tumour angiogenesis, tissue remodelling and tumour progression [32]. Interestingly, the frequency of TAMs has also been associated with clinical treatment response and disease progression [33,34]. In murine tumour models, low-dose gamma irradiation (LDI; 2 Gy) has been shown to promote repolarisation of M2-like TAMs towards M1-like inducible nitric oxide synthase (iNOS)-expressing TAMs, contributing to T-cell recruitment and tumour regression (Figure 1) [35]. TAMs and MDSCs are dependent on colony-stimulating factor (CSF1) signalling for recruitment into the TME. In murine models of breast cancer, blocking CSF1/CSF1R signalling inhibited TAM recruitment and delayed tumour regrowth following RT (5 Gy), an effect associated with an increase in CD8+ T cells and a reduction in CD4+ (helper) T cells [36]. Similar effects were observed following CSF1R signalling blockade in combination with RT (3 Gy, five fractions) in syngeneic models of prostate cancer in vivo. Furthermore, serum levels of CSF1 were also shown to be elevated in prostate cancer patients following RT [37]. Clinically, in patients with T3 rectal cancer, a short course of radiotherapy (neoadjuvant hyperfractionated 25 Gy in 10 fractions; surgery performed on day 2–5) promoted TAM repolarisation towards an M1-like proinflammatory phenotype. Interestingly, ex vivo modelling of this response suggested that HMGB1 in EVs from irradiated tumour cells could be responsible for this effect on TAM polarisation [38].
2.2. Cancer-Associated Fibroblasts
The stromal compartment of the TME plays an integral role in the response to treatment, including RT (Figure 1). Radiotherapy-induced tissue fibrosis is a late side effect where myofibroblast transformation leads to the excess production of collagen and deposition of components of the extracellular matrix (ECM) [39]. RT can also lead to the release of the pleotropic cytokine transforming growth factor beta (TGFβ), which can modulate fibroblast phenotype and function [40]. Fibroblasts recruited into the TME are transformed into cancer-associated fibroblasts (CAFs), where they play a role in regulating the extracellular matrix [41]. Furthermore, CAFs are responsible for the secretion of a number of cytokines (including interleukin 6 (IL6) and IL8), chemokines (including C-X-C motif ligand 12 (CXCL12)) and growth factors (including TGF-β and platelet-derived growth factor (PDGF)) that can influence immune cell fate and tumour progression, often contributing to the immunosuppressive TIME [42]. However, the effects of RT on the stromal compartment of the TME including CAFs are less well understood and they appear to have contradictory roles, contributing to both tumour growth and suppression [43]. Coimplantation of A549 lung tumour xenografts with preirradiated CAFs (at both 18 Gy × 1 fraction or 6 Gy × 3 fractions) abrogated the protumour growth effect observed in tumours coimplanted with nonirradiated CAFs [44]. In contrast, irradiated fibroblasts (1, 6 or 12 Gy) have been shown to express high levels of TGF-β1 and promote human T3M-1 squamous cell carcinoma (SCC) invasion and growth [45]. Furthermore, EVs derived from CAFs were shown to contribute to colorectal cancer cell stemness and radioresistance (6 Gy) in vitro, through the activation of the TGF-β signalling pathway [46]. It is therefore clear that more work is needed to understand the complex role of CAFs in the tumour response to RT.
2.3. Tumour Vasculature
The integrity of the tumour vasculature differs significantly from that of physiologically normal vessels, characterised by abnormal recruitment of pericytes, leading to increased tortuosity and porosity. This, in part, contributes to treatment failure through poor drug penetration into the TME, establishing local hypoxia gradients and increasing the yield of reactive oxygen species [47]. The effect of RT on the tumour vasculature has been well studied, with tumour blood vessels and their endothelial cells proven to exhibit increased sensitivity to radiation, a response likely dependent on total radiation dose and fractionation schedule [5,48,49]. Vascular damage is mainly witnessed at radiation doses exceeding 5 Gy. Conversely, individual, low-dose fractions have been shown to temporarily stimulate blood flow, while at higher or cumulative doses, the vascular network is disrupted, promoting hypoxic stress that can trigger tumour cell death [50,51]. In a recent dose-escalation study, single administration of 2, 4 or 8 Gy doses were shown to compromise the tumour vasculature in a dose-dependent manner, prolonging the survival of mice bearing CT-2A (high-grade glioma) tumours. Interestingly, this was also associated with changes in the TIME, promoting an increase in CD8+ T cells and a reduction in M2-like TAMs [52]. Potiron et al. [53] reported that RT (at both 10 × 2 Gy and 2 × 12 Gy) induces tumour vasculature normalisation and remodelling, thus improving the distribution and efficacy of the anticancer drug doxorubicin (DOX) [53]. Further evidence of the effects of RT effects on endothelial cell permeability has been demonstrated in vitro. Monotherapy radiation doses to primary human umbilical vein endothelial cells (HUVECs) increased permeability and transmigration of tumour cells, owing to altered metalloprotease ADAM10 expression and degradation of VE-cadherin, both of which play an integral role in maintaining intercellular junctions and vascular integrity [54]. High radiation doses (>20 Gy) were also found to cause transient endothelial dysfunction, platelet leukocyte adhesion and increased expression of hypoxia-inducible factor-1α (HIF-1α) in pancreatic tumours [55]. However, a recent study indicated that high-dose RT (>8 Gy) induced expression of Notch1 signalling in HUVEC monolayers. Consequently, in vivo high-dose RT, in combination with inhibition of Notch1 signalling, resulted in a significant reduction in tumour vessel endothelial cell coverage in comparison to high-dose RT alone, suggesting Notch1 signalling may protect tumour vessels from radiation-induced damage [56]. Furthermore, it is also well understood that oxygenated tumour cells are preferentially killed by RT, due to oxygen-induced fixation of radiation-induced DNA damage. However, this effect has been proven to accelerate the production of proangiogenic cytokines, inhibiting treatment-induced apoptosis, stimulating a postradiotherapy angiogenic burst that can contribute to eventual tumour regrowth [57].
3. Predictive Biomarkers of Radiation Response
Precision medicine based on common tumour-specific alterations, emerging from high-throughput molecular profiling, has become a reality in recent years. This approach underpins the discovery of clinically validated prognostic and/or predictive biomarkers, allowing for stratification of patients based either on those most likely to derive benefit or have treatment-related harm limited. This strategy gained significant momentum in the chemotherapy field with the development of various commercially produced kits such as Prosigna (NanoString Technologies, Inc., Seattle, USA) and MammaPrint (Agendia, Amsterdam, The Netherlands), designed to aid clinical decision-making [58,59]. However, equivalence in radiotherapy has not yet been achieved due to the variability in radiation response, an effect attributed to tumour heterogeneity. Heterogeneity is an umbrella term used to describe both intra- and intertumour variability at the morphological, physiological and more recently, genetic levels. Divergence of these features exerts a profound influence on localised factors such as vascular integrity, tumour oxygenation and immune infiltrate, ultimately influencing treatment outcome (detailed in Section 2 [5,13,48]). In an effort to address the issue of heterogeneity, research efforts have shifted from focusing on macroscopic phenotypic or environmental variation to the identification of commonality at the molecular level. Table 1 provides an outline of biomarkers for radiotherapy response in a number of tumour types (summarised in Figure 1); these are discussed further in the sections below.
Table 1.
Biomarkers of radiotherapy response.
| Year | Cancer Type | Biomarker | Results | Ref | |
|---|---|---|---|---|---|
| Gene signatures | 2012 | NCI-60 human tumor cell lines screen | A 31-gene signature developed from meta-analysis of microarray data correlated with clonogenic assay data to identify radiosensitive or radioresistant cells | Genes involved in cell cycle progression (CCNA2, CDK6, CCND1) and DNA damage repair were associated with increased radiosensitivity | [60] |
| 2014 | Breast cancer | A 7-gene signature applied to the Danish Breast Cancer Cooperative Group (DBCG82bc) cohort to stratify patients into either high-risk locoregional recurrence (LRR) or low-risk LRR | Identified that post-mastectomy RT would benefit only those identified as high risk, providing no benefit to low-risk patients | [61] | |
| 2015 | Breast cancer | Radiation sensitivity gene signature developed from correlating radiation sensitivity (SF2) of a panel of breast cancer models against gene expression changes | Gene signature significantly predicted loco-regional recurrence; beating all clinicopathologic features used in clinical practice | [62] | |
| 2016 | Prostate cancer | A 24-gene signature applied to prostate cancer patients who had undergone radical prostatectomy to identify those most likely to benefit from postoperative radiotherapy | Retrospective analysis identified that those patients with a high PROTOS (post-operative radiation therapy outcomes score), indicative of radiation sensitive tumours, were less likely to develop metastasis at 10 years post-RT. In the low PROTOS score group, radiotherapy proved detrimental | [63] | |
| 2020 | HNSCC | A 12-gene signature | Classified patients with a higher radiosensitivity for whom RT would be beneficial and could predict overall survival. | [64] | |
| DNA-damage response | 2010 | Breast cancer | Gene expression signature associated with DDR, correlated against publicly available breast cancer microarray data | DDR-associated genes induced by radiation correlated positively with those who responded favourably to radiation treatment | [65] |
| 2014 | Breast cancer | Radiation-induced 30-gene DDR signature | Gene signature was capable of discriminating between breast cancer patients likely to achieve a pathological complete response (pCR) to neoadjuvant chemotherapy and poor-responding patients | [66] | |
| Hypoxia | 2013 | Laryngeal cancer | A 26-hypoxia gene signature | Could predict those patients receiving RT for whom hypoxia-modifying ARCON (accelerated radiotherapy with carbogen and nicotinamide) therapy would be of benefit | [67] |
| 2012 | HNSCC | A 15-gene hypoxia signature | Classified patients who would benefit from combining RT with hypoxia modification (nimorazole) | [68] | |
| Liquid biopsies | 2011 | Prostate cancer | Altered miRNA expression: developed through screening of miRNAs in prostate cancer cells (LNCaP) in response to RT | Suppressed miR-221 expression linked with increased radiation sensitivity: data subsequently correlated in clinical datasets where low serum levels of miR-221 are indicative of low-risk prostate cancer | [69] |
| 2018 | Nonmetastatic rectal cancer and head and neck cancers | miRNA expression rations: prediction classifier | The expressions of three miRNAs—miR-374a-5p, miR-342-5p and miR-519d-3p—were significantly different between responsive and poor-responsive RT groups. miRNA classifier successfully predicted radiotherapy outcomes | [70] | |
| Immune signature | 2018 | Breast cancer | Combined radiation sensitivity (RS) gene signature with an antigen-presentation (AP) immune signature | Both RS and AP signatures capable of predicting increased disease specific survival (DSS) in patients identified with either radio-sensitive or immune-effective tumours | [71] |
3.1. Gene Signatures of Radiation Sensitivity
An early example of this approach used the clonogenic assay to profile radiation sensitivity, based on survival fraction data at 2 Gy (SF2), of the NCI-60 cancer cell line panel [60]. This was then correlated against gene expression data from four published microarray platforms, identifying significant alterations in expression profiles for 31 genes, common to each microarray dataset. Unsurprisingly, significant suppression of genes which regulate cell cycle progression (CCNA2, CDK6, CCND1) and DNA damage repair were associated with increased radiosensitivity. CCND1, the gene encoding for cyclin D1, stalls cell cycle progression, providing time for DNA damage repair, ultimately suppressing radiation-induced apoptosis [72]. Therefore, suppressed CCND1 and other cell cycle regulatory genes may contribute, in part, towards a genetic signature for identifying radiosensitive tumours. A second set of genes common to the top 10% most radiosensitive (SF2 < 0.2) cells, and totally absent from the most radioresistant (SF2 > 0.8), were those involved in integrin signalling, cell adhesion and cytoskeletal remodelling. Cell-adhesion complexes and integrin signalling act both directly and indirectly to influence radiation response [73]. Cell-to-cell contact and adhesion with the extracellular matrix are central features of the protumour phenotypes of migration and invasion. Along with integrin β1, the 31-gene profile identified downregulation of ITGB5, the gene encoding integrin β5, as a highly significant indicator of radiosensitivity [60]. Indeed, radiosensitisation achieved through the antagonism of αvβ5 integrin using a cyclic-RGD (arginine-glycine-aspartate) containing peptide was the focus of a large phase III clinical trial for the treatment of glioblastoma multiforme [74]. This was based on the rationale that αvβ5 antagonism suppresses tumour angiogenesis and metastasis, an effect in part attributed to the dampening of major cancer-related signalling pathways, including Wnt and PI3K [75]. Developed as a universal predicator of radiation sensitivity, independent of tumour type, many of the 31 genes identified likely hold predictive value in relation to radiation response. However, stringent application using only the most radiosensitive or radioresistant cells again highlights the problem of heterogeneity, where 80% tumour models analysed exhibited intermediary gene expression alterations, diluting the predictive power of the signature.
Recent approaches adopting a similar strategy tend to focus on a specific disease type. Breast cancer radiotherapy is most commonly used in the adjuvant setting to improve treatment outcomes, forming a core strategy of breast conservation surgery and mastectomy. However, not all patients benefit from adjuvant radiotherapy and some experience significant debilitating late effects [76]. The importance of identifying those who will benefit most from adjuvant radiotherapy was neatly demonstrated in a study using FFPE tumour tissue from the Danish Breast Cancer Cooperative Group (DBCG82bc) cohort. Applying a seven-gene signature to stratify patients into either high-risk loco regional recurrence (LRR) or low-risk LRR, the authors were able to establish that postmastectomy radiotherapy would benefit only those identified as high risk, providing no benefit to low-risk patients [61]. Adopting a similar strategy to the 31-gene signature, Speers et al. [62] correlated the radiation sensitivity (SF2) of a panel of breast cancer models against gene expression changes, developing a radiation sensitivity signature (RSS), which was subsequently shown to be the most significant factor in prediction of loco-regional recurrence, beating all clinicopathologic features used in clinical practice [62]. While a clear step forward, RRS remains a prognostic signature for loco-regional control, and not predictive of radiation response. Similar predictive gene signatures have been developed, including a six-gene signature (including genes such as HOXB13 and NKX2-2) that was also shown to predict radiotherapy sensitivity in breast cancer [77]. Applying a 24-gene signature to prostate cancer patients who had undergone radical prostatectomy to identify those most likely to benefit from postoperative radiotherapy similarly found that those with a high PROTOS (postoperative radiation therapy outcomes score), indicative of radiation-sensitive tumours, significantly benefited from radiotherapy, with a 10-year metastasis rate of 4% (95% CI 0–10) versus 35% (CI 7–54) for those not receiving radiotherapy. However, in the low PROTOS score group, radiotherapy proved detrimental (HR 2.5 (CI 1.6–4.1); p < 0.0001) in the 157-patient cohort training group and of no benefit in the 248-patient validation cohort [63]. Liu et al. [64] recently used multiple omics data to develop a prediction model of sensitivity to radiation in head and neck squamous cell carcinoma (HNSCC) tumours. A 12-gene signature was established from differentially expressed genes in patients treated with or without RT and used to develop a scoring system. Those HNSCC patients with a low score had a higher radiosensitivity and were shown to benefit from RT [64].
3.2. DNA Damage Response Biomarkers
The antitumour effects of radiotherapy are directly proportional to the degree to which potentially lethal DNA DSBs are both induced by radiation and are sustained by the cell following activation of DDR processes. Continual refinements to the delivery of radiotherapy have ensured that the DNA-damaging properties of the most commonly utilised radiation sources, such as X-rays and γ-rays, minimise dose to surrounding healthy tissue, while focusing dose on the target volume. In parallel, intensive research efforts have led to the development of numerous small-molecule inhibitors targeting key DNA damage repair proteins, thus sustaining radiation-induced damage, resulting in increased tumour cell death. This is the fundamental basis of many radiosensitising strategies. Key targets of the DNA damage response pathways for which clinically utilised inhibitors have been developed include the ATM/ATR (ataxia–telangiectasia mutated and Rad3-related) signalling pathways, PARP (poly (ADP-ribose) polymerase), DNA-PKcs (DNA-dependent protein kinase, catalytic subunit), BRAC1 (breast cancer1 C terminal) and HIF-1, amongst others. While reviewing the full therapeutic potential of these inhibitors is beyond the scope of the current article, several recent publications provide comprehensive details of this field [27,78,79]. Herein, we aim to focus on the utility of gene expression alterations in DDR genes as prognostic/predictive indicators of radiation response. Piening et al. [65] developed an early radiation-derived gene signature, evaluated for prognostic utility in breast cancer. The signature was derived from gene expression alterations following a 5 Gy dose across a panel of nontumour lymphoblast cells, a relevant point given that genomic instability in tumours support aberrant DDR activity. Expression levels of 219 genes were altered with 160 being induced and 59 repressed by radiation. Using a gene set enrichment algorithm [80], the prognostic utility of the signature was evaluated against publicly available breast cancer microarray data. With respect to the repressed genes, tumour samples neatly clustered into two groups, aligning with gene repression or not, where the former strongly correlated with increased proliferation and poor overall treatment outcomes. Similarly, genes induced by radiation correlated positively with those who responded favourably to radiation treatment, promoting the expression of genes involved in negative regulation of the cell cycle, apoptosis (e.g., caspases) and DNA damage repair proteins. Importantly, applying the same approach but using the NCI-60 cancer cell line panel to derive the radiation signature failed to discriminate between favourable and poor outcomes, with no overlap between the altered gene set signature [65]. This clearly illustrates the impact of genomic instability in influencing the DDR response and an important point for consideration in the development of radiation biomarkers.
Another study exploited the overlapping DNA damage responses activated by both chemotherapy and radiotherapy, producing a radiation-induced 30-gene signature. This signature was proven capable of discriminating between breast cancer patients likely to achieve a pathological complete response (pCR) to neoadjuvant chemotherapy and poor-responding patients. Importantly, pCR represents the most relevant clinical end point for predicting improved overall and disease-free survival [81]. In addition to genes clearly linked to DNA damage pathways, such as the extracellular signal-regulated kinase (ERK) pathway, AKT, mTOR and NF-ĸB, radiation significantly elevated the expression of metabolism processing genes, in particular PDHA1 and LDHB. These genes encode for key proteins driving pyruvate metabolism and energy production, along with the catalytic conversion of pyruvate to lactate, thus indicating that tumours with a high metabolic demand are more likely to prove sensitive to the effects of chemo- and radiotherapy [66].
3.3. Hypoxia Biomarkers
As outlined previously, hypoxia resulting from aberrant tumour vasculature can influence RT resistance. As such, there is a strong rationale for identifying robust biomarkers of tumour hypoxia that predict response to RT [82]. Traditionally, tumour hypoxia was measured using oxygen electrode probes, endogenous HIF-1α levels, physiological markers such as pimonidazole staining or other imaging methodologies (MRI). However, gene signatures may better represent the nuances of hypoxia within the TME that might predict response to RT. To this end, Eustace et al. [67] developed a 26-hypoxia gene signature (informed by a 121-gene hypoxia meta-signature derived from datasets of head and neck, breast and lung cancers [83]) predicting treatment response in laryngeal cancer. This hypoxia signature, composed of genes involved in glucose metabolism (ALDOA, ENO1, LDHA), cell proliferation (CDKN3, FOSL1) and angiogenesis (VEGFA), could predict those patients receiving RT for whom hypoxia-modifying ARCON (accelerated radiotherapy with carbogen and nicotinamide) therapy would be of benefit in laryngeal carcinomas [67]. The approach of stratifying patients for hypoxic modification of RT has also been performed by Troustrup et al. [68] to classify HNSCC tumours as “more” or “less” hypoxic [84]. A 15-gene hypoxic signature including genes for stress response (ADM, HIG2), cell proliferation (FOSL2, IGFBP3) and glucose metabolism (ALDOA, FKBP3) was developed from HNSCC cell lines under hypoxic conditions, and subsequently validated in patients that had previously been hypoxia-evaluated [85,86]. The predictive power of this gene signature was validated in a clinical cohort of HPV-negative HNSCC tumours, with those classified as having “more” hypoxic tumours having more favourable outcomes (loco-regional tumour control and disease-specific survival) after combining RT with hypoxia modification using nimorazole [68].
3.4. Liquid Biopsies
Minimally invasive liquid biopsies represent an area of intense research interest. While the field is in relative infancy, with no commercially validated tests, the identification of circulating biomarkers predicative of radiation response holds tremendous potential. MicroRNAs (miRNAs) are differentially regulated in a number of disease types and following exposure to ionizing radiation; they therefore offer a potential biomarker to predict treatment response in cancer [87,88,89]. A radiotherapeutic response predication was developed for patients with lower-grade glioma (LGG), based on the expression of five miRNAs. The signature was capable of classifying those as low-risk or high-risk in terms of survival and radiation response, based on the analysis of miRNA expression profiles in 624 patients. This signature was found to be superior to isocitrate dehydrogenase (IDH) mutational status in predicting survival in LGG [90]. Of particular interest is free plasma or exosome secretion of miRNAs predictive of radiation response: Li et al. [69] linked low-level miR-221 expression with increased radiation sensitivity, a finding subsequently correlated with several patient studies reporting that low serum levels of miR-221 and miR-125b are indicative of low-risk prostate cancer [69,91,92]. Furthermore, Li et al. [70] associated the levels of three miRNAs (miR-374a-5p, miR-342-5p and miR-519d-3p) with radiation responses in the plasma of patients with nonmetastatic rectal cancer and head and neck cancers. Prediction classifiers were developed from miRNA signatures in pre- and postradiotherapy samples and could significantly distinguish between radiation responders and poor responders 6 months postradiotherapy [70]. The importance of effective biomarkers, particularly in the prostate cancer setting, is evident considering that prostate-specific antigen (PSA) screening has formed the bedrock of prostate cancer diagnosis for over 25 years—a test lacking in specificity—resulting in significant treatment related morbidities from overdiagnosis and overtreatment [93]. Given the role of the TIME in influencing tumour fate postradiotherapy (detailed in Section 2), immune infiltrate composition in the TME may predict radiotherapy response and prognosis in cancer patients [5,94]. Cui et al. [71] pioneered a combined radiation sensitivity (RS) gene signature with an antigen-presentation (AP) immune signature, establishing a dual-modality approach with predictive capabilities of radiation response. Independently, both RS and AP signatures were proven capable of predicting increased disease-specific survival (DSS) in patients identified with either radiosensitive or immune-effective tumours, with the reverse observed in radioresistant and immune-defective individuals. Importantly, integration of both signatures further strengthened the predictive capabilities of either signature used independently [71].
4. Conclusions and Future Perspectives
RT is the treatment of choice for a number of cancer, designed to target and kill tumour cells; however, it triggers a myriad of effects on other components of the TME, including the vasculature, stroma and the immune compartment [5]. The immunomodulatory effects of RT are complex, with reported changes to the proportions and functionality of T cells and antigen-presenting dendritic cells, and effects on TAM polarisation within the TME. This effect is further complicated by clinical observations of an increase in the abscopal effect reported in patients receiving RT in combination with immunotherapeutics. RT has also been shown to affect tumour vascular architecture, inducing tissue fibrosis. It is important to note that the majority of responses to RT in the TME reported above are in the context of conventional X-ray or photon radiation therapy. Recent advances in the clinical delivery of RT, including high-energy proton beam therapy and heavy ion therapy, have the improvement of delivering more dose in the Bragg peak with a lower dependence on tissue oxygenation and improved biological effectiveness [95]. While these newer treatment modalities are likely to have biological effects on the components of the TME outlined in this review, their response has been less well characterised [96,97]. Therefore, it is of critical importance to take into consideration the role of the TME when considering radiobiological responses and disease recurrence. As RT techniques have evolved over the last two decades, so too have their physical precision, aided by improved imaging guidance and technological advancements. However, genomic precision has lagged, as most RT treatment planning is designed around the tumour and local tissue architecture, with the aim to deliver the maximum dose to the tumour while sparing healthy tissue. However, as highlighted above, genomic signatures could allow for a greater prediction of those patients for whom RT would be of benefit as a single therapy or in combination with radiation sensitizers or hypoxia modifiers [6]. Yet, of critical importance, these findings further stress the necessity for a precision medicine approach, in that not only do patients with radioresistant tumours fail to experience radiotherapy benefit, but that treatment is actually detrimental both in terms of DSS and toxicities associated with radiation-induced late effects [71]. Taking a more “personalised” approach to RT could ensure patients receive the most benefit from their treatment.
Acknowledgments
Figures created using MedART (creative commons license): https://smart.servier.com/.
Author Contributions
Conceptualization, N.M.B. and J.A.C.; writing—original draft preparation, review and editing, N.M.B., P.T. and J.A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bernier J., Hall E.J., Giaccia A. Radiation oncology: A century of achievements. Nat. Rev. Cancer. 2004;4:737–747. doi: 10.1038/nrc1451. [DOI] [PubMed] [Google Scholar]
- 2.Barcellos-Hoff M.H., Park C., Wright E.G. Radiation and the microenvironment—tumorigenesis and therapy. Nat. Rev. Cancer. 2005;5:867–875. doi: 10.1038/nrc1735. [DOI] [PubMed] [Google Scholar]
- 3.De Palma M., Biziato D., Petrova T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer. 2017;17:457–474. doi: 10.1038/nrc.2017.51. [DOI] [PubMed] [Google Scholar]
- 4.Quail D.F., Joyce J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker H.E., Paget J.T., Khan A.A., Harrington K.J. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer. 2015;15:409–425. doi: 10.1038/nrc3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bratman S.V., Milosevic M.F., Liu F.F., Haibe-Kains B. Genomic biomarkers for precision radiation medicine. Lancet Oncol. 2017;18:e238. doi: 10.1016/S1470-2045(17)30263-2. [DOI] [PubMed] [Google Scholar]
- 7.Scott J.G., Berglund A., Schell M.J., Mihaylov I., Fulp W.J., Yue B., Welsh E., Caudell J.J., Ahmed K., Strom T.S., et al. A genome-based model for adjusting radiotherapy dose (GARD): A retrospective, cohort-based study. Lancet Oncol. 2017;18:202–211. doi: 10.1016/S1470-2045(16)30648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ni J., Bucci J., Malouf D., Knox M., Graham P., Li Y. Exosomes in Cancer Radioresistance. Front. Oncol. 2019;9:869. doi: 10.3389/fonc.2019.00869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szatmári T., Hargitai R., Sáfrány G., Lumniczky K. Extracellular Vesicles in Modifying the Effects of Ionizing Radiation. Int. J. Mol. Sci. 2019;20:5527. doi: 10.3390/ijms20225527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Binnewies M., Roberts E.W., Kersten K., Chan V., Fearon D.F., Merad M., Coussens L.M., Gabrilovich D.I., Ostrand-Rosenberg S., Hedrick C.C., et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gajewski T.F. The Next Hurdle in Cancer Immunotherapy: Overcoming the Non-T-Cell-Inflamed Tumor Microenvironment. Semin. Oncol. 2015;42:663–671. doi: 10.1053/j.seminoncol.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fridman W.H., Pagès F., Sautès-Fridman C., Galon J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 14.Bonaventura P., Shekarian T., Alcazer V., Valladeau-Guilemond J., Valsesia-Wittmann S., Amigorena S., Caux C., Depil S. Cold Tumors: A Therapeutic Challenge for Immunotherapy. Front. Immunol. 2019;10:168. doi: 10.3389/fimmu.2019.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan Q., Zhang H., Zheng J., Zhang L. Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends Cancer. 2020;6:605–618. doi: 10.1016/j.trecan.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Galon J., Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019;18:197–218. doi: 10.1038/s41573-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 17.Dudzinski S.O., Cameron B.D., Wang J., Rathmell J.C., Giorgio T.D., Kirschner A.N. Combination immunotherapy and radiotherapy causes an abscopal treatment response in a mouse model of castration resistant prostate cancer. J. Immunother. Cancer. 2019;7:218. doi: 10.1186/s40425-019-0704-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynders K., Illidge T., Siva S., Chang J.Y., De Ruysscher D. The abscopal effect of local radiotherapy: Using immunotherapy to make a rare event clinically relevant. Cancer Treat. Rev. 2015;41:503–510. doi: 10.1016/j.ctrv.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gong J., Le T.Q., Massarelli E., Hendifar A.E., Tuli R. Radiation therapy and PD-1/PD-L1 blockade: The clinical development of an evolving anticancer combination. J. Immunother. Cancer. 2018;6:46. doi: 10.1186/s40425-018-0361-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roger A., Finet A., Boru B., Beauchet A., Mazeron J.J., Otzmeguine Y., Blom A., Longvert C., de Maleissye M.F., Fort M., et al. Efficacy of combined hypo-fractionated radiotherapy and anti-PD-1 monotherapy in difficult-to-treat advanced melanoma patients. Oncoimmunology. 2018;7:e1442166. doi: 10.1080/2162402X.2018.1442166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demaria S., Kawashima N., Yang A.M., Devitt M.L., Babb J.S., Allison J.P., Formenti S.C. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin. Cancer Res. 2005;11:728–734. [PubMed] [Google Scholar]
- 22.Postow M.A., Callahan M.K., Barker C.A., Yamada Y., Yuan J., Kitano S., Mu Z., Rasalan T., Adamow M., Ritter E., et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chandra R.A., Wilhite T.J., Balboni T.A., Alexander B.M., Spektor A., Ott P.A., Ng A.K., Hodi F.S., Schoenfeld J.D. A systematic evaluation of abscopal responses following radiotherapy in patients with metastatic melanoma treated with ipilimumab. Oncoimmunology. 2015;4:e1046028. doi: 10.1080/2162402X.2015.1046028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garelli E., Rittmeyer A., Putora P.M., Glatzer M., Dressel R., Andreas S. Abscopal effect in lung cancer: Three case reports and a concise review. Immunotherapy. 2019;11:1445–1461. doi: 10.2217/imt-2019-0105. [DOI] [PubMed] [Google Scholar]
- 25.Lin W., Xu Y., Chen X., Liu J., Weng Y., Zhuang Q., Lin F., Huang Z., Wu S., Ding J., et al. Radiation-induced small extracellular vesicles as "carriages" promote tumor antigen release and trigger antitumor immunity. Theranostics. 2020;10:4871–4884. doi: 10.7150/thno.43539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodríguez-Ruiz M.E., Vanpouille-Box C., Melero I., Formenti S.C., Demaria S. Immunological Mechanisms Responsible for Radiation-Induced Abscopal Effect. Trends Immunol. 2018;39:644–655. doi: 10.1016/j.it.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang R.X., Zhou P.K. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct. Target. Ther. 2020;5:60. doi: 10.1038/s41392-020-0150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golden E.B., Apetoh L. Radiotherapy and immunogenic cell death. Semin. Radiat. Oncol. 2015;25:11–17. doi: 10.1016/j.semradonc.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Golden E.B., Frances D., Pellicciotta I., Demaria S., Helen Barcellos-Hoff M., Formenti S.C. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology. 2014;3:e28518. doi: 10.4161/onci.28518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma A., Bode B., Wenger R.H., Lehmann K., Sartori A.A., Moch H., Knuth A., Boehmer L., Broek M. γ-Radiation promotes immunological recognition of cancer cells through increased expression of cancer-testis antigens in vitro and in vivo. PLoS ONE. 2011;6:e28217. doi: 10.1371/journal.pone.0028217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deng L., Liang H., Xu M., Yang X., Burnette B., Arina A., Li X.D., Mauceri H., Beckett M., Darga T., et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 2014;41:843–852. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mantovani A., Marchesi F., Malesci A., Laghi L., Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao X., Qu J., Sun Y., Wang J., Liu X., Wang F., Zhang H., Wang W., Ma X., Gao X., et al. Prognostic significance of tumor-associated macrophages in breast cancer: A meta-analysis of the literature. Oncotarget. 2017;8:30576–30586. doi: 10.18632/oncotarget.15736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuri P., Shigemura K., Kitagawa K., Hadibrata E., Risan M., Zulfiqqar A., Soeroharjo I., Hendri A.Z., Danarto R., Ishii A., et al. Increased tumor-associated macrophages in the prostate cancer microenvironment predicted patients’ survival and responses to androgen deprivation therapies in Indonesian patients cohort. Prostate Int. 2020;8:62–69. doi: 10.1016/j.prnil.2019.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klug F., Prakash H., Huber P.E., Seibel T., Bender N., Halama N., Pfirschke C., Voss R.H., Timke C., Umansky L., et al. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24:589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 36.Shiao S.L., Ruffell B., DeNardo D.G., Faddegon B.A., Park C.C., Coussens L.M. TH2-Polarized CD4(+) T Cells and Macrophages Limit Efficacy of Radiotherapy. Cancer Immunol. Res. 2015;3:518–525. doi: 10.1158/2326-6066.CIR-14-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu J., Escamilla J., Mok S., David J., Priceman S., West B., Bollag G., McBride W., Wu L. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013;73:2782–2794. doi: 10.1158/0008-5472.CAN-12-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stary V., Wolf B., Unterleuthner D., List J., Talic M., Laengle J., Beer A., Strobl J., Stary G., Dolznig H., et al. Short-course radiotherapy promotes pro-inflammatory macrophages via extracellular vesicles in human rectal cancer. J. Immunother. Cancer. 2020;8 doi: 10.1136/jitc-2020-000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Straub J.M., New J., Hamilton C.D., Lominska C., Shnayder Y., Thomas S.M. Radiation-induced fibrosis: Mechanisms and implications for therapy. J. Cancer Res. Clin. Oncol. 2015;141:1985–1994. doi: 10.1007/s00432-015-1974-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dancea H.C., Shareef M.M., Ahmed M.M. Role of Radiation-induced TGF-beta Signaling in Cancer Therapy. Mol. Cell. Pharmacol. 2009;1:44–56. doi: 10.4255/mcpharmacol.09.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sahai E., Astsaturov I., Cukierman E., DeNardo D.G., Egeblad M., Evans R.M., Fearon D., Greten F.R., Hingorani S.R., Hunter T., et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer. 2020;20:174–186. doi: 10.1038/s41568-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ganguly D., Chandra R., Karalis J., Teke M., Aguilera T., Maddipati R., Wachsmann M.B., Ghersi D., Siravegna G., Zeh H.J., 3rd, et al. Cancer-Associated Fibroblasts: Versatile Players in the Tumor Microenvironment. Cancers (Basel) 2020;12:2652. doi: 10.3390/cancers12092652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Z., Tang Y., Tan Y., Wei Q., Yu W. Cancer-associated fibroblasts in radiotherapy: Challenges and new opportunities. Cell Commun. Signal. 2019;17:47. doi: 10.1186/s12964-019-0362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grinde M.T., Vik J., Camilio K.A., Martinez-Zubiaurre I., Hellevik T. Ionizing radiation abrogates the pro-tumorigenic capacity of cancer-associated fibroblasts co-implanted in xenografts. Sci. Rep. 2017;7:46714. doi: 10.1038/srep46714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kamochi N., Nakashima M., Aoki S., Uchihashi K., Sugihara H., Toda S., Kudo S. Irradiated fibroblast-induced bystander effects on invasive growth of squamous cell carcinoma under cancer-stromal cell interaction. Cancer Sci. 2008;99:2417–2427. doi: 10.1111/j.1349-7006.2008.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu L., Zhang Z., Zhou L., Hu L., Yin C., Qing D., Huang S., Cai X., Chen Y. Cancer associated fibroblasts-derived exosomes contribute to radioresistance through promoting colorectal cancer stem cells phenotype. Exp. Cell Res. 2020;391:111956. doi: 10.1016/j.yexcr.2020.111956. [DOI] [PubMed] [Google Scholar]
- 47.Colton M., Cheadle E.J., Honeychurch J., Illidge T.M. Reprogramming the tumour microenvironment by radiotherapy: Implications for radiotherapy and immunotherapy combinations. Radiat. Oncol. 2020;15:254. doi: 10.1186/s13014-020-01678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown J.M. Radiation Damage to Tumor Vasculature Initiates a Program That Promotes Tumor Recurrences. Int. J. Radiat. Oncol. Biol. Phys. 2020;108:734–744. doi: 10.1016/j.ijrobp.2020.05.028. [DOI] [PubMed] [Google Scholar]
- 49.Castle K.D., Kirsch D.G. Establishing the Impact of Vascular Damage on Tumor Response to High-Dose Radiation Therapy. Cancer Res. 2019;79:5685–5692. doi: 10.1158/0008-5472.CAN-19-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arnold K.M., Flynn N.J., Raben A., Romak L., Yu Y., Dicker A.P., Mourtada F., Sims-Mourtada J. The Impact of Radiation on the Tumor Microenvironment: Effect of Dose and Fractionation Schedules. Cancer Growth Metastasis. 2018;11:1179064418761639. doi: 10.1177/1179064418761639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park H.J., Griffin R.J., Hui S., Levitt S.H., Song C.W. Radiation-induced vascular damage in tumors: Implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS) Radiat. Res. 2012;177:311–327. doi: 10.1667/RR2773.1. [DOI] [PubMed] [Google Scholar]
- 52.Riva M., Wouters R., Nittner D., Ceuster J., Sterpin E., Giovannoni R., Himmelreich U., Gsell W., Van Ranst M., Coosemans A. Radiation dose-escalation and dose-fractionation modulate the immune microenvironment, cancer stem cells and vasculature in experimental high-grade gliomas. J. Neurosurg. Sci. 2020 doi: 10.1093/neuros/nyaa421. [DOI] [PubMed] [Google Scholar]
- 53.Potiron V., Clément-Colmou K., Jouglar E., Pietri M., Chiavassa S., Delpon G., Paris F., Supiot S. Tumor vasculature remodeling by radiation therapy increases doxorubicin distribution and efficacy. Cancer Lett. 2019;457:1–9. doi: 10.1016/j.canlet.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 54.Kouam P.N., Rezniczek G.A., Adamietz I.A., Bühler H. Ionizing radiation increases the endothelial permeability and the transendothelial migration of tumor cells through ADAM10-activation and subsequent degradation of VE-cadherin. BMC Cancer. 2019;19:958. doi: 10.1186/s12885-019-6219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maeda A., Chen Y., Bu J., Mujcic H., Wouters B.G., DaCosta R.S. In Vivo Imaging Reveals Significant Tumor Vascular Dysfunction and Increased Tumor Hypoxia-Inducible Factor-1α Expression Induced by High Single-Dose Irradiation in a Pancreatic Tumor Model. Int. J. Radiat. Oncol. Biol. Phys. 2017;97:184–194. doi: 10.1016/j.ijrobp.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 56.Banerjee D., Barton S.M., Grabham P.W., Rumeld A.L., Okochi S., Street C., Kadenhe-Chiweshe A., Boboila S., Yamashiro D.J., Connolly E.P. High-Dose Radiation Increases Notch1 in Tumor Vasculature. Int. J. Radiat. Oncol. Biol. Phys. 2020;106:857–866. doi: 10.1016/j.ijrobp.2019.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stapleton S., Jaffray D., Milosevic M. Radiation effects on the tumor microenvironment: Implications for nanomedicine delivery. Adv. Drug Deliv. Rev. 2017;109:119–130. doi: 10.1016/j.addr.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 58.Jensen M.B., Lænkholm A.V., Balslev E., Buckingham W., Ferree S., Glavicic V., Dupont Jensen J., Søegaard Knoop A., Mouridsen H.T., Nielsen D., et al. The Prosigna 50-gene profile and responsiveness to adjuvant anthracycline-based chemotherapy in high-risk breast cancer patients. NPJ Breast Cancer. 2020;6:7. doi: 10.1038/s41523-020-0148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cardoso F., van’t Veer L.J., Bogaerts J., Slaets L., Viale G., Delaloge S., Pierga J.Y., Brain E., Causeret S., DeLorenzi M., et al. 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N. Engl. J. Med. 2016;375:717–729. doi: 10.1056/NEJMoa1602253. [DOI] [PubMed] [Google Scholar]
- 60.Kim H.S., Kim S.C., Kim S.J., Park C.H., Jeung H.C., Kim Y.B., Ahn J.B., Chung H.C., Rha S.Y. Identification of a radiosensitivity signature using integrative metaanalysis of published microarray data for NCI-60 cancer cells. BMC Genom. 2012;13:348. doi: 10.1186/1471-2164-13-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tramm T., Mohammed H., Myhre S., Kyndi M., Alsner J., Børresen-Dale A.L., Sørlie T., Frigessi A., Overgaard J. Development and validation of a gene profile predicting benefit of postmastectomy radiotherapy in patients with high-risk breast cancer: A study of gene expression in the DBCG82bc cohort. Clin. Cancer Res. 2014;20:5272–5280. doi: 10.1158/1078-0432.CCR-14-0458. [DOI] [PubMed] [Google Scholar]
- 62.Speers C., Zhao S., Liu M., Bartelink H., Pierce L.J., Feng F.Y. Development and Validation of a Novel Radiosensitivity Signature in Human Breast Cancer. Clin. Cancer Res. 2015;21:3667–3677. doi: 10.1158/1078-0432.CCR-14-2898. [DOI] [PubMed] [Google Scholar]
- 63.Zhao S.G., Chang S.L., Spratt D.E., Erho N., Yu M., Ashab H.A., Alshalalfa M., Speers C., Tomlins S.A., Davicioni E., et al. Development and validation of a 24-gene predictor of response to postoperative radiotherapy in prostate cancer: A matched, retrospective analysis. Lancet Oncol. 2016;17:1612–1620. doi: 10.1016/S1470-2045(16)30491-0. [DOI] [PubMed] [Google Scholar]
- 64.Liu J., Han M., Yue Z., Dong C., Wen P., Zhao G., Wu L., Xia J., Bin Y. Prediction of Radiosensitivity in Head and Neck Squamous Cell Carcinoma Based on Multiple Omics Data. Front. Genet. 2020;11:960. doi: 10.3389/fgene.2020.00960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Piening B.D., Wang P., Subramanian A., Paulovich A.G. A radiation-derived gene expression signature predicts clinical outcome for breast cancer patients. Radiat. Res. 2009;171:141–154. doi: 10.1667/RR1223.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oh D.S., Cheang M.C., Fan C., Perou C.M. Radiation-induced gene signature predicts pathologic complete response to neoadjuvant chemotherapy in breast cancer patients. Radiat. Res. 2014;181:193–207. doi: 10.1667/RR13485.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eustace A., Mani N., Span P.N., Irlam J.J., Taylor J., Betts G.N., Denley H., Miller C.J., Homer J.J., Rojas A.M., et al. A 26-gene hypoxia signature predicts benefit from hypoxia-modifying therapy in laryngeal cancer but not bladder cancer. Clin. Cancer Res. 2013;19:4879–4888. doi: 10.1158/1078-0432.CCR-13-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Toustrup K., Sørensen B.S., Lassen P., Wiuf C., Alsner J., Overgaard J. Gene expression classifier predicts for hypoxic modification of radiotherapy with nimorazole in squamous cell carcinomas of the head and neck. Radiother. Oncol. 2012;102:122–129. doi: 10.1016/j.radonc.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 69.Li B., Shi X.B., Nori D., Chao C.K., Chen A.M., Valicenti R., White Rde V. Down-regulation of microRNA 106b is involved in p21-mediated cell cycle arrest in response to radiation in prostate cancer cells. Prostate. 2011;71:567–574. doi: 10.1002/pros.21272. [DOI] [PubMed] [Google Scholar]
- 70.Li A.L., Chung T.S., Chan Y.N., Chen C.L., Lin S.C., Chiang Y.R., Lin C.H., Chen C.C., Ma N. microRNA expression pattern as an ancillary prognostic signature for radiotherapy. J. Transl. Med. 2018;16:341. doi: 10.1186/s12967-018-1711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cui Y., Li B., Pollom E.L., Horst K.C., Li R. Integrating Radiosensitivity and Immune Gene Signatures for Predicting Benefit of Radiotherapy in Breast Cancer. Clin. Cancer Res. 2018;24:4754–4762. doi: 10.1158/1078-0432.CCR-18-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Q., He G., Hou M., Chen L., Chen S., Xu A., Fu Y. Cell Cycle Regulation by Alternative Polyadenylation of CCND1. Sci. Rep. 2018;8:6824. doi: 10.1038/s41598-018-25141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goel H.L., Mercurio A.M. VEGF targets the tumour cell. Nat. Rev. Cancer. 2013;13:871–882. doi: 10.1038/nrc3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stupp R., Hegi M.E., Gorlia T., Erridge S.C., Perry J., Hong Y.K., Aldape K.D., Lhermitte B., Pietsch T., Grujicic D., et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1100–1108. doi: 10.1016/S1470-2045(14)70379-1. [DOI] [PubMed] [Google Scholar]
- 75.Gvozdenovic A., Boro A., Meier D., Bode-Lesniewska B., Born W., Muff R., Fuchs B. Targeting αvβ3 and αvβ5 integrins inhibits pulmonary metastasis in an intratibial xenograft osteosarcoma mouse model. Oncotarget. 2016;7:55141–55154. doi: 10.18632/oncotarget.10461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Noal S., Levy C., Hardouin A., Rieux C., Heutte N., Ségura C., Collet F., Allouache D., Switsers O., Delcambre C., et al. One-year longitudinal study of fatigue, cognitive functions, and quality of life after adjuvant radiotherapy for breast cancer. Int J. Radiat. Oncol. Biol. Phys. 2011;81:795–803. doi: 10.1016/j.ijrobp.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 77.Chen X., Zheng J., Zhuo M.L., Zhang A., You Z. A six-gene-based signature for breast cancer radiotherapy sensitivity estimation. Biosci. Rep. 2020;40 doi: 10.1042/BSR20202376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hu Y., Guo M. Synthetic lethality strategies: Beyond BRCA1/2 mutations in pancreatic cancer. Cancer Sci. 2020;111:3111–3121. doi: 10.1111/cas.14565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reichert Z.R., Wahl D.R., Morgan M.A. Translation of Targeted Radiation Sensitizers into Clinical Trials. Semin. Radiat. Oncol. 2016;26:261–270. doi: 10.1016/j.semradonc.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 80.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., Paulovich A., Pomeroy S.L., Golub T.R., Lander E.S., et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rastogi P., Anderson S.J., Bear H.D., Geyer C.E., Kahlenberg M.S., Robidoux A., Margolese R.G., Hoehn J.L., Vogel V.G., Dakhil S.R., et al. Preoperative chemotherapy: Updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J. Clin. Oncol. 2008;26:778–785. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 82.Yang L., West C.M. Hypoxia gene expression signatures as predictive biomarkers for personalising radiotherapy. Br. J. Radiol. 2019;92:20180036. doi: 10.1259/bjr.20180036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Buffa F.M., Harris A.L., West C.M., Miller C.J. Large meta-analysis of multiple cancers reveals a common, compact and highly prognostic hypoxia metagene. Br. J. Cancer. 2010;102:428–435. doi: 10.1038/sj.bjc.6605450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Toustrup K., Sørensen B.S., Nordsmark M., Busk M., Wiuf C., Alsner J., Overgaard J. Development of a hypoxia gene expression classifier with predictive impact for hypoxic modification of radiotherapy in head and neck cancer. Cancer Res. 2011;71:5923–5931. doi: 10.1158/0008-5472.CAN-11-1182. [DOI] [PubMed] [Google Scholar]
- 85.Nordsmark M., Overgaard M., Overgaard J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother. Oncol. 1996;41:31–39. doi: 10.1016/S0167-8140(96)91811-3. [DOI] [PubMed] [Google Scholar]
- 86.Nordsmark M., Overgaard J. A confirmatory prognostic study on oxygenation status and loco-regional control in advanced head and neck squamous cell carcinoma treated by radiation therapy. Radiother. Oncol. 2000;57:39–43. doi: 10.1016/S0167-8140(00)00223-1. [DOI] [PubMed] [Google Scholar]
- 87.Małachowska B., Tomasik B., Stawiski K., Kulkarni S., Guha C., Chowdhury D., Fendler W. Circulating microRNAs as Biomarkers of Radiation Exposure: A Systematic Review and Meta-Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2020;106:390–402. doi: 10.1016/j.ijrobp.2019.10.028. [DOI] [PubMed] [Google Scholar]
- 88.Asakura K., Kadota T., Matsuzaki J., Yoshida Y., Yamamoto Y., Nakagawa K., Takizawa S., Aoki Y., Nakamura E., Miura J., et al. A miRNA-based diagnostic model predicts resectable lung cancer in humans with high accuracy. Commun. Biol. 2020;3:134. doi: 10.1038/s42003-020-0863-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hamam R., Hamam D., Alsaleh K.A., Kassem M., Zaher W., Alfayez M., Aldahmash A., Alajez N.M. Circulating microRNAs in breast cancer: Novel diagnostic and prognostic biomarkers. Cell Death Dis. 2017;8:e3045. doi: 10.1038/cddis.2017.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang J.H., Hou R., Pan Y., Gao Y., Yang Y., Tian W., Zhu Y.B. A five-microRNA signature for individualized prognosis evaluation and radiotherapy guidance in patients with diffuse lower-grade glioma. J. Cell Mol. Med. 2020;24:7504–7514. doi: 10.1111/jcmm.15377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shen J., Hruby G.W., McKiernan J.M., Gurvich I., Lipsky M.J., Benson M.C., Santella R.M. Dysregulation of circulating microRNAs and prediction of aggressive prostate cancer. Prostate. 2012;72:1469–1477. doi: 10.1002/pros.22499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zedan A.H., Hansen T.F., Assenholt J., Madsen J.S., Osther P.J.S. Circulating miRNAs in localized/locally advanced prostate cancer patients after radical prostatectomy and radiotherapy. Prostate. 2019;79:425–432. doi: 10.1002/pros.23748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cary K.C., Cooperberg M.R. Biomarkers in prostate cancer surveillance and screening: Past, present, and future. Ther. Adv. Urol. 2013;5:318–329. doi: 10.1177/1756287213495915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen D.S., Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 95.Kirkby K.J., Kirkby N.F., Burnet N.G., Owen H., Mackay R.I., Crellin A., Green S. Heavy charged particle beam therapy and related new radiotherapy technologies: The clinical potential, physics and technical developments required to deliver benefit for patients with cancer. Br. J. Radiol. 2020;93:20200247. doi: 10.1259/bjr.20200247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lupu-Plesu M., Claren A., Martial S., N’Diaye P.D., Lebrigand K., Pons N., Ambrosetti D., Peyrottes I., Feuillade J., Hérault J., et al. Effects of proton versus photon irradiation on (lymph)angiogenic, inflammatory, proliferative and anti-tumor immune responses in head and neck squamous cell carcinoma. Oncogenesis. 2017;6:e354. doi: 10.1038/oncsis.2017.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Durante M., Formenti S. Harnessing radiation to improve immunotherapy: Better with particles? Br. J. Radiol. 2020;93:20190224. doi: 10.1259/bjr.20190224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable.