Abstract
Simple Summary
Hyperprogressive disease (HPD) is a pattern of paradoxical tumor progression that has been reported in patients treated with immune checkpoint inhibitors (ICIs). Although a large number of studies have investigated HPD and several associated factors have been reported, the mechanisms that drive the acceleration of tumor growth remain unknown. In this review, we discuss the possible role of IFN-γ and CD38 in the development of HPD, and we report the main findings in the scientific literature on the signaling pathways in which these factors take part, and their involvement in response and resistance to ICI therapy.
Abstract
Immune checkpoint inhibitors (ICIs) improve the survival of patients with multiple types of cancer. However, low response rates and atypical responses limit their success in clinical applications. The paradoxical acceleration of tumor growth after treatment, defined as hyperprogressive disease (HPD), is the most difficult problem facing clinicians and patients alike. The mechanisms that underlie hyperprogression (HP) are still unclear and controversial, although different factors are associated with the phenomenon. In this review, we propose two factors that have not yet been demonstrated to be directly associated with HP, but upon which it is important to focus attention. IFN-γ is a key cytokine in antitumor response and its levels increase during ICI therapy, whereas CD38 is an alternative immune checkpoint that is involved in immunosuppressive responses. As both factors are associated with resistance to ICI therapy, we have discussed their possible involvement in HPD with the conclusion that IFN-γ may contribute to HP onset through the activation of the inflammasome pathway, immunosuppressive enzyme IDO1 and activation-induced cell death (AICD) in effector T cells, while the role of CD38 in HP may be associated with the activation of adenosine receptors, hypoxia pathways and AICD-dependent T-cell depletion.
Keywords: hyperprogression, hyperprogressive disease, cancer, immune checkpoint inhibitors, immunotherapy, IFN-γ, CD38, macrophage, tumor microenvironment
1. Introduction
Cancer immunotherapy aims to strengthen the immune system against tumors. The introduction of immunotherapy into clinical practice has provided clinicians with an innovative tool for the treatment of various solid and hematologic malignancies [1]. One of the most successful strategies is the administration of immune checkpoint inhibitors (ICIs), which are a wide range of monoclonal antibodies directed toward immune checkpoint (IC) proteins that are expressed on tumor cell and/or immune cell surfaces. The targeting of ICs reverses tumor-mediated immunosuppression and awakens immune responses [2]. The use of ICIs, either as monotherapy or combo-therapy, has shown favorable outcomes and remarkably long-term responses in patients with a large variety of cancer types, especially malignant melanoma and lung cancer [3,4,5]. The Food and Drug Administration (FDA) has so far approved seven ICIs that target cytotoxic T-lymphocyte antigen 4 (CTLA-4), programmed death-1 (PD-1) and PD-ligand 1 (PD-L1) for the treatment of several tumor types [5]. Nevertheless, response rates, according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, for IC blockade in patients with solid tumors, range from 18 to 40% [6,7], as the beneficial clinical effects of ICI therapy are not long-lasting in some cases [8]. Moreover, the unique mechanism of action of ICIs leads to unconventional responses, making IC blockade harmful to a subset of patients. Among these novel responses, the most relevant, in terms of negative clinical outcome, is hyperprogressive disease (HPD), a paradoxical acceleration of tumor growth induced by ICI therapy [8].
1.1. Clinical Evidence of Hyperprogression and Associated Factors
HPD was first reported in retrospective studies in which patients showed rapid disease progression after the initiation of ICI therapy. Evidence suggests that a subset (4–29%) of cancer patients develops HPD [9], with a particular reference to patients with non-small-cell lung cancer (NSCLC) (from 8 to 21%), advanced gastric cancer (AGC) (from 10 to 29%), head and neck squamous cell carcinoma (HNSCC) (29%) and melanoma (9%) [9,10,11,12,13,14,15]. After Champiat et al. had introduced the concept of hyperprogression (HP) in a retrospective study of cancer patients receiving PD-1/PD-L1 inhibitors [12], several groups assessed the phenomenon and proposed different criteria for its definition, resulting in notable variations in HPD rates (Table 1). The lack of a univocal definition makes the phenomenon controversial and adds to the difficulty of comparing different studies [16]. Nevertheless, Kas et al. have recently suggested an optimized and homogenized definition of HPD, based on the previously used criteria [17].
Table 1.
The incidence of hyperprogression after ICI therapy and definitions of HPD in different studies.
| Authors | Tumor Types | Treatment | Rate of HPD (%) | Criteria Used to Define HPD |
|---|---|---|---|---|
| Champiat et al. (2017) [12] | Multiple tumor types 1 | PD-1/PD-L1 inhibitors | 9.2 |
|
| Kanjanapan et al. (2019) [18] | Multiple tumor types 2 | CTLA-4, PD-1/PD-L1 inhibitors | 7 | |
| Aoki et al. (2019) [9] |
AGC | PD-1 inhibitors | 29.4 |
|
| Ferrara et al. (2018) [13] | NSCLC | PD-1/PD-L1 inhibitors | 14 |
|
| Saâda-Bouzid et al. (2017) [14] | HNSCC | PD-1/PD-L1 inhibitors | 29.4 |
|
| Kim et al. (2019) [15] |
NSCLC | PD-1/PD-L1 inhibitors | 17 | |
| Sasaki et al. (2019) [10] |
AGC | PD-1 inhibitors | 21 |
|
| Kato et al. (2017) [19] |
Multiple tumor types 3 | CTLA-4, PD-1/PD-L1 inhibitors | 3.9 |
|
| Kamada et al. (2019) [11] | AGC | PD-1 inhibitors | 10 | |
| Matos et al. (2018) [20] |
Multiple tumor types 4 | PD-1/PD-L1 inhibitors | 15 |
|
| Lo Russo et al. (2019) [21] | NSCLC | PD-1/PD-L1 inhibitors | 25.7 |
|
1 Melanoma, renal carcinoma, colorectal cancer, urothelial cancer, hepatocellular carcinoma, head and neck carcinoma, cutaneous squamous cell carcinoma, breast cancer, ovarian cancer, glioblastoma endometrium cancer, glioblastoma, cervix cancer, gastric and esophagus cancer, mesothelioma, pancreas cancer, sarcoma. 2 Head and neck carcinoma, gynecological tumors, lung cancer, gastrointestinal tumors, genitourinary tumors, melanoma, sarcoma. 3 Melanoma, non-small-cell lung cancer, squamous cell carcinoma of head and neck, cutaneous squamous cell carcinoma, renal cell carcinoma, and colorectal cancer. 4 Melanoma, lung cancer, breast cancer, colon cancer, others. HPD—Hyperprogressive disease; NSCLC—Non-small-cell lung cancer; HNSCC—Head and neck squamous cell carcinoma; AGC—Advanced Gastric Cancer; RECIST — Response Evaluation Criteria in Solid Tumors; TGR—Tumor growth rate; ΔTGR — TGR on treatment minus the TGR before treatment; TGK—tumor growth kinetics; TTF—time-to-treatment failure; ECOG-PS—Eastern Cooperative Oncology Group performance status.
Older age (≥65) was one of the factors identified by various studies in association with an increased risk of developing HPD during ICI therapy [12,22,23,24]. Age-related immune dysfunction (ARID) leads to a decrease in the quantity and activity of T cells [25,26], which are essential for the success of ICI therapy. Previous irradiation was also associated with higher incidence of hyperprogression [14]. Alterations in the tumor infiltrate after radiotherapy, which promotes the production of neoantigens, may facilitate rapid progression in the irradiated area [27]. In a retrospective study involving patients with NSCLC treated with ICIs, hyperprogression was significantly associated with the presence of more than two metastatic sites before the treatment [13]. Mouse double minute homolog (MDM2/MDM4) amplification and epidermal growth factor receptor (EGFR) alterations were indicated as genomic markers of increased risk of hyperprogression [19,28]. Furthermore, patients with oncogene-addicted NSCLC, e.g., ALK, EGFR and STK11, did not benefit from IC blockade therapy, probably because of the “cold” nature of these tumors [29,30,31,32,33,34]. Hyperprogression was also associated with elevated serum lactate dehydrogenase (LDH) concentration [10,12,15,35]. High levels of LDH may be indicative of a hypoxic and acid microenvironment, which reduces the functionality of infiltrating T lymphocytes (TILs) and natural killer (NK) cells [36,37].
In contrast, no association between HPD and tumor histology, baseline tumor size and previous lines of therapy has been reported [12,14,19]. Concerning the association of PD-L1 expression with HPD, studies have shown discordant results [38,39,40]. Nevertheless, a significant inverse correlation between PD-L1 expression in tumor cells and HPD has been detected in NSCLC patients [21].
1.2. Hypothesized Mechanisms of HPD
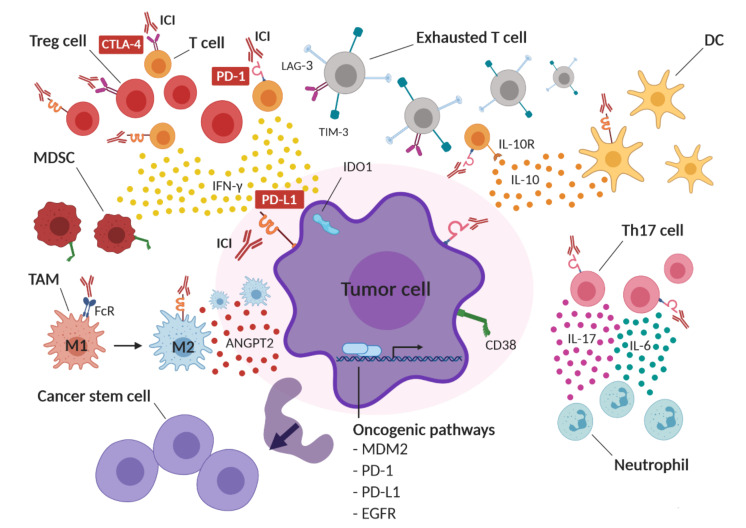
The mechanisms underlying hyperprogression after ICI therapy are still unknown; however, alterations in T-cell subpopulations, cytokine secretion, inflammation and tumor cells have been proposed (Figure 1).
Figure 1.
Possible mechanisms of hyperprogressive disease (HPD) in cancer after immune checkpoint blockade therapy. ICI therapy may functionally activate infiltrating regulatory T cells (Tregs), leading to an immunosuppressive tumor microenvironment. At the same time, compensatory upregulation of alternative immune checkpoints, such as lymphocyte-activation gene 3 (LAG-3), T-cell immunoglobulin and mucin domain-3 (TIM-3) and CTLA-4, on effector T cells after ICI therapy may induce T-cell exhaustion. IC inhibition might also induce the expression of cluster of differentiation 38 (CD38) on tumor cells and IFN-γ-dependent recruitment of CD38-expressing myeloid-derived suppressor cells (MDSCs), resulting in immune suppression. Moreover, ICI therapy may induce the upregulation of the immunosuppressive enzyme indoleamine 2,3-dioxygenase (IDO1) and the increased secretion of immunosuppressive cytokines and soluble molecules, such as interleukin 10 (IL-10), angiopoietin-2 (ANGPT2) and interferon-gamma (IFN-γ) into the tumor microenvironment. IC blockade might also functionally boost T helper 1 (not shown) and T helper 17 (Th17) lymphocytes, resulting in neutrophil recruitment and in an inflammatory immunosuppressive tumor microenvironment enriched in interleukin 6 (IL-6) and interleukin 17 (IL-17). In addition, ICIs may bind to Fc receptors (FcR) on tumor-associated macrophages (TAMs), resulting in a shift from M1 to an immunosuppressive M2 phenotype. IC blockade may also induce an increase in cancer stem cells (CSC) and may activate oncogenic pathways, such as MDM2, PD-1, PD-L1 and EGFR, thus promoting tumor proliferation. DC: dendritic cell.
ICs, such as CTLA-4, PD-1 and PD-L1, can be overexpressed on immunosuppressive tumor-specific regulatory T (Treg) cells [41,42,43]. A rapid expansion of forkhead box P3+ (Foxp3+) Treg cells has been observed in the tumors of HPD patients with advanced gastric cancer treated with nivolumab. Moreover, PD-1 blockade enhances the proliferation and suppressive activity of human Treg cells in vitro [11].
IC blockade therapy can also induce compensatory mechanisms, leading to T-cell exhaustion, local immune suppression and tumor escape. Two independent studies have reported the compensatory upregulation of ICs, including lymphocyte activation gene-3 (LAG-3), T-cell immunoglobulin and mucin domain-3 (TIM-3) and CTLA-4, on CD8+ T cells after PD-1 blockade in immunocompetent murine models of ovarian cancer and lung adenocarcinoma [44,45]. ICI therapy can also upregulate cluster of differentiation 38 (CD38) on tumor cells, leading to immune suppression and resistance to therapy [46]. In addition, the aberrant expansion of peripheral exhausted CD4+ memory T cells has been reported to occur after the first administration of anti-PD-1/PD-L1 antibodies in patients with HPD, unlike non-HPD patients [47].
The compensatory immune response triggered by IC blockade can induce the production of immunosuppressive cytokines and other soluble mediators. In preclinical studies, PD-1/PD-L1 blockade increased IL-10 secretion by tumor infiltrating dendritic cells (DCs) and upregulated PD-L1 on DCs, leading to tumor immune escape [48]. In addition, tumor-specific CD8+PD-1+ T cells, in patients with advanced melanoma under PD-1 blockade therapy, overexpressed the IL-10 receptor (IL-10R). The inhibition of IL-10 strengthened the effect of anti-PD-1 antibodies in expanding tumor-specific CD8+ T cells, and thus reinforced their antitumor action [49].
Angiopoietin-2 (ANGPT2) has been proposed as a predictive and prognostic marker in ICI-treated patients with advanced melanoma. High levels of ANGPT2 in serum before treatment were associated with reduced response and/or overall survival and with higher levels of immunosuppressive M2 macrophages in ICI-treated patients [50,51].
Aberrant inflammation that is caused by increased T helper 1 (Th1) and Th17-dependent secretion of inflammatory cytokines, such as IFN-γ, IL-6 and IL-17, which are associated with neutrophil recruitment, has been observed in patients with prostate cancer and melanoma that were treated with PD-1/PD-L1 inhibitors [52]. Interestingly, neutrophil depletion and IL-6 blockade resulted in more effective antitumor immune responses in mouse models [53,54,55]. Moreover, the interaction between the Fc domain of ICIs and Fc-receptors (FcR) induces macrophage reprogramming, from the M1 to M2 phenotype, in patients with HPD [21].
Tumor-specific non-lytic CD8+ T cells induce the overexpression of PD-L1 and IDO1, which are associated with adaptive immune resistance and stemness phenotype in tumor cells [56].
MDM2 is an oncoprotein that is involved in the degradation and inhibition of p53. The amplification of this gene has frequently been observed in HPD patients [19]. IFN-γ-induced interferon regulatory factor 8 (IRF-8) induces MDM2 overexpression by binding to its promoter [19,57].
The activation of the PD-1 axis on T cells reduces T-cell proliferation [58], while PD-1 inhibition on neoplastic T cells accelerates tumor growth [59,60,61]. PD-1 expression on tumor cells drives melanoma tumorigenesis via PD-1/PD-L1 interaction [62]. Conversely, preclinical data suggest that PD-1 blockade may increase tumor growth by interfering with the PD-1-dependent upregulation of proapoptotic proteins, e.g., BIM, p15INK4 and cyclin-dependent kinase 2 [63].
PD-1/PD-L1 interaction transmits antiapoptotic signals to cancer cells, leading to resistance to T-cell-mediated cytolysis and Fas-mediated apoptosis. The elimination of the intracellular domain of PD-L1 ablated cancer resistance to immune response, leading to tumor regression [64]. In addition, PD-L1 can transmit protumorigenic intracellular signaling, even in a PD-1-independent manner, by interfering with the autophagy process or activating mTOR [65,66]. PD-L1 expression also protects tumor cells from IFN-antitumor action by inhibiting STAT3-caspase 7 signaling [67]. Finally, activating mutations of EGFR drive PD-L1 expression on several tumor types, including NSCLC, HNSCC and breast cancer [68]. The EGFR-dependent mechanism of PD-L1 regulation involves post-translational modifications. Indeed, the inhibition of EGFR signaling destabilizes PD-L1 expression in mouse models, thus enhancing the therapeutic efficacy of PD-1 blockade [69,70].
2. Role of IFN-γ in HPD
IFN-γ is a major regulatory and effector cytokine predominantly produced by T and NK cells in response to inflammatory and immune stimuli. In the tumor microenvironment (TME), IFN-γ, mainly produced by TILs, is a key player in tumor immunosurveillance. The antitumor action includes antiproliferative, antiangiogenic and proapoptotic effects [71,72,73,74,75], in addition to the upregulation of major histocompatibility complex (MHC) class I molecules on tumor cells [76,77]. Moreover, IFN-γ activates CD8+ cytotoxic T lymphocytes, CD4+ Th1 cells, NK cells, DCs and macrophages, and stimulates the latter to switch towards the tumoricidal and proinflammatory M1 phenotype [77,78,79]. Conversely, IFN-γ inhibits Treg cell differentiation and function [80].
On the other hand, IFN-γ exerts a paradoxical immunosuppressive role that supports tumor progression and dissemination [81,82,83]. The activation of the IFN-γ receptor (IFNGR) on tumor cells activates the JAK/STAT signaling pathway, resulting in PD-L1 upregulation [84,85,86]. Nevertheless, alterations in the JAK/STAT pathway have been frequently associated with resistance to ICI therapy [87,88,89]. For instance, human melanoma cell lines with loss-of-function mutations in either JAK1 or JAK2 do not express IFN-γ-response genes after IFN-γ exposure. The analysis of the transcriptome of advanced melanoma under ICI therapy has highlighted an association between clinical response to treatment and the expression of IFN-γ-response genes involved in MHC class I and II upregulation [90]. Resistance to ICI therapy may therefore be due both to the incapacity of tumor cells to induce the full set of IFN-γ-response genes and to the loss of sensitivity to IFN-γ signaling. Interestingly, prolonged IFN-γ receptor signaling in tumor cells can also mediate resistance to ICIs through epigenomic changes in the JAK/STAT pathway [91].
IFN-γ can even support tumorigenesis by influencing the TME. The IFN-γ signaling pathway can indeed increase angiogenesis in the TME by inhibiting the expression of vascular endothelial growth inhibitor (VEGI) [92]. IFN-γ also suppresses the action of immune effector cells via the upregulation of immunosuppressive cytokines, including IL-21, IL-27 and IL-35 [93,94,95,96,97,98], and by the recruitment and differentiation of Treg cells and MDSCs [99,100,101,102,103,104,105,106,107].
All of the above-reported examples highlight the role that IFN-γ plays in tumor resistance to ICI therapy. Based on this evidence, it is reasonable to assume that IFN-γ is worthy of investigation in the context of HPD.
2.1. IFN-γ and Inflammasome
Several studies reported a negative correlation between MDSCs and the response to ICIs [108,109,110], leading to the suggestion that MDCSs can be a negative predictive marker for ICI therapy [111]. A few case reports noted a correlation between HPD development and the number of MDSCs in the peripheral blood and in the TME [112,113]. Moreover, the recruitment of granulocytic MDSCs to the TME following ICI therapy, through the IFN-γ-dependent activation of the inflammasome pathway in cancer cells, has been reported [114]. PD-L1 upregulation by IFN-γ after ICIs and the activation of the PD-L1 intrinsic signaling pathway in tumor cells trigger the activation of NLR family pyrin domain containing 3 (NLRP3), which leads to the downstream activation of the heat-shock proteins 70 (HSP70)/Toll-like receptor 4 (TLR4) signaling pathway and Wnt5a production. This signaling cascade ultimately leads to C-X-C motif chemokine ligand 5 (CXCL5) release, resulting in chemokine-dependent recruitment of polymorphonuclear-like MDSCs [114]. PD-L1 triggers NLRP3 activation by repressing STAT3, which is a transcription factor involved in IFN-cytotoxicity. Mutations in the intracytoplasmic DTSSK domain of PD-L1, which is a conserved sequence that acts as a negative regulator of PD-L1 functions, lead to hyperactive PD-L1 molecules in human tumors, enhancing the capacity of the PD-L1 intracellular pathway to interfere with STAT3 expression and phosphorylation [67]. Thus, in patients carrying mutations in the intracytoplasmic domain of PD-L1, the molecules of the inflammasome may be further augmented by PD-L1 upregulation after IFN-γ secretion in response to ICIs, eventually leading to HPD. Interestingly, a mutational analysis performed on tumors after pembrolizumab treatment highlighted the presence of missense or indel mutations in genes involved in the negative regulation of NLRP3 activation and inflammasome pathway [113], including the caspase recruitment domain (CARD8 and CARD11), protein flightless-1 homolog (FLII) and nuclear factor erythroid 2-related factor 2 (NFE2L2) [115,116,117,118]. Moreover, hyperprogressive tumors show mutations in NOTCH1, which seems to be involved in NLRP3 activation and inflammasome pathway [19,113,119,120,121]. In addition, the mechanism of MDSC recruitment in HPD may also be related to the impairment of effector T-cell activity, resulting in the expansion of Treg cells, the inhibition of NK cells and the secretion of immunosuppressive cytokines [122]. IFN-γ-mediated recruitment of MDSCs in the TME may therefore be a relevant aspect of HPD.
2.2. IFN-γ and IDO1
Paracrine Wnt5a signaling is also involved in DC upregulation and enzymatic activity of IDO1, resulting in the promotion of DC-mediated Treg differentiation [123,124]. IDO1 is a cytosolic enzyme that contributes to immune regulation by inducing metabolic changes in the local microenvironment. The enzyme catalyzes the rate-limiting step of tryptophan metabolism, which converts tryptophan (trp) into the downstream catabolite kynurenine (kyn). IDO1 is physiologically expressed by professional antigen-presenting cells (APCs), as well as by epithelial cells, the vascular endothelium and peripheral lymphoid organs, and acts as a peripheral IC, contributing to host defense against infection, peripheral immune tolerance, inhibition of local inflammation and autoimmunity [125,126]. Tumor cells use IDO1 expression as a mechanism of immune escape [127,128,129]. Trp depletion indeed results in a blockade of T-cell protein synthesis [130,131], while kyn and its derivatives induce PD-1 expression on activated T cells, and the differentiation of Treg cells and tolerogenic DCs through aryl hydrocarbon receptor (AhR) activation [132,133,134,135]. In addition, IDO1 expression is induced, as is that of PD-L1, when some degree of inflammation occurs in the tumor, e.g., the presence of proinflammatory mediators, such as IFN-γ, as a mechanism of adaptive resistance against infiltrating T cells [136,137,138]. This aspect clearly has negative implications for ICI therapy, since increased IDO1 activity may increase tumor-infiltrating Treg cells, decrease TILs and accelerate tumor growth [139,140,141,142,143]. In NSCLC patients treated with nivolumab, serum kyn/trp ratio was higher in early progressors with intrinsic resistance to anti-PD-1 therapy. Moreover, patients with high kyn/trp ratios showed a progression-free survival of three months, which is very similar to that of hyperprogressive patients in a study by Champiat et al. [12,144]. This evidence suggests that IDO1 induction by IFN-γ after ICI therapy may counteract the effectiveness of an otherwise beneficial treatment. Combination treatment with ICIs and IDO1 inhibitors in preclinical studies has been observed to enhance the infiltration and proliferation of effector T cells in the TME [145,146]. However, despite encouraging clinical results in early phase trials [147,148,149], in a randomized phase III study, patients with metastatic melanoma treated with both the IDO1 inhibitor epacadostat and pembrolizumab, had no benefit from the combined therapy, in comparison to pembrolizumab monotherapy [150]. This negative result may, however, be caused by several factors, including limited preclinical data and either the incomplete inhibition of IDO1 or the compensatory expression of other trp-degrading enzymes [151]. Further clinical studies are therefore needed to understand whether this combined therapy may have therapeutic potential.
Interestingly, IDO1 upregulation is inversely correlated with p53 [152], whose expression can be suppressed by IDO1 via the c-Jun N-terminal kinase (JNK) pathway [153]. The downregulation of p53 has already been suggested as a HPD mechanism in patients with MDM2 amplification [19]. Moreover, IDO1 expression is modulated by transforming growth factor-beta (TGF-β) via the Fyn-dependent phosphorylation of immunoreceptor tyrosine-based inhibition motifs (ITIMs) in IDO1, and the activation of the NF-κB pathway, leading to a tolerogenic phenotype in DCs [154]. TGF-β can also activate the JNK pathway through TGF-β activated kinase 1 (TAK1) and c-Jun phosphorylation [155,156]. The TGF-β signaling pathway has been found to be transcriptionally upregulated in HPD tumors following IC blockade, as compared to treatment-naive tumors [113]. Therefore, HPD patients with IDO1-expressing tumors may present a hyperactivated JNK pathway which may result in p53 suppression. Moreover, post-therapy HPD tumors have also displayed transcriptional upregulation of PI3K/AKT and MAPK/ERK pathways, and it has been shown that PI3K and MAPK oncogenic mutations can favor constitutive IDO1 expression on tumor cells [113,157,158].
2.3. IFN-γ and Activation-Induced Cell Death
A further hypothesis that involves IFN-γ focuses on the differential immunological actions of IC blockade that occur depending on tumor burden. The combination of anti-CTLA-4 and anti-PD-1 therapy in mice with high tumor burden (HTB) leads to improved tumor control and to the generation of more activated antigen-specific T cells, as compared to mice with low tumor burden (LTB), in which combination treatment compromised antitumor immune response, inducing the loss of antigen-specific T cells [159]. This finding was supported by retrospective clinical data from metastatic melanoma patients who received either monotherapy or combination therapy. Patients treated with dual IC blockade showed significantly lower response rates than those treated with monotherapy in low disease settings, but not in higher disease settings. The detrimental effect of combined therapy in the LTB state was associated with higher IFN-γ production, which was responsible for tumor-reactive CD8+ T-cell apoptosis via activation-induced cell death (AICD). AICD physiologically takes place in the early CD4+ and CD8+ T-cell priming stage, and leads to cell apoptosis to prevent immune hyperactivation. IFN-γ signaling is the key factor in activating this process, together with IL-2 [160]. The induction of IFN-γ secretion after dual-blockade treatments can promote the apoptosis of tumor-reactive CD8+ T cells in the LTB setting, limiting the formation of effector memory antitumor responses. In the HTB state, prolonged antigen exposure may lead to T cells with a markedly exhausted phenotype, which may be more prone to reinvigoration after IC blockade. By contrast, in LTB or in the early tumor setting, short-term antigen exposure may be unable to induce a fully exhausted phenotype in T cells. Therefore, the activation of T-cell-receptor signaling against tumor antigens, in combination with dual IC blockade, may result in immune hyperactivation, triggering the AICD process. These findings appear to indicate that the paradoxical effect of IFN-γ in tumor response might derive from the differential exhaustion status of T cells in response to ICIs. Although the mechanisms underlying AICD have not yet been fully understood, Fas seems to be the major death receptor responsible for triggering the AICD pathway in CD4+ T cells [161]. Moreover, STAT1 and caspase 8, which are activated by the IFN-γ pathway, may be involved in the process [160]. The possible role of the activation of the AICD pathway by IFN-γ in HPD is supported by a study in which two hyperprogressive patients displayed depletion of the immune-cell populations involved in tumor clearance, including monocytes, central memory CD4+ T cells, NK cells and activated DCs [113]. In these patients, anti-PD-1 therapy had probably induced an accelerated AICD process in the antitumor activating lymphocytes, as suggested by the activation of apoptosis gene sets and the upregulation of marker genes in the bcl-2 pathway after treatment. These studies suggest that, in some patients, ICI therapy may be responsible for excessive activation of the immune response, which could trigger regulatory mechanisms and hinder therapeutic antitumor effects.
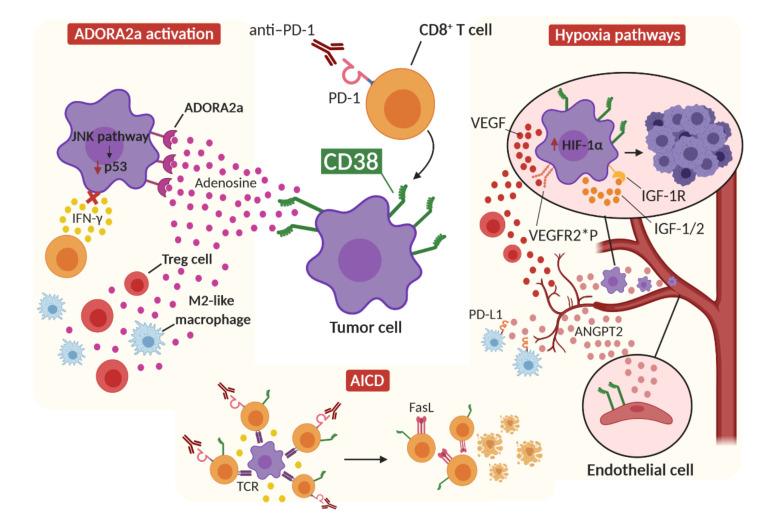
In conclusion it may be suggested that IFN-γ contributes to HPD onset in predisposed patients via the induction of the inflammasome pathway and consequent MDSC recruitment, the induction of IDO1 activity, which may result in the downregulation of p53 in tumor cells, and the activation of AICD, which leads to T-cell depletion (Figure 2).
Figure 2.
Proposed IFN-γ-dependent mechanisms of hyperprogression. The release of IFN-γ from CD8+ T cells after ICI therapy can activate the inflammasome pathway by upregulating PD-L1 expression on tumor cells and activating NLRP3 signaling, resulting in immunosuppressive MDSC recruitment in the tumor microenvironment. At the same time, IFN-γ can induce IDO1 activity in tumor cells, which activates the JNK pathway, leading to p53 downregulation and tumor growth. Finally, the concomitant stimulation of tumor-specific CD8+ T cells by ICI therapy and T-cell-receptor (TCR) activation results in a hyperactivated immune environment in which IFN-γ triggers the activation-induced cell death (AICD) mechanism and T-cell Fas-mediated apoptosis.
3. Role of CD38 in HPD
CD38 is a multifunctional ectoenzyme that is widely expressed on the surface of multiple immune populations, including T cells, NK cells, DCs and myeloid-derived cells. This molecule, which performs both enzyme and receptor activity, is involved in the regulation of intracellular Ca2+, cell adhesion, infection, tumorigenesis and immunosuppression [162,163]. As far as the enzymatic activity of CD38 is concerned, it acts as a NADase, catalyzing nicotinamide adenine dinucleotide (NAD+) hydrolysis and creating immunosuppressive byproducts such as adenosine diphosphate-ribose (ADPR) and cyclic ADPR (cADPR). Moreover, under acidic conditions, CD38 catalyzes the formation of nicotinic acid adenine dinucleotide phosphate (NAADP) from nicotinamide adenine dinucleotide phosphate (NADP+) [163]. CD38 NADase activity contributes to T-cell exhaustion via the reduction of NAD+ levels in T cells. Exhausted T cells indeed show high PD-1 and CD38 expression [164,165].
Concerning the function of CD38 as a receptor, its short cytoplasmatic tail makes CD38 unable to activate a downstream signaling pathway [166]. CD38 indeed depends on other signaling receptors and cell-surface molecules, such as CD31, which is an adhesion protein that is mainly expressed on endothelial cells. The interaction between CD38 and CD31 induces dynamic interactions between CD38+ immune cells and endothelial cells, promoting cell migration and homing [167,168,169]. In T cells, however, the interaction between CD38 and CD31 induces cell activation, which is mediated by the CD38-dependent phosphorylation of the intracellular proteins involved in T-cell-receptor (TCR)/CD3 signaling pathway and MAPK-pathway activation [170]. CD38 ligation in peripheral blood mononuclear cells (PBMCs) induces the expression of IL-1β, IL-6, IL-10 and IFN-γ [171], while in NK cells, CD38 association with CD16 promotes degranulation and IFN-γ production [172,173]. In B cells, CD38 induces either proliferation or apoptosis, depending on differentiation stage, thanks to its association with B-cell-receptor (BCR) downstream pathway [174].
Despite the cooperation of CD38 in effector cytotoxic responses, the enzyme is involved in immune escape and tumor growth. CD38 has been widely investigated in chronic lymphocytic leukemia (CLL) and multiple myeloma (MM). In CLL, CD38 is a marker of both poor prognosis and aggressive tumor phenotype [175,176]. In CLL cells, CD38 expression, which works in synergy with the BCR/CD19 complex, is associated with greater proliferative and antiapoptotic activity as well as with enhanced migratory ability [177,178,179]. CD38 is highly expressed on MM cells as well [180,181]. This finding has made CD38 a target for therapeutic antibodies in MM, including daratumumab, a human anti-CD38 antibody that has been approved by the FDA both as monotherapy and in combination with standards of care, and isatuximab, a human anti-CD38 antibody that has recently been approved in combination with pomalidomide and dexamethasone [182,183]. Both daratumumab and isatuximab have been shown to effectively induce myeloma cell death via Fc-dependent mechanisms of action and the depletion of CD38+ immunosuppressive cells [183,184,185,186,187,188].
In solid tumors, CD38 is involved in immune evasion and tumor progression. In the TME, CD38 can be upregulated on MDSCs, inducing a more immature and immunosuppressive phenotype [189]. In gliomas, the expression of CD38 in the TME has been associated with F4/80 infiltrating microglia and macrophages, which favor tumor growth, and suggest that CD38 may play a role in the recruitment and survival of these immune subpopulations [190]. Other studies have demonstrated that CD38 has a role in tumor growth that is independent of the expression of CD38 on immune cells, and is rather due to its expression on tumor cells. The knockout of CD38 on tumor cells reduced the invasion and progression of tumors in nude mice models, while the expression of CD38 by tumor cells enhanced in vitro and in vivo growth [191,192]. Furthermore, CD38, when expressed on tumor cells, can inhibit CD8+ T cells via adenosine production and the activation of adenosine receptor signaling on T cells [46]. Based on the above-reported evidence, it can be stated that CD38 exerts a protumorigenic role, both through its enzymatic activity and its receptor functions, by activating downstream pathways in tumor cells that enhance their proliferative, antiapoptotic and migratory ability, and promoting immune evasion through the recruitment of immunosuppressive cells and T-cell exhaustion.
CD38 expression has also been correlated with resistance to ICI therapy. PD-1/PD-L1 blockade has been reported to upregulate CD38, not only on CD8+ cytotoxic T cells, but also on tumor cells [46,193]. CD38 expression can be induced in monocytes, bone-marrow progenitor cells and CLL cells by IFN-γ [194,195,196]. Treatment with the anti-CD38 antibody in mice that are resistant to IC blockade therapy inhibits tumor growth, enhances effector CD8+ and CD4+ T-cell responses and reduces CD4+ Treg cells and MDSCs [46]. CD38 expression in tumors has been recognized as a biomarker of poor response to ICI therapy [197].
3.1. CD38 and Adenosine Receptor Activation
CD38 mediates IC-blockade resistance through the modulation of adenosine receptor signaling in the TME, which leads to the inhibition of T-cell proliferation and function [46]. As briefly mentioned above, CD38 enzymatic activity includes the production of ADPR and cADPR. ADPR can be further metabolized by CD203 and CD73 to produce adenosine, which is implicated in immune suppression through the activation of adenosine receptors (ADORA) [198]. The activation of ADORA on tumor cells regulates cell proliferation, apoptosis, cytoprotection and migration [199,200]. In particular, the adenosine A2A receptor (ADORA2a) has been found to be overexpressed in HNSCC [201,202] and in several melanoma and breast cancer cell lines [203,204,205]. The ADORA2a downstream pathway inhibits JAK/STAT signaling, either by preventing phosphorylation or inducing the proteasomal degradation of STAT proteins [206]. ADORA2a-driven STAT degradation may be involved in HPD along with the JAK/STAT dysfunctions that were previously described in patients who are resistant to IC blockade therapy. Although CD38 upregulation has not been directly linked to hyperprogressive tumors, it is interesting to highlight the reported mutations of NOTCH genes in HPD patients [19,113] and the correlation between NOTCH signaling activation and CD38 expression [207]. Furthermore, ADORA2a physically interacts with the fibroblast growth factor receptor (FGFR) and the concomitant activation of these receptors induces the activation of the MAPK/ERK pathway in neural tissue [208]. The amplification of FGF genes and the upregulation of the TGF-β pathway have been reported in hyperprogressive tumors [28,113]. These data suggest that, in hyperprogressive tumors, these alterations may lead to hyperactivation of the presumed ADORA2a-FGFR-dependent pathway, possibly resulting in accelerated tumor growth. Moreover, the expression of ADORA2a by tumor cells can induce the JNK signaling pathway, leading to p53 downregulation [209,210]. Furthermore, the activation of ADORA2a by extracellular adenosine results in the accumulation of intracellular cAMP, which induces the upregulation of inhibitory receptors, such as PD-1, increased Foxp3 expression in CD4+ T cells, the induction of effector T-cell anergy and the upregulation of inhibitory cytokines, including TGF-β [211]. Since Treg cells express high levels of CD39 and CD73, which catabolize adenosine triphosphate (ATP) and adenosine diphosphate (ADP) into adenosine, ADORA2a can lead to an immunosuppressive amplification circuit in which increasing amounts of adenosine are created [178]. It can be hypothesized that, in a context of high CD38 activity and, consequently, high adenosine levels in TME, hyperprogressive tumors may display a hyperactivated ADORA2a pathway, which may confer resistance to IFN-γ cytotoxic action, enhance oncogenic pathways, such as MAPK/ERK and TGF-β, and induce a strong immunosuppressive microenvironment through the shift of CD4+ T cells towards a Treg phenotype. Hyperprogressive patients have indeed demonstrated a marked increase in effector Foxp3high Treg cells with proliferative capacity compared to non-hyperprogressive patients, who, on the contrary, showed a significant reduction [212]. Adenosine signaling can even have an impact on DC maturation, inducing the secretion of tolerogenic factors such as vascular endothelial growth factor (VEGF), IL-10, TGF-β, arginase and IDO1 [213]. In addition, adenosine signaling triggers the increased expression of other IC pathways, such as PD-1, CTLA-4 and LAG-3, in both effector and Treg cells [214,215]. Finally, adenosine receptor stimulation promotes M2 polarization in tumor-associated macrophages (TAMs), which are known to be involved in HPD [21,216]. These findings demonstrate that exposure to high concentrations of adenosine, which are induced by CD38 activation, can dampen the antitumor function of immune cells, contributing to a more immunosuppressive microenvironment that may interfere with the therapeutic response to ICIs.
3.2. CD38 and Activation-Induced Cell Death
CD38 expression seems to occur in settings with active intratumoral cytolytic immune-cell infiltrates and immune-inflammatory signatures, e.g., Foxp3, CTLA-4, PD-1, PD-L1, LAG-3, TIM-3, IDO1 and C-C Motif Chemokine Ligand 2 (CCL2). Thus, CD38 upregulation may be a consequence of intratumoral T-cell infiltration and associated changes in the cytokine/metabolite milieu after IC blockade [46]. ICI therapy may exert prolonged pressure that may induce CD38 upregulation as a compensatory mechanism of immune regulation. Over time, the immunosuppressive effect of highly CD38-expressing tumor cells may become dominant over the expression of PD-1 and PD-L1, resulting in resistance to treatment. Interestingly, in a study of microglial activation in the central nervous system, CD38 promoted the AICD process via cADPR production [217]. CD38−/− primary microglia was more resistant to AICD than wild-type microglia. Moreover, CD38 can promote nitric oxide production, which is an essential mediator for AICD. Nevertheless, CD38 does not seem to independently regulate the AICD process, since it is not able to regulate caspase 11 expression, which is an important mediator of AICD. It can therefore be stated that CD38 participates in AICD activation, which, however, is also mediated by other pathways. Interestingly, IC blockade therapy induces not only CD38 upregulation, but also increased expression of IRF-1, which upregulates Fas ligand (FasL) transcription by directly binding to its promoter [46,218]. As Fas/FasL signaling is the main mechanism of AICD, it can be concluded that ICI therapy, and CD38 upregulation after treatment, seem to be associated with the activation of AICD, which may in turn lead to the depletion of activated cytotoxic CD8+ T cells in the TME and, consequently, tumor growth.
3.3. CD38 and Hypoxia
Increased CD38 expression has also been associated with a markedly hypoxic microenvironment, which stimulates angiogenesis events and immunosuppressive actions. Hypoxia upregulates inhibitory signals for antitumor immune response, including PD-L1, IDO1, IL-6 and IL-10, and promotes the polarization of TAMs towards a M2-like phenotype. Moreover, tumor hypoxia recruits Treg cells in the TME via the upregulation of chemokines, while the increased expression of FasL on the tumor endothelial barrier in hypoxic conditions can selectively eliminate effector CD8+ T cells. Increased expression of ANGPT2 mRNA in mononuclear cells of untreated CLL patients has been associated with high CD38 expression [219], which influences the expression of hypoxia-inducible factor 1-alpha (HIF-1α) [220]. The overexpression of HIF-1α has been associated with poor prognosis and an aggressive phenotype in several malignant tumors [221,222,223]. HIF-1α takes part in tumor angiogenesis via the regulation of VEGF expression [224,225]. The ability of CD38 to promote HIF-1α expression may be related to the onset of hyperprogression. Post-therapy tumors of HPD patients show somatic mutations in the von Hippel–Lindau tumor suppressor gene (VHL), which is a master regulator of HIF activity [113,226]. Biallelic VHL inactivation in renal cell carcinoma (RCC) is indeed associated with increased HIF-1α, which is responsible for the highly vascular nature of RCC [113,227]. A nonsense mutation in the BRCA1 associated protein 1 (BAP1) gene has been reported in the post-therapy tumor of a hyperprogressive patient [113], and the loss of BAP1 expression was highly correlated with HIF-1α expression in uveal melanoma. It has been proposed that BAP1 may lead to HIF-1α upregulation via the NF-κB pathway [228]. Furthermore, hyperprogressive patients show the transcriptional upregulation of the oncogenic insulin-like growth factor 1 (IGF-1) signaling pathway and somatic mutation in the IGF-binding protein 2 (IGFBP2) gene [113]. Interestingly, a study has reported that HIF-1α overexpression in tumor cells takes part in an autocrine growth factor loop, which involves the HIF-1α-dependent expression of genes encoding IGF-2, IGFBP-2 and IGFBP-3. Therefore, HIF-1α may be involved in tumor growth, resistance to apoptosis and migration via the activation of IGF pathways [229]. HIF-1α is also a driver of VEGF expression in tumors and may help to establish autocrine and paracrine signaling networks in the TME. For instance, VEGF acts as a chemoattractant for CD4+Foxp4+ Treg cells into the tumor [230]. Moreover, the autocrine signaling of VEGF in tumor cells, through the activation of the VEGF receptor (VEGFR), contributes to tumorigenesis, tumor dedifferentiation and invasion [231,232]. Hyperprogression has been significantly associated with VEGFR2 rs1870377 A/T and A/A polymorphisms [233], which are able to increase VEGF-A binding and activity, inducing increased microvessel density in tumors [234]. Hyperprogressive tumors may show strong binding between VEGF and VEGFR, either on endothelial cells or on tumor cells, leading to increased angiogenesis and rapid tumor growth. The association between CD38 expression and the induction of a hypoxic environment is further confirmed by the strong correlation between higher serum LDH and CD38 expression in CLL patients, in which CD38 expression was also associated with shorter overall survival compared to patients without CD38 expression [235]. Based on the above-reported evidence, the association between CD38 and hypoxic responses may be an important part of HPD development.
In conclusion, CD38 upregulation after IC blockade therapy may contribute to the development of hyperprogression through the release of high levels of adenosine into the TME and the consequent activation of the ADORA2a pathway, which may lead to tumor insensitivity to IFN-γ action, to the downregulation of p53 with consequent tumor growth, and to strong immunosuppression. CD38 upregulation may also be an adaptive immune response to a hyperactivated immune setting induced by ICI therapy. In this context, CD38 may promote the apoptosis of effector T cells via the AICD process, leading to a protumorigenic setting. Finally, CD38 may enhance hypoxia signaling pathways in tumor cells or endothelial cells, leading to increased angiogenesis, immunosuppression and tumor proliferation (Figure 3).
Figure 3.
Proposed CD38-dependent mechanisms of hyperprogression. The upregulation and activation of CD38 after IC blockade therapy, together with the action of enzymes that catabolize ATP and ADP (not shown), lead to the release of adenosine into the tumor microenvironment. The activation of ADORA2a receptors on tumor cells by adenosine leads to the blockade of the IFN-γ pathway, making the tumor cell resistant to cytotoxic IFN-γ action, as well as the activation of oncogenic pathways, such as the JNK signaling pathway, which leads to the downregulation of p53. The action of adenosine also increases Treg cells and the polarization of M2 macrophages in the tumor microenvironment. CD38 also promotes the activation of AICD after the hyperactivation of tumor-specific CD8+ T cells by ICI therapy, and induces the expression of FasL on T cells. CD38 also induces the release of Angiopoietin-2 from endothelial cells, leading to increased angiogenesis, PD-L1 expression on M2-like macrophages and enhanced tumor invasion. Finally, CD38 induces the expression of HIF-1α in tumor cells, which can, in turn, induce the production of VEGF and IGF factors. VEGF can establish paracrine signaling, promoting the recruitment of Treg cells, and autocrine signaling on tumor cells by binding VEGFR2, promoting tumorigenesis and invasion. The polymorphisms rs1870377 A/T and A/A may make this binding stronger. HIF-1α can also induce the expression of IGF-pathway components, leading to autocrine signaling that results in tumor growth and survival. ANGPT2: Angiopoietin 2; IGF: insulin-like growth factor; IGF-1R: IGF-1 receptor; VEGFR2*P: VEGFR2 receptor with rs1870377 A/T or A/A polymorphisms.
4. Conclusions
HPD is surely a challenge in the context of atypical therapeutic responses to ICI therapy. In this review, we focused on two factors that often recur in the landscape of clinical resistance to ICI: the cytokine IFN-γ and the alternative immune checkpoint CD38. We have discussed the detrimental role that IFN-γ secretion and CD38 upregulation can play in the TME after ICIs, and have suggested that a possible interplay exists between these factors, in the context of resistance to therapy and HPD.
Based on the reported data, patients with a high risk of developing hyperprogression may have specific traits, such as mutations on the PD-L1 intracytoplasmic domain and molecules involved in downstream inflammasome signaling pathways. Increased levels of secreted IFN-γ during ICI therapy may further stimulate this activated path, thus inducing the formation of an inflammatory microenvironment that stimulates CD38 upregulation. The combined activation of the inflammasome pathway, which leads to substantial MDSC recruitment, and CD38 activation, which reduces the sensitivity of tumor cells to IFN-γ through ADORA2a activation, may lead to a loss of therapeutic efficacy and uncontrolled tumor progression. Moreover, in some patients, ICI therapy may, at first, hyperactivate the antitumor immune response, either because of tumor-intrinsic characteristics or differential responses of T cells to treatment. Immune hyperactivation may trigger negative feedback mechanisms that have the aim of preventing excessive immune responses, and thus lead to the upregulation of CD38 and the activation of the AICD mechanism, which can be induced both by IFN-γ and by CD38 itself. Hyperprogressive patients, who have been reported to experience AICD, have displayed a dampening of effector T-cell responses and consequent tumor progression.
Finally, an important role is played by the immunosuppressive responses induced by IFN-γ and CD38 through the induction of IDO1 and hypoxic pathways, respectively. Mutations that are associated with the constitutive expression of IDO1, the activation of oncogenic pathways and HIF-1α expression have been reported in hyperprogressive patients and may play a role in HPD. An increase in angiogenesis and the recruitment of immunosuppressive cells, such as M2-like macrophages and Treg cells, serve as a framework for this hypothesized HPD mechanism.
As only a few targeted studies on the role of IFN-γ and CD38 in HPD mechanisms have been conducted, new effort should be invested in collecting further data about these two factors and their interplay. The chance to treat HPD patients with CD38 inhibitors is a real opportunity to overcome tumor acceleration. The correlation between CD38 and angiogenesis also suggests the possible use of anti-angiogenic inhibitors in HPD patients. IFN-γ signature/key mediators could also be investigated and monitored over time in order to prevent AICD. The immune infiltrate of re-biopsies should also be monitored and compared to the baseline to quantify the presence of immunosuppressive populations. Moreover, the evaluation of the genetic signature of each tumor and the identification of polymorphisms associated with immune response in patients, especially those involved in IFN-γ and CD38-dependent mechanisms, may provide a relevant tool to identify patients at risk of developing HPD.
Author Contributions
Conceptualization: S.A., A.P. and P.-L.L.; Writing—Original Draft Preparation: S.A., A.P., P.-L.L. and P.N.; Figures and Tables: S.A.; Writing—Review and Editing: S.A., F.R., L.L., L.S., F.G., A.A., P.N., P.-L.L. and A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ricerca Finalizzata Ministero della Salute 2018 grant, number GR-2018-12368031 (to F.G. and A.P.); the Department of Experimental, Diagnostic and Specialty Medicine of the University of Bologna (“Pallotti” Fund to P.N., P.-L.L.); the University of Bologna, Fundamentally Oriented Research funds (to P.N., P.-L.L.); CARISBO Foundation, grant 2019-0543 (to P.-L.L.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Darvin P., Toor S.M., Sasidharan Nair V., Elkord E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018;50:1–11. doi: 10.1038/s12276-018-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topalian S.L., Sznol M., McDermott D.F., Kluger H.M., Carvajal R.D., Sharfman W.H., Brahmer J.R., Lawrence D.P., Atkins M.B., Powderly J.D., et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahamadi M., Freshwater T., Prohn M., Li C., de Alwis D., de Greef R., Elassaiss-Schaap J., Kondic A., Stone J. Model-Based Characterization of the Pharmacokinetics of Pembrolizumab: A Humanized Anti-PD-1 Monoclonal Antibody in Advanced Solid Tumors. CPT Pharmacomet. Syst. Pharmacol. 2017;6:49–57. doi: 10.1002/psp4.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaddepally R.K., Kharel P., Pandey R., Garje R., Chandra A.B. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers. 2020;12:738. doi: 10.3390/cancers12030738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robert C., Long G.V., Brady B., Dutriaux C., Maio M., Mortier L., Hassel J.C., Rutkowski P., McNeil C., Kalinka-Warzocha E., et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 7.Herbst R.S., Baas P., Kim D.W., Felip E., Pérez-Gracia J.L., Han J.Y., Molina J., Kim J.H., Arvis C.D., Ahn M.J., et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 8.Borcoman E., Kanjanapan Y., Champiat S., Kato S., Servois V., Kurzrock R., Goel S., Bedard P., le Tourneau C. Novel patterns of response under immunotherapy. Ann. Oncol. 2019;30:385–396. doi: 10.1093/annonc/mdz003. [DOI] [PubMed] [Google Scholar]
- 9.Aoki M., Shoji H., Nagashima K., Imazeki H., Miyamoto T., Hirano H., Honma Y., Iwasa S., Okita N., Takashima A., et al. Hyperprogressive disease during nivolumab or irinotecan treatment in patients with advanced gastric cancer. ESMO Open. 2019;4:e000488. doi: 10.1136/esmoopen-2019-000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sasaki A., Nakamura Y., Mishima S., Kawazoe A., Kuboki Y., Bando H., Kojima T., Doi T., Ohtsu A., Yoshino T., et al. Predictive factors for hyperprogressive disease during nivolumab as anti-PD1 treatment in patients with advanced gastric cancer. Gastric Cancer. 2019;22:793–802. doi: 10.1007/s10120-018-00922-8. [DOI] [PubMed] [Google Scholar]
- 11.Kamada T., Togashi Y., Tay C., Ha D., Sasaki A., Nakamura Y., Sato E., Fukuoka S., Tada Y., Tanaka A., et al. PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc. Natl. Acad. Sci. USA. 2019;116:9999–10008. doi: 10.1073/pnas.1822001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Champiat S., Dercle L., Ammari S., Massard C., Hollebecque A., Postel-Vinay S., Chaput N., Eggermont A., Marabelle A., Soria J.C., et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin. Cancer Res. 2017;23:1920–1928. doi: 10.1158/1078-0432.CCR-16-1741. [DOI] [PubMed] [Google Scholar]
- 13.Ferrara R., Mezquita L., Texier M., Lahmar J., Audigier-Valette C., Tessonnier L., Mazieres J., Zalcman G., Brosseau S., le Moulec S., et al. Hyperprogressive Disease in Patients with Advanced Non-Small Cell Lung Cancer Treated with PD-1/PD-L1 Inhibitors or with Single-Agent Chemotherapy. JAMA Oncol. 2018;4:1543–1552. doi: 10.1001/jamaoncol.2018.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saâda-Bouzid E., Defaucheux C., Karabajakian A., Coloma V.P., Servois V., Paoletti X., Even C., Fayette J., Guigay J., Loirat D., et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann. Oncol. 2017;28:1605–1611. doi: 10.1093/annonc/mdx178. [DOI] [PubMed] [Google Scholar]
- 15.Kim C.G., Kim K.H., Pyo K.-H., Xin C.-F., Hong M.H., Ahn B.-C., Kim Y., Choi S.J., Yoon H.I., Lee J.G., et al. Hyperprogressive disease during PD-1/PD-L1 blockade in patients with non-small-cell lung cancer. Ann. Oncol. 2019;30:1104–1113. doi: 10.1093/annonc/mdz123. [DOI] [PubMed] [Google Scholar]
- 16.Okan Cakir M., Kirca O., Gunduz S., Ozdogan M. Hyperprogression after immunotherapy: A comprehensive review. JBUON. 2019;24:2232–2241. [PubMed] [Google Scholar]
- 17.Kas B., Talbot H., Ferrara R., Richard C., Lamarque J.-P., Pitre-Champagnat S., Planchard D., Balleyguier C., Besse B., Mezquita L., et al. Clarification of Definitions of Hyperprogressive Disease During Immunotherapy for Non–Small Cell Lung Cancer. JAMA Oncol. 2020;6:1039–1046. doi: 10.1001/jamaoncol.2020.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanjanapan Y., Day D., Wang L., Al-Sawaihey H., Abbas E., Namini A., Siu L.L., Hansen A., Razak A.A., Spreafico A., et al. Hyperprogressive disease in early-phase immunotherapy trials: Clinical predictors and association with immune-related toxicities. Cancer. 2019;125:1341–1349. doi: 10.1002/cncr.31999. [DOI] [PubMed] [Google Scholar]
- 19.Kato S., Goodman A., Walavalkar V., Barkauskas D.A., Sharabi A., Kurzrock R. Hyperprogressors after immunotherapy: Analysis of genomic alterations associated with accelerated growth rate. Clin. Cancer Res. 2017;23:4242–4250. doi: 10.1158/1078-0432.CCR-16-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matos I., Martin-Liberal J., Hierro C., Ochoa De Olza M., Viaplana C., Costa M., Felip-Falg’s E., Mur-Bonet G., Vieito M., Brana I., et al. Incidence and clinical implications of a new definition of hyperprogression (HPD) with immune checkpoint inhibitors (ICIs) in patients treated in phase 1 (Ph1) trials. J. Clin. Oncol. 2018;36:3032. doi: 10.1200/JCO.2018.36.15_suppl.3032. [DOI] [Google Scholar]
- 21.Lo Russo G., Moro M., Sommariva M., Cancila V., Boeri M., Centonze G., Ferro S., Ganzinelli M., Gasparini P., Huber V., et al. Antibody-Fc/FcR interaction on macrophages as a mechanism for hyperprogressive disease in non-small cell lung cancer subsequent to PD-1/PD-L1 blockade. Clin. Cancer Res. 2019;25:989–999. doi: 10.1158/1078-0432.CCR-18-1390. [DOI] [PubMed] [Google Scholar]
- 22.Brahmer J., Reckamp K.L., Baas P., Crinò L., Eberhardt W.E.E., Poddubskaya E., Antonia S., Pluzanski A., Vokes E.E., Holgado E., et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motzer R.J., Escudier B., McDermott D.F., George S., Hammers H.J., Srinivas S., Tykodi S.S., Sosman J.A., Procopio G., Plimack E.R., et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borghaei H., Paz-Ares L., Horn L., Spigel D.R., Steins M., Ready N.E., Chow L.Q., Vokes E.E., Felip E., Holgado E., et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hakim F.T., Flomerfelt F.A., Boyiadzis M., Gress R.E. Aging, immunity and cancer. Curr. Opin. Immunol. 2004;16:151–156. doi: 10.1016/j.coi.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Hurez V., Padrón S., Svatek R.S., Curiel T.J. Considerations for successful cancer immunotherapy in aged hosts. Clin. Exp. Immunol. 2017;187:53–63. doi: 10.1111/cei.12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fields E.C., McGuire W.P., Lin L., Temkin S.M. Radiation treatment in women with ovarian cancer: Past, present, and future. Front. Oncol. 2017;7:177. doi: 10.3389/fonc.2017.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singavi A.K., Menon S., Kilari D., Alqwasmi A., Ritch P.S., Thomas J.P., Martin A.L., Oxencis C., Ali S., George B. Predictive biomarkers for hyper-progression (HP) in response to immune checkpoint inhibitors (ICI) – analysis of somatic alterations (SAs) Ann. Oncol. 2017;28:v405. doi: 10.1093/annonc/mdx376.006. [DOI] [Google Scholar]
- 29.Gainor J.F., Shaw A.T., Sequist L.V., Fu X., Azzoli C.G., Piotrowska Z., Huynh T.G., Zhao L., Fulton L., Schultz K.R., et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: A retrospective analysis. Clin. Cancer Res. 2016;22:4585–4593. doi: 10.1158/1078-0432.CCR-15-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berghoff A.S., Bellosillo B., Caux C., de Langen A., Mazieres J., Normanno N., Preusser M., Provencio M., Rojo F., Wolf J., et al. Immune checkpoint inhibitor treatment in patients with oncogene- addicted non-small cell lung cancer (NSCLC): Summary of a multidisciplinary round-table discussion. Esmo Open. 2019;4:e000498. doi: 10.1136/esmoopen-2019-000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skoulidis F., Goldberg M.E., Greenawalt D.M., Hellmann M.D., Awad M.M., Gainor J.F., Schrock A.B., Hartmaier R.J., Trabucco S.E., Gay L., et al. STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma. Cancer Discov. 2018;8:822–835. doi: 10.1158/2159-8290.CD-18-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazieres J., Drilon A., Lusque A., Mhanna L., Cortot A.B., Mezquita L., Thai A.A., Mascaux C., Couraud S., Veillon R., et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: Results from the IMMUNOTARGET registry. Ann. Oncol. 2019;30:1321–1328. doi: 10.1093/annonc/mdz167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garassino M.C., Cho B.-C., Kim J.-H., Mazières J., Vansteenkiste J., Lena H., Corral Jaime J., Gray J.E., Powderly J., Chouaid C., et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): An open-label, single-arm, phase 2 study. Lancet Oncol. 2018;19:521–536. doi: 10.1016/S1470-2045(18)30144-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamberti G., Andrini E., Sisi M., Rizzo A., Parisi C., di Federico A., Gelsomino F., Ardizzoni A. Beyond EGFR, ALK and ROS1: Current evidence and future perspectives on newly targetable oncogenic drivers in lung adenocarcinoma. Crit. Rev. Oncol./Hematol. 2020;156:103119. doi: 10.1016/j.critrevonc.2020.103119. [DOI] [PubMed] [Google Scholar]
- 35.Tunali I., Gray J.E., Qi J., Abdalah M., Jeong D.K., Guvenis A., Gillies R.J., Schabath M.B. Novel clinical and radiomic predictors of rapid disease progression phenotypes among lung cancer patients treated with immunotherapy: An early report. Lung Cancer. 2019;129:75–79. doi: 10.1016/j.lungcan.2019.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koukourakis M.I., Giatromanolaki A., Sivridis E., Bougioukas G., Didilis V., Gatter K.C., Harris A.L. Lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis. Br. J. Cancer. 2003;89:877–885. doi: 10.1038/sj.bjc.6601205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brand A., Singer K., Koehl G.E., Kolitzus M., Schoenhammer G., Thiel A., Matos C., Bruss C., Klobuch S., Peter K., et al. LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metab. 2016;24:657–671. doi: 10.1016/j.cmet.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 38.Teixidó C., González-Cao M., Karachaliou N., Rosell R. Predictive factors for immunotherapy in melanoma. Ann. Transl. Med. 2015;3:208. doi: 10.3978/j.issn.2305-5839.2015.05.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Passiglia F., Bronte G., Bazan V., Natoli C., Rizzo S., Galvano A., Listì A., Cicero G., Rolfo C., Santini D., et al. PD-L1 expression as predictive biomarker in patients with NSCLC: A pooled analysis. Oncotarget. 2016;7:19738–19747. doi: 10.18632/oncotarget.7582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis A.A., Patel V.G. The role of PD-L1 expression as a predictive biomarker: An analysis of all US food and drug administration (FDA) approvals of immune checkpoint inhibitors. J. Immunother. Cancer. 2019;7:278. doi: 10.1186/s40425-019-0768-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simpson T.R., Li F., Montalvo-Ortiz W., Sepulveda M.A., Bergerhoff K., Arce F., Roddie C., Henry J.Y., Yagita H., Wolchok J.D., et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti–CTLA-4 therapy against melanoma. J. Exp. Med. 2013;210:1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Simone M., Arrigoni A., Rossetti G., Gruarin P., Ranzani V., Politano C., Bonnal R.J.P., Provasi E., Sarnicola M.L., Panzeri I., et al. Transcriptional Landscape of Human Tissue Lymphocytes Unveils Uniqueness of Tumor-Infiltrating T Regulatory Cells. Immunity. 2016;45:1135–1147. doi: 10.1016/j.immuni.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lowther D.E., Goods B.A., Lucca L.E., Lerner B.A., Raddassi K., van Dijk D., Hernandez A.L., Duan X., Gunel M., Coric V., et al. PD-1 marks dysfunctional regulatory T cells in malignant gliomas. JCI Insight. 2016;1:e85935. doi: 10.1172/jci.insight.85935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koyama S., Akbay E.A., Li Y.Y., Herter-Sprie G.S., Buczkowski K.A., Richards W.G., Gandhi L., Redig A.J., Rodig S.J., Asahina H., et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat. Commun. 2016;7:10501. doi: 10.1038/ncomms10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang R.-Y., Francois A., McGray A.R., Miliotto A., Odunsi K. Compensatory upregulation of PD-1, LAG-3, and CTLA-4 limits the efficacy of single-agent checkpoint blockade in metastatic ovarian cancer. OncoImmunology. 2017;6:e1249561. doi: 10.1080/2162402X.2016.1249561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen L., Diao L., Yang Y., Yi X., Rodriguez B.L., Li Y., Villalobos P.A., Cascone T., Liu X., Tan L., et al. CD38-Mediated Immunosuppression as a Mechanism of Tumor Cell Escape from PD-1/PD-L1 Blockade. Cancer Discov. 2018;8:1156–1175. doi: 10.1158/2159-8290.CD-17-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zuazo-Ibarra M., Arasanz H., Fernández-Hinojal G., María G.-C., Hernández-Marín B., Martínez-Aguillo M., Jose Lecumberri M., Fernández A., Teijeira L., Vera R., et al. Highly differentiated CD4 T cells Unequivocally Identify Primary Resistance and Risk of Hyperprogression to PD-L1/PD-1 Immune Checkpoint Blockade in Lung Cancer. bioRxiv. 2018:320176. doi: 10.1101/320176. [DOI] [Google Scholar]
- 48.Lamichhane P., Karyampudi L., Shreeder B., Krempski J., Bahr D., Daum J., Kalli K.R., Goode E.L., Block M.S., Cannon M.J., et al. IL10 Release upon PD-1 Blockade Sustains Immunosuppression in Ovarian Cancer. Cancer Res. 2017;77:6667–6678. doi: 10.1158/0008-5472.CAN-17-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun Z., Fourcade J., Pagliano O., Chauvin J.-M., Sander C., Kirkwood J.M., Zarour H.M. IL10 and PD-1 Cooperate to Limit the Activity of Tumor-Specific CD8+ T Cells. Cancer Res. 2015;75:1635–1644. doi: 10.1158/0008-5472.CAN-14-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scholz A., Lang V., Henschler R., Czabanka M., Vajkoczy P., Chavakis E., Drynski J., Harter P.N., Mittelbronn M., Dumont D.J., et al. Angiopoietin-2 promotes myeloid cell infiltration in a β2-integrin–dependent manner. Blood. 2011;118:5050–5059. doi: 10.1182/blood-2011-03-343293. [DOI] [PubMed] [Google Scholar]
- 51.Wu X., Giobbie-Hurder A., Liao X., Connelly C., Connolly E.M., Li J., Manos M.P., Lawrence D., McDermott D., Severgnini M., et al. Angiopoietin-2 as a Biomarker and Target for Immune Checkpoint Therapy. Cancer Immunol. Res. 2017;5:17–28. doi: 10.1158/2326-6066.CIR-16-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dulos J., Carven G.J., van Boxtel S.J., Evers S., Driessen-Engels L.J.A., Hobo W., Gorecka M.A., de Haan A.F.J., Mulders P., Punt C.J.A., et al. PD-1 Blockade Augments Th1 and Th17 and Suppresses Th2 Responses in Peripheral Blood From Patients With Prostate and Advanced Melanoma Cancer. J. Immunother. 2012;35:169–178. doi: 10.1097/CJI.0b013e318247a4e7. [DOI] [PubMed] [Google Scholar]
- 53.Akbay E.A., Koyama S., Liu Y., Dries R., Bufe L.E., Silkes M., Alam M.M., Magee D.M., Jones R., Jinushi M., et al. Interleukin-17A Promotes Lung Tumor Progression through Neutrophil Attraction to Tumor Sites and Mediating Resistance to PD-1 Blockade. J. Thorac. Oncol. 2017;12:1268–1279. doi: 10.1016/j.jtho.2017.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Y., Cheng Y., Xu Y., Wang Z., Du X., Li C., Peng J., Gao L., Liang X., Ma C. Increased expression of programmed cell death protein 1 on NK cells inhibits NK-cell-mediated anti-tumor function and indicates poor prognosis in digestive cancers. Oncogene. 2017;36:6143–6153. doi: 10.1038/onc.2017.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang T., Song X., Xu L., Ma J., Zhang Y., Gong W., Zhang Y., Zhou X., Wang Z., Wang Y., et al. The binding of an anti-PD-1 antibody to FcγRΙ has a profound impact on its biological functions. Cancer Immunol. Immunother. 2018;67:1079–1090. doi: 10.1007/s00262-018-2160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stein R.G., Ebert S., Schlahsa L., Scholz C.J., Braun M., Hauck P., Horn E., Monoranu C.-M., Thiemann V.J., Wustrow M.P., et al. Cognate Nonlytic Interactions between CD8 + T Cells and Breast Cancer Cells Induce Cancer Stem Cell–like Properties. Cancer Res. 2019;79:1507–1519. doi: 10.1158/0008-5472.CAN-18-0387. [DOI] [PubMed] [Google Scholar]
- 57.Zhou J.X., Lee C.H., Qi C.F., Wang H., Naghashfar Z., Abbasi S., Morse H.C. IFN Regulatory Factor 8 Regulates MDM2 in Germinal Center B Cells. J. Immunol. 2009;183:3188–3194. doi: 10.4049/jimmunol.0803693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yao H., Wang H., Li C., Fang J.-Y., Xu J. Cancer Cell-Intrinsic PD-1 and Implications in Combinatorial Immunotherapy. Front. Immunol. 2018;9:1774. doi: 10.3389/fimmu.2018.01774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ludin A., Zon L.I. The dark side of PD-1 receptor inhibition. Nature. 2017;552:41–42. doi: 10.1038/nature24759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wartewig T., Kurgyis Z., Keppler S., Pechloff K., Hameister E., Öllinger R., Maresch R., Buch T., Steiger K., Winter C., et al. PD-1 is a haploinsufficient suppressor of T cell lymphomagenesis. Nature. 2017;552:121–125. doi: 10.1038/nature24649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ratner L., Waldmann T.A., Janakiram M., Brammer J.E. Rapid Progression of Adult T-Cell Leukemia–Lymphoma after PD-1 Inhibitor Therapy. N. Engl. J. Med. 2018;378:1947–1948. doi: 10.1056/NEJMc1803181. [DOI] [PubMed] [Google Scholar]
- 62.Kleffel S., Posch C., Barthel S.R., Mueller H., Schlapbach C., Guenova E., Elco C.P., Lee N., Juneja V.R., Zhan Q., et al. Melanoma Cell-Intrinsic PD-1 Receptor Functions Promote Tumor Growth. Cell. 2015;162:1242–1256. doi: 10.1016/j.cell.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Du S., McCall N., Park K., Guan Q., Fontina P., Ertel A., Zhan T., Dicker A.P., Lu B. Blockade of Tumor-Expressed PD-1 promotes lung cancer growth. OncoImmunology. 2018;7:e1408747. doi: 10.1080/2162402X.2017.1408747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Azuma T., Yao S., Zhu G., Flies A.S., Flies S.J., Chen L. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;111:3635–3643. doi: 10.1182/blood-2007-11-123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang C.-H., Qiu J., O’Sullivan D., Buck M.D., Noguchi T., Curtis J.D., Chen Q., Gindin M., Gubin M.M., van der Windt G.J.W., et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell. 2015;162:1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clark C.A., Gupta H.B., Sareddy G., Pandeswara S., Lao S., Yuan B., Drerup J.M., Padron A., Conejo-Garcia J., Murthy K., et al. Tumor-Intrinsic PD-L1 Signals Regulate Cell Growth, Pathogenesis, and Autophagy in Ovarian Cancer and Melanoma. Cancer Res. 2016;76:6964–6974. doi: 10.1158/0008-5472.CAN-16-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gato-Cañas M., Zuazo M., Arasanz H., Ibañez-Vea M., Lorenzo L., Fernandez-Hinojal G., Vera R., Smerdou C., Martisova E., Arozarena I., et al. PDL1 Signals through Conserved Sequence Motifs to Overcome Interferon-Mediated Cytotoxicity. Cell Rep. 2017;20:1818–1829. doi: 10.1016/j.celrep.2017.07.075. [DOI] [PubMed] [Google Scholar]
- 68.Sun C., Mezzadra R., Schumacher T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity. 2018;48:434–452. doi: 10.1016/j.immuni.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li C.-W., Lim S.-O., Xia W., Lee H.-H., Chan L.-C., Kuo C.-W., Khoo K.-H., Chang S.-S., Cha J.-H., Kim T., et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat. Commun. 2016;7:12632. doi: 10.1038/ncomms12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Akbay E.A., Koyama S., Carretero J., Altabef A., Tchaicha J.H., Christensen C.L., Mikse O.R., Cherniack A.D., Beauchamp E.M., Pugh T.J., et al. Activation of the PD-1 Pathway Contributes to Immune Escape in EGFR-Driven Lung Tumors. Cancer Discov. 2013;3:1355–1363. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chin Y.E., Kitagawa M., Kuida K., Flavell R.A., Fu X.Y. Activation of the STAT signaling pathway can cause expression of caspase 1 and apoptosis. Mol. Cell. Biol. 1997;17:5328–5337. doi: 10.1128/MCB.17.9.5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu X., Fu X.-Y., Plate J., Chong A.S.-F. IFN-y Induces Cell Growth Inhibition by Fas-mediated Apoptosis: Requirement of STATI Protein for Up-Regulation of Fas and FasL Expression. Cancer Res. 1998;58:2832–2837. [PubMed] [Google Scholar]
- 73.Hobeika A.C., Etienne W., Torres B.A., Johnson H.M., Subramaniam P.S. IFN-gamma Induction of p21WAF1 Is Required for Cell Cycle Inhibition and Suppression of Apoptosis. J. Interferon Cytokine Res. 1999;19:1351–1361. doi: 10.1089/107999099312812. [DOI] [PubMed] [Google Scholar]
- 74.Fulda S., Debatin K.-M. IFNγ sensitizes for apoptosis by upregulating caspase-8 expression through the Stat1 pathway. Oncogene. 2002;21:2295–2308. doi: 10.1038/sj.onc.1205255. [DOI] [PubMed] [Google Scholar]
- 75.Kammertoens T., Friese C., Arina A., Idel C., Briesemeister D., Rothe M., Ivanov A., Szymborska A., Patone G., Kunz S., et al. Tumour ischaemia by interferon-γ resembles physiological blood vessel regression. Nature. 2017;545:98–102. doi: 10.1038/nature22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lollini P.-L., de Giovanni C., del Re B., Nicoletti G., Prodi G., Nanni P. Interferon-mediated enhancement of metastasis. Are MHC antigens involved? Clin. Exp. Metastasis. 1987;5:277–287. doi: 10.1007/BF00120723. [DOI] [PubMed] [Google Scholar]
- 77.Castro F., Cardoso A.P., Gonçalves R.M., Serre K., Oliveira M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018;9:847. doi: 10.3389/fimmu.2018.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Y., Apilado R., Coleman J., Ben-Sasson S., Tsang S., Hu-Li J., Paul W.E., Huang H. Interferon γ Stabilizes the T Helper Cell Type 1 Phenotype. J. Exp. Med. 2001;194:165–172. doi: 10.1084/jem.194.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Curtsinger J.M., Agarwal P., Lins D.C., Mescher M.F. Autocrine IFN-γ Promotes Naive CD8 T Cell Differentiation and Synergizes with IFN-α To Stimulate Strong Function. J. Immunol. 2012;189:659–668. doi: 10.4049/jimmunol.1102727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Overacre-Delgoffe A.E., Chikina M., Dadey R.E., Yano H., Brunazzi E.A., Shayan G., Horne W., Moskovitz J.M., Kolls J.K., Sander C., et al. Interferon-γ Drives T reg Fragility to Promote Anti-tumor Immunity. Cell. 2017;169:1130–1141. doi: 10.1016/j.cell.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mojic M., Takeda K., Hayakawa Y. The Dark Side of IFN-γ: Its Role in Promoting Cancer Immunoevasion. Int. J. Mol. Sci. 2017;19:89. doi: 10.3390/ijms19010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lollini P.-L., Nanni P., de Giovanni C., Nicoletti G., Landuzzi L. Re: Randomized Trial of Adjuvant Human Interferon Gamma Versus Observation in High-Risk Cutaneous Melanoma: A Southwest Oncology Group Study. JNCI J. Natl. Cancer Inst. 1996;88:926–927. doi: 10.1093/jnci/88.13.926. [DOI] [PubMed] [Google Scholar]
- 83.Lollini P.-L., Bosco M.C., Cavallo F., de Giovanni C., Giovarelli M., Landuzzi L., Musiani P., Modesti A., Nicoletti G., Palmieri G., et al. Inhibition of tumor growth and enhancement of metastasis after transfection of the γ-interferon gene. Int. J. Cancer. 1993;55 doi: 10.1002/ijc.2910550224. [DOI] [PubMed] [Google Scholar]
- 84.Ribas A., Butler M., Lutzky J., Lawrence D.P., Robert C., Miller W., Linette G.P., Ascierto P.A., Kuzel T., Algazi A.P., et al. Phase I study combining anti-PD-L1 (MEDI4736) with BRAF (dabrafenib) and/or MEK (trametinib) inhibitors in advanced melanoma. J. Clin. Oncol. 2015;33:3003. doi: 10.1200/jco.2015.33.15_suppl.3003. [DOI] [Google Scholar]
- 85.Garcia-Diaz A., Shin D.S., Moreno B.H., Saco J., Escuin-Ordinas H., Rodriguez G.A., Zaretsky J.M., Sun L., Hugo W., Wang X., et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep. 2017;19:1189–1201. doi: 10.1016/j.celrep.2017.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lamberti G., Sisi M., Andrini E., Palladini A., Giunchi F., Lollini P.-L., Ardizzoni A., Gelsomino F. The Mechanisms of PD-L1 Regulation in Non-Small-Cell Lung Cancer (NSCLC): Which Are the Involved Players? Cancers. 2020;12:3129. doi: 10.3390/cancers12113129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zaretsky J.M., Garcia-Diaz A., Shin D.S., Escuin-Ordinas H., Hugo W., Hu-Lieskovan S., Torrejon D.Y., Abril-Rodriguez G., Sandoval S., Barthly L., et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N. Engl. J. Med. 2016;375:819–829. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Manguso R.T., Pope H.W., Zimmer M.D., Brown F.D., Yates K.B., Miller B.C., Collins N.B., Bi K., LaFleur M.W., Juneja V.R., et al. In vivo CRISPR screening identifies Ptpn2 as a cancer immunotherapy target. Nature. 2017;547:413–418. doi: 10.1038/nature23270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kalbasi A., Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat. Rev. Immunol. 2020;20:25–39. doi: 10.1038/s41577-019-0218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grasso C.S., Tsoi J., Onyshchenko M., Abril-Rodriguez G., Ross-Macdonald P., Wind-Rotolo M., Champhekar A., Medina E., Torrejon D.Y., Shin D.S., et al. Conserved Interferon-γ Signaling Drives Clinical Response to Immune Checkpoint Blockade Therapy in Melanoma. Cancer Cell. 2020;38:500–515. doi: 10.1016/j.ccell.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Benci J.L., Xu B., Qiu Y., Wu T.J., Dada H., Twyman-Saint Victor C., Cucolo L., Lee D.S.M., Pauken K.E., Huang A.C., et al. Tumor Interferon Signaling Regulates a Multigenic Resistance Program to Immune Checkpoint Blockade. Cell. 2016;167:1540–1554.e12. doi: 10.1016/j.cell.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lu Y., Gu X., Chen L., Yao Z., Song J., Niu X., Xiang R., Cheng T., Qin Z., Deng W., et al. Interferon-γ produced by tumor-infiltrating NK cells and CD4+ T cells downregulates TNFSF15 expression in vascular endothelial cells. Angiogenesis. 2014;17:529–540. doi: 10.1007/s10456-013-9397-y. [DOI] [PubMed] [Google Scholar]
- 93.Jauch D., Martin M., Schiechl G., Kesselring R., Schlitt H.J., Geissler E.K., Fichtner-Feigl S. Interleukin 21 controls tumour growth and tumour immunosurveillance in colitis-associated tumorigenesis in mice. Gut. 2011;60:1678–1686. doi: 10.1136/gutjnl-2011-300612. [DOI] [PubMed] [Google Scholar]
- 94.Carbotti G., Barisione G., Airoldi I., Mezzanzanica D., Bagnoli M., Ferrero S., Petretto A., Fabbi M., Ferrini S. IL-27 induces the expression of IDO and PD-L1 in human cancer cells. Oncotarget. 2015;6:43267–43280. doi: 10.18632/oncotarget.6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Turnis M.E., Sawant D.V., Szymczak-Workman A.L., Andrews L.P., Delgoffe G.M., Yano H., Beres A.J., Vogel P., Workman C.J., Vignali D.A.A. Interleukin-35 Limits Anti-Tumor Immunity. Immunity. 2016;44:316–329. doi: 10.1016/j.immuni.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhao Z., Chen X., Hao S., Jia R., Wang N., Chen S., Li M., Wang C., Mao H. Increased interleukin-35 expression in tumor-infiltrating lymphocytes correlates with poor prognosis in patients with breast cancer. Cytokine. 2017;89:76–81. doi: 10.1016/j.cyto.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 97.Zou J.-M., Qin J., Li Y.-C., Wang Y., Li D., Shu Y., Luo C., Wang S.-S., Chi G., Guo F., et al. IL-35 induces N2 phenotype of neutrophils to promote tumor growth. Oncotarget. 2017;8:33501–33514. doi: 10.18632/oncotarget.16819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huang C., Li N., Li Z., Chang A., Chen Y., Zhao T., Li Y., Wang X., Zhang W., Wang Z., et al. Tumour-derived Interleukin 35 promotes pancreatic ductal adenocarcinoma cell extravasation and metastasis by inducing ICAM1 expression. Nat. Commun. 2017;8:14035. doi: 10.1038/ncomms14035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang B., Pan P.-Y., Li Q., Sato A.I., Levy D.E., Bromberg J., Divino C.M., Chen S.-H. Gr-1+ CD115+ Immature Myeloid Suppressor Cells Mediate the Development of Tumor-Induced T Regulatory Cells and T-Cell Anergy in Tumor-Bearing Host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 100.Nishibori T., Tanabe Y., Su L., David M. Impaired Development of CD4+ CD25+ Regulatory T Cells in the Absence of STAT1. J. Exp. Med. 2004;199:25–34. doi: 10.1084/jem.20020509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang Z., Hong J., Sun W., Xu G., Li N., Chen X., Liu A., Xu L., Sun B., Zhang J.Z. Role of IFN-g in induction of Foxp3 and conversion of CD4+CD25- T cells to CD4+ Tregs. J. Clin. Investig. 2006;116:2434–2441. doi: 10.1172/JCI25826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sawitzki B., Kingsley C.I., Oliveira V., Karim M., Herber M., Wood K.J. IFN-γ production by alloantigen-reactive regulatory T cells is important for their regulatory function in vivo. J. Exp. Med. 2005;201:1925–1935. doi: 10.1084/jem.20050419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wei B., Baker S., Wieckiewicz J., Wood K.J. IFN-γ Triggered STAT1-PKB/AKT Signalling Pathway Influences the Function of Alloantigen Reactive Regulatory T Cells. Am. J. Transplant. 2010;10:69–80. doi: 10.1111/j.1600-6143.2009.02858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Feng T., Cao A.T., Weaver C.T., Elson C.O., Cong Y. Interleukin-12 Converts Foxp3+ Regulatory T Cells to Interferon–γ-Producing Foxp3+ T Cells That Inhibit Colitis. Gastroenterology. 2011;140:2031–2043. doi: 10.1053/j.gastro.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Koenecke C., Lee C.-W., Thamm K., Föhse L., Schafferus M., Mittrücker H.-W., Floess S., Huehn J., Ganser A., Förster R., et al. IFN-γ Production by Allogeneic Foxp3 + Regulatory T Cells Is Essential for Preventing Experimental Graft-versus-Host Disease. J. Immunol. 2012;189:2890–2896. doi: 10.4049/jimmunol.1200413. [DOI] [PubMed] [Google Scholar]
- 106.Greifenberg V., Ribechini E., Rößner S., Lutz M.B. Myeloid-derived suppressor cell activation by combined LPS and IFN-γ treatment impairs DC development. Eur. J. Immunol. 2009;39:2865–2876. doi: 10.1002/eji.200939486. [DOI] [PubMed] [Google Scholar]
- 107.Shime H., Maruyama A., Yoshida S., Takeda Y., Matsumoto M., Seya T. Toll-like receptor 2 ligand and interferon-γ suppress anti-tumor T cell responses by enhancing the immunosuppressive activity of monocytic myeloid-derived suppressor cells. OncoImmunology. 2018;7:e1373231. doi: 10.1080/2162402X.2017.1373231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Weide B., Martens A., Zelba H., Stutz C., Derhovanessian E., di Giacomo A.M., Maio M., Sucker A., Schilling B., Schadendorf D., et al. Myeloid-Derived Suppressor Cells Predict Survival of Patients with Advanced Melanoma: Comparison with Regulatory T Cells and NY-ESO-1- or Melan-A-Specific T Cells. Clin. Cancer Res. 2014;20:1601–1609. doi: 10.1158/1078-0432.CCR-13-2508. [DOI] [PubMed] [Google Scholar]
- 109.Sade-Feldman M., Kanterman J., Klieger Y., Ish-Shalom E., Olga M., Saragovi A., Shtainberg H., Lotem M., Baniyash M. Clinical Significance of Circulating CD33+CD11b+HLA-DR- Myeloid Cells in Patients with Stage IV Melanoma Treated with Ipilimumab. Clin. Cancer Res. 2016;22:5661–5672. doi: 10.1158/1078-0432.CCR-15-3104. [DOI] [PubMed] [Google Scholar]
- 110.Martens A., Wistuba-Hamprecht K., Foppen M.G., Yuan J., Postow M.A., Wong P., Romano E., Khammari A., Dreno B., Capone M., et al. Baseline Peripheral Blood Biomarkers Associated with Clinical Outcome of Advanced Melanoma Patients Treated with Ipilimumab. Clin. Cancer Res. 2016;22:2908–2918. doi: 10.1158/1078-0432.CCR-15-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Meyer C., Cagnon L., Costa-Nunes C.M., Baumgaertner P., Montandon N., Leyvraz L., Michielin O., Romano E., Speiser D.E. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol. Immunother. 2014;63:247–257. doi: 10.1007/s00262-013-1508-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Faure M., Rochigneux P., Olive D., Taix S., Brenot-Rossi I., Gilabert M. Hyperprogressive Disease in Anorectal Melanoma Treated by PD-1 Inhibitors. Front. Immunol. 2018;9:797. doi: 10.3389/fimmu.2018.00797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xiong D., Wang Y., Singavi A.K., Mackinnon A.C., George B., You M. Immunogenomic Landscape Contributes to Hyperprogressive Disease after Anti-PD-1 Immunotherapy for Cancer. iScience. 2018;9:258–277. doi: 10.1016/j.isci.2018.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Theivanthiran B., Evans K.S., DeVito N.C., Plebanek M., Sturdivant M., Wachsmuth L.P., Salama A.K.S., Kang Y., Hsu D., Balko J.M., et al. A tumor-intrinsic PD-L1/NLRP3 inflammasome signaling pathway drives resistance to anti–PD-1 immunotherapy. J. Clin. Investig. 2020;130:2570–2586. doi: 10.1172/JCI133055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ito S., Hara Y., Kubota T. CARD8 is a negative regulator for NLRP3 inflammasome, but mutant NLRP3 in cryopyrin-associated periodic syndromes escapes the restriction. Arthritis Res. Ther. 2014;16:R52. doi: 10.1186/ar4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Burger D., Fickentscher C., de Moerloose P., Brandt K.J. F-actin dampens NLRP3 inflammasome activity via Flightless-I and LRRFIP2. Sci. Rep. 2016;6:29834. doi: 10.1038/srep29834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Clipman S.J., Henderson-Frost J., Fu K.Y., Bern C., Flores J., Gilman R.H. Genetic association study of NLRP1, CARD, and CASP1 inflammasome genes with chronic Chagas cardiomyopathy among Trypanosoma cruzi seropositive patients in Bolivia. PLoS ONE. 2018;13:e0192378. doi: 10.1371/journal.pone.0192378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hennig P., Garstkiewicz M., Grossi S., di Filippo M., French L., Beer H.D. The Crosstalk between Nrf2 and Inflammasomes. Int. J. Mol. Sci. 2018;19:562. doi: 10.3390/ijms19020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yue S., Li C., Ke M., Lu L., Busuttil R., Ying Q., Kupiec-Weglinski J., Ke B. Notch Signal Regulates Inflammasome NLRP3 Activation in Liver Ischemia and Reperfusion Injury. J. Hepatol. 2016;64:S508–S509. doi: 10.1016/S0168-8278(16)00883-7. [DOI] [Google Scholar]
- 120.Jiang L., Ke M., Yue S., Xiao W., Yan Y., Deng X., Ying Q.L., Li J., Ke B. Blockade of Notch signaling promotes acetaminophen-induced liver injury. Immunol. Res. 2017;65:739–749. doi: 10.1007/s12026-017-8913-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jin Y., Li C., Xu D., Zhu J., Wei S., Zhong A., Sheng M., Duarte S., Coito A.J., Busuttil R.W., et al. Jagged1-mediated myeloid Notch1 signaling activates HSF1/Snail and controls NLRP3 inflammasome activation in liver inflammatory injury. Cell. Mol. Immunol. 2019:17. doi: 10.1038/s41423-019-0318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Weber R., Fleming V., Hu X., Nagibin V., Groth C., Altevogt P., Utikal J., Umansky V. Myeloid-Derived Suppressor Cells Hinder the Anti-Cancer Activity of Immune Checkpoint Inhibitors. Front. Immunol. 2018;9:1310. doi: 10.3389/fimmu.2018.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Holtzhausen A., Zhao F., Evans K.S., Tsutsui M., Orabona C., Tyler D.S., Hanks B.A. Melanoma-Derived Wnt5a Promotes Local Dendritic-Cell Expression of IDO and Immunotolerance: Opportunities for Pharmacologic Enhancement of Immunotherapy. Cancer Immunol. Res. 2015;3:1082–1095. doi: 10.1158/2326-6066.CIR-14-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhao F., Xiao C., Evans K.S., Theivanthiran T., DeVito N., Holtzhausen A., Liu J., Liu X., Boczkowski D., Nair S., et al. Paracrine Wnt5a-β-Catenin Signaling Triggers a Metabolic Program that Drives Dendritic Cell Tolerization. Immunity. 2018;48:147–160. doi: 10.1016/j.immuni.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Badawy A.A.B. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int. J. Tryptophan Res. 2017;10:117864691769193. doi: 10.1177/1178646917691938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hornyák L., Dobos N., Koncz G., Karányi Z., Páll D., Szabó Z., Halmos G., Székvölgyi L. The Role of Indoleamine-2,3-Dioxygenase in Cancer Development, Diagnostics, and Therapy. Front. Immunol. 2018;9:151. doi: 10.3389/fimmu.2018.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Uyttenhove C., Pilotte L., Théate I., Stroobant V., Colau D., Parmentier N., Boon T., van den Eynde B.J. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat. Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 128.Katz J.B., Muller A.J., Prendergast G.C. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol. Rev. 2008;222:206–221. doi: 10.1111/j.1600-065X.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 129.Munn D.H., Mellor A.L. IDO in the Tumor Microenvironment: Inflammation, Counter-Regulation, and Tolerance. Trends Immunol. 2016;37:193–207. doi: 10.1016/j.it.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Munn D.H., Sharma M.D., Baban B., Harding H.P., Zhang Y., Ron D., Mellor A.L. GCN2 Kinase in T Cells Mediates Proliferative Arrest and Anergy Induction in Response to Indoleamine 2,3-Dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 131.Metz R., Rust S., DuHadaway J.B., Mautino M.R., Munn D.H., Vahanian N.N., Link C.J., Prendergast G.C. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: A novel IDO effector pathway targeted by D-1-methyl-tryptophan. OncoImmunology. 2012;1:1460–1468. doi: 10.4161/onci.21716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fallarino F., Grohmann U., You S., McGrath B.C., Cavener D.R., Vacca C., Orabona C., Bianchi R., Belladonna M.L., Volpi C., et al. The Combined Effects of Tryptophan Starvation and Tryptophan Catabolites Down-Regulate T Cell Receptor ζ-Chain and Induce a Regulatory Phenotype in Naive T Cells. J. Immunol. 2006;176:6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 133.Mezrich J.D., Fechner J.H., Zhang X., Johnson B.P., Burlingham W.J., Bradfield C.A. An Interaction between Kynurenine and the Aryl Hydrocarbon Receptor Can Generate Regulatory T Cells. J. Immunol. 2010;185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gutiérrez-Vázquez C., Quintana F.J. Regulation of the Immune Response by the Aryl Hydrocarbon Receptor. Immunity. 2018;48:19–33. doi: 10.1016/j.immuni.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu M., Wang X., Wang L., Ma X., Gong Z., Zhang S., Li Y. Targeting the IDO1 pathway in cancer: From bench to bedside. J. Hematol. Oncol. 2018;11:100. doi: 10.1186/s13045-018-0644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Taube J.M., Young G.D., McMiller T.L., Chen S., Salas J.T., Pritchard T.S., Xu H., Meeker A.K., Fan J., Cheadle C., et al. Differential Expression of Immune-Regulatory Genes Associated with PD-L1 Display in Melanoma: Implications for PD-1 Pathway Blockade. Clin. Cancer Res. 2015;21:3969–3976. doi: 10.1158/1078-0432.CCR-15-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bilir C., Sarisozen C. Indoleamine 2,3-dioxygenase (IDO): Only an enzyme or a checkpoint controller? J. Oncol. Sci. 2017;3:52–56. doi: 10.1016/j.jons.2017.04.001. [DOI] [Google Scholar]
- 138.Spranger S., Spaapen R.M., Zha Y., Williams J., Meng Y., Ha T.T., Gajewski T.F. Up-Regulation of PD-L1, IDO, and Tregs in the Melanoma Tumor Microenvironment Is Driven by CD8+ T Cells. Sci. Transl. Med. 2013;5 doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Curti A., Pandolfi S., Valzasina B., Aluigi M., Isidori A., Ferri E., Salvestrini V., Bonanno G., Rutella S., Durelli I., et al. Modulation of tryptophan catabolism by human leukemic cells results in the conversion of CD25− into CD25+ T regulatory cells. Blood. 2007;109:2871–2877. doi: 10.1182/blood-2006-07-036863. [DOI] [PubMed] [Google Scholar]
- 140.Yu G., Dai H., Chen J., Duan L., Gong M., Liu L., Xiong P., Wang C.Y., Fang M., Gong F. Gene delivery of indoleamine 2,3-dioxygenase prolongs cardiac allograft survival by shaping the types of T-cell responses. J. Gene Med. 2008;10:754–761. doi: 10.1002/jgm.1201. [DOI] [PubMed] [Google Scholar]
- 141.Witkiewicz A., Williams T.K., Cozzitorto J., Durkan B., Showalter S.L., Yeo C.J., Brody J.R. Expression of Indoleamine 2,3-Dioxygenase in Metastatic Pancreatic Ductal Adenocarcinoma Recruits Regulatory T Cells to Avoid Immune Detection. J. Am. Coll. Surg. 2008;206:849–854. doi: 10.1016/j.jamcollsurg.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 142.Mansfield A.S., Heikkila P.S., Vaara A.T., von Smitten K.A.J., Vakkila J.M., Leidenius M.H.K. Simultaneous Foxp3 and IDO expression is associated with sentinel lymph node metastases in breast cancer. BMC Cancer. 2009;9:231. doi: 10.1186/1471-2407-9-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Baban B., Chandler P.R., Sharma M.D., Pihkala J., Koni P.A., Munn D.H., Mellor A.L. IDO Activates Regulatory T Cells and Blocks Their Conversion into Th17-Like T Cells. J. Immunol. 2009;183:2475–2483. doi: 10.4049/jimmunol.0900986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Botticelli A., Cerbelli B., Lionetto L., Zizzari I., Salati M., Pisano A., Federica M., Simmaco M., Nuti M., Marchetti P. Can IDO activity predict primary resistance to anti-PD-1 treatment in NSCLC? J. Transl. Med. 2018;16:219. doi: 10.1186/s12967-018-1595-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Holmgaard R.B., Zamarin D., Munn D.H., Wolchok J.D., Allison J.P. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J. Exp. Med. 2013;210:1389–1402. doi: 10.1084/jem.20130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Spranger S., Koblish H.K., Horton B., Scherle P.A., Newton R., Gajewski T.F. Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8+ T cells directly within the tumor microenvironment. J. Immunother. Cancer. 2014;2:3. doi: 10.1186/2051-1426-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Perez R.P., Riese M.J., Lewis K.D., Saleh M.N., Daud A., Berlin J., Lee J.J., Mukhopadhyay S., Zhou L., Serbest G., et al. Epacadostat plus nivolumab in patients with advanced solid tumors: Preliminary phase I/II results of ECHO-204. J. Clin. Oncol. 2017;35:3003. doi: 10.1200/JCO.2017.35.15_suppl.3003. [DOI] [Google Scholar]
- 148.Mitchell T.C., Hamid O., Smith D.C., Bauer T.M., Wasser J.S., Olszanski A.J., Luke J.J., Balmanoukian A.S., Schmidt E.V., Zhao Y., et al. Epacadostat Plus Pembrolizumab in Patients With Advanced Solid Tumors: Phase I Results From a Multicenter, Open-Label Phase I/II Trial (ECHO-202/KEYNOTE-037) J. Clin. Oncol. 2018;36:3223–3230. doi: 10.1200/JCO.2018.78.9602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gibney G.T., Hamid O., Lutzky J., Olszanski A.J., Mitchell T.C., Gajewski T.F., Chmielowski B., Hanks B.A., Zhao Y., Newton R.C., et al. Phase 1/2 study of epacadostat in combination with ipilimumab in patients with unresectable or metastatic melanoma. J. Immunother. Cancer. 2019;7:80. doi: 10.1186/s40425-019-0562-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Long G.V., Dummer R., Hamid O., Gajewski T., Caglevic C., Dalle S., Arance A., Carlino M.S., Grob J.J., Kim T.M., et al. Epacadostat (E) plus pembrolizumab (P) versus pembrolizumab alone in patients (pts) with unresectable or metastatic melanoma: Results of the phase 3 ECHO-301/KEYNOTE-252 study. J. Clin. Oncol. 2018;36:108. doi: 10.1200/JCO.2018.36.15_suppl.108. [DOI] [PubMed] [Google Scholar]
- 151.Van den Eynde B.J., van Baren N., Baurain J.F. Is There a Clinical Future for IDO1 Inhibitors After the Failure of Epacadostat in Melanoma? Annu. Rev. Cancer Biol. 2020;4:241–256. doi: 10.1146/annurev-cancerbio-030419-033635. [DOI] [Google Scholar]
- 152.Tang D., Yue L., Yao R., Zhou L., Yang Y., Lu L., Gao W. P53 prevent tumor invasion and metastasis by down-regulating IDO in lung cancer. Oncotarget. 2017;8:54548–54557. doi: 10.18632/oncotarget.17408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mei J., Li M.-Q., Ding D., Li D.-J., Jin L.-P., Hu W.-G., Zhu X.-Y. Indoleamine 2,3-dioxygenase-1 (IDO1) enhances survival and invasiveness of endometrial stromal cells via the activation of JNK signaling pathway. Int. J. Clin. Exp. Pathol. 2013;6:431–444. [PMC free article] [PubMed] [Google Scholar]
- 154.Pallotta M.T., Orabona C., Volpi C., Vacca C., Belladonna M.L., Bianchi R., Servillo G., Brunacci C., Calvitti M., Bicciato S., et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat. Immunol. 2011;12:870–878. doi: 10.1038/ni.2077. [DOI] [PubMed] [Google Scholar]
- 155.Hammaker D.R., Boyle D.L., Inoue T., Firestein G.S. Regulation of the JNK pathway by TGF-beta activated kinase 1 in rheumatoid arthritis synoviocytes. Arthritis Res. Ther. 2007;9:R57. doi: 10.1186/ar2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wu S., Kasisomayajula K., Peng J., Bancalari E. Inhibition of JNK Enhances TGF-β1-Activated Smad2 Signaling in Mouse Embryonic Lung. Pediatric Res. 2009;65:381–386. doi: 10.1203/PDR.0b013e3181991c67. [DOI] [PubMed] [Google Scholar]
- 157.Hennequart M., Pilotte L., Cane S., Hoffmann D., Stroobant V., Plaen E., van den Eynde B.J. Constitutive IDO1 Expression in Human Tumors Is Driven by Cyclooxygenase-2 and Mediates Intrinsic Immune Resistance. Cancer Immunol. Res. 2017;5:695–709. doi: 10.1158/2326-6066.CIR-16-0400. [DOI] [PubMed] [Google Scholar]
- 158.Xu Z., Chen L., Zheng L., Yang Q., Chen M., Wang J., Zhu G., Chen Z., Sun J. Hyperprogressive Disease In Cervical Small Cell Carcinoma Treated By Immune Checkpoint Inhibitor. Oncotargets Ther. 2019;12:8873–8877. doi: 10.2147/OTT.S213436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pai C.-C.S., Huang J.T., Lu X., Simons D.M., Park C., Chang A., Tamaki W., Liu E., Roybal K.T., Seagal J., et al. Clonal Deletion of Tumor-Specific T Cells by Interferon-γ Confers Therapeutic Resistance to Combination Immune Checkpoint Blockade. Immunity. 2019;50:477–492. doi: 10.1016/j.immuni.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Refaeli Y., van Parijs L., Alexander S.I., Abbas A.K. Interferon γ is Required for Activation-induced Death of T Lymphocytes. J. Exp. Med. 2002;196:999–1005. doi: 10.1084/jem.20020666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ju S.-T., Panka D.J., Cui H., Ettinger R., EI-Khatib M., Sherr D.H., Stanger B.Z., Marshak-Rothstein A. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 162.Malavasi F., Deaglio S., Funaro A., Ferrero E., Horenstein A.L., Ortolan E., Vaisitti T., Aydin S. Evolution and Function of the ADP Ribosyl Cyclase/CD38 Gene Family in Physiology and Pathology. Physiol. Rev. 2008;88:841–886. doi: 10.1152/physrev.00035.2007. [DOI] [PubMed] [Google Scholar]
- 163.Hogan K.A., Chini C.C.S., Chini E.N. The Multi-faceted Ecto-enzyme CD38: Roles in Immunomodulation, Cancer, Aging, and Metabolic Diseases. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.01187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bengsch B., Ohtani T., Khan O., Setty M., Manne S., O’Brien S., Gherardini P.F., Herati R.S., Huang A.C., Chang K.-M., et al. Epigenomic-Guided Mass Cytometry Profiling Reveals Disease-Specific Features of Exhausted CD8 T Cells. Immunity. 2018;48:1029–1045. doi: 10.1016/j.immuni.2018.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chatterjee S., Daenthanasanmak A., Chakraborty P., Wyatt M.W., Dhar P., Selvam S.P., Fu J., Zhang J., Nguyen H., Kang I., et al. CD38-NAD+Axis Regulates Immunotherapeutic Anti-Tumor T Cell Response. Cell Metab. 2018;27:85–100. doi: 10.1016/j.cmet.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mehta K., Shahid U., Malavasi F. Human CD38, a cell-surface protein with multiple functions. Faseb J. 1996;10:1408–1417. doi: 10.1096/fasebj.10.12.8903511. [DOI] [PubMed] [Google Scholar]
- 167.Dianzani U., Funaro A., DiFranco D., Garbarino G., Bragardo M., Redoglia V., Buonfiglio D., de Monte L.B., Pileri A., Malavasi F. Interaction between endothelium and CD4+ CD45RA+ lymphocytes. Role of the human CD38 molecule. J. Immunol. 1994;153:952–959. [PubMed] [Google Scholar]
- 168.Deaglio S., Dianzani U., Horenstein A.L., Fernandez J.E., van Kooten C., Bragardo M., Funaro A., Garbarino G., di Virgilio F., Banchereau J., et al. Human CD38 ligand. A 120-KDA protein predominantly expressed on endothelial cells. J. Immunol. 1996;156:727–734. [PubMed] [Google Scholar]
- 169.Estrada-Figueroa L.A., Ramirez-Jimenez Y., Osorio-Trujillo C., Shibayama M., Navarro-Garcia F., Garcia-Tovar C., Talamas-Rohana P. Absence of CD38 delays arrival of neutrophils to the liver and innate immune response development during hepatic amoebiasis by Entamoeba histolytica. Parasite Immunol. 2011;33:661–668. doi: 10.1111/j.1365-3024.2011.01333.x. [DOI] [PubMed] [Google Scholar]
- 170.Zubiaur M., Izquierdo M., Terhorst C., Malavasi F., Sancho J. CD38 ligation results in activation of the Raf-1/mitogen-activated protein kinase and the CD3-zeta/zeta-associated protein-70 signaling pathways in Jurkat T lymphocytes. J. Immunol. 1997;159:193–205. [PubMed] [Google Scholar]
- 171.Ausiello C.M., Urbani F., la Sala A., Funaro A., Malavasi F. CD38 ligation induces discrete cytokine mRNA expression in human cultured lymphocytes. Eur. J. Immunol. 1995;25:1477–1480. doi: 10.1002/eji.1830250554. [DOI] [PubMed] [Google Scholar]
- 172.Deaglio S., Zubiaur M., Gregorini A., Bottarel F., Ausiello C.M., Dianzani U., Sancho J., Malavasi F. Human CD38 and CD16 are functionally dependent and physically associated in natural killer cells. Blood. 2002;99:2490–2498. doi: 10.1182/blood.V99.7.2490. [DOI] [PubMed] [Google Scholar]
- 173.Sconocchia G., Titus J.A., Mazzoni A., Visintin A., Pericle F., Hicks S.W., Malavasi F., Segal D.M. CD38 triggers cytotoxic responses in activated human natural killer cells. Blood. 1999;94:3864–3871. doi: 10.1182/blood.V94.11.3864. [DOI] [PubMed] [Google Scholar]
- 174.Funaro A., Morra M., Calosso L., Zini M.G., Ausiello C.M., Malavasi F. Role of the human CD38 molecule in B cell activation and proliferation. Tissue Antigens. 1997;49:7–15. doi: 10.1111/j.1399-0039.1997.tb02703.x. [DOI] [PubMed] [Google Scholar]
- 175.Damle R.N., Wasil T., Fais F., Ghiotto F., Valetto A., Allen S.L., Buchbinder A., Budman D., Dittmar K., Kolitz J., et al. Ig V Gene Mutation Status and CD38 Expression As Novel Prognostic Indicators in Chronic Lymphocytic Leukemia. Blood. 1999;94:1840–1847. doi: 10.1182/blood.V94.6.1840. [DOI] [PubMed] [Google Scholar]
- 176.Matrai Z. CD38 as a prognostic marker in CLL. Hematology. 2005;10:39–46. doi: 10.1080/10245330400020470. [DOI] [PubMed] [Google Scholar]
- 177.Hambley R., Payne T., Taylor H., Fegan C., Mills K., Pepper C. CD38+ and CD38-B-CLL subsets from patients with bimodal expression of the CD38 antigen exhibit distinct cell cycle distribution and Bcl-2 family protein expression. Br. J. Haematol. 2004;125:10. [Google Scholar]
- 178.Deaglio S., Dwyer K.M., Gao W., Friedman D., Usheva A., Erat A., Chen J.-F., Enjyoji K., Linden J., Oukka M., et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Vaisitti T., Aydin S., Rossi D., Cottino F., Bergui L., D’Arena G., Bonello L., Horenstein A.L., Brennan P., Pepper C., et al. CD38 increases CXCL12-mediated signals and homing of chronic lymphocytic leukemia cells. Leukemia. 2010;24:958–969. doi: 10.1038/leu.2010.36. [DOI] [PubMed] [Google Scholar]
- 180.Lin P., Owens R., Tricot G., Wilson C.S. Flow Cytometric Immunophenotypic Analysis of 306 Cases of Multiple Myeloma. Am. J. Clin. Pathol. 2004;121:482–488. doi: 10.1309/74R4TB90BUWH27JX. [DOI] [PubMed] [Google Scholar]
- 181.Harada H., Kawano M.M., Huang N., Harada Y., Iwato K., Tanabe O., Tanaka H., Sakai A., Asaoku H., Kuramoto A. Phenotypic difference of normal plasma cells from mature myeloma cells. Blood. 1993;81:2658–2663. doi: 10.1182/blood.V81.10.2658.2658. [DOI] [PubMed] [Google Scholar]
- 182.McKeage K. Daratumumab: First Global Approval. Drugs. 2016;76:275–281. doi: 10.1007/s40265-015-0536-1. [DOI] [PubMed] [Google Scholar]
- 183.Dhillon S. Isatuximab: First Approval. Drugs. 2020;80:905–912. doi: 10.1007/s40265-020-01311-1. [DOI] [PubMed] [Google Scholar]
- 184.Krejcik J., Casneuf T., Nijhof I.S., Verbist B., Bald J., Plesner T., Syed K., Liu K., van de Donk N.W.C.J., Weiss B.M., et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016;128:384–394. doi: 10.1182/blood-2015-12-687749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Van de Donk N.W.C.J., Usmani S.Z. CD38 Antibodies in Multiple Myeloma: Mechanisms of Action and Modes of Resistance. Front. Immunol. 2018;9:2134. doi: 10.3389/fimmu.2018.02134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Martin T.G., Corzo K., Chiron M., van de Velde H., Abbadessa G., Campana F., Solanki M., Meng R., Lee H., Wiederschain D., et al. Therapeutic Opportunities with Pharmacological Inhibition of CD38 with Isatuximab. Cells. 2019;8:1522. doi: 10.3390/cells8121522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Deckert J., Wetzel M.-C., Bartle L.M., Skaletskaya A., Goldmacher V.S., Vallee F., Zhou-Liu Q., Ferrari P., Pouzieux S., Lahoute C., et al. SAR650984, A Novel Humanized CD38-Targeting Antibody, Demonstrates Potent Antitumor Activity in Models of Multiple Myeloma and Other CD38+ Hematologic Malignancies. Clin. Cancer Res. 2014;20:4574–4583. doi: 10.1158/1078-0432.CCR-14-0695. [DOI] [PubMed] [Google Scholar]
- 188.Jiang H., Acharya C., An G., Zhong M., Feng X., Wang L., Dasilva N., Song Z., Yang G., Adrian F., et al. SAR650984 directly induces multiple myeloma cell death via lysosomal-associated and apoptotic pathways, which is further enhanced by pomalidomide. Leukemia. 2016;30:399–408. doi: 10.1038/leu.2015.240. [DOI] [PubMed] [Google Scholar]
- 189.Karakasheva T.A., Waldron T.J., Eruslanov E., Kim S.-B., Lee J.-S., O’Brien S., Hicks P.D., Basu D., Singhal S., Malavasi F., et al. CD38-Expressing Myeloid-Derived Suppressor Cells Promote Tumor Growth in a Murine Model of Esophageal Cancer. Cancer Res. 2015;75:4074–4085. doi: 10.1158/0008-5472.CAN-14-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Levy A., Blacher E., Vaknine H., Lund F.E., Stein R., Mayo L. CD38 deficiency in the tumor microenvironment attenuates glioma progression and modulates features of tumor-associated microglia/macrophages. Neuro-Oncology. 2012;14:1037–1049. doi: 10.1093/neuonc/nos121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Liao S., Xiao S., Chen H., Zhang M., Chen Z., Long Y., Gao L., Zhu G., He J., Peng S., et al. CD38 enhances the proliferation and inhibits the apoptosis of cervical cancer cells by affecting the mitochondria functions. Mol. Carcinog. 2017;56:2245–2257. doi: 10.1002/mc.22677. [DOI] [PubMed] [Google Scholar]
- 192.Bu X., Kato J., Hong J.A., Merino M.J., Schrump D.S., Lund F.E., Moss J. CD38 knockout suppresses tumorigenesis in mice and clonogenic growth of human lung cancer cells. Carcinogenesis. 2018;39:242–251. doi: 10.1093/carcin/bgx137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Feng X., Zhang L., Acharya C., An G., Wen K., Qiu L., Munshi N.C., Tai Y.-T., Anderson K.C. Targeting CD38 Suppresses Induction and Function of T Regulatory Cells to Mitigate Immunosuppression in Multiple Myeloma. Clin. Cancer Res. 2017;23:4290–4300. doi: 10.1158/1078-0432.CCR-16-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Musso T., Deaglio S., Franco L., Calosso L., Badolato R., Garbarino G., Dianzani U., Malavasi F. CD38 expression and functional activities are up-regulated by IFN-γ on human monocytes and monocytic cell lines. J. Leukoc. Biol. 2001;69:605–612. doi: 10.1189/jlb.69.4.605. [DOI] [PubMed] [Google Scholar]
- 195.Bürgler S., Gimeno A., Parente-Ribes A., Wang D., Os A., Devereux S., Jebsen P., Bogen B., Tjønnfjord G.E., Munthe L.A. Chronic Lymphocytic Leukemia Cells Express CD38 in Response to Th1 Cell–Derived IFN-γ by a T-bet–Dependent Mechanism. J. Immunol. 2015;194:827–835. doi: 10.4049/jimmunol.1401350. [DOI] [PubMed] [Google Scholar]
- 196.Snoeck H.W., Lardon F., Lenjou M., Nys G., Van D.B., Peetermans M.E. Differential regulation of the expression of CD38 and human leukocyte antigen-DR on CD34+ hematopoietic progenitor cells by interleukin-4 and interferon-gamma. Exp. Hematol. 1993;21:1480–1486. [PubMed] [Google Scholar]
- 197.Yi M., Jiao D., Xu H., Liu Q., Zhao W., Han X., Wu K. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol. Cancer. 2018;17:129. doi: 10.1186/s12943-018-0864-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Konen J.M., Fradette J.J., Gibbons D.L. The Good, the Bad and the Unknown of CD38 in the Metabolic Microenvironment and Immune Cell Functionality of Solid Tumors. Cells. 2019;9:52. doi: 10.3390/cells9010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kazemi M.H., Raoofi Mohseni S., Hojjat-Farsangi M., Anvari E., Ghalamfarsa G., Mohammadi H., Jadidi-Niaragh F. Adenosine and adenosine receptors in the immunopathogenesis and treatment of cancer. J. Cell. Physiol. 2018;233:2032–2057. doi: 10.1002/jcp.25873. [DOI] [PubMed] [Google Scholar]
- 200.Sek K., Mølck C., Stewart G., Kats L., Darcy P., Beavis P. Targeting Adenosine Receptor Signaling in Cancer Immunotherapy. Int. J. Mol. Sci. 2018;19:3837. doi: 10.3390/ijms19123837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ma S.-R., Deng W.-W., Liu J.-F., Mao L., Yu G.-T., Bu L.-L., Kulkarni A.B., Zhang W.-F., Sun Z.-J. Blockade of adenosine A2A receptor enhances CD8+ T cells response and decreases regulatory T cells in head and neck squamous cell carcinoma. Mol. Cancer. 2017;16:99. doi: 10.1186/s12943-017-0665-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Vogt T.J., Gevensleben H., Dietrich J., Kristiansen G., Bootz F., Landsberg J., Goltz D., Dietrich D. Detailed analysis of adenosine A2a receptor (ADORA2A) and CD73 (5′-nucleotidase, ecto, NT5E) methylation and gene expression in head and neck squamous cell carcinoma patients. OncoImmunology. 2018;7:e1452579. doi: 10.1080/2162402X.2018.1452579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Merighi S., Mirandola P., Milani D., Varani K., Gessi S., Klotz K.-N., Leung E., Baraldi P.G., Borea P.A. Adenosine Receptors as Mediators of Both Cell Proliferation and Cell Death of Cultured Human Melanoma Cells. J. Investig. Dermatol. 2002;119:923–933. doi: 10.1046/j.1523-1747.2002.00111.x. [DOI] [PubMed] [Google Scholar]
- 204.Etique N., Grillier-Vuissoz I., Lecomte J., Flament S. Crosstalk between adenosine receptor (A2A isoform) and ERα mediates ethanol action in MCF-7 breast cancer cells. Oncol. Rep. 2009;21:977–981. doi: 10.3892/or_00000311. [DOI] [PubMed] [Google Scholar]
- 205.Koszałka P., Gołuńska M., Urban A., Stasiłojć G., Stanisławowski M., Majewski M., Składanowski A.C., Bigda J. Specific Activation of A3, A2A and A1 Adenosine Receptors in CD73-Knockout Mice Affects B16F10 Melanoma Growth, Neovascularization, Angiogenesis and Macrophage Infiltration. PLoS ONE. 2016;11:e0151420. doi: 10.1371/journal.pone.0151420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Milne G.R., Palmer T.M. Anti-Inflammatory and Immunosuppressive Effects of the A2A Adenosine Receptor. Sci. World J. 2011;11:320–339. doi: 10.1100/tsw.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sharma N., Dev R., de Dios Ruiz-Rosado J., Partida-Sanchez S., Guerau-de-Arellano M., Dhakal P., Kuivaniemi H., Hans C.P. Pharmacological inhibition of Notch signaling regresses pre-established abdominal aortic aneurysm. Sci. Rep. 2019;9:13458. doi: 10.1038/s41598-019-49682-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Flajolet M., Wang Z., Futter M., Shen W., Nuangchamnong N., Bendor J., Wallach I., Nairn A.C., Surmeier D.J., Greengard P. FGF acts as a co-transmitter through adenosine A2A receptor to regulate synaptic plasticity. Nat. Neurosci. 2008;11:1402–1409. doi: 10.1038/nn.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Das M., Jiang F., Sluss H.K., Zhang C., Shokat K.M., Flavell R.A., Davis R.J. Suppression of p53-dependent senescence by the JNK signal transduction pathway. Proc. Natl. Acad. Sci. USA. 2007;104:15759–15764. doi: 10.1073/pnas.0707782104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Gessi S., Bencivenni S., Battistello E., Vincenzi F., Colotta V., Catarzi D., Varano F., Merighi S., Borea P.A., Varani K. Inhibition of A2A Adenosine Receptor Signaling in Cancer Cells Proliferation by the Novel Antagonist TP455. Front. Pharmacol. 2017;8:888. doi: 10.3389/fphar.2017.00888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Leone R.D., Lo Y.-C., Powell J.D. A2aR antagonists: Next generation checkpoint blockade for cancer immunotherapy. Comput. Struct. Biotechnol. J. 2015;13:265–272. doi: 10.1016/j.csbj.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Togashi Y., Kamada T., Sasaki A., Nakamura Y., Fukuoka S., Tada Y., Kawazoe A., Shitara K., Nishikawa H. Clinicopathological, genomic and immunological features of hyperprogressive disease during PD-1 blockade in gastric cancer patients. J. Clin. Oncol. 2018;36:4106. doi: 10.1200/JCO.2018.36.15_suppl.4106. [DOI] [Google Scholar]
- 213.Novitskiy S.V., Ryzhov S., Zaynagetdinov R., Goldstein A.E., Huang Y., Tikhomirov O.Y., Blackburn M.R., Biaggioni I., Carbone D.P., Feoktistov I., et al. Adenosine receptors in regulation of dendritic cell differentiation and function. Blood. 2008;112:1822–1831. doi: 10.1182/blood-2008-02-136325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Cekic C., Linden J. Purinergic regulation of the immune system. Nat. Rev. Immunol. 2016;16:177–192. doi: 10.1038/nri.2016.4. [DOI] [PubMed] [Google Scholar]
- 215.Allard D., Turcotte M., Stagg J. Targeting A2 adenosine receptors in cancer. Immunol. Cell Biol. 2017;95:333–339. doi: 10.1038/icb.2017.8. [DOI] [PubMed] [Google Scholar]
- 216.Vijayan D., Young A., Teng M.W.L., Smyth M.J. Targeting immunosuppressive adenosine in cancer. Nat. Rev. Cancer. 2017;17:709–724. doi: 10.1038/nrc.2017.86. [DOI] [PubMed] [Google Scholar]
- 217.Mayo L., Jacob-Hirsch J., Amariglio N., Rechavi G., Moutin M.-J., Lund F.E., Stein R. Dual Role of CD38 in Microglial Activation and Activation-Induced Cell Death. J. Immunol. 2008;181:92–103. doi: 10.4049/jimmunol.181.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhang J., Xu X., Liu Y. Activation-Induced Cell Death in T Cells and Autoimmunity. Cell. Mol. Immunol. 2004;1:186–192. [PubMed] [Google Scholar]
- 219.Vrbacky F., Smolej L., Vroblova V., Pekova S., Hrudkova M., Cervinka M., Pecka M., Krejsek J., Maly J. Angiopoietin-2 mRNA expression is increased in chronic lymphocytic leukemia patients with poor prognostic features. Hematology. 2010;15:210–214. doi: 10.1179/102453309X12583347113898. [DOI] [PubMed] [Google Scholar]
- 220.Ge Y., Long Y., Xiao S., Liang L., He Z., Yue C., Wei X., Zhou Y. CD38 affects the biological behavior and energy metabolism of nasopharyngeal carcinoma cells. Int. J. Oncol. 2018;54:585–599. doi: 10.3892/ijo.2018.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhong H., De Marzo A.M., Laughner E., Lim M., Hilton D.A., Zagzag D., Buechler P., Isaacs W.B., Semenza G.L., Simons J.W. Overexpression of hypoxia-inducible factor 1α in common human cancers and their metastases. Cancer Res. 1999;59:5830–5835. [PubMed] [Google Scholar]
- 222.Madu C., Li L., Lu Y. Selection, Analysis and Improvement of Anti-Angiogenesis Compounds Identified by an Anti-HIF-1α Screening and Validation System. J. Cancer. 2016;7:1926–1938. doi: 10.7150/jca.15603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Guo Y., Han B., Luo K., Ren Z., Cai L., Sun L. NOX2-ROS-HIF-1α signaling is critical for the inhibitory effect of oleanolic acid on rectal cancer cell proliferation. Biomed. Pharmacother. 2017;85:733–739. doi: 10.1016/j.biopha.2016.11.091. [DOI] [PubMed] [Google Scholar]
- 224.Cheng R., Yong H., Xia Y., Xie Q., Gao G., Zhou X. Chemotherapy regimen based on sorafenib combined with 5-FU on HIF-1α and VEGF expression and survival in advanced gastric cancer patients. Oncol. Lett. 2017;13:2703–2707. doi: 10.3892/ol.2017.5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kim M.-H., Jeong Y.-J., Cho H.-J., Hoe H.-S., Park K.-K., Park Y.-Y., Choi Y.H., Kim C.-H., Chang H.-W., Park Y.-J., et al. Delphinidin inhibits angiogenesis through the suppression of HIF-1α and VEGF expression in A549 lung cancer cells. Oncol. Rep. 2017;37:777–784. doi: 10.3892/or.2016.5296. [DOI] [PubMed] [Google Scholar]
- 226.Haase V. The VHL Tumor Suppressor: Master Regulator of HIF. Curr. Pharm. Des. 2009;15:3895–3903. doi: 10.2174/138161209789649394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hsieh J.J., Le V.H., Oyama T., Ricketts C.J., Ho T.H., Cheng E.H. Chromosome 3p Loss–Orchestrated VHL, HIF, and Epigenetic Deregulation in Clear Cell Renal Cell Carcinoma. J. Clin. Oncol. 2018;36:3533–3539. doi: 10.1200/JCO.2018.79.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Brouwer N.J., Wierenga A.P.A., Gezgin G., Marinkovic M., Luyten G.P.M., Kroes W.G.M., Versluis M., van der Velden P.A., Verdijk R.M., Jager M.J. Ischemia Is Related to Tumour Genetics in Uveal Melanoma. Cancers. 2019;11:1004. doi: 10.3390/cancers11071004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Denduluri S.K., Idowu O., Wang Z., Liao Z., Yan Z., Mohammed M.K., Ye J., Wei Q., Wang J., Zhao L., et al. Insulin-like growth factor (IGF) signaling in tumorigenesis and the development of cancer drug resistance. Genes Dis. 2015;2:13–25. doi: 10.1016/j.gendis.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hansen W., Hutzler M., Abel S., Alter C., Stockmann C., Kliche S., Albert J., Sparwasser T., Sakaguchi S., Westendorf A.M., et al. Neuropilin 1 deficiency on CD4+Foxp3+ regulatory T cells impairs mouse melanoma growth. J. Exp. Med. 2012;209:2001–2016. doi: 10.1084/jem.20111497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Perrot-Applanat M., di Benedetto M. Autocrine functions of VEGF in breast tumor cells. Cell Adhes. Migr. 2012;6:547–553. doi: 10.4161/cam.23332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Goel H.L., Mercurio A.M. VEGF targets the tumour cell. Nat. Rev. Cancer. 2013;13:871–882. doi: 10.1038/nrc3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Refae S., Gal J., Brest P., Giacchero D., Borchiellini D., Ebran N., Peyrade F., Guigay J., Milano G., Saada-Bouzid E. Hyperprogression under Immune Checkpoint Inhibitor: A potential role for germinal immunogenetics. Sci. Rep. 2020;10:3565. doi: 10.1038/s41598-020-60437-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Glubb D.M., Cerri E., Giese A., Zhang W., Mirza O., Thompson E.E., Chen P., Das S., Jassem J., Rzyman W., et al. Novel Functional Germline Variants in the VEGF Receptor 2 Gene and Their Effect on Gene Expression and Microvessel Density in Lung Cancer. Clin. Cancer Res. 2011;17:5257–5267. doi: 10.1158/1078-0432.CCR-11-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Xu W., Li J.-Y., Wu Y.-J., Yu H., Shen Q.-D., Tian T., Li L., Qiu H.-X. CD38 as a prognostic factor in Chinese patients with chronic lymphocytic leukaemia. Leuk. Res. 2009;33:237–243. doi: 10.1016/j.leukres.2008.06.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.