INTRODUCTION
Asthma impacts over 19,000,000 (7.7%) U.S. adults.1 Despite advances in asthma care, asthma prevalence has increased over the past decade.2 Asthma imposes a significant burden on society, with $50.3 billion in annual medical costs, 500,000 hospitalizations, and 2 million emergency department visits annually.3–5 Consequently, identification of modifiable health behaviors that may improve patient outcomes and reduce healthcare utilization is needed.
Sleep is a modifiable behavior and it is recommended that adults regularly get 7 or more hours of sleep per night.6 In the general population, both short (i.e., less than 6 hours) and long (i.e., 9 or more hours) sleep duration have well-established associations with adverse health outcomes including chronic diseases, functional decline, and mortality.7–11 Among older adults, short and long sleep duration is associated with an increased risk for hospitalizations and emergency department visits.12, 13 Most studies of sleep in adults with asthma have focused on insufficient or poor quality sleep.14–17 An analysis of National Health Nutrition Examination Survey (NHANES) data found those with asthma to be significantly more likely to sleep less than 6 hours (23% vs. 13%) or 9 or more hours (7% vs. 6%) as compared to those without asthma.18 These findings suggest that, in addition to insufficient sleep, sleeping too long may also be a problem in adults with asthma.
Among adolescents, evidence has demonstrated a negative effect of short sleep, induced by partial sleep restriction, on asthma symptoms and health-related quality of life.19 No studies to date have examined the impact of sleep duration on health outcomes in adults with asthma. The present study analyzed data from the NHANES that includes a large nationally representative sample of adults in the United States. Using data from the 2007–2012 waves of NHANES, we examined the associations between sleep duration and patient-reported outcomes (i.e., asthma symptoms and attacks, activity limitation, and health-related quality of life) and healthcare use (i.e., asthma-specific and general). We hypothesized that both short and long sleep duration will be associated with worse patient-reported outcomes and greater healthcare use.
METHODS
NHANES dataset
The NHANES is a continuous in-person survey of health indicators of the civilian, noninstitutionalized U.S. population administered by the National Center for Health Statistics of the Centers for Disease Control and Prevention. Households were selected and recruited using a stratified multistage probability sampling design. The data was collected by face-to-face interviews that included demographics, socioeconomic, and health-related questions and by clinical examinations conducted in a mobile assessment unit including physical measurement and laboratory testing. The survey was approved by the National Center for Health Statistics Research Ethics Review Board and all study participants provided informed consent and only de-identified data was used for the current analysis.
Analytic sample
This study was a cross-sectional analysis of adults at least 20 years and older with asthma drawn from six years (2007–2012) of NHANES survey data. Participants who answered ‘yes’ to the questions, “Has a doctor or other health professional ever told you that you had asthma?” and “Do you still have asthma?” were defined as currently having asthma. Participants with a prior physician diagnosis of asthma but who reported that they no longer had asthma were excluded from the analyses.
Sleep Duration
Sleep duration was measured by a single question, “How much sleep do you usually get at night on weekdays or workdays?” with allowable responses in whole numbers between 1 and 24 hours. Habitual or typical hours of sleep were categorized as short sleep duration (5 or less hours per night), normal sleep duration (6 to 8 hours per night), and long sleep duration (9 or more hours per night).
Patient-Reported Outcomes
Asthma symptoms and attacks.
Asthma symptoms included the presence of wheezing and coughing. The presence of wheezing was defined as an affirmative response to the question: “In the past 12 months, have you had wheezing or whistling in your chest?” The presence of a cough was defined as an affirmative response to the question: “In the past 12 months, have you had a dry cough at night not counting a cough associated with a cold or chest infection lasting 14 days or more?” The presence of an asthma attack was identified as an affirmative answer to the question: “During the past 12 months, have you had an episode of asthma or an asthma attack?” The percentage of ‘yes’ answers from each question are reported separately.
Activity limitation.
Activity limitation due to wheezing was measured by the question: “During the past 12 months, how much did you limit your usual activities due to wheezing or whistling?” Answers were on a 5-point Likert scale (1= not at all, 5= a lot). Responses were collapsed into three categories: not at all, a little, and frequent.
Health-related quality of life.
Health-related quality of life was assessed by over the past 30 days by asking the following questions: “how many days was mental health which includes stress, depression, and problems with emotions not good,” “how many days physical health including physical illness and injury not good,” and “how many days did poor physical and mental health keep you from doing usual activities, such as self-care, work, school, or recreation.”
Healthcare Use
Asthma-related.
Two questions asked about asthma-related healthcare use. Participants were asked whether they had, within the previous 12 months, visited an emergency room or an urgent care center because of asthma (Yes/No) and the frequency of medical visits (doctor’s office or the hospital emergency room) during this time period because of “attacks of wheezing or whistling?”
General.
Participants were asked two questions about their general healthcare use in the past 12 months including their frequency of seeing a healthcare provider (i.e., doctor, clinic, or emergency room, but not including an overnight hospitalization) and whether they had an overnight hospitalization (Yes/No).
Demographic and Clinical Data
Demographic data collected from the participants included age, sex, race/ethnicity, educational attainment, and socioeconomic status. Age at screening was recoded with persons older than 79 years coded as “80”. Race/ethnicity was categorized as non-Hispanic White, non-Hispanic Black, Hispanic, and other. Marital status was collapsed into three categories of married/partnered, divorced/separated, or widowed/single. Education, originally evaluated by NHANES as the highest grade or degree received from “less than 9th grade” to “college graduate or above,” was collapsed into two categories of less than high school education and greater equal or greater than high school education. Socioeconomic status was estimated by NHANES using the ratio of total annual household income to family size according to the US poverty threshold for those cohort years with the range of values collapsed into four categories: < 133%, 134–299%, 300–400% and > 500%. Relevant clinical indicators include smoking status, body mass index (BMI) (kg/m2), and pulmonary function tests. Self-reported smoking status was categorized as either as a nonsmoker, current smoker, or former smoker. Nonsmoker was defined by a non-affirmative response to the question: “Have you smoked at least 100 cigarettes in your entire life?” Former smoker was defined by an affirmative response to this question but a non-affirmative response to the question: “Do you now smoke cigarettes?” Current smoker was defined as an affirmative response to both of the aforementioned questions. BMI was calculated from measured weight and height. Spirometry testing had been conducted at the NHANES Mobile Examination Center according to a standardized protocol and obtained forced expiratory airflow volume in the first second (FEV1) and the forced vital capacity (FVC). Normative reference equations developed from NHANES III data were used to determine predicted spirometry values.20 Baseline spirometry data and predicted values were used to calculate percent predicted values which was used to characterize the study sample. Spirometry data was available for 96% (n = 1334) of participants.
Data Analysis
All analyses included the complex multistage sampling and sampling weights provided by NHANES. Analyses were computed with SAS v.9.4 (SAS Institute Inc. Cary, NC). Demographic and clinical characteristics, patient-reported outcomes (asthma symptoms and attacks, activity limitation, and health-related quality of life), and healthcare use (asthma-related and general) were presented as weighted proportions for categorical variables and mean and standard deviation for continuous variables in US adults with asthma with short (≤ 5 hours), normal (6–8 hours), and long (≥ 9 hours) sleep duration. Estimates for sleep duration groups were compared using chi-square test or analysis of variance with SAS SURVEY procedures. Despite non-normally distributed data for some of the continuous variables, analysis of variance was still used as it is not sensitive to moderate deviations from normality.21
Multivariate analyses using linear, logistic, and multinomial logistic regression were used to examine the associations between sleep duration and patient-reported outcomes and healthcare use. Separate models were run using each patient-reported outcome and healthcare use variables as dependent variables and sleep duration groups as independent variables. All models were adjusted for age, sex, race/ethnicity, socioeconomic status, and smoking status and were weighted to account for the complex sampling design of NHANES.
The survey instruments for the asthma and respiratory outcomes use skip patterns, in which only individuals with asthma and wheezing are asked subsequent related questions. Missing data were recoded as intended “no” responses for individuals who were administered these questions. Less than 10% of missing data was observed on the variables of interest so missing data was ignored.
RESULTS
Sample
There were 1389 adults 20 years and older in the national sample from 2007–2012 who self-identified as currently having asthma. Weighted estimates of demographic characteristics of the sample stratified by individuals reporting 5 or fewer, 6 to 8, and 9 or more hours of sleep per night are presented in Table 1. Of the sample, 25.9% slept ≤ 5 hours, 65.9% slept 6–8 hours, and 8.2% slept ≥ 9 hours. Short sleepers were more likely to be younger and to be non-White while long sleepers were more likely to be older, female, and a current smoker (all p-values <.001). Compared to normal sleepers, both the short and long sleeper groups were characterized by persons who are more likely to have a lower income (27.9% versus 45.5% and 45%, respectively) and a lower education level (17.1% versus 27.3% and 27.7, respectively). Marital status, BMI, and lung function except for FEV/FVC ratio were similar across all the three sleep duration groups.
Table 1.
Weighted demographic and clinical characteristics for all adults with asthma by sleep duration, NHANES 2007–2012a
| Sleep Duration | |||||
|---|---|---|---|---|---|
| Total (n = 1389) | ≤ 5 (n = 359) | 6–8 (n = 916) | ≥ 9 (n = 114) | P value | |
| Age, mean (SD) | 47.2 (0.6) | 44.7 (1.2) | 47.7 (0.7%) | 49.6 (2.3) | 0.001 |
| Male | 513 (36.4%) | 137 (38.8%) | 339 (37.1%) | 37 (22.9%) | 0.02 |
| Race/ethnicity | |||||
| White | 674 (71.0%) | 143 (19.6%) | 459 (72.3%) | 72 (8.2%) | <0.001 |
| Black | 360 (14.2%) | 112 (31.7%) | 226 (62.6%) | 22 (5.8%) | |
| Hispanic | 156 (5.5%) | 48 (26.3%) | 93 (62.6%) | 15 (11.1%) | |
| Other | 199 (9.3%) | 56 (30.3%) | 138 (67.6%) | 5 (2.1%) | |
| Education | |||||
| < High school | 409 (20.2%) | 118 (27.3%) | 246 (17.1%) | 45 (27.7%) | <0.001 |
| ≥ High school | 980 (79.8%) | 241 (72.7%) | 670 (82.9%) | 69 (72.3%) | |
| Smoking status | |||||
| Current smoker | 633 (47.1%) | 140 (37.8%) | 441 (49.5%) | 52 (52.9%) | <0.001 |
| Former smoker | 365 (26.2%) | 83 (19.7%) | 252 (28.6%) | 30 (23.4%) | |
| Nonsmoker | 390 (26.7%) | 136 (42.5%) | 222 (21.9%) | 32 (23.8%) | |
| Marital status | |||||
| Married | 703 (55.5%) | 178 (20.9%) | 465 (71.3%) | 60 (7.7%) | 0.66 |
| Divorced/separated | 257 (15.7%) | 75 (26.4%) | 165 (67.7%) | 17 (5.8%) | |
| Widowed/single | 429 (28.7%) | 106 (23.9%) | 286 (68.4%) | 37 (7.6%) | |
| Poverty Level | |||||
| ≤ 133 | 582 (33.1%) | 174 (45.5%) | 350 (27.9%) | 58 (45.0%) | <0.001 |
| 134–299 | 329 (24.8%) | 89 (27.5%) | 214 (23.7%) | 26 (27.1%) | |
| 300–499 | 187 (19.4%) | 37 (16.9%) | 137 (20.6%) | 13 (16.0%) | |
| ≥ 500 | 164 (22.6%) | 21 (10.1%) | 136 (27.8%) | 7 (11.9%) | |
| BMI, mean (SD) | 30.8 (0.3) | 31.8 (0.5) | 30.4 (0.4) | 31.1 (0.7) | 0.09 |
| FEV1% predicted, mean (SD) | 88.6 (0.0) | 87.6 (0.0) | 89.2 (0.0) | 85.5 (0.0) | 0.89 |
| FVC% predicted, mean (SD) | 95.8 (0.0) | 94.0 (0.0) | 96.5 (0.0) | 94.7 (0.0) | 0.17 |
| FEV1/FVC% predicted, mean (SD) | 94.9 (0.0) | 96.6 (0.0) | 94.6 (0.0) | 92.1 (0.0) | 0.02 |
Abbreviations: BMI, body mass index; FEV1, forced expiratory volume at 1 second; FVC, forced vital capacity, SD, standard deviation.
Data is presented as n (%), unless indicated.
Patient-reported outcomes and healthcare use by sleep duration
The weighted prevalence of patient-reported outcomes and healthcare use among the sleep duration groups is reported in Table 2 and Figure 1. Compared to normal sleepers, short and long sleepers had a higher proportion of persons who reported having an asthma attack in the past year (45% versus 59% and 51%, respectively) and had more days with an impaired health-related quality of life as indicated by more days of poor physical and mental health and inactive days due to poor physical or mental health (all p-values <.05). Short sleepers also had more persons who reported wheezing (67% versus 59% and 56%, respectively), dry cough (22% versus 12% and 14%), and activity limitation due to wheezing (40% versus 30% and 31%) in the last 12 months and who had an overnight hospitalization (28% versus 16% and 19%) compared to normal and long sleepers (all p-values <.05). There were no significant differences between sleep duration groups in regard to the proportion having an emergency room visit for asthma or the frequency of general healthcare utilization and emergency room visits for wheezing in the last 12 months.
Table 2.
Patient-reported outcomes and healthcare use by sleep duration in adults with asthma: Weighted bivariate analysisa
| Sleep Duration | |||||
|---|---|---|---|---|---|
| Total (n = 1389) | ≤ 5 (n = 359) | 6–8 (n = 916) | ≥ 9 (n = 114) | P value | |
| Activity limitation due to wheezing in past yr, n (%) | |||||
| None | 902 (67.9) | 210 (60.45) | 614 (70.3) | 78 (68.6) | 0.001 |
| A little | 222 (16.7) | 56 (17.0) | 154 (17.1) | 12 (12.1) | |
| Frequent | 265 (15.4) | 93 (22.6) | 148 (12.6) | 24 (19.3) | |
| Health-related quality of life | |||||
| Days physical health was not good | 6.1 (0.4) | 8.6 (0.8) | 5.0 (0.3) | 8.1 (0.3) | 0.003 |
| Days mental health was not good | 6.1 (0.4) | 9.8 (0.7) | 4.8 (0.4) | 7.0 (0.9) | <0.001 |
| Inactive days due to health | 4.0 (0.3) | 6.7 (0.8) | 2.8 (0.3) | 6.6 (1.1) | 0.001 |
| Number of times received healthcare in past yr | 2.6 (0.0) | 2.8 (0.1) | 2.5 (0.0) | 3.0 (0.2) | 0.58 |
| Number of times received healthcare for wheezing in past yr | 0.7 (0.1) | 0.7 (0.1) | 0.6 (0.1) | 0.9 (0.2) | 0.83 |
Data is presented as mean (standard deviation), unless otherwise indicated.
Figure 1.
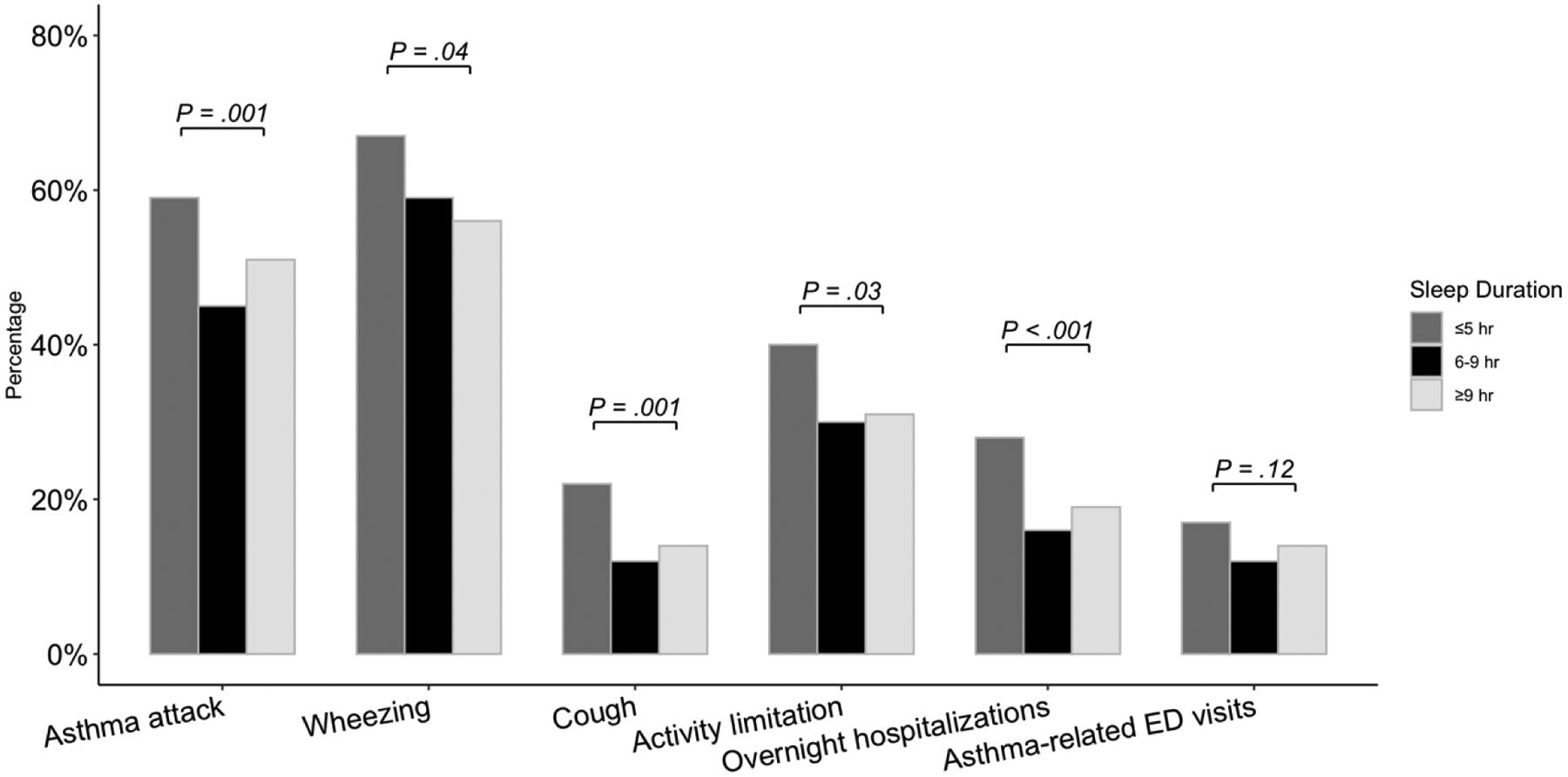
Weighted proportions of sample with asthma attack, wheezing, cough, activity limitation, overnight hospitalizations, and asthma-related emergency room visits during the past year by sleep duration.
Note: ER, emergency room.
Associations between sleep duration and patient-reported outcomes and healthcare use
Table 3 shows the results of the weighted linear and logistic regression models of the likelihood of adverse patient-reported outcomes and healthcare use among adults with asthma with short (≤ 5 hours), normal (6–8 hours), and long (≥ 9 hours) sleep duration. Individuals with short sleep duration, as compared to normal sleep duration, had a greater likelihood of an asthma attack (aOR 1.58, 95% CI 1.13–2.21), dry cough (aOR 1.95, 95% CI 1.32–2.87), and an overnight hospitalization (aOR 2.14, 95% CI 1.37–3.36) during the past year after controlling for age, sex, race, socioeconomic status, and smoking status. Short sleepers had significantly worse health-related quality of life including days of poor physical and mental health and inactive days due to poor health, and more frequent general healthcare use during the past year as compared to normal sleepers. After adjustment for the above-mentioned covariates, wheezing, activity limitation due to wheezing, emergency room visits for asthma, and frequency of healthcare use for wheezing did not differ between the short and normal sleepers. The odds for long sleepers to have a little activity limitation due to wheezing was higher when compared to normal sleepers (aOR 1.82, 95% CI 1.13–2.91). No significant differences in other patient-reported outcomes and healthcare use were observed between the long and normal sleepers.
Table 3.
Weighted multivariate regression models of sleep duration predicting patient-reported outcomes and healthcare use among adults with asthma (n = 1389)
| Multivariate Analysesa,b | |||||
|---|---|---|---|---|---|
| ≤ 5 h of sleep vs. 6–8 h of sleep | ≥ 9 h of sleep vs. 6–8 h of sleep | ||||
| β or ORc (95% CI) | P value | β or ORc (95% CI) | P value | ||
| Patient-reported outcomes | |||||
| Asthma attack in past yr | 1.58 (1.13–2.21) | 0.008 | 1.27 (0.83–1.95) | 0.26 | |
| Wheezing in past yr | 1.31 (0.91–1.90) | 0.14 | 0.88 (0.55–1.43) | 0.61 | |
| Dry cough in past yr | 1.95 (1.32–2.87) | 0.001 | 1.15 (0.56–2.36) | 0.70 | |
| Activity limitation due to wheezing in past yr | |||||
| None | 1.00 | 1.00 | |||
| A little | 1.09 (0.71–1.69) | 0.64 | 1.82 (1.13–2.91) | 0.01 | |
| Frequent | 0.67 (0.30–1.48) | 0.31 | 1.25 (0.70–2.24) | 0.44 | |
| Health-related quality of life | |||||
| Days physical health was not good | 2.70 (0.90–4.51) | 0.004 | 1.70 (−0.60–3.99) | 0.15 | |
| Days mental health was not good | 3.43 (2.07–4.79) | <0.001 | 1.62 (−0.71–3.97) | 0.16 | |
| Inactive days due to health | 2.53 (0.74–4.32) | 0.007 | 2.23 (0.00–4.46) | 0.05 | |
| Healthcare use | |||||
| Emergency room visits for asthma in past yr | 1.19 (0.79–1.79) | 0.39 | 1.16 (0.62–2.17) | 0.49 | |
| Number of times received healthcare for wheezing in past yr | 0.09 (−0.12–0.31) | 0.40 | 0.21 (−0.17–0.59) | 0.27 | |
| Number of times received general healthcare in past yr | 0.34 (0.11–0.57) | 0.005 | 0.43 (−0.01–0.86) | 0.06 | |
| Overnight hospitalizations in past yr | 2.14 (1.37–3.36) | 0.001 | 1.17 (0.62–2.22) | 0.63 | |
Analyses used linear regression (for continuous dependent variables: days physical health was not good, days mental health was not good, inactive days due to health, number of times received healthcare for wheezing, number of times received healthcare), logistic regression (for dichotomous dependent variables), or multinomial logistic regression (for categorical dependent variable).
Adjusted for age, sex, race/ethnicity, socioeconomic status, and smoking status.
β = standardized beta coefficient from linear regression; OR = odds ratio from logistic regression
DISCUSSION
This is the first study to examine the impact of sleep duration on asthma outcomes and healthcare use in a nationally representative sample of adults with asthma. We found that over one third of adults with asthma are short or long sleepers. It was hypothesized that both short and long sleep duration would be associated with worse patient-reported outcomes and greater healthcare use. In support of our hypothesis, our results indicated that short sleep duration was associated with worse patient-reported outcomes, particularly asthma symptoms and attacks, health-related quality of life, and greater general healthcare use, while long sleep duration was associated with activity limitation due to wheezing.
The current study found adults with asthma and short sleep duration are 1.5 times as likely to have an asthma attack as compared to those with normal sleep duration after controlling for age, sex, race, socioeconomic status, and smoking status. Moreover, those with short sleep duration were more likely to have dry cough and poorer health-related quality of life compared to normal sleepers. Poor sleep in adults with asthma may be contributable to a number of factors including obstructive sleep apnea, gastroesophageal reflux disease, anxiety, sex, corticosteroid use, asthma severity, and inadequate asthma control which can result in nocturnal awakenings.22
Our work is consistent with prior investigations exploring an association of asthma and sleep. After controlling for age, sex, BMI, oral corticosteroid use, gastroesophageal reflux disease, and sleep apnea, poor sleep quality remained significantly associated with worse asthma control and asthma-related quality of life.15, 16 Poor sleep in asthma may not solely be attributable to nighttime awakenings.15, 16 Sleep disturbances and sleep loss have been shown to increase inflammation and decrease lung function.23, 24 Experimental sleep deprivation in healthy adults has been found to significantly elevate pro-inflammatory cytokine levels.25,26 Short-term sleep deprivation in rodents exacerbated lung inflammation.27,29 Thus, short sleep may negatively impact asthma through decreased lung function and increased lung inflammation. More research is needed to determine mechanisms linking poor sleep and asthma outcomes.
We found that as compared to those with normal sleep duration, adults with asthma and short sleep duration were twice as likely to have an overnight hospitalization and more frequent general healthcare use in the past year, although they did not have significantly different asthma-related healthcare use. In the general adult population, poor sleep, in particular short sleep duration and insomnia, has been shown to be associated with increased healthcare utilization including emergency department visits and hospitalizations.12, 13, 29–35 In a prior study of adults with physician-confirmed asthma, those with insomnia had an increased risk for asthma-related healthcare use (i.e., unscheduled doctor visits, emergency department visits, hospital admissions, and intensive care unit admissions) in the past year.15 In addition, those with insomnia were more likely to have not well-controlled asthma compared with those without insomnia. It is important to note that asthma was self-identified in NHANES and that asthma control was not assessed, thus severity of asthma and level of asthma control could not be controlled for in the analyses and could have influenced the association between sleep duration and asthma-related healthcare use. The effect of poor sleep on healthcare utilization in adults with asthma requires further investigation.
Long sleep duration has not been extensively examined in adults with asthma. In the current study, 8% of adults with asthma reported sleeping 9 or more hours per night. Comparatively, data from a national survey of U.S. adults identified self-reported long sleep duration (≥ 9 hours per night) in approximately 8% of respondents.36 Our study found long sleep to be associated with a little activity limitation due to wheezing when compared to normal sleep. However, no differences in other patient-reported outcomes or healthcare use was found between long and normal sleepers. A recent meta-regression revealed associations between long sleep and health outcomes such as mortality, obesity, and incident cardiovascular disease; however, these associations vary depending on age and sex.10 Choi et al. reported older adults with short sleep had a greater odds of emergency room visits in the past year as compared to normal sleepers, whereas long sleepers had a greater odds of an overnight hospitalization compared to normal sleepers.13 In contrast, Paudel et al. found older women with short sleep had an increased risk of hospitalization compared to those with normal sleep duration, but no difference in odds of hospitalization between those with long and normal sleep duration.12 Further research on the effect of long sleep on asthma outcomes is warranted.
This study has several limitations. The cross-sectional nature of NHANES precludes the inference of causal relationships. Furthermore, available NHANES data does not allow for the examination of the impact of asthma symptoms on sleep duration, thus it is possible that the results could alternatively be explained by asthma symptoms interfering with sleep duration. Errors in recall on the health surveys may have affected participants’ responses. Self-report of sleep duration is less accurate as objective measures such as actigraphy or polysomnography. In addition, the findings could not be adjusted for specific asthma medication use, asthma control, or for sleep-specific factors including insomnia, sleep quality, and obstructive sleep apnea, which are possible clinical confounders in the relationship between sleep duration and patient-reported outcomes and healthcare use. Furthermore, this study did not control for other factors that could impact sleep and asthma such as chronic obstructive pulmonary disease, gastroesophageal reflux disease, nasal and sinus symptoms, and obesity. The study sample is a small representation of the large number of adults with asthma in the U.S., yet the sample selected for NHANES is representative of the U.S. adult population.
In conclusion, short sleep duration was associated with worse patient-reported outcomes, in particular more frequent asthma attacks and coughing and poorer health-related quality of life, and increased risk of overnight hospitalizations and general healthcare utilization. Long sleep was associated with some activity limitation. Further investigation into factors independent of asthma symptoms that may contribute to short and long sleep duration (e.g., insomnia, sleep quality, obstructive sleep apnea, use of sleep medications, and comorbidities like gastroesophageal reflux disease, chronic nasal and sinus symptoms, and chronic obstructive lung disease) in adults with asthma is warranted. However, evaluation for sleep disorders should be considered in adults with asthma as these conditions could potentially contribute to inadequate sleep duration and proper treatment may improve health status.
Acknowledgments
Funding Source: This work was supported by funding from the National Institutes of Health K24 NR016685.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to report.
REFERENCES
- 1.Center for Disease Control and Prevention. National Health Interview Survey (NHIS) data. https://www.cdc.gov/asthma/nhis/2018/data.htm. Published 2018. Accessed February 21, 2020.
- 2.Centers for Disease Control Prevention. Vital signs: asthma prevalence, disease characteristics, and self-management education: United States, 2001–-2009. MMWR Morb Mortal Wkly Rep. 2011;60(17):547–552. [PubMed] [Google Scholar]
- 3.Nurmagambetov T, Kuwahara R, Garbe PJ. The economic burden of asthma in the United States, 2008–2013. Ann Am Torac Soc. 2018;15(3):348–356. [DOI] [PubMed] [Google Scholar]
- 4.Akinbami OJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005–2009. Natl Health Stat Report. 2011;32:1–14. [PubMed] [Google Scholar]
- 5.Moorman JE, Akinbami LJ, Bailey C, et al. National Surveillance of Asthma: United States, 2001–2010. Vital Health Stat. 2012;35:1–57. [PubMed] [Google Scholar]
- 6.Watson N, Badr M, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;11(6):591–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dam TTL, Ewing S, Ancoli‐Israel S, et al. Association between sleep and physical function in older men: the osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2008;56(9):1665–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldman SE, Stone KL, Ancoli-Israel S, et al. Poor sleep is associated with poorer physical performance and greater functional limitations in older women. Sleep. 2007;30(10):1317–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Itani O, Jike M, Watanabe N, Kaneita Y. Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med. 2017;32:246–256. [DOI] [PubMed] [Google Scholar]
- 10.Jike M, Itani O, Watanabe N, Buysse DJ, Kaneita Y. Long sleep duration and health outcomes: A systematic review, meta-analysis and meta-regression. Sleep Med Rev. 2018;39:25–36. [DOI] [PubMed] [Google Scholar]
- 11.Stenholm S, Kronholm E, Sainio P, et al. Sleep-related factors and mobility in older men and women. J Gerontl A Biol Sci Med Sci. 2010;65(6):649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paudel ML, Taylor BC, Vo TN, et al. Sleep disturbances and risk of hospitalization and inpatient days among older women. Sleep. 2017;40(2):zsx037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi NG, DiNitto DM, Marti CN, Choi BY. Too little sleep and too much sleep among older adults: Associations with self‐reported sleep medication use, sleep quality and healthcare utilization. Geriatr Gerontol Int. 2017;17(4):545–553. [DOI] [PubMed] [Google Scholar]
- 14.Braido F, Baiardini I, Ferrando M, et al. The prevalence of sleep impairments and predictors of sleep quality among patients with asthma [published online ahead of print (January 2020)]. J Asthma. 2020;12:1–7. doi: 10.1080/02770903.2019.1711391. [DOI] [PubMed] [Google Scholar]
- 15.Luyster FS, Strollo PJ, Holguin F, et al. Association between insomnia and asthma burden in the Severe Asthma Research Program (SARP) III. Chest. 2016;150(6):1242–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luyster FS, Teodorescu M, Bleecker E, et al. Sleep quality and asthma control and quality of life in non-severe and severe asthma. Sleep Breath. 2012;16(4):1129–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanz de Burgoa V, Rejas J, Ojeda P, et al. Self-perceived sleep quality and quantity in adults with asthma: findings from the CosteAsma Study. J Investig Allergol Clinc Immunol. 2016;26(4):256–262. [DOI] [PubMed] [Google Scholar]
- 18.Yang G, Han Y-Y, Sun T, et al. Sleep duration, current asthma, and lung function in a nationwide study of US adults. Am J Respir Crit Care Med. 2019;200(7):926–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meltzer LJ, Beebe DW, Jump S, et al. Impact of sleep opportunity on asthma outcomes in adolescents. Sleep Med. 2020;65:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159(1):179–187. [DOI] [PubMed] [Google Scholar]
- 21.Lix LM, Keselman JC, Keselman H. Consequences of assumption violations revisited: A quantitative review of alternatives to the one-way analysis of variance F test. Rev Edu Res. 1996;66(4):579–619. [Google Scholar]
- 22.Kavanagh J, Jackson DJ, Kent BD. Sleep and asthma. Curr Opin Pulm Med. 2018;24(6):569–73. [DOI] [PubMed] [Google Scholar]
- 23.Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80(1):40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krouse HJ, Krouse JH. Diurnal variability of lung function and its association with sleep among patients with asthma. J Asthma. 2007;44(9):759–63. [DOI] [PubMed] [Google Scholar]
- 25.Frey DJ, Fleshner M, Wright KP. The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun. 2007;21(8):1050–1057. [DOI] [PubMed] [Google Scholar]
- 26.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166(16):1756–1762. [DOI] [PubMed] [Google Scholar]
- 27.Kim J-Y, Lee Y-D, Kim B-J, et al. Melatonin improves inflammatory cytokine profiles in lung inflammation associated with sleep deprivation. Mol Med Report. 2012;5(5):1281–1284. [DOI] [PubMed] [Google Scholar]
- 28.Nunes JOF, Apostolico JS, Andrade DAG, et al. Sleep deprivation predisposes allergic mice to neutrophilic lung inflammation. J Allergy Clin Immunol. 2018;141(3):1018–1027. [DOI] [PubMed] [Google Scholar]
- 29.Daley M, Morin CM, LeBlanc M, Grégoire J-P, Savard J, Baillargeon L. Insomnia and its relationship to health-care utilization, work absenteeism, productivity and accidents. Sleep Med. 2009;10(4):427–438. [DOI] [PubMed] [Google Scholar]
- 30.Ensrud KE, Kats AM, Schousboe JT, et al. Multidimensional sleep health and subsequent health-care costs and utilization in older women. Sleep. 2020;43(2):zsz230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayward R, Jordan KP, Croft P. Healthcare use in adults with insomnia: a longitudinal study. Br J Gen Pract. 2010;60(574):334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaufmann CN, Canham SL, Mojtabai R, et al. Insomnia and health services utilization in middle-aged and older adults: results from the Health and Retirement Study. J Gerontol A Biol Sci Med Sci. 2013;68(12):1512–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wickwire EM, Tom SE, Scharf SM, Vadlamani A, Bulatao IG, Albrecht JS. Untreated insomnia increases all-cause health care utilization and costs among Medicare beneficiaries. Sleep. 2019;42(4):zsz007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H-S, Mai Y-B, Li W-D, et al. Sleep quality and health service utilization in Chinese general population: A cross-sectional study in Dongguan, China. Sleep Med. 2016;27–28:9–14. [DOI] [PubMed] [Google Scholar]
- 35.Novak M, Mucsi I, Shapiro CM, Rethelyi J, Kopp MS. Increased utilization of health services by insomniacs—an epidemiological perspective. J Psychosom Res. 2004;56(5):527–536. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Wheaton AG, Chapman DP, et al. Prevalence of healthy sleep duration among adults—United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65(6):137–141. [DOI] [PubMed] [Google Scholar]


