Abstract
Purpose:
Urologists face a dilemma when a lesion identified on multiparametric magnetic resonance imaging is benign on image guided fusion biopsy. We investigated the detection rate of prostate cancer on repeat fusion biopsy in multiparametric magnetic resonance imaging lesions initially found to be pathologically benign on fusion biopsy.
Materials and Methods:
We reviewed the records of all patients from 2007 to 2014 who underwent multiparametric magnetic resonance imaging and image guided fusion biopsy. We identified men who underwent rebiopsy of the same discrete lesion after initial fusion biopsy results were benign. Data were documented on a per lesion basis. We manually reviewed UroNav system (Invivo, Gainesville, Florida) needle tracking to verify accurate image registration. Multivariate analysis was used to identify clinical and imaging factors predictive of prostate cancer detection at repeat fusion biopsy.
Results:
A total of 131 unique lesions were rebiopsied in 90 patients. Of these 131 resampled lesions 21 (16%) showed prostate cancer, which in 13 (61.9%) was Gleason 3 + 3. On multivariate analysis only lesion growth on repeat multiparametric magnetic resonance imaging was significantly associated with prostate cancer detection at repeat biopsy (HR 3.274, 95% CI 1.205–8.896, p = 0.02).
Conclusions:
Pathologically benign multiparametric magnetic resonance imaging lesions on initial image guided fusion biopsy are rarely found to harbor clinically significant prostate cancer on repeat biopsy. When prostate cancer
was identified, most disease was low risk. An increase in lesion diameter was an independent predictor of prostate cancer detection. While these data are retrospective, they may provide some confidence in the reliability of negative initial image guided fusion biopsies despite a positive multiparametric magnetic resonance imaging finding.
Keywords: prostatic neoplasms; magnetic resonance imaging; ultrasound, high-intensity focused, transrectal; image-guided biopsy; watchful waiting
The addition of mpMRI of the prostate and subsequent FB, which registers mpMRI to TRUS at biopsy, has been shown to improve the detection rate in patients with intermediate and high risk PCa while reducing the detection rate of low risk PCa.1–3 FB yields a PCa detection rate approaching 60% as validated across multiple studies compared to historical ranges of 27% to 40% for systematic 12-core biopsies.4,5 To our knowledge the outcome of the remaining 40% of FB samples that initially demonstrate benign pathology is unknown. Thus, urologists are faced with the dilemma of how to manage persistently identified lesions on mpMRI that were previously found to be benign.
A previous group at our institution investigated the PCa detection rate at repeat FB in men initially shown to have completely benign pathology on systematic 12-core biopsy and FB.6 We sought to investigate the PCa detection rate in initially benign lesions with time. Furthermore, in prior studies repeat mpMRI and FB have demonstrated potential benefit for treating patients on AS for confirmatory and protocol surveillance sampling.7,8
Our aim was to investigate the prostate cancer detection rate of repeat FB for lesions initially found to be pathologically benign and determine clinical or imaging factors associated with PCa in these lesions.
MATERIALS AND METHODS
Patient Cohort
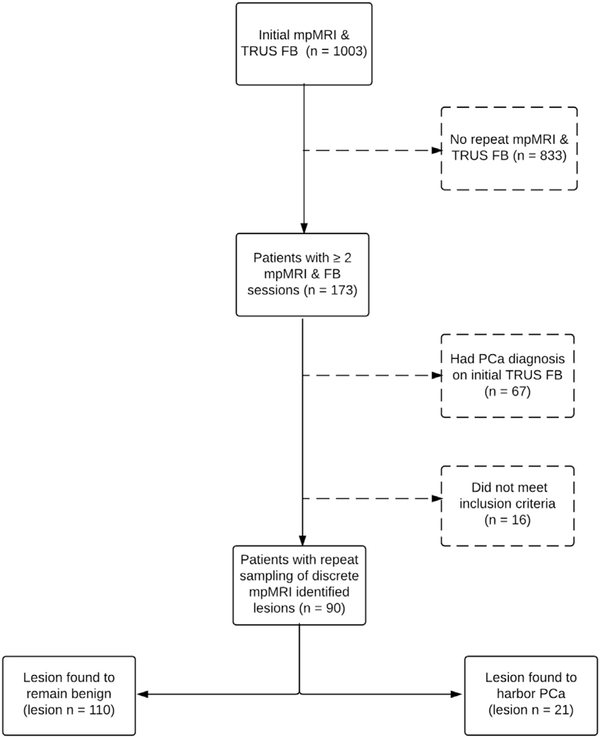
We retrospectively reviewed the records of all 1,003 patients who presented to NIH (National Institutes of Health) for mpMRI and FB between August 2007 and February 2014 (fig. 1). These patients had been enrolled in an institutional review board approved prospective trial of mpMRI and FB (ClinicalTrials.gov Identifier NCT00102544). This cohort was evaluated for evidence of rebiopsy of specific lesions identified on initial mpMRI. Some patients with multiple repeat FB sessions were excluded from our cohort, including 67 in whom PCa was diagnosed in the mpMRI lesion at the initial session, 10 with no repeat biopsy of the same anatomical space, 4 in whom mpMRI was not repeated and 2 with biopsy that was judged to be technically inadequate upon review of the prior image registration or biopsy tract. A number of men in the cohort of 10 who were excluded from analysis due to a lack of rebiopsy of initially benign lesions had multiple FB sessions. They were excluded for reasons such as a lesion with low clinical suspicion based on imaging findings or a lesion that was not found on repeat mpMRI.
Figure 1.
Data analysis inclusion and exclusion criteria for repeat mpMRI and FB sessions.
Clinically significant PCa was defined as 4 + 3 or greater.2,9 All pathological specimens were reviewed by a single genitourinary pathologist (MJM).
Imaging and Interpretation
All patients underwent diagnostic 3.0 Tesla mpMRI using an Achieva device (Philips Healthcare, Andover, Massachusetts). A BPX-30 endorectal coil (Medrad®) was placed along with a 16-channel SENSE cardiac surface coil (Philips Healthcare) positioned over the pelvis as described previously.10 Scans were reviewed by 2 genitourinary radiologists with a combined more than 20 years of experience with prostate MRI (BT and PLC) in consensus to identify lesions and assign suspicion scores based on previously published criteria.1
Biopsy Methodology
Patients who harbored lesions suspicious for PCa on mpMRI were enrolled in an ongoing prospective clinical trial to undergo FB followed by systematic 12-core biopsy using the UroNav FB system as described previously.2 Briefly, suspicious lesions were marked and segmented on T2 images. The software registered mpMRI with annotated lesions to TRUS and the same lesions were shown on the real-time TRUS image. All FB targeted lesions were sampled in the axial and sagittal planes.11 At our practice sampling is not varied by the size of the target. The quality of registration between mpMRI and TRUS as well as biopsy needle trajectory was recorded. Because prior iterations of the software did not have needle tracking capability, it did not allow for re-review of 3 of the 21 imaged lesions.
Lesion Selection and Study Design
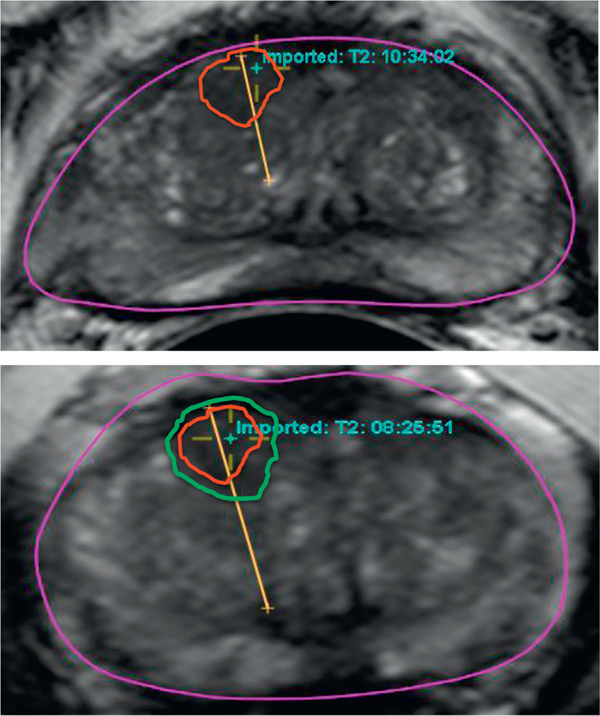
Validation that the same lesion was biopsied on initial and repeat FB was done by reviewing the description of the core location on the pathology reports as well as by correlating the location with mpMRI findings. MRI findings, including lesion diameter measured on T2, diffusion weighted imaging and dynamic contrast enhancement, were collected on a per lesion basis. For lesions found to have PCa at repeat biopsy we recalled saved data from the original FB session archived in the UroNav platform.12 Each fusion biopsy needle tracking trajectory, registration quality and location information were reviewed independently by a multidisciplinary group of attending physicians from urology, radiology and interventional radiology to assure a technically adequate biopsy that resampled the same lesion (fig. 2). Two lesions were excluded for this reason.
Figure 2.
Lesions and needle tracking of patient initially biopsied in 2013 and found to have pathologically benign prostate tissue (red circle). He was placed on AS and rebiopsied in 2014 in same anatomical space due to increased lesion size (green circle). Lesion harbored Gleason 4 + 3 disease.
Clinical, imaging and pathological variables collected included age, race, initial PSA, initial lesion size measured on T2 MRI, maximum initial GS for the total organ, initial systematic 12-core biopsy pathology, clinical stage, lesion location, PSA at repeat biopsy, and time to followup mpMRI and to followup biopsy. All data were defined on a per lesion basis. The first biopsy session that sampled a lesion was designated as the initial session. The repeat session was defined as the most recent biopsy session. If a patient underwent more than 2 FBs, the interval FB sessions were also examined for lesion imaging or pathological continuity compared to the initial and repeat sessions.
Statistical Methods
Statistical analysis was performed using Prism®, version 6.0 for Mac® and IBM® SPSS®, version 21.0. The Fisher exact and Mann-Whitney U tests were run to compare proportions and continuous variables, respectively. Univariate and multivariate logistic regression analysis was done to identify predictors of positive repeat biopsy. We included plausible indicators for PCa detection in our multivariate model, including an increase in lesion size, a PSA increase or a change in mpMRI suspicion score. Statistical significance was considered at p <0.05.
RESULTS
Of 1,003 patients 173 presented for repeat mpMRI and FB (fig. 1). Of these 173 men 90 harbored a total of 131 initially benign lesions that were rebiopsied. Of the 90 men 69 were enrolled in AS and 21 were rebiopsied secondary to persistently elevated PSA. Median age of the total cohort was 60.0 years and median initial PSA was 4.99 ng/ml. Digital rectal examination was negative in 89% of all patients. Median time to repeat imaging in the entire cohort was 16.3 months and the median time to repeat FB was 19.2 months.
Table 1 lists patient, imaging and biopsy characteristics by PCa detection on repeat FB. There was no difference in clinical data, including racial distribution (p = 0.42) or imaging characteristics such as initial mpMRI suspicion score (p = 0.82) and anterior lesion location (p = 0.28). In terms of initial biopsy status 33.3% of patients with a positive repeat biopsy had a completely negative initial biopsy. A PCa diagnosis in other lesions on initial FB was not associated with a positive repeat FB of an initially negative FB lesion (p = 0.23).
Table 1.
Patient, imaging and biopsy characteristics by PCa diagnosis at repeat FB
| PCa on Rebiopsy |
|||
|---|---|---|---|
| No | Yes | p Value | |
| No. lesions/total No. (%) | 110/131 (84) | 21/131 (16) | – |
| Median age (range) | 60.0 (45.0–78.0) | 62.0 (47.0–74.0) | 0.451 |
| Median ng/ml prebiopsy PSA (range) | 5.12 (1.23–24.80) | 4.97 (1.67–13.22) | 0.205 |
| Median cm initial lesion diameter (range) | 0.80 (0.30–3.0) | 0.90 (0.30–2.1) | 0.232 |
| No. max initial GS/total No. (%): | 0.121 | ||
| Benign | 55/110 (50) | 7/21 (33.3) | |
| 3 + 3 | 42/110 (38.2) | 13/21 (61.9) | |
| 3 + 4 | 13/110 (11.8) | 1/21 (4.8) | |
| No. anterior lesions/total No. (%) | 31/131 (28.2) | 4/21 (19.0) | 0.282 |
| No. initial MRI suspicion score/total No. (%): | 0.821 | ||
| Low | 35/110 (31.8) | 7/21 (33.3) | |
| Moderate | 73/110 (66.4) | 14/21 (66.7) | |
| High | 2/110 (1.8) | 0/21 (0) | |
| Median ng/ml repeat biopsy PSA (range) | 5.31 (0.83–36.62) | 4.82 (1.28–21.35) | 0.422 |
| Median days to repeat (range): | |||
| mpMRI | 497.5 (240–1,694) | 475.0 (364–2,742) | 0.537 |
| Biopsy | 530.0 (108–2,581) | 517.0 (364–2,418) | 0.900 |
| Median cm final lesion size (range) | 0.750 (0.30–3.10) | 1.10 (0.30–2.30) | 0.060 |
| Mean ± SD lesion size increase (mm) | −0.316 ± 1.993 | 0.710 ± 2.444 | 0.104 |
| Mean ± SD growth rate (mm/yr) | −0.203 ± 1.082 | 0.143 ± 1.573 | 0.143 |
| Mean ± SD PSA increase (ng/ml) | 0.25 ± 3.53 | 0.45 ± 2.58 | 0.115 |
Systematic 12-Core Biopsy
At repeat FB 121 lesions remained benign, including 64 of 110 with benign initial systematic 12-core biopsy findings, and 36 and 10 with 3 + 3 and 3 + 4 systematic 12-core biopsy findings, respectively. At repeat FB PCa was found in 21 lesions, including 7 with initially benign systematic 12-core biopsy, 13 with GS 3 + 3 and 1 with GS 3 + 4.
Repeat Fusion Biopsy
Of 131 lesions 21 (16.0%) showed PCa at repeat biopsy after initial benign histology and 110 (84.0%) remained negative. In the 21 lesions with PCa 13 (61.9%) were GS 3 + 3. Two lesions (9.52% or 1.52% overall) harbored clinically significant PCa, 1 was GS 4 + 3 and 1 was GS 4 + 4. The remaining 6 lesions were GS 3+4. The median size of lesions found to have PCa at repeat biopsy was 1.1 cm. The mean rate of growth was 0.143 mm per year in lesions found to harbor PCa as opposed to a mean negative growth rate for lesions with pathology findings that remained benign on repeat FB (table 1).
Univariate and multivariate logistic regression analysis was done to identify predictors of positive repeat biopsy (table 2). On multivariate analysis an increase in lesion diameter on repeat mpMRI was a significant predictor of PCa detection at repeat biopsy (OR 3.274, 95% CI 1.205–8.896, p = 0.02). PSA increase at repeat FB and an increased mpMRI suspicion score were not significant.
Table 2.
Association of imaging and clinical noninvasive predictive parameters with PCa detection at repeat FB
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| PSA increase | 1.511 (0.58–3.935) | 0.398 | 1.397 (0.522–3.341) | 0.522 |
| Upgraded MRI suspicion score/lesion | 1.667 (0.417–6.654) | 0.470 | 1.728 (0.412–7.257) | 0.412 |
| Lesion diameter increase | 3.375 (1.253–9.090) | 0.016 | 3.274 (1.205–8.896) | 0.020 |
DISCUSSION
As mpMRI guided fusion biopsy becomes more established in the diagnosis of PCa13,14 the significance of a negative biopsy with a positive mpMRI has become more closely scrutinized. It was previously reported that there is a lower estimated PCa detection rate per patient when FB is used for repeat biopsy.6 This initial report of patients with a completely negative initial FB and systematic 12-core biopsy sessions identified that 14 of 34 (41%) had PCa at repeat FB, which was lower than the initial PCa detection rate of 55.3%.
In the current study we sought to evaluate on a lesion level the evolution of mpMRI identified lesions initially found to be pathologically benign in patients rebiopsied a median of 19.2 months later. We also sought to identify potential predictive factors associated with progression that would inform the need for repeat sampling. Furthermore, small lesions (less than 0.7 cm) initially detected on imaging tend to be benign or represent low risk disease15 and it was shown that the overall risk of PCa detection after a totally negative (but nonmpMRI targeted) biopsy session was low at 7.0%.16 Our finding that 16% of rebiopsied lesions were found to harbor PCa supports a low false-negative rate for initial negative FBs. Most of these lesions were Gleason 3 + 3 and only 1.5% of all lesions harbored clinically significant PCa upon rebiopsy.
Our data provide some confidence that most negative initial FBs represent benign or low grade prostate cancer on followup, although continued suspicion is always warranted. The presence of low risk cancer cannot be ignored but as a shift in the treatment paradigm of low volume, low/intermediate risk cancer continues to evolve, the focus is becoming the identification of patients with clinically significant disease.17 There are a number of indications for repeat FB, including the protocol FB during AS, continued suspicion of PCa in the face of rising PSA or a high suspicion score on mpMRI. Other groups have investigated the natural history of initially benign cancer suspicious regions on mpMRI and shown results similar to ours but their lack of followup pathological data limit the clinical usefulness of their findings.18 We determined that if a lesion is found to have benign pathology after initial FB, there is a low likelihood of finding pathologically proven, clinically actionable disease if repeat FB of the same lesion is done.
We examined a number of factors that might predict conversion of a lesion from benign to malignant, including an increase in PSA, an increase in mpMRI suspicion score and an increase in lesion size. These parameters are readily obtained during routine care and would plausibly reflect biological progression if present. Among these factors PSA has previously been shown to be an independent predictor of PCa detection after an initially negative, nonmpMRI guided biopsy session.19 Based on univariate and multivariate analysis only an increase in lesion size on mpMRI was predictive of detecting PCa on repeat FB. This discrepant finding may be due to the fact that in our series repeat FB was often prompted by a persistent lesion on imaging as opposed to a large PSA increase.
These findings are congruent with and add to prior studies in an AS population regarding potentially extending the followup biopsy interval to 2 years while using mpMRI in addition to PSA to monitor for disease progression,20 given reports that FB combined with systematic 12-core biopsy matches to final pathology in 81% of cases.21 In a nonmpMRI AS cohort after a GS upgrade cases that continued to be followed were unlikely to be upgraded subsequently and few cases were upgraded upon the final pathology evaluation.22 If such outcomes could be replicated with mpMRI substituting for multiple biopsies, it would be of great use for managing PCa.
To address potential confounders we evaluated whether the anatomical location of the lesion influenced the rate of conversion of FB from negative to positive. There is some rationale for this since anterior lesions may represent a location in the prostate that is more challenging to sample and, thus, prone to registration or sampling error.23 However, we were unable to find such an association. Additionally, time to rebiopsy was comparable between men who did and did not have PCa at repeat FB. A main concern was whether our targets that were found to have PCa on resampling simply represented registration or sampling error in the initial biopsy. We manually reviewed all needle trackings that we could obtain. The available 18 of 21 lesions were deemed by independent review to sample the same target adequately at both sessions.
One limitation of this study is our sample size. Data on only 131 initially negative lesions were available, making generalizability difficult. Additionally, our study was subject to selection bias as not every patient in our total cohort was resampled and repeat biopsies were based on the mentioned criteria. Also, this analysis only applies to lesions that can be visualized on mpMRI and, thus, it offers no guidance for resampling in the context of systematic 12-core biopsy. Furthermore, we do not know whether detecting PCa at repeat biopsy illustrates the limitations of this technology or whether we are witnessing biological transformation and progression. We intend to address this question by molecular biological analysis of samples obtained from these patients. Finally, the NIH suspicion score was used in this cohort, although it is not widely used. However, it was validated in prior studies and prospective reports of PI-RADS (Prostate Imaging Reporting and Data System), version 2 are being collected and evaluated at our institution.24
CONCLUSIONS
FB negative mpMRI lesions are rarely found to be PCa on repeat biopsy. Even when PCa is identified on repeat FB, the majority represent low risk disease. Any increase in lesion size measured on mpMRI should be a trigger to consider repeating a biopsy of that lesion as there is an association between an increase in lesion size and conversion of FB from negative to positive. While understanding that this is a retrospective study in a limited cohort, these data may provide some confidence in the reliability of negative initial FBs despite a positive mpMRI finding.
Acknowledgments
Supported by the Intramural Research Program of NIH, National Cancer Institute, Center for Cancer Research, Center for Interventional Oncology, Philips Healthcare, NIH Medical Research Scholars Program, a public-private partnership supported jointly by NIH and contributions to Foundation for the NIH from Pfizer Inc., The Doris Duke Charitable Foundation, The Alexandria Real Estate Equities, Inc., Mr. and Mrs. Joel S. Marcus, the Howard Hughes Medical Institute and other private donors (http://fnih.org/work/education-training-0/medical-research-scholars-program).
Abbreviations and Acronyms
- AS
active surveillance
- FB
image guided fusion biopsy
- GS
Gleason score
- mpMRI
multiparametric MRI
- MRI
magnetic resonance imaging
- PCa
prostate cancer
- PSA
prostate specific antigen
- TRUS
transrectal ultrasound
Footnotes
The corresponding author certifies that, when applicable, a statement(s) has been included in the manuscript documenting institutional review board, ethics committee or ethical review board study approval; principles of Helsinki Declaration were followed in lieu of formal ethics committee approval; institutional animal care and use committee approval; all human subjects provided written informed consent with guarantees of confidentiality; IRB approved protocol number; animal approved project number.
No direct or indirect commercial incentive associated with publishing this article.
Contributor Information
Raju Chelluri, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Amichai Kilchevsky, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Arvin K. George, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
Abhinav Sidana, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Thomas P. Frye, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
Daniel Su, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Michele Fascelli, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Richard Ho, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Steven F. Abboud, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
Baris Turkbey, Molecular Imaging Program, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Maria J. Merino, Laboratory of Pathology, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
Peter L. Choyke, Molecular Imaging Program, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
Bradford J. Wood, Center for Interventional Oncology, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
Peter A. Pinto, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
REFERENCES
- 1.Siddiqui MM, Rais-Bahrami S, Turkbey B et al. : Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015; 313: 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siddiqui MM, Rais-Bahrami S, Truong H et al. : Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol 2013; 64: 713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rastinehad AR, Turkbey B, Salami SS et al. : Improving detection of clinically significant prostate cancer: magnetic resonance imaging/ transrectal ultrasound fusion guided prostate biopsy. J Urol 2014; 191: 1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinto PA, Chung PH, Rastinehad AR et al. : Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol 2011; 186: 1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hambrock T, Somford DM, Hoeks C et al. : Magnetic resonance imaging guided prostate biopsy in men with repeat negative biopsies and increased prostate specific antigen. J Urol 2010; 183: 520. [DOI] [PubMed] [Google Scholar]
- 6.Hong CW, Walton-Diaz A, Rais-Bahrami S et al. : Imaging and pathology findings after an initial negative MRI-US fusion-guided and 12-core extended sextant prostate biopsy session. Diagn Interv Radiol 2014; 20: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walton Diaz A, Shakir NA, George AK et al. : Use of serial multiparametric magnetic resonance imaging in the management of patients with prostate cancer on active surveillance. Urol Oncol 2015; 33: 202.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okoro C, George AK, Siddiqui MM et al. : Magnetic resonance imaging/transrectal ultrasonography fusion prostate biopsy significantly outperforms systematic 12-core biopsy for prediction of total magnetic resonance imaging tumor volume in active surveillance patients. J Endourol 2015; 29: 1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore CM, Kasivisvanathan V, Eggener S et al. : Standards of reporting for MRI-targeted biopsy studies (START) of the prostate: recommendations from an International Working Group. START Consortium. Eur Urol 2013; 54: 544. [DOI] [PubMed] [Google Scholar]
- 10.Sankineni S, George AK, Brown AM et al. : Posterior subcapsular prostate cancer: identification with mpMRI and MRI/TRUS fusion-guided biopsy. Abdom Imaging 2015; 40: 2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong CW, Rais-Bahrami S, Walton-Diaz A et al. : Comparison of magnetic resonance imaging and ultrasound (MRI-US) fusion-guided prostate biopsies obtained from axial and sagittal approaches. BJU Int 2015; 115: 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raskolnikov D, Rais-Bahrami S, George AK et al. : The role of image guided biopsy targeting in patients with atypical small acinar proliferation. J Urol 2015; 193: 473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frye TP, Pinto PA and George AK: Optimizing patient population for MP-MRI and fusion biopsy for prostate cancer detection. Curr Urol Rep 2015; 16: 50. [DOI] [PubMed] [Google Scholar]
- 14.George AK, Pinto PA and Rais-Bahrami S: Multiparametric MRI in the PSA screening era. Biomed Res Int 2014; 2014: 465816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rais-Bahrami S, Turkbey B, Rastinehad AR et al. : Natural history of small index lesions suspicious for prostate cancer on multiparametric MRI: recommendations for interval imaging follow-up. Diagn Interv Radiol 2014; 20: 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ploussard G, Nicolaiew N, Marchand C et al. : Risk of repeat biopsy and prostate cancer detection after an initial extended negative biopsy: longitudinal follow-up from a prospective trial. BJU Int 2013; 111: 988. [DOI] [PubMed] [Google Scholar]
- 17.Eggener SE, Badani K, Barocas DA et al. : Gleason 6 prostate cancer: translating biology into population health. J Urol 2015; 194: 626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bryk DJ, Llukani E, Huang WC et al. : Natural history of pathologically benign cancer suspicious regions on multiparametric magnetic resonance imaging following targeted biopsy. J Urol 2015; 194: 1234. [DOI] [PubMed] [Google Scholar]
- 19.Gann PH, Fought A, Deaton R et al. : Risk factors for prostate cancer detection after a negative biopsy: a novel multivariable longitudinal approach. J Clin Oncol 2010; 28: 1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fascelli M, George AK, Frye TP et al. : The role of MRI in active surveillance for prostate cancer. Curr Urol Rep 2015; 16: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le JD, Stephenson S, Brugger M et al. : Magnetic resonance imaging-ultrasound fusion biopsy for prediction of final prostate pathology. J Urol 2014; 192: 1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hussein AA, Welty CJ, Ameli N et al. : Untreated Gleason grade progression on serial biopsies during prostate cancer active surveillance: clinical course and pathological outcomes. J Urol 2015; 194: 85. [DOI] [PubMed] [Google Scholar]
- 23.Ouzzane A, Puech P, Lemaitre L et al. : Combined multiparametric MRI and targeted biopsies improve anterior prostate cancer detection, staging, and grading. Urology 2011; 78: 1356. [DOI] [PubMed] [Google Scholar]
- 24.Muller BG, Shih JH, Sankineni S et al. : Prostate cancer: interobserver agreement and accuracy with the revised prostate imaging reporting and data system at multiparametric MR imaging. Radiology 2015; 277: 741. [DOI] [PMC free article] [PubMed] [Google Scholar]