Abstract
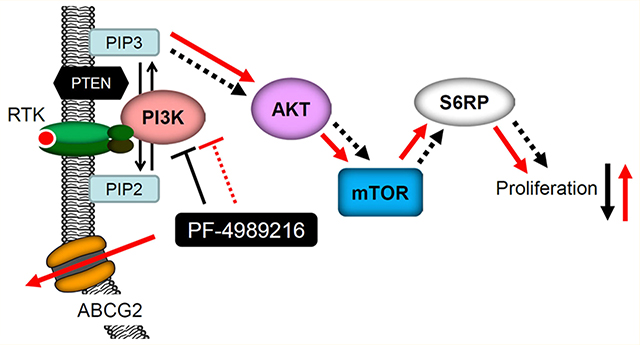
Deregulated activation of phosphoinositide 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway is frequently found in human cancers, which plays a key role in promoting cancer proliferation and resistance to anticancer therapies. Therefore, developing inhibitors targeting key components of the PI3K/Akt/mTOR signaling pathway has great clinical significance. PF-4989216 is a novel, orally available small-molecule drug that was developed to selectively inhibit the PI3K/Akt/mTOR signaling pathway and subsequent cancer cell proliferation. PF-4989216 exhibited potent and selective inhibition against PI3K kinase activity in preclinical small-cell lung cancer (SCLC) models, and was especially effective against the proliferation of SCLCs harboring PIK3CA mutation. Unfortunately, in addition to innate resistance mechanisms, drug extrusion by the efflux pumps may also contribute to the development of acquired resistance to PI3K inhibitors in cancer cells. The overexpression of ATP-binding cassette (ABC) drug transporters ABCB1 and ABCG2 is one of the most common mechanisms for reducing intracellular drug concentration and developing multidrug resistance, which remains a substantial challenge to the effective treatment of cancer. In this study, we report the discovery of ABCG2 overexpression as a mechanism of resistance to PI3K inhibitor PF-4989216 in human cancer cells. We demonstrated that the inhibition of Akt and downstream S6RP phosphorylation by PF-4989216 were significantly reduced in ABCG2-overexpressing human cancer cells. Moreover, overexpression of ABCG2 in various cancer cell lines confers significant resistance to PF-4989216, which can be reversed by an inhibitor or competitive substrate of ABCG2, indicating that ABCG2-mediated transport alone can sufficiently reduce the intracellular concentration of PF-4989216.
Keywords: multidrug resistance, ABCG2, PI3K, PF-4989216
Graphical Abstract
INTRODUCTION
The phosphoinositide 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) signaling pathway has a central role in regulating cell proliferation and survival. Constitutive activation of the PI3K/Akt/mTOR (PAM) pathway is common in various cancer types through amplification or mutations of receptor tyrosine kinases, amplification of PI3K, PIK3CA mutations, overexpression of Akt, PTEN mutation/inactivation, or KRAS mutation.1 Consequently, key signaling proteins in this pathway present attractive targets for cancer therapy. Preclinical development and clinical trials of many novel PI3K inhibitors, including pan-PI3K inhibitors and isoform-selective PI3K inhibitors, are currently ongoing.2 Notably, the U.S. Food and Drug Administration (FDA) approved idelalisib, a PI3K inhibitor, to treat patients with relapsed follicular B-cell non-Hodgkin lymphoma, relapsed small lymphocytic lymphoma, or chronic lymphocytic leukemia.3 Moreover, considering that the activation of the PAM pathway is often associated with chemotherapeutic resistance in various cancers, the use of potent PI3K inhibitors is therefore of importance when administered in combination with conventional cytotoxic chemotherapeutic agents.4,5
Cancer cells often develop multidrug resistance (MDR) after chronic treatment with conventional chemotherapeutic agents.6 MDR cancer cells are insensitive to multiple structurally and mechanically unrelated anticancer drugs, which frequently lead to failure in chemotherapy and cancer relapse.7,8 The overexpression of ATP-binding cassette (ABC) transporters is one of the many mechanisms that are known to contribute to the development of MDR in cancer cells.7,8 In particular, MDR mediated by the overexpression of ABCB1 (P-glycoprotein/MDR1) or ABCG2 (BCRP, MXR) is often considered to be a major obstacle in cancer chemotherapy.9 Structurally, ABCB1 protein is composed of two transmembrane domains and two nucleotide-binding domains, whereas ABCG2 protein is half transporter composed of one transmembrane domain and one nucleotide-binding domains, and functions as a homodimer or oligomer in cancer cells.10 Both transporters are capable of transporting and conferring resistance to a majority of conventional anticancer drugs as well as tyrosine kinase inhibitors.11,12 Importantly, ABCG2 has been show to transport a range of protein kinase inhibitors, including gefitinib, sorafenib, sunitinib, imatinib, vemurafenib, and CUDC-907,13–22 and is linked to drug resistance in patients with acute lymphocytic leukemia (ALL) and acute myelogenous leukemia (AML).23–25 Both ABCB1 and ABCG2 utilize energy derived from ATP hydrolysis to actively efflux structurally diverse anticancer agents out of cancer cells, which lead to reduced intracellular drug concentration and cytotoxicity, rendering chemotherapy ineffective.9 Furthermore, ABCB1 and ABCG2 have an important endogenous protective role, limiting xenobiotics from crossing the intestinal walls, liver canalicular membrane, and the blood-brain barrier (BBB).9,12 Therefore, their transport function can significantly alter the adsorption, distribution, metabolism, elimination, and toxicity of most anticancer drugs.26
PF-4989216 is a novel, orally available small molecule PI3K inhibitor that demonstrated potent and selective inhibition against PI3K kinase activity.27 PF-4989216 has been subsequently tested in preclinical small-cell lung cancer (SCLC) models and showed promising antitumor activity against SCLCs harboring PIK3CA mutation, one of the most commonly mutated oncogenes in human cancer.28 Many small-molecule PI3K inhibitors, including PF-4989216, have shown promising preclinical activity against human cancers, both in vitro and in vivo. However, the emergence of drug resistance is likely to limit their clinical use in the future. Although numerous resistance mechanisms have already been discovered to negatively affect the activity of PI3K inhibitors,29 such as the presence of extensive cross-talk between the PAM pathway and other signaling pathways,5 drug extrusion by the efflux pumps may also contribute to the development of acquired resistance to PI3K inhibitors in cancer cells. Considering that the overexpression of ABCB1 and/or ABCG2 are among the most common mechanisms for developing drug resistance in cancer cells,30 we decided to examine the impact of ABCB1 and ABCG2 on the effectiveness of PF-4989216 in human cancer cells.
In this study, we discovered that the overexpression of ABCG2 significantly reduced the ability of PF-4989216 to inhibit the phosphorylation of PI3K downstream molecules and subsequent cancer cell proliferation. Moreover, the activity and cytotoxicity of PF-4989216 in ABCG2-overexpressing cells were fully restored in the presence of an inhibitor or a competitive substrate of ABCG2. In summary, we provide experimental evidence that the drug efflux function of ABCG2 can contribute significantly to the development of acquired resistance to PF-4989216 in cancer cells, and there is an urgent need for developing a drug combination strategy to overcome multidrug resistance in cancer therapy.
EXPERIMENTAL SECTION
Chemicals and Cell cultures.
Dulbecco’s Modified Eagle’s medium (DMEM), RPMI medium, fetal calf serum (FCS), phosphate-buffered saline (PBS), trypsin-EDTA, penicillin, and streptomycin were purchased from Gibco, Invitrogen (CA, USA). PF-4989216 was purchased from Selleckchem (Houston, TX, USA). [125I]-Iodoarylazidoprazosin (IAAP) (2200 Ci/mmol) was purchased from PerkinElmer Life Sciences. All other chemicals were purchase from Sigma-Aldrich (St. Louis, MO, USA). OVCAR-8, NCI-ADR-RES, NIH3T3, NIH3T3-G185, S1, and S1-M1–80 cells were cultured in RPMI-1640 (Gibco, Invitrogen), supplemented with 10% FCS, 2 mM L-glutamine, and 100 units of penicillin/streptomycin/mL. H460, H460-MX20, MCF-7, MCF7-FLV1000, MCF7-AdVp3000, KB-3–1, KB-V-1, pcDNA3.1-HEK293, ABCB1-transected MDR19-HEK293, and ABCG2-transfected R482-HEK293 were cultured in DMEM supplemented with 10% FCS, 2 mM L-glutamine, 100 units of penicillin/streptomycin/mL. S1-M1–80 cells were cultured in RPMI-1640 with addition of 80 μM mitoxantrone,31 HEK293 and HEK293 transfected lines were maintained in DMEM with addition of 2 mg/mL G418,31 KB-V-1 cells were cultured in DMEM with addition of 1 mg/mL vinblastine,32 MCF7-FLV1000 cells were cultured in DMEM with addition of 1 μg/mL of flavopiridol, whereas MCF7-AdVp3000 cells were cultured DMEM with addition of 3 μg/mL doxorubicin and 5 μg/mL verapamil.33 All cell lines were maintained at 37 °C in 5% CO2 humidified air and placed in drug-free medium 7 days prior to assay.
Cytotoxicity Assay.
MTT and CCK-8 assays were performed to determine the general sensitivities of cells to the tested drugs according to the method described by Ishiyama et al.34 For the reversal of cytotoxicity assays, PF-4989216 or Ko143 or lapatinib at a nontoxic concentration was added into the cytotoxicity assay, and the extent of reversal was then calculated as described previously.35
Immunoblotting.
Antibodies antiphospho-Akt (Thr308), antiphospho-Akt (Ser473), antitotal-Akt, antiphospho-S6RP, antitotal-S6RP, BXP-21, and anti-α-tubulin were used to detect phosphorylated Akt at Thr308, phosphorylated Akt at Ser473, total Akt, phosphorylated S6 ribosomal protein, total S6 ribosomal protein, ABCG2, and tubulin, which was used as positive control for Western blotting. Horseradish peroxidase-conjugated goat antimouse IgG and antirabbit IgG were used as secondary antibodies. Signals were detected as described previously.20,28,31,36
Fluorescent Drug Accumulation Assay.
The accumulation of fluorescent dyes in cancer cells were determined using a FACSort flow cytometer equipped with Cell Quest software (Becton-Dickinson) as described previously.37 Briefly, cells were first trypsinized and resuspended in Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 5% FCS, then pheophorbide A (PhA) was added to 3 × 105 cells in 4 mL of IMDM in the absence or presence of PF-4989216 or Ko143 to determine ABCG2-mediated PhA efflux according to the method described by Gribar et al.38
ATPase Assay and Photoaffinity Labeling of ABCG2 with [125I]IAAP.
Vanadate (Vi)-sensitive ABCG2 ATPase activity in High-Five cell crude membranes was measured by end point Pi assay as described previously.39 To perform photoaffinity labeling assays, membrane vesicles (500 μg protein/mL) made from MCF7-FLV1000 cells expressing ABCG2 were prepared as described previously.39 Membranes were first incubated with PF-4989216 for 10 min at room temperature in 50 mM Tris-HCl and 150 mM NaCl, pH 7.5 before 3–6 nmol/L [125I]IAAP (2200 Ci/mmole) was added. The samples were then processed as described previously.31
Statistical Analysis.
All experiments were performed independent at least three times. Results were presented as mean ± standard error of the mean (S.E.M), and the IC50 values were calculated as mean ± standard deviation (SD). Two-sided Student’s t test method was used to determined the differences between any mean values, and results were considered statistically significant at P < 0.05.
RESULTS
ABCG2-Overexpressing Cells Are Resistant to PF-4989216.
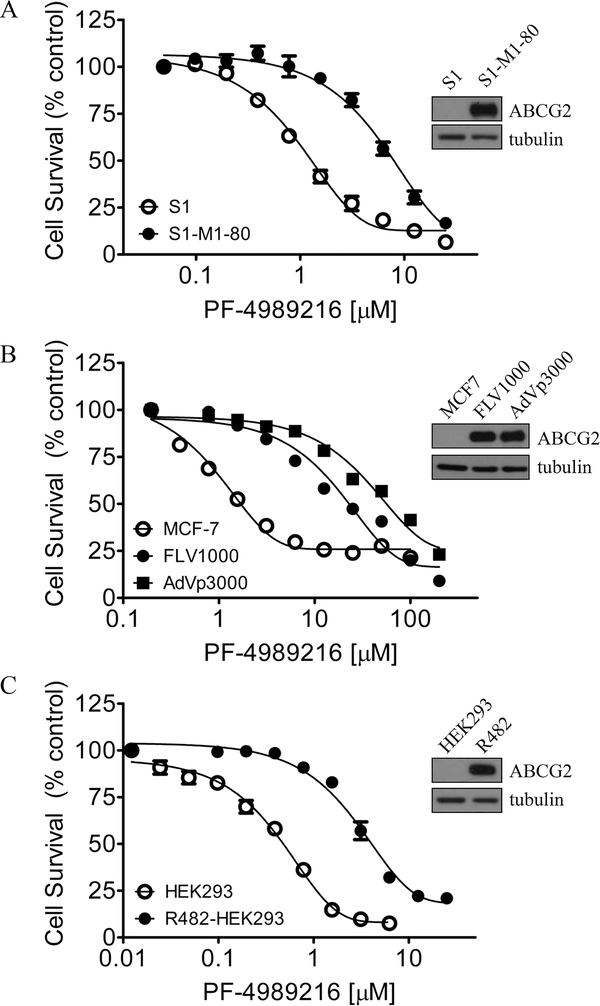
Given that the overexpression of MDR-linked ABC drug transporters ABCB1 and ABCG2 in cancer cells is known to lead to acquired resistance to a variety of molecularly targeted anticancer agents,13,20,40–43 we determined the toxicity of PF-4989216 in several drug-sensitive and MDR cancer cell lines, including cells overexpressing ABCB1 or ABCG2, and in HEK293 cells transfected with human ABCB1 or ABCG2. We noticed that ABCG2-overexpressing S1-M1–80 (Figure 1A, closed circles), human breast cancer MCF7-FLV1000 (Figure 1B, closed circles), and MCF7-AdVp3000 (Figure 1B, closed squares), as well as human ABCG2-transfected HEK293cells, R482-HEK293 (Figure 1C, closed circles) were all resistant to PF-4989216 as compared to the ABCG2-negative parental cells (Figure 1, open circles). The calculated IC50 and resistance factor (RF) values of PF-4989216 are summarized in Table 1. The RF value corresponds to the extent of cellular resistance to PF-4989216 caused by the overexpression of a particular ABC drug transporter in a cell lines, which is calculated by dividing the IC50 value of a particular MDR subline by the IC50 value of the parental line. In contrast to ABCG2-overexpressing cells, cancer cells overexpressing ABCB1, or cells transfected with human ABCB1 were equally sensitive to PF-4989216 as their drug-sensitive parental cells (Table 1). Our results here show that PF-4989216 is significantly less effective against the proliferation of cells overexpressing human ABCG2, with RF values ranging from 4 to 27 (Table 1).
Figure 1.
Cytotoxic effect of PF-4989216 in cancer cells is reduced by the overexpression of human ABCG2 protein. The cytotoxicity of PF-4989216 in (A) human colon carcinoma S1 cell line (○) and ABCG2-overexpressing subline S1-M1–80 (●); (B) human breast carcinoma MCF-7 (○) and ABCG2-overexpressing sublines MCF7-FLV1000 (●) and MCF7-AdVp3000 (■); as well as in (C) parental HEK293 (○) and ABCG2-tranfected R482-HEK293 (●) cells, was determined as described previously.20 The representative immunoblots of ABCG2 and tubulin as loading control are shown (inset). Points, mean from at least three independent experiments; bars, SEM.
Table 1.
Cytotoxicity of PF-4989216 in Drug-Sensitive Parental and Respective MDR Cell Lines
| cell line | cancer origin | transporter overexpressed | IC50 (μM)a | RFb |
|---|---|---|---|---|
| KB-3-1 | epidermal | 4.37 ± 0.51 | 1 | |
| KB-V-1 | epidermal | ABCB1 | 3.07 ± 0.44 | 1 |
| OVCAR-8 | ovarian | 15.28 ± 5.55 | 1 | |
| NCI-ADR-RES | ovarian | ABCB1 | 17.52 ± 3.62 | 1 |
| NIH3T3 | 1.35 ± 0.18 | 1 | ||
| NIH3T3-G185 | ABCB1 | 1.33 ± 0.18 | 1 | |
| S1 | colon | 1.11 ± 0.09 | 1 | |
| S1-M1-80 | colon | ABCG2 | 6.79 ± 1.00***c | 6 |
| H460 | lung | 0.93 ± 0.11 | 1 | |
| H460-MX20 | lung | ABCG2 | 4.03 ± 0.51*** | 4 |
| MCF7 | breast | 2.30 ± 0.68 | 1 | |
| MCF7-FLV1000 | breast | ABCG2 | 23.26 ± 2.94*** | 10 |
| MCF7-AdVp3000 | breast | ABCG2 | 62.57 ± 5.46*** | 27 |
| pcDNA-HEK293 | 0.44 ± 0.05 | 1 | ||
| MDR19-HEK293 | ABCB1 | 0.38 ± 0.06 | 1 | |
| R482-HEK293 | ABCG2 | 5.05 ± 0.89*** | 11 |
IC50 values are mean ± SD calculated from dose–response curves obtained from three independent experiments using cytotoxicity assay as described in Experimental Section.
Abbreviation: RF, resistance factor. RF values were calculated by dividing IC50 values of ABC transporter overexpressing cells by IC50 values of respective parental cells.
P < 0.05
P < 0.01
P < 0.001.
Inhibition of PI3K Downstream Signaling in Human Cancer Cells by PF-4989216 Is Reduced by the Efflux Function of ABCG2.
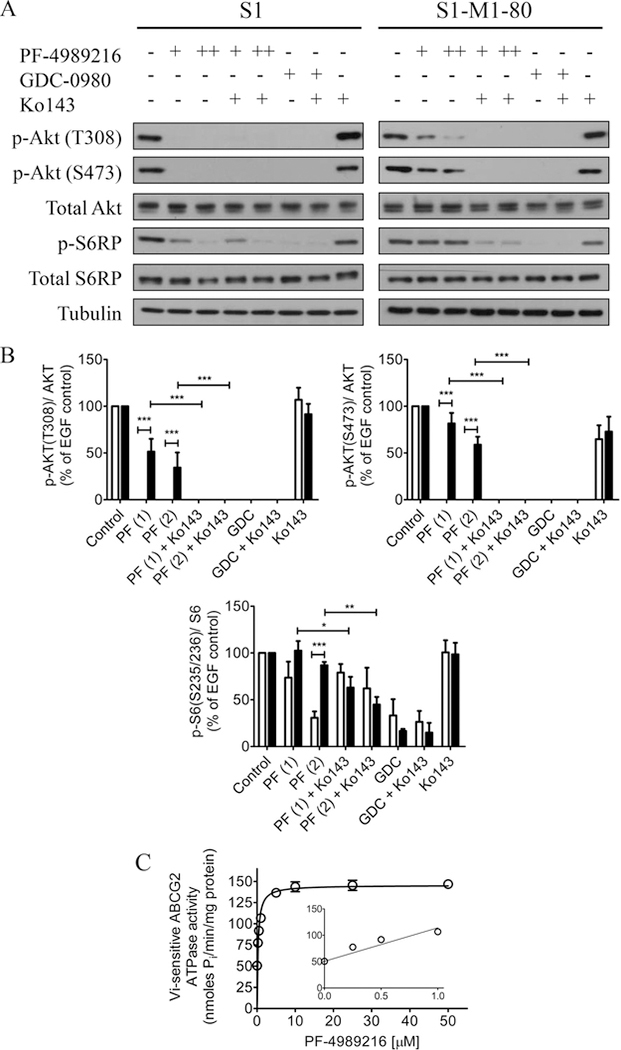
Given that PF-4989216 is a potent PI3K inhibitor,28 we compared the inhibitory effect of PF-4989216 on the phosphorylation of PI3K downstream molecules in drug-sensitive human colon S1 cancer cell line and in its ABCG2-overexpressing S1-M1–80 MDR subline. As expected, phosphorylation of Akt at threonine 308 (T308) and serine 473 (S473) were completely inhibited by PF-4989216 in drug-sensitive S1 cells. Moreover, downstream phosphorylation of S6RP was also inhibited by PF-4989216, in a dose-dependent manner (Figure 2A, left panels and 2B). However, PF-4989216 was considerably less effective in inhibiting Akt and S6RP phosphorylation in ABCG2-overexpressing S1-M1–80 cells (Figure 2A, right panels). In contrast, GDC-0980, a known PI3K/mTOR kinase inhibitor,44 was equally effective in inhibiting Akt and S6RP phosphorylation in both parental S1 and ABCG2 expressing S1-M1–80 cells (Figure 2B). Of note, since the positive control GDC-0980 has been reported as a substrate for ABCG2,45 a saturating concentration of GDC-0980 was used to ensure the complete inhibition of Akt and S6RP phosphorylation. Interestingly, we discovered that in the presence of ABCG2 reference inhibitor Ko143, the inhibitory activity of PF-4989216 on PI3K signaling in S1-M1–80 increased significantly to a comparable level as in drug-sensitive S1 cancer cells (Figure 2B). In addition, treatment with PF-4989216 or GDC-0980 alone or in combination with Ko143 did not affect the level of total Akt or total S6RP in these cells (Figure 2A and 2B). In order to further confirm the role of ABCG2 function in mediating cellular resistance to PF-4989216, we evaluated the resensitization effect of Ko143 and lapatinib, a known competitive inhibitor of ABCG246 on ABCG2-mediated resistance to PF-4989216. As shown in Table 2, by inhibiting the transport function of ABCG2, Ko143 and lapatinib significantly reversed PF-4989216 resistance in ABCG2-overexpressing S1-M1–80 cells and H460-MX20 cell, as well as ABCG2-transfected R482-HEK293 cells. Collectively, our results demonstrate that the function of ABCG2 negatively affects the ability of PF-4989216 to inhibit PI3K signaling and the proliferation of cancer cells.
Figure 2.
PF-4989216 inhibits PI3K signaling in S1 cancer cells and stimulates vanadate (Vi)-sensitive ABCG2 ATPase activity. (A) Drug-sensitive S1 and the ABCG2-overexpressing MDR subline S1-M1–80 were treated with DMSO (control), 1 μM of PF-4989216 (PF-4989216+), 2 μM of PF-4989216 (PF-4989216++), or 10 μM of GDC-0980 in the absence or presence of 1 μM of Ko 143 as indicated for 2 h before processed for immunoblotting. Human EGF (50 ng/mL) was added to the culture medium for 5 min to stimulate phosphorylation. GDC-0980 is a dual PI3K/mTOR inhibitor used here as a control, whereas Ko 143 is a reference inhibitor of ABCG2 used here to inhibit the function of ABCG2. (B) Western blot quantification of relative levels of Akt and S6 ribosomal protein phosphorylation in S1 (open bars) and S1-M1–80 (closed bars) cells. Representative Western blots are shown and values are presented as mean ± SD calculated from more than three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001, versus the same treatment in parental cells or in the presence of Ko143. (C) Crude membrane protein (50–100 μg protein/mL) from High-Five insect cells expressing ABCG2 was incubated at 37 °C with 0–50 μM and 0–1 μM of PF-4989216 (inset) in the presence or absence of vanadate. The ABCG2 ATPase activity was measured as described previously.31
Table 2.
Reference Inhibitor and Competitive Substrate of ABCG2 Restore Sensitivity of PF-4989216 to ABCG2-Overexpressing Cell Lines
| IC50 (μM)a |
|||
|---|---|---|---|
| cell line | PF-4989216 | PF-4989216 + Ko143 (1 μM) | PF-4989216 + lapatinib (0.3 μM) |
| S1 | 1.11 ± 0.09 | 1.62 ± 0.11 | 0.96 ± 0.11 |
| S1-M1-80 | 6.79 ± 1.00 | 2.36 ± 0.49**b | 1.56 ± 0.25*** |
| H460 | 0.93 ± 0.11 | 1.04 ± 0.18 | 0.86 ± 0.11 |
| H460-MX20 | 4.03 ± 0.51 | 1.12 ± 0.22*** | 1.20 ± 0.16*** |
| pcDNA-HEK293 | 0.44 ± 0.05 | 0.40 ± 0.09 | 0.41 ± 0.07 |
| R482-HEK293 | 5.05 ± 0.89 | 0.82 ± 0.17*** | 2.26 ± 0.50** |
IC50 values are mean ± SD of PF-4989216 in the presence and absence of a reference inhibitor of ABCG2. The IC50 values were calculated from dose–response curves obtained from at least three independent experiments.
P < 0.05
P < 0.01
P < 0.001.
PF-4989216 Stimulates ATPase Activity of ABCG2.
Next, to gain insight into the relationship between PF-4989216 and ABCG2-mediated transport, we studied the effect of PF-4989216 on vanadate (Vi)-sensitive ATPase activity of ABCG2 in crude membranes isolated from High-Five cells expressing human ABCG2 protein. We discovered that PF-4989216 stimulated ABCG2 ATP hydrolysis in a dose-dependent manner. PF-4989216 produced a maximum stimulation of approximately 3-fold, and the concentration required to produce 50% of stimulation was 0.71 ± 0.06 μM (Figure 2C). Since substrate transport mediated by ABCG2 is known to couple to stimulation of ABCG2 ATP hydrolysis,47 our results suggest that PF-4989216 is a transport substrate of ABCG2, which is in agreement with the cytotoxicity data (Figure 1).
PF-4989216 Antagonizes Transport Mediated by ABCG2.
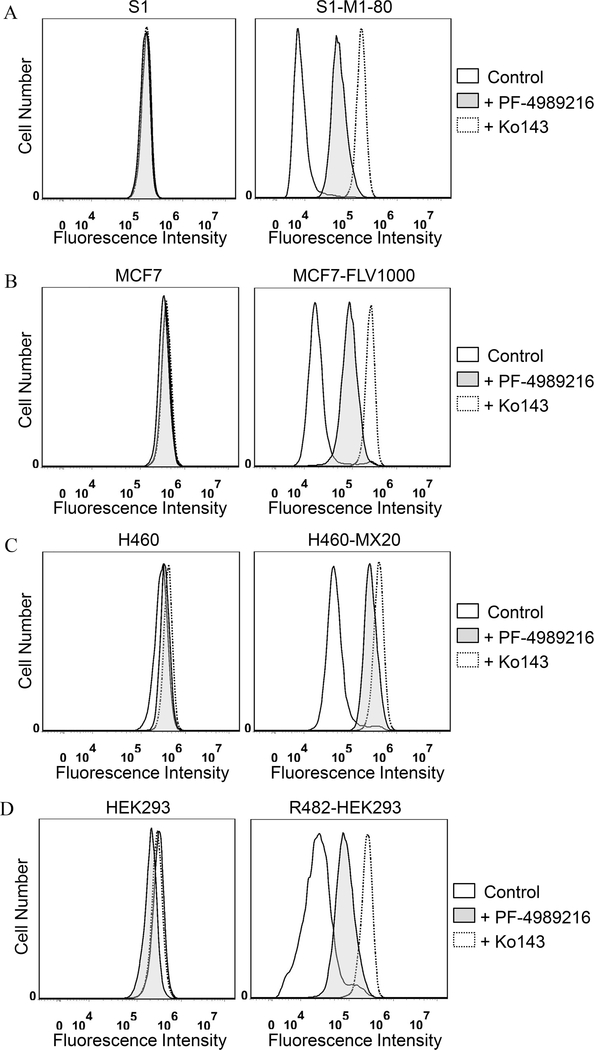
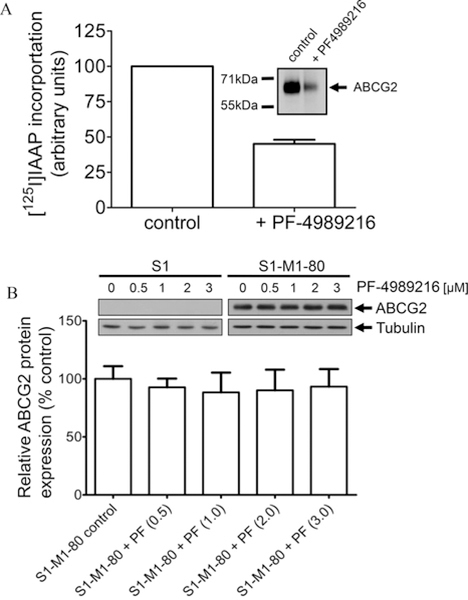
We evaluated the interaction between PF-4989216 and ABCG2 by examining the effect of PF-4989216 on ABCG2 function and protein expression. The effect of PF-4989216 on ABCG2-mediated efflux of PhA, a known fluorescent substrate of ABCG237 was determined in ABCG2-overexpressing S1-M1–80 cells (Figure 3A), MCF7-FLV1000 cells (Figure 3B) and H460-MX20 nonsmall human lung cancer cells (Figure 3C), as well as ABCG2-transfected R482-HEK293 cells (Figure 3D). PF-4989216 significantly inhibited ABCG2-mediated PhA transport from ABCG2-overexpressing cancer cells (Figure 3A–C, right panels) and cells transfected with ABCG2 (Figure 3D, right panel). Moreover, PF-4989216 had no significant effect on the accumulation of PhA in drug-sensitive parental cells (Figure 3A–D, left panels). Next, we examined the effect of PF-4989216 on photoaffinity labeling of [125I]IAAP in isolated membrane vesicles from ABCG2-overexpressing MCF7-FLV1000 cells. [125I]IAAP is a known transport substrate of ABCG2 and binds directly to the substrate binding site of ABCG2,48 for this reason any substrate that binds to the same site will inhibit the photolabeling.31,49 We discovered that 10 μM of PF-4989216 inhibited [125I]IAAP photoaffinity labeling of ABCG2 by approximately 55% (Figure 4A). Our result confirms that PF-4989216 interacts, similar to IAAP, at the substrate-binding site of ABCG2.
Figure 3.
Effect of PF-4989216 on the transport function of ABCG2. The accumulation of fluorescent pheophorbide A (PhA) in drug-sensitive S1 (A, left panel) and ABCG2-overexpressing S1-M1–80 (A, right panel); drug-sensitive MCF7 (B, left panel) and ABCG2-overexpressing MCF7-FLV1000 (B, right panel); drug-sensitive human nonsmall lung carcinoma H460 (C, left panel) and ABCG2-overexpressing H460-MX20 MDR (C, right panel) cell lines; or parental HEK293 (D, left panel) and ABCG2-transfected R482-HEK293 cells (D, right panel), were measured in the absence (solid lines) or presence of 40 μM PF-4989216 (shaded, solid lines) or 1 μM ABCG2 reference inhibitor Ko143 (dotted lines), and analyzed immediately by flow cytometry. Representative histograms of three independent experiments are shown.
Figure 4.
Effect of PF-4989216 on photoaffinity labeling of ABCG2 with [125I]IAAP and protein expression of ABCG2. (A) Membrane vesicles (500 μg protein/mL) from MCF7-FLV1000 cells expressing ABCG2 was incubated in the presence or absence of 10 μM of PF-4989216 at 21 °C in 50 μmol/L Tris-HCL (pH 7.5) and 150 mM NaCl. [125I]IAAP (2,200 Ci/mmol) at 3 to 6 nmol/L was added to the samples and incubated for 5 min under subdued light before illuminated with an UV lamp (365 nm) for 10 min at room temperature. The labeled ABCG2 was immunoprecipitated with BXP-21 antibody and visualized as described previously.31 (B) Immunoblot detection of human ABCG2 (upper panel) and quantification (lower panel) of total lysate protein (10 μg) from drug-sensitive S1 and drug-resistant S1-M1–80 cells treated with increasing concentrations (0–3 μM) of PF-4989216 for 72 h as indicated. α-Tubulin was used as loading control. Representative autoradiogram and values represent mean ± SD calculated from three independent experiments.
Previous reports have demonstrated that some drug substrates of ABCG2 can reverse ABCG2-mediated MDR in cancer cells by competing directly with the transport of another drug substrate.31,46,50,51 Therefore, we examined the effect of PF-4989216 on ABCG2-mediated resistance to mitoxantrone, a well-established anticancer drug substrate of ABCG2,10 in S1-M1–80 and R482-HEK293 cells.
The fold-reversal (FR) value46 shown in Table 3 represents the extent of MDR reversal of cells to mitoxantrone caused by a reversing agent. We found that at the highest nontoxic concentration of 200 nM (Figure 1), PF-4989216 had no significant effect on ABCG2-mediated mitoxantrone resistance in any of the cell lines tested (Table 3). Of note, Ko143 was used as positive control for complete inhibition of ABCG2 function (Figure 3A–D, dashed lines) and reversal of drug resistance conferred by ABCG2 (Table 3). The protein expression of ABCG2 in S1 and S1-M1–80 cells was also examined after treating these cells with increasing concentrations of PF-4989216 (0.5–3 μM) for 72 h and processed for immunoblotting as described in Experimental Section. Our results indicate that short-term exposure to PF-4989216 has no significant effect on the total protein expression level of ABCG2 in these cancer cells (Figure 4B).
Table 3.
Effect of PF-4989216 on ABCG2-Mediated Mitoxantrone Resistance in ABCG2-Overexpressing S1-M1-80 Cells and ABCG2-Transfected R482-HEK293 Cells
| IC50a (FR)b |
|||
| drug | concentration (nM) | S1 (nM) | S1-M1-80 (μM) |
| mitoxantrone | 5.05 ± 1.13 (1.0) | 68.37 ± 3.01 (1.0) | |
| +PF-4989216 | 100 | 4.21 ± 0.87 (1.2) | 73.97 ± 4.15 (1.0) |
| +PF-4989216 | 200 | 3.67 ± 0.92 (1.4) | 79.19 ± 7.12 (0.9) |
| +Ko143 | 1000 | 4.76 ± 1.15 (1.0) | 0.29 ± 0.06***c (235.8) |
| pcDNA-HEK293 (nM) | R482-HEK293 (nM) | ||
| mitoxantrone | 1.34 ± 0.20 (1.0) | 112.78 ± 15.94 (1.0) | |
| +PF-4989216 | 100 | 1.51 ± 0.26 (0.9) | 69.01 ± 14.41* (1.6) |
| +PF-4989216 | 200 | 1.19 ± 0.23 (1.1) | 69.61 ± 12.02* (1.6) |
| +Ko143 | 1000 | 1.38 ± 0.23 (1.0) | 10.27 ± 2.17*** (11.0) |
IC50 values are mean ± SD calculated from dose–response curves obtained from three independent experiments using cytotoxicity assay as described in Experimental Section.
Abbreviation: FR, fold-reversal. FR values were obtained by dividing IC50 values of cells treated with a particular anticancer drug in the absence of an inhibitor by IC50 values of cells treated with the same anticancer drug in the presence of an inhibitor.
P < 0.05
P < 0.01
P < 0.001.
DISCUSSION
The activated PI3K signaling pathway is an attractive therapeutic target for many types of cancers, including nonsmall cell lung carcinoma, breast cancer, lymphoma, and glioblastoma.52 However, the overexpression of ABCB1 and ABCG2 has been shown to significantly reduce the therapeutic efficacy of PI3K signaling pathway inhibitors, such as GDC-0980,45 CUDC-101,42 CUDC-907,22 and AZD8055.53 Moreover, the presence of ABCB1 and ABCG2 at the BBB also restricts the brain penetration of many PI3K inhibitors that can be used to treat glioblastoma. For instance, NVP-BEZ235 and pictilisib (GDC-0941) are potent inhibitors of the PI3K signaling pathway with similar properties to PF-4989216, both inhibiting cancer cell proliferation in the nanomolar range54,55 and are highly active against cancer cells harboring PIK3CA mutations.55–58 However, studies found that NVP-BEZ235 and pictilisib are substrates of ABCB1 and ABCG2, and the brain penetration of both PI3K inhibitors were negatively affected by the transport function of ABCB1 and ABCG2 at the BBB, restricting the use of both agents to treat patients with brain tumors.53,59
The recently developed PI3K inhibitor PF-4989216 has demonstrated promising preclinical activity, both in vitro and in vivo, targeting the PAM signaling pathway27 and subsequent cancer cell proliferation.28 Unfortunately, the risk of developing resistance to PF-4989216 mediated by ABCB1 and ABCG2 can one day become a therapeutic challenge to clinicians in the clinic. Therefore, we first evaluated the cytotoxicity of PF-4989216 in multiple cancer cell lines of different origins, including ABCB1 and ABCG2-overexpressing cancer cell lines. We observed that PF-4989216 was significantly less cytotoxic to ABCG2-overexpressing cells and cells transfected with human ABCG2 as compared to the ABCG2-negative cells. We were able to restore the sensitivity of ABCG2-overexpressing cells to PF-4989216 by cotreatment with an inhibitor (Ko143) or a competitive substrate (lapatinib) of ABCG2,46 demonstrating ABCG2 as a mechanism of resistance for PF-4989216. Knowing that ABCG2-expressing cells are resistant to PF-4989216, we next examined the efficacy of PF-4989216 to inhibit the phosphorylation of PI3K downstream molecules, which is a key characteristic of PF-4989216 to inhibit cancer cell proliferation,28 in ABCG2-negative human S1 colon cancer cell line and its ABCG2-overexpressing S1-M1–80 MDR subline. Consistent with the cytotoxicity data, we found that PF-4989216 failed to inhibit the phosphorylation of Akt at threonine 308 (T308) and serine 473 (S473), as well as downstream S6RP in ABCG2-overexpressing S1-M1–80 cancer cells to the same extent as in S1 cancer cells. Furthermore, we discovered that ABCG2 inhibitor Ko143 was able to restore the activity of PF-4989216 in S1-M1–80 cells, suggesting the direct involvement of ABCG2 in reducing the potency of PF-4989216 in cancer cells and the need for developing a drug combination strategy to overcome multidrug resistance in cancer therapy. Taking into account that ABCG2 negatively affects the distribution and oral bioavailability of a wide variety of therapeutic drugs,60 it remains to be determined whether the overall absorption and distribution of PF-4989216 could be altered by the overexpression of ABCG2 at the intestinal walls and BBB. It is worth noting that we also demonstrated that PF-4989216 stimulates vanadate-sensitive ATPase activity of ABCG2. Since the stimulation of ABCG2 ATP hydrolysis is known to couple to substrate transport by ABCG247 and PF-4989216 stimulated the ATPase activity of ABCG2 in a similar manner as other well-established substrates,47 our data indicate that PF-4989216 is a transport substrate of ABCG2.
Numerous protein kinase inhibitors, including PI3K inhibitors, have been reported capable of reversing ABCG2-mediated drug resistance in MDR cancer cells20,46,50,61–63 through direct inhibition of ABCG2 function63,64 or transient downregulation of ABCG2 expression.65,66 For instance, the PI3K inhibitor LY294002 has been shown to competitively inhibit the function of ABCG2, and reverse MDR mediated by ABCG2 in a dose-dependent manner.63 For that reason, we investigated the effect of PF-4989216 on ABCG2-mediated drug transport and drug resistance, as well as ABCG2 protein expression in ABCG2-overexpressing cells. We discovered that PF-4989216 strongly inhibited ABCG2-mediated PhA efflux and the photoaffinity labeling of [125I]IAAP to ABCG2, confirming direct and competitive binding of PF-4989216 to the substrate-binding site(s) of ABCG2. However, we found that due to high toxicity, PF-4989216 failed to reverse ABCG2-mediated drug resistance at low, nontoxic concentrations, despite its ability to inhibit ABCG2-mediated efflux of fluorescent substrates in a short-term assay at higher concentrations. Moreover, we found that PF-4989216 had no significant effect on the protein expression of ABCG2 in cancer cells over a period of 72 h. However, the clinical effect of prolonged treatment of cancer patients with PF-4989216 remains to be determined.
In summary, the results of our study indicate that ABCG2 confers resistance to PF-4989216, reducing its intracellular concentration and ability to inhibit PAM signaling pathway to suppress cancer cell proliferation. Moreover, we found that by introducing a competitive inhibitor or drug substrate of ABCG2, the activity of PF-4989216 in ABCG2-overexpressing MDR cancer cells can be fully restored. Our results are consistent with published work showing that ABCG2 is known to reduce the bioavailability and efficacy of many molecularly targeted drugs,13,15,17,20,42,67–69 and the overexpression of ABCG2 in cancer cells remains as a common mechanism of drug resistance. Thus, acquired resistance to PF-4989216 mediated by ABCG2 could have clinical implications for patients receiving this drug treatment for cancer, and should be further investigated.
ACKNOWLEDGMENTS
This work was supported by funds from the Ministry of Science and Technology of Taiwan (MOST-105-2320-B-182-018), the Chang Gung Medical Research Program (CMRPD190653, CMRPD1D0153, CMRPD1G0111, BMRPC17), and a grant to Chang Gung University from the Ministry of Education (EMRPD1G0121). Drs M. Murakami and S.V. Ambudkar were supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
ABBREVIATIONS:
- MDR
multidrug resistance
- ABC
ATP-binding cassette
- PI3K
phosphatidylinositol 3-kinase
- FCS
fetal calf serum
- PBS
phosphate-buffered saline
- CCK-8
Cell Counting Kit-8
- IMDM
Iscove’s Modified Dulbecco’s Medium
- MTT
3-(4,5-dimethylthiazol-yl)-2,5-diphenyllapatinibrazolium bromide
- Vi
sodium orthovanadate
- IAAP
iodoarylazidoprazosin
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Dienstmann R; Rodon J; Serra V; Tabernero J Picking the point of inhibition: a comparative review of PI3K/AKT/mTOR pathway inhibitors. Mol. Cancer Ther. 2014, 13 (5), 1021–31. [DOI] [PubMed] [Google Scholar]
- (2).Garcia-Echeverria C; Sellers WR Drug discovery approaches targeting the PI3K/Akt pathway in cancer. Oncogene 2008, 27 (41), 5511–26. [DOI] [PubMed] [Google Scholar]
- (3).Greenwell IB; Flowers CR; Blum KA; Cohen JB Clinical use of PI3K inhibitors in B-cell lymphoid malignancies: today and tomorrow. Expert Rev. Anticancer Ther. 2017, 17, 271–279. [DOI] [PubMed] [Google Scholar]
- (4).West KA; Castillo SS; Dennis PA Activation of the PI3K/Akt pathway and chemotherapeutic resistance. Drug Resist. Updates 2002, 5 (6), 234–248. [DOI] [PubMed] [Google Scholar]
- (5).Burris HA 3rd. Overcoming acquired resistance to anticancer therapy: focus on the PI3K/AKT/mTOR pathway. Cancer Chemother. Pharmacol. 2013, 71 (4), 829–42. [DOI] [PubMed] [Google Scholar]
- (6).Lynch TJ; Bell DW; Sordella R; Gurubhagavatula S; Okimoto RA; Brannigan BW; Harris PL; Haserlat SM; Supko JG; Haluska FG; Louis DN; Christiani DC; Settleman J; Haber DA Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004, 350 (21), 2129–39. [DOI] [PubMed] [Google Scholar]
- (7).Gillet JP; Gottesman MM Mechanisms of multidrug resistance in cancer. Methods Mol. Biol. 2010, 596, 47–76. [DOI] [PubMed] [Google Scholar]
- (8).Wu CP; Hsieh CH; Wu YS The emergence of drug transporter-mediated multidrug resistance to cancer chemotherapy. Mol. Pharmaceutics 2011, 8 (6), 1996–2011. [DOI] [PubMed] [Google Scholar]
- (9).Szakacs G; Paterson JK; Ludwig JA; Booth-Genthe C; Gottesman MM Targeting multidrug resistance in cancer. Nat. Rev. Drug Discovery 2006, 5 (3), 219–34. [DOI] [PubMed] [Google Scholar]
- (10).Doyle LA; Yang W; Abruzzo LV; Krogmann T; Gao Y; Rishi AK; Ross DD A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. U. S. A. 1998, 95 (26), 15665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Wu C-P; Hsieh C-H; Wu Y-S The Emergence of Drug Transporter-Mediated Multidrug Resistance to Cancer Chemotherapy. Mol. Pharmaceutics 2011, 8 (6), 1996–2011. [DOI] [PubMed] [Google Scholar]
- (12).Gottesman MM; Fojo T; Bates SE Multidrug resistance in cancer: role of ATP-dependent transporters. Nat. Rev. Cancer 2002, 2 (1), 48–58. [DOI] [PubMed] [Google Scholar]
- (13).Nakanishi T; Shiozawa K; Hassel BA; Ross DD Complex interaction of BCRP/ABCG2 and imatinib in BCR-ABL-expressing cells: BCRP-mediated resistance to imatinib is attenuated by imatinibinduced reduction of BCRP expression. Blood 2006, 108 (2), 678–684. [DOI] [PubMed] [Google Scholar]
- (14).Hegedus C; Ozvegy-Laczka C; Apati A; Magocsi M; Nemet K; Orfi L; Keri G; Katona M; Takats Z; Varadi A; Szakacs G; Sarkadi B Interaction of nilotinib, dasatinib and bosutinib with ABCB1 and ABCG2: implications for altered anti-cancer effects and pharmacological properties. Br. J. Pharmacol. 2009, 158 (4), 1153–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Burger H; van Tol H; Brok M; Wiemer EA; de Bruijn EA; Guetens G; de Boeck G; Sparreboom A; Verweij J; Nooter K Chronic imatinib mesylate exposure leads to reduced intracellular drug accumulation by induction of the ABCG2 (BCRP) and ABCB1 (MDR1) drug transport pumps. Cancer Biol. Ther. 2005, 4 (7), 747–52. [DOI] [PubMed] [Google Scholar]
- (16).Tang C; Schafranek L; Watkins DB; Parker WT; Moore S; Prime JA; White DL; Hughes TP Tyrosine kinase inhibitor resistance in chronic myeloid leukemia cell lines: investigating resistance pathways. Leuk. Lymphoma 2011, 52 (11), 2139–47. [DOI] [PubMed] [Google Scholar]
- (17).Tang SC; Lagas JS; Lankheet NA; Poller B; Hillebrand MJ; Rosing H; Beijnen JH; Schinkel AH Brain accumulation of sunitinib is restricted by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and can be enhanced by oral elacridar and sunitinib coadministration. Int. J. Cancer 2012, 130 (1), 223–33. [DOI] [PubMed] [Google Scholar]
- (18).Lagas JS; van Waterschoot RA; van Tilburg VA; Hillebrand MJ; Lankheet N; Rosing H; Beijnen JH; Schinkel AH Brain accumulation of dasatinib is restricted by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and can be enhanced by elacridar treatment. Clin. Cancer Res. 2009, 15 (7), 2344–51. [DOI] [PubMed] [Google Scholar]
- (19).Lagas JS; van Waterschoot RA; Sparidans RW; Wagenaar E; Beijnen JH; Schinkel AH Breast cancer resistance protein and P-glycoprotein limit sorafenib brain accumulation. Mol. Cancer Ther. 2010, 9 (2), 319–26. [DOI] [PubMed] [Google Scholar]
- (20).Wu CP; Sim HM; Huang YH; Liu YC; Hsiao SH; Cheng HW; Li YQ; Ambudkar SV; Hsu SC Overexpression of ATP-binding cassette transporter ABCG2 as a potential mechanism of acquired resistance to vemurafenib in BRAF(V600E) mutant cancer cells. Biochem. Pharmacol. 2013, 85 (3), 325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Noguchi K; Katayama K; Sugimoto Y Human ABC transporter ABCG2/BCRP expression in chemoresistance: basic and clinical perspectives for molecular cancer therapeutics. Pharmacogenomics Pers. Med. 2014, 7, 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Wu CP; Hsieh YJ; Hsiao SH; Su CY; Li YQ; Huang YH; Huang CW; Hsieh CH; Yu JS; Wu YS Human ATP-Binding Cassette Transporter ABCG2 Confers Resistance to CUDC-907, a Dual Inhibitor of Histone Deacetylase and Phosphatidylinositol 3-Kinase. Mol. Pharmaceutics 2016, 13 (3), 784–94. [DOI] [PubMed] [Google Scholar]
- (23).Ross DD; Karp JE; Chen TT; Doyle LA Expression of breast cancer resistance protein in blast cells from patients with acute leukemia. Blood 2000, 96 (1), 365–8. [PubMed] [Google Scholar]
- (24).Steinbach D; Sell W; Voigt A; Hermann J; Zintl F; Sauerbrey A BCRP gene expression is associated with a poor response to remission induction therapy in childhood acute myeloid leukemia. Leukemia 2002, 16 (8), 1443–7. [DOI] [PubMed] [Google Scholar]
- (25).Uggla B; Stahl E; Wagsater D; Paul C; Karlsson MG; Sirsjo A; Tidefelt U BCRP mRNA expression v. clinical outcome in 40 adult AML patients. Leuk. Res. 2005, 29 (2), 141–6. [DOI] [PubMed] [Google Scholar]
- (26).Paez JG; Janne PA; Lee JC; Tracy S; Greulich H; Gabriel S; Herman P; Kaye FJ; Lindeman N; Boggon TJ; Naoki K; Sasaki H; Fujii Y; Eck MJ; Sellers WR; Johnson BE; Meyerson M EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004, 304 (5676), 1497–1500. [DOI] [PubMed] [Google Scholar]
- (27).Liu KK; Zhu J; Smith GL; Yin MJ; Bailey S; Chen JH; Hu Q; Huang Q; Li C; Li QJ; Marx MA; Paderes G; Richardson PF; Sach NW; Walls M; Wells PA; Zou A Highly Selective and Potent Thiophenes as PI3K Inhibitors with Oral Antitumor Activity. ACS Med. Chem. Lett. 2011, 2 (11), 809–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Walls M; Baxi SM; Mehta PP; Liu KK; Zhu J; Estrella H; Li C; Zientek M; Zong Q; Smeal T; Yin MJ Targeting small cell lung cancer harboring PIK3CA mutation with a selective oral PI3K inhibitor PF-4989216. Clin. Cancer Res. 2014, 20 (3), 631–43. [DOI] [PubMed] [Google Scholar]
- (29).Leroy C; Amante RJ; Bentires-Alj M Anticipating mechanisms of resistance to PI3K inhibition in breast cancer: a challenge in the era of precision medicine. Biochem. Soc. Trans. 2014, 42 (4), 733–41. [DOI] [PubMed] [Google Scholar]
- (30).Gottesman MM; Ambudkar SV Overview: ABC transporters and human disease. J. Bioenerg. Biomembr. 2001, 33 (6), 453–8. [DOI] [PubMed] [Google Scholar]
- (31).Wu CP; Shukla S; Calcagno AM; Hall MD; Gottesman MM; Ambudkar SV Evidence for dual mode of action of a thiosemicarbazone, NSC73306: a potent substrate of the multidrug resistance linked ABCG2 transporter. Mol. Cancer Ther. 2007, 6 (12), 3287–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Shen DW; Fojo A; Chin JE; Roninson IB; Richert N; Pastan I; Gottesman MM Human multidrug-resistant cell lines: increased mdr1 expression can precede gene amplification. Science 1986, 232 (4750), 643–645. [DOI] [PubMed] [Google Scholar]
- (33).Honjo Y; Hrycyna CA; Yan QW; Medina-Perez WY; Robey RW; van de Laar A; Litman T; Dean M; Bates SE Acquired mutations in the MXR/BCRP/ABCP gene alter substrate specificity in MXR/BCRP/ABCP-overexpressing cells. Cancer Res. 2001, 61 (18), 6635–6639. [PubMed] [Google Scholar]
- (34).Ishiyama M; Tominaga H; Shiga M; Sasamoto K; Ohkura Y; Ueno K A combined assay of cell viability and in vitro cytotoxicity with a highly water-soluble tetrazolium salt, neutral red and crystal violet. Biol. Pharm. Bull. 1996, 19 (11), 1518–1520. [DOI] [PubMed] [Google Scholar]
- (35).Hsiao SH; Lu YJ; Li YQ; Huang YH; Hsieh CH; Wu CP Osimertinib (AZD9291) Attenuates the Function of Multidrug Resistance-Linked ATP-Binding Cassette Transporter ABCB1 in Vitro. Mol. Pharmaceutics 2016132117. 10.1021/acs.molpharmaceut.6b00249 [DOI] [PubMed] [Google Scholar]
- (36).Lu JC; Chang YT; Wang CT; Lin YC; Lin CK; Wu ZS Trichostatin A modulates thiazolidinedione-mediated suppression of tumor necrosis factor alpha-induced lipolysis in 3T3-L1 adipocytes. PLoS One 2013, 8 (8), e71517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Robey RW; Steadman K; Polgar O; Morisaki K; Blayney M; Mistry P; Bates SE Pheophorbide a is a specific probe for ABCG2 function and inhibition. Cancer Res. 2004, 64 (4), 1242–1246. [DOI] [PubMed] [Google Scholar]
- (38).Gribar JJ; Ramachandra M; Hrycyna CA; Dey S; Ambudkar SV Functional characterization of glycosylation-deficient human P-glycoprotein using a vaccinia virus expression system. J. Membr. Biol. 2000, 173 (3), 203–14. [DOI] [PubMed] [Google Scholar]
- (39).Ambudkar SV Drug-stimulatable ATPase activity in crude membranes of human MDR1-transfected mammalian cells. Methods Enzymol. 1998, 292, 504–514. [DOI] [PubMed] [Google Scholar]
- (40).Thomas J; Wang L; Clark RE; Pirmohamed M Active transport of imatinib into and out of cells: implications for drug resistance. Blood 2004, 104 (12), 3739–3745. [DOI] [PubMed] [Google Scholar]
- (41).Hegedus C; Ozvegy-Laczka C; Szakacs G; Sarkadi B Interaction of ABC multidrug transporters with anticancer protein kinase inhibitors: substrates and/or inhibitors? Curr. Cancer Drug Targets 2009, 9 (3), 252–272. [DOI] [PubMed] [Google Scholar]
- (42).Wu CP; Hsiao SH; Su CY; Luo SY; Li YQ; Huang YH; Hsieh CH; Huang CW Human ATP-Binding Cassette transporters ABCB1 and ABCG2 confer resistance to CUDC-101, a multi-acting inhibitor of histone deacetylase, epidermal growth factor receptor and human epidermal growth factor receptor 2. Biochem. Pharmacol. 2014, 92 (4), 567–76. [DOI] [PubMed] [Google Scholar]
- (43).Wu CP; Hsieh CH; Hsiao SH; Luo SY; Su CY; Li YQ; Huang YH; Huang CW; Hsu SC Human ATP-Binding Cassette Transporter ABCB1 Confers Resistance to Volasertib (BI 6727), a Selective Inhibitor of Polo-like Kinase 1. Mol. Pharmaceutics 2015, 12 (11), 3885–95. [DOI] [PubMed] [Google Scholar]
- (44).Wallin JJ; Edgar KA; Guan J; Berry M; Prior WW; Lee L; Lesnick JD; Lewis C; Nonomiya J; Pang J; Salphati L; Olivero AG; Sutherlin DP; O’Brien C; Spoerke JM; Patel S; Lensun L; Kassees R; Ross L; Lackner MR; Sampath D; Belvin M; Friedman LS GDC-0980 is a novel class I PI3K/mTOR kinase inhibitor with robust activity in cancer models driven by the PI3K pathway. Mol. Cancer Ther. 2011, 10 (12), 2426–36. [DOI] [PubMed] [Google Scholar]
- (45).Salphati L; Pang J; Plise EG; Lee LB; Olivero AG; Prior WW; Sampath D; Wong S; Zhang X Preclinical assessment of the absorption and disposition of the phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor GDC-0980 and prediction of its pharmacokinetics and efficacy in human. Drug Metab. Dispos. 2012, 40 (9), 1785–96. [DOI] [PubMed] [Google Scholar]
- (46).Dai CL; Tiwari AK; Wu CP; Su XD; Wang SR; Liu DG; Ashby CR Jr.; Huang Y; Robey RW; Liang YJ; Chen LM; Shi CJ; Ambudkar SV; Chen ZS; Fu LW Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2. Cancer Res. 2008, 68 (19), 7905–7914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Ozvegy C; Litman T; Szakacs G; Nagy Z; Bates S; Varadi A; Sarkadi B Functional characterization of the human multidrug transporter, ABCG2, expressed in insect cells. Biochem. Biophys. Res. Commun. 2001, 285 (1), 111–7. [DOI] [PubMed] [Google Scholar]
- (48).Shukla S; Robey RW; Bates SE; Ambudkar SV The calcium channel blockers, 1,4-dihydropyridines, are substrates of the multidrug resistance-linked ABC drug transporter, ABCG2. Biochemistry 2006, 45 (29), 8940–51. [DOI] [PubMed] [Google Scholar]
- (49).Sauna ZE; Ambudkar SV Evidence for a requirement for ATP hydrolysis at two distinct steps during a single turnover of the catalytic cycle of human P-glycoprotein. Proc. Natl. Acad. Sci. U. S. A. 2000, 97 (6), 2515–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Sodani K; Tiwari AK; Singh S; Patel A; Xiao ZJ; Chen JJ; Sun YL; Talele TT; Chen ZS GW583340 and GW2974, human EGFR and HER-2 inhibitors, reverse ABCG2- and ABCB1-mediated drug resistance. Biochem. Pharmacol. 2012, 83 (12), 1613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Kuang YH; Patel JP; Sodani K; Wu CP; Liao LQ; Patel A; Tiwari AK; Dai CL; Chen X; Fu LW; Ambudkar SV; Korlipara VL; Chen ZS OSI-930 analogues as novel reversal agents for ABCG2-mediated multidrug resistance. Biochem. Pharmacol. 2012, 84 (6), 766–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Massacesi C; Di Tomaso E; Urban P; Germa C; Quadt C; Trandafir L; Aimone P; Fretault N; Dharan B; Tavorath R; Hirawat S PI3K inhibitors as new cancer therapeutics: implications for clinical trial design. OncoTargets Ther. 2016, 9, 203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Lin F; de Gooijer MC; Hanekamp D; Chandrasekaran G; Buil LC; Thota N; Sparidans RW; Beijnen JH; Wurdinger T; van Tellingen O PI3K-mTOR Pathway Inhibition Exhibits Efficacy Against High-grade Glioma in Clinically Relevant Mouse Models. Clin. Cancer Res. 2017, 23, 1286. [DOI] [PubMed] [Google Scholar]
- (54).Liu TJ; Koul D; LaFortune T; Tiao N; Shen RJ; Maira SM; Garcia-Echevrria C; Yung WK NVP-BEZ235, a novel dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor, elicits multifaceted antitumor activities in human gliomas. Mol. Cancer Ther. 2009, 8 (8), 2204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Junttila TT; Akita RW; Parsons K; Fields C; Lewis Phillips GD; Friedman LS; Sampath D; Sliwkowski MX Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell 2009, 15 (5), 429–40. [DOI] [PubMed] [Google Scholar]
- (56).Brachmann SM; Hofmann I; Schnell C; Fritsch C; Wee S; Lane H; Wang S; Garcia-Echeverria C; Maira SM Specific apoptosis induction by the dual PI3K/mTor inhibitor NVP-BEZ235 in HER2 amplified and PIK3CA mutant breast cancer cells. Proc. Natl. Acad. Sci. U. S. A. 2009, 106 (52), 22299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Mueller A; Bachmann E; Linnig M; Khillimberger K; Schimanski CC; Galle PR; Moehler M Selective PI3K inhibition by BKM120 and BEZ235 alone or in combination with chemotherapy in wild-type and mutated human gastrointestinal cancer cell lines. Cancer Chemother. Pharmacol. 2012, 69 (6), 1601–15. [DOI] [PubMed] [Google Scholar]
- (58).Zheng J; Wang H; Yao J; Zou X More antitumor efficacy of the PI3K inhibitor GDC-0941 in breast cancer with PIK3CA mutation or HER2 amplification status in vitro. Pharmazie 2014, 69 (1), 38–42. [PubMed] [Google Scholar]
- (59).Salphati L; Lee LB; Pang J; Plise EG; Zhang X Role of P-glycoprotein and breast cancer resistance protein-1 in the brain penetration and brain pharmacodynamic activity of the novel phosphatidylinositol 3-kinase inhibitor GDC-0941. Drug Metab. Dispos. 2010, 38 (9), 1422–6. [DOI] [PubMed] [Google Scholar]
- (60).Shukla S; Ohnuma S; Ambudkar SV Improving cancer chemotherapy with modulators of ABC drug transporters. Curr. Drug Targets 2011, 12 (5), 621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Tiwari AK; Sodani K; Dai CL; Abuznait AH; Singh S; Xiao ZJ; Patel A; Talele TT; Fu L; Kaddoumi A; Gallo JM; Chen ZS Nilotinib potentiates anticancer drug sensitivity in murine ABCB1-, ABCG2-, and ABCC10-multidrug resistance xenograft models. Cancer Lett. 2013, 328 (2), 307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Shi Z; Tiwari AK; Shukla S; Robey RW; Singh S; Kim IW; Bates SE; Peng X; Abraham I; Ambudkar SV; Talele TT; Fu LW; Chen ZS Sildenafil reverses ABCB1- and ABCG2-mediated chemotherapeutic drug resistance. Cancer Res. 2011, 71 (8), 3029–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Imai Y; Yamagishi H; Ono Y; Ueda Y Versatile inhibitory effects of the flavonoid-derived PI3K/Akt inhibitor, LY294002, on ATP-binding cassette transporters that characterize stem cells. Clinical and translational medicine 2012, 1 (1), 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Robey RW; Obrzut T; Shukla S; Polgar O; Macalou S; Bahr JC; Di Pietro A; Ambudkar SV; Bates SE Becatecarin (rebeccamycin analog, NSC 655649) is a transport substrate and induces expression of the ATP-binding cassette transporter, ABCG2, in lung carcinoma cells. Cancer Chemother. Pharmacol. 2009, 64 (3), 575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Cuestas ML; Castillo AI; Sosnik A; Mathet VL Downregulation of mdr1 and abcg2 genes is a mechanism of inhibition of efflux pumps mediated by polymeric amphiphiles. Bioorg. Med. Chem. Lett. 2012, 22 (21), 6577–9. [DOI] [PubMed] [Google Scholar]
- (66).Natarajan K; Bhullar J; Shukla S; Burcu M; Chen ZS; Ambudkar SV; Baer MR The Pim kinase inhibitor SGI-1776 decreases cell surface expression of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and drug transport by Pim-1-dependent and -independent mechanisms. Biochem. Pharmacol. 2013, 85 (4), 514–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Elkind NB; Szentpetery Z; Apati A; Ozvegy-Laczka C; Varady G; Ujhelly O; Szabo K; Homolya L; Varadi A; Buday L; Keri G; Nemet K; Sarkadi B Multidrug transporter ABCG2 prevents tumor cell death induced by the epidermal growth factor receptor inhibitor Iressa (ZD1839, Gefitinib). Cancer Res. 2005, 65 (5), 1770–1777. [DOI] [PubMed] [Google Scholar]
- (68).Mittapalli RK; Vaidhyanathan S; Sane R; Elmquist WF Impact of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) on the brain distribution of a novel BRAF inhibitor: vemurafenib (PLX4032). J. Pharmacol. Exp. Ther. 2012, 342 (1), 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Wu CP; Hsiao SH; Sim HM; Luo SY; Tuo WC; Cheng HW; Li YQ; Huang YH; Ambudkar SV Human ABCB1 (P-glycoprotein) and ABCG2 mediate resistance to BI 2536, a potent and selective inhibitor of Polo-like kinase 1. Biochem. Pharmacol. 2013, 86 (7), 904–13. [DOI] [PMC free article] [PubMed] [Google Scholar]