Summary
We report a case of intestinal indolent T-cell lymphoproliferative disease (TCLPD) occurring after the initiation of tumor necrosis factor‒α (TNF-α) inhibitor therapy for resistant Crohn’s disease. A prominent T-cell infiltrate positive for CD8, TIA-1, and T-cell receptor–βF1 was associated with the foci of active inflammation. T-cell receptor gene clonality studies (BIOMED-2) demonstrated monoclonality. After the TNF-α inhibitor treatment was withdrawn, the T-cell infiltrates regressed, but 2 years later, the same monoclonal T-cell infiltrate reappeared at the only site of active inflammation. To the best of our knowledge, this report is the first to show a link between active inflammation and the TCLPD. In addition, it suggests a possible influence of the TNF-α inhibitor treatment on the evolution of the TCLPD. A high degree of suspicion is required in the presence of any unusual lymphoid infiltrate in inflammatory bowel disease to avoid over-looking an indolent TCLPD or misdiagnose an aggressive lymphoma.
Keywords: Indolent T-cell lymphoproliferative disease, Immunosuppression, Inflammatory bowel disease, Tumor necrosis factor–α inhibitor
1. Introduction
Primary T-cell lymphomas of the gastrointestinal tract (GIT), the most common of which is enteropathy-associated T-cell lymphoma, account for almost 15% of all intestinal lymphomas and are aggressive neoplasms that do not respond to current therapies.
We describe an indolent CD8-positive cytotoxic αβ T-cell lymphoproliferative disease (TCLPD) in a patient treated with tumor necrosis factor–α (TNF-α) inhibitor for resistant Crohn’s disease. TNF-α inhibitors are increasingly used in the setting of autoimmune and chronic inflammatory disease in patients unresponsive to conventional treatments, including idiopathic inflammatory bowel disease (IBD). Data from the FDA adverse events reporting system and a review of published cases demonstrate an increased risk for the development of malignant lymphoma, especially hepatosplenic T-cell lymphoma (HSTCL), in Crohn’s disease treated by immunosuppressive therapies including TNF-α inhibitors [1]. In contrast, most studies do not find any significant increase in the incidence of lymphoma in the absence of immunosuppressive therapy, neither in the total IBD population nor in patients with Crohn’s colitis [2,3].
Indolent TCLPD of the GIT is a recently described rare entity with only a few cases reported since the 1990s [4–9]. Several cases occurred in patients with idiopathic IBD including Crohn’s disease, and some of them were previously treated by immunosuppressive therapy [7,8]. These cases have variable phenotypes including CD4-positive T-cells, CD4/CD8 double negative, whereas most are CD8-positive, but nearly all are αβ T-cells. The distinction between indolent TCLPD and an HSTCL of the GIT is still mostly based on the clinicopathological features. The histologic features of this entity, in conjunction with the clonality of the T-cell infiltrate in the context of an immunosuppressive treatment, can lead to confusion with HSTCL and to an unnecessarily aggressive therapy.
2. Materials and methods
2.1. Clinical story
A 27-year-old woman presented with a 15-year history of IBD diagnosed as Crohn’s disease after several relapses. She did not undergo any surgical intervention during the course of her disease. She was initially treated with 5-aminosalycilic acid (Rafassal), and steroid enemas were added later. After 9 years, she became unresponsive; azathioprine was added, and after 21 months, discontinued due to leukopenia and patient intolerance. Endoscopic biopsies during this period were consistent with IBD. As a consequence of a poor response to the treatment, TNF-α inhibitor (adalimumab, Humira) was initiated without improvement after 7 months of therapy. The colonoscopy performed then showed a small inflammatory pseudopolyp with severe inflammation in the sigmoid colon, polypoid prominences in the ascending colon, edema and inflammation of the cecum without noticeable mass. Multiple biopsies were taken from the cecum to the rectum. At that time, her blood count and LDH levels were within normal limits. No hepatosplenomegaly was demonstrated by physical examination or imaging studies. Serologic studies were negative for Epstein-Barr virus, cytomegalovirus, and human immunodeficiency virus.
2.2. Immunohistochemistry
Three-micrometer formalin-fixed paraffin-embedded (FFPE) sections were processed using an automated immunostainer (Benchmark; Ventana Medical Systems, Phoenix, AZ, USA) and stained with antibodies against CD3, CD2, CD4, CD8, CD5, CD7, CD10, Granzyme B, TIA-1, CD56, CD57, TdT, Bcl2, CD20, CD23, and T-cell receptor (TCR)–βF1.
2.3. Clonality studies
Molecular analysis for TCRG, TCRB, and TCRD genes rearrangement was performed after DNA isolation from the paraffin blocks of the intestinal biopsies, using the polymerase chain reaction (PCR) and GeneScan analysis, according to the recommendations and the established BIOMED-2 protocols of the EuroClonality consortium [10].
2.4. Genetic study
Mutational analysis was performed on DNA extracted from selected FFPE biopsies with prominent infiltration by CD8-positive and clonal T-cell populations, using next-generation sequencing approach targeting 40 genes involved in T-cell lymphomagenesis. Among the genes included in the panel are JAK1, JAK2, JAK3, STAT3 STAT5B, TET2, DMNT3A, RHOA, IDH1, IDH2, NOTCH1, NRAS, KRAS, HRAS, BRAF, and PIK3CA.
2.5. Histological, immunophenotypic, and molecular findings
The biopsies demonstrated foci of active chronic inflammation in the cecum, ascending colon, and sigmoid. The foci of active IBD were associated with a dense infiltrate of small lymphocytes widening the lamina propria without crypts destruction, necrosis, or ulcerations. The normal mucosa was devoid of lymphoid infiltrates. Most of the small lymphocytes were positive for CD3, CD2, CD5, CD7, CD8, TCR-βF1, and TIA-1, and negative for Granzyme-B, CD56, and CD57, with few CD4-positive T-cells (CD4/CD8 ratio approximately 1:4) and tiny aggregates of CD20+ B-cells (Fig. 1). Those findings initially raised a consideration for HSTCL, a recognized complication of immunosuppressive treatment [1,11]. Immunostain for cytomegalovirus was negative. In situ hybridization revealed no evidence of Epstein-Barr virus EBER-1 DNA. GeneScan analyses of the BIOMED-2 PCR studies for T-cell clonality on TCRG, TCRB, and TCRD genes, performed on lymphoid infiltrates associated with the foci of inflammation, disclosed a monoclonal population positive for TCRG and TCRB (Fig. 1 H–I) and negative for TCRD (data not shown), consistent for αβ T-cells. The normal mucosa was free of lymphoid infiltrates and then no clonality studies were performed on these biopsies. The bone marrow biopsy showed a small interstitial infiltrate of CD8- and TIA-1–positive T-cells, only a few CD4-positive T-cells, and no intrasinusoidal T-cell infiltrate. The molecular studies for TCRG gene rearrangement performed on the FFPE tissue from the bone marrow biopsy demonstrated the presence of the same αβ T-cell clone (data not shown). An upper GIT endoscopy was subsequently performed and did not reveal involvement by the IBD or by the TCLPD. Review of previous biopsies up to 6 years before did not show TCR gene rearrangement (Fig. 2A), although the CD4/CD8 ratio was abnormal with a predominance of CD8-positive T-cells in the infiltrates associated with the active inflammation (Fig. 2A). Targeted sequencing of 40 genes associated with several subgroups of T-cell lymphoma was performed on selected FFPE biopsies showing a high percentage of clonal CD8-positive T-cells but failed to detect any mutations. In particular, mutations associated with HSTCL and aggressive enteropathy-associated T-cell lymphoma of both γ/δ and α/β T-cell origin lymphomas (including STAT5B, STAT3, JAK1, and JAK3) were not detected ([12] and unpublished data–MR).
Fig. 1.
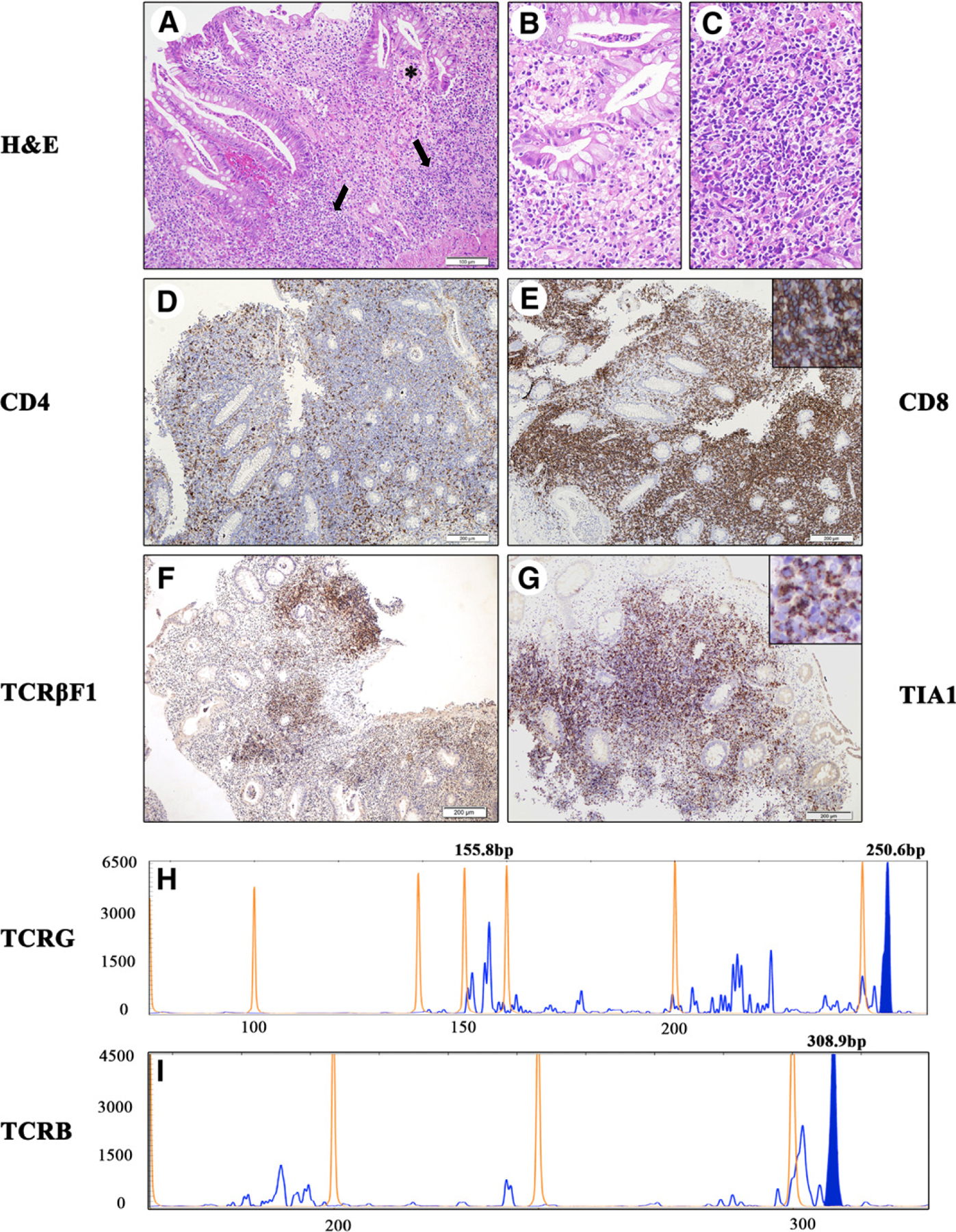
Representative features of the endoscopic colonic biopsy with foci of indolent T-cell LPD at diagnosis (2013). A-I, Histology and immunohistochemistry. A, Infiltrates of indolent TCLPD (arrows) are associated with the foci of active IBD (*). The colonic mucosa shows the classic picture of active chronic IBD with numerous eosinophils and proliferation of small blood vessels with prominent endothelial cells in the lamina propria. The crypts are deformed and infiltrated by neutrophils with epithelial reactive changes and formation of crypt abscesses. B, Multiple infiltrates of small lymphocytes expand the lamina propria of the inflamed mucosa without crypt destruction. The indolent TCLPD infiltrate is composed by small lymphocytes with irregular nucleus and scant clear cytoplasm; few lymphocytes infiltrate the crypts without destruction of the epithelium. C, Hematoxylin and eosin stain, original magnification, ×200; scale bar, 100 μm. D-G, Most small lymphocytes in the infiltrate express (E) CD8, (F) TCRβ-F1, and (G) TIA-1, a phenotype of inactivated cytotoxic αβ T-cells. Only a few cells express (D) CD4 with an inverted CD4/CD8 ratio. (D-G, original magnification, ×100; scale bar, 200 μm). H-I, The BIOMED-2 PCR studies for (H) TCRG and (I) TCRB gene rearrangement after TNF-α inhibitor therapy revealed a reproducible monoclonal peak, which is observed at 309 bp for TCRB (I, expected range, 170–210 bp, 285–325 bp) and 250 bp for TCRG (H, expected range, 145–255 bp).
Fig. 2.
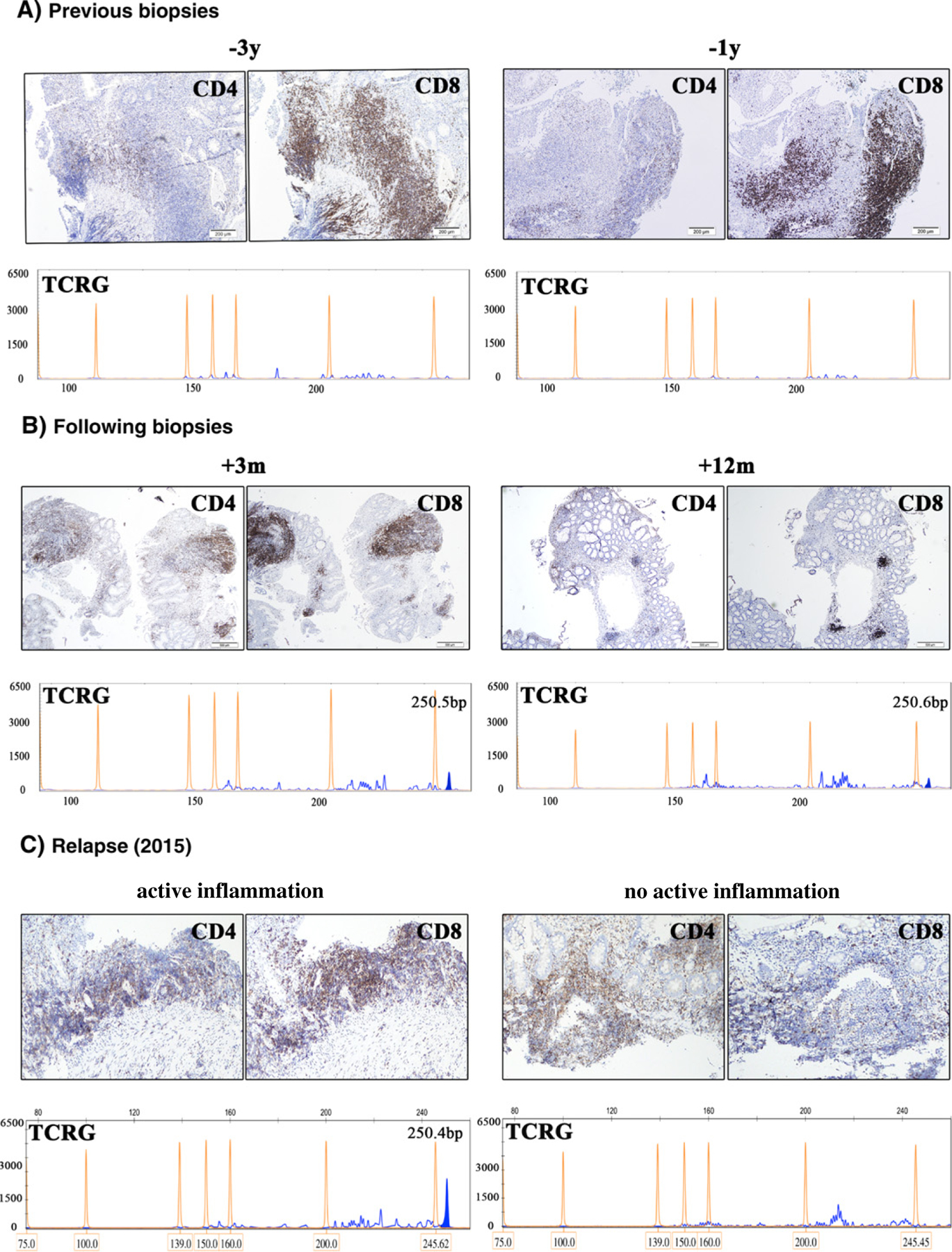
CD4 and CD8 immunostains of the lymphoid infiltrates and PCR studies for TCRG gene rearrangement before and after TNF-α inhibitor treatment. A, Before the occurrence of the indolent TCLPD. The colonic biopsies taken 3 years (2010) and 1 year (2012) prior the development of the indolent TCLPD show the same picture of a more or less prominent lymphocytic infiltrate closely associated with the foci of active inflammation and predominantly composed of CD8-positive T-cells with a marked inversed CD4/CD8 ratio. (Original magnification, ×100; scale bar, 200 μm). The BIOMED-2 PCR studies for TCRG gene rearrangement made from the infiltrates of CD8+ T-cells do not show evidence of monoclonality. B, After withdrawal of the TNF-α inhibitor treatment. Three months and 1 year after the cessation of the TNF-α inhibitor, the follow-up biopsies demonstrate a progressive attenuation of the T-cells infiltrates with a less prominent CD8-positive population and slight increase in the number of CD4-positive T-cells (original magnification, ×40; scale bar, 500 μm). The TCRG clone at 250 bp progressively regressed to the level of the polyclonal background. C, Representative colonic and terminal ileum biopsies at recurrence of the indolent TCLPD (2015). The active IBD inflammation in sigmoid colon is associated with a predominantly CD8-positive T-cells infiltrate. The TCRG gene rearrangement of this focus demonstrates the recurrence of the clonal peak at 250 bp, identical to the previously observed peak of the indolent TCLPD (2013). The terminal ileum biopsy shows a prominent lymphoid infiltrate without active inflammation. The CD4/CD8 ratio is within normal limits (original magnification, ×100; scale bar, 200 μm). The TCRG gene rearrangement of this infiltrate shows a reactive polyclonal T-cell population.
HSTCL was ruled out because of the lack of B-signs and hepatosplenomegaly at physical examination confirmed by CT scan and abdominal ultrasonography, normal peripheral blood count, absence of intrasinusoidal T-cell infiltrate in the bone marrow biopsy, CD5/CD8/TCR-βF1–positive phenotype, and the TCRB-positive/TCRD-negative gene rearrangements. Assuming that the TCLPD was related to the TNF-α inhibitor treatment, adalimumab prescription was withdrawn after 7 months of treatment. The patient was left with Prednisone treatment only (40 mg) and a “wait and see” follow-up was adopted without any other immunomodulatory medication. The subsequent endoscopic biopsies up to 12 months after the TCLPD diagnosis demonstrate a decrease of the CD8 T-cell infiltrate (Fig. 2B). The clonality analysis shows the regression of the monoclonal peak of TCRG (Fig. 2B) and TCRB gene rearrangements to the background level after the withdrawal of the TNF-α inhibitor. Two years after the cessation of adalimumab (April 2015), 4 months after interruption of the steroid treatment, the endoscopic biopsies from colonoscopy showed a single focus of active inflammation associated with a CD8 T-cell infiltrate (Fig. 2C, left panel). Other biopsies taken from the terminal ileum and along the colon did not demonstrate active inflammation or significant T-cell infiltrate (Fig. 2C, right panel). A PCR study for TCRG gene rearrangement performed from the biopsy of the T-lymphocytes infiltrate in the sigmoid colon showed a monoclonal peak at the same location (250 bp) seen 2 years before, consistent with a relapse of the TCLPD (Fig. 2C, left panel). Lymphoid hyperplasia with no active inflammation was seen in terminal ileum without clonal TCRG gene rearrangement (Fig. 2C, right panel). At the last follow-up in April 2015 with no treatment, an extensive clinical investigation did not reveal any systemic disease or evidence for an aggressive T-cell lymphoma in the GIT, allowing the diagnosis of a still indolent TCLPD.
3. Discussion
We describe an indolent TCLPD with an inactivated CD8-positive cytotoxic αβ T-cell phenotype in a patient with resistant Crohn’s disease treated with TNF-α inhibitor.
Indolent TCLPD of the GIT is a recently described entity with morphologic features mimicking HSTCL, an aggressive disease with a dire prognosis [7]. Only a few cases have been documented, mostly case reports with heterogeneous T-cells phenotypes (CD4+, CD4−/CD8−, CD8+) [4–9], suggesting the existence of different entities with a close clinical behavior. Some reported cases occurred in patients with Crohn’s disease [7,8] at least 2 after TNF-α inhibitor therapy (adalimumab and certolizumab) [7]. In addition to these previous reports, to the best of our knowledge, our case is the first to demonstrate a correlation between the active inflammation and the CD8-positive T-cells infiltrates (Figs. 1 and 2), and suggests a possible association between the adalimumab treatment and the development of the TCLPD.
The CD8 polyclonal T-cell aggregates observed in the biopsies before the initiation of the TNF-α inhibitor treatment were only associated with the IBD active inflammation, pointing to a link between the acute inflammatory process and the CD8 T-cells infiltrates. The development of a clonal subpopulation with an identical phenotype in the T-cell infiltrates, shortly after the introduction of the TNF-α inhibitor therapy, suggests that an inflammation-related TNF-pathway could be a key factor in the development of the TCLPD. Consistent with this hypothesis, a recent study of colitis in mice demonstrated the protective role of TNF-receptor 2 (TNFR2, TNFR1b) through the growth inhibition of the colonic mucosal CD8 T-cells [13,14]. TNFR2 has been associated with susceptibility to IBD, through the control of the expression of genes that regulate CD8 T-cells [15,16]. It has been demonstrated that the CD8 T-cells present in the lamina propria of the colonic mucosa play a critical role in the protection against inflammation [17] and in the pathogenesis of resistant Crohn’s disease [18]. Disturbance of TNFR2 signaling might therefore be associated with the pathogenesis of IBD, via unrestricted proliferation of intramucosal CD8 T-cells in reaction to the inflammatory stimulus [13].
Of note, the patient showed a consistent inverted CD4/CD8 ratio of the T-cells associated with the active inflammation before the development of the TCLPD (Fig. 2A), as shown also by the flow cytometry studies of her peripheral blood and bone marrow aspiration, suggesting a dysfunctional CD8 T-cell population. In 20 Crohn’s disease control cases we examined, the CD4/CD8 ratio of the lymphoid aggregates associated with acute inflammation was within the normal limits (data not shown). Therefore, we postulate that in our reported case, the intramucosal CD8 T-cells are dysfunctional and not normally responding to the TNF-α regulatory pathway. Thus, the observed inverted CD4/CD8 ratio leads to the development of the colitis and the close association of the CD8 T-cell infiltrates with the foci of active inflammation. Hence, introduction of TNF-α inhibitor in this context could have led to the emergence of the CD8 T-cell clone. However, further studies should be performed to confirm or invalidate this hypothesis, particularly genomic studies for specific mutations aimed at the genes involved in the TNF-α/TNFR1/TNFR2 pathway for inflammatory response regulation of the CD8 T-cells in resistant IBD.
The prevalence of IBD is now roughly 0.5% of the total population, Crohn’s disease representing nearly 50% of the IBD [19]. In a recent European publication about management of IBD, 20% of the patients are reported to be treated with TNF-α inhibitors [20], and this number is still growing. Our report suggests the need for a screening of the CD4/CD8 ratio and to exclude any dysfunction of the CD8 T-lymphocytes, before initiating a treatment with TNF-α inhibitors in IBD patients. Moreover, any unusually prominent lymphoid infiltrate needs to be analyzed with a high index of suspicion to exclude a monoclonal TCLPD.
Acknowledgments
We thank the team of the Institute of Tissue Diagnostics and Cancer Research laboratory for excellent technical assistance.
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest. No funds were received for this work.
References
- [1].Selvaraj SA, Chairez E, Wilson LM, Lazarev M, Bass EB, Hutfless S. Use of case reports and the adverse event reporting system in systematic reviews: overcoming barriers to assess the link between Crohn’s disease medications and hepatosplenic T-cell lymphoma. Syst Rev 2013;2:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Vos AC, Bakkal N, Minnee RC, et al. Risk of malignant lymphoma in patients with inflammatory bowel diseases: a Dutch nationwide study. Inflamm Bowel Dis 2011;17:1837–45. [DOI] [PubMed] [Google Scholar]
- [3].Lewis JD. Risk of lymphoma in patients with inflammatory bowel disease. Gastroenterol Hepatol 2012;8:45–7. [PMC free article] [PubMed] [Google Scholar]
- [4].Egawa N, Fukayama M, Kawaguchi K, et al. Relapsing oral and colonic ulcers with monoclonal T-cell infiltration. A low grade mucosal T-lymphoproliferative disease of the digestive tract. Cancer 1995;75: 1728–33. [DOI] [PubMed] [Google Scholar]
- [5].Hirakawa K, Fuchigami T, Nakamura S, et al. Primary gastrointestinal T-cell lymphoma resembling multiple lymphomatous polyposis. Gastroenterology 1996;111:778–82. [DOI] [PubMed] [Google Scholar]
- [6].Ranheim EA, Jones C, Zehnder JL, Warnke R, Yuen A. Spontaneously relapsing clonal, mucosal cytotoxic T-cell lymphoproliferative disorder: case report and review of the literature. Am J Surg Pathol 2000;24: 296–301. [DOI] [PubMed] [Google Scholar]
- [7].Perry AM, Warnke RA, Hu Q, et al. Indolent T-cell lymphoproliferative disease of the gastrointestinal tract. Blood 2013;122:3599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Leventaki V, Manning JT Jr, Luthra R, et al. Indolent peripheral T-cell lymphoma involving the gastrointestinal tract. HUM PATHOL 2014;45:421–6. [DOI] [PubMed] [Google Scholar]
- [9].Margolskee E, Jobanputra V, Lewis SK, Alobeid B, Green PH, Bhagat G. Indolent small intestinal CD4+ T-cell lymphoma is a distinct entity with unique biologic and clinical features. PLoS One 2013;8:e68343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].van Dongen JJ, Langerak AW, Bruggemann M, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 concerted action BMH4-CT98–3936. Leukemia 2003;17:2257–317. [DOI] [PubMed] [Google Scholar]
- [11].Schmidt LA, Lim MS. T cell lymphoproliferative disorders associated with anti-tumor necrosis factor alpha antibody therapy for ulcerative colitis: literature summary. J Hematop 2009;2:121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kucuk C, Jiang B, Hu X, et al. Activating mutations of STAT5B and STAT3 in lymphomas derived from gammadelta-T or NK cells. Nat Commun 2015;6:6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Punit S, Dube PE, Liu CY, Girish N, Washington MK, Polk DB. Tumor necrosis factor receptor 2 restricts the pathogenicity of CD8(+) T cells in mice with colitis. Gastroenterology 2015;149:993–1005 [e1002]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dube PE, Punit S, Polk DB. Redeeming an old foe: protective as well as pathophysiological roles for tumor necrosis factor in inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol 2015;308:G161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Twu YC, Gold MR, Teh HS. TNFR1 delivers pro-survival signals that are required for limiting TNFR2-dependent activation-induced cell death (AICD) in CD8+ T cells. Eur J Immunol 2011;41:335–44. [DOI] [PubMed] [Google Scholar]
- [16].Kim EY, Teh SJ, Yang J, Chow MT, Teh HS. TNFR2-deficient memory CD8 T cells provide superior protection against tumor cell growth. J Immunol 2009;183:6051–7. [DOI] [PubMed] [Google Scholar]
- [17].Brimnes J, Allez M, Dotan I, Shao L, Nakazawa A, Mayer L. Defects in CD8+ regulatory T cells in the lamina propria of patients with inflammatory bowel disease. J Immunol 2005;174:5814–22. [DOI] [PubMed] [Google Scholar]
- [18].Lee JC, Lyons PA, McKinney EF, et al. Gene expression profiling of CD8+ T cells predicts prognosis in patients with Crohn disease and ulcerative colitis. J Clin Invest 2011;121:4170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142:46–54, e42 [quiz e30]. [DOI] [PubMed] [Google Scholar]
- [20].Rahier JF, Magro F, Abreu C, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis 2014;8: 443–68. [DOI] [PubMed] [Google Scholar]


