Abstract
Background
Persistent air leaks after thoracic trauma are associated with significant morbidity. To evaluate a novel pectin sealant in a swine model of traumatic air leaks, we compared a pectin biopolymer to standard surgical and fibrin-based interventions.
Methods
A standardized lung injury was created in male Yorkshire swine. Interventions were randomized to stapled wedge resection (N=5), topical fibrin glue (N=5), fibrin patch (N=5) and a pectin sealant (N=6, Figure). Baseline, pre- and post-intervention tidal volumes (TV) were recorded. Early success was defined as the return to near-normal TV (≥ 95% of baseline). Late success was defined as no detectable air leak in the chest tube after chest closure.
Results
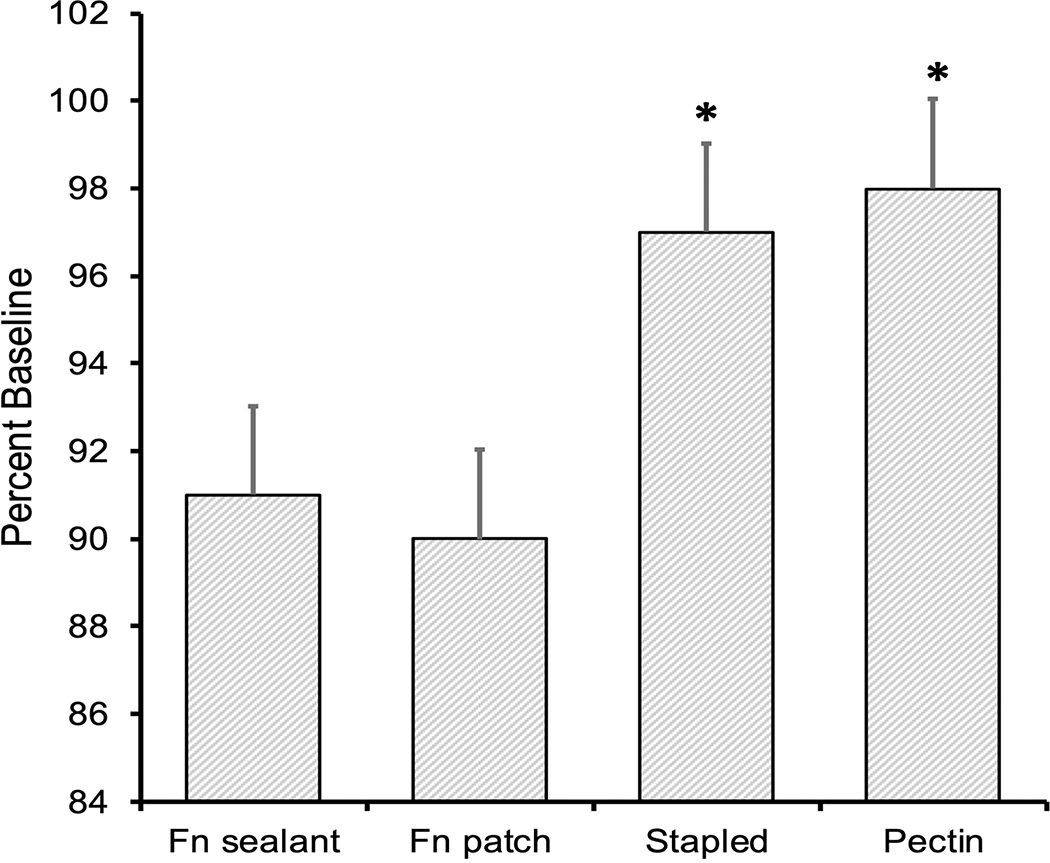
There were no differences in injury severity between groups (mean TV loss = 62ml±17, p =0.2). Early success was appreciated in 100% (N=6) of the pectin interventions which was significantly better than the fibrin sealant (20%, N=1), fibrin patch (20%, N=1) and stapled groups (80%, N=4, p=0.01). The percent of return to baseline TV after sealant intervention was significantly increased in the pectin (98%) and staple arms (97%) compared to the fibrin sealant (91%) and fibrin patch arms (90%) (p = 0.02; p = 0.03). Late success was also improved with the pectin sealant: no air leak was detected in 83% of the pectin group compared to 40% in the stapled group (p=0.008)—90% of the fibrin-based interventions resulted in continuous air leaks (p=0.001).
Conclusion
Pectin-based bioadhesives effectively seal traumatic air leaks upon application in a porcine model. Further testing is warranted as they may provide a superior parenchymal-sparing treatment option for traumatic air leaks.
Level of Evidence
Not applicable as an animal study
Study Type
Orginal Article
Keywords: Pectin, Mesothelium, Air leak, Pleural injury, Parenchymal sparing
INTRODUCTION
Pleural injury from traumatic laceration of the lung leads to an associated “air leak,” progressive pneumothorax and eventual cardiopulmonary compromise.(1) Air leaks have been observed in nearly 50% of patients presenting with hemothorax or pneumothorax after thoracic trauma.(2) The majority of air leaks will resolve with conservative management but those lasting longer than 48 hours to 7 days are considered persistent air leaks. Although more common in spontaneous pneumothorax and post-operative settings, they can occur after either blunt and penetrating chest injuries. They currently represent a significant clinical challenge with various treatment strategies employed and no current guideline recommendations.(3, 4) According the Joint Theater Trauma Registry (www.jts.amedd.army.mil), combat related injury -including blast injuries- account for 6% of all trauma treated in the military.(5, 6) One third of these injuries will have a lung injury requiring operative intervention—most often managed with wedge resection or suture repair.(7) Although treatment with wedge resections have been advocated early after lung trauma,(2) parenchymal resections are associated with increased morbidity correlating with the amount of lung resected.(8)
Currently, there is no FDA approved or commonly used sealant of visceral organ injuries. Sealants fail because of 1) the nonadhesive surface of the visceral mesothelium, and 2) the disruptive movement of visceral organs. Pressure-sensitive or “tacky” adhesives do not adhere to visceral organs because of the slippery surface glycocalyx. Similarly, sealants based on fibrin (Evicel, Ethicon, Somerville, NJ)(9) or albumin and polyethylene glycol (Progel, Bard-Davol, Freemont, CA)(10) demonstrate weak adhesion to the mesothelium. Albumin-glutaraldehyde adhesives (BioGlue, CryoLife, Kennesaw, GA)(11–13) or cyanoacrylate derivatives (Histoacryl, B. Braun, Melsungen, Germany)(14–16) fail because they have significantly greater cohesion (like-to-like stickiness) than adhesion (like-to-unlike stickiness);(17) that is, the sealants stick to themselves more than they stick to the glycocalyx. Consequently, movement such as changes in organ volume results in the sealant simply peeling off the mesothelial surface.(18)
We have identified a new class of high performance bioadhesives based on the structural heteropolysaccharide called pectin. In contrast to bioadhesives relying on surface chemistry, our pectin-based bioadhesives use the otherwise slippery carbohydrate surface layer (glycocalyx) as an anchor for bioadhesion. The branched-chain structure of pectin heteropolysaccharides mirrors the structure of the glycocalyx and—likely because of these shared physicochemical properties—rapidly entangles with the glycocalyx. The physical entanglement of these branched polymers is analogous to the hook-and-loop structure of commercial Velcro™. Moreover, pectin has flexural properties capable of accommodating changes in lung volume.
Our hypothesis is that the physical entanglement of pectin with the glycocalyx will be superior to chemical or mechanical approaches to the control of visceral pleural air leaks. To test this hypothesis, we created traumatic pleural lacerations in a large animal (porcine) model and compared the sealant properties of pectin biopolymers to fibrin-based products and surgical stapling.
MATERIALS AND METHODS
Animals
Research grade (www.usda.gov) male Yorkshire Swine 3–5-months-old (40–50 kg) were used for all experiments. To maximize utilization, animals were used for unrelated pharmacologic experiments for 2–4 hours then humanely euthanized for post-mortem thoracic investigations. Institutional Animal Care and Use Committee application approval was granted prior to all experiments. The care and treatment of the animals uniformly conformed to the Guide for the Care and Use of Laboratory Animals published by the Institute of Laboratory Animal Research established in the Department of Clinical Investigation at Madigan Army Medical Center (Tacoma, WA, USA).
Anesthesia, Intubation, and Euthanasia
Animals were sedated with intramuscular ketamine (15–33 mg/kg, Par Pharmaceutical, Woodcliff Lake, NJ) and midazolam (400–500 μg/kg, Par Pharmaceutical, Woodcliff Lake, NJ) prior to the induction of general anesthesia. After adequate general anesthesia, the animal was intubated with a 6.5 French cuffed endotracheal tube (Medtronic, Minneapolis, MN); anesthesia was maintained with 1–3% Isoflurane (Baxter, Deerfield, IL). Ventilation was set to maintain normal airway pressures of 18–22 cm H2O at a rate of 14 breaths/minute and tidal volumes (TV) were recorded (Drager, Lubeck, Germany). Ventilation was sustained for a 5–10 minute prior to intervention to establish a baseline TV and to ensure ventilation and compliance stability. Unrelated pharmacologic experiments, without relevant pulmonary effects, were performed followed by animal euthanasia using intravenous FatalPlus (≥100 mg/kg, Vortech Pharmaceuticals, Dearborn, MI). Euthanasia ensured complete chest wall relaxation and avoided anesthesia-related respiratory variation in subsequent experiments. The euthanized animals were maintained on their baseline ventilatory parameters throughout the experimental period.
Pleural Injury
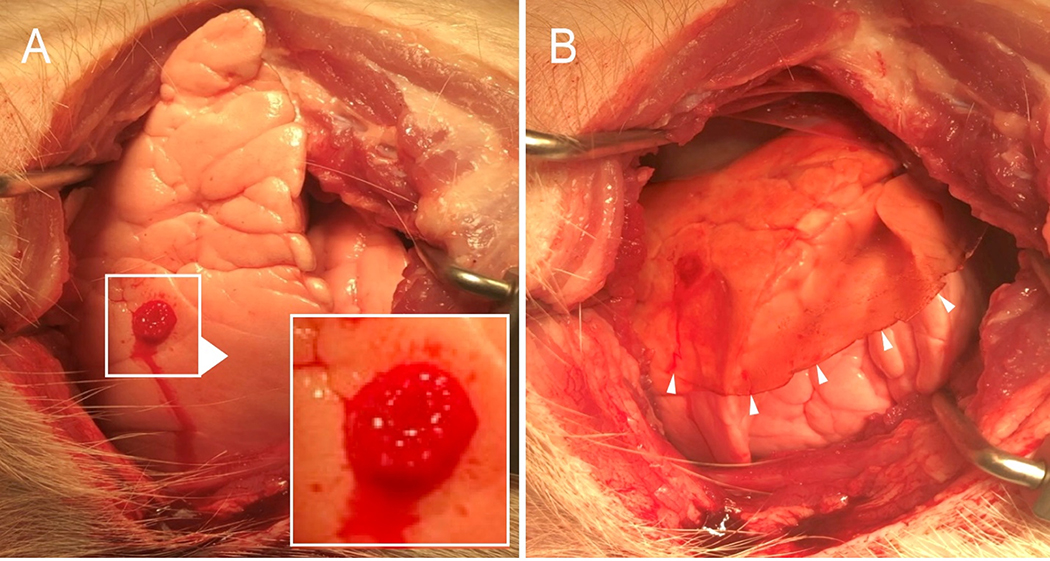
After euthanasia, a 4th interspace anterolateral thoracotomy was performed and the cranial lobe was identified. A standard depth 2–3cm-long pleural laceration was created in the anterior surface of the cranial lobe using a #10 blade (Aspen Surgical, Caledonia, MI). The pleural injury resulted in an immediate decrement in tidal volume up of approximately 10– 15% from baseline TV.
Interventions
After pleural injury, computer randomization was used to assign the animal to one of four interventions: 1) a novel pectin based bioadhesive, 2) fibrin sealant patches (EVARREST™, Ethicon, Somerville, NJ), 3) fibrin sealant bio-adhesive glue (EVICEL™ Fibrin Sealant [Human], Ethicon, Somerville, NJ), or 4) surgically stapled wedge resection of the effected lung. The standardized parenchymal resection removed only the lacerated tissue. Manufacturer guidelines for use were strictly followed during use of the commercially available products. Once interventions were applied, gross evidence of air leak seal and changes in tidal volume were observed and recorded. Following the initial assessment, defined as early success, a 20Fr chest tube (Cardinal Health, Dublin, OH) was placed in the left hemithorax and the thoracotomy was closed using running #0 silk suture (Ethicon, Somerville, NJ) sutures. Animals were observed and ventilated for at least 30 minutes to detect the subsequent development of a late air leak; absence of an air leak was defined as late success. Chest tubes were placed on water seal using an Atrium Oasis™ chest drainage system (Getinge AB, Wayne, NJ). The water seal chamber was used for semi-quantitative air leak detection and trending.
Pectins
Pectin is a naturally occurring heteropolysaccharide represents the structural glue between plant cells responsible for the tensile strength and shear resistance of plants. The preparation and chemical characterization has been documented elsewhere. (19) Briefly, the high-methoxyl pectins (HMP) used in these studies demonstrated a greater than 50% degree of methoxylation. The pectin powder was stored in low humidity at 25oC. The pectin patch was prepared using step-wise fluidization and dispersion with a 10,000rpm rotor-stator mixer (L5M-A, Silverson, East Longmeadow, MA USA) as previously described,(19) The patch was poured into a 5cm diameter circular mold and cured in a 20% relative humidity environment. The cured patch was 100um in thickness, translucent and readily applied to the pleural surface with gentle pressure for up to 1 minute until grossly adhered. The patch was formed in Boston, MA, shipped in sealed petri dishes, and then used in the animal lab in Tacoma, WA.
Data Collection
Effective sealing of the pleural injury after treatment was defined as a return to near-baseline tidal volumes (≥95%). Acute restoration of effective ventilation was considered an early success. The maintenance of the seal was monitored during closure of the thoracotomy and a 30-minute observation period during which ventilatory time tidal volumes and chest drain air leaks were monitored. Late success was defined by effective ventilation (≥95% tidal volume) and minimal chest tube air leak during this second observation period. Air leak severity was further stratified into intermittent and continuous.
Statistics
This study is a pilot study utilizing 5–6 animals in each group. Given that there is no prior data available on the use of pectin biopolymer patches for traumatic pleural air leaks, no power analysis was performed. All data was electronically collected and stored. Linear data with normal distribution was evaluated using analysis of variance (ANOVA) with Tukey post-hoc analysis. Categorical variable were compared using Chi square analysis and Fischer’s exact testing were appropriate. Comparisons were made between the four experimental groups for all data points. Statistical significance was set at a p-value of < 0.05. Statistical analyses were performed using IBM® SPSS® Statistics 25 (IBM Corp., Armonk, NY).
RESULTS
No differences were seen between groups for weight, baseline TV or injury severity (Table 1). Tidal volume improvement after intervention was significantly improved in both the pectin and staple groups when compared to the fibrin-based groups (Table 1). Early success, defined as no air leak after initial sealant application, was appreciated in 100% (N=6) of pectin interventions (Figure 1)—significantly better than the fibrin sealant (N=1, 20%), fibrin patch (N=1, 20%) and stapled groups (N=4, 80%, p=0.01). Late success, defined as no air leak after chest closure, was also improved with pectin at 83% compared to 40% in the stapled group (p=0.008, Table 2). Percent volume improvement (calculated as (TV after treatment/baseline TV)x100) after intervention was significantly better in the pectin (98%) compared to the fibrin sealant (91%) and the fibrin patch (90%, p = 0.02 and p = 0.03 respectively when, see Figure 2). This was also true for the stapled arm (97% improvement) when compared to the sealant (91%, p=0.02) and patch (90%, p = 0.03). Only 1 intermittent air leak was seen in the pectin arm (N=1, 17%) compared to 3 intermittent leaks in the staple group (60%) and 90% (N=9) of the fibrin- based interventions resulting in continuous air leaks (p=0.001, Table 2).
| Table 1. a | Fn Sealant (N=5) | Fn Patch (N=5) | Staple (N=5) | Pectin (N=6) | P Value |
|---|---|---|---|---|---|
| Weight (kg) | 48.2 (SD 2.1) | 48 (SD 1.9) | 47.5 (SD 2.8) | 48.9 (SD 1.4) | 0.76 |
| Baseline TV (ml) | 510 (SD 36) | 543 (SD 53) | 564.8 (SD 62) | 587.3 (SD 47) | 0.25 |
| TV (ml/kg) | 10.6 (SD 1.5) | 11.3 (SD 1.1) | 11.8 (SD 0.9) | 12.01 (SD 1) | 0.21 |
| Air Leak (%)b | 10 (SD 1.8) | 11 (SD 2.1) | 12.4 (SD 2.7) | 11 (SD 1.6) | 0.69 |
| Post Injury TV (ml) | 462 (SD 41) | 479 (SD 48) | 495 (SD 61) | 523 (SD 51) | 0.47 |
| Post Intervention TV (ml) | 464 (SD 90) | 489 (SD 44) | 549 (SD 56) | 575 (SD 48.6) | 0.03 |
| Post treatment TV change from baseline (ml) | 46 (SD 19) | 53 (SD 29) | 15 (SD 6.6) | 12.3 (SD 8) | 0.005 |
Animal weight as well as baseline and post injury tidal volumes with post treatment tidal volumes for each group. P values represent result of ANOVA analysis.
Percent air leak is calculated as 100-[(Post injury TV/Baseline TV)x100]
Fn =Fibrin, kg=kilogram, ml=milliliter, SD=standard deviation
FIGURE 1:
Pleural injury and pectin sealant application. A) Standardized pleural injury with air bubbles visible on the pleural surface. Higher magnification is shown in inset. B) The translucent pectin patch, applied to the injury, is shown. Arrows highlight the pectin margin.
Table 2.
Late Success after Interventiona
| Air leak | Fn Sealant N (%) | Fn Patch N (%) | Staple N (%) | Pectin N (%) |
|---|---|---|---|---|
| Continuous | 5 (100) | 4 (80) | 0 | 0 |
| Intermittent | 0 | 1 (20) | 3 (60) | 1 (17) |
| None | 0 | 0 | 2 (40) | 5 (83)* |
Air leak detected in the water seal chamber after chest closure and 30 minutes of ventilation was graded as continuous, intermittent or absent (none) for each animal in the 4 experimental conditions.
The pectin patch demonstrated significant benefit when compared to the other 3 conditions (p<0.05).
Fn=Fibrin, N=number
FIGURE 2:
Early success after sealant application. The pleural injuries were treated and the efficacy was immediately evaluated by change in tidal volume. The percent return to baseline ventilation is shown. The stapled repair and the pectin patch were significantly improved relative to the fibrin-based sealant and patch (*, p<.01).
DISCUSSION
In this report, we tested surgical repair, fibrin-based sealants and a novel pectin biopolymer for their effectiveness in sealing acute pleural injuries in a large animal (porcine) experimental model. We found that the pectin biopolymer demonstrated effective binding with minimal application pressure and limited development time. Importantly, the pectin biopolymer demonstrated superior control of the air leak both early after application and late after chest closure. We conclude that the pectin-based bioadhesives effectively seal traumatic air leaks in the short term in our porcine model and may provide a superior parenchymal-sparing treatment option for traumatic air leaks.
Penetrating and blunt trauma to the chest occurs in 60% of polytrauma patients and accounts for approximately 25% of mortality in the US.(20, 21) Air leaks related to parenchymal injuries account for roughly one fifth of these injuries with the majority of these patients being successfully managed with chest tube drainage.(22) However, up to 20% of these patients may develop persistent air leaks.(2, 23) Evidence has shown that early operative intervention with lung resection for these patients may lead to shorter hospital stays with improved outcomes.(2, 24–26)
In addition to their relevance in trauma, air leaks are the most common complication after civilian thoracic surgery.(14, 27, 28) Air leaks are important clinical problems because they have a significant impact on patient morbidity and hospital resources. Air leaks are the primary reason for increased hospital length of stay after pulmonary resection.(29, 30) Pleural air leaks increase length of stay by 5 to 13 days; more than doubling of the cost of hospitalization.(31) Economic analyses indicate that air leaks after pulmonary surgery leads to additional complications in both the lung and the pleural space, such as atelectasis, pneumonia, empyema, and the need for chest drains.(32)
Constituted as a 100um thick film, the pectin-based sealant was easily prepared and readily applied to the injured pleura—requiring only a minute of gentle pressure to seal the air leak. In mice, there is clinical and histologic evidence that the sealant persists for the typical 7 day healing process without secondary complications such as pleural effusion or adhesions.(33)
Pectin-based films represent a new class of high-performance sealants and adhesives. In contrast to most pressure-sensitive adhesives, the pectin polymer is not “tacky” to the touch; but with contact to the pleural surface, the pectin polymer became strongly adherent to the pleural surface. In most circumstances, the densely adherent pectin film cannot be removed from the lung surface. The reason for this distinctive behavior is likely a novel mechanism of adhesion; namely, the unique interactions between the pectin and the pleural surface. The pleural glycocalyx is a 12um thick mesopolysaccharide layer anchored to the mesothelial surface. Similar to the structure of pectin, the glycocalyx is comprised of branched-chain polysaccharides. The mutual entanglement of the pectin polysaccharides and glycocalyceal mesopolysaccharide is likely responsible for the adhesive strength observed here.
Finally, the pectin has clinically relevant physical properties; namely, the pectin sealant is a patch and not injectable or sprayable; nonetheless, we note the flexural properties of the pectin are likely sufficient for delivery through a trocar. We speculate that pectin and a minimally-invasive delivery device would have broad application in both military and civilian settings for the control of pleural air leaks.
This study has several important limitations. First, the standardized lung laceration was designed to produce a reproducible air leak between animals, but was representative of only one type of pulmonary injury. Clinical trials will be necessary to assess the performance of pectin patches in a variety of clinical scenarios and adverse conditions. Second, our experiments tested only the short-term efficacy of the pectin at controlling acute air leaks. These tests were compared to currently available fibrin based sealants that do not carry FDA approval for the treatment of air leaks. Future large animal work will need to study the performance of pectin biopolymers over a more clinically relevant 7–10 day time course.CONCLUSIONS
Pectin-based bio adhesives appear to be effective in the control of significant pulmonary air leaks with superiority to fibrin based sealant techniques. This product is a viable potential option for use as a parenchymal sparing technique for persistent air leaks in the posttraumatic setting. Additional investigation of the long-term effect of this novel material is necessary, however, these promising early findings suggest unique pectin based biopolymers may be superior to current treatments ways used to mitigate and may have potential applications even beyond pulmonary parenchymal sealing.
Acknowledgments
Funding: No funding was provided for the completion of this project.
Abbreviations
- LOS
length of stay
- TV
Tidal volume
Footnotes
Conflicts of Interests: No authors have any conflicts of interest to report.
Conflicts of Interest: No authors have any financial disclosures or any conflict of interests to report.
Disclosures: No disclosures
REFERENCES
- 1.Mentzer SJ, Tsuda A, Loring SH. Pleural mechanics and the pathophysiology of air leaks. J Thor Cardiovasc Surg. 2018;155:2182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schermer CR, Matteson BD, Demarest GB, Albrecht RM, Davis VH. A prospective evaluation of video-assisted thoracic surgery for persistent air leak due to trauma. Am J Surg. 1999;177(6):480–4. [DOI] [PubMed] [Google Scholar]
- 3.Lazarus DR, Casal RF. Persistent air leaks: a review with an emphasis on bronchoscopic management. J Thorac Dis. 2017;9(11):4660–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dugan KC, Laxmanan B, Murgu S, Hogarth DK. Management of Persistent Air Leaks. Chest. 2017;152(2):417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Littlejohn LF. Treatment of Thoracic Trauma: Lessons From the Battlefield Adapted to All Austere Environments. Wilderness Environ Med. 2017;28(2):S69–S73. [DOI] [PubMed] [Google Scholar]
- 6.Turner CA, Stockinger ZT, Gurney JM. Combat surgical workload in Operation Iraqi Freedom and Operation Enduring Freedom: The definitive analysis. J Trauma Acute Care Surg. 2017;83(1):77–83. [DOI] [PubMed] [Google Scholar]
- 7.Ivey KM, White CE, Wallum TE, Aden JK, Cannon JW, Chung KK, McNeil JD, Cohn SM, Blackbourne LH. Thoracic injuries in US combat casualties: A 10-year review of Operation Enduring Freedom and Iraqi Freedom. J Trauma Acute Care Surg. 2012;73:S514–S9. [PubMed] [Google Scholar]
- 8.Stewart KC, Urschel JD, Nakai SS, Gelfand ET, Hamilton SM. Pulmonary resection for lung trauma. Ann Thorac Surg. 1997;63(6):1587–8. [DOI] [PubMed] [Google Scholar]
- 9.Pedersen TB, Honge JL, Pilegaard HK, Hasenkam JM. Comparative study of lung sealants in a porcine ex vivo model. Ann Thorac Surg. 2012;94(1):234–40. [DOI] [PubMed] [Google Scholar]
- 10.Fuller C Reduction of intraoperative air leaks with Progel in pulmonary resection: a comprehensive review. J Cardiothorac Surg. 2013;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ennker J, Ennker IC, Schoon D, Schoon HA, Dorge S, Meissler M, Rimpler M, Hetzer R. The impact of gelatin-resorcinol glue on aortic tissue - a histomorphologic evaluation. J Vasc Surg. 1994;20(1):34–43. [DOI] [PubMed] [Google Scholar]
- 12.Hewitt CW, Marra SW, Kann BR, Tran HS, Puc MM, Chrzanowski FA, Tran JLV, Lenz SD, Cilley JH, Simonetti VA, et al. BioGlue surgical adhesive for thoracic aortic repair during coagulopathy: Efficacy and histopathology. Ann Thorac Surg. 2001;71(5):1609–12. [DOI] [PubMed] [Google Scholar]
- 13.Chao HH, Torchiana DF. BioGlue: Alburnin/glutaraldehyde sealant in cardiac surgery. J Cardiac Surg. 2003;18(6):500–3. [DOI] [PubMed] [Google Scholar]
- 14.Petrella F, Borri A, Brambilla D, Calanca G, Vezzani N, Colantoni A, Gasparetto A, Spaggiari L. Efficacy and safety of Innoseal for air leak after pulmonary resection: a case-control study. J Surg Res. 2016;206(1):22–6. [DOI] [PubMed] [Google Scholar]
- 15.Toriumi DM, Raslan WF, Friedman M, Tardy ME. Histotoxicity of cyanoacrylate tissue adhesives. A comparative study. Arch Otolaryngol Head Neck Surg. 1990;116(5):546–50. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan M, Bozkurt S, Kut MS, Kullu S, Demirtas MM. Histopathological effects of ethyl 2-cyanoacrylate tissue adhesive following surgical application: an experimental study. Eur J Cardiothorac Surg. 2004;25(2):167–72. [DOI] [PubMed] [Google Scholar]
- 17.Smith AM, Callow JA. Biological Adhesives. Berlin: Springer-Verlag; 2006. [Google Scholar]
- 18.Annabi N, Yue K, Tamayol A, Khademhosseini A. Elastic sealants for surgical applications. Eur J Pharm Biopharm. 2015;95:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pierce A, Zheng Y, Wagner WL, Scheller HV, Mohnen D, Tsuda A, Ackermann M, Mentzer SJ. Pectin biopolymer mechanics and microstructure associated with polysaccharide phase transitions. J Biol Mat Res Part A. 2019;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feenstra TM, Dickhoff C, Deunk J. Systematic review and meta-analysis of tube thoracostomy following traumatic chest injury; suction versus water seal. Eur J Trauma Emerg Surg. 2018;44(6):819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khandhar SJ, Johnson SB, Calhoon JH. Overview of thoracic trauma in the United States. Thorac Surg Clin. 2007;17(1):1–9. [DOI] [PubMed] [Google Scholar]
- 22.Di Bartolomeo S, Sanson G, Nardi G, Scian F, Michelutto V, Lattuada L. A population-based study on pneumothorax in severely traumatized patients. J Trauma. 2001;51(4):677–82. [DOI] [PubMed] [Google Scholar]
- 23.Carrillo EH, Kozloff M, Saridakis A, Bragg S, Levy J. Thoracoscopic application of a topical sealant for the management of persistent posttraumatic pneumothorax. J Trauma. 2006;60(1):111–4. [DOI] [PubMed] [Google Scholar]
- 24.Carrillo EH, Schmacht DC, Gable DR, Spain DA, Richardson JD. Thoracoscopy in the management of posttraumatic persistent pneumothorax. J Am Coll Surg. 1998;186(6):636–9; discussion 9–40. [DOI] [PubMed] [Google Scholar]
- 25.Fabbrucci P, Nocentini L, Secci S, Manzoli D, Bruscino A, Fedi M, Paroli GM, Santoni S. Video-assisted thoracoscopy in the early diagnosis and management of post-traumatic pneumothorax and hemothorax. Surg Endosc. 2008;22(5):1227–31. [DOI] [PubMed] [Google Scholar]
- 26.Smith JW, Franklin GA, Harbrecht BG, Richardson JD. Early VATS for blunt chest trauma: a management technique underutilized by acute care surgeons. J Trauma. 2011;71(1):102–5; discussion 5–7. [DOI] [PubMed] [Google Scholar]
- 27.Malapert G, Abou Hanna H, Pages PB, Bernard A. Surgical sealant for the prevention of prolonged air leak after lung resection: Meta-analysis. Ann Thorac Surg. 2010;90(6):1779–85. [DOI] [PubMed] [Google Scholar]
- 28.Pompili C, Miserocchi G. Air leak after lung resection: pathophysiology and patients’ implications. J Thorac Dis. 2016;8:S46–S54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irshad K, Feldman LS, Chu VF, Dorval JF, Baslaim G, Morin JE. Causes of increased length of hospitalization on a general thoracic surgery service: a prospective observational study. Can J Surg. 2002;45(4):264–8. [PMC free article] [PubMed] [Google Scholar]
- 30.Bardell T, Petsikas D. What keeps postpulmonary resection patients in hospital? Can Respir J. 2003;10(2):86–9. [DOI] [PubMed] [Google Scholar]
- 31.Varela G, Jimenez MF, Novoa N, Aranda JL. Estimating hospital costs attributable to prolonged air leak in pulmonary lobectomy. Eur J Cardio-Thorac Surg. 2005;27(2):329–33. [DOI] [PubMed] [Google Scholar]
- 32.Brunelli A, Beretta E, Cassivi SD, Cerfolio RJ, Detterbeck F, Kiefer T, Miserocchi G, Shrager J, Singhal S, Van Raemdonck D, et al. Consensus definitions to promote an evidence-based approach to management of the pleural space. A collaborative proposal by ESTS, AATS, STS, and GTSC. Eur J Cardio-Thorac Surg. 2011;40(2):291–7. [DOI] [PubMed] [Google Scholar]
- 33.Servais AB, Kienzle A, Ysasi AB, Valenzuela CD, Wagner WL, Tsuda A, Ackermann M, Mentzer SJ. Structural heteropolysaccharides as air-tight sealants of the human pleura. J Biol Mat Res. 2018;107:799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]