Abstract
The translational value of osteoarthritis (OA) models is often debated because numerous studies have shown that animal models frequently fail to predict the efficacy of therapies in humans. In part, this failing may be due to the paucity of preclinical studies that include behavioral assessments in their metrics. Behavioral assessments of animal OA models can provide valuable data on the pain and disability associated with disease—sequelae of significant clinical relevance. Clinical definitions of efficacy for OA therapeutics often center on their palliative effects. Thus, the widespread inclusion of behaviors indicative of pain and disability in preclinical animal studies may contribute to greater success identifying clinically relevant interventions. Unfortunately, studies that include behavioral assays still frequently encounter pitfalls in assay selection, protocol consistency, and data/methods transparency. Targeted selection of behavioral assays, with consideration of the array of clinical OA phenotypes and the limitations of individual behavioral assays, is necessary to identify clinically relevant outcomes in OA animal models appropriately. Furthermore, to facilitate accurate comparisons across research groups and studies, it is necessary to improve the transparency of methods. Finally, establishing agreed-upon and clear definitions of behavioral data will reduce the convolution of data both within and between studies. Improvement in these areas is critical to the continued benefit of preclinical animal studies as translationally relevant data in OA research. As such, this review highlights the current state of behavioral analyses in preclinical OA models.
Keywords: behavior, pain assessment, osteoarthritis, disease model, rodents
Résumé
La valeur translationnelle des modèles de l’arthrose est souvent débattue, car de nombreuses études ont montré que les modèles animaux ne parvenaient souvent pas à prévoir l’efficacité des traitements chez les humains. Cela peut être dû en partie à la rareté des études précliniques qui comprennent des évaluations de comportement dans leurs données. Les évaluations de comportement des modèles animaux de l’arthrose peuvent fournir des données valables sur la douleur et le handicap associés à la maladie - des séquelles ayant une pertinence clinique significative. Les définitions cliniques de l’efficacité des traitements de l’arthrose sont souvent axées sur leurs effets palliatifs: l’inclusion très répandue des comportements indicateurs de la douleur et du handicap dans les études précliniques peut donc contribuer à une identification plus réussie des interventions cliniquement pertinentes. Les études qui comprennent des essais de comportement rencontrent malheureusement toujours des problèmes lors de sélection des tests, de cohérence de protocole et de transparence des données/méthodes. La sélection ciblée des tests de comportement, en tenant compte de l’éventail des phénotypes d’arthrose cliniques et des limitations des tests eux-mêmes, est nécessaire afin d’identifier correctement les résultats pertinents du point de vue clinique dans les modèles animaux de l’arthrose. De plus, afin de faciliter des comparaisons exactes entre les groupes de recherche et les études, il est nécessaire d’améliorer la transparence des méthodes utilisées. Enfin, établir des définitions claires et convenues de données comportementales permettra de réduire la convolution des données à la fois au sein des études et entre elles. Des améliorations dans ces domaines sont essentielles pour que les études animales précliniques continuent d’apporter des bénéfices translationnels en ce qui concerne les données de recherche sur l’arthrose. La présente analyse met ainsi en lumière l’état actuel des analyses comportementales dans les modèles précliniques de l’arthrose.
Abstract
Über den translationalen Wert von Osteoarthritis (OA)-Modellen wird viel diskutiert, da zahlreiche Studien gezeigt haben, dass Tiermodelle die Wirksamkeit von entsprechenden Therapien beim Menschen oft nicht vorhersagen können. Teilweise dürfte dieses Problem auf die geringe Zahl präklinischer Studien zurückzu-führen sein, die Verhaltensbewertungen in ihre Metriken aufnehmen. Verhaltensbewertungen von tierischen OA-Modellen können wertvolle Daten über die mit Krankheit verbundenen Schmerzen und Behinderungen liefern - Folgen von erheblicher klinischer Relevanz. Klinische Definitionen der Wirksamkeit von OA-Therapeutika konzentrieren sich oft auf ihre palliativen Effekte, daher trägt eine umfassende Einbeziehung von Verhaltensweisen, die auf Schmerzen und Behinderungen hinweisen, in präklinischen Tierstudien möglicherweise zu einem größeren Erfolg bei der Identifizierung klinisch relevanter Interventionen bei. Leider stoßen Studien, die Verhaltensanalysen beinhalten, immer noch häufig auf Tücken bei Assayauswahl, Protokollkonsistenz und Transparenz von Daten/Methoden. Um klinisch relevante Ergebnisse in Open-Access-Tiermodellen angemessen zu identifizieren, ist eine gezielte Auswahl von Verhaltensassays unter Berücksichtigung der Vielzahl klinischer OA-Phänotypen und der Grenzen einzelner Verhaltensassays erforderlich. Um genaue Vergleiche zwischen Forschungsgruppen und Studien zu ermö-glichen, ist es außerdem notwendig, die Transparenz der Methoden zu verbessern. Schließlich dürfte die Festlegung vereinbarter und klarer Definitionen von Verhaltensdaten die Datenfaltung sowohl innerhalb als auch zwischen den Studien verringern. Eine Verbesserung in diesen Bereichen ist entscheidend für den weiteren Nutzen präklinischer Tierversuche für translational relevante Daten in der OA-Forschung. In diesem Sinne unterstreicht dieser Bericht den aktuellen Stand der Verhaltensanalysen in präklinischen OA-Modellen.
Resumen
El valor traslacional de los modelos de osteoartritis (OA) es a menudo objeto de debate, ya que numerosos estudios han demostrado que los modelos animales a menudo no predicen la eficacia de las terapias en humanos. En parte, este fallo puede deberse a la escasez de estudios preclínicos que incluyan evaluaciones del comportamiento en sus mediciones. Las evaluaciones conductuales de los modelos de OA animal pueden proporcionar datos valiosos sobre el dolor y la discapacidad asociados con la enfermedad, secuelas de importancia clínica significativa. Las definiciones clínicas de la eficacia de la terapia de la OA a menudo se centran en sus efectos paliativos; por lo tanto, la inclusión generalizada de conductas indicativas del dolor y la discapacidad en estudios preclínicos en animales puede contribuir a un mayor éxito en la identificación de intervenciones clínicamente relevantes. Desafortunadamente, los estudios que incluyen ensayos conductuales todavía encuentran con frecuencia dificultades en la selección de ensayos, la consistencia del protocolo y la transparencia de los datos/métodos. Es necesario realizar una selección selectiva de los ensayos conductuales, teniendo en cuenta la variedad de fenotipos clínicos de la OA y las limitaciones de los ensayos conductuales individuales, para identificar adecuadamente los resultados clínicamente relevantes en los modelos animales de la OA. Además, para facilitar comparaciones precisas entre grupos de investigación y estudios, es necesario mejorar la transparencia de los métodos. Por último, el establecimiento de definiciones claras y consensuadas de los datos sobre el comportamiento reducirá la convolución de los datos tanto dentro de los estudios como entre ellos. La mejora en estas áreas es crítica para el beneficio continuo de los estudios preclínicos en animales como datos relevantes para la traducción en la investigación de la agricultura biológica. Como tal, esta revisión destaca el estado actual de los análisis conductuales en los modelos preclínicos de OA.
Introduction
In biomedical research, animal models are a crucial step in the translational pipeline. However, some discordance exists between animal studies and clinical trials. The predictive value of animal studies varies among the body’s biologic systems,1–7 and currently, less than one in ten promising basic scientific discoveries significantly impact clinical practice within 20 years of discovery.8 This trend extends to osteoarthritis (OA) research, where there has been little success in translating promising preclinical findings to clinical therapies. While shortcomings in preclinical research are not exclusively responsible for this problem, challenges in conducting animal studies can contribute to the lack of clinical impact.
OA presents as a collection of disease phenotypes with shared features.9 Thus, developing models of OA requires balancing clinically relevant etiology, measurable sequelae, and the target OA phenotype for a potential therapy. Furthermore, preclinical researchers must identify critical OA symptoms without a direct means of communicating with their subjects. However, even in clinical studies, patient reports of pain can vary and be subject to confounding factors.10–14 Attempting to establish similar metrics in animals is nontrivial.
Behavioral assays should allow researchers to examine the symptomatic changes in animals in a controlled, repeatable manner. However, animal behavior is complex, quantifying behaviors is challenging, and inadequate reporting can make it difficult to compare behavioral data between studies. To be clear, behavioral analysis in animal models has been a powerful tool for preclinical researchers, as preclinical research affords a level of experimental control that is difficult to achieve in clinical studies. As such, animal models can provide a platform for in-depth studies of specific OA etiologies, and repeatable behavioral assay design, execution, and reporting would help to improve the robustness of these studies.
Just as a single OA animal model cannot equally replicate all OA phenotypes, so too “OA symptoms” cannot be adequately quantified by a single behavioral test. In her seminal book on behavioral phenotyping in mice, What’s Wrong with My Mouse?,15 Dr. Jacqueline Crawley describes the importance of putting multiple assays together in behavioral testing to form “an optimal constellation of behavioural tests to address specific hypotheses.” This includes considerations of animal numbers, strain, controls, timing, multiple uses and interactions with the same animal, and experimenter workload. As such, the primary objective of this review is to highlight findings from the most used behavioral assessments in the most common rodent OA models. In so doing, we aim to highlight how and why animal behavioral measures can deviate across OA models and experiments. In addition, we suggest steps that may improve the reliability of behavioral assays, since many factors can confound the outcome of behavioral assays. Most importantly, behavioral assays should aim to mitigate the number of possible variables influencing test outcomes and be transparent in the methodologies used. Improving the quality of behavioral assay design, execution, and reporting will benefit animal model utility, promoting robust assessment of OA diagnostics and therapies. Combined, this review serves to highlight the current state of behavioral analysis of OA-related pain and disability in rodent models.
Literature review criteria
A Pubmed search was conducted for the following: osteoarthritis, animal model(s), mice OR mouse, rat OR rats, pain, disability, behavioral analysis, and ARRIVE guidelines. Additional detailed searches were conducted for common behavioral assessments of rodent OA models, including: gait analysis, open field test, running wheel, Rotorod, incapacitance meter, static weight bearing, mechanical allodynia, and Von Frey testing.
Intersection of OA model and behavioral assay selection
OA animal models include chemical, surgical, spontaneous, or genetic models. The aim of this review is not to review different OA models extensively, as several full-length reviews have been written on these differences.16–19 Instead, we aim to highlight the intersection of OA pathology and behavior, and to discuss why these differences and inconsistencies may be occurring. Table 1 briefly summarizes the pathologic differences and behaviors reported for common OA models.
Table 1.
Common types of OA models and the behaviors reported within these OA phenotypes.
| Model type | Reported histology | Reported behaviors |
|---|---|---|
| Surgical | Cartilage lesions, cartilage thinning, osteophyte formation, loss of proteoglycan staining, synovitis40,89,90 | Mechanical allodynia, altered gait, changes in static weight bearing and other behaviors20,22,55,71,91,92 |
| Tibial overload and noninvasive ACL rupture | Cartilage fibrillation and clefts, proteoglycan loss, subchondral bone sclerosis93 | No behavioral data available to date |
| Mechanical loading | Cartilage thinning, subchondral bone sclerosis, osteophyte formation (at higher loads), cartilage lesions, meniscal ossification, synovial hyperplasia, synovial fibrosis94 | No behavioral data available to date |
| Chemical (MIA and collagenase) | Osteophyte formation (certain models), cartilage lesions (certain models), chondrocyte death, cartilage fibrillation, collapse of cartilaginous matrix95,96,89,97 | Thermal and mechanical sensitivity (thermal or mechanical hyperalgesia, mechanical allodynia), altered gait, altered sleep patterns20,96,98 |
| Spontaneous/genetic | Cartilage lesions, cartilage fibrillation, focal cell loss, proteoglycan loss, apoptosis of deep zone chondrocytes90,99,100 | Behaviors vary by model, but common behaviors include mechanical allodynia, altered gait, sensorimotor dyscoordination, thermal sensitivity, decreased grip strength100–105 |
| High-fat diet/obesity | Cartilage fibrillation, proteoglycan loss, synovitis, focal cell loss, acceleration of histological damage seen when combined with other OA models106,107 | Decreased grip strength (associated with obesity, not OA progression), decreased peak vertical force, mechanical allodynia/hypersensitivity, anxiety-like behavior108 |
OA: osteoarthritis; ACL: anterior cruciate ligament; MIA: monoiodoacetate.
First, in humans, OA is often idiopathic, and while several underlying risk factors are known, there is significant heterogeneity in the etiology, progression, and presentation of OA. Complicating matters further, there is a lack of consensus on what qualifies as an OA model20,21 and which etiological roots different OA models are meant to represent (though there has been some effort to specify OA models as either primary or post-traumatic).17 For example, despite prevalent use in studies of OA-related pain, chemical injection models are known to cause joint damage that is not necessarily characteristic of clinical OA.20 An excellent review by Little and Zaki highlights the need for precision when extrapolating OA model data to the clinical condition, as the molecular mechanisms influencing pain and pathophysiology may be distinct for different OA etiologies.20 Ultimately, there is not a “gold standard” OA model, nor should there be, given the heterogeneity of OA. Different models can provide insight into different aspects of OA. As such, there is a need for specificity in study design, clearly framing the intent behind, and limitations of, a particular OA model.
Applicability of different OA models to human OA
Intra-articular monoiodoacetate (MIA) injection is a widely used model of OA-related pain.22,23 The MIA model produces robust behavioral changes that appear before histological signs of OA, which is consistent with some patient reports.24,25 However, the reasons for clinical development of OA pain prior to joint damage are not fully understood, and whether the MIA model effectively recapitulates these pathways is not known. Additionally, while many OA patients report pain without severe joint damage, many individuals also present severe joint damage without pain.24,26 Finally, the MIA model shares no common etiology with clinical OA, causes severe structural histopathology without fully emulating human OA,27 and has little transcriptional overlap with human OA cartilage.28 Thus, the MIA model (and other chemically induced models) may have limited utility when extending the analysis beyond joint-related pain.
Conversely, joint trauma is a known clinical etiology of post-traumatic OA. As such, partial meniscectomy/transection, meniscus destabilization, and anterior cruciate ligament (ACL) transection are often used as OA models. Several surgically induced models of post-traumatic OA reasonably mimic clinical OA etiologies.27 However, these models have sometimes failed to produce robust behavioral modifications.24,25 A study examining the MIA and partial medial meniscectomy models found partial meniscectomy resulted in less severe pain-related behaviors, despite similar levels of joint damage.24 Finally, surgically induced models tend to cause focal damage, a histopathology reminiscent of post-traumatic OA, rather than the widespread joint damage characteristic of primary OA.29 In addition, surgical models introduce a surgical injury that can be difficult to separate from the modeled joint injury. As such, recently developed noninvasive models of post-traumatic OA, like the noninvasive ACL injury model,30–32 have some advantages over traditional surgical models, as these noninvasive injuries mimic clinical etiologies and avoid surgery-associated damage. Nonetheless, little behavioral data have been collected on these models to date.
Selecting behavioral assays for different OA models
Pain is a complex experience subject to biological, psychological, and social influences.33 There are many approaches for assessing behaviors indicative of pain in animals.5,7,18,34–37 Table 2 describes the most common behavioral assays used in OA models, the typical time commitment for each test, common risks and concerns for the assay, and the relationship (if any) between these behaviors and common clinical symptoms. These assays also have utility in other musculoskeletal diseases, including fracture healing, bone regeneration, tendon and ligament injury, and joint contracture. Moreover, this table is not an exhaustive list. New assays are developed each year, and several assays, like the grimace scale, have not been thoroughly explored in OA models. Importantly, absence of evidence for certain behaviors in OA models should not be considered evidence of absence. Additionally, some behavioral assays are more sensitive than others. For OA models in particular, pain-related behaviors can be very subtle, and highly sensitive assays are necessary. As such, the intersection of animal model and behavioral assay selection can quickly obfuscate meaningful data.
Table 2.
Common OA behavioral tests, typical time commitment for the assay, and the relationship between these behaviors and common OA symptoms.
| Behavioral assay | Time commitment | Risks and concerns | Relation to human OA symptoms |
|---|---|---|---|
| Static weight bearing (incapacitance meter) | 5–10 minutes per animal | Little risk of injury; the enclosures and awkward handling postures may be stressful for the animal | Weight bearing during stance |
| Gait analysis | 20–30 minutes per animal | For overground testing, there is minimal injury risk, and this risk increases for treadmill testing; stress considerations include exposure to bright lights in some systems, prompting motion by the experiment (for non-voluntary exploration), and exposure to a treadmill (for treadmill testing). | Gait characteristics (though quadrupedal gait varies from that of bipedal humans) |
| Motor coordination (Rotorod) | 30–60 minutes for four animals | Some risk to the animal, as the endpoint of the assay is often falling from the rotating rod treadmill; the assay is stressful for the animal, as the animal is trying to stay on a rotating rod | A direct corollary to human symptoms of OA is unknown, though the assay measures sensorimotor coordination in rodents |
| Open field and LABORAS test | 12–24 hours per animal, often requiring individual animal housing | Little risk of injury; the enclosures may be stressful for the animal, as the arena may be unfamiliar to the animal; rodents typically live in colonies, and individual housing can be stressful for a rodent | Spontaneous activity |
| Mechanical allodynia (Von Frey test) | 30–60 minutes for 6–12 animals (depending on cage setup) | Little risk of injury. The enclosures may be stressful for the animal; moreover, the grate and wire floors may affect the animals’ behavior | Heightened sensitivity to light touch |
| Thermal hyperalgesia (Hargreaves’ method, hot plate, and/or tail flick test) | 5–10 minutes per animal | Risks of injury include minor burns and soreness; these assays distinctly cause pain in the animal, and thus are stressful for the animal; moreover, the enclosures for these tests can affect the animals’ behavior | Heightened sensitivity to heat |
As an example of behavioral inconsistencies between OA models, several studies have shown static weight-bearing changes in OA. Statistically significant static weight-bearing asymmetry was identified with an ACL transection (male Sprague Dawley rat),38 medial meniscus transection (male Lewis rat),39 destabilized medial meniscus (male 129/SvEv mouse),40 and the MIA (male Wistar rat) models.24 In contrast, Fernihough et al.24 reported that partial meniscectomy in the male Wistar rat resulted in an insignificant change in static weight bearing. Similarly, Knights et al.41 found no change in static weight bearing in a female C57Bl/6 mouse partial meniscectomy model. However, the same study noted changes in tactile sensitivity and vocalizations subsequent to knee compression, where fully meniscectomized mice displayed persistent sensitivity post surgery with these measures.41 Overall, the differences in behavioral outcomes across rodent OA models may be related to the type of OA model selected, natural differences between rats and mice, variability between different animal strains, and differences between male and female animals.
Simply put, a single assay is unlikely to capture all behavioral changes in all possible OA models. Thus, when characterizing pain-related behaviors in an OA model, a variety of behavioral assays is recommended, when possible. Of course, the number of behavioral tests must balance animal stress and fatigue (see Table 2). In the future, our goal should be to identify the best behavioral assays for specific OA models. However, at this point, behavioral assays have not been consistently evaluated within or broadly evaluated across different models. As such, evaluation of multiple behaviors can help lead to improved experimental design, behavioral assay selection, and scientific robustness in future studies.
Considerations for common OA behavioral assays
Increasing transparency in methods reporting for behavioral assays
With provisions such as the ARRIVE guidelines, methods reporting in animal models has moved toward better transparency.42–44 However, seemingly benign variables can still have significant effects on behavioral outcomes, such as the biological sex of the researcher conducting the test45 or the surface on which the animal stands.46 While the ARRIVE guidelines suggest including details such as housing and husbandry conditions,42–44 these guidelines cannot reasonably cover all possible variables that can impact behavioral assays. Furthermore, although widely endorsed, the ARRIVE guidelines have yet to be thoroughly implemented.47,48 Thus, not only is it difficult (or functionally impossible) to report all potential sources of variation for a behavioral test, many studies still fail to follow currently available guidelines.
As an example, in 1994, Chaplan et al. described a method for evaluating tactile sensitivity in the rat.49 Their method was based on previous tests using Von Frey filaments50–53 and is widely used today. In 1996, Pitcher et al. reported that the surface on which animals stand for Von Frey testing could significantly affect the animals’ withdrawal thresholds.46 Pitcher additionally reported that most studies did not report floor size or material used for Von Frey testing.46 More than 20 years later, floor size and the material of the testing enclosures is still not regularly reported, despite the continued popularity of Chaplan’s method.
One change in the research community that may improve transparency in behavioral assays is the move toward open-source methods and technologies. Many research groups share testing protocols and software on Web sites such as protocols.io and github.com, among others. Our own group hosts a Web site detailing our custom gait analysis system and its associated software (see gaitor.org). Expanding access to shared assays, or at least providing transparency in how the assay is conducted, has the potential to foster community-wide improvements in behavioral testing.
Controlling for common sources of variation
Reporting control data should also be standard for behavioral tests, and internal controls, such as sham procedures, are essential to assess the internal validity of an experiment. However, as OA progresses over long time scales, animals within the experiment may experience several weeks or months of aging. Notably, because of these long time scales, baseline controls are rarely appropriate for OA behavioral testing. Several animal behaviors correlate to animal age, weight, and size. As a primary example, most gait data correlate to the animal’s size.54 Failure to account for these natural confounders can markedly decrease the sensitivity of gait measures in both sham and experimental groups. For these age-, weight-, or size-related effects, historical naïve data may be used to reduce the variability associated with known correlates.55 That said, while historical naïve data can help account for these covariates, historical data cannot replace internal controls, such as sham procedures, which are essential to the assessment of the internal validity of a study.
Reducing subjectivity in behavioral assays
One approach to improving behavioral assays is to reduce potential sources of variation. Chaplan’s up-down method of Von Frey testing calculates a 50% withdrawal threshold based on a series of applied filaments. While the paw withdrawal threshold has proven useful, the Chaplan paper itself reports variability in animal responsiveness over time.56 Furthermore, Chaplan’s method describes paw withdrawal in possibly ambiguous terms: “apparent resolution” of behavioral responses, “brisk withdrawal,” and “flinching.”56 In contrast, behavioral assays such as gait analysis, certain activity monitors, and static weight bearing provide quantitative measures of natural behaviors, removing the need for researchers to interpret a behaviour.57–61 Of course, even assays with quantitative outputs still encounter failures in protocol agreement, as Walsh and Cummins note in their review of open field tests (Table 3).62
Table 3.
Considerations for selecting an open field assay.
| Apparatus design |
|
| Outcome measures (not including different means of reporting similar measures) |
|
| Methods |
|
To be clear, the level of subjectivity in a behavioral assay, by itself, does not mean the assay is more or less valuable for behavioral analysis in OA. In other words, behavioral changes detected by a Von Frey test may have a larger effect size than behavioral changes detected by gait analysis. Nonetheless, reducing the subjectivity within a test can help provide better comparisons between similar behaviors measured in different studies or by different groups.
Reducing animal stress
Another means of improving the reliability of behavioral assays is removing sources of animal stress. For any behavioral test, animals should be acclimated to their housing conditions, the researchers who will be handling them, and the testing equipment and procedures.63 Stress effects have been demonstrated, particularly in behavioral tests requiring animals to be restrained.64,65 Simply incorporating handling methods such as tunnel handling to transport animals in and out of behavioral testing equipment can markedly reduce animal stress.66 Moreover, utilizing behavioral assays without animal immobilization or restraint can potentially reduce animal stress.
For example, activity monitoring systems require neither researcher interaction nor animal restraint,59,60,67 providing quantitative measures of animal activity.59–61,67,68 Additionally, many rodents are most active in the evening, and activity monitoring can be scheduled to account for light/dark cycles. Finally, activity monitoring can often be conducted in the animals’ home cages, allowing researchers to collect behavioral data in a familiar space.59–61,68 Opting to use less stressful behavioral assays, when possible, may reduce data variability related to animals’ natural stress responses.
Adapting to operant testing methods
Operant behavioral tests allow animals to choose to participate, often measuring participation rates as an outcome. Changing a behavioral test to utilize an operant paradigm can affect measured behaviors. As an example, the voluntarily accessed static incapacitance chamber allows animals to self-select participation in the weight-bearing test for an allotted time (30 minutes).69 Here, a water bottle is placed high in a cage, requiring the animal to rear to access the bottle. While drinking, weight bearing is dynamically recorded by an underlying force plate.69 In contrast, incapacitance meters typically place animals in confined spaces but only require the animal to contact the force panels for 30-second intervals.70 Moreover, the cages used for the incapacitance meter range from forced, squat-like postures to extended reaching postures (Figure 1). While similar metrics may be obtained, the environment is clearly different, as is the animal’s posture. Hence, reporting the enclosure and posture characteristics is critical, and utilization of operant methods, when available, may improve behavioral assessments and reduce animal stress.
Figure 1.
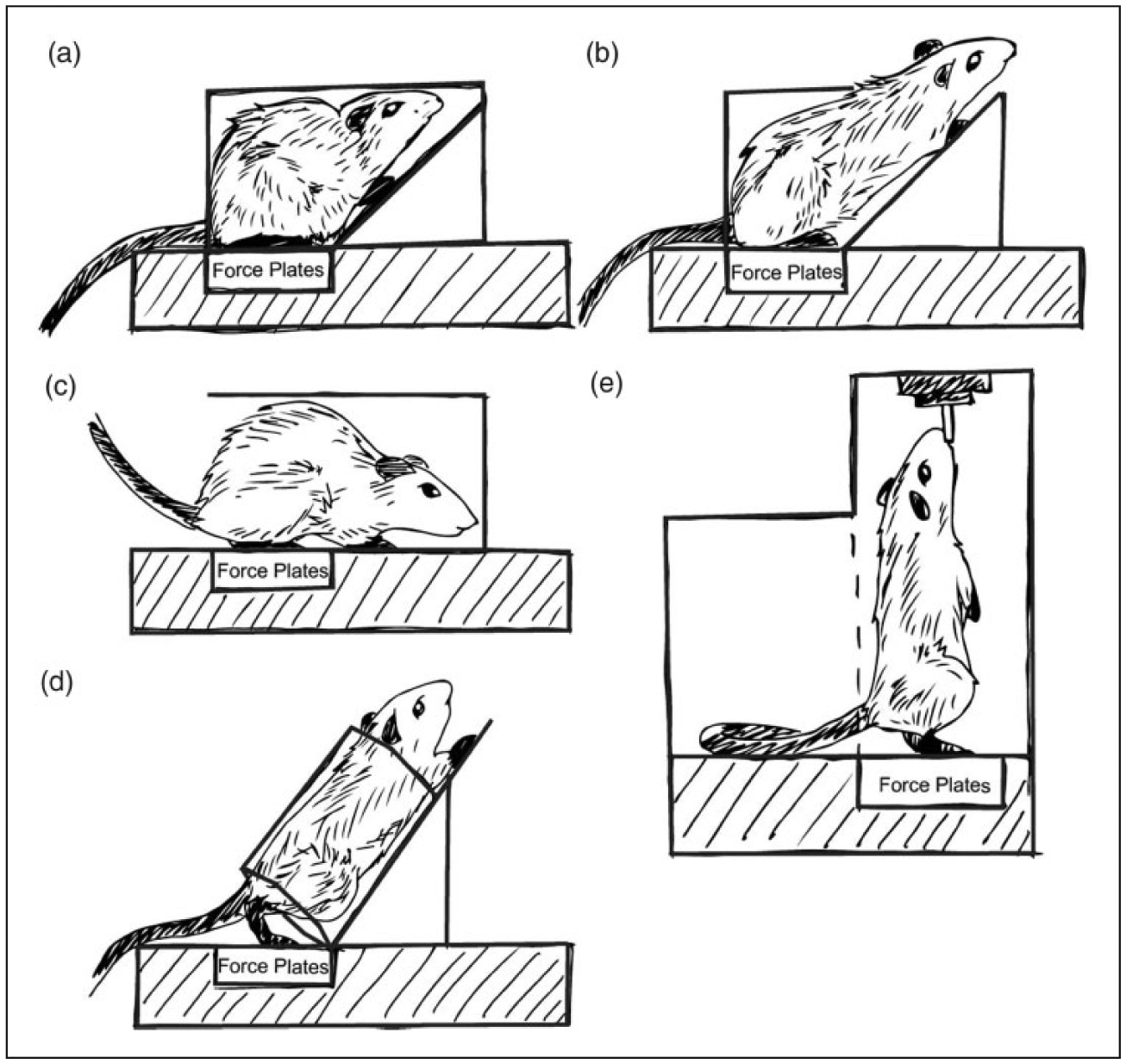
The incapacitance meter, or static weight-bearing assay, is a commonly used tool for assessing changes related to pain and dysfunction in the rodent joint. Despite sharing the same name, static weight-bearing tests can be conducted in a number of apparatuses, as shown. The variations in the animal’s positioning and restraint may affect both the animal’s weight distribution (e.g. more ability to offload weight to the forepaws) and stress.
Evolving as best practices form
While behavioral testing may be improved by increasing methodological transparency, reducing subjectivity within specific assays, and adapting to less stressful methods for the animal, standardization will remain difficult to achieve due to the rapid evolution of the field. Advancing behavioral assays to temper sources of variance further or address other potential limitations is important to the evolution of the field. Moreover, it is important to pair behavioral assays to confirm a symptomatic state in the animal. For example, while Von Frey tests can be prone to subjectivity, the test provides very useful data on paw sensitivity. Thus, pairing Von Frey tests with static weight bearing, activity monitoring, or gait data can provide complementary data indicative of the animals overarching symptomatic state. By working toward establishing broadly accepted tests for OA models, we will be better able to evaluate how and why OA-related symptoms vary as a result of different biological and physiologic factors.
Considerations for reporting behavioral testing results
Failure to adapt similar terminology across studies
In addition to refining behavioral assays, refining data presentation from behavioral assays is also critical. Consensus on which measures are meaningful (and what they mean) has not been reached for all assays. Unfortunately, many behavioral measures are either uncommonly used or have been defined in conflicting ways. While it is entirely valid for groups to employ different behavioral assays, it reduces the utility of these studies when the metrics remain “niche.”
As an example, much like OA may refer to many different etiologies of joint degeneration, “gait analysis” can refer to assays using treadmill walking or overground walking, which may additionally include spatiotemporal and/or dynamic assessments.49,54,58,71–75 Moreover, many gait systems approach data collection in different ways, and the outputs from different systems and software do not always agree or report the same measures.54,76 Some groups have also reported gait changes based on qualitative observation.77–82 These measures may share some terminology with quantitative measures (i.e. stance score vs. stance time), despite lacking any quantitative assessment.
As another example, weight bearing may refer to static or dynamic weight bearing. Bioseb’s kinetic weight-bearing assay is described as a “refined” dynamic weight-bearing test, whereas incapacitance meters typically assess static weight distribution. Further conflating terminology, both static and dynamic weight bearing have been referred to as incapacitance meters, and “dynamic weight bearing” is also a metric obtained by certain gait analysis systems. Furthermore, descriptively similar measures (weight bearing on a given hind limb) may be presented in terms of weight distribution of ipsilateral relative to contralateral limb,70 percent body weight,57 or percent difference between “baseline reading and post-treatment,”22 among other potentially derived ratios.
From these examples, the need for consensus in data presentation becomes apparent. Variation in data presentation poses a few issues. First, a lack of consensus on which measures to report (and how to report them) can hamper both inter- and intra-field communication. Second, as discussed next, reporting variables that are not independent measures can con-volute the data.
Relative independence of behavioral measures
Returning to the example of gait analysis, the DigiGait system (Mouse Specifics, Inc., Quincy, MA) reports more than 50 gait measures.54 In a study comparing DigiGait to TreadScan (CleverSys, Inc., Reston, VA), only five gait measures were reported for comparison, as they were the five measures “often used in existing publications of gait analysis in preclinical models.”76 Consistent differences were observed between the two systems, and it was suggested that the lack of agreement might be due to belt speed.76
Of course, walking velocity correlates with the majority of gait variables, and failure to account for velocity will increase the variability of gait measures.58 A study by Batka et al.83 identified more than 90% of gait variables reported by the CatWalk method were velocity dependent. However, few studies using the system control for speed. A study by Cendelin et al.84 found that most gait differences identified in their study became insignificant when corrected for velocity. Furthermore, while treadmill-based gait systems allow for velocity control, overground walking reduces animal stress. Combined, it is difficult to compare velocity-dependent variables without presenting these data in a manner that accounts for velocity (such as residualizing the data).55 Presenting data as relatively independent measures, such as velocity normalized gait data, could improve the utility of study results to the field at large.
Standardizing definitions
While developing new metrics can be beneficial to characterizing behavioral changes, “novel” measures have sometimes proven to be just new presentations of existing measures. Returning to the example of gait analysis, swing time ratio has been presented as a sensitive parameter defined as the swing time of the ipsilateral limb divided by the subsequent contralateral limb swing time.85 However, swing time is not independent of stance time.86 Thus, swing time ratio provides only a different presentation of stance time imbalance.
Furthermore, accepted metrics are sometimes misinterpreted and consequently presented in misleading ways. In gait analysis, stride length refers to the distance between subsequent footsteps on the same limb, while step length refers to the distance between a left and right footstep along the direction of travel.58 Occasionally, stride length has been incorrectly presented as step length, and comparisons between left and right “strides” are assessed.87 One would anticipate these results to be insignificant, as strides measured by either the right or left limb should be equal unless the animal is moving in a circle. However, step length, with its proper definition of distance between left and right footsteps, can be a significant measure of gait modification.88 Recommendations on conducting and presenting rodent gait data have been published previously by our group.54,58 Similar recommendations for other behavioral assays, for both quantitative and qualitative reporting, could assist with improving these measures across the field. Furthermore, aligning terminology and definitions with clinical and historical definitions, where possible, would help to communicate study results to the broader research community.
Conclusions
Behavioral analysis serves an important role in preclinical studies, allowing researchers to assess clinically poignant consequences of disease. To improve the utility of behavioral tests, researchers should seek parity in their methods, and transparent methods reporting is essential to this goal. Additionally, studies should critically examine the intersection of behavioral assays and the OA model, seeking to understand behaviors indicative of pain and disability in the context of the human OA condition and various OA phenotypes. Finally, there is a need to improve our reporting of behavioral data, using clear definitions and calculations. This is not to suggest that there should be a singular standard array of behavioral assays for OA research at this time. We recognize that many of these assays are still relatively new to the field and have yet to be widely adopted across research groups. Rather, there is a need for pre-clinical researchers to push for broader dissemination of behavioral assay technology, protocols, and techniques to improve the quality of preclinical studies and the reliability of these critical symptomatic data.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the NIH (grant number R01AR071431-01).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Perel P, Roberts I, Sena E, et al. Comparison of treatment effects between animal experiments and clinical trials: systematic review. BMJ 2007; 334: 197–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greaves P, Williams A and Eve M. First dose of potential new medicines to humans: how animals help. Nat Rev Drug Discov 2004; 3: 226–236. [DOI] [PubMed] [Google Scholar]
- 3.Olson H, Betton G, Robinson D, et al. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul Toxicol Pharm 2000; 32: 56–67. [DOI] [PubMed] [Google Scholar]
- 4.Berge OG. Predictive validity of behavioural animal models for chronic pain. Br J Pharmacol 2011; 164: 1195–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whiteside GT, Adedoyin A and Leventhal L. Predictive validity of animal pain models? A comparison of the pharmacokinetic-pharmacodynamic relationship for pain drugs in rats and humans. Neuropharmacology 2008; 54: 767–775. [DOI] [PubMed] [Google Scholar]
- 6.Villanueva L. Is there a gap between preclinical and clinical studies of analgesia? Trends Pharmacol Sci 2000; 21: 461–462; author reply 465. [DOI] [PubMed] [Google Scholar]
- 7.Blackburn-Munro G. Pain-like behaviours in animals – how human are they? Trends Pharmacol Sci 2004; 25: 299–305. [DOI] [PubMed] [Google Scholar]
- 8.Contopoulos-Ioannidis DG, Ntzani EE and Ioannidis JPA. Translation of highly promising basic science research into clinical applications. Am J Med 2003; 114: 477–484. [DOI] [PubMed] [Google Scholar]
- 9.Lane NE, Brandt K, Hawker G, et al. OARSI-FDA initiative: defining the disease state of osteoarthritis. Osteoarthritis Cartilage 2011; 19: 478–482. [DOI] [PubMed] [Google Scholar]
- 10.Young EE, Lariviere WR and Belfer I. Genetic basis of pain variability: recent advances. J Med Genet 2012; 49: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Brien EM, Waxenberg LB, Atchison JW, et al. Intraindividual variability in daily sleep and pain ratings among chronic pain patients. Clin J Pain 2011; 27: 425–433. [DOI] [PubMed] [Google Scholar]
- 12.Stone AA, Schwartz JE, Broderick JE, et al. Variability of momentary pain predicts recall of weekly pain: a consequence of the peak (or salience) memory heuristic. Pers Soc Psychol Bull 2005; 31: 1340–1346. [DOI] [PubMed] [Google Scholar]
- 13.Harris RE, Williams DA, McLean SA, et al. Characterization and consequences of pain variability in individuals with fibromyalgia. Arthritis Rheum 2005; 52: 3670–3674. [DOI] [PubMed] [Google Scholar]
- 14.Schneider S, Junghaenel DU, Keefe FJ, et al. Individual differences in the day-to-day variability of pain, fatigue, and well-being in patients with rheumatic disease: associations with psychological variables. Pain 2012; 153: 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crawley JN. What’s wrong with my mouse? Behavioral phenotyping of transgenic and knockout mice. 2nd ed. Hoboken, NJ, Wiley-Interscience. [Google Scholar]
- 16.D’Souza NW, Ng YG, Youngblood DB, et al. A review of current animal models of osteoarthritis pain. Curr Pharm Biotechnol 2011; 12: 1596–1612. [DOI] [PubMed] [Google Scholar]
- 17.Kuyinu EL, Narayanan G, Nair LS, et al. Animal models of osteoarthritis: classification, update, and measurement of outcomes. J Orthop Surg Res 2016; 11: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teeple E, Jay GD, Elsaid KA, et al. Animal models of osteoarthritis: challenges of model selection and analysis. AAPS J 2013; 15: 438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bendele AM, Pelletier JP, Kapoor M, et al. Animal models of osteoarthritis. J Musculoskelet Neuronal Interact 2001; 1: 363–376. [PubMed] [Google Scholar]
- 20.Little CB and Zaki S. What constitutes an ‘animal model of osteoarthritis’ – the need for consensus? Osteoarthritis Cartilage 2012; 20: 261–267. [DOI] [PubMed] [Google Scholar]
- 21.Malfait A-M and Little CB. On the predictive utility of animal models of osteoarthritis. Arthritis Res Ther 2015; 17: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guzman RE, Evans MG, Bove SE, et al. Mono-iodoace-tate-induced histologic changes in subchondral bone and articular cartilage of rat femorotibial joints: an animal model of osteoarthritis. Toxicol Pathol 2003; 31: 619–624. [DOI] [PubMed] [Google Scholar]
- 23.Liu P, Okun A, Ren J, et al. Ongoing pain in the MIA model of osteoarthritis. Neurosci Lett 2011; 493: 72–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bove SE, Calcaterra SL, Brooker RM, et al. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthritis Cartilage 2003; 11: 821–830. [DOI] [PubMed] [Google Scholar]
- 25.Combe R, Bramwell S and Field MJ. The monosodium iodoacetate model of osteoarthritis: a model of chronic nociceptive pain in rats? Neurosci Lett 2004; 370: 236–240. [DOI] [PubMed] [Google Scholar]
- 26.Fernihough J, Gentry C, Malcangio M, et al. Pain related behaviour in two models of osteoarthritis in the rat knee. Pain 2004; 112: 83–93. [DOI] [PubMed] [Google Scholar]
- 27.Ferland CE, Laverty S, Beaudry F, et al. Gait analysis and pain response of two rodent models of osteoarthritis. Pharmacol Biochem Behav 2011; 97: 603–610. [DOI] [PubMed] [Google Scholar]
- 28.Hannan MT, Felson DT and Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumetol 2000; 27: 1513–1517. [PubMed] [Google Scholar]
- 29.O’Brien M, Philpott HT and McDougall JJ. Understanding osteoarthritis pain through animal models. Clin Exp Rheumatol 2017; 35: S47–S52. [PubMed] [Google Scholar]
- 30.Barve RA, Minnerly JC, Weiss DJ, et al. Transcriptional profiling and pathway analysis of monosodium iodoace-tate-induced experimental osteoarthritis in rats: relevance to human disease. Osteoarthritis Cartilage 2007; 15: 1190–1198. [DOI] [PubMed] [Google Scholar]
- 31.Ameye LG and Young MF. Animal models of osteoarthritis: lessons learned while seeking the ‘Holy Grail’. Curr Opin Rheumatol 2006; 18: 537–547. [DOI] [PubMed] [Google Scholar]
- 32.Maerz T, Kurdziel MD, Davidson AA, et al. Biomechanical characterization of a model of noninvasive, traumatic anterior cruciate ligament injury in the rat. Ann Biomed Eng 2015; 43: 2467–2476. [DOI] [PubMed] [Google Scholar]
- 33.Tang Z, Yang L, Wang Y, et al. Contributions of different intraarticular tissues to the acute phase elevation of synovial fluid MMP-2 following rat ACL rupture. J Orthop Res 2009; 27: 243–248. [DOI] [PubMed] [Google Scholar]
- 34.Christiansen BA, Anderson MJ, Lee CA, et al. Musculoskeletal changes following non-invasive knee injury using a novel mouse model of post-traumatic osteoarthritis. Osteoarthritis Cartilage 2012; 20: 773–782. [DOI] [PubMed] [Google Scholar]
- 35.Gatchel RJ, Peng YB, Peters ML, et al. The biopsycho-social approach to chronic pain: scientific advances and future directions. Psychol Bull 2007; 133: 581–624. [DOI] [PubMed] [Google Scholar]
- 36.Neugebauer V, Han JS, Adwanikar H, et al. Techniques for assessing knee joint pain in arthritis. Mol Pain 2007; 3: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poole R, Blake S, Buschmann M, et al. Recommendations for the use of preclinical models in the study and treatment of osteoarthritis. Osteoarthritis Cartilage 2010; 18: S10–S16. [DOI] [PubMed] [Google Scholar]
- 38.Yu YC, Koo ST, Kim CH, et al. Two variables that can be used as pain indices in experimental animal models of arthritis. J Neurosci Methods 2002; 115: 107–113. [DOI] [PubMed] [Google Scholar]
- 39.Gegout-Pottie P, Philippe L, Simonin MA, et al. Biotelemetry: an original approach to experimental models of inflammation. Inflamm Res 1999; 48: 417–424. [DOI] [PubMed] [Google Scholar]
- 40.Hamilton CB, Pest MA, Pitelka V, et al. Weight-bearing asymmetry and vertical activity differences in a rat model of post-traumatic knee osteoarthritis. Osteoarthritis Cartilage 2015; 23: 1178–1185. [DOI] [PubMed] [Google Scholar]
- 41.Bove SE, Laemont KD, Brooker RM, et al. Surgically induced osteoarthritis in the rat results in the development of both osteoarthritis-like joint pain and secondary hyperalgesia. Osteoarthritis Cartilage 2006; 14: 1041–1048. [DOI] [PubMed] [Google Scholar]
- 42.Glasson SS, Blanchet TJ and Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage 2007; 15: 1061–1069. [DOI] [PubMed] [Google Scholar]
- 43.Knights CB, Gentry C and Bevan S. Partial medial meniscectomy produces osteoarthritis pain-related behaviour in female C57BL/6 mice. Pain 2012; 153: 281–292. [DOI] [PubMed] [Google Scholar]
- 44.Kilkenny C, Browne W, Cuthill IC, et al. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 2010; 160: 561–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kilkenny C and Altman DG. Improving bioscience research reporting: ARRIVE-ing at a solution. Lab Anim 2010; 44: 377–378. [DOI] [PubMed] [Google Scholar]
- 46.Kilkenny C, Browne W, Cuthill IC, et al. Editorial: Animal research: reporting in vivo experiments – the ARRIVE guidelines. J Cereb Blood Flow Metab 2011; 31: 991–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sorge RE, Martin LJ, Isbester KA, et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods 2014; 11: 629–632. [DOI] [PubMed] [Google Scholar]
- 48.Pitcher GM, Ritchie J and Henry JL. Paw withdrawal threshold in the Von Frey hair test is influenced by the surface on which the rat stands. J Neurosci Methods 1999; 87: 185–193. [DOI] [PubMed] [Google Scholar]
- 49.Baker D, Lidster K, Sottomayor A, et al. Two years later: journals are not yet enforcing the ARRIVE guidelines on reporting standards for pre-clinical animal studies. PLoS Biol 2014; 12: e1001756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwarz F, Iglhaut G and Becker J. Quality assessment of reporting of animal studies on pathogenesis and treatment of peri-implant mucositis and peri-implantitis. A systematic review using the ARRIVE guidelines. J Clin Periodontol 2012; 39: 63–72. [DOI] [PubMed] [Google Scholar]
- 51.Kupers RC and Gybels JM. Electrical stimulation of the ventroposterolateral thalamic nucleus (VPL) reduces mechanical allodynia in a rat model of neuropathic pain. Neurosci Lett 1993; 150: 95–98. [DOI] [PubMed] [Google Scholar]
- 52.Hao JX, Xu XJ, Aldskogius H, et al. Allodynia-like effects in rat after ischaemic spinal cord injury photo-chemically induced by laser irradiation. Pain 1991; 45: 175–185. [DOI] [PubMed] [Google Scholar]
- 53.Lewin GR, Ritter AM and Mendell LM. Nerve growth factor-induced hyperalgesia in the neonatal and adult rat. J Neurosci 1993; 13: 2136–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shir Y and Seltzer Z. A-fibers mediate mechanical hyperesthesia and allodynia and C-fibers mediate thermal hyperalgesia in a new model of causalgiform pain disorders in rats. Neurosci Lett 1990; 115: 62–67. [DOI] [PubMed] [Google Scholar]
- 55.Lakes EH and Allen KD. Gait analysis methods for rodent models of arthritic disorders: reviews and recommendations. Osteoarthritis Cartilage 2016; 24: 1837–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kloefkorn HE, Jacobs BY, Loye AM, et al. Spatiotemporal gait compensations following medial col-lateral ligament and medial meniscus injury in the rat: correlating gait patterns to joint damage. Arthritis Res Ther 2015; 17: 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 58.Schött E, Berge OG, Ängeby-Möller K, et al. Weight bearing as an objective measure of arthritic pain in the rat. J Pharmacol Toxicol Methods 1994; 31: 79–83. [DOI] [PubMed] [Google Scholar]
- 59.Jacobs BY, Kloefkorn HE and Allen KD. Gait analysis methods for rodent models of osteoarthritis. Curr Pain Headache Rep 2014; 18: 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quinn LP, Stean TO, Trail B, et al. LABORAS: initial pharmacological validation of a system allowing continuous monitoring of laboratory rodent behaviour. J Neurosci Methods 2003; 130: 83–92. [DOI] [PubMed] [Google Scholar]
- 61.Zurn JB, Jiang X and Motai Y. Video-based rodent activity measurement using near-infrared illumination In: IEEE Instrumentation and Measurement Technology Conference Proceedings. Ontario, Canada, 17–19 May 2005, pp.17–19. Piscataway, NJ: IEEE Publishing. [Google Scholar]
- 62.Dewsbury DA. Wheel-running behaviour in 12 species of muroid rodents. Behav Processes 1980; 5: 271–280. [DOI] [PubMed] [Google Scholar]
- 63.Walsh RN and Cummins RA. The open-field test: a critical review. Psychol Bull 1976; 83: 482–504. [PubMed] [Google Scholar]
- 64.Chesler EJ, Wilson SG, Lariviere WR, et al. Influences of laboratory environment on behaviour. Nat Neurosci 2002; 5: 1101–1102. [DOI] [PubMed] [Google Scholar]
- 65.Abbott FV, Franklin KBJ and Connell B. The stress of a novel environment reduces formalin pain: possible role of serotonin. Eur J Pharmacol 1986; 126: 141–144. [DOI] [PubMed] [Google Scholar]
- 66.Porro CA and Carli G. Immobilization and restraint effects on pain reactions in animals. Pain 1988; 32: 289–307. [DOI] [PubMed] [Google Scholar]
- 67.Hurst JL and West RS. Taming anxiety in laboratory mice. Nat Methods 2010; 7: 825–826. [DOI] [PubMed] [Google Scholar]
- 68.Gould TD, Dao DT and Kovacsics CE. The open field test In: Gould TD (ed) Mood and anxiety related phenotypes in mice: characterization using behavioral tests. Totowa, NJ: Humana Press, 2009, pp.1–20. [Google Scholar]
- 69.Jud C, Schmutz I, Hampp G, et al. A guideline for analyzing circadian wheel-running behavior in rodents under different lighting conditions. Biol Proced Online 2005; 7: 101–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim HT, Uchimoto K, Duellman T, et al. Automated assessment of pain in rats using a voluntarily accessed static weight-bearing test. Physiol Behav 2015; 151: 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Quadros AU, Pinto LG, Fonseca MM, et al. Dynamic weight bearing is an efficient and predictable method for evaluation of arthritic nociception and its pathophysiological mechanisms in mice. Sci Rep 2015; 5: 14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Allen KD, Mata BA, Gabr MA, et al. Kinematic and dynamic gait compensations resulting from knee instability in a rat model of osteoarthritis. Arthritis Res Ther 2012; 14: R78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vrinten DH and Hamers FT. ‘CatWalk’ automated quantitative gait analysis as a novel method to assess mechanical allodynia in the rat: a comparison with Von Frey testing. Pain 2003; 102: 203–209. [DOI] [PubMed] [Google Scholar]
- 74.Gabriel AF, Marcus MA, Walenkamp GH, et al. The CatWalk method: assessment of mechanical allodynia in experimental chronic pain. Behav Brain Res 2009; 198: 477–480. [DOI] [PubMed] [Google Scholar]
- 75.Gabriel AF, Marcus MA, Honig WM, et al. The CatWalk method: a detailed analysis of behavioural changes after acute inflammatory pain in the rat. J Neurosci Methods 2007; 163: 9–16. [DOI] [PubMed] [Google Scholar]
- 76.Berryman ER, Harris RL, Moalli M, et al. DigiGait quantitation of gait dynamics in rat rheumatoid arthritis model. J Musculoskelet Neuronal Interact 2009; 9: 89–98. [PubMed] [Google Scholar]
- 77.Min SS, Han JS, Kim YI, et al. A novel method for convenient assessment of arthritic pain in voluntarily walking rats. Neurosci Lett 2001; 308: 95–98. [DOI] [PubMed] [Google Scholar]
- 78.Dorman CW, Krug HE, Frizelle SP, et al. A comparison of DigiGait and TreadScan imaging systems: assessment of pain using gait analysis in murine monoarthritis. J Pain Res 2013; 7: 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boettger MK, Leuchtweis J, Schaible H-G, et al. Videoradiographic analysis of the range of motion in unilateral experimental knee joint arthritis in rats. Arthritis Res Ther 2011; 13: R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boettger MK, Weber K, Schmidt M, et al. Gait abnormalities differentially indicate pain or structural joint damage in monoarticular antigen-induced arthritis. Pain 2009; 145: 142–150. [DOI] [PubMed] [Google Scholar]
- 81.Dawane JS, Pandit VA and Rajopadhye BD. Experimental evaluation of antiinflammatory effect of topical application of entada phaseoloides seeds as paste and ointment. N Am J Med Sci 2011; 3: 513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heilborn U, Berge OG, Arborelius L, et al. Spontaneous nociceptive behaviour in female mice with Freund’s complete adjuvant- and carrageenan-induced monoarthritis. Brain Res 2007; 1143: 143–149. [DOI] [PubMed] [Google Scholar]
- 83.Dief AE, Mostafa DK, Sharara GM, et al. Hydrogen sulfide releasing naproxen offers better anti-inflammatory and chondroprotective effect relative to naproxen in a rat model of zymosan induced arthritis. Eur Rev Med Pharmacol Sci 2015; 19: 1537–1546. [PubMed] [Google Scholar]
- 84.Anderson S, Krug H, Dorman C, et al. Analgesic effects of intra-articular botulinum toxin type B in a murine model of chronic degenerative knee arthritis pain. J Pain Res 2010; 3: 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Batka RJ, Brown TJ, Mcmillan KP, et al. The need for speed in rodent locomotion analyses. Anat Rec 2014; 297: 1839–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cendelín J, Voller J and Vozěh F. Ataxic gait analysis in a mouse model of the olivocerebellar degeneration. Behav Brain Res 2010; 210: 8–15. [DOI] [PubMed] [Google Scholar]
- 87.Orito K, Kurozumi S, Ishii I, et al. A sensitive gait parameter for quantification of arthritis in rats. J Pharmacol Sci 2007; 103: 113–116. [DOI] [PubMed] [Google Scholar]
- 88.Clarke KA. Swing time changes contribute to stride time adjustment in the walking rat. Physiol Behav 1991; 50: 1261–1262. [DOI] [PubMed] [Google Scholar]
- 89.Olmarker K, Iwabuchi M, Larsson K, et al. Walking analysis of rats subjected to experimental disc herniation. Eur Spine J 1998; 7: 394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roerdink M and Beek PJ. Understanding inconsistent step-length asymmetries across hemiplegic stroke patients. Neurorehab Neural Repair 2011; 25: 253–258. [DOI] [PubMed] [Google Scholar]
- 91.Mapp PI, Sagar DR, Ashraf S, et al. Differences in structural and pain phenotypes in the sodium monoiodoacetate and meniscal transection models of osteoarthritis. Osteoarthritis Cartilage 2013; 21: 1336–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McNulty MA, Loeser RF, Davey C, et al. Histopathology of naturally occurring and surgically induced osteoarthritis in mice. Osteoarthritis Cartilage 2012; 20: 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jacobs BY, Dunnigan K, Pires-Fernandes M, et al. Unique spatiotemporal and dynamic gait compensations in the rat monoiodoacetate injection and medial meniscus transection models of knee osteoarthritis. Osteoarthritis Cartilage 2017; 25: 750–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miller RE, Tran PB, Das R, et al. CCR2 chemokine receptor signaling mediates pain in experimental osteoarthritis. Proc Natl Acad Sci U S A 2012; 109: 20602–20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Furman BD, Strand J, Hembree WC, et al. Joint degeneration following closed intraarticular fracture in the mouse knee: a model of posttraumatic arthritis. J Orthop Res 2007; 25: 578–592. [DOI] [PubMed] [Google Scholar]
- 96.Ko FC, Dragomir C, Plumb DA, et al. In vivo cyclic compression causes cartilage degeneration and subchondral bone changes in mouse tibiae. Arthritis Rheum 2013; 65: 1569–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Silva A, Araujo P, Zager A, et al. Sex differences in sleep pattern of rats in an experimental model of osteoarthritis. Eur J Pain 2011; 15: 545–553. [DOI] [PubMed] [Google Scholar]
- 98.Takahashi I, Matsuzaki T and Hoso M. Long-term histopathological developments in knee-joint components in a rat model of osteoarthritis induced by monosodium iodoacetate. J Phys Ther Sci 2017; 29: 590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bendele AM and Hulman JF. Spontaneous cartilage degeneration in guinea pigs. Arthritis Rheum 1988; 31: 561–565. [DOI] [PubMed] [Google Scholar]
- 100.Zamli Z, Adams MA, Tarlton JF, et al. Increased chondrocyte apoptosis is associated with progression of osteoarthritis in spontaneous Guinea pig models of the disease. Int J Mol Sci 2013; 14: 17729–17743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Allen KD, Griffin TM, Rodriguiz RM, et al. Decreased physical function and increased pain sensitivity in mice deficient for type IX collagen. Arthritis Rheum 2009; 60: 2684–2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Javaheri B, Poulet B, Aljazzar A, et al. Stable sulforaphane protects against gait anomalies and modifies bone microarchitecture in the spontaneous STR/Ort model of osteoarthritis. Bone 2017; 103: 308–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Piles M, Poulet B, de Souza R, et al. Gait asymmetry and imbalance as potential markers of natural osteoarthritis development in mice. Osteoarthritis Cartilage 2014; 22: S121. [Google Scholar]
- 104.Poulet B, De Souza R, Knights CB, et al. Modifications of gait as predictors of natural osteoarthritis progression in Str/Ort mice. Arthritis Rheum 2014; 66: 1832–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Costello KE, Guilak F, Setton LA, et al. Locomotor activity and gait in aged mice deficient for type IX collagen. J Appl Physiol 2010; 109: 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Datta P, Zhang Y, Parousis A, et al. High-fat dietinduced acceleration of osteoarthritis is associated with a distinct and sustained plasma metabolite signature. Sci Rep 2017; 7: 8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Louer CR, Furman BD, Huebner JL, et al. Diet-induced obesity significantly increases the severity of posttraumatic arthritis in mice. Arthritis Rheum 2012; 64: 3220–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Griffin TM, Fermor B, Huebner JL, et al. Diet-induced obesity differentially regulates behavioural, biomechanical, and molecular risk factors for osteoarthritis in mice. Arthritis Res Ther 2010; 12: R130. [DOI] [PMC free article] [PubMed] [Google Scholar]


