Abstract
Background:
Massive transfusion protocols to treat post-injury hemorrhage are based on pre-defined blood product transfusion ratios followed by goal-directed transfusion based on patient’s clinical evolution. However, it remains unclear how these transfusion ratios impact patient outcomes over time from injury.
Methods:
The Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) is a phase 3, randomized controlled trial, across 12 level-I trauma centers in North America. From 2012 to 2013, 680 severely injured patients required massive transfusion. We used semi-parametric machine learning techniques and causal inference methods to augment the intent-to-treat analysis of PROPPR, estimating the dynamic relationship between transfusion ratios and outcomes: mortality and hemostasis at different time-points during the first 24 hours after admission.
Results:
In the intention-to-treat analysis, the 1:1:1 group tended to have decreased mortality, but with no statistical significance. For patients in whom hemostasis took longer than 2 hours, the 1:1:1 ratio was associated with a higher probability of hemostasis, statistically significant from the 4th hour on. In the per-protocol, actual-transfusion-ratios-received analysis, during four successive time intervals, no significant association was found between the actual ratios and mortality. When comparing patient groups who received both high plasma:PRBC and high platelet:PRBC ratios to the group of low ratios in both, the relative risk of achieving hemostasis was 2.49 (95% CI = 1.19–5.22) during the 3rd hour after admission, suggesting a significant beneficial impact of higher transfusion ratios of plasma and platelets on hemostasis.
Conclusions:
Our results suggest that the impact of transfusion ratios on hemostasis is dynamic. Overall, the transfusion ratios had no significant impact on mortality over time. However, receiving higher ratios of platelets and plasma relative to red blood cells hastens hemostasis in subjects who have yet to achieve hemostasis within 3 hours after hospital admission.
Keywords: Blood transfusion, Trauma, Hemorrhage, Post-injury hemostasis
Background
Trauma is the leading cause of death among younger Americans.1 After major trauma, early mortality typically relates to bleeding and central nervous system lesions.2 Hemorrhage is the predominant cause of preventable deaths in trauma patients, accounting for 30 to 40% of the overall mortality.3
Up to one-third of hemorrhaging trauma patients have disordered coagulation termed trauma-induced coagulopathy (TIC). The immediate consequences of TIC are exsanguination and death, and the delayed consequences are multiple organ dysfunction and secondary mortality. The management of hemorrhage following trauma with damage control resuscitation principles including: i) surgical control of bleeding, ii) restoring adequate organ perfusion, and iii) treating acquired coagulopathy via transfusion of packed red blood cells (PRBC), coagulation factors (plasma), and platelets have been linked to improved outcomes.4, 5
The optimal plasma:PRBC transfusion ratio have long been studied, but remain uncertain. Military medicine was the first to suggest the potential benefit of high plasma:PRBC ratios in injured patients. In 2004, the United States (US) Department of Defense recommended the use of high plasma:PRBC ratios as part of damage control resuscitation. The current US military resuscitation practice is to use 1:1:1 ratio for units of plasma to platelets to packed red blood cells (plasma:platelets:PRBC),6 as the closest approximation to whole blood for resuscitation of the most seriously injured casualties.3 Accordingly, the 2016 National Clinical Guideline Centre of United Kingdom published guidelines for adult trauma care in which they also recommended the use of 1:1 ratio of plasma:PRBC to replace volume.7,8 These recommendations are widely applied in both civilian and military trauma centers.5, 9
However, most observational studies supporting the use of high plasma:PRBC blood product transfusion ratios are potentially biased because they compare the outcome before/after implementing a massive transfusion protocol, or because they compare plasma:PRBC ratio between survivors and non survivors without adequately accounting for confounding factors. These studies are frequently under-powered, have conflicting results, and most systematically discard patients with less than 10 units of PRBCs transfused during the first 24 hours.3, 7, 9–13
The Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) trial was designed to further the Prospective Observational Multicenter Major Trauma Transfusion (PROMMTT)11 study. Whereas PROMMTT studied ratios “closer” to 1:1:1 vs. “closer” to 1:1:2, PROPPR was designed to specifically study two different high fixed ratios of 1:1:1 and 1:1:2 blood product transfusion for resuscitation, addressing the effectiveness and safety of 1:1:1 ratio compared to the 1:1:2 ratio in trauma patients for whom massive transfusion was indicated (see3 for details on design). This study failed to show any statistically significant benefit of 1:1:1 blood product transfusion ratio on mortality at 24 hours or at 30 days. However, the authors found that fewer patients experienced death due to exsanguination by 24 hours, and that more patients in the 1:1:1 group achieved hemostasis, a secondary outcome of the study.3
However, resuscitation of hemorrhaging trauma patients is a highly dynamic process. Massive transfusion protocols to treat post-injury hemorrhage rely on pre-defined blood product transfusion ratios followed by goal-directed transfusion based on patient’s clinical evolution and hemostasis.14 We hypothesize that the impact of blood product transfusion ratios on time-to-hemostasis and mortality evolves over time, and that patients who achieve hemostasis quickly after hospital admission are less likely to benefit from high transfusion ratios, while patients in whom hemostasis is difficult to achieve and takes longer, high transfusion ratios are more likely to be beneficial. We augment the original publication of the intervention impact in PROPPR by using machine learning and causal inference methods15 to estimate the relationship between the actual transfusion ratios in specific intervals of time and outcomes (mortality and hemostasis) at different time-points during the first 24 hours.
Methods
The study was registered with clinicaltrials.gov with the identifier NCT01545232
Data: The PROPPR Study
The PROPPR study was a pragmatic, phase 3, multicenter, randomized clinical trial of 680 severely injured patients who arrived at one of 12 level I trauma centers in the US and in Canada between August 2012 and December 2013.3 Participants met all of the following criteria: 1) required the highest trauma team activation; 2) estimated age 15 years or older or greater than/equal to weight of 50 kg if age unknown; 3) received directly from the injury scene; 4) initiated transfusion of at least one unit of blood within the first hour of arrival or during pre-hospital transport; 5) predicted need for massive transfusion based on the assessment of blood consumption (ABC score;16) or the attending trauma physician’s judgment. Patients with devastating injuries expected to die within one hour of admission to the emergency department (ED) were excluded.
The study used US Food and Drug guidance for exception from informed consent requirements for emergency research.17 Eligible patients were randomized into two groups to receive one of two blood product transfusion ratios. The randomized blood products, prepared in the blood bank, were delivered in sealed coolers. For the 1:1:1 group, patients received blood in the following order: a pooled 6 units of platelets, then alternating PRBCs and plasma up to 6 times. For the 1:1:2 group, patients received blood as: alternating 2 units of PRBCs with 1 unit of plasma up to 3 times, a pooled 6 units of platelets, and again alternating 2 units of PRBCs with 1 unit of plasma up to 3 times. Patients were transfused until one of the following occurred: hemostasis, death, protocol violations, or no other clinical need for further transfusion. After being screened and enrolled in the trial, patients were followed until discharge or up to the 30th day of hospitalization.
Because of the order of how the blood products were given, if the transfusion was stopped early, then the patients would not receive the intended ratios. The actual received blood ratios were dynamic over time while the patients were being transfused. Time scale in this study started at admittance into the ED (t = 0). Data from patient assessments were collected at multiple time-points, including pre-ED time for baseline variables. The primary outcomes in the original study were 24-hour and 30-day all-cause mortality.3 Hemostasis was one of the pre-specified ancillary outcomes.
Study Goals and Outcome Measures
The goal of this study was to estimate the impact of blood product transfusion ratios on the probabilities of death and hemostasis at different time-points during the first 24 hours following hospital admission. To do so, we performed two separate analyses, i) an Intention-to-Treat analysis (i.e, examining the impact of the assigned treatment based on randomization); ii) a per protocol analysis, referred to as Actual Treatment Analysis, to estimate the impact of transfusion ratios actually received during each time interval.
The actual ratios in specific time-intervals were not cumulative but based on the amount of products transfused within the interval. In PROPPR, anatomic hemostasis in the operating room was an objective assessment by the surgeon indicating that bleeding within the surgical field was controlled and no further hemostatic interventions were anticipated. In the interventional radiology suite, anatomic hemostasis was defined as achieving resolution of contrast blush after embolization.
Intention-to-Treat Analysis
Our Intention-to-Treat analysis was closely aligned with the analysis originally reported based on randomized treatments.3 We adjusted the estimates using variables such as: vital signs, lab values, demographics, injury-related, and diagnostic scores (see Table 1, Supplemental Digital Content 2). In PROPPR, patients were randomized into either the 1:1:1 or 1:1:2 blood product transfusion ratio groups. We estimated the association between allocated blood product transfusion ratio, (denoted by A), and the risk of outcomes occurring at 20 consecutive time intervals t of 30 minutes, starting from the first hour up to the 10th hour, and including 30 minutes before the 24th hour since ED arrivals. We evaluated two separate primary outcomes of interest, mortality and hemostasis at 24 hours.
Table 1: Summaries of the cross-sectional transfused blood product ratios and counts of outcomes (mortality and hemostasis) within 4 time-intervals (in minutes).
Treatment groups indicate ratio levels (High or Low) of Plasma/RBC – Platelets/RBC.
| Time interval | Treatment group | Count | Average Plasma/RBC ratio | Average Platelets/RBC ratio | Proportion in 1:1:1 (%) | Proportion Hemostasis | Proportion Death |
|---|---|---|---|---|---|---|---|
| 30–89 minute | LL | 387 | 0.11 | 0.0014 | 0.32 | 0.22 | 0.04 |
| HL | 32 | 0.99 | < 0.0001 | 0.41 | 0.03 | < 0.0001 | |
| LH | 106 | 0.28 | 2.48 | 0.95 | 0.24 | 0.06 | |
| HH | 42 | 1 | 2.56 | 1 | 0.21 | 0.07 | |
| 90–119 minute | LL | 260 | 0.22 | 0.03 | 0.21 | 0.35 | 0.03 |
| HL | 114 | 1.12 | 0.01 | 0.75 | 0.39 | 0.05 | |
| LH | 98 | 0.35 | 2.74 | 0.62 | 0.34 | 0.02 | |
| HH | 80 | 0.98 | 2.48 | 0.86 | 0.3 | 0.04 | |
| 120–179 minute | LL | 117 | 0.33 | 0.04 | 0.25 | 0.42 | 0.03 |
| HL | 107 | 1.14 | 0.02 | 0.8 | 0.46 | < 0.0001 | |
| LH | 32 | 0.39 | 2.41 | 0.28 | 0.31 | 0.09 | |
| HH | 57 | 1.05 | 3.21 | 0.77 | 0.54 | 0.04 | |
| 180–239 minute | LL | 62 | 0.26 | 0.06 | 0.27 | 0.37 | 0.13 |
| HL | 57 | 1.05 | 0.01 | 0.68 | 0.44 | 0.04 | |
| LH | 23 | 0.42 | 2.28 | 0.35 | 0.35 | 0.13 | |
| HH | 31 | 1.08 | 3.01 | 0.81 | 0.61 | 0.06 |
LL = Low ratio of Plasma/RBC – Low ratio of Platelets/RBC
HL = High ratio of Plasma/RBC – Low ratio of Platelets/RBC
LH = Low ratio of Plasma/RBC – High ratio of Platelets/RBC
HH = High ratio of Plasma/RBC – High ratio of Platelets/RBC
Actual Treatment Analysis
For the actual blood product transfusion ratios that patients received, we defined a composite treatment A(t) = (a1,a2) of plasma:PRBC and platelets:PRBC ratios based on the actual units of blood products transfused, over 4 consecutive time periods. We used the cut-off of 0.75 to categorize the ratios into low and high. The 0.75 cut-off value was chosen to be clinically meaningful, and, at the same time, resulting in ratio-defined groups that were large enough to conduct meaningful statistical analyses. The cut-off is also frequently used in several papers including a recent meta-analysis.18–20 Hence, in A(t) = (a1,a2), a1 is the plasma:PRBC ratio and a2 is the platelets:PRBC ratio, and the ratios a1 and a2 can assume either of two values: L=low (ratio ≤ 0.75), and conversely H=high (ratio > 0.75). Thus A(t) = (a1,a2) can attain 4 possible values: (H,H), (H,L), (L,H), and (L,L) ratio.21
The intervention during t, A(t) is the actual blood product transfusion ratio that patients received 30 minutes prior to the duration of outcomes. These intervention windows are: t = 1: 0 – 29 minutes, t = 2: 30 – 59 minutes, t = 3: 90 – 119 minutes, and t = 4: 150 – 179 minutes following admission. We examined the association between A(t) and the risks of mortality and hemostasis within the following four different time intervals. The time intervals for evaluation of the outcomes are: t = 1: 60 – 89 minutes, t = 2: 90 – 119 minutes, t = 3: 120 – 179 minutes, and t = 4: 180 – 239 minutes following admission.
Statistical Analyses
All analyses were done in the statistical programming language R22, software version 3.4.4. A detailed description of the statistical analysis is provided (see text document, Supplemental Digital Content 1, statistical analysis).
Results
Description of the Population
Between August 2012 and December 2013, 680 patients were included in the study: 338 were assigned to the blood product transfusion ratio of 1:1:1 and 342 to 1:1:2 ratio. Seven patients died too early to enter the study and were dropped, leaving 673 patients (Figure 1). We provide a summary of the baseline variables in Table 1, Supplemental Digital Content 2. As illustrated in Figures 1 & 2, Supplemental Digital Content 2, there was a substantial difference in average values for some time-varying variables for those that died (or achieved hemostasis) versus not. These variables include blood pressures (both diastolic and systolic), coagulation labs (INR, PT, and aPTT), fibrinogen, lactate, and pH.
Figure 1:
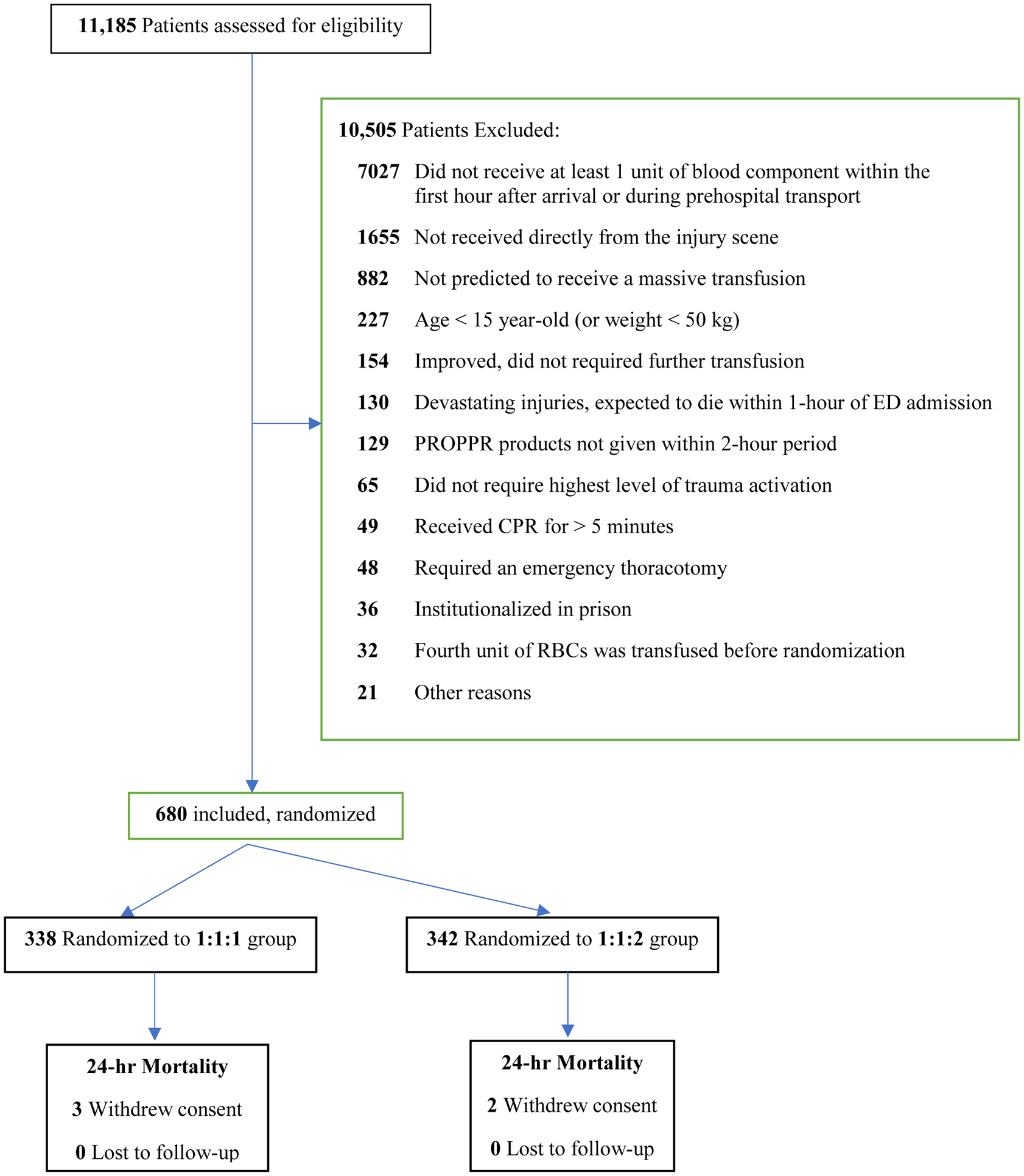
Flow diagram of patient process through the Pragmatic Randomized Optimal Platelet and Plasma Ratios (PROPPR) trial.
Figure 2:
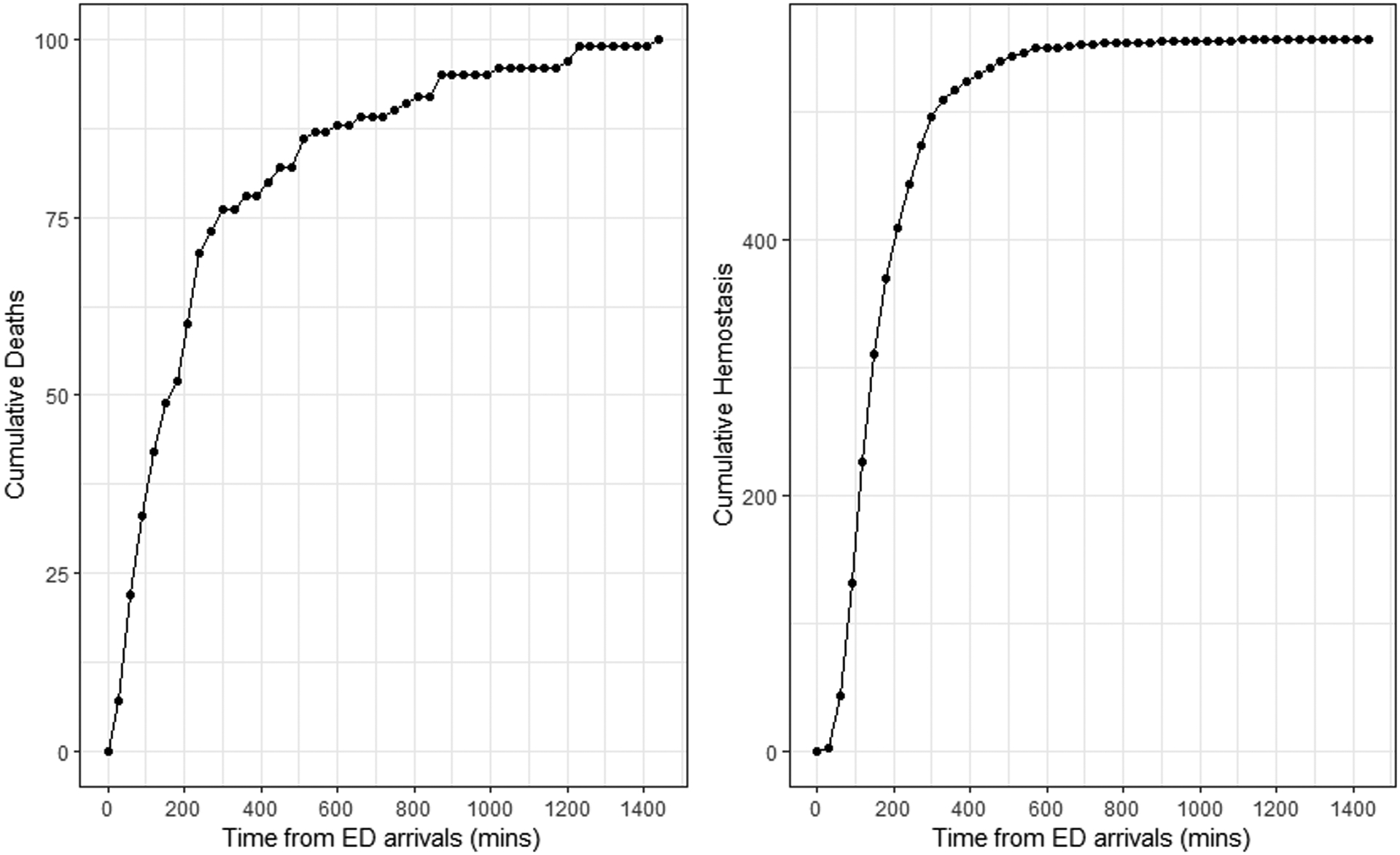
Cumulative counts of deaths and hemostasis over 24 hours since patient arrivals at the Emergency Departments
Figure 2 shows the cumulative counts of deaths and hemostasis over time. At the 24th hour, the total number of deaths was 100 (15%) and hemostasis events was 553 (82%). Whereas the deaths occurred over the entire 24 hours after admission, almost all of the hemostasis occurred by 6 hours, with higher rates in the first 4 hours. By 24 hours, the number of deaths plus the number of those achieving hemostasis were 663 (98.5%), suggesting that very few patients who did not achieve hemostasis were alive at 24 hours.
Intention to Treat
Figure 3 shows the actual cumulative blood products transfused ratios by randomized treatment group Overall, after 90 minutes since ED arrivals, there is a clear separation in the actual cumulative transfused ratios between the 2 randomized groups. The difference is remarkable with the platelets:PRBC ratio. Therefore, it is reasonable to consider the intention-to-treat analysis. Results reported in this section parallel that reported in the original publication11 but Targeted Maximum Likelihood Estimator (TMLE) was used to estimate the adjusted treatment effects. Figure 4 displays the results of the estimated causal relative risks with 95% confidence intervals (95% CI) for mortality and hemostasis by time.
Figure 3:
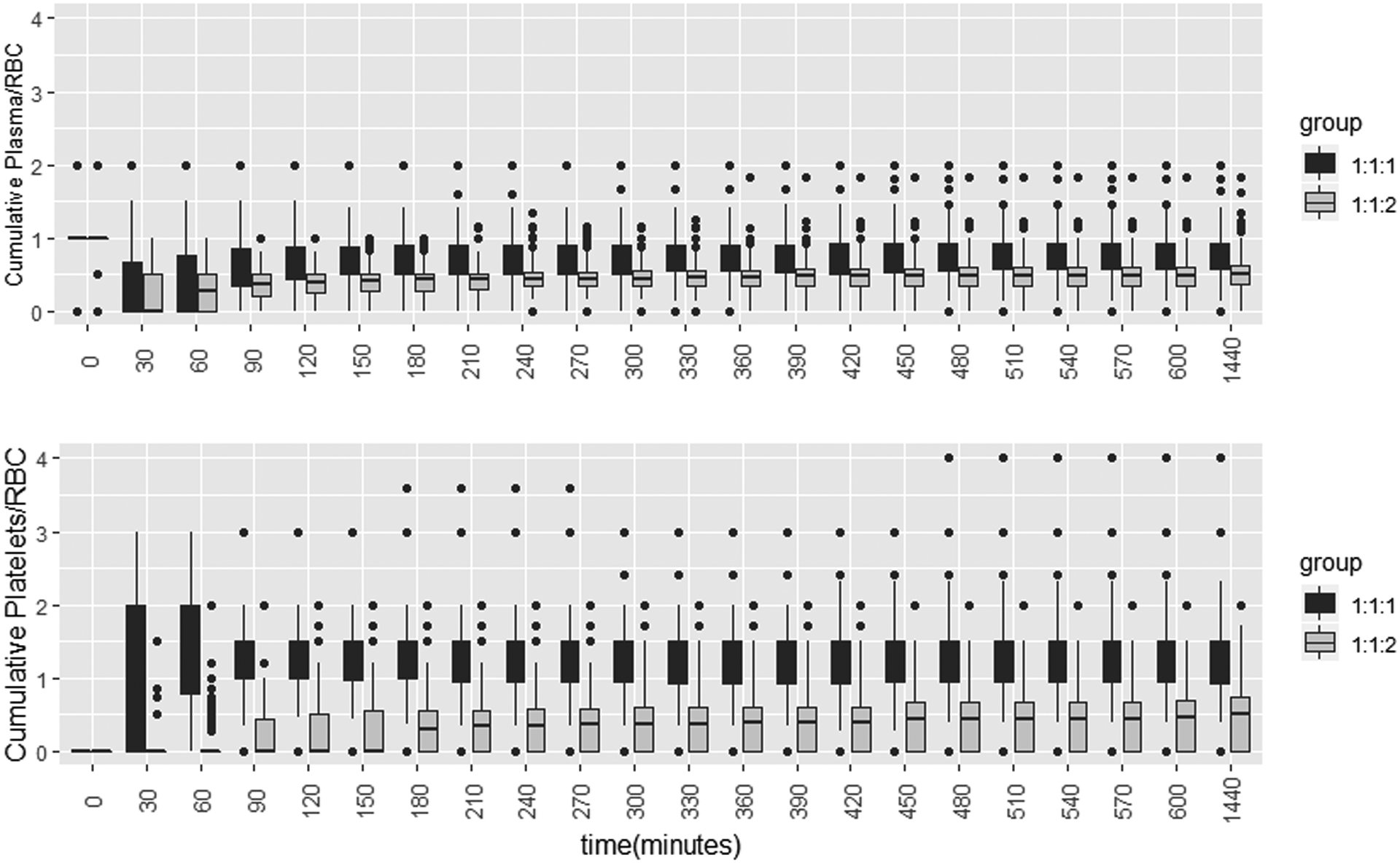
Cumulative actual transfused Plasma:RBC and Platelets:RBC ratios by assigned treatment groups 1:1:1 vs. 1:1:2
Figure 4:
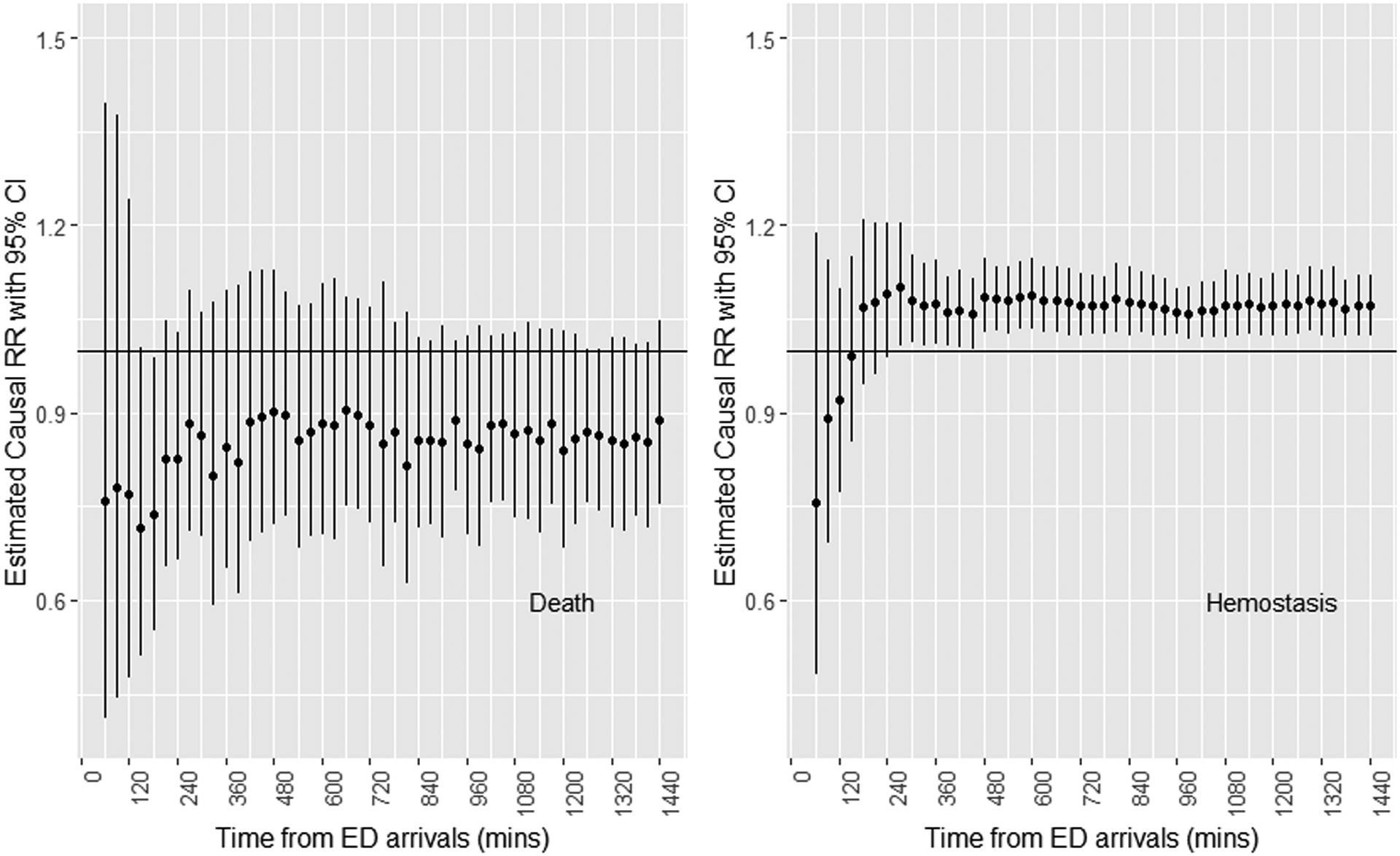
Adjusted causal relative risk (RR) estimates, 1:1:1 group / 1:1:2 group, with 95% confidence intervals for mortality and hemostasis over 24 hours since patient arrivals at the Emergency Departments.
Mortality.
All point estimates showed that the 1:1:1 group has lower risk of dying, which could suggest a beneficial impact of 1:1:1 ratio on mortality. However, the adjusted relative risk of death at the 24th hour was not statistically significant (relative risk = 0.89, 95%CI = 0.75–1.05).
Hemostasis.
The association between the randomized blood product transfusion ratios and the risk of having reached hemostasis by time t was significant for all time-points after 240 minutes. The adjusted relative risk of having reached hemostasis at the 24th hour was 1.07 (95%CI = 1.02–1.12), suggesting that high blood product transfusion ratios help patients achieve hemostasis among those who did not reach it earlier.
Time-dependent Actual Treatment Received
Despite the presence of a strong correlation between the randomized treatments and the actual ratios received, initially within the first 90 minutes, there was substantial overlap in the distribution of plasma:PRBC ratio between the randomized treatment groups.
Table 1 summarizes the distribution of some key variables within the four groups (HH, HL, LH and LL). We chose LL (Plasma:PRBC < 0.75, Platelets:RBC < 0.75) as the baseline group to compare to the other groups. Significant differences existed early in groups that received high platelets:PRBC ratio (LH and HH) compared to those with low platelets:PRBC ratio (HL and LL). The mean ratios in the high platelet groups were much larger. For the two high ratio groups (HL and HH), the mean plasma:PRBC ratios were above or close to 1, whereas this ratio was below 1/2 for all time intervals in the two low ratio groups (LH and LL). In the first time-interval, all patients who received HH ratios came from the 1:1:1 group. However, at the last time interval, most (81%) but not all of the HH group was assigned to 1:1:1.
As in the original study11, there was no significant unadjusted or adjusted association between the actual transfused ratio groups and mortality (see Table 2, Supplemental Digital Content 2). Likewise, there were no significant adjusted nor unadjusted associations found between the actual blood product ratios transfused and hemostasis in the earliest 3 time-intervals, up to 179 minutes. However, after 179 minutes, there was significant impact on hemostasis. We found a significant unadjusted and adjusted beneficial impact of the high plasma:PRBC and high platelets:PRBC (HH group) ratios compared to the LL group (adjusted relative risk = 2.49, 95% CI = 1.19–5.22), as well as a significant adjusted impact of the high ratio of platelets:PRBC ratio in the LH group compared to the LL group (adjusted relative risk = 1.94, 95% CI = 1.10–3.43). Table 2 shows the results for adjusted and unadjusted probabilities of hemostasis as well as the relative risk ratios in all actual interventions, at 4 time-intervals.
Table 2: Estimation results of the adjusted Targeted Maximum Likelihood (TMLE) and unadjusted risk ratio (RR) for hemostasis within 4 outcome time-intervals (in minutes).
Treatment groups indicate ratio levels (High or Low) of Plasma/RBC – Platelets/RBC.
| Time Interval | Treatment group | Count | Unadjusted RR | 95% CI Unadjusted RR | Proportion Hemostasis | (Adjusted) TMLE Proportion Hemostasis | TMLE RR | 95% CI TMLE RR |
|---|---|---|---|---|---|---|---|---|
| 30–89 minute | LL | 392 | 1 | 0.21 | 0.19 | 1 | ||
| HL | 32 | 0.15 | (0.02, 1.01) | 0.03 | 0.04 | 0.2 | (0.02, 1.74) | |
| LH | 105 | 1.02 | (0.67, 1.54) | 0.22 | 0.20 | 1.03 | (0.68, 1.54) | |
| HH | 42 | 1 | (0.54, 1.84) | 0.21 | 0.21 | 1.1 | (0.41, 2.89) | |
| 90–119 minute | LL | 249 | 1 | 0.31 | 0.29 | 1 | ||
| HL | 111 | 1.03 | (0.74, 1.44) | 0.32 | 0.30 | 1.03 | (0.71, 1.50) | |
| LH | 95 | 0.93 | (0.64, 1.35) | 0.28 | 0.28 | 0.95 | (0.62, 1.47) | |
| HH | 77 | 0.89 | (0.59, 1.35) | 0.27 | 0.31 | 1.08 | (0.72, 1.62) | |
| 120–179 minute | LL | 102 | 1 | 0.31 | 0.29 | 1 | ||
| HL | 89 | 1.07 | (0.71, 1.62) | 0.34 | 0.32 | 1.07 | (0.75, 1.52) | |
| LH | 33 | 0.87 | (0.46, 1.63) | 0.27 | 0.42 | 1.41 | (0.88, 2.25) | |
| HH | 48 | 1.33 | (0.85, 2.06) | 0.42 | 0.37 | 1.26 | (0.79, 2.02) | |
| 180–239 minute | LL | 48 | 1 | 0.15 | 0.19 | 1 | ||
| HL | 45 | 1.98 | (0.87, 4.52) | 0.29 | 0.25 | 1.35 | (0.82, 2.24) | |
| LH | 21 | 1.96 | (0.75, 5.13) | 0.29 | 0.37 | 1.94 | (1.10, 3.43) | |
| HH | 22 | 3.12 | (1.37, 7.10) | 0.45 | 0.46 | 2.49 | (1.19, 5.22) |
LL = Low ratio of Plasma/RBC – Low ratio of Platelets/RBC
HL = High ratio of Plasma/RBC – Low ratio of Platelets/RBC
LH = Low ratio of Plasma/RBC – High ratio of Platelets/RBC
HH = High ratio of Plasma/RBC – High ratio of Platelets/RBC
Discussion
High plasma:PRBC transfusion ratio is thought to be more beneficial for trauma patients in hemorrhagic shock because it approximates the ratio of clotting factors to red blood cells contained in shed whole blood. Damage control resuscitation includes not only early aggressive blood product transfusion to restore blood volume, but also early correction of coagulopathies and hemodynamic stabilization.23 These principles evolved from the original concepts of damage control surgery, shown to improve outcomes following severe trauma.24 As part of damage control resuscitation protocols, the balancing blood component transfusion is intended to approach whole blood transfusion.25 Although whole blood transfusion is not routine in civilian clinical practice, it is in military medicine.26, 27 As such, approximating whole blood with a 1:1 transfusion ratio of plasma:PRBC is considered a fundamental principle for clinical practice. Indeed, military medicine was the first to report a benefit of a higher plasma:PRBC ratio in patients with penetrating trauma.10 Consequently, it has been suggested that early administration of plasma and in comparable amount to PRBCs may prevent the development of coagulopathy and thereby improve early survival.28 In 2008, Duchesne et al. published the first retrospective observational civilian study on this topic.29 Subsequently, several retrospective and prospective civilian and military studies have evaluated the impact of high transfusion ratio on outcomes after trauma and have generated conflicting results.30, 31 Yet, these recommendations are now widely applied in both civilian and military trauma centers.32, 33
PROPPR was the first and only multicenter randomized controlled trial to assess the difference in two blood product transfusion ratios of 1:1:1 and 1:1:2 (plasma:platelets:PRBC) in trauma patients for whom massive transfusion was predicted. Previously, it was found that there was no statistical difference between groups in mortality at 24 hours or at 30 days. However, significantly more patients achieved hemostasis in the 1:1:1 group versus 1:1:2 group.11 This suggests that a high blood product transfusion ratio helps patients better achieve hemostasis. In the Intention-to-Treat analysis, although the results were not statistically significant for mortality benefit, high blood product transfusion ratio tended to be protective after 120 minutes of ED arrivals. For hemostasis, high blood product transfusion ratios were beneficial with significant differences from 240 minutes on. This suggests that high blood product transfusion ratios are essentially beneficial in patients in whom hemostasis is long and complicated. Although PROPPR was a well-designed randomized controlled trial, the first analysis did not take into account the time factor, nor the actual practice when the patients were still hemorrhaging, especially in more critical conditions during the first 4 – 6 hours. Our study augmented the original study in this aspect, and therefore provided a significantly better understanding of the consequences of the current transfusion practices.
Within randomization groups, there was sufficient variability in the timing of when blood products where given that we could examine the impacts of different patterns of transfusion. In Table 1, the proportion in 1:1:1 group shows that, except for the first interval, there is a mix of subjects in the 1:1:1 and 1:1:2 groups in each of the groups defined by ratios received within the interval. Thus, whereas the cumulative blood product transfusion ratios converge to their desired ratios over time, there is sufficient experimentation over time to examine impacts of relative volumes by time. Therefore, it is important to explore the actual treatments. Consistent with the Intention-to-Treat analysis, our results from analysis of actual treatments suggest that there are some benefits to giving patients high blood product transfusion ratios in both plasma:PRBC and platelets:PRBC, especially at later stages, i.e., when hemostasis is long and difficult to achieve. The PROMMTT study showed that transfusion ratios fluctuated during the first 24 hours, which is consistent with our result. PROMMTT concluded that after 24 hours, plasma and platelet ratios were unassociated with mortality but higher ratios of plasma:RBCs and platelets:RBCs were independently associated with decreased 6-hour mortality. According to the PROMMTT authors, such a discrepancy might be related to the fact that deaths due to hemorrhage were predominant during the first 6 hours, while competing causes of death were predominant thereafter. We added to this interpretation the concept that, the longer it takes to achieve hemostasis, the more likely high blood product transfusion ratios are to be beneficial, both in terms of hemostasis and survival.
This study carries limitations. Although there are suggestions of impacts from differing transfusion ratios, the PROPPR study may be under-powered to detect these impacts. Given the observed proportions of mortality in the 1:1:1 group over time, the smallest required sample size to achieve a power of 90% or 80% for detecting a 8% difference in average treatment effects are about 1100 or 850 respectively, and the average total sample size required is 2350 or 1750. Relative to mortality, for hemostasis, smaller sample sizes are required to achieve the same powers. Given the observed proportions of hemostasis in the 1:1:1 group over time, the smallest sample size needed to achieve a power of 90% or 80% for detecting a 6% difference in average treatment effects are 950 or 700 respectively, and the average sample size required is 1800 and 1350.
In conclusion, despite the lack of significant benefit on mortality with high blood product transfusion ratios (plasma:PRBC or platelets:PRBC ratio > 0.75) in the PROPPR study, our results suggest that the impact of high blood product transfusion ratios is dynamic over time, with more benefit in terms of hemostasis evaluated 3 hours after ED arrival. This suggests that patients whose hemostasis is difficult to achieve (still bleeding after 2 hours) are more likely to benefit from high blood product transfusion ratios. For further studies, larger samples and additional collection of time-varying variables over time would be helpful to raise the power of the study and allow longitudinal analyses.
Supplementary Material
Supplemental Digital Content 1. Text document that provides detailed description of the statistical analysis .txt
Supplemental Digital Content 2. Tables and Figures that show detailed information on some important variables and results.
Group Acknowledgement:
The PROPPR Study Group: Clinical Coordinating Center, University of Texas Health Science Center, Houston: John B. Holcomb, MD, Charles E. Wade, PhD, Deborah J. del Junco, PhD, Erin E. Fox, PhD, Nena Matijevic, PhD (laboratory committee co-chair), Jeanette M. Podbielski, RN, Angela M. Beeler, BS. Data Coordinating Center, University of Texas Health Science Center, Houston: Barbara C. Tilley, PhD, Sarah Baraniuk, PhD, Stacia M. DeSantis, PhD, Hongjian Zhu, PhD, Joshua Nixon, MS, Roann Seay, MS, Savitri N. Appana, MS, Hui Yang, MS, Michael O. Gonzalez, MS. Core Laboratory, University of Texas Health Science Center, Houston: Lisa Baer, MS, Yao-Wei Willa Wang, MD, Brittany S. Hula, MS, Elena Espino, BS, An Nguyen, BS, Nicholas Pawelczyk, BS, Kisha D. Arora-Nutall, BS, Rishika Sharma, MD, Jessica C. Cardenas, PhD, Elaheh Rahbar, PhD, Tyrone Burnett Jr, BS, David Clark, BS. Resuscitation Outcomes Consortium, University of Washington: Gerald van Belle, PhD, Susanne May, PhD, Brian Leroux, PhD, David Hoyt, MD, Judy Powell, BSN, RN, Kellie Sheehan, BSN. Systems Biology Committee, University of California, Berkeley: Alan Hubbard, PhD (co-chair), Adam P. Arkin, PhD. Transfusion Committee: John R. Hess, MD, MPH (co-chair, University of Washington), Jeannie L. Callum, MD (co-chair, Sunnybrook Health Sciences Centre). Anesthesiology Committee: Jean-Francois Pittet, MD (chair, University of Alabama, Birmingham). Emergency Medicine Committee: Christopher N. Miller, MD (chair, University of Cincinnati). PROPPR Clinical Sites (listed in order of number of patients enrolled): University of Texas Health Science Center, Houston: Bryan A. Cotton, MD, MPH, Laura Vincent, BSN, RN, CCRP, Timothy Welch, Tiffany Poole, DC, Evan G. Pivalizza, MD, Sam D. Gumbert, MD, Yu Bai, MD, PhD, James J. McCarthy, MD, Amy Noland, MD, Rhonda Hobbs, MT(ASCP)SBB. University of Washington: Eileen M. Bulger, MD, Patricia Klotz, RN, Lindsay Cattin, BA, Keir J. Warner, BS, Angela Wilson, BA, David Boman, BA, Nathan White, MD, MS, Andreas Grabinsky, MD, Jennifer A. Daniel-Johnson, MBBS. University of California, San Francisco: Mitchell Jay Cohen, MD (systems biology and laboratory committee co-chair), Rachael A. Callcut, MD, MSPH, Mary Nelson, RN, MPA, Brittney Redick, BA, Amanda Conroy, BA, Marc P. Steurer, MD, DESA, Preston C. Maxim, MD, Eberhard Fiebig, MD, Joanne Moore, Eireen Mallari, MT. University of Cincinnati: Peter Muskat, MD, Jay A. Johannigman, MD, Bryce R. H. Robinson, MD, Richard D. Branson, MSc, RRT, Dina Gomaa, BS, RRT, Christopher Barczak, BS, MT (ASCP), Suzanne Bennett, MD, Patricia M. Carey, MD, Helen Hancock, BS, MT(ASCP), Carolina Rodriguez, BA. University of Southern California: Kenji Inaba, MD, Jay G. Zhu, MD, Monica D. Wong, MS, Michael Menchine, MD, MPH, Kelly Katzberg, MD, FACEP, Sean O. Henderson, MD, Rodney McKeever, MD, Ira A. Shulman, MD, Janice M. Nelson, MD, Christopher W. Tuma, BA, MT(ASCP), SBB, Cheryl Y. Matsushita, BS, MT(ASCP). Shock, Trauma and Anesthesiology Research-Organized Research Center, R. Adams Cowley Shock Trauma Center, University of Maryland Medical Center: Thomas M. Scalea, MD, Deborah M. Stein, MD, MPH, Cynthia K. Shaffer, MS, MBA, Christine Wade, BA, Anthony V. Herrera, MS, Seeta Kallam, MBBS, Sarah E. Wade, BS, Samuel M. Galvagno Jr, DO, PhD, Magali J. Fontaine, MD, PhD, Janice M. Hunt, BS, MT(ASCP) SBB, Rhonda K. Cooke, MD. University of Tennessee Health Science Center, Memphis: Timothy C. Fabian, MD, Jordan A. Weinberg, MD, Martin A. Croce, MD, Suzanne Wilson, RN, Stephanie Panzer-Baggett, RN, Lynda WaddleSmith, BSN, Sherri Flax, MD. Medical College of Wisconsin: Karen J. Brasel, MD, MPH, Pamela Walsh, AS, CCRC, David Milia, MD, Allia Nelson, BS, BA, Olga Kaslow, MD, PhD, Tom P. Aufderheide, MD, MS, Jerome L. Gottschall, MD, Erica Carpenter, MLS(ASCP). University of Arizona: Terence O’Keeffe, MBChB, MSPH, Laurel L. Rokowski, RN, BSN, MKT, Kurt R. Denninghoff, MD, Daniel T. Redford, MD, Deborah J. Novak, MD, Susan Knoll, MS, MT(ASCP)SBB. University of Alabama, Birmingham: Jeffrey D. Kerby, MD, PhD, Patrick L. Bosarge, MD, Albert T. Pierce, MD, Carolyn R. Williams, RN, BSN, BSME, Shannon W. Stephens, EMTP, Henry E. Wang, MD, MS, Marisa B. Marques, MD. Oregon Health & Science University: Martin A. Schreiber, MD, Jennifer M. Watters, MD, Samantha J. Underwood, MS, Tahnee Groat, MPH, Craig Newgard, MD, MPH, Matthias Merkel, MD, PhD, Richard M. Scanlan, MD, Beth Miller, MT(ASCP)SBB. Sunnybrook Health Science Center: Sandro Rizoli, MD, PhD, Homer Tien, MD, Barto Nascimento, MD, MSc, CTBS, Sandy Trpcic, Skeeta Sobrian-Couroux, RN, CCRP, BHA, Marciano Reis, Adic Pérez, MD, Susan E. Belo, MD, PhD, Lisa Merkley, BA, MLT, CBTS, Connie Colavecchia, BSc, MLT.
Special Acknowledgments: We thank Jonathan Levy, PhD for theoretical and implementation insights; and Robert Talley, MA, for helping with proofreading.
Funding/Support:
This work was supported with grant U01HL077863 from the US National Heart, Lung, and Blood Institute and funding from the US Department of Defense, the Defense Research and Development Canada in partnership with the Canadian Institutes of Health Research-Institute of Circulatory and Respiratory Health (grant CRR120612).
Role of the Funder/Sponsor: The US National Heart, Lung, and Blood Institute (NHLBI) and the US Department of Defense had a role in the study design but had no role in the conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Disclosure:
The authors declare no conflicts of interest.
Publisher's Disclaimer: (copied from original paper) Disclaimer: The content is the sole responsibility of the authors and should not be construed as official or as reflecting the views of any of the sponsors nor of non-authors in the PROPPR Study Group.
Contributor Information
Minh Nguyen, Division of Epidemiology and Biostatistics, School of Public Health, University of California, Berkeley; Center for Biomedical Informatics Research, Department of Biomedical Data Science, School of Medicine, Stanford University.
Romain Pirracchio, Department of Anesthesia and Perioperative Care, School of Medicine, University of California, San Francisco.
Erin E. Fox, Center for Translational Injury Research, Division of Acute Care Surgery, Department of Surgery, Medical School, University of Texas Health Science Center, Houston.
Charles E. Wade, Center for Translational Injury Research, Department of Surgery, Medical School, University of Texas Health Science Center, Houston.
Martin Schreiber, Division of Trauma, Critical Care and Acute Care Surgery, School of Medicine, Oregon Health & Science University, Portland.
John B. Holcomb, Center for Translational Injury Research, Department of Surgery, Medical School, University of Texas Health Science Center, Houston.
Jeremy Coyle, Division of Epidemiology and Biostatistics, School of Public Health, University of California, Berkeley.
Mitchell Cohen, Department of Surgery, School of Medicine, University of Colorado.
Alan Hubbard, Division of Epidemiology and Biostatistics, School of Public Health, University of California, Berkeley.
References:
- 1.Heron M Deaths: Leading Causes for 2015. National Vital Statistics Reports, 66(5), 2017. Available at: https://www.cdc.gov/nchs/data/nvsr/nvsr66/nvsr66_05.pdf. Accessed December 18, 2019. [PubMed] [Google Scholar]
- 2.Callcut RA, Kornblith LZ, Conroy AS, Robles AJ, Meizoso JP, Namias N, Meyer DE, Haymaker A, Truitt SM, Agrawal V, et al. The why and how our trauma patients die: A prospective Multicenter Western Trauma Association study. J Trauma Acute Care Surg. 2019;86(5): 864–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holcomb JB, Tilley BC, Baraniuk S, Fox EE; Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cotton BA, Reddy N, Hatch QM, LeFebvre E, Wade CE, Kozar RA, Gill BS, Albarado R, McNutt MK, Holcomb JB. Damage control resuscitation is associated with a reduction in resuscitation volumes and improvement in survival in 390 damage control laparotomy patients. Ann Surg. 2011;254(4):598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langan NR, Eckert M, Martin MJ. Changing patterns of in-hospital deaths following implementation of damage control resuscitation practices in us forward military treatment facilities. JAMA Surg. 2014;149(9):904–912. [DOI] [PubMed] [Google Scholar]
- 6.US Department of Defense Center of Excellence for Trauma. Joint trauma system clinical practice guideline on damage control resuscitation. July 12, 2019. Available at: https://jts.amedd.army.mil/assets/docs/cpgs/JTS_Clinical_Practice_Guidelines_(CPGs)/Damage_Control_Resuscitation_12_Jul_2019_ID18.pdf. Accessed July 21, 2019.
- 7.Johansson PI, Sørensen AM, Larsen CF, Windeløv NA, Stensballe J, Perner A, Rasmussen LS, Ostrowski SR. Low hemorrhage-related mortality in trauma patients in a Level I trauma center employing transfusion packages and early thromboelastography-directed hemostatic resuscitation with plasma and platelets. Transfusion. 2013;53(12):3088–3099. [DOI] [PubMed] [Google Scholar]
- 8.Cannon JW, Khan MA, Raja AS, Cohen MJ, Como JJ, Cotton BA, Dubose JJ, Fox EE, Inaba K, Rodriguez CJ, et al. Damage control resuscitation in patients with severe traumatic hemorrhage: a practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2017;82(3):605–617. [DOI] [PubMed] [Google Scholar]
- 9.Scalea TM, Bochicchio KM, Lumpkins K, Hess JR, Dutton R, Pyle A, Bochicchio GV. Early aggressive use of fresh frozen plasma does not improve outcome in critically injured trauma patients. Ann Surg. 2008;126:221–227. [DOI] [PubMed] [Google Scholar]
- 10.Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, Sebesta J, Jenkins D, Wade CE, Holcomb JB. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63(4):805–813. [DOI] [PubMed] [Google Scholar]
- 11.Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, Alarcon LH, Bai Y, Brasel KJ, Bulger EM, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148(2):127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson JL, Moore EE, Kashuk JL, Banerjee A, Cothren CC, Biffl WL, Sauaia A. Effect of blood products transfusion on the development of postinjury multiple organ failure. Arch Surg. 2010;145(10):973–977. [DOI] [PubMed] [Google Scholar]
- 13.Shaz BH, Dente CJ, Nicholas J, MacLeod JB, Young AN, Easley K, Ling Q, Harris RS, Hillyer CD. Increased number of coagulation products in relationship to red blood cell products transfused improves mortality in trauma patients. Transfusion. 2010;50(2):493–500. [DOI] [PubMed] [Google Scholar]
- 14.Johansson PI, Stensballe J, Oliveri R, Wade CE, Ostrowski SR, Holcomb JB. How I treat patients with massive hemorrhage. Blood. 2014;124(20):3052–3058 [DOI] [PubMed] [Google Scholar]
- 15.Laan MJVD., Rose S. Targeted learning: causal inference for observational and experimental data. Springer, 2011. [Google Scholar]
- 16.Nunez TC, Voskresensky IV, Dossett LA, Shinall R, Dutton WD, Cotton BA. Early prediction of massive transfusion in trauma: simple as ABC (Assessment of Blood Consumption)? J Trauma. 2009;66(2):346–352. [DOI] [PubMed] [Google Scholar]
- 17.US Department of Health and Human Services; US Food and Drug Administration. Guidance for institutional review boards, clinical investigators, and sponsors: exception from informed consent requirements for emergency research, 2013. Available at: http://www.fda.gov/downloads/RegulatoryInformation/Guidances/UCM249673.pdf Accessed September 1, 2014.
- 18.Peralta R, Vijay A, El-Menyar A, Consunji R, Abdelrahman H, Parchani A, Afifi I, Zarour A, Al-Thani H, Latifi R. Trauma resuscitation requiring massive transfusion: a descriptive analysis of the role of ratio and time. World J Emerg Surg. 2015;10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bui E, Inaba K, Ebadat A, Karamanos E, Byerly S, Okoye O, Shulman I, Rhee P, Demetriades D. The impact of increased plasma ratios in massively transfused trauma patients: a prospective analysis. Eur J Trauma Emerg Surg. 2016;42(4):519–525. [DOI] [PubMed] [Google Scholar]
- 20.Rahouma M, Kamel M, Jodeh D, Kelley T, Ohmes LB, de Biasi AR, Abouarab AA, Benedetto U, Guy TS, Lau C, et al. Does a balanced transfusion ratio of plasma to packed red blood cells improve outcomes in both trauma and surgical patients? A meta-analysis of randomized controlled trials and observational studies. Am J Surg. 2018;216(2):342–350. [DOI] [PubMed] [Google Scholar]
- 21.Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal LA, Schreiber MA, Gonzalez EA, Pomper GJ, Perkins JG, Spinella PC, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248(3):447–458. [DOI] [PubMed] [Google Scholar]
- 22.Ihaka R, Gentleman R. R: A language for data analysis and graphics. J Comput Graph Stat. 1996;5(3):299–314. [Google Scholar]
- 23.Cap AP, Pidcoke HF, Spinella P, Strandenes G, Borgman MA, Schreiber M, Holcomb J, Tien HC, Beckett AN, Doughty H, et al. Damage control resuscitation. Military medicine. 2018;183(1Suppl):36–43 [DOI] [PubMed] [Google Scholar]
- 24.Stone HH, Strom PR, Mullins RJ. Management of the major coagulopathy with onset during laparotomy. Ann Surg. 1983;197(5):532–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murdock AD, Berséus O, Hervig T, Strandenes G, Lunde TH. Whole blood: the future of traumatic hemorrhagic shock resuscitation. Shock. 2014;41(1Suppl): 62–69. [DOI] [PubMed] [Google Scholar]
- 26.Spinella PC, Perkins JG, Grathwohl KW, Beekley AC, Holcomb JB. Warm fresh whole blood is independently associated with improved survival for patients with combat-related traumatic injuries. J Trauma. 2009;66(4Suppl):S69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holcomb JB, Hess JR. Early massive trauma transfusion: state of the art: editors’ introduction. J Trauma. 2006;60(6):S1–2 [Google Scholar]
- 28.Gonzalez EA, Moore FA, Holcomb JB, Miller CC, Kozar RA, Todd SR, Cocanour CS, Balldin BC, McKinley BA.. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007;62(1):112–119. [DOI] [PubMed] [Google Scholar]
- 29.Duchesne JC, Hunt JP, Wahl G, Marr AB, Wang YZ, Weintraub SE, Wright MJ, McSwain NE Jr. Review of current blood transfusions strategies in a mature level I trauma center: were we wrong for the last 60 years? J Trauma. 2008;65(2):272–276. [DOI] [PubMed] [Google Scholar]
- 30.Ho AMH, Dion PW, Yeung JH, Holcomb JB Critchley LA, Ng CS, Karmakar MK, Cheung CW, Rainer TH. Prevalence of survivor bias in observational studies on fresh frozen plasma erythrocyte ratios in trauma requiring massive transfusion. Anesthesiology. 2012;116(3):716–728. [DOI] [PubMed] [Google Scholar]
- 31.Roquet F, Neuschwander A, Hamada S, Favé G, Follin A, Marrache D, Cholley B, Pirracchio R; Traumabase Group. Association of early, high plasma-to–red blood cell transfusion ratio with mortality in adults with severe bleeding atter trauma. JAMA Netw Open. 2019;2(9): e1912076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Clinical Guideline Centre (UK). Major Trauma: Assessment and initial management. London: National Institute for Health and Care Excellence (UK); 2016. Available at http://www.ncbi.nlm.nih.gov/books/NBK344252/. Accessed June 24, 2017. [PubMed] [Google Scholar]
- 33.Malone DL, Hess JR, Fingerhut A. Massive transfusion practices around the globe and a suggestion for a common massive transfusion protocol. J Trauma. 2006;60(6Suppl):S91–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Text document that provides detailed description of the statistical analysis .txt
Supplemental Digital Content 2. Tables and Figures that show detailed information on some important variables and results.


