Abstract
Purpose
Although strong associations between self-reported health and mortality exist, quality of life is not conceptualized as a cardiovascular disease (CVD) risk factor. Our objective was to assess the independent association between health-related quality of life (HRQOL) and incident CVD.
Methods
This study used the REasons for Geographic And Racial Differences in Stroke data, which enrolled 30,239 adults from 2003 to 2007 and followed them over 10 years. We included 22,229 adults with no CVD history at baseline. HRQOL was measured using the SF-12 Physical Component Summary (PCS) and Mental Component Summary (MCS) scores, which range from 0 to 100, with higher scores indicating better HRQOL. Scores were normed to the general US population with mean 50 and standard deviation 10. We constructed a four-level HRQOL variable: (1) individuals with PCS & MCS < 50, (2) PCS < 50 & MCS ≥ 50, (3) MCS < 50 & PCS ≥ 50, and (4) PCS & MCS ≥ 50, which was the reference. The primary outcome was incident CVD (non-fatal myocardial infarction (MI), fatal MI or coronary heart disease (CHD) death, fatal and non-fatal stroke). Cox proportional hazards models examined associations between HRQOL and CVD.
Results
Median follow-up was 8.4 (IQR 5.9–10.0) years. We observed 1766 CVD events. Compared to having PCS & MCS ≥ 50, having MCS & PCS < 50 was associated with increased CVD risk (aHR 1.46; 95% 1.24–1.70), adjusting for demographics, comorbidities, and CVD risk factors. Associations between MCS & PCS < 50 and CVD were consistent for CHD (aHR 1.54 [1.26–1.89]) and stroke (aHR 1.35 [1.05–1.72]) endpoints.
Conclusions
Given strong, adjusted associations between poor HRQOL and incident CVD, self-reported health may be an excellent complement to current approaches to CVD risk identification.
Keywords: Quality of life, Cardiovascular events, Risk assessments
Introduction
The burden of cardiovascular disease (CVD) among adults in the USA is high [1]. Estimates suggest that by 2030, 44% of the population will have some type of CVD [1]. As the prevalence of vascular disease grows, it is important to identify factors that may be used to detect individuals who are at high risk in order to appropriately target prevention efforts.
It is well accepted that hyperlipidemia, hypertension, diabetes, and smoking are causally linked to CVD risk [2]. However, several non-causal and non-modifiable factors such as age, sex, and race are also included in traditional CVD risk prediction tools. While risk prediction tools incorporating these factors are well-calibrated, some studies found that they may over- or underestimate risk depending on the population studied [3, 4]. Additionally, while CVD risk prediction tools include demographic and clinical characteristics, they do not incorporate patients’ perspectives of their own well-being [2, 5]. This is problematic since prior studies found that poor self-reported health is independently associated with increased risk of mortality, even after adjusting for age, functional status, and comorbidities [6, 7]. Yet, although it is easily assessed, patients’ perceived health is not commonly conceptualized as a CVD risk factor [2, 5]. A better understanding of the association between health-related quality of life (HRQOL) and incident CVD would offer a more comprehensive view of the role of health status in CVD risk and could potentially identify a group of individuals who may benefit from increased clinical attention, should independent associations exist.
We used data from the national Reasons for Geographic and Racial Differences in Stroke (REGARDS) cohort study to determine associations between baseline HRQOL and incident CVD events among adults with no history of stroke or heart disease. We also examined whether associations between HRQOL and CVD varied by physical and mental HRQOL domains.
Methods
REGARDS study design
REGARDS is a national, prospective cohort study evaluating racial and geographic disparities in stroke mortality, with an ancillary study examining disparities in myocardial infarction (MI). REGARDS recruited 30,239 community dwelling, English-speaking individuals ≥ 45 years of age from 2003 to 2007 and is following participants longitudinally [8]. Given that one of the primary objectives of the REGARDS study was to assess and explain regional and racial variation in stroke outcomes, Blacks and individuals from the Southeast were over-sampled [8].
Participants completed a 45-min telephone interview at baseline, which ascertained sociodemographic and medical history. Additionally, study participants underwent an in-home physical exam and medication inventory. During the in-home visit, laboratory data and electrocardiogram (ECGs) were collected. At 6-month intervals, participants were contacted by phone to ascertain CVD outcomes [8]. Potential CVD events were adjudicated by experts. This study was approved by the participating institutions’ Institutional Review Boards. All participants provided written informed consent.
Study Cohort
REGARDS participants with baseline Short-Form (SF)-12 scores and without a history of self-reported stroke or heart disease (MI, coronary artery bypass grafting [CABG], angioplasty, percutaneous coronary intervention [PCI]) or evidence of MI by ECG at baseline.
HRQOL
HRQOL includes an individual’s perceived sense of well-being and was measured using the SF-12, a 12-item instrument that has been psychometrically validated to assess generic self-reported health [9]. The SF-12 captures physical and mental HRQOL through the Physical Component Summary (PCS) and Mental Component Summary (MCS) scores [9]. The PCS includes physical functioning, physical limitations, bodily pain, and general health, whereas the MCS includes concepts related to mental health, emotional limitations, vitality, and social functioning. MCS and PCS scores range from 0 to 100 with higher scores representing better HRQOL. Subscales were normalized with mean of 50 and standard deviation (SD) of 10. Scores above or below 50 give a sense of HRQOL compared to the general US population [9].
We constructed a 4-category HRQOL variable: individuals with low HRQOL scores (MCS < 50 for and PCS < 50), individuals with scores PCS < 50 and MCS ≥ 50, individuals with scores MCS < 50 and PCS ≥ 50, and individuals with high HRQOL scores (MCS ≥ 50 and PCS ≥ 50).
Incident CVD events
Incident CVD events were defined as: (1) first non-fatal MI (definite or probable), (2) fatal MI (if deceased within 28 days after adjudicated event) or coronary heart disease (CHD) death (sudden cardiac death or death from coronary disease that did not meet criteria for fatal MI), and (3) fatal and non-fatal stroke.
CHD events were expertly adjudicated by clinicians using published guidelines [10, 11] which consider clinical signs and symptoms consistent with ischemia; rising and/ or falling pattern of cardiac biomarkers over ≥ 6 h with a peak at least twice the upper limit of normal; and/or ECG or other imaging findings consistent with ischemia based on the Minnesota code [12]. Only definite or probable MIs were considered MIs. Cause of death was assessed through next of kin interviews, the National Death Index (NDI) [8], death certificates, medical records, and autopsies.
Stroke was determined in four possible ways: (1) the World Health Organization’s definition of strokes (focal neurological deficit lasting ≥ 24 h, confirmed with medical records), (2) clinically defined strokes (focal or nonfocal neurological deficit with positive imaging, may or may not lasting 24 h, confirmed with medical records), (3) stroke determined from the NDI as cause of death from stroke without medical records, and (4) strokes determined through a next of kin interview (possible stroke identified, medical records were unavailable).
Baseline characteristics of study participants
Demographic covariates included sex, age at baseline, race (White or Black), education, relationship status, access to care, income, health insurance, living in Health Professional Shortage Area (HPSA) [13], stroke-belt region (belt/buckle, defined as North Carolina, South Carolina, Georgia, Tennessee, Mississippi, Alabama, Louisiana and Arkansas; or non-stroke belt), cigarette smoking, and sex-specific alcohol consumption defined by The National Institute on Alcohol Abuse and Alcoholism.
Clinical covariates included history of diabetes, defined as fasting blood glucose ≥ 126 mL/dL (glucose > 200 mL/ dL for those failing to fast) or oral hypoglycemic or insulin use; hypertension, defined as systolic blood pressure (BP) ≥ 140 mm Hg or diastolic BP of ≥ 90 mm Hg or self-reported medication use to control BP; atrial fibrillation (AF) self-reported or by ECG at in-home exam; left ventricular hypertrophy (LVH), defined as presence of LVH on 12-lead ECG using the Sokolow criteria [14]; medication use (pulmonary, antihypertensive, statins [yes/no]); body mass index (BMI) based on measured height and weight; high density lipoprotein cholesterol (HDL), low density lipoprotein cholesterol (LDL), log-transformed high sensitivity C-reactive protein (hsCRP); log-transformed urinary albumin/creatinine ratio (ACR); estimated glomerular filtration rate (eGFR), calculated using the Chronic Kidney Disease (CKD) Epidemiology Collaboration formula [15], with CKD defined as eGFR as < 60 ml/min/1.732.
We implemented multiple imputation of missing baseline covariates to reduce bias [16]. The highest proportion of missingness was observed for LVH (29%) and income (12%). Other covariates had < 6% missing. We employed multivariate imputation by chained equations (MICE) and used classification and regression trees (CART) as an imputation engine because it captures potential nonlinear effects [17, 18]. We obtained 20 imputed data sets with 20 iterations. Data imputation procedures were performed in R version 3.4.1 “mice” package.
Statistical analyses
We estimated Kaplan–Meier survival functions for all four HRQOL groups and compared them using the log-rank test. Individuals with MCS > 50 and PCS > 50 were the reference group. We assessed unadjusted differences in cohort characteristics across HRQOL groups using ANOVA, Kruskal–Wallis or Chi-square tests. To examine associations between HRQOL groups and CVD, we fit Cox Proportional Hazards models, first examining crude associations and then adjusting for demographics, socioeconomics, health behaviors, and comorbid conditions. Given that CVD risk prediction tools often perform worse in older adults, we examined HRQOL and age (< 75 and ≥ 75 years) interactions [19]. Additionally, because CVD risk varies considerably between Blacks and Whites, we also explored interactions between HRQOL and race. The proportional hazards assumption was assessed for all models. Unadjusted and adjusted hazard ratios (aHR) and 95% confident intervals (CI) were calculated.
We conducted numerous sensitivity analyses. First, we estimated adjusted Cox models using continuous HRQOL as the primary explanatory variables (PCS only, MCS only, and both PCS and MCS). Second, as 5 points is considered a clinically meaningful SF-12 difference, we examined associations between 5-point decrements in PCS and MCS and CVD. Finally, we examined associations between the first SF-12 question alone (i.e., SF-1: “In general, would you say your health is excellent, very good, good, fair, poor?”) and CVD. As a single-item question can be quickly and easily administered in clinical practice, determining the association between the SF-1 and incident CVD events has potentially high clinical utility.
We assessed model discrimination using Harrell’s C-index [20–22] designed specifically for right-censored data. As the data were multiply imputed, in this manuscript we reported the median and range of c-statistics across the 20 imputed datasets [23]. Finally, we evaluated the functional forms of the continuous versions of PCS and MCS and tested the linearity assumption by using cumulative Martingale residuals. Analyses were conducted in SAS version 9.4 with 2-sided statistical tests and significance levels of 5%.
Results
Of the 30,239 REGARDS participants, 56 individuals were excluded due to consent errors and 469 individuals were excluded due to lack of follow-up. Of the remaining participants, 1899 (6%) were excluded because they self-reported a history of stroke, leaving 27,815 participants from which 4631 (17%) were excluded due to a history of heart disease. Of the remaining 23,184 eligible individuals, 955 (4%) had missing SF-12 values and were excluded from our study. As such, our final sample included 22,229 participants (Supplementary Fig. 1).
Baseline characteristics of participants
Overall, 58% of participants were female, 41% Black, and the mean age at enrollment was 63.8 years (SD 9.2) (Table 1). At baseline, 18% had diabetes and 55% had hypertension. Mean PCS and MCS scores were 47.6 (SD = 10.0) and 54.3 (SD = 8.1), respectively.
Table 1.
Participant characteristics by health-related quality of life group
| HRQOL groups |
|||||
|---|---|---|---|---|---|
| PCS < 50, MCS < 50 | PCS < 50, MCS ≥ 50 | PCS ≥ 50, MCS < 50 | PCS ≥ 50, MCS ≥ 50 | p value | |
| N | 2466 | 7706 | 1936 | 10,121 | |
| Key predictors | |||||
| SF-12 physical component score, median (IQR)a | 37.8 (30.1, 44.3) | 42.4 (34.1, 47.1) | 56.0 (53.3, 58.9) | 54.8 (52.8, 56.2) | <0.001 |
| SF-12 mental component score, median (IQR)b | 41.7 (35.3, 46.9) | 58.6 (55.5, 61.2) | 43.3 (36.4, 47.5) | 57.8 (55.5, 58.8) | <0.001 |
| Sociodemographic factors Age, mean (SD) | 62.4 (9.5) | 65.4 (9.3) | 61.4 (9.0) | 63.3 (8.9) | <0.001 |
| Black race, N (%) | 1364 (55.3%) | 3387 (44.0%) | 762 (39.4%) | 3687 (36.4%) | <0.001 |
| Male, N (%) | 682 (27.7%) | 2902 (37.7%) | 672 (34.7%) | 4966 (49.1%) | <0.001 |
| HPSA status (partial or complete), N (%)c | 1038 (42.1%) | 3268 (42.4%) | 858 (44.3%) | 4165 (41.2%) | 0.053 |
| Southeast region (stroke belt or buckle), N (%)d | 1519 (61.6%) | 4390 (57.0%) | 995 (51.4%) | 5417 (53.5%) | <0.001 |
| Low annual household income (< $35,000), N (%) | 1496 (60.7%) | 3545 (46.0%) | 768 (39.7%) | 2989 (29.5%) | <0.001 |
| Lack of health insurance, N (%) | 289 (11.7%) | 489 (6.3%) | 203 (10.5%) | 613 (6.1%) | <0.001 |
| Low education (< high school), N (%) | 562 (22.8%) | 954 (12.4%) | 171 (8.8%) | 647 (6.4%) | <0.001 |
| Relationship status, N (%) | |||||
| Married | 1088 (44.1%) | 4384 (56.9%) | 1002 (51.8%) | 6732 (66.5%) | <0.001 |
| Widowed | 573 (23.2%) | 1588 (20.6%) | 343 (17.7%) | 1404 (13.9%) | |
| Divorced | 506 (20.5%) | 1160 (15.1%) | 374 (19.3%) | 1351 (13.3%) | |
| Single | 183 (7.4%) | 409 (5.3%) | 160 (8.3%) | 476 (4.7%) | |
| Health behaviors | |||||
| Cigarette smoking (current smoker), N (%) | 546 (22.1%) | 1004 (13.0%) | 389 (20.1%) | 1136 (11.2%) | <0.001 |
| Alcohol use, N (%) | |||||
| Moderate drinking | 604 (24.5%) | 2302 (29.9%) | 724 (37.4%) | 3912 (38.7%) | <0.001 |
| Heavy drinking | 79 (3.2%) | 257 (3.3%) | 124 (6.4%) | 488 (4.8%) | |
| Medical risk factors | |||||
| Diabetes, N (%) | 710 (28.8%) | 1769 (23.0%) | 245 (12.7%) | 1213 (12.0%) | <0.001 |
| Hypertension, N (%)e | 1654 (67.1%) | 4875 (63.3%) | 902 (46.6%) | 4728 (46.7%) | <0.001 |
| Atrial fibrillation, N (%) | 256 (10.4%) | 640 (8.3%) | 98 (5.1%) | 414 (4.1%) | <0.001 |
| Left ventricular hypertrophy, N (%) | 104 (4.2%) | 232 (3.0%) | 41 (2.1%) | 196 (1.9%) | <0.001 |
| Medication use | |||||
| Pulmonary medications, N (%) | 337 (13.7%) | 839 (10.9%) | 129 (6.7%) | 591 (5.8%) | <0.001 |
| Antihypertensive medications, N (%) | 1641 (66.5%) | 4878 (63.3%) | 848 (43.8%) | 4455 (44.0%) | <0.001 |
| Statins, N (%) | 681 (27.6%) | 2160 (28.0%) | 390 (20.1%) | 2312 (22.8%) | <0.001 |
| Physiological factors | |||||
| Body mass index, mean (SD)f | 31.5 (7.4) | 30.6 (6.7) | 28.5 (5.7) | 27.9 (5.1) | <0.001 |
| High density lipoprotein cholesterol, median (IQR) | 50.0 (41.0, 61.0) | 50.0 (41.0, 61.0) | 52.0 (42.0, 64.0) | 51.0 (41.0, 63.0) | <0.001 |
| Low density lipoprotein cholesterol, median (IQR) | 114.0 (93.0, 138.0) | 112.0 (91.0, 136.0) | 118.0 (96.0, 141.0) | 116.0 (95.0, 138.0) | <0.001 |
| C-reactive protein, mean (SD)g | 1.2 (1.2) | 0.9 (1.2) | 0.7 (1.2) | 0.6 (1.1) | <0.001 |
| Urinary albumin/creatinine ratio (mg/g), mean (SD)g | 2.5 (1.4) | 2.4 (1.2) | 2.2 (1.0) | 2.1 (1.0) | <0.001 |
| Estimated GFR, median (IQR)h | 92.8 (75.4, 103.6) | 87.9 (73.1, 98.9) | 92.4 (78.7, 101.4) | 89.5 (77.5, 98.9) | <0.001 |
p values correspond with ANOVA for continuous variables that are normally distributed, Wilcoxon rank-sum (two groups) or Kruskal–Wallis (> 2 groups) for continuous variables that are skewed, and Pearson’s Chi squared or Fisher’s exact test for binary and categorical variables
Ranges from 0 to 100 and a higher score indicate better physical health
Ranges from 0 to 100 and a higher score indicate better mental health
HPSA—Health Professional Shortage Area
REGARDS study oversampled residents from the stroke belt (Alabama, Arkansas, Louisiana, Mississippi, Tennessee, and the non-coastal regions in North Carolina, South Carolina, and Georgia) and the stroke buckle (the coastal regions within North Carolina, South Carolina, and Georgia)
Calculated based on self-reported hypertension, in-home visit SBP ≥ 140 or DBP ≥ 90, or self-reported current medication use to control blood pressure
Calculated as weight in kilograms divided by height in meters squared
Log-transformed variable
Estimated GFR from the CKD-Epi equation
There were 10,121 (45%) individuals in the PCS ≥ 50 & MCS ≥ 50 group, 1936 (9%) in the PCS ≥ 50 & MCS < 50 group, 7706 (35%) in the PCS < 50 & MCS ≥ 50 group, and 2466 (11%) in PCS < 50 & MCS < 50 group. Participants with both PCS and MCS scores below 50 were more likely to be Black or female; have low income, have no health insurance, have diabetes or hypertension, and have a BMI > 30.
Incident CVD events
Over a median follow-up time of 8.4 years with interquartile range of 5.9–10.0 years, 1766 incident CVD events were observed. Of these, 1051 (60%) were CHD and 715 (40%) strokes.
Associations between HRQOL and incident CVD events
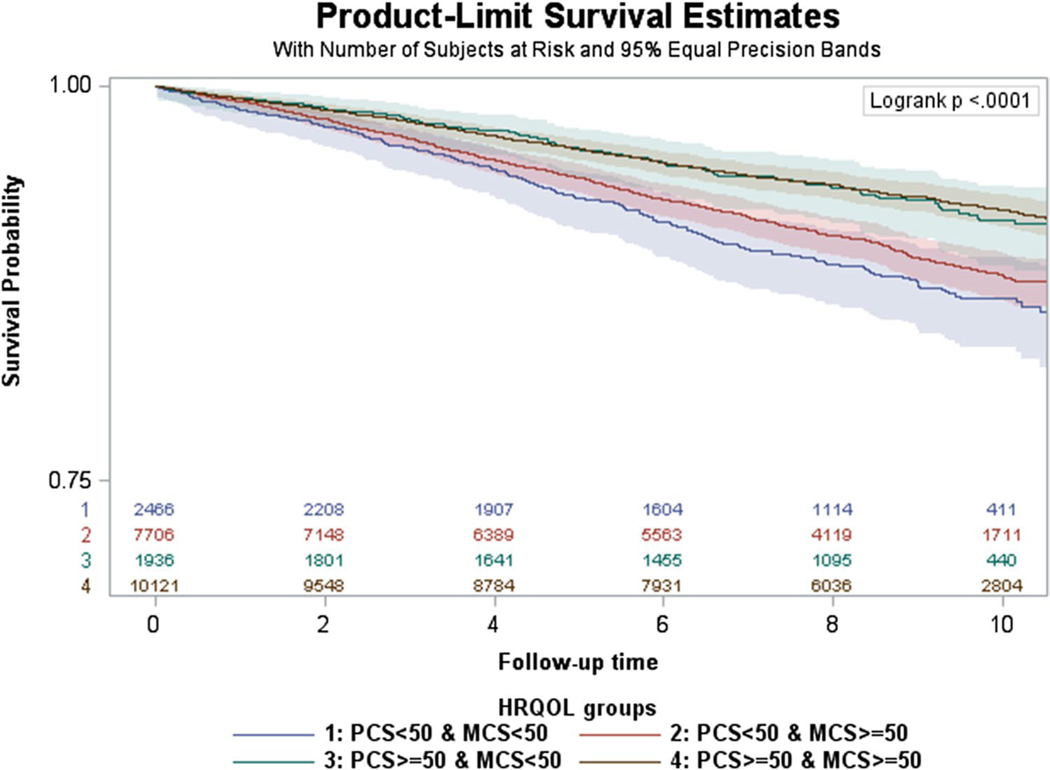
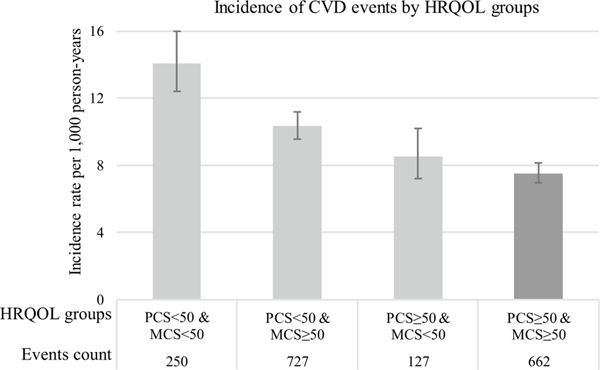
Figure 1 shows unadjusted Kaplan Meier curves for incident CVD events by the four HRQOL group. The log-rank test indicated significant differences between survival functions of the HRQOL groups (χ32 = 100.9, p < 0.0001). To further explore this, we ran the Dunnett’s modified Tukey–Kramer pairwise multiple comparison test (designed for unequal sample sizes and variances) which showed significant differences between the reference group (PCS ≥ 50 & MCS ≥ 50) and the other three HRQOL groups. Figure 2 shows age-adjusted CVD incidence rates per 1000 person-years (PYs) by HRQOL group. The Dunnett–Hsu multiple comparisons test suggests that significant differences between the reference group (PCS ≥ 50 & MCS ≥ 50) and the two HRQOL groups with PCS < 50 exist. Kaplan–Meier curves and incidence rates for CHD and stroke, separately, are presented in Supplementary Figs. 2–5.
Fig. 1.
Unadjusted Kaplan–Meier curves for incident CVD events. Note HRQOL groups are mutually exclusive
Fig. 2.
Age-adjusted incident CVD events by health-related quality of life group. Note Incidence rate per 1000 person-years were adjusted for participant age at baseline
In minimally adjusted Cox models controlling only for age, race, and sex, having PCS & MCS < 50 was associated with twofold higher CVD risk (aHR 2.11; 95% CI 1.82–2.45), compared to having PCS & MCS ≥ 50 (Table 2). Compared to the reference group, having PCS < 50 & MCS ≥ 50 or PCS ≥ 50 & MCS < 50 was also associated with increased CVD risk aHR 1.47; 95% CI 1.32–1.63) and (aHR 1.24; 95% CI 1.02–1.50), respectively. We observed similar associations when CHD and stroke outcomes were examined separately, but aHRs were larger for CHD.
Table 2.
Minimally adjusted associations between baseline health-related quality of life and incident CVD events
| Effects | aHR (95% CI) |
||
|---|---|---|---|
| CVD events | CHD events | Stroke events | |
| HRQOL groups | |||
| PCS ≥ 50 & MCS ≥ 50 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| PCS ≥ 50 & MCS < 50 | 1.24 (1.02, 1.50) | 1.27 (0.99, 1.64) | 1.19 (0.89, 1.59) |
| PCS < 50 & MCS ≥ 50 | 1.47 (1.32, 1.63) | 1.69 (1.48, 1.95) | 1.19 (1.00, 1.40) |
| PCS < 50 & MCS < 50 | 2.11 (1.82, 2.45) | 2.37 (1.95, 2.88) | 1.79 (1.42, 2.26) |
| Sociodemographic factors | |||
| Age | 1.06 (1.05, 1.06) | 1.05 (1.05, 1.06) | 1.07 (1.06, 1.08) |
| Black race | 1.13 (1.03, 1.24) | 1.08 (0.95, 1.22) | 1.20 (1.04, 1.40) |
| Male | 1.70 (1.54, 1.87) | 1.97 (1.74, 2.23) | 1.36 (1.17, 1.58) |
aHR adjusted hazard ratios, 95% CI 95% confidence intervals
When participant demographics, socioeconomics, health behaviors, and comorbid conditions were added to CVD models, aHRs attenuated but remained statistically significant for the PCS & MCS < 50 (aHR 1.46; 95% CI 1.24–1.70) and PCS < 50 & MCS ≥ 50 (aHR 1.21; 95% CI 1.08–1.35) groups compared to the PCS ≥ 50 & MCS ≥ 50 group (Table 3). For CVD events, only being male (aHR 1.86; 95% CI 1.65–2.09), smoking (aHR 1.69; 95% CI 1.48–1.93) and atrial fibrillation (aHR 1.48; 95% CI 1.25–1.73) had larger effects than having PCS & MCS < 50.
Table 3.
Fully adjusted associations between baseline health-related quality of life and incident CVD events Effects
| Effects | aHR (95% CI) |
||
|---|---|---|---|
| CVD events | CHD events | Stroke events | |
| HRQOL groups | |||
| PCS ≥ 50 & MCS ≥ 50 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| PCS ≥ 50 & MCS < 50 | 1.14 (0.94, 1.38) | 1.17 (0.91, 1.51) | 1.04 (0.87, 1.24) |
| PCS < 50 & MCS ≥ 50 | 1.21 (1.08, 1.35) | 1.34 (1.16, 1.55) | 1.10 (0.82, 1.47) |
| PCS < 50 & MCS < 50 | 1.46 (1.24, 1.70) | 1.54 (1.26, 1.89) | 1.35 (1.05, 1.72) |
| Sociodemographic factors | |||
| Age | 1.05 (1.04, 1.06) | 1.04 (1.03, 1.05) | 0.96 (0.81, 1.13) |
| Black race | 0.88 (0.79, 0.99) | 0.84 (0.73, 0.97) | 1.06 (1.05, 1.07) |
| Male | 1.86 (1.65, 2.09) | 2.16 (1.85, 2.52) | 1.50 (1.25, 1.80) |
| HPSA status (partial or complete) | 1.03 (0.94, 1.14) | 1.04 (0.91, 1.18) | 1.03 (0.88, 1.20) |
| Southeast region (stroke belt or buckle) | 1.11 (1.01, 1.23) | 1.15 (1.01, 1.31) | 1.07 (0.91, 1.24) |
| Low annual household income (< $35,000) | 1.10 (0.98, 1.23) | 1.05 (0.90, 1.21) | 1.19 (0.99, 1.42) |
| Lack of health insurance | 1.27 (1.04, 1.55) | 1.34 (1.04, 1.72) | 1.18 (0.85, 1.63) |
| Low education (< high school) | 1.15 (1.00, 1.32) | 1.23 (1.03, 1.47) | 1.04 (0.83, 1.30) |
| Relationship status (widowed) | 1.12 (0.98, 1.28) | 1.14 (0.95, 1.36) | 1.10 (0.89, 1.36) |
| Relationship status (divorced) | 0.99 (0.84, 1.15) | 0.87 (0.70, 1.07) | 1.16 (0.91, 1.46) |
| Relationship status (single) | 1.03 (0.82, 1.30) | 1.02 (0.76, 1.39) | 1.05 (0.72, 1.51) |
| Health behaviors | |||
| Smoking | 1.69 (1.48, 1.93) | 1.82 (1.54, 2.16) | 1.51 (1.22, 1.87) |
| Alcohol consumption (heavy) | 0.94 (0.73, 1.21) | 0.91 (0.66, 1.26) | 0.99 (0.68, 1.44) |
| Alcohol consumption (moderate) | 0.88 (0.79, 0.98) | 0.86 (0.75, 0.99) | 0.91 (0.76, 1.08) |
| Medical risk factors | |||
| Diabetes | 1.30 (1.15, 1.47) | 1.39 (1.19, 1.62) | 1.17 (0.96, 1.42) |
| Hypertension | 1.37 (1.20, 1.56) | 1.26 (1.07, 1.50) | 1.53 (1.25, 1.88) |
| Atrial fibrillation | 1.48 (1.27, 1.73) | 1.43 (1.17, 1.75) | 1.56 (1.23, 1.99) |
| Left ventricular hypertrophy | 1.29 (1.02, 1.63) | 1.43 (1.07, 1.90) | 1.11 (0.76, 1.64) |
| Medication use | |||
| Pulmonary medications | 1.04 (0.88, 1.23) | 1.19 (0.97, 1.46) | 0.82 (0.62, 1.10) |
| Antihypertensive medications | 1.02 (0.89, 1.16) | 1.09 (0.92, 1.30) | 0.92 (0.75, 1.12) |
| Statins | 0.99 (0.88, 1.11) | 1.00 (0.86, 1.16) | 0.97 (0.81, 1.17) |
| Physiological factors | |||
| Body mass index | 0.99 (0.99, 1.00) | 1.00 (0.99, 1.01) | 0.98 (0.97, 1.00) |
| High density lipoprotein cholesterol | 1.00 (0.99, 1.00) | 1.00 (0.99, 1.00) | 1.00 (0.99, 1.00) |
| Low density lipoprotein cholesterol | 1.00 (1.00, 1.01) | 1.00 (1.00, 1.01) | 1.00 (1.00, 1.01) |
| C-reactive proteina | 1.12 (1.07, 1.17) | 1.13 (1.07, 1.20) | 1.09 (1.02, 1.17) |
| Urinary albumin/creatinine ratio (mg/g)a | 1.20 (1.16, 1.24) | 1.19 (1.14, 1.25) | 1.21 (1.15, 1.29) |
| Estimated GFR | 1.00 (0.99, 1.00) | 0.99 (0.99, 1.00) | 1.00 (0.99, 1.00) |
Log-transformed variable
When we examined associations between HRQOL and CHD and stroke outcomes, separately, we observed significant associations for both CHD (aHR 1.54; 95% CI 1.26–1.89) and stroke (aHR 1.35; 95% CI 1.05–1.72) outcomes. For CHD, having PCS < 50 & MCS ≥ 50 was significantly associated with increased CVD risk (aHR 1.34; 95% CI 1.16–1.55), but the association was not statistically significant for stroke (aHR 1.04; 95% CI 0.87–1.24). Although estimates of adjusted HR were above 1.0, we did not observe statistically significant associations between having PCS ≥ 50 & MCS < 50 and stroke or CHD events. Finally, in fully adjusted CVD models, HRQOL-race and HRQOL-age interactions were not statistically significant. The p values for interactions by race were: p = 0.78, p = 0.58 and p = 0.79 for PCS & MCS < 50, PCS ≥ 50 & MCS < 50 and PCS < 50 & MCS ≥ 50 groups, respectively. The p values for interactions by age were: p = 0.46, p = 0.39 and p = 0.49 for PCS & MCS < 50, PCS ≥ 50 & MCS < 50 and PCS < 50 & MCS ≥ 50 groups, respectively.
Sensitivity analyses
Continuous PCS and MCS scores were significantly associated with increased CVD risk in minimally and fully adjusted Cox models. In fully adjusted models, 5-point PCS and MCS decreases were associated with higher CVD risk (aHR 1.06; 95% 1.04–1.09) and (aHR 1.04; 95% 1.02–1.08), respectively. Finally, in adjusted models, the SF-1 (“In general, would you say your health is excellent, very good, good, fair, poor?”) was significantly associated with increased CVD risk. Compared to individuals who reported “excellent” health, reporting “poor” or “fair” health was associated with higher CVD risk (aHR 1.68; 95% CI 1.26–2.25) and (aHR 1.25; 95% CI 1.04–1.50), respectively. We did not observe significant differences for individuals who reported “good” or “very good” compared to “excellent” health (aHR 1.11, 95% CI 0.95–1.29; and aHR 0.93, 95% CI 0.80–1.08, respectively).
Model discrimination
The fully adjusted Cox model with the 4-category HRQOL variable had a median Harrell’s C-statistic of 0.7262 (0.7256–0.7268) across 20 imputed datasets. Median c-statistics for CHD and stroke were 0.7368 (0.7356–0.7377) and 0.7250 (0.7243–0.7263), respectively. Upon formal evaluation, PCS and MCS scores suggest log-linear relationships with incident CVD. In a model including only PCS score, the c-statistic was 0.5760. Similarly, in a model including only MCS score, the c-statistic was 0.4980. In a fully adjusted model including PCS (not MCS), the c-statistic rose to 0.7269 (0.7264–0.7275). In a fully adjusted model including MCS (not PCS), the c-statistic rose to 0.7254 (0.7248–0.7260).
Discussion
We found that poor HRQOL was significantly associated with higher risk of incident CVD events overall, and for CHD and stroke events, separately. Associations persisted after adjustment for demographics, social determinants of health, health behaviors, comorbidities, and traditional CVD risk factors. Finally, associations between HRQOL and incident CVD did not vary by race or age.
Our findings contribute to the literature in several ways. First, our results support prior studies which have found poor HRQOL to be significantly associated with adverse outcomes such as survival, CHD events, and stroke [24–26]. The magnitude of the association between poor HRQOL and incident CVD that we found extends previous work. In fact, associations between HRQOL and CVD events were comparable to that of Framingham CVD risk factors, demonstrating the powerful effect that self-reported HRQOL can have on incident CVD events. For incident CHD, poor HRQOL was associated with a magnitude of risk similar to diabetes and hypertension. For incident stroke, poor HRQOL demonstrated independent associations after full adjustment, whereas some traditional CVD risk factors, like diabetes, did not. These findings demonstrate that HRQOL can serve as an important independent predictors for incident CVD events, compared to well-recognized risk factors.
Second, we found that the association between poor HRQOL and CVD events was much stronger for poor physical health (reported at baseline), rather than for mental well-being. This is consistent with previous studies that concluded that physical HRQOL was a strong predictor of CVD risk [24]. For example, a study in the UK found that physical functioning was associated with greater CHD risk, independent of age, sex, BMI, and smoking, and another study reported associations between poor physical HRQOL and incident stroke [26]. Notably though, previous work has centered around quantifiable aspects of physical health (e.g., physical activity, functioning, and mobility) [27, 28]. We assessed physical health from the patient’s own perspective at baseline, which is both novel in this context and patient-centered. Clinically, identifying individuals who self-report poor physical HRQOL is important, as these patients who voice and acknowledge poor HRQOL may be more amendable to interventions which seek to mitigate pain and discomfort, compared to patients who do not report poor physical HRQOL. In a clinical setting, patients reporting poor physical HRQOL could be connected with physical therapists or rehabilitation medicine to help improve their physical well-being, if medically indicated. Furthermore, self-reported HRQOL is known to be representative of patient preferences and priorities, which is consistent with national efforts to provide patient-centered care [29].
Notably, using the 4-category HRQOL variable, we did not observe significant associations between poor mental health and incident stroke or CHD. However, we did see an increase in CVD risk when examining the 5-point decrement in MCS. Literature over the last decade suggests that psychological factors including depressive symptoms and stress are associated with CVD [30–34]. The putative mechanism underlying this association is that chronic exposure to psychological stress and poor mood may activate physiologic processes involving the hypothalamic–pituitary–adrenal axis, the sympathetic-parasympathetic systems, and inflammatory cascades, all of which can have downstream consequences for cardiovascular health [34, 35]. The relatively small effect between mental HRQOL declines and incident CVD in our study may be partially because we examined mental HRQOL at baseline, which may not represent one’s mental health close to their CVD event. We would possibly observe stronger associations if we examined time-varying mental HRQOL or sustained mental distress over time [36]. Additionally, the SF-12 is a generic instrument intended to measure broad constructs of mental health, emotional limitations, vitality, and social functioning in the general population [9]. Therefore, the MCS may not be sensitive enough to show varying levels of depression, which might be more closely related to CVD events than baseline HRQOL.
Interestingly, although age and race are important predictors of CVD events, associations between HRQOL and CVD did not vary significantly (p > 0.10) by either, suggesting that the strong, independent, inverse relationship between HRQOL and CVD is consistent across adults 45 + years and between Whites and Blacks. This is an unforeseen finding, as we anticipated that HRQOL might be a better predictor for CVD events among older adults who have numerous chronic conditions and who may place a greater importance on subjective well-being and quality of life. However, lack of variation by race was consistent with another REGARDS study, which did not find associations between mental health and incident CHD to differ by race [36].
Our findings have important clinical implications, particularly for screening and CVD risk prediction. CVD risk prediction often relies on tools that include physiologic and disease parameters, but not patients’ perceived health. Given our findings, providers might consider incorporating the SF-12 or even the single-item SF-1 into clinical encounters as a way to complement current history taking. This may seem challenging, as office visits are limited in time, particularly for patients with multiple chronic conditions. However, studies have shown that the SF-12 is easy to complete, requiring 2–3 min, and relatively easy for clinicians to interpret [9, 37]. Asking patients without history of CVD to fill the SF-12 out prior to their office visit (e.g., by email or patient portal) or in the waiting room could enhance information that providers gather during a patient’s history and physical examination, especially since HRQOL measures pick up on aspects of information which may not be concretely measured or observed by the physician during the visit [38, 39]. A single-item question such as the SF-1 might also be useful for quickly flagging patients with poor self-reported HRQOL who may require additional attention.
Due to possible stigma associated with poor physical and mental HRQOL, patients may be reluctant to initiate conversations with providers [40, 41]. Thus, the SF-12 and SF-1 may allow providers to identify patients with poor HRQOL, which may not have previously been recognized. Identifying patients with poor HRQOL may enable a subset of patients who are vulnerable to incident CVD events to be targeted with additional attention and support. A heightened understanding of a patient’s elevated incident CVD risk would likely prompt physicians to more aggressively manage known modifiable risk factors (i.e., diabetes, hypertension, smoking status), and also encourage providers to engage in counseling around physical activity and mental health.
Limitations
The SF-12 does not allow for domain-specific analyses, which could provide more granular insights regarding HRQOL. Additionally, both physical and mental HRQOL were collected at one timepoint (baseline). HRQOL patterns observed at baseline may change over the 10-year follow-up. Because the SF-12 is normed to have a mean of 50 and standard deviation of 10, we chose 50 as our cutoff for low and high HRQOL. However, we recognize limitations in selecting 50 as a cutoff and explored additional cutoffs including tertiles and quartiles and found consistent results. Furthermore, biases inherent to self-report of other domains and variables in this study are well known. Finally, given that the mean age at study enrollment was 64 years, these results have limited generalizability to younger populations.
Strengths
This large, community-based study examined associations between HRQOL and incident, expert-adjudicated CVD events. The racial and geographical diversity of the REGARDS dataset increases the generalizability of these findings beyond studies conducted in clinical trials or singlesite, academic medical institutions. Furthermore, our ability to control for self-reported and physiological factors contributes to the HRQOL and CVD literature.
Conclusions
We observed that HRQOL was significantly and independently associated with increased incident CVD risk among adults without a history of CHD or stroke. The magnitude of the independent association was greater than that of several established CVD risk factors. Using a short, inexpensive, and psychometrically validated HRQOL instrument may be warranted in future clinical encounters, as it offers opportunities to incorporate patients’ perspectives into CVD prevention efforts. Given the relationship between poor HRQOL and CVD, self-reported health may be an excellent complement to current approaches to CVD risk identification and treatment.
Supplementary Material
Acknowledgements
The authors thank the other investigators, the staff, and participants of the REGARDS study for all of their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
Funding This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, and R01 HL80477 from the National Heart Lung and Blood Institute, National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data.
Footnotes
Compliance with ethical standards
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. This study was approved by the participating institutions’ Institutional Review Boards. All authors have read and approved the manuscript for submission to Quality of Life Research.
Informed consent For this type of study, formal consent is not required. All participants provided written informed consent.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s11136-019-02103-1) contains supplementary material, which is available to authorized users.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Conflict of interest The authors have no conflicts of interest or financial disclosures.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, & Muntner P. (2017). Heart disease and stroke statistics-2017 update: A report From the American Heart Association. Circulation, 135(10), e146–e603. 10.1161/cir.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goff DC Jr., Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB Sr., Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr., Sorlie P, Stone NJ, & Wilson PW (2014). 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology, 63(25 Pt B), 2935–2959. 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colantonio LD, Richman JS, Carson AP, Lloyd-Jones DM, Howard G, Deng L, Howard VJ, Safford MM, Muntner P, & Goff DC Jr. (2017) Performance of the atherosclerotic cardiovascular disease pooled cohort risk equations by social deprivation status. Journal of the American Heart Association. 10.1161/jaha.117.005676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook NR, & Ridker PM (2014). Further insight into the cardiovascular risk calculator: The roles of statins, revascularizations, and underascertainment in the Women’s Health Study. JAMA Internal Medicine, 174(12), 1964–1971. 10.1001/jamainternmed.2014.5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Agostino RB Sr., Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, & Kannel WB (2008). General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation, 117(6), 743–753. 10.1161/circulationaha.107.699579. [DOI] [PubMed] [Google Scholar]
- 6.DeSalvo KB, Bloser N, Reynolds K, He J, & Muntner P. (2006). Mortality prediction with a single general self-rated health question. A meta-analysis. Journal of General Internal Medicine, 21(3), 267–275. 10.1111/j.1525-1497.2005.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominick KL, Ahern FM, Gold CH, & Heller DA (2002). Relationship of health-related quality of life to health care utilization and mortality among older adults. Aging Clinical and Experimental Research, 14(6), 499–508. [DOI] [PubMed] [Google Scholar]
- 8.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, & Howard G. (2005). The reasons for geographic and racial differences in stroke study: Objectives and design. Neuroepidemiology, 25(3), 135–143. 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 9.Ware J Jr., Kosinski M, & Keller SD (1996). A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Medical Care, 34(3), 220–233. [DOI] [PubMed] [Google Scholar]
- 10.Zhang ZM, Prineas RJ, & Eaton CB (2010). Evaluation and comparison of the Minnesota Code and Novacode for electrocardiographic Q-ST wave abnormalities for the independent prediction of incident coronary heart disease and total mortality (from the Women’s Health Initiative). The American Journal of Cardiology, 106(1), 18–25.e12. 10.1016/j.amjcard.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, Goldberg RJ, Hand MM, Jaffe AS, Julian DG, Levy D, Manolio T, Mendis S, Mensah G, Pajak A, Prineas RJ, Reddy KS, Roger VL, Rosamond WD, Shahar E, Sharrett AR, Sorlie P, & Tunstall-Pedoe H. (2003) Case definitions for acute coronary heart disease in epidemiology and clinical research studies: A statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation 108(20):2543–2549. 10.1161/01.cir.0000100560.46946.ea. [DOI] [PubMed] [Google Scholar]
- 12.Prineas RCR, & Blackburn H. (1982). The Minnesota code manual of electrocardiographic findings: Standards and procedures for measurement and classification. Boston: Wright-OSG. [Google Scholar]
- 13.Brown TM, Parmar G, Durant RW, Halanych JH, Hovater M, Muntner P, Prineas RJ, Roth DL, Samdarshi TE, & Safford MM (2011). Health Professional Shortage Areas, insurance status, and cardiovascular disease prevention in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Journal of Health Care for the Poor and Underserved, 22(4), 1179–1189. 10.1353/hpu.2011.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sokolow M, & Lyon TP (1949). The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. American Heart Journal, 37(2), 161–186. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, & Coresh J. (2009). A new equation to estimate glomerular filtration rate. Annals of Internal Medicine, 150(9), 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schroff P, Gamboa CM, Durant RW, Oikeh A, Richman JS, & Safford MM (2017) Vulnerabilities to health disparities and statin use in the REGARDS (reasons for geographic and racial differences in stroke) study. Journal of the American Heart Association. 10.1161/jaha.116.005449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burgette LF, & Reiter JP (2010). Multiple imputation for missing data via sequential regression trees. American Journal of Epidemiology, 172(9), 1070–1076. 10.1093/aje/kwq260. [DOI] [PubMed] [Google Scholar]
- 18.Doove LL, Van Buuren S, & Dusseldorp E. (2014). Recursive partitioning for missing data imputation in the presence of interaction effects. Computational Statistics and Data Analysis, 72, 92–104. 10.1016/j.csda.2013.10.025. [DOI] [Google Scholar]
- 19.Rich MW, Chyun DA, Skolnick AH, Alexander KP, Forman DE, Kitzman DW, Maurer MS, McClurken JB, Resnick BM, Shen WK, & Tirschwell DL (2016). Knowledge gaps in cardiovascular care of older adults: A scientific statement from the American Heart Association, American College of Cardiology, and American Geriatrics Society: Executive Summary. Journal of the American Geriatrics Society, 64(11), 2185–2192. 10.1111/jgs.14576. [DOI] [PubMed] [Google Scholar]
- 20.Harrell FE Jr., Lee KL, Mark DB (1996) Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Statistics in Medicine 15(4):361–387. [DOI] [PubMed] [Google Scholar]
- 21.Barkhordari M, Padyab M, Sardarinia M, Hadaegh F, Azizi F, & Bozorgmanesh M. (2016). Survival regression modeling strategies in CVD prediction. International Journal of Endocrinology and Metabolism, 14(2), e32156. 10.5812/ijem.32156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrell FE (1986) The PHGLM Procedure. In: SUGI Supplemental Library Guide. Version 5 edn. SAS Institute Cary, NC. [Google Scholar]
- 23.Clark TG, & Altman DG Developing a prognostic model in the presence of missing data. Journal of Clinical Epidemiology 56(1):28–37. 10.1016/S0895-4356(02)00539-5. [DOI] [PubMed] [Google Scholar]
- 24.Ul-Haq Z, Mackay DF, & Pell JP (2014). Association between physical and mental health-related quality of life and adverse outcomes: A retrospective cohort study of 5,272 Scottish adults. BMC Public Health, 14, 1197 10.1186/1471-2458-14-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li CL, Chang HY, Hsu CC, Lu JF, & Fang HL (2013). Joint predictability of health related quality of life and leisure time physical activity on mortality risk in people with diabetes. BMC Public Health, 13, 67 10.1186/1471-2458-13-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myint PK, Surtees PG, Wainwright NW, Luben RN, Welch AA, Bingham SA, Wareham NJ, & Khaw KT (2007). Physical health-related quality of life predicts stroke in the EPIC-Norfolk. Neurology, 69(24), 2243–2248. 10.1212/01.wnl.0000296010.21252.78. [DOI] [PubMed] [Google Scholar]
- 27.Sattelmair J, Pertman J, Ding EL, Kohl HW III, Haskell W, & Lee IM (2011). Dose response between physical activity and risk of coronary heart disease: A meta-analysis. Circulation, 124(7), 789–795. 10.1161/circulationaha.110.010710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, & Sone H. (2009). Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: A meta-analysis. JAMA, 301(19), 2024–2035. 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 29.Fredericksen RJ, Edwards TC, Merlin JS, Gibbons LE, Rao D, Batey DS, Dant L, Paez E, Church A, Crane PK, Crane HM, & Patrick DL (2015). Patient and provider priorities for self-reported domains of HIV clinical care. AIDS Care, 27(10), 1255–1264. 10.1080/09540121.2015.1050983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Backe EM, Seidler A, Latza U, Rossnagel K, & Schumann B. (2012). The role of psychosocial stress at work for the development of cardiovascular diseases: A systematic review. International Archives of Occupational and Environmental Health, 85(1), 67–79. 10.1007/s00420-011-0643-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicholson A, Kuper H, & Hemingway H. (2006). Depression as an aetiologic and prognostic factor in coronary heart disease: A meta-analysis of 6362 events among 146,538 participants in 54 observational studies. European Heart Journal, 27(23), 2763–2774. 10.1093/eurheartj/ehl338. [DOI] [PubMed] [Google Scholar]
- 32.Richardson S, Shaffer JA, Falzon L, Krupka D, Davidson KW, & Edmondson D. (2012). Meta-analysis of perceived stress and its association with incident coronary heart disease. The American Journal of Cardiology, 110(12), 1711–1716. 10.1016/j.amjcard.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rugulies R. (2002). Depression as a predictor for coronary heart disease. A review and meta-analysis. American Journal of Preventive Medicine, 23(1), 51–61. [DOI] [PubMed] [Google Scholar]
- 34.Henderson KM, Clark CJ, Lewis TT, Aggarwal NT, Beck T, Guo H, Lunos S, Brearley A, Mendes de Leon CF, Evans DA, & Everson-Rose SA (2013). Psychosocial distress and stroke risk in older adults. Stroke, 44(2), 367–372. 10.1161/strokeaha.112.679159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stewart JC, Rand KL, Muldoon MF, & Kamarck TW (2009). A prospective evaluation of the directionality of the depression-inflammation relationship. Brain, Behavior, and Immunity, 23(7), 936–944. 10.1016/j.bbi.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moise N, Khodneva Y, Richman J, Shimbo D, Kronish I, & Safford MM (2016) Elucidating the association between depressive symptoms, coronary heart disease, and stroke in black and white adults: The REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Journal of the American Heart Association. 10.1161/jaha.116.003767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burstrom B, & Fredlund P. (2001). Self rated health: Is it as good a predictor of subsequent mortality among adults in lower as well as in higher social classes? Journal of Epidemiology and Community Health, 55(11), 836–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pakhomov SV, Jacobsen SJ, Chute CG, & Roger VL (2008). Agreement between patient-reported symptoms and their documentation in the medical record. The American Journal of Managed Care, 14(8), 530–539. [PMC free article] [PubMed] [Google Scholar]
- 39.Basch E. (2017). Patient-reported outcomes—Harnessing patients’ voices to improve clinical care. The New England Journal of Medicine, 376(2), 105–108. 10.1056/NEJMp1611252. [DOI] [PubMed] [Google Scholar]
- 40.Cagle J, & Bunting M. (2017). Patient reluctance to discuss pain: Understanding stoicism, stigma, and other contributing factors. Journal of Social Work in End-of-Life & Palliative Care, 13(1), 27–43. 10.1080/15524256.2017.1282917. [DOI] [PubMed] [Google Scholar]
- 41.Hatzenbuehler ML, Phelan JC, & Link BG (2013). Stigma as a fundamental cause of population health inequalities. American Journal of Public Health, 103(5), 813–821. 10.2105/ajph.2012.301069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.