Abstract
Clinical characteristics are essential for the correct diagnosis of diseases. The current review aimed to summarize the global clinical characteristics of the COVID-19 patients systematically and identify their diagnostic challenges to help the medical practitioners properly diagnose and for better management of COVID-19 patients. We conducted a systematic search in PubMed, Web of Science, Scopus, Science Direct, and Google Scholar databases for original articles containing clinical information of COVID-19 published up to 7th May 2020. Two researchers independently searched the databases to extract eligible articles. A total of 34 studies from 8 different countries with 10889 case-patients were included for clinical characteristics. The most common clinical symptoms were cough 59.6, fever 46.9, fatigue 27.8, and dyspnea 20.23%. The prominent laboratory findings were lymphocytopenia 55.9, elevated levels of CRP 61.9, aspartate aminotransferase 53.3, LDH 40.8, ESR 72.99, serum ferritin 63, IL-6 52, and prothrombin time 35.47%, and decreased levels of platelets 17.26, eosinophils 59.0, hemoglobin 29, and albumin 38.4%. CT scan of the chest showed an abnormality in 93.50% cases with bilateral lungs 71.1%, ground-glass opacity 48%, lesion in lungs 78.3%, and enlargement of lymph node 50.7%. Common comorbidities were hypertension, diabetes, obesity, and cardiovascular diseases. The estimated median incubation period was 5.36 days, and the overall case fatality rate was 16.9% (Global case fatality outside China was 22.24%: USA 21.24%, Italy 25.61%, and others 0%; whereas the case fatality inside the Hubei Province of China was found to be 11.71%). Global features on the clinical characteristics of COVID-19 obtained from laboratory tests and CT scan results will provide useful information to the physicians to diagnose the disease and for better management of the patients as well as to address the diagnostic challenges to control the infection.
Keywords: clinical characteristics, SARS coronavirus (SARS-CoV-2), novel coronavirus diseases (COVID-19), diagnostic challenges, systematic review
Introduction
In late December 2019, the Chinese government officially announced the novel coronavirus (2019-nCoV) outbreak in Wuhan. To contain the virus, Wuhan and eventually the whole Hubei province was put into massive lockdown. The virus, which was later named SARS-CoV-2, got out anyway in other countries of the world. Although limited to China initially, the novel strain of the coronavirus being super contagious compared to the previously known strains has quickly spread across the globe. WHO declared the virus as a global pandemic on March 11, 2020. By then the epicenter of the virus had been shifted from China to Eastern Europe followed by the USA. As updated by the World Health Organization (WHO) on May 28, 2020, the COVID-19 outbreak has reached 213 countries and territories worldwide with 5,593,631 confirmed cases and 353,334 deaths (1).
The virus is yet to be peaked in most of the countries that got infected, and on top of that public health experts have warned that the newest epicenter of the virus could be shifted to the densely populated south and south-east Asia (2). Despite the concerted efforts by every stakeholder, tens of thousands of new cases of COVID-19 have been arising every day. Suddenly the world is facing a severe crisis of ICU beds and artificial respirators. From frontline doctors and nurses to pharmacists, all healthcare professionals are now facing an intense workload. It would take years to develop a vaccine for this novel coronavirus considering the fact that we are yet to see any effective vaccines for the previous coronavirus outbreaks (e.g., SARS & MERS) (3). Besides no well-established and specific anti-viral drug for this virus is available right now. Under this context, it is essential to better elucidate the clinical characteristics of the virus for a clear understanding of the disease and proper management of the patients by the health care providers. Moreover, the diagnosis also coincides with many challenges that include false test results, sampling errors, asymptomatic cases, insufficient testing facilities and lack of consciousness among mass people. Due to higher cost for RT-PCR testing facilities and lack of skilled manpower, many countries could not make it available across every regions, specially remote areas. In addition, due to the unwillingness of the patients arising out of fear of contamination and tendency to avoid procedural complexities, many suspected cases remain undiagnosed. To get the actual scenario of the prevalence and incidences as well as for the proper management of COVID-19 addressing all the diagnostic challenges is of utmost importance. This systematic review presents the summary of published reports up to 7th May 2020 on the clinical characteristics of COVID-19 for a better understanding of the frontiers team (especially to the medical doctors) involved in the treatment and management of COVID-19 patients. Additionally, the challenges for the prevention, treatment and management of COVID-19 have been discussed in the review.
Methodology
Protocol
This systematic review protocol is designed following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Statement-2009 (4).
Literature Search Strategy
We conducted a systematic and comprehensive search of important online databases—PubMed, Web of Science, Science Direct, Scopus, Wiley Online Library, and Google Scholar. The articles published and available online up to 7th May 2020 were considered to include in this study. The keywords used for the searches are included in the Table 1. All the searches were done in the English language only.
Table 1.
Search strategy.
| Database | PubMed, Web of Science, Science Direct, Scopus, Wiley Online Library, Google Scholar |
| Articles included | Articles published up to 7th May 2020 were included in the study |
| Keywords used for the search | Clinical characteristics, Clinical features, Clinical symptoms, Findings, Diagnosis, Novel coronavirus 2019, COVID-19, SARS-CoV-2, Challenges |
| Language used | English |
| Inclusion criteria | i) Articles containing clinical characteristics of COVID-19 ii) Original articles iii) Case series iv) Published in English language |
| Exclusion criteria | i) Reviews ii) Meta-analysis iii) Case reports iv) Expert opinions v) Newspaper articles vi) Commentaries vii) Prospective viii) Correspondence |
Eligibility Criteria
The search results were subjected to a range of inclusion and exclusion criteria, as listed in Table 1. The inclusion criteria mainly include original peer-reviewed articles (articles based on direct clinical data of the COVID-19 patients in various clinical settings). We opted out case reports, meta-analyses, expert opinions, newspaper articles, and commentaries.
Screening and Study Selection
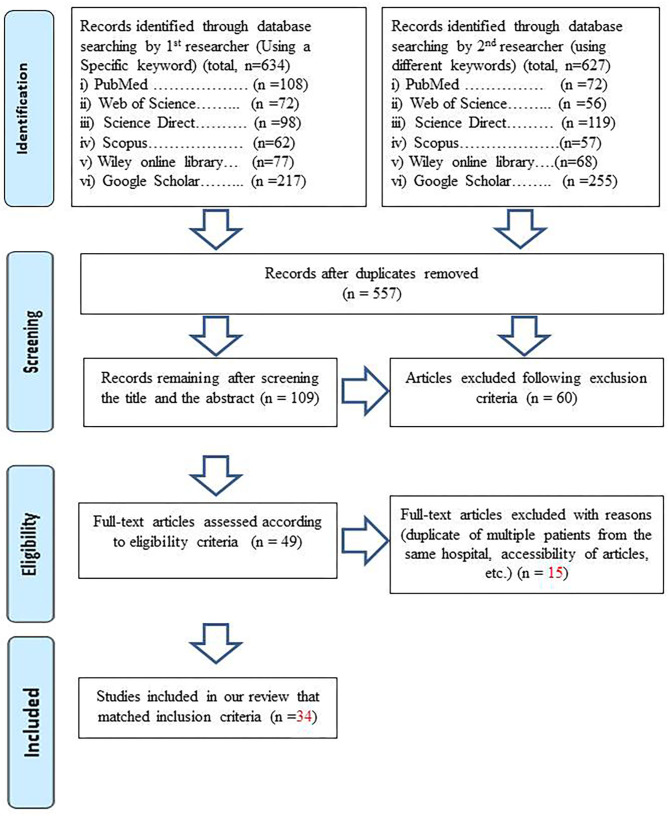
Following the eligibility criteria, two researchers were collaboratively involved in the screening and selection procedure of the articles of interest. The screening procedure was based on the PRISMA-2009 flow diagram, as presented in Figure 1. To present a global overview of the clinical trends of COVID-19, we have selected original studies from different hospitals across the world. The time period of each study and other related data were carefully screened to avoid any duplicates. Multiple studies from the same institutions were mainly analyzed and included avoiding data duplication. Observational studies reporting desired clinical parameters like symptoms, laboratory characteristics, and risk factors were included in the review. Case reports were discarded because they do not add significant value when stacked up against case studies. The whole procedure was overseen by two experienced researchers (an expert clinician and supervisor of this project).
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) for the identification and screening of articles to include in the study according to eligible criteria.
Data Extraction and Entry
The full text of the selected articles was thoroughly examined for relevant data of this systematic review that included the author's name, name of the hospital where the research had been conducted, number of case-patients and their clinical features, etc. We divided the case studies into three categories, namely, (i) Case studies in Hubei province only (China) (ii) Case studies in China (outside Hubei) (iii) Global case studies outside China (Table 2). We recorded and summarized the extracted data in Microsoft Excel. The whole process was carefully monitored and adjusted, had there been any discrepancies, by two independent expert researchers, including a clinician.
Table 2.
Studies included in the review.
| Region | References | Journal | Hospital/Region | Total case patients (n) |
|---|---|---|---|---|
| Case studies in China (inside Hubei) | (5) | Allergy: European Journal of Allergy and Clinical Immunology | No.7 hospital of Wuhan | 140 |
| (6) | SSRN Electronic Journal | Zhongnan Hospital of Wuhan University | 221 | |
| (7) | Clinical Infectious Diseases | Union hospital in Wuhan | 69 | |
| (8) | The Lancet | Outpatients of 30 hospitals in Wuhan designated for covid-19 treatment | 124 | |
| (9) | The Lancet Respiratory Medicine | Wuhan Jin Yin-tan hospital | 52 | |
| (10) | SSRN Electronic Journal | Renmin Hospital of Wuhan University | 101 | |
| (11) | Journal of the American Medical Association | Department of Critical Care Medicine, Zhongnan Hospital of Wuhan University | 138 | |
| (12) | The Lancet | Jin Yin-tan Hospital, Wuhan | 41 | |
| (13) | Chinese Medical Journal | 9 tertiary hospitals of Hubei Province | 137 | |
| (14) | The Lancet | Jinyintan Hospital in Wuhan, China | 99 | |
| (14) | MedRxiv | Mobile Cabin Hospital of Optical Valley and Tongji Hospital of Huazhong University of Science and Technology in Wuhan | 534 | |
| Case studies in China (outside Hubei) | (15) | Investigative Radiology | N/A | 80 |
| (16) | Journal of Infection | 57 hospitals across Beijing | 262 | |
| (17) | Journal of Infection | Wenzhou central hospital,Ruian people's hospital, Yueqing people's hospital | 149 | |
| (18) | China | Suzhou Fifth People's Hospital | 69 | |
| (19) | Clinical infectious diseases. | 3 Grade IIIA hospitals of Jiangsu | 80 | |
| (20) | The BMJ | 7 hospitals in Zhejiang | 62 | |
| (21) | Journal of Infection | Shanghai Public Health Clinical Center (SPHCC), Shanghai, China | 249 | |
| (22) | European Journal of Radiology | 6 hospitals across Anhui province | 73 | |
| (23) | European journal of nuclear medicine and molecular imaging | Guangzhou Eighth People's Hospital | 90 | |
| (24) | European Respiratory Journal | First Affiliated Hospital of Zhengzhou University | 18 | |
| (14) | MedRxiv | 5 hospitals across Zhejiang province | 91 | |
| (25) | European Review for Medical and Pharmacological Sciences | North Hospital of Changsha First Hospital | 161 | |
| Global case studies outside China | (26) | MedRxiv | N/A | 12 |
| (27) | Jama | Evergreen Hospital, USA | 21 | |
| (28) | Jama | 12 hospitals in New York City, Long Island, and Westchester County, New York, within the Northwell Health system | 5700 | |
| (29) | Jama | 72 hospitals of Lombardy ICU Network, Milan, Italy | 1591 | |
| (30) | The Lancet | N/A | 17 | |
| (31) | Journal of the American Medical Association | National Centre for Infectious Diseases, Singapore General Hospital, Changi General Hospital, and Sengkang General Hospital, Singapore |
18 | |
| (32) | International Journal of Biological Sciences | Centro Hospitalar Conde de São Januário, Macau | 10 | |
| (33) | Influenza and Other Respiratory Viruses. | Castle Hill Hospital, UK | 68 | |
| (34) | Travel Medicine and Infectious Disease | The Mediterranean Infection University Hospital Institute, France | 280 | |
| (35) | The Lancet | Self-Defense Forces Central Hospital, Japan | 104 | |
| (36) | Osong Public Health Res Perspect | N/A | 28 |
Quality Assessment
The validation instrument called “methodological index for non-randomized studies” (MINORS) was used to assess the methodological quality and bias risk of our data (Table 3). It is based on eight criteria for non-comparative clinical reviews, and the global acceptance score had been set to 16 (37).
Table 3.
Quality ratings of included studies according to MINORS*.
| References | ① | ② | ③ | ④ | ⑤ | ⑥ | ⑦ | ⑧ | Total score | Overall | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| rating | |||||||||||
| Case studies in China (inside Hubei) | (5) | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 14 | Good |
| (6) | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 14 | Good | |
| (7) | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 13 | Good | |
| (8) | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 14 | Good | |
| (9) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 14 | Good | |
| (10) | 2 | 2 | 2 | 2 | 2 | 0 | 1 | 1 | 12 | Satisfactory | |
| (11) | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 13 | Good | |
| (12) | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 0 | 13 | Good | |
| (13) | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 14 | Good | |
| (14) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 14 | Good | |
| (14) | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 14 | Good | |
| Case studies in China (outside Hubei) | (15) | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 0 | 13 | Good |
| (16) | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 15 | Excellent | |
| (17) | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 13 | Good | |
| (18) | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 0 | 12 | Satisfactory | |
| (19) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 14 | Good | |
| (20) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 14 | Good | |
| (21) | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 14 | Good | |
| (22) | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 13 | Good | |
| (23) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 14 | Good | |
| (24) | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 13 | Good | |
| (14) | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 14 | Good | |
| (25) | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 13 | Good | |
| Global case studies outside China | (26) | 2 | 2 | 2 | 2 | 2 | 0 | 1 | 0 | 11 | Satisfactory |
| (27) | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 13 | Good | |
| (28) | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 0 | 12 | Satisfactory | |
| (29) | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 0 | 12 | Satisfactory | |
| (30) | 2 | 2 | 2 | 2 | 2 | 0 | 1 | 0 | 11 | Satisfactory | |
| (31) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 14 | Good | |
| (32) | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 0 | 12 | Satisfactory | |
| (33) | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 13 | Good | |
| (5) | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 15 | Excellent | |
| (6) | 2 | 2 | 2 | 2 | 2 | 0 | 1 | 1 | 12 | Satisfactory | |
| (7) | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 13 | Good |
①A clearly stated aim; ②Inclusion of consecutive patients; ③Prospective collection of data; ④Endpoints appropriate to the aim of the study; ⑤Unbiased assessment of the study endpoint; ⑥Follow-up period appropriate to the aim of the study; ⑦Loss to follow up less than 5%; ⑧Prospective calculation of the study size.
The items are scored 0 (not reported), 1 (reported but inadequate) or 2 (reported and adequate). The global ideal score being 16 for non-comparative studies. The scoring was characterized as follows: 15-16, Excellent, 13-14, good, 11-12, Satisfactory, 9-10, Weak, <9, Unacceptable.
Results
Study Selection and Characterization
The result of our screening and selection protocol has been shown in Figure 1. We initially retrieved 557 articles after removing duplicates that were later shortened to 34 following the screening protocol for the selection of eligible articles. The relevant information of these finally selected articles along, with associated hospital or medical institution names, has been given in Table 2. The total number of case-patients in these studies is 10,889. We retrieved a total of 176 variables regarding these patients that included demographic features, laboratory findings, symptoms, comorbidities, etc. (Tables 4–9) from these articles for the review. Most of the studies were from China, particularly from the Hubei province. For convenience, the data were split into three groups as mentioned earlier. But we inferred our final results after combining them into a single dataset.
Table 4.
Demographic and epidemiologic features.
| Region | Global (Excluding China) | Only Hubei (China) | Outside Hubei (China) | Total/Mean | Percentage (%) on total |
|---|---|---|---|---|---|
| Number of studies | 11 | 11 | 12 | 34 | – |
| Number of case patients | 7849 | 1656 | 1384 | 10889 | – |
| Demographic features | |||||
| Median Age (in years) (Range) | 51.3 (0.5–84) |
55.6 (16–87) |
45.1 (1–94) |
49.8 (0.5–94) |
– |
| Sex | |||||
| Male | 5000/7849 | 867/ 1656 |
700/1384 | 6567/10889 | 60.3 |
| Female | 2849/7849 | 789/1656 | 684/1384 | 4422/10889 | 39.7 |
| Smoking (current) | 577/3683 | 38/767 | 26/80 | 641/4530 | 14.2 |
| Severity on diagnosis/admission | |||||
| Severe/ ICU/ critical patient | 2042/4640 | 212/592 | 154/1214 | 2408/6446 | 37.4 |
| Non-severe/non-ICU patient | 2598/4640 | 380/592 | 1060/1214 | 4038/6446 | 62.6 |
| Epidemiological Data (Transmission Pattern) | |||||
| Travel/residence history in Wuhan | 53/365 | – | 411/816 | 464/1181 | 39.2 |
| Contact with people from Hubei Province/stay in Hubei | – | – | 210/238 | 210/238 | 88.2 |
| Contact with COVID-19 patient in family or community | 10/292 | 395/1486 | 275/649 | 680/2427 | 28 |
| No obvious contact history with COVID-19 patient | – | – | 8/254 | 417/667 | 62.5 |
| Resident of the infected area | 280/347 | – | 137/320 | 19/308 | 6.2 |
| Contact with person with fever | 19/308 | – | – | 159/1486 | 10.70 |
| Infection during hospitalization | – | 159/1486 | – | 43/1486 | 2.89 |
| Hospital staff | - | 43/1486 | - | 43/1486 | 2.89 |
| Huanan seafood wholesale market exposure | - | 43/1486 | - | 118/1486 | 7.94 |
| No obvious contact history with COVID-19 patient | - | 118/1486 | - | 8/254 | 3.1 |
Demographic Characteristics
The mean age of the case population was 50.6 years (0.5–94). The percentage of the male population was 60.3% (6567/10889), whereas that of the female population was 39.7% (4322/10889). The case population includes Asian, European and North American patients. Around 14.2% (641/4530) of them were found to be current smokers (Table 4).
Clinical Symptoms
The most frequently observed clinical symptoms (Table 5) of the COVID-19 patients were cough/ dry cough 59.6 (2146/3598), fever 46.9 (4342/9242), fatigue 27.8 (1000/3598), dyspnea/shortness of breath 20.23 (728/3598), muscle ache/myalgia 12.64 (455/3598), diarrhea 11.95 (430/3598), headache 10.8 (389/3598), anorexia 9.9 (356/3598), sore throat 7.5 (270/3598), expectoration 7.48 (269/3598), upper airway congestion 6.67 (240/3598), and rhinitis 5.86 (211/3598). The lesser observed symptoms were pneumonia 2.89% (104/3598), abdominal Pain 2.31% (83/3598), nausea/vomiting 2.28 (82/3598), pharyngitis/pharyngalgia 1.83 (66/3598), chest pain 1.81 (65/3598), dizziness 1.56 (56/3598), chest tightness 1.25 (45/3598), malaise 0.61 (22/3598), chill 0.78 (28/3598), hemoptysis 0.42 (15/3598), heart palpitations 0.28 (10/3598), confusion 0.25 (9/3598), ARDS 0.22 (8/3598), belching 0.2 (7/3598), back discomfort 0.1 (3/3598), and arthralgia 0.03 (1/3598). A minor portion, 0.56% (20/3598), of the patients of the case population was found to be asymptomatic as well.
Table 5.
Clinical symptoms of COVID-19 patients.
| Symptoms | Global (Excluding China) (symptomaic patients/case patients) | Only Hubei (China) (symptomatic patients/case patients) | Outside Hubei (China) (symptomatic patients/case patients) | Patients with symptoms/Total no. of case patients | Percentage (%) on total |
|---|---|---|---|---|---|
| Cough/dry cough | 338/558 | 1016/1656 | 792/1384 | 2146/3598 | 59.6 |
| Fever | 2020/6202 | 1200/1656 | 1122/1384 | 4342/9242 | 46.9 |
| Highest temperature (°C) | 37.34 (35.3–39.2) | 38.5 (37.6–39.14) | 39.0(36.99–41) | 38.3 (35.3–41) | – |
| Fatigue | 3/558 | 679/1656 | 318/1384 | 1000/3598 | 27.8 |
| Dyspnea/shortness of breath | 70/558 | 542/1656 | 116/1384 | 728/3598 | 20.23 |
| Muscle ache/myalgia | 75/558 | 287/1656 | 93/1384 | 455/3598 | 12.64 |
| Diarrhea | 29/558 | 284/1656 | 117/1384 | 430/3598 | 11.95 |
| Headache | 76/558 | 189/1656 | 124/1384 | 389/3598 | 10.8 |
| Anorexia | – | 305/1656 | 51/1384 | 356/3598 | 9.9 |
| Sore throat | 84/558 | 114/1656 | 72/1384 | 270/3598 | 7.5 |
| Rhinitis/rhinorrhea | 186/558 | – | 25/1384 | 211/3598 | 5.86 |
| Upper airway congestion | 22/558 | 218/1656 | – | 240/3598 | 6.67 |
| Expectoration/sputum production | – | 95/1656 | 174/1384 | 269/3598 | 7.48 |
| Pneumonia | 21/558 | 7/1656 | 76/1384 | 104/3598 | 2.89 |
| Abdominal pain | 2/558 | 74/1656 | 7/1384 | 83/3598 | 2.31 |
| Nausea and vomiting | 8/558 | 59/1656 | 15/1384 | 82/3598 | 2.28 |
| Pharyngitis/pharyngalgia | – | 57/1656 | 9/1384 | 66/3598 | 1.83 |
| Chest pain | 35/558 | 17/1656 | 13/1384 | 65/3598 | 1.81 |
| Dizziness | 2/558 | 18/1656 | 36/1384 | 56/3598 | 1.56 |
| Chest tightness | – | – | 45/1384 | 45/3598 | 1.25 |
| Malaise | 22/558 | – | – | 22/3598 | 0.61 |
| Chill | 1/558 | – | 27/1384 | 28/3598 | 0.78 |
| Hemoptysis | – | 9/1656 | 6/1384 | 15/3598 | 0.42 |
| Heart palpitations | – | 10/1656 | – | 10/3598 | 0.28 |
| Confusion | – | 9/1656 | – | 9/3598 | 0.25 |
| ARDS | – | – | 8/1384 | 8/3598 | 0.22 |
| Belching | – | 7/1656 | – | 7/3598 | 0.2 |
| Back discomfort | – | – | 3/1384 | 3/3598 | 0.1 |
| Arthralgia | – | 1/1656 | – | 1/3598 | 0.03 |
| Others | 3/558 | – | – | 3/3598 | 0.1 |
| Asymptomatic | 4/558 | – | 16/1384 | 20/3598 | 0.56 |
Laboratory Findings
The laboratory findings (Table 6) included RT-PCR assay results, routine blood tests, and tests for various other blood-based biomarkers (e.g., coagulation factors, infection-related biomarkers, etc.). Among 8,650 patients, 8,253 (95.4%) were tested positive for SARS-CoV-2 in RT-PCR assay. Among the blood-based examinations, lymphocytopenia 55.9% (4177/7470) was most frequently observed. Other major lab findings include elevated levels of C-reactive protein 61.9% (830/1340), Aspartate aminotransferase 53.3% (3481/6537), Alanine aminotransferase 35.64% (2318/6503), lactate dehydrogenase 40.8% (392/973), ESR 72.99% (173/237), serum ferritin 63% (62/99), Interleukin-6 (IL-6) 52% (51/99), prothrombin time 35.47% (102/286), and D-dimer 28.06% (179/638). On the contrary, the levels of platelets 17.26% (160/927), eosinophils 59.0% (121/205), hemoglobin 29% (125/431), albumin 38.4% (187/487), and blood urea nitrogen (BUN) 20.9% (19/91) were reported to decrease.
Table 6.
Laboratory findings and physical examinations.
| Laboratory findings | Global (Excluding China) | Only Hubei (China) | Outside Hubei (China) | Total/Mean (Range) | Total percentage (%) |
|---|---|---|---|---|---|
| SARS-CoV-2 RT-PCR assay + (%) | 7147/7330 | 928/1139 | 178 / 181 | 8253/8650 | 95.4 |
| Blood Routine | |||||
| Leukocytes (× 109/L, normal range 3.5–9.5) | 8.3 (1.7–16.9) |
6.1 (3.2–13) |
4.7 (2.48–6.95) |
6.4 (1.7-16.9) |
- |
| Increased–No./total No. (%) | 5/68 | 103/702 | 38/637 | 146/1407 | 10.38 |
| Decreased–No./total No. (%) | 21/114 | 206/702 | 119/637 | 346/1453 | 23.81 |
| Neutrophils (× 109/L, normal range 1.8–6.3) | 2.65 (0.7–4.5) |
4.6 (1.62–9.13) | 3.3 (2.0–5.9) | 3.52 (0.7-9.13) | - |
| Increased–No. /total No. (%) | - | 84/290 | 64/485 | 148/775 | 19.1 |
| Decreased–No. /total No. (%) | 1/10 | - | 52/ 389 | 53/399 | 13.28 |
| Lymphocytes (× 109/L, normal range 1.1–3.2) | 1.1 (0.2–1.7) | 0.85 (0.6–1.46) | 1.1 (0.4–1.66) | 1.02 (0.2–1.7) | – |
| Increased–No./total No. (%) | - | - | 70/222 | 70/222 | 31.5 |
| Decreased–No./total No. (%) | 3407/5745 | 551/1017 | 219/708 | 4177/7470 | 55.9 |
| Platelets (× 109/L, normal range 125–350) | 158 (116–217) | 176 (127–263) | 173.9 (78.25–238) | 169.7 (78.25–263) | - |
| Increased–No./total No. (%) | - | 14/223 | 14/382 | 28/605 | 4.63 |
| Decreased–No./total No. (%) | 12/121 | 37/263 | 111/543 | 160/927 | 17.26 |
| Monocytes (%, normal range 3–8) | 2.83 (0.5–12.22) | 0.37 (0.23–0.55) | 0.44 (0.27–0.7) | 1.21 (0.23–12.22 | – |
| Increased–No./total No. (%) | - | - | 41/251 | 41/251 | 16.3 |
| Decreased–No. /total No. (%) | - | - | 1/171 | 1/171 | 0.6 |
| Hemoglobin (g/L, normal range 130–175) | 12.67 (8–17.2) | 130 (118–146) | 131.8 (120–152.3) | 92.5 (8–152.3) | - |
| Decreased–No./total No. (%) | – | 50/99 | 75/332 | 125/431 | 29 |
| Eosinophils (× 109/L, normal range 0.02–0.52) | – | 0.011 (0.00–0.05) | – | 0.011 (0.00–0.05) | – |
| Decreased–No./total No. (%) | – | 121/205 | – | 121/205 | 59.00 |
| Blood biochemistry | |||||
| Aspartate aminotransferase (U/L, normal range 15–40) | 33.33 (9.0–71) | 31.2 (22–48) | 25.3 (15.75–39) | 29.9 (9.0–71) | – |
| Increased–No. /total No. (%) | 3283/5700 | 69/209 | 129/628 | 3481/6537 | 53.3 |
| Decreased–No./total No. (%) | – | – | 19/153 | 19/153 | 12.4 |
| Alanine aminotransferase (U/L, normal range 9–50) |
36.6 (19.3–55.0) | 28.6 (16–53) | 22.7 (12.0–39.5) | 29.3 (12.0–55.0) | – |
| Increased–No. /total No. (%) | 2197/5769 | 51/168 | 70/566 | 2318/6503 | 35.64 |
| Decreased–No./total No. (%) | – | – | 8/240 | 8/240 | 3.3 |
| Albumin (g/L, normal range 35–57) | – | 32.04 | 39.8(5.48–46.3) | 35.92(5.48–46.3) | – |
| Increased–No./total No. (%) | – | – | 3/240 | 3/240 | 1.3 |
| Decreased–No./total No. (%) | – | 97/98 | 90/389 | 187/487 | 38.4 |
| Creatinine (μmol/L, normal range 64–104) | 83.3 (7.6–343.1) | 72.7 (56–87) | 69.4(51.28–90) | 75.13(7.6–343.1) | – |
| Increased–No./total No. (%) | – | 7/140 | 116/451 | 123/591 | 20.8 |
| Decreased–No./total No. (%) | – | 21/99 | 81/371 | 102/470 | 21.7 |
| Serum Creatinine Kinase (mmol/L, normal range 40–200) |
117.67 (45–1290) | 90.1 (51–219) | 81.5(40.5–191) | 96.42(40.5–1290 | – |
| Increased–No./total No. (%) | 1/10 | 30/199 | 35/291 | 66/500 | 13.2 |
| Decreased–No./total No. (%) | – | 23/99 | 76/211 | 99/310 | 31.9 |
| Lactate dehydrogenase (U/L, range12–250) |
381.3 (206.5–796) | 266.8 (174–447) | 246.02(94.5–554) | 298.04(94.5–796) | – |
| Increased–No. /total No. (%) | 33/114 | 184/338 | 179/521 | 392/973 | 40.8 |
| Glucose (mmol/L; normal range 3·9–6·1) |
– | 8.2 | 6.3(1.97–7.7) | 7.3(1.97–7.7) | – |
| Increased–No./total No. (%) | – | 51/99 | 78/229 | 129/328 | 39.3 |
| Decreased–No./total No. (%) | – | – | 1/149 | 1/149 | 0.7 |
| Total Bilirubin (μmol/L, normal value 4.0–17.1) |
8.13 (4–18.81) | 14.8 (8.4–31.7) | 9.3 (5.4–15.43) | 10.74 (4–31.7) | – |
| Increased–No. /total No. (%) | – | 18/99 | 25/459 | 43/558 | 7.71 |
| Decreased–No./total No. (%) | – | – | 9/218 | 9/218 | 4.1 |
| Blood urea nitrogen (mmol/L; normal range 2.8–7.6) |
– | 6.6 (3.4–13.03) | 4.4(3.3–5.9) | 5.5(3.3–13.03 | – |
| Increased–No. /total No. (%) | – | – | 3/171 | 3/171 | 1.8 |
| Decreased–No./total No. (%) | – | – | 19/91 | 19/91 | 20.9 |
| Lactate concentration (mmol/L normal range 0.5–1.6) |
1.5 (1.1–2.1) | 1.5 (0.7–3.2) | 1.4(1.1–2.1) | 1.47(0.7–3.2) | – |
| Hematocrit (%, normal range M: 40–50, F: 37–48) | 12.67 (8–17.2) | 40.3 (36.5–43.5) | – | 40.3 (36.5–43.5) | – |
| Coagulation function | – | – | – | – | – |
| Activated partial thromboplastin time in seconds (normal range 21–37) | – | 27.3 | 26(17.2–38.27) | 26.65 (17.2–38.27) | – |
| Increased–No. /total No. (%) | – | 6/99 | 40/149 | 46/248 | 18.55 |
| Decreased–No./total No. (%) | – | 16/99 | 2/80 | 18/179 | 10.05 |
| Prothrombin time in seconds (9.4–12.5) | – | 12.6 (10.1–17.39) | 11.5(9.3–13.6) | 12.05 (9.3–17.39) | – |
| Increased–No. (>1.00)/total No. (%) | – | 85/137 | 17/149 | 102/286 | 35.47 |
| Decreased–No./total No. (%) | – | 30/99 | 7/229 | 37/328 | 11.28 |
| D–dimer (ng/L; normal range 0–500) | 438 (262–872) | 712.2 (100–2800) | 368 (106–2400) | 506.1 (100–2800) | – |
| Increased–No./total No. (%) | 113/249 | 56/389 | 179/638 | 28.06 | |
| Infection–related biomarkers | |||||
| Procalcitonin (PCT) (ng/ml, normal range 0–0.1) |
0.7 (0.03–9.59) | 0.24 | 0.35(0–2.6) | 0.43(0.0–9.59) | – |
| Increased–No. (>1.00)/total No. (%) | – | 125/677 | 58/329 | 183/1006 | 18.2 |
| ESR (mm/h normal range 0–20) | – | 20 (8–31) | 32.95(9–90) | 26.5(8–90 | – |
| Increased–No. (≥20)/total No. (%) | – | 114/157 | 59/80 | 173/237 | 72.99 |
| Serum ferritin (ng/mL, normal range 21.0–274.7) | – | 808.7 | – | 808.7 | – |
| Increased–No. /total No. (%) | – | 62/99 | – | 62/99 | 63.00 |
| C–reactive protein (CRP) (mg/L, normal range <10) | 30.94 (0.1–97.5) | 37.8 (6.78–67.4) | 10.3 (1.8–50.61) | 26.35(0.1–97.5) | – |
| Increased–No./total No. (%) | 17/96 | 379/508 | 434/736 | 830/1340 | 61.9 |
| Interleukin−6 (pg/mL, normal range 0.0–7.0) | – | – | 0/181 | 7.9 (6.1–10.6) | – |
| Increased–No. /total No. (%) | – | 7.9 (6.1–10.6) | – | 51/99 | 52.00 |
| Decreased–No./total No. (%) | – | 51/99 | – | 0/181 | 0 |
| Physical Examinations | |||||
| Respiratory rate, breaths/min. (Normal range: 12–20) | 16.33 (13.5–21) | 20.5 (19–30) | 22.5 | 19.8(13.5–30.0) | – |
| O2 saturation,% (Normal range: 75–100) | 96.9 (91–100) | – | 91.78 | 94.34(91–100) | – |
| Mean Systolic pressure (mmHg) (Normal range−100) | 128 (96–180) | 91.5 (83–105) | 114.5 (80–145.42) | 111.3(80–180) | – |
| Heart rate /min (Normal range: 60–100) | 92.4 | – | 88.44 | 90.42(52–125) | – |
Radiological Findings
The results of chest CT scans (Table 7) showed abnormality of at least one kind in 93.5% (1668/1785) of the case-patients. The predominant abnormality had been bilateral lungs found in 71.1% (1581/2223) of the patients. Other significant findings of the lung characteristics from CT scan result include Ground-glass opacity 48% (432/900), consolidation 21.88% (140/640), pleural effusion 20.6% (195/947), the lesion in lung 78.3% (180/230), enlargement of lymph node 50.7% (153/302), thickening of bronchial wall 30.3% (80/264), thickening of lung texture 84.9% (62/73), and thickening of Interlobular septal 47.1% (80/170).
Table 7.
Radiological findings (Chest CT scan results).
| Radiological findings | Global (Excluding China) (n) | Only Hubei (China) (n) | Outside Hubei (China) (n) | Total (n) | Percentage (%) in total |
|---|---|---|---|---|---|
| Chest CT | |||||
| Normal | 49/164 | 22/1292 | 38/329 | 109/1785 | 6.11 |
| Abnormality | 115/164 | 1262/1292 | 291/329 | 1668/1785 | 93.5 |
| Unilateral lungs | 144/1292 | 137/306 | 281/1598 | 17.6 | |
| Bilateral lungs | 57/122 | 1187/1529 | 337/572 | 1581/2223 | 71.1 |
| Density and Inner Features | |||||
| Ground–glass opacity | 53/112 | 69/236 | 310/552 | 432/900 | 48 |
| Consolidation | 32/112 | 25/137 | 83/391 | 140/640 | 21.88 |
| Mixed | – | – | 35.4/132 | 35.4/132 | 26.8 |
| Other Features | |||||
| Pleural effusion | 6/21 | 31/534 | 158/392 | 195/947 | 20.6 |
| Lung lesion | – | – | 180/230 | 180/230 | 78.3 |
| Interlobular septal thickening | – | – | 80/170 | 80/170 | 47.1 |
| Crazy paving pattern | – | – | 34/170 | 34/170 | 20 |
| Spider web sign | – | – | 20/80 | 20/80 | 25 |
| Sub–pleural line | – | – | 16/80 | 16/80 | 20 |
| Bronchial wall thickening | 5/21 | – | 75/243 | 80/264 | 30.3 |
| Lymph node enlargement | – | – | 153/302 | 153/302 | 50.7 |
| Pericardial effusion | – | – | 5/170 | 5/170 | 2.9 |
| Paving stone sign | – | – | 25/73 | 25/73 | 34.25 |
| Thickening of lung texture | – | – | 62/73 | 62/73 | 84.9 |
| Pulmonary edema | 2/21 | – | – | 2/21 | 9.5 |
| Venous congestion | 1/21 | – | – | 1/21 | 4.8 |
| Atelectasis | 1/22 | – | – | 1/22 | 4.55 |
Physical Examinations
The results of the physical examinations (Table 6) that included respiratory rate 19.8 breaths/min (13.5–30.0), pulse oximeter O2 saturation 94.34%, mean systolic pressure 111.3 mmHg (80–180), and heart rate 90.42/min (52–125) were found to be slightly higher than their respective normal range.
Comorbidities
Various underlying medical conditions (Table 8) were found in the case-patients, the most significant ones being hypertension 35.9% (3909/10889), diabetes 20.17% (2196/10889), obesity 15.95% (1735/10889), cardiovascular disease 13.92% (1516/10889), asthma 4.42% (481/10889), COPD 4.31% (469/10889) and malignancy 3.99% (435/10889). Several patients were also found to be co-infected with other viruses, 9.1% (244/2684), Bacteria 4.99% (24/481), and Fungus 3.43% (11/320).
Table 8.
Comorbidities.
| Comorbidities | Global (Excluding China) (n = 7849) | Only Hubei (China) (n = 1656) | Outside Hubei (China) (n = 384) | Total (n = 10889) | Percentage (%) in total |
|---|---|---|---|---|---|
| Hypertension | 3541/7849 | 296/1656 | 72/1384 | 3909/10889 | 35.9 |
| Diabetes | 2003/7849 | 160/1656 | 33/1384 | 2196/10889 | 20.17 |
| Obesity (BMI ≥30) | 1737/7849 | – | – | 1737/10889 | 15.95 |
| Cardiovascular disease | 1231/7849 | 164/1656 | 121/1384 | 1516/10889 | 13.92 |
| Asthma | 481/7849 | – | – | 481/10889 | 4.42 |
| COPD | 344/7849 | 110/1656 | 15/1384 | 469/10889 | 4.31 |
| Malignancy/cancer | 401/7849 | 25/1656 | 9/1384 | 435/10889 | 3.99 |
| Chronic renal insufficiency | 304/7849 | 26/1656 | 4/1384 | 334/10889 | 3.07 |
| End-stage kidney disease | 188/7849 | – | – | 188/10889 | 1.73 |
| Hyperlipidemia | 192/7849 | – | – | 192/10889 | 1.76 |
| Cerebrovascular Disease | – | 46/1656 | 116/1384 | 162/10889 | 1.49 |
| Pneumonia | – | 7/1656 | 76/1384 | 83/10889 | 0.76 |
| Chronic liver disease | 40/7849 | 31/1656 | 15/1384 | 86/10889 | 0.79 |
| Chronic gastritis and gastric ulcer | 0/7849 | 47/1656 | 11/1384 | 58/10889 | 0.53 |
| History of solid organ transplant | 57/7849 | – | – | 57/10889 | 0.52 |
| Fatty liver and abnormal liver function | 5/7849 | 43/1656 | – | 48/10889 | 0.44 |
| Endocrine diseases | – | 5/1656 | 39/1384 | 44/10889 | 0.40 |
| HIV infection | 43/7849 | 2/1656 | – | 45/10889 | 0.41 |
| ARDS | 8/7849 | 17/1656 | 2/1384 | 27/10889 | 0.25 |
| Acute kidney injury | 2/7849 | 22/1656 | – | 24/10889 | 0.22 |
| Autoimmune disease | 1/7849 | 10/1656 | – | 11/10889 | 0.1 |
| Immunosuppression | 3/7849 | 3/1656 | 3/1384 | 9/10889 | 0.08 |
| Co-infection | |||||
| Other viruses | 211/2364 | 33/320 | – | 244/2684 | 9.10 |
| Bacteria | 1/21 | 23/460 | – | 24/481 | 4.99 |
| Fungus | – | 11/320 | – | 11/320 | 3.43 |
Clinical Progression Data
The median incubation period of the patients is found to be 5.36 days (1.5–15) based on 12 studies involving 1080 patients. The median hospital stay of the patients is eight based on three studies. Median days from onset of illness to hospital admission is 4.83 (0–11) based on 13 studies. Global (outside China) case fatality rate was found to be 22.24% (969/4357), among which 21.24% (564/2655) was in the USA, 25.61% (405/1581) in Italy, and 0% (0/121) was in other countries (Singapore and Diamond Princess Cruise Ship while it was staying in Japan). In comparison the case fatality inside the Hubei Province of China was 11.71% (194/1656) (Table 9).
Table 9.
Disease progression and other clinical data.
| Disease progression and other clinical data | Global (Excluding China) | Only Hubei (China) | Outside Hubei (China) | Total/Mean (Range) | Percentage (%) |
|---|---|---|---|---|---|
| Median hospital stay of patients (days) | – | 8 | – | 8 | – |
| Median Incubation Period (days) | 4.05 (2–9) |
6 (5–13) | 6.3 (1.5–15) |
5.36 (1.5–15) | – |
| Days from onset of illness to dyspnea | 4 (1–20) | – | – | 4 (1–20) | – |
| Days from onset of illness to ICU | 4.7 (1–14) |
– | – | 4.7 (1–14) | – |
| Days from Onset of symptoms to hospital admission | 4 (0–11) | 7 (4–11) | 3.5 (0.8–8.2) |
4.83 (0–11) | – |
| Days From hospital admission to death | – | 4 (2–7) | – | 4 (2–7) | – |
| Days from onset of illness to ARDS | – | 8 (6–12) | – | 8 (6–12) | – |
| Length of follow-up days | 7.6 (1–15) |
– | – | 7.6 (1–15) | – |
| Total Death (Case Fatality Rate)* | Global: 969/4357 (22.24%) [USA: 564/2655 (21.24%), Italy: 405/1581 (25.61%), Other countries (Singapore, and Diamond Princess Cruise Ship while it was staying in Japan: 0/121(0%)] |
194/1656 (11.71%) |
5/893 (0.56%) |
1168/6906 | 16.9 |
ARDS, Acute respiratory distress syndrome; ICU, intensive care unit.
Based on the available follow-up data on patients from the selected case studies.
Exposure Pattern
A significant portion of the study cases has their exposure history linked to family members or their respective community 28% (680/2427) (Table 4) which is particularly the true for the study patients from China. Our review found that the exposure pattern in the global arena (outside China) has been largely centered on the imported cases 80.7% (280/347). This review also revealed that the hospital staff's exposure risk is 2.89% (43/1486) and the in-patients of the hospitals 2.89% (43/1486).
Age-Dependent Effects of COVID-19
It is assumed that, the age of the patients may influence the clinical features, disease progression, complexities, and fatality of the COVID-19. However, we have analyzed and included the available data on this aspect. The notable findings include faster disease progression, higher mortality rate, progressively lower lymphocyte count, higher ICU admission rate, higher mortality rate for those receiving mechanical ventilation, and higher risk of severe heart attack among the older patients (Table 10).
Table 10.
Age dependent effects of COVID-19.
| Parameter | Age related impact | References |
|---|---|---|
| Severity of disease | Median age of severe patients (62.0) were significantly older than non-severe ones (51.0) | Zhang et al. (6) |
| Disease progression | Disease progressed rapidly in older patients with ARDS and septic shock | Zhang et al. (6) |
| Survivors vs. non-survivors | Non-survivors were older (mean age: 64.5 years) compared to the survivors (mean age: 51.9 years) Mortality rate increases in older (<65 years) patients with comorbidities | Yang et al. (17) |
| Severe heart attack/death | Older patients (>70 years) with chronic medical conditions are likely to suffer a severe heart attack and death. | Shi et al. (10) |
| ICU admission | Patients treated in ICU were significantly older (median age: 66 years) than patients not treated in ICU (median age: 51 years) | Wang et al. (11) |
| Mortality rate of the patients who received mechanical ventilation | Mortality rate were found to be higher (97.2%) in older patients (>65 years) compared to 76.4% in case of younger patients (18–65 years). | Richardson et al. (28) |
| Lowest absolute lymphocyte count | Progressively lower for older patients | Grasselli et al. (29) |
| Median fraction of inspired oxygen (FiO2) | Lower (60%) in younger patient compared to the older patients (70%) | Grasselli et al. (29) |
Discussion
This systematic review is based on a random effect model. As such, it contains data from studies that took place in different countries, including China, the USA, Japan, South Korea, France, and Singapore. All the studies were published in peer-reviewed articles with patient data between late December 2019 and May 7, 2020.
We managed to retrieve data on clinical characteristics of COVID-19 from 10,889 infected patients. Various studies and reports worldwide showed that COVID-19 seems to infect the male population more frequently than it does to female population. Our review also corroborates this claim as 60.3% (6487/10889) of our case population are male and the rest 39.7% (4241/10889) are female. Previously older age had been predicted to be an essential factor for higher mortality in SARS and MERS patients (38). Several studies in our review have reported the same. During the initial transmission period, COVID-19 seemed to affect the elderly more, as reflected by the mean age of 49.8 years (0.5–94) of the case population included in the review attributing to the higher frequency of comorbidities observed among the elderly (39). But in the current situation, it seems that the virus can equally affect everyone irrespective of age. However, it is evident that the clinical characteristics and prognosis of the disease greatly vary among patients with different age groups. Patients over 60 years tend to show more severe clinical manifestations and relatively longer disease duration, meaning they would require more careful monitoring and more comprehensive medical interventions (40).
The Chinese Center for Disease Control and Prevention (CDC) reported that the vast majority (81%) of COVID-19 patients will develop only mild symptoms, and the rest develop severe illness i.e., they will require oxygen therapy (14%) or require ICU treatment (5%) (41). Usually, COVID-19 patients are diagnosed with signs of severe pneumonia. Our review found that the most commonly reported symptoms are dry cough (59.6%), fever (46.9%), fatigue (27.8%), and dyspnea/shortness of breath (20.23%). These symptoms, together with prior contact history with suspected patients, immediately would require medical attention. Other less frequent symptoms include myalgia, diarrhea, headache, anorexia, sore throat, rhinitis, upper airway congestion, expectoration, pneumonia, etc. The symptoms are similar to those of SARS and MERS (42, 43). In most cases, the symptoms are mild during the initial days of infection but can be very severe, particularly for the elderly and patients with underlying respiratory diseases. Unlike SARS and MERS, SARS-CoV-2 behaves mildly during the initial stage of infection, making it significantly more contagious than the previous coronaviruses since the condition can go unnoticed in some cases (44). New shreds of evidence emerging from across the globe suggest that the asymptomatic cases of COVID-19 infections are rising (45, 46). But our review found that only a small percentage (0.56%) (20/3598) of the patients were asymptomatic which may be attributed to the fact that the initial tests for COVID-19 were only being conducted for patients with a distinct illness. To screen out the asymptomatic cases, experts have emphasized on comprehensive epidemiological investigations of the suspects by disease control specialists. Besides, the asymptomatic patients have shown consistent abnormalities in their CT scan reports for which radiological investigations can be a useful diagnostic tool to find the asymptomatic variants (47).
The most notable laboratory finding of our review is lymphocytopenia found in 55.9% cases (4177/7470). It was also reasonably expected in the influenza virus (H5N1), SARS, and MERS (48). In H5N1 influenza the fall in lymphocyte count had been attributed to dendritic cell (DC) dysfunction suggesting that a similar mechanism related to dysfunctional adaptive immunity can cause the same in COVID-19 patients (49). Besides, another study has demonstrated the correlation between the lymphocytopenia and the clinical severity of SARS-CoV-2 infection. The study also infers that the lymphocytopenia might be an outcome of the death of lymphocytes or the damage of lymphatic organs like thymus or spleen directly by the virus itself (25).
Another significant finding of our review is the elevated level of CRP 61.9% (830/1340) which supports a study that demonstrated elevated CRP levels in COVID-19 patients even though they did not have any kind of coinfections (50). It is to be noted that the CRP level is usually increased in bacterial or viral infections. However, it does not demonstrate a significant elevation in the case of mild viral infections. Another study claimed the potentiality of CRP as a comprehensive predictive factor of COVID-19 prior to the changes in other inflammatory-related blood parameters e.g., leucocytes, lymphocytes, and neutrophils (5). Besides, CDC guidelines have also reported an elevated CRP level in COVID-19 patients with higher CRP levels indicative of the severity of the infection and poor prognosis (51). Previously it has been associated with Influenza H1N1 & H7N9 and the SARS epidemic (52, 53). In COVID-19 patients, this significantly elevated level of CRP can be explained by the excessive production of inflammatory cytokines due to immune response as well as the damage of the lung alveoli (50).
LDH level has been found to be increased by 40.8% (392/973) in COVID-19 patients. Although it is abundant in tissues, LDH level is low in blood circulation. Elevated LDH, as reported in SARS, reflects tissue necrosis corresponding to hyperactive immunity (54). Besides, viral infections can cause lung tissue damage, which in turn raises the LDH level in blood circulation (55).
Our review revealed abnormal liver function tests in the case of patients with elevated ALT 36.65% (2318/6503) & AST 53.3% (3481/6537) level and lower albumin (38.4%) (187/487) and serum creatinine kinase level (31.9%) (99/310). One report has proposed that the hepatic dysfunction of COVID-19 patients can be linked to direct liver injury via viral hepatitis or due to abnormal levels of blood coagulative and infection-related functions, such as, elevated prothrombin time, D-dimer level, and serum ferritin, as found in our review (56).
We could not find sufficient data on the immunological parameters of the case patients. But one of the studies included in our review reported a higher IL-6 count in 51 (50%) of 99 patients (14). Besides, two recent studies have shown that IL-6 level was substantially increased in both severe and moderate patients. In addition to that, other pro-inflammatory parameters like IL10, IL2, IFN-γ, and TNF-α were found to be higher in more severe patients than in the moderate ones (21, 22). The magnitude of the cytokine storm or, more particularly, elevated IL-6 level has been associated with disease severity (13). It has been proposed as an essential parameter to predict respiratory failure risk based on a study that found a significantly higher IL-6 level in patients requiring ventilation (57).
The radiological findings showed that COVID-19 patients are accompanied by an abnormality in their CT scan report in 93.5% (1668/1785) cases. So CT scanning can provide an important base for early diagnosis of the virus. The typical CT scan features include bilateral lung 71.1% (1581/2223) and unilateral lung 17.6% (281/1598). The density characteristics of the lung lesions, found in 78.3% (180/230) cases, was mostly uneven, with ground-glass opacity 48% (432/900) as the primary presentation accompanied by consolidation in 21.88% (140/640) cases. CT scan reports have been demonstrated an association between disease progression and a higher rate of consolidative opacities (58). CT scan reports and the RT-PCR tests are generally found to be harmonious with a few exceptions. CT scan results can be a more specific diagnostic tool for the suspected COVID-19 cases since even the asymptomatic patients have shown abnormalities in their CT scan reports (46, 47). The less common CT findings include pleural effusion 20.6% (195/947), pericardial effusion 2.9% (5/170), bronchial wall thickening 30.3% (80/264), and interlobular septal thickening 47.1% (80/170) which have been reported as the disease progresses.
The median incubation period of the SARS-CoV-2 virus has been found to be 5.36 days (1.5–15). National Health Commission of China previously reported an incubation period of 1 to 14 days. The CDC has updated the mean incubation period to be between 2 to 14 days (51). However, various studies together with our findings suggest that the incubation period of SARS-CoV-2 is highly variable and requires more concrete statistically significant results. This high variability in the incubation period of SARS-CoV-2 has warranted a suitable quarantine period of at least 3 weeks to reduce the community transmission of the virus (59).
A significant portion of our case patients had one or more comorbidities indicating that a significant portion of our case patients is elderly. They are more likely to develop various chronic or acute clinical conditions on aging. Hypertension (35.9%) (3909/10889), diabetes (20.17%) (2196/10889), obesity (15.95%s) (1737/10889), cardiovascular diseases (13.92%) (1516/10889) and respiratory diseases (e.g., asthma-4.42%; COPD-4.31%) are the most frequent comorbidities found in the case patients of our review. The prevalence of comorbidities can be a major risk factor for severe patients compared to non-severe patients due to higher case fatality and poor prognosis (17). Thus, a proper medical history of the COVID-19 patients, particularly elderly patients, should be documented. Moreover, adequate clinical facilities should be made available for this group of patients.
Age-specific patient data was not sufficiently available in the case of most of our included studies. Still, for some crucial parameters, such as mortality rate and severity of the disease, a pattern was observed across the studies involving the older patients who, in general, possess a higher risk. Table 10 has listed all of the available age-related effects that we were able to retrieve. One of the recent studies has indicated that the children's overall risk factors are not significantly affected by the age and sex (60). Unlike adults, the children are at a lower risk of developing severe symptoms or death.
Diagnostic Challenges of COVID-19
Article search strategy: For the collection of information on diagnostic challenges of COVID-19, we have extensively searched by using the keywords “COVID-19 and diagnostic challenges,” “challenges for the diagnosis of novel coronavirus diseases,” on Pubmed, Web of Science, Science Direct, Scopus, Wiley Online Library, Google Scholar databases and other online websites of reliable sources such as Gavi-The Vaccine Alliance (gavi.org), World Health Organization, US-FDA, etc.
Review Findings on the Diagnosis Challenges of COVID-19
Every day the novel coronavirus is presenting us with new clinical challenges. As countries worldwide are considering to curb the limits on social distancing measures, general testing and rapid diagnosis of the disease have become of utmost importance. Even though various efforts are undertaken both by the government agencies and international bodies, the diagnosis of COVID-19 is still challenging. For convenience, the information on the current challenges for the diagnosis of COVID-19 have been summarized as follows:
Although RT-PCR has been regarded as the gold standard for viral detection of SARS-CoV-2, it also comes with few challenges. The equipment and lab facilities required for this testing can be expensive. The testing environment requires a certain biosafety level e.g., BSL-2 cabinets for sample preparation or ideally a negative pressure room (61). Most of the traditional testing centers all around the globe do not have these facilities. On top of that, these facilities require technicians with enough expertise and experience to perform the tests smoothly and flawlessly (62). Therefore, lack of testing centers and expert technicians are the primary diagnostic challenges faced by most of the countries all over the world. For this very reason, many countries failed to start coronavirus testing early in its transmission phase. Even when they had started testing, it was strictly restricted to suspected individuals with clear symptoms of the viral infection (63). Moreover, as countries all over the world are trying to increase their number of testing each day, this has put a serious pressure on the ready availability of reagents for the PCR reactions. Thus, shortage of testing kits is next big challenge to the rapid diagnosis of the disease.
Another crucial diagnostic challenge with RT-PCR testing is the risk of eliciting false-negative and false-positive results. It has been found that many patients with typical COVID-19 symptoms and CT features have shown a negative influence in RT-PCR testing (64). Therefore, a negative result should not preclude the patient as a COVID-19 suspect, and it should not be used as the only criterion of diagnosis. Besides, a false result due to human error cannot be overruled as under-trained technicians are performing many of these tests.
The collection of the patient sample (throat swab) presents another diagnostic challenge as this requires specific procedures to be followed for the sake of proper sample preparation for RT-PCR testing. Wrong sampling procedures can cause errors in RT-PCR testing (65). Moreover, a global shortage of personal protective equipment (PPE) has put additional challenges as adequate protective measures have to be made available for the staff involved in this sample collection procedure.
RT-PCR testing also faces another challenge that arises from the susceptibility of the viral mutations in the SARS-CoV-2 genome. Various studies have shown a rapid evolution of the virus (66, 67). Consequently, a false negative result may arise due to mutation in the primer or probe target regions (61). Although they are based on the most conserved regions of the viral genome, a slight variability can decrease the assay performance.
Asymptomatic patients have become a major challenge for both the clinicians and the administration. They are difficult to diagnose and isolate and they also pose a more significant threat of rapid and unchecked viral transmission. A recent study has found a similar viral load in asymptomatic patients compared to the symptomatic ones, indicative of their transmission potential (68). Another big concern is that most of these asymptomatic patients will not report to the hospitals or testing centers. Under such circumstances, their diagnosis solely depends on contact tracing or cluster screening by the disease control experts (68). Diagnosis of asymptomatic children is also challenging as their diagnosis is only limited to tracing their family history (69, 70). But this task is getting increasingly tricky both due to an overwhelming number of new cases arising each day and the limited workforce of the disease control centers.
Although COVID-19 patients have clear chest CT scan manifestations, several studies have reported usual CT scan reports despite being RT-PCR confirmed COVID-19 patients (9, 71). One study has found a false negative result of over 12% when attempted to predict the infection based on CT scan reports only (72). These reports clearly mean that clinicians have to be more vigilant in diagnosing the suspected cases of COVID-19 patients and consider multidimensional factors including laboratory parameters, CT scan reports, and other tests.
Additionally, most countries do not have a sufficient number of CT scan machines to support widespread testing of the rising number of COVID-19 patients (73). Besides, it takes a lot of effort to disinfect the machines after each test to prevent unwanted viral transmission.
Another challenge is that it requires multiple scans (at least 2, 6 days apart) for maximum accuracy in the diagnostic results meaning this would put additional pressure on the already scarce CT scan machines (74).
Sometimes suspected patients of the virus are showing unwillingness to come to test centers to confirm the presence of the virus. Some of the developing nations have reported these incidents, which were mostly attributed to the lack of concern, illiteracy, and the fear of social stigma (75). These patients remain undiagnosed for a considerable time till their symptoms get severe to the extent that they need hospitalization. So this group of patients presents us with a severe diagnostic challenge.
Serological detection tools utilize the detection of specific antibodies (IgM and IgG) against COVID-19 infections. These antibody tests have the advantage of low cost, fast detection and easy availability but suffer from low sensitivity as is seen with the antibody tests deployed for other coronavirus and influenza virus (76–78). However, IgM responses vary from person to person and require days to develop once they get infected. Considering that this may not be an useful tool for the accurate diagnosis of the viral infection except in confirming the late cases of COVID-19 and immunity of the recovered individuals (79). Due to these concerns, on April 1, 2020, the FDA granted Emergency Use Authorization (EUA). Still, they repeatedly expressed their doubts about the effectiveness of these tests in detecting the virus early during infection (80).
Strength and Limitations
To our knowledge, this is one of the first attempts to summarize the clinical characteristics and diagnostic challenges of COVID-19 taking into considerations of all the available data from global studies and variabilities so that the researchers, health care workers, policy makers and related stakeholders can get a contemporary overview of the COVID-19 situation.
The most noticeable limitation of our review is the lack of consistent data on every variable across the studies that we have included, which happened due to underreporting of symptoms, comorbidities, laboratory results, or exposure patterns of the case patients. These initially published studies have some issues related to lack of adequate laboratory testing, which could be attributed to the fact that these studies had to be completed as fast as possible to keep people around the world up to date about the virus and to prepare them fast to manage the disease. Besides, we were able to fetch only a limited amount of data regarding the clinical progression and other epidemiologic characteristics that would otherwise come in handy in defining clinical characteristics. In addition, most of our studies were from China, as no data were available from other countries. It would be better to broaden the geographical scope of our review to get a more global scenario of the clinical characteristics of the outbreak.
Conclusion
This systematic review summarized the latest clinical findings of the novel coronavirus outbreak that has virtually affected the whole world. To the best of our knowledge, this is the first large scale review comprising of studies from across the globe focusing on the clinical features of the COVID-19 patients. Laboratory tests and CT scan reports, together with clinical symptoms will provide useful information for the correct diagnosis and better management of the patients. This review provides a comprehensive overview and clear features of the clinical characteristics of COVID-19 which will help the physicians to make proper clinical decisions and correct assessment regarding the patients. Additionally, we have reviewed the challenges being faced for the diagnosis of the disease across the world. Overcoming these obstacles to the fast and prompt diagnosis of COVID-19 will be crucial for the proper containment of the disease.
Data Availability Statement
All data generated or analyzed in this study have been included in the article. The raw data will be provided by the corresponding and/or by the first author on reasonable request.
Author Contributions
SMHI and MMRS: conceived and designed the study; SMHI and PTR: Collected data through article search in different online databases; KAK and MMRS: Checked and verified collected data; SMHI, PTR, MMRS, FK, and SA: Analysis and interpretation of data; SMHI and MMRS: prepared the manuscript draft; AAT, CLP, INM, and LCM critically reviewed the manuscript. LCM was also involved with the planning of the study. All authors read the manuscript, agreed to be accountable for all aspects of the work, and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful to the Department of Pharmacy, State University of Bangladesh, and Health Med Science Research Limited for providing necessary supports to perform this systematic review.
Glossary
Abbreviations
- COVID-19
Coronavirus diseases
- Novel coronavirus 2019
2019n-CoV
- SARS-CoV-2
Severe Acute Respiratory Syndrome coronavirus 2
- WHO
World Health Organization
- MERS-CoV
Middle East Respiratory Syndrome Coronavirus
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- CRP
C-Reactive Protein
- ICU
Intensive Care Unit
- MINORS
Methodical Index for Non-randomized Studies
- CDC
Center for Disease Control and Prevention
- LDH
Lactate dehydrogenase
- INF
Interferon
- TNF
Tumor necrosis factor
- ESR
Erythrocyte sedimentation rate
- ARDS
Acute respiratory distress syndrome
- IL
Interleukins
- RT-PCR
Reverse transcription polymerase chain reaction
- BUN
Blood urea nitrogen
- CT Scan
computed tomography scan.
References
- 1.COVID-19 Situation Reports. (2020). Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed May 29, 2020).
- 2.Tang B, Bragazzi NL, Li Q, Tang S, Xiao Y, Wu J. An updated estimation of the risk of transmission of the novel coronavirus (2019-nCov). Infect Dis Model. (2020) 5:248–55. 10.1016/j.idm.2020.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pacific J Aller Immunol. (2020) 38:1–9. 10.12932/AP-200220-0772 [DOI] [PubMed] [Google Scholar]
- 4.Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. (2009) 6:e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mo P, Xing Y, Xiao Y, Deng L, Zhao Q, Wang H, et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. (2020). 10.1093/cid/ciaa270. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang G, Hu C, Luo L, Fang F, Chen Y, Li J, et al. Clinical Features and short-term outcomes of 221 Patients with COVID-19 in Wuhan, China. J Clin Virol. (2020) 127:104364. 10.1016/j.jcv.2020.104364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China. Clin Infect Dis. (2020) 71: 769–77. 10.1093/cid/ciaa272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin Y, Ji C, Weng W, Xu P, Hu Y, Liang W, Pu J, Zhang H. Epidemiological and Clinical Characteristics of 124 Elderly Outpatients with COVID-19 in Wuhan, China. Available online at: https://ssrn.com/abstract=3543596. 10.2139/ssrn.3543596 [DOI]
- 9.Yang W, Cao Q, Qin L, Wang X, Cheng Z, Pan A, et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19):A multi-center study in Wenzhou city, Zhejiang, China. J Infect. (2020) 80:388–93. 10.1016/j.jinf.2020.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi Q, Zhao K, Yu J, Feng J, Zhao K, Zhang X, et al. Clinical Characteristics of 101 Non-Survivors Hospitalized with COVID-19 - A Single Center, Retrospective Study. SSRN Electron J. (2020). 10.2139/ssrn.3550014 [DOI] [Google Scholar]
- 11.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. (2020) 323:1061–9. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu T, Zhang J, Yang Y, Ma H, Li Z, Zhang J, et al. The potential role of IL-6 in monitoring coronavirus disease 2019. medRxiv [Preprint]. (2020). 10.1101/2020.03.01.20029769 [DOI] [Google Scholar]
- 14.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. (2020) 395:507–13. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu J, Wu X, Zeng W, Guo D, Fang Z, Chen L, et al. Chest CT findings in patients with coronavirus disease 2019 and its relationship with clinical features. Invest Radiol. (2020) 55:257–61. 10.1097/RLI.0000000000000670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian S, Hu N, Lou J, Chen K, Kang X, Xiang Z, et al. Characteristics of COVID-19 infection in Beijing. J Infect. (2020) 80:401–6. 10.1016/j.jinf.2020.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: a systematic review and meta-analysis. Int J Infect Dis. (2020) 94:91–5. 10.1016/j.ijid.2020.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Yao L, Zhang J-P, Tang P-J, Ye Z-J, Shen X-H, et al. Clinical characteristics and laboratory indicator analysis of 69 COVID-19 pneumonia patients in Suzhou, China. Lancet [Pre-print]. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu J, Liu J, Zhao X, Liu C, Wang W, Wang D, et al. Clinical characteristics of imported cases of COVID-19 in Jiangsu province: a multicenter descriptive study. Clin Infect Dis. (2020) 71:706–12. 10.1093/cid/ciaa199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: Retrospective case series. BMJ. (2020) 368:1–7. 10.1136/bmj.m606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunologic features in severe and moderate coronavirus disease 2019. J Clin Invest. (2020) 130:2620–9. 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Li S, Liu J, Liang B, Wang X, Wang H, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMed. (2020) 55:2763. 10.1016/j.ebiom.2020.102763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao Y, Liu X, Xiong L, Cai K. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2: A systematic review and meta-analysis. J Med Virol. (2020) 92:1449–59. 10.1002/jmv.25822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Gao YH, Lou LL, Zhang GJ. The clinical dynamics of 18 cases of COVID-19 outside of Wuhan, China. Eur Respir J. (2020) 55:2000398. 10.1183/13993003.00398-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng Y, Huang Z, Yin G, Zhang X, Ye W, Hu Z, et al. Study of the lymphocyte change between COVID-19 and non-COVID-19 pneumonia cases suggesting other factors besides uncontrolled inflammation contributed to multi-organ injury. medRxiv [Preprint]. (2020). 10.1101/2020.02.19.20024885 [DOI] [Google Scholar]
- 26.Kujawski S, Wong K, Collins J, Epstein L, Killerby M, Midgley C, et al. First 12 patients with coronavirus disease 2019 (COVID-19) in the United States. medRxiv. (2020). 10.1101/2020.03.09.20032896 [DOI] [PubMed] [Google Scholar]
- 27.Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. (2020) 323:1612–4. 10.1001/jama.2020.4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized With COVID-19 in the New York City area. JAMA. (2020) 10022:1–8. 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. (2020) 323:1574–81. 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pung R, Chiew CJ, Young BE, Chin S, Chen MIC, Clapham HE, et al. Investigation of three clusters of COVID-19 in Singapore: implications for surveillance and response measures. Lancet. (2020) 395:1039–46. 10.1016/S0140-6736(20)30528-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young BE, Ong SWX, Kalimuddin S, Low JG, Tan SY, Loh J, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. (2020) 323:1488–94. 10.1001/jama.2020.3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lo IL, Lio CF, Cheong HH, Lei CI, Cheong TH, Zhong X, et al. Evaluation of SARS-CoV-2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID-19 in Macau. Int J Biol Sci. (2020) 16:1698–707. 10.7150/ijbs.45357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Easom N, Moss P, Barlow G, Samson A, Taynton T, Adams K, et al. Sixty-eight consecutive patients assessed for COVID-19 infection: experience from a UK Regional infectious diseases Unit. Influenza Other Respir Viruses. (2020) 14:374–9. 10.1111/irv.12739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amrane S, Tissot-Dupont H, Doudier B, Eldin C, Hocquart M, Mailhe M, et al. Rapid viral diagnosis and ambulatory management of suspected COVID-19 cases presenting at the infectious diseases referral hospital in Marseille, France, - January 31st to March 1st, 2020: a respiratory virus snapshot. Travel Med Infect Dis. (2020) 36:101632. 10.1016/j.tmaid.2020.101632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tabata S, Imai K, Kawano S, Ikeda M, Kodama T, Miyoshi K, et al. Non-severe vs severe symptomatic COVID-19: 104 cases from the outbreak on the cruise ship “Diamond Princess” in Japan. medRxiv. (2020). 10.1101/2020.03.18.20038125 [DOI] [Google Scholar]
- 36.Kong I, Park Y, Woo Y, Lee J, Cha J, Choi J, et al. Early epidemiological and clinical characteristics of 28 cases of coronavirus disease in South Korea. Osong Public Heal Res Perspect. (2020) 11:8–14. 10.24171/j.phrp.2020.11.1.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (Minors): development and validation of a new instrument. ANZ J. Surg. (2003) 73:712–6. 10.1046/j.1445-2197.2003.02748.x [DOI] [PubMed] [Google Scholar]
- 38.Miller EJ, Linge HM. Age-related changes in immunological and physiological responses following pulmonary challenge. Int J Mol Sci. (2017) 18:18061294. 10.3390/ijms18061294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guan W, Liang W, Zhao Y, Liang H, Chen Z, Li Y, et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J. (2020) 2020:2000547. 10.1183/13993003.00547-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y, Mao B, Liang S, Yang J, Lu H, Chai Y, et al. Association between age and clinical characteristics and outcomes of coronavirus disease 2019. SSRN Electron J. (2020) 2019:3556689. 10.2139/ssrn.355668932312864 [DOI] [Google Scholar]
- 41.Team T. N. C. P. E. R. E The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) — China. China CDC Wkly. (2020) 2:113–22. 10.46234/ccdcw2020.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hui DS, Wong PC, Wang C. SARS: clinical features and diagnosis. Respirology. (2003) (Suppl 1):S20–4. 10.1046/j.1440-1843.2003.00520.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raj VS, Osterhaus ADME, Fouchier RAM, Haagmans BL. MERS: Emergence of a novel human coronavirus. Curr Opin Virol. (2014) 5:58–62. 10.1016/j.coviro.2014.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Day M. Covid-19: four fifths of cases are asymptomatic, China figures indicate. BMJ. (2020) 369:m1375. 10.1136/bmj.m1375 [DOI] [PubMed] [Google Scholar]
- 46.Singhal T. A review of coronavirus disease-2019 (COVID-19). Indian J Pediatr. (2020) 87:281–6. 10.1007/s12098-020-03263-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. (2020) 382:727–33. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boonnak K, Vogel L, Feldmann F, Feldmann H, Legge KL, Subbarao K. Lymphopenia associated with highly virulent H5N1 virus infection due to plasmacytoid dendritic cell-mediated apoptosis of T cells. J Immunol. (2014) 192:5906–12. 10.4049/jimmunol.1302992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. (2020) 63:364–74. 10.1007/s11427-020-1643-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ji W, Bishnu G, Cai Z, Shen X. Analysis clinical features of COVID-19 infection in secondary epidemic area and report potential biomarkers in evaluation. medRxiv. [Preprint] (2020). 10.1101/2020.03.10.20033613 [DOI] [Google Scholar]
- 51.CDC Management of Patients with Confirmed 2019-nCoV. (2020). Available online at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html (accessed May 11, 2020).
- 52.Wang JT, Sheng WH, Fang CT, Wang JL, Yu CJ. Clinical manifestations, laboratory findings, and treatment outcomes of SARS patients. Emerg Infect Dis. (2004) 10:818–24. 10.3201/eid1005.030640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ko J.-H., Park GE, Lee JY, Lee JY, Cho SY, et al. Predictive factors for pneumonia development and progression to respiratory failure in MERS-CoV infected patients. J Infect. (2016) 73:468–75. 10.1016/j.jinf.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsui PT, Kwok ML, Yuen H, Lai ST. Severe acute respiratory syndrome: clinical outcome and prognostic correlates. Emerg Infect Dis. (2003) 9:1064–9. 10.3201/eid0909.030362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.UF Health LDH Isoenzyme Blood Test. University of Florida Health; (2020). Available online at: https://ufhealth.org/ldh-isoenzyme-blood-test?fbclid=IwAR2wYAlUBHM0vR0rqPG2YNITjIS5pEDZeXJp0xZVwjpIqPDf7fq-m03hOhU (accessed May 11, 2020). [Google Scholar]
- 56.Bangash MN, Patel J, Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. (2020) 1253:20–1. 10.1016/S2468-1253(20)30084-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Surbatovic M, Popovic N, Vojvodic D, Milosevic I, Acimovic G, Stojicic M, et al. Cytokine profile in severe gram-positive and gram-negative abdominal sepsis. Sci Rep. (2015) 5:1–12. 10.1038/srep11355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song F, Shi N, Shan F, Zhang Z, Shen J, Lu H, et al. Emerging 2019 novel coronavirus (2019-NCoV) pneumonia. Radiology. (2020) 295:210–7. 10.1148/radiol.2020200274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leung C. The difference in the incubation period of 2019 novel coronavirus (SARS-CoV-2) infection between travelers to Hubei and non-travelers: the need of a longer quarantine period. Infect Control Hosp Epidemiol. (2020) 81:1–3. 10.1017/ice.2020.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Castagnoli R, Votto M, Licari A, Brambilla I, Bruno R, Perlini S, et al. Severe Acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. (2020) 174:882–9. 10.1001/jamapediatrics.2020.1467 [DOI] [PubMed] [Google Scholar]
- 61.Tahamtan A, Ardebili A. Real-time RT-PCR in COVID-19 detection: issues affecting the results. Expert Rev Mol Diagn. (2020) 37:1–2. 10.1080/14737159.2020.1757437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dheda K, Davids M, Chang J.-W., Gina P, Pooran A, et al. Diagnosis of COVID-19: considerations, controversies and challenges in South Africa. Wits J Clin Med. (2020) 2:3 10.18772/26180197.2020.v2nsia1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coronavirus. Why is India Testing so Little? BBC News; (20 March, 2020). Available online at: https://www.bbc.com/news/world-asia-india-51922204 (accessed May 18, 2020). [Google Scholar]
- 64.Tang Y-W, Schmitz JE, Persing DH, Stratton CW. The laboratory diagnosis of COVID-19 infection: current issues and challenges. J Clin Microbiol. (2020) 58:e00512–20. 10.1128/JCM.00512-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu E. COVID-19 testing: challenges, limitations and suggestions for improvement. Preprints. (2020) 2020040155 10.20944/preprints202004.0155.v1 [DOI] [Google Scholar]
- 66.Phan T. Genetic diversity and evolution of SARS-CoV-2. Infect Genet Evol. (2020) 81:104260. 10.1016/j.meegid.2020.104260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shen Z, Xiao Y, Kang L, Ma W, Shi L, Zhang L, et al. Genomic diversity of SARS-CoV-2 in Coronavirus Disease 2019 patients. Clin Infect Dis. (2020) 71:713–20. 10.1093/cid/ciaa203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. (2020) 382:1177–9. 10.1056/NEJMc2001737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carlotti AP, de CP, Carvalho, de WB, Johnston C, Rodriguez IS. COVID-19 diagnostic and management protocol for pediatric patients. Clinics. (2020) 75:e1894. 10.6061/clinics/2020/e1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chang TH, Wu JL, Chang LY. Clinical characteristics and diagnostic challenges of pediatric COVID-19: a systematic review and meta-analysis. J Formos Med Assoc. (2020) 119:982–9. 10.1016/j.jfma.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang W, Yan F. Patients with RT-PCR-confirmed COVID-19 and normal chest CT. Radiology. (2020) 295:E3. 10.1148/radiol.2020200702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. (2020) 2020:200642. 10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.NHS Scanner and Radiologist Shortages Contributing to Thousands of Avoidable Heart Attack Deaths, Experts Warn The Independent. (2020). Available online at: https://www.independent.co.uk/news/health/heart-attack-deaths-mri-nhs-scanner-radiologist-shortages-budget-a8622461.html (accessed May 18, 2020).
- 74.Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. Am J Roentgenol. (2020) 2020:1–7. 10.2214/AJR.20.23034 [DOI] [PubMed] [Google Scholar]
- 75.Why Coronavirus Testing in the U.S. Is So Delayed - The Atlantic. (2020). Available online at: https://www.theatlantic.com/health/archive/2020/03/why-coronavirus-testing-us-so-delayed/607954/ (accessed May 18, 2020).
- 76.Lau SKP, Woo PCY, Wong BHL, Tsoi H.-W., Woo GKS, et al. Detection of severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein in sars patients by enzyme-linked immunosorbent assay. J Clin Microbiol. (2004) 42:2884–9. 10.1128/JCM.42.7.2884-2889.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu IJ, Chen PJ, Yeh SH, Huang LM, Chang MF. Immunofluorescence assay for detection of the nucleocapsid antigen of the severe acute respiratory syndrome (SARS)-associated coronavirus in cells derived from throat wash samples of patients with SARS. J Clin Microbiol. (2005) 43:2444–8. 10.1128/JCM.43.5.2444-2448.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sastre P, Dijkman R, Camuñas A, Ruiz T, Jebbink MF, van der Hoek L, et al. Differentiation between human coronaviruses NL63 and 229E using a novel double-antibody sandwich enzyme-linked immunosorbent assay based on specific monoclonal antibodies. Clin Vaccine Immunol. (2011) 18:113–8. 10.1128/CVI.00355-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abbasi J. The promise and peril of antibody testing for COVID-19. JAMA. (2020) 323:1881–3. 10.1001/jama.2020.6170 [DOI] [PubMed] [Google Scholar]
- 80.EUA Authorized Serology Test Performance. FDA; (2020). Available online at: https://www.fda.gov/medical-devices/emergency-situations-medical-devices/eua-authorized-serology-test-performance (accessed May 18, 2020). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed in this study have been included in the article. The raw data will be provided by the corresponding and/or by the first author on reasonable request.