Abstract
Background: Impella CP is a left ventricular pump which may serve as a circulatory support during cardiopulmonary resuscitation (CPR) for cardiac arrest (CA). Nevertheless, the survival rate and factors associated with survival in patients undergoing Impella insertion during CPR for CA are unknown. Methods: We performed a retrospective multicenter international registry of patients undergoing Impella insertion during on-going CPR for in- or out-of-hospital CA. We recorded immediate and 30-day survival with and without neurologic impairment using the cerebral performance category score and evaluated the factors associated with survival. Results: Thirty-five patients had an Impella CP implanted during CPR for CA. Refractory ventricular arrhythmias were the most frequent initial rhythm (65.7%). In total, 65.7% of patients immediately survived. At 30 days, 45.7% of patients were still alive. The 30-day survival rate without neurological impairment was 37.1%. In univariate analysis, survival was associated with both an age < 75 years and a time from arrest to CPR ≤ 5 min (p = 0.035 and p = 0.008, respectively). Conclusions: In our multicenter registry, Impella CP insertion during ongoing CPR for CA was associated with a 37.1% rate of 30-day survival without neurological impairment. The factors associated with survival were a young age and a time from arrest to CPR ≤ 5 min.
Keywords: Impella, cardiac arrest, refractory cardiac arrest, hemodynamic support device, cardiopulmonary resuscitation
1. Introduction
Sudden cardiac arrest (CA) is a major cause of death worldwide [1]. Survival following cardiopulmonary resuscitation (CPR) remains limited and the overall rate of survival to hospital discharge is about 10% for both in-hospital and out-of-hospital CA [2,3,4,5]. Despite advances in CPR, survival remains limited [6]. Following reports of a benefit of extracorporeal membrane oxygenation (ECMO) in patients with refractory CA, mechanical circulatory support (MCS) has emerged as a tool of potential interest [4,7]. Standardized protocols to better select patients who may benefit from ECMO and prevent futile use were proposed in the most recent guidelines [4,8]. Although ECMO support is associated with improved survival in selected patients, it is a resource-intensive therapy requiring a dedicated staff and therefore has a limited availability in centers without on-site ECMO [9,10]. The Impella CP device is a continuous flow pump inserted percutaneously into the left ventricle (LV), ensuring up to 3.5 L of blood flow per minute [11]. This device is available in cath-labs and could be quickly implemented during CPR. In recent years, case reports and monocenter registries have suggested a potential role for Impella CP in the setting of CA [12,13,14]. However, in these reports, the inclusion criteria and survival rates were variable, the sample size was limited, and predictors of success could not be determined. We therefore initiated an international multicenter registry of Impella implantation during on-going CPR for CA related to acute coronary syndrome (ACS), in order to investigate the outcome and the factors associated with survival.
2. Experimental Section
2.1. Participants and Informed Consent
The Impella CP registry is a retrospective multicenter registry from April 2014 to January 2020 of 35 patients who required Impella CP (Abiomed, Inc., Danvers, MA, USA) implantation during on-going CPR with manual or mechanical chest compression for CA presumably related to an ACS based on electrocardiography (ECG) and clinical features (ST segment elevation on ECG, shockable initial rhythm, or chest pain prior to the cardiac arrest). Only patients who had continuous on-going CPR when the Impella device was inserted were included. Patients with both in- or out-of-hospital witnessed CA were eligible. In out of hospital cardiac arrest (OHCA) situations, patients were transferred during ongoing CPR in hospital. If return of spontaneous circulation (ROSC) and hemodynamic stability were obtained before the Impella device was implanted and started pumping, cardiac arrest was not considered refractory and the patient was not included [4]. Patients without ongoing CA presenting with hemodynamic instability due to cardiogenic shock were not included in the analysis. The present retrospective registry conformed to the ethical guidelines of the 1975 Declaration of Helsinki and received institutional review board approval, and informed written consent was obtained from each patient before hospital discharge. When the patient was deceased, the consent was obtained from the family.
2.2. Study Procedure
The device was inserted during CPR in the catheterization laboratory via the femoral artery under vascular ultrasound guidance when possible and as specified by the manufacturer. Coronary angiography was conducted in all patients after Impella insertion and start. If required, revascularization was performed, depending on the clinical context. Chest compressions were continued until sufficient Impella flow could be achieved or until the patients was considered deceased. The neurological status was assessed using the cerebral performance category score (CPC score) collected from the medical health records. The weaning of the hemodynamic support was conducted according to each center’s protocols. Clinical data and survival at one month were collected retrospectively from electronic medical records in a dedicated database. Vascular complications were all complications requiring surgical care (including access site infection). Bleeding complications include all bleeding requiring transfusion. Time from arrest to CPR was defined as the time between collapse and the beginning of CPR (also called the “no flow time”). The CPR duration was the time of continuous manual or mechanical chest compressions (also called the “low flow time”). If a sustained and continuous ROSC (>30 min) was achieved, CA was not considered refractory and the patient was not eligible. When many short periods of unsustained ROSC were achieved, this time was deduced from the CPR duration.
2.3. Statistical Analysis
Statistical analyses were performed using IBM SPSS Statistics 20.0 (IBM Inc., New York, NY, USA). Continuous data are reported as the mean ± standard deviation. Categorical data are reported as absolute counts (percentages). Continuous data were compared using a Mann–Whitney test. Categorical data were compared using an χ2 test or a Fisher’s exact test. The Kaplan–Meier method was used to describe the probability of survival over time in the whole population. All tests were two-sided. Differences were considered to be statistically significant when the p value was less than 0.05. Figures were drawn using the GraphPad Prism 8 (GraphPad Software Inc., San Diego, CA, USA) software system.
3. Results
The registry included 35 patients from five different tertiary centers in four different countries, including the USA, Denmark, France, and The Netherlands, requiring ongoing CPR for CA.
3.1. Patients’ Characteristics
The main characteristics of the study population are described in Table 1. Patients had a mean age of 66 ± 9 years and the majority had suffered in-hospital CA (80.6%). The cardiac rhythm at the time of CA was a refractory ventricular arrhythmia in 65.7% of patients. The mean time from arrest to CPR was 3 ± 4 min. The mean time from CA to implantation of the Impella CP device and the delivery of blood flow was 46 ± 33 min. The duration of CPR for intra-hospital cardiac arrest (IHCA) patients was 39 ± 32 min, whereas the time of CPR for OHCA patients was 75 ± 40 min (p = 0.04). In all patients, an Impella CP device was used. The mean first in-hospital arterial pH and lactate levels were 7.0 ± 0.1 and 11.8 ± 3.8 mmol/L, respectively. Extended CPR was required following Impella start in 48.6% of patients. Coronary revascularization was performed in 85.7% of patients after device implantation. Four patients needed ECMO support after Impella insertion.
Table 1.
Characteristics of the population.
| Variables | Entire Population (n = 35) |
| Age (years) | 66 ± 9/65 (59–72) |
| Male | 28 (80.0) |
| Location of cardiac arrest | |
| IHCA | 29 (80.6) |
| OHCA | 6 (17.1) |
| Initial rhythm | |
| VF/VT | 23 (65.7) |
| PEA/asystole | 12 (34.3) |
| CPR time courses | |
| Time from arrest to CPR (min) | 3 ± 4/0 (0–5) |
| CPR duration (min) | 45 ± 36/30 (20–55) |
| Time between CA and Impella insertion (min) | 46 ± 33/35 (26–60) |
| Extended CPR after Impella insertion | 17 (48.6) |
| Initial blood gas | |
| Lactates | 11 ± 3.8 |
| Arterial pH | 7.0 ± 0.1 |
| Use of catecholamines | |
| Adrenaline | 23 (65.7) |
| Dobutamine | 12 (34.3) |
| Noradrenaline | 23 (65.7) |
| Duration of Impella support (hours) | 43.1 ± 48/24 (1–72) |
| Duration of inotropic support (hours) | 151 ± 238.8/45 (1–200) |
| Outcomes | |
| Median hospital length of stay (days) mean ± SD | 17 ± 20/11 (1–26) |
| Vascular complications | 6 (17.1) |
| Immediate survival | 23 (65.7) |
| Survival at 1-month | 16 (45.7) |
Values are the mean ± SD/median (IQR) for continuous data or n (%). Legend: CA: cardiac arrest; CPR: cardiopulmonary resuscitation; IHCA: intra-hospital cardiac arrest; OHCA: out-of-hospital cardiac arrest; PEA: pulseless electrical activity; VF: ventricular fibrillation; and VT: ventricular tachycardia.
3.2. Outcome
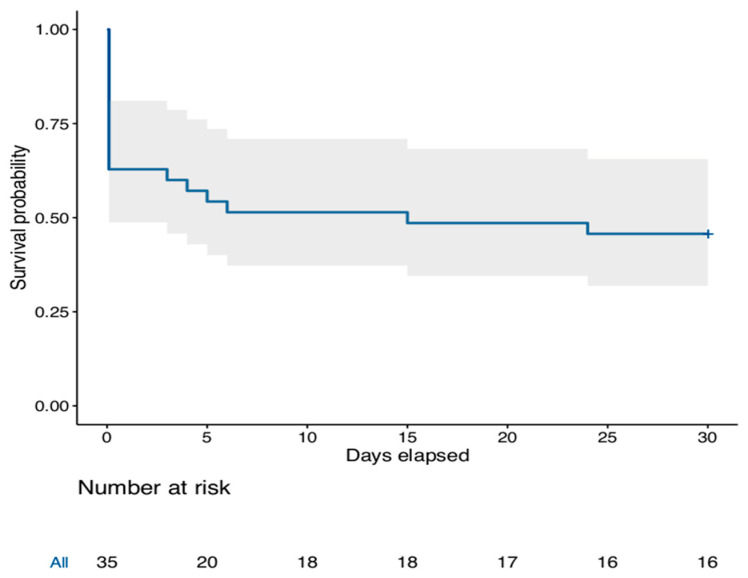
Twelve patients (34.3%) died in the cath-lab and 23 (65.7%) survived the procedure and were transported to the intensive care unit. Following the procedure, all patients were under catecholamines (Table 1). The mean in-hospital length of stay was 17 ± 20 days (median 11 days IQR (1–26)). At 30-day follow-up, 16 patients (45.7%) were alive (Figure 1). Three patients out of the 16 survivors at 30-days had neurological sequelae (CPC score > 2). Overall, the 30-day survival rate without neurological sequelae (CPC score 1 or 2) was 37.1%. The cause of death was multi organ failure or refractory CA in all patients. Vascular and bleeding complications occurred in six patients (17.1%), of which two required vascular surgery, but none led to death. When considering survival at each center, we found a non-significant heterogeneity between participating hospitals (I2 = 43%, p = 0.149, Figure S1).
Figure 1.
Kaplan–Meier curve showing the probability of survival over time.
3.3. Factors Associated with Immediate and 30-Day Survival
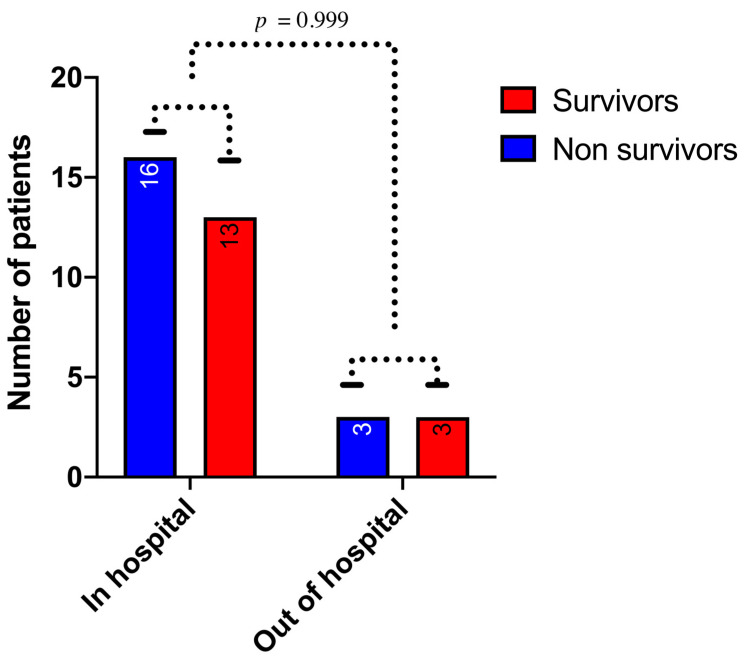
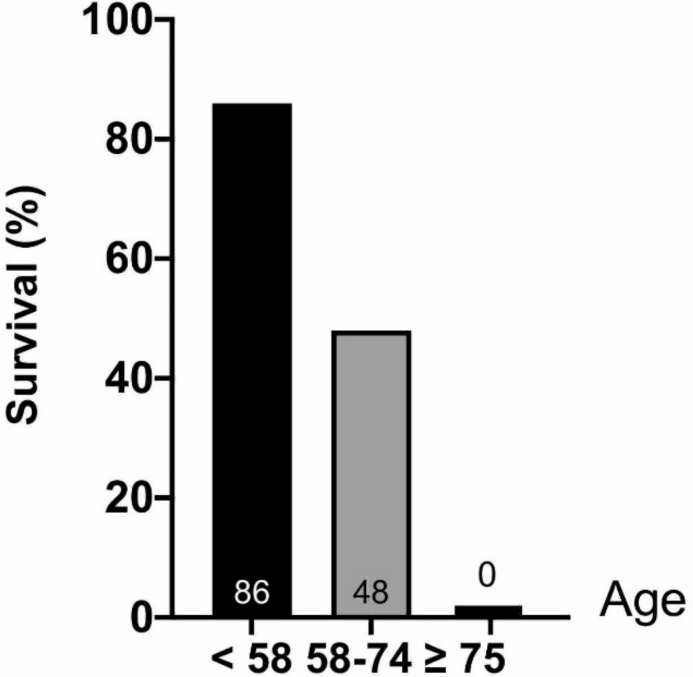
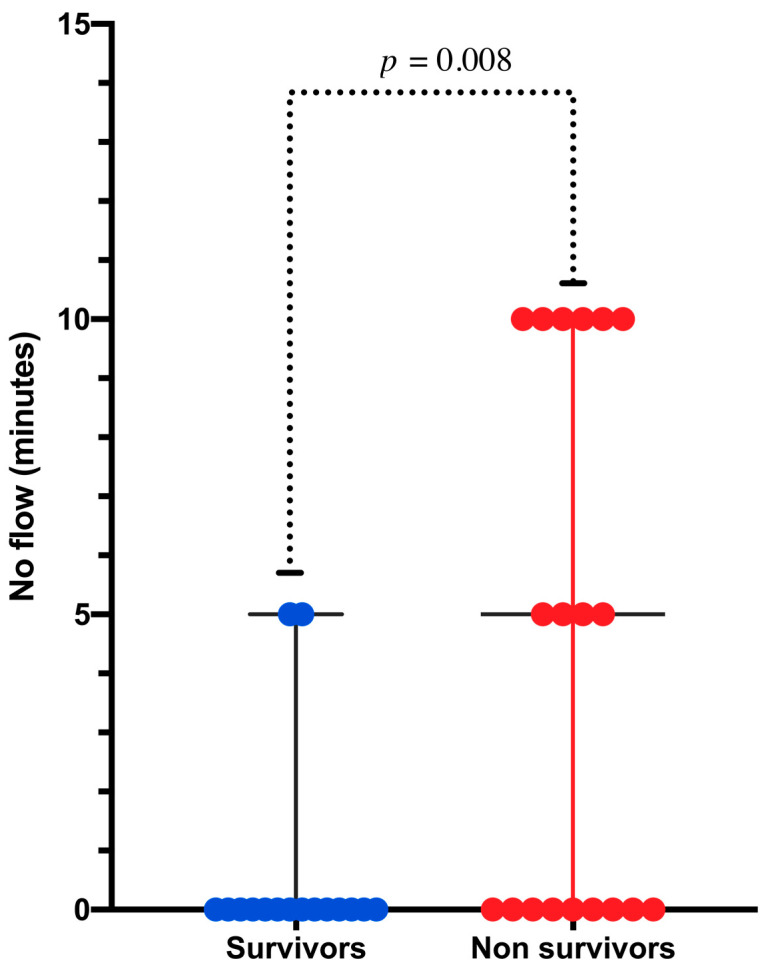
As illustrated in Table 2, patients who survived the procedure were significantly younger (p = 0.02) and displayed a short time between CA and the initiation of CPR (p < 0.01). Although it did not reach statistical significance, there was a strong association between immediate survival and the presence of an initial shockable rhythm compared to pulseless electric activity or asystole (p = 0.059). Revascularization was not associated with immediate survival (p = 0.57). Regarding 30-day survival, we observed no difference in survival between in- and out-of-hospital CA (p = 0.99) (Figure 2). We observed a significant association between age and 30-day survival (p = 0.035). Among the seven patients over 75 years old, none survived to 30 days (Figure 3). The time between CA and CPR was also a factor associated with survival (p = 0.008) (Figure 4). Patients with a time from CA to CRP > 5 min did not survive. Again, although not significant, a trend toward a better 30-day survival was observed in patients with an initial shockable rhythm (p = 0.076).
Table 2.
Univariate predictors of survival.
| Successful Resuscitation n, (%) | Deceased Patients (n = 12) | Survivors (n = 23) | p |
| Age and mean ± SD | 71 ± 7 | 63 ± 9 | 0.023 |
| VF/VT | 5 (41.7) | 18 (78.3) | 0.059 |
| Time from arrest to CPR (min) | 6 ± 5 | 1 ± 2 | 0.01 |
| pH at initiation of CPR | 7.0 | 7.02 | 0.487 |
| Lactates level at initiation of CPR (mmol/L) | 12.2 | 10.6 | 0.333 |
| 1-month n, (%) | Deceased Patients (n = 19) | Survivors (n = 16) | p |
| Age y mean ± SD | 69 ± 8 | 62 ± 9 | 0.035 |
| VF/VT | 10 (52.6) | 13 (81.2) | 0.076 |
| Time from arrest to CPR (min) | 4 ± 4 | 1 ± 2 | 0.008 |
| pH at initiation of CPR | 7.04 | 6.98 | 0.298 |
| Lactates level at initiation of CPR (mmol/L) | 10.8 | 11.3 | 0.696 |
Values are the mean ± SD or n (%). Legend: VF: ventricular fibrillation, and VT: ventricular tachycardia.
Figure 2.
Relationship between the cardiac arrest location and 1-month survival (p value [Fisher’s exact test] = 0.999).
Figure 3.
Relationship between age and 30-day survival.
Figure 4.
Scatter plot of time from arrest to cardiopulmonary resuscitation (CPR) (no flow time) in survivors and non survivors at 1 month (p value [Mann–Whitney] = 0.008).
4. Discussion
The Impella CP registry suggests that the early implantation of an Impella CP during ongoing-CPR for CA related to ACS is feasible and is associated with a 30-day survival rate of 45.7% and 37.1% without neurological sequelae. Previous monocenter studies have suggested that such an intervention could be successful. However, they had various inclusion criteria, involved a small sample size, and reported a large survival rate ranging between 5% and 50% [14,15]. The relatively high survival rate reported in our study was obtained despite the fact that the Impella was inserted as a salvage therapy during ongoing CPR without ROSC. However, because of the retrospective nature of the study and small sample size, these results should be considered preliminary and considered as a feasibility and safety analysis.
Although Impella is only an LV support device, it enabled successful resuscitation in a significant number of patients with ongoing CPR for CA. The feasibility and efficacy of only supporting the LV, with the Impella CP, during resuscitation in the present study are consistent with recent experimental and clinical evidence. Lotun et al. were able to resuscitate swine specimens in CA thanks to the combination of chest compressions and Impella, with favorable neurological recovery [16]. In their experimental model, the survival rate of Impella-facilitated resuscitation was superior to conventional CPR. Furthermore, in humans, consistent with our findings, previous reports have underlined the feasibility of LV support with only the Impella in CA [12,13,14,15].
In our study, the 1-month survival rate was 45.7% and 37.1% without neurological impairment. These results, although based on a limited number of patients, are promising and similar to those obtained with ECMO [17,18]. To date, few therapies have improved the outcome of CA [19]. ECMO is currently used in refractory CA based on promising registry data. Although there are no randomized studies, meta-analysis has suggested that ECMO utilization results in an overall survival rate of 22%, including 13% of patients with a good neurological recovery in refractory CA [7]. Furthermore, in another meta-analysis, ECMO was associated with a 13% absolute increase in 30-day survival compared to standard CPR [20]. However, the selection of patients is key, as demonstrated by the lack of overall benefit of ECMO compared to standard CPR in a propensity match analysis [21]. Lamhaut et al. reported that the appropriate selection of patients with CA for MCS is critical to preventing futile use [22]. Overall, these studies are in favor of a benefit of MCS in CA in selected patients. Accordingly, despite the relatively small sample size of our study, we were able to identify the duration of time from arrest to CPR and age as factors associated with survival. This result is original, since previous studies with the Impella could not assess factors associated with survival given their small sample size. Our findings are consistent with previous observations with ECMO [23] regarding these predictors, which are commonly used to select patients for MCS during CA [4]. Of note, our revascularization rate is close to those observed in the same setting in the literature [24]. Although the duration of CPR was not identified as a factor associated with survival in our study, it is well-recognized that a sustained time of CPR is associated with a poor outcome [25]. The lack of a significant impact of the duration of CPR in our study is probably related to the limited sample size. However, the factors associated with survival evidence in the present registry are useful for accurately selecting patients for Impella CP insertion during on-going CPR for CA and can help in the design of future trials in this field. Our results suggest that the implantation of Impella in patients over 75 years old or with a time from arrest to CPR > 5 min in the setting of ongoing CPR for CA may be futile.
In the setting of refractory CA, there are potential advantages of the Impella device over ECMO. First, Impella seems more available and quicker to insert by the interventional cardiologist than ECMO, which may ensure greater access to MCS for patients [26]. In addition, it reduces the time to full hemodynamic support and CPR duration, which are also critical for success in the setting of CA. Therefore, the Impella device may play a role in centers without ECMO or when the time to ECMO is expected to be long. Furthermore, the use of the Impella device may result in a lower rate of device-related vascular complications compared to ECMO [26,27]. Finally, ECMO-generated blood flow is continuous and retrograde, increasing ventricular stroke work and making this system efficient in terms of supporting peripheral organs, but responsible for LV overload [28,29]. On the other hand, Impella, despite a lower blood flow, is associated with several favorable properties of the heart by unloading the LV, limiting the infarct size, and promoting recovery [30]. However, there are no studies comparing ECMO and Impella in CA.
Limitations
There are limitations to the present findings. First, the sample size is small, which may limit the generalizability of our results and prevented multivariate analysis for predictors of survival. Second, there is an obvious selective bias when using such an invasive and costly strategy for specific patients who may be considered as having a higher chance of survival and lower co-morbidities. Third, the majority of reported cases had good prognosis factors, such as in-hospital cardiac arrest and a limited period of time from arrest to CPR, and our result may not apply in a larger population. Additionally, the number of patients suffering OHCA is rather small and their time of CPR was longer than IHCA. Fourth, this is a retrospective observational study with inherent limitations and some clinical data were not available.
Nevertheless, the present study is the largest to date and suggests that in selected patients, Impella CP may be a lifesaving tool during CPR for CA related to an ACS. Prospective and larger studies are required to confirm these preliminary results, perform multivariate analysis for obtaining more reliable results, and help better define the patients likely to benefit from biventricular support rather than LV support alone during CPR for CA related to ACS.
5. Conclusions
In this international registry, in patients undergoing Impella CP insertion during on-going CPR for CA, the 30-day survival rate was 45.7% overall and 37.1% without neurological impairment. Impella CP insertion may be lifesaving in patients with CA younger than 75 years old with a time from arrest to CPR ≤ 5 min. Prospective studies are required to confirm these encouraging but preliminary data and should help to accurately define patient selection criteria.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/10/2/339/s1, Figure S1: Hetereogeneity between participating centers.
Author Contributions
Conceptualization, V.P. and L.B.; methodology, J.M.; software, J.M.; validation, J.M., V.P. and L.B.; formal analysis, J.M.; investigation, V.P., H.V., S.P.S., M.B.B., H.K., S.B., M.L., H.E., S.C., M.K., F.P., J.P.S.H. and L.B.; resources, V.P., H.V., S.P.S., M.B.B., H.K., S.B., M.L., H.E., S.C., M.K., F.P., J.P.S.H. and L.B.; data curation, J.M.; writing—original draft preparation, V.P. and L.B.; writing—review and editing, V.P. and L.B.; visualization, L.B.; supervision, L.B.; project administration, L.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of each center included.
Informed Consent Statement
An informed written consent was obtained from each patient before hospital discharge. When the patient was deceased, the consent was obtained from the family.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gräsner J.-T., Lefering R., Koster R.W., Masterson S., Böttiger B.W., Herlitz J., Wnent J., Tjelmeland I.B.M., Ortiz F.R., Maurer H., et al. EuReCa ONE-27 Nations, ONE Europe, ONE Registry: A prospective one month analysis of out-of-hospital cardiac arrest outcomes in 27 countries in Europe. Resuscitation. 2016;105:188–195. doi: 10.1016/j.resuscitation.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Berdowski J., Berg R.A., Tijssen J.G.P., Koster R.W. Global incidences of out-of-hospital cardiac arrest and survival rates: Systematic review of 67 prospective studies. Resuscitation. 2010;81:1479–1487. doi: 10.1016/j.resuscitation.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Gregers E., Kjærgaard J., Lippert F., Thomsen J.H., Køber L., Wanscher M., Hassager C., Søholm H. Refractory out-of-hospital cardiac arrest with ongoing cardiopulmonary resuscitation at hospital arrival—Survival and neurological outcome without extracorporeal cardiopulmonary resuscitation. Crit Care. 2018;22:242. doi: 10.1186/s13054-018-2176-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conseil Français de Réanimation Cardiopulmonaire. Société Française D’anesthésie et de Réanimation. Société Française de Cardiologie. Société Française de Chirurgie Thoracique et Cardiovasculaire. Société Française de Médecine D’urgence. Société Française de Pédiatrie. Groupe Francophone de Réanimation et D’urgence Pédiatriques. Société Française de Perfusion. Société de Réanimation de Langue Française . Guidelines for Indications for the Use of Extracorporeal Life Support in Refractory Cardiac Arrest. Volume 28. French Ministry of Health; Paris, France: 2009. pp. 182–190. [DOI] [PubMed] [Google Scholar]
- 5.Sandroni C., Nolan J., Cavallaro F., Antonelli M. In-hospital cardiac arrest: Incidence, prognosis and possible measures to improve survival. Intensive Care Med. 2007;33:237–245. doi: 10.1007/s00134-006-0326-z. [DOI] [PubMed] [Google Scholar]
- 6.Quitzau L.H., Ullerup-Aagaard H., Brabrand M. No change in survival after cardiac arrest in 2007 and 2012 at a hospital in Denmark. Resuscitation. 2015;87:e11. doi: 10.1016/j.resuscitation.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 7.Ortega-Deballon I., Hornby L., Shemie S.D., Bhanji F., Guadagno E. Extracorporeal resuscitation for refractory out-of-hospital cardiac arrest in adults: A systematic review of international practices and outcomes. Resuscitation. 2016;101:12–20. doi: 10.1016/j.resuscitation.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 8.Le Guen M., Nicolas-Robin A., Carreira S., Raux M., Leprince P., Riou B., Langeron O. Extracorporeal life support following out-of-hospital refractory cardiac arrest. Crit Care. 2011;15:R29. doi: 10.1186/cc9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pozzi M., Armoiry X., Achana F., Koffel C., Pavlakovic I., Lavigne F., Fellahi J.L., Obadia J.F. Extracorporeal Life Support for Refractory Cardiac Arrest: A 10-Year Comparative Analysis. Ann. Thorac. Surg. 2019;107:809–816. doi: 10.1016/j.athoracsur.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Barbaro R.P., Odetola F.O., Kidwell K.M., Paden M.L., Bartlett R.H., Davis M.M., Annich G.M. Association of Hospital-Level Volume of Extracorporeal Membrane Oxygenation Cases and Mortality. Analysis of the Extracorporeal Life Support Organization Registry. Am. J. Respir. Crit. Care Med. 2015;191:894–901. doi: 10.1164/rccm.201409-1634OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thiele H., Ohman E.M., Desch S., Eitel I., de Waha S. Management of cardiogenic shock. Eur. Heart J. 2015;36:1223–1230. doi: 10.1093/eurheartj/ehv051. [DOI] [PubMed] [Google Scholar]
- 12.Asrress K.N., Marciniak M., Briceno N., Perera D. Cardiac Arrest in Acute Myocardial Infarction: Concept of Circulatory Support With Mechanical Chest Compression and Impella to Facilitate Percutaneous Coronary Intervention. Heart Lung Circ. 2017;26:e37–e40. doi: 10.1016/j.hlc.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Støttrup N.B., Jakobsen L., Krusell L.R., Terkelsen C.J. Utility of Impella® left ventricular assist device during cardiac arrest: A case report. Int. J. Cardiol. 2016;225:111–112. doi: 10.1016/j.ijcard.2016.09.074. [DOI] [PubMed] [Google Scholar]
- 14.Davidsen C., Packer E.J.S., Løland K.H., Rotevatn S., Nygreen E.L., Eriksen E., Øksnes A., Herstad J., Haaverstad R., Bleie Ø., et al. Impella use in acute myocardial infarction complicated by cardiogenic shock and cardiac arrest: Analysis of 10 years registry data. Resuscitation. 2019;140:178–184. doi: 10.1016/j.resuscitation.2019.04.022. [DOI] [PubMed] [Google Scholar]
- 15.Vase H., Christensen S., Christiansen A., Therkelsen C.J., Christiansen E.H., Eiskjær H., Poulsen S.H. The Impella CP device for acute mechanical circulatory support in refractory cardiac arrest. Resuscitation. 2017;112:70–74. doi: 10.1016/j.resuscitation.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Lotun K., Truong H.T., Cha K.-C., Alsakka H., Gianotto-Oliveira R., Smith N., Rao P., Bien T., Chatelain S., Kern M.C., et al. Cardiac Arrest in the Cardiac Catheterization Laboratory: Combining Mechanical Chest Compressions and Percutaneous LV Assistance. JACC Cardiovasc. Interv. 2019;12:1840–1849. doi: 10.1016/j.jcin.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Debaty G., Babaz V., Durand M., Gaide-Chevronnay L., Fournel E., Blancher M., Bouvaist H., Chavanon O., Maignan M., Bouzat P., et al. Prognostic factors for extracorporeal cardiopulmonary resuscitation recipients following out-of-hospital refractory cardiac arrest. A systematic review and meta-analysis. Resuscitation. 2017;112:1–10. doi: 10.1016/j.resuscitation.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 18.D’Arrigo S., Cacciola S., Dennis M., Jung C., Kagawa E., Antonelli M., Sandroni C. Predictors of favourable outcome after in-hospital cardiac arrest treated with extracorporeal cardiopulmonary resuscitation: A systematic review and meta-analysis. Resuscitation. 2017;121:62–70. doi: 10.1016/j.resuscitation.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Patil K.D., Halperin H.R., Becker L.B. Cardiac arrest: Resuscitation and reperfusion. Circ. Res. 2015;116:2041–2049. doi: 10.1161/CIRCRESAHA.116.304495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouweneel D.M., Schotborgh J.V., Limpens J., Sjauw K.D., Engström A.E., Lagrand W.K., Cherpanath T.G.V., Driessen A.H.G., de Mol B.A.J.M., Henriques J.P.S. Extracorporeal life support during cardiac arrest and cardiogenic shock: A systematic review and meta-analysis. Intensive Care Med. 2016;42:1922–1934. doi: 10.1007/s00134-016-4536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi D.S., Kim T., Ro Y.S., Ahn K.O., Lee E.J., Hwang S.S., Song S.W., Song K.J., Shin S.D. Extracorporeal life support and survival after out-of-hospital cardiac arrest in a nationwide registry: A propensity score-matched analysis. Resuscitation. 2016;99:26–32. doi: 10.1016/j.resuscitation.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Lamhaut L., Hutin A., Puymirat E., Jouan J., Raphalen J.-H., Jouffroy R., Jaffry M., Dagron C., An K., Dumas F., et al. A Pre-Hospital Extracorporeal Cardio Pulmonary Resuscitation (ECPR) strategy for treatment of refractory out hospital cardiac arrest: An observational study and propensity analysis. Resuscitation. 2017;117:109–117. doi: 10.1016/j.resuscitation.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Murakami N., Kokubu N., Nagano N., Nishida J., Nishikawa R., Nakata J., Suzuki Y., Tsuchihashi K., Narimatsu E., Miura T. Prognostic Impact of No-Flow Time on 30-Day Neurological Outcomes in Patients With Out-of-Hospital Cardiac Arrest Who Received Extracorporeal Cardiopulmonary Resuscitation. Circ. J. 2020;84:1097–1104. doi: 10.1253/circj.CJ-19-1177. [DOI] [PubMed] [Google Scholar]
- 24.Yannopoulos D., Bartos J.A., Raveendran G., Conterato M., Frascone R.J., Trembley A., John R., Connett J., Benditt D.G., Lurie K.G., et al. Coronary Artery Disease in Patients with Out-of-Hospital Refractory Ventricular Fibrillation Cardiac Arrest. J. Am. Coll. Cardiol. 2017;70:1109–1117. doi: 10.1016/j.jacc.2017.06.059. [DOI] [PubMed] [Google Scholar]
- 25.Wengenmayer T., Rombach S., Ramshorn F., Biever P., Bode C., Duerschmied D., Staudacher D.L. Influence of low-flow time on survival after extracorporeal cardiopulmonary resuscitation (eCPR) Crit Care. 2017;21:157. doi: 10.1186/s13054-017-1744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karami M., den Uil C.A., Ouweneel D.M., Scholte N.T., Engström A.E., Akin S., Lagrand W.K., Vlaar A.P., Jewbali L.S., Henriques J.P. Mechanical circulatory support in cardiogenic shock from acute myocardial infarction: Impella CP/5.0 versus ECMO. Eur. Heart J. Acute Cardiovasc. Care. 2020;9:164–172. doi: 10.1177/2048872619865891. [DOI] [PubMed] [Google Scholar]
- 27.Cheng R., Hachamovitch R., Kittleson M., Patel J., Arabia F., Moriguchi J., Esmailian F., Azarbal B. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: A meta-analysis of 1866 adult patients. Ann. Thorac. Surg. 2014;97:610–616. doi: 10.1016/j.athoracsur.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Ouattara A., Rémy A., Quessard A. ExtraCorporeal Life support for refractory cardiogenic shock: “An efficient system support of peripheral organs more than real ventricular assist device…”. Anaesth. Crit. Care Pain Med. 2018;37:195–196. doi: 10.1016/j.accpm.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Kawashima D., Gojo S., Nishimura T., Itoda Y., Kitahori K., Motomura N., Morota T., Murakami A., Takamoto S., Kyo S., et al. Left ventricular mechanical support with Impella provides more ventricular unloading in heart failure than extracorporeal membrane oxygenation. ASAIO J. 2011;57:169–176. doi: 10.1097/MAT.0b013e31820e121c. [DOI] [PubMed] [Google Scholar]
- 30.Kapur N.K., Alkhouli M.A., DeMartini T.J., Faraz H., George Z.H., Goodwin M.J., Hernandez-Montfort J.A., Iyer V.S., Josephy N., Kalra S., et al. Unloading the Left Ventricle Before Reperfusion in Patients With Anterior ST-Segment–Elevation Myocardial Infarction. Circulation. 2019;139:337–346. doi: 10.1161/CIRCULATIONAHA.118.038269. [DOI] [PubMed] [Google Scholar]