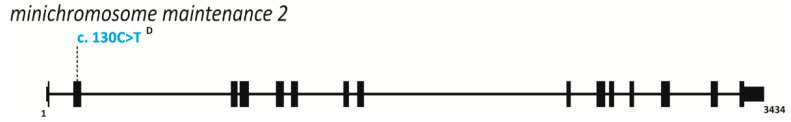Abstract
Deoxyribonucleic acid (DNA) replication can be divided into three major steps: initiation, elongation and termination. Each time a human cell divides, these steps must be reiteratively carried out. Disruption of DNA replication can lead to genomic instability, with the accumulation of point mutations or larger chromosomal anomalies such as rearrangements. While cancer is the most common class of disease associated with genomic instability, several congenital diseases with dysfunctional DNA replication give rise to similar DNA alterations. In this review, we discuss all congenital diseases that arise from pathogenic variants in essential replication genes across the spectrum of aberrant replisome assembly, origin activation and DNA synthesis. For each of these conditions, we describe their clinical phenotypes as well as molecular studies aimed at determining the functional mechanisms of disease, including the assessment of genomic stability. By comparing and contrasting these diseases, we hope to illuminate how the disruption of DNA replication at distinct steps affects human health in a surprisingly cell-type-specific manner.
Keywords: Meier-Gorlin syndrome, natural killer cell deficiency, X-linked pigmentary reticulate disorder, Van Esch-O’Driscoll disease, IMAGe syndrome, FILS syndrome, Rothmund-Thomson syndrome, Baller-Gerold syndrome, RAPADILINO
1. Introduction
1.1. Replication Initiation
Accurate and efficient replication of our genome is critical to human health and development. Deoxyribonucleic acid (DNA) replication is composed of three main processes: initiation, elongation and termination. Initiation of DNA replication can be further divided into origin licensing and origin firing. Origin licensing begins at the end of mitosis (M phase) in the previous cell cycle and continues throughout gap 1 (G1) phase [1]. Licensing is the process by which a subset of replication components is assembled at an origin of replication in preparation for the initiation of DNA synthesis. Origin firing begins at the transition from G1-to-synthesis (S) phase of the cell cycle and this marks the time when double-stranded (ds) DNA begins to melt and separate into single-stranded (ss) DNA. Interestingly, more origins are licensed than are activated during the subsequent S phase [2]. Origins that are licensed, but do not fire, are termed dormant origins and serve as back-up origins if adjacent replication forks stall and cannot be reactivated [2]. In eukaryotic cells, origins are not all fired at the same time, with origins in more transcriptionally active regions characterized by open chromatin generally firing earlier than those in less active, heterochromatic regions [3]. Recent studies suggest that distinct replisome components operate at early and late replicating origins [4]. For instance, downstream neighbor of SON (DONSON), the homolog of the Drosophila humpty dumpty gene that is thought to promote replication fork stability may preferentially associate with early replicating origins [5,6]. Both origin licensing and firing are highly regulated processes to ensure that DNA is not re-replicated.
Origin licensing begins with the assembly of the origin recognition complex (ORC) on the DNA in an ATP-dependent manner [7,8]. ORC is a hetero-hexameric complex comprised of ORC1-6 subunits that encircle the DNA as an incomplete ring (Figure 1) [8]. ORC then recruits and binds cell division cycle 6 (CDC6) protein, which fills the gap in the ORC ring structure (Figure 1) [9]. Together, ORC and CDC6 recruit chromatin licensing and DNA replication factor 1 (CDT1) and one minichromosome maintenance 2-7 (MCM2-7) complex (Figure 1) [10,11]. MCM2-7 is another hetero-hexameric ring that functions as the core of the replicative helicase and CDT1 acts to hold this ring open at the interface between MCM2 and MCM5 (the MCM2/5 gate) [12]. It is thought that the ring-like structure of ORC and CDC6 enables loading of MCM2-7 onto dsDNA [9]. Additionally, ORC induces DNA bending, which may facilitate DNA interactions with the MCM2/5 gate in MCM2-7 [8]. After the first MCM2-7 ring is loaded, a second MCM2-7 complex is recruited in a cooperative manner [13,14,15]. The exact mechanism underlying the loading of the second MCM2-7 hexamer is debated; however, a quality control mechanism ensures that both hexamer complexes are loaded in a head-to-head orientation, bringing the N-termini of each hexamer together [13,16]. In a poorly understood process, the complexes can then bypass one another [16]. This is an additional regulatory step in replication initiation requiring subsequent origin firing factors to determine which origins of replication are activated [16].
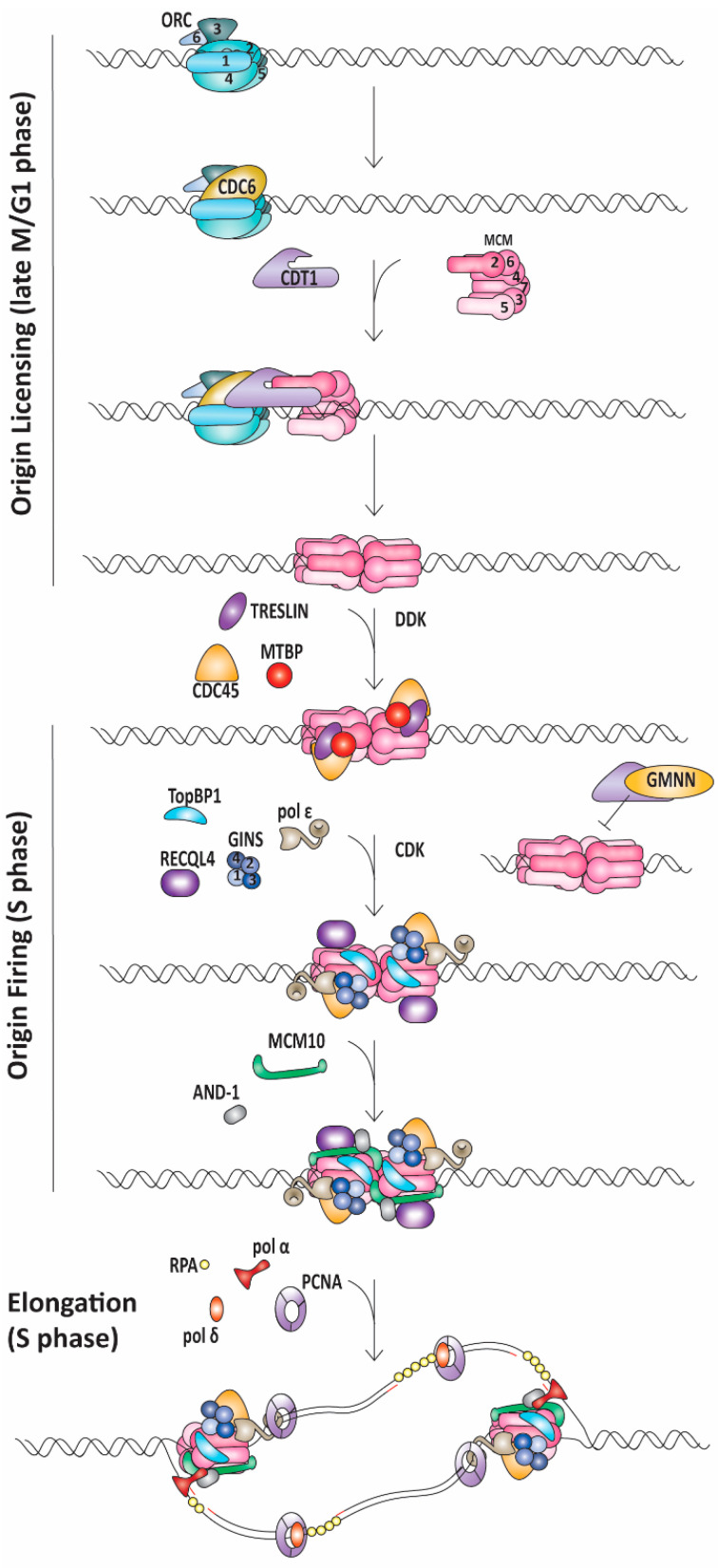
Figure 1.
Origin licensing and firing require the coordinated action of multiple protein complexes. Origin licensing and firing require the coordinated action of multiple protein complexes. This cartoon depicts the steps and essential proteins involved in DNA replication origin licensing and firing, drawing from knowledge gained by studying yeast and human systems. Origin licensing occurs in the late M and G1 phase of the cell cycle when ORC1-6 binds to origins of replication. Together, CDC6 and ORC recruit CDT1 and MCM2-7, loading two MCM2-7 complexes onto dsDNA. In budding yeast, CDT1 binds to the MCM2-7 hexamer; however, these proteins have not been identified in a soluble complex without DNA in human cells. As cells transition into the S phase, Treslin, CDC45 and MTBP are recruited in a DDK-dependent manner. Pol ε and the GINS complex are recruited together with TopBP1 and RECQL4 in a CDK-dependent manner. During the S phase, licensing of additional origins is prevented by multiple mechanisms including sequestering of CDT1 by GMNN. Activation of DNA replication or origin firing requires MCM10, which aids in the bypass of the two CMG helicases past each other. MCM10 and AND-1 anchor pol α-primase to initiate DNA synthesis. As the CMG helicases progress in opposite directions, two replication forks form with pol α-primase, pol δ, pol ε and PCNA to promote DNA synthesis. Pol ε synthesizes the leading strand, while RPA binds single-stranded DNA on the lagging strand template until Okazaki fragments are produced by the consecutive action of pol α-primase and pol δ. Abbreviations: ORC, origin recognition complex; CDC, cell division cycle; CDT1, chromatin licensing and DNA replication factor 1; MCM, minichromosome maintenance; MTBP, Mdm2-binding protein; DDK, Dbf4-dependent kinase; GINS, go-ichi-ni-san; TopBP1, DNA topoisomerase II binding protein 1; RECQL4, ATP-dependent DNA helicase Q4; CDK, cyclin-dependent kinases; GMNN, geminin; AND-1, acidic nucleoplasmic DNA-binding protein 1; PCNA, proliferating cell nuclear antigen; RPA, replication protein A.
Origin licensing and subsequent firing are tightly controlled by cyclin-dependent kinases (CDKs) to prevent re-replication after DNA synthesis is initiated (Figure 1) [1]. To be active, CDKs must bind a cyclin partner which also directs substrate specificity. Cyclin expression varies in a cell-cycle-dependent manner. In yeast, a single CDK partners with specific cyclins to regulate each cell cycle transition, whereas in humans, multiple cyclin/CDK pairs govern each phase of the cell cycle [1]. Both in yeast and humans, the active S phase CDKs prevent re-replication by phosphorylating components of the licensing machinery, including the ORC subunits, CDC6 and CDT1 [1]. This phosphorylation leads to protein degradation or exclusion from the nucleus, thus preventing licensing of new origins after the G1-to-S phase transition [1]. CDT1 is further controlled by Geminin (GMNN) [17]. GMNN is a protein that binds CDT1 and prevents its interaction with the MCM2-7 complex, thereby inhibiting origin licensing (Figure 1) [17]. GMNN levels fluctuate with cell cycle progression as well [18]. Specifically, GMNN is degraded during M phase and then slowly accumulates in S phase when its abundance is sufficiently high to sequester CDT1 [1,17,18].
As cells progress into S phase, firing factors assemble at replication origins to allow for the initiation of DNA unwinding and synthesis. The proteins that catalyze DNA replication are highly conserved across eukaryotes and much of the field’s understanding comes from studying model organisms, such as Saccharomyces cerevisiae. Many of these findings are consistent with observations in human cells and we will note where the two systems diverge. Utilizing purified proteins, Yeeles and coworkers demonstrated which firing factors were required for DNA replication initiation in an in vitro system [19]. These essential firing factors are DNA polymerase B II (Dpb11), synthetically lethal with Dpb11 2 (Sld2), Sld3, Sld7, cell division cycle 45 (CDC45), go-ichi-ni-san (GINS) complex, DNA polymerase epsilon (pol ε) and MCM10 [19]. Sld3, Sld7, Sld2 and Dpb11 do not have homologous genes in humans; however, they have orthologs or analogs: Treslin, Mdm2-binding protein (MTBP), ATP-dependent DNA helicase Q4 (RECQL4) and DNA topoisomerase II binding protein 1 (TopBP1), respectively (Figure 1) [20,21,22,23,24].
The recruitment of these factors is regulated by S phase CDK and Dbf4-dependent kinase (DDK) [1,19,25,26]. As cells transition into S phase, DDK accumulates and phosphorylates MCM2, 4 and 6 [1,19,26]. These phosphorylations likely lead to a conformational change, facilitating the recruitment of CDC45 and Sld3/Sld7 [19,27]. Sld3/Sld7 are needed to stabilize CDC45 binding to MCM2-7 until the GINS complex is recruited with Sld3, Sld2, Dpb11 and pol ε following S-CDK phosphorylation of Sld3 and Sld2 [19,28,29]. Similarly, in humans, TopBP1 and MTBP interact with Treslin in a CDK-dependent manner and are required for CDC45 recruitment and stabilization [23,24,30,31]. Unlike the role that Sld2 plays in GINS recruitment in yeast, RECQL4 has not been shown to interact with GINS but is required for replication initiation, possibly by stabilizing the CMG complex after GINS loading [22,31,32]. Furthermore, whereas Sld3, Sld7, Sld2 and Dpb11 are thought to dissociate from the replication fork after DNA melting, in humans, TopBP1 continues to travel with the elongating replication fork (Figure 1) [33].
Both CDC45 and the GINS complex are helicase coactivators. With the loading of the GINS complex, the CDC45-Mcm2-7-GINS (CMG) helicase is completely assembled, and a conformational change occurs in which each CMG complex encircles ssDNA by extruding the lagging strand template [34,35]. It remains unclear if the same MCM2/5 gate that facilitates dsDNA loading also serves as the ssDNA gate for lagging strand template extrusion [34,35,36]. What is known is that CDC45 and the GINS complex bind across the MCM2/5 gate and may be needed to facilitate ssDNA extrusion or prevent leading strand template escape [34,35,36]. MCM10 is required for significant ssDNA generation and subsequent replication protein A (RPA) binding (Figure 1) [37,38,39]. MCM10′s role in generating ssDNA during origin firing may be to enable the head-to-head oriented CMG complexes to separate and slide past each other to initiate unwinding [40]. As DNA replication progresses, topoisomerase II eases super coiling caused by strand unwinding [19]. With origin firing complete, the two nascent forks move away from the origin and MCM10 remains associated with CMG during elongation [41].
1.2. Replication Elongation
Elongation is the process in which DNA polymerases catalyze the formation of the phosphodiester bond between the 3′ hydroxyl group of the growing strand and the 5′ phosphate group of the incoming nucleotide while using the parental strand as a template. DNA polymerases add nucleotides to a primed template in the 5′ to 3′ direction [42]. As the CMG helicase unwinds parental DNA, the strand with 3′ to 5′ polarity serves as the leading stand template and can be duplicated continuously. The other strand, the lagging strand template, is copied in a discontinuous manner by the repeated synthesis of Okazaki fragments. For DNA synthesis to begin, MCM10 and acidic nucleoplasmic DNA-binding protein 1 (AND-1) recruit polymerase alpha-primase (pol α-primase), which synthesizes a short ribonucleic acid (RNA)-DNA primer (Figure 1) [43,44,45]. On the lagging strand, replication factor C (RFC) recognizes the ds-to-ss junction at the RNA-DNA primer:template with a recessed 3′ end as a substrate onto which it loads proliferating cell nuclear antigen (PCNA), the tether for DNA polymerase delta (pol δ) (Figure 1) [19,46,47]. Pol ε is loaded onto DNA before origin firing and is already present at the replication fork to take over synthesis of the leading strand [43]. Further processing of Okazaki fragments is needed to remove the RNA primers. This occurs when pol δ, aided by PCNA, encounters the previous Okazaki fragment and displaces the RNA portion to generate a 5′ flap [48,49,50]. This displaced 5′ flap can then be cleaved by flap endonuclease 1 (FEN1) or DNA replication helicase/nuclease 2 (DNA2) depending on the length of the 5′ flap [48,49,51,52]. The flap cleavage results in a DNA nick that is sealed by DNA ligase 1 [53].
1.3. Replication Termination
Termination of replication occurs when two forks converge. One proposed model for termination has CMG helicases pass each other and transition from encircling ssDNA to dsDNA when they reach the 5′ end of the previously synthesized Okazaki fragment [54,55]. Synthesis of the leading strand is completed when pol ε reaches this fragment shortly thereafter. The replisome then needs to be disassembled. CRL2Lrr1 (a Cul2-based RING E3 ubiquitin ligase) polyubiquitylates MCM7, leading to CMG unloading by CDC48/p97 segregase [56,57]. The CMG components are not degraded but rather recycled [58]. Currently, there are no known congenital diseases caused by disruption of replication termination.
As summarized above, DNA replication is a multistep process involving a multitude of protein complexes. In this review, we describe inherited genetic diseases caused by defects of proteins essential for DNA replication. For each disease, we describe the clinical presentation, report associated genetic mutations and discuss studies of their pathophysiologies. As we explore diseases caused by defects in the core replication machinery, we highlight both expected commonalities, such as severe growth defects, as well as tissue-specific abnormalities. Interestingly, mutations in individual replication factors can cause glaringly unique and disease-specific symptoms. The field of DNA replication has yet to fully understand why mutations in different DNA replication proteins cause these diverse symptoms. We propose that different cell types have specific thresholds for replication factors that govern proper differentiation. Specifically, each disease and associated genetic defect can be broadly grouped into two categories that display some homogeneity in symptomology—defects in origin licensing or defects in origin firing/elongation.
2. Diseases
2.1. Meier-Gorlin Syndrome Is Caused by Defects in Origin Licensing
Meier-Gorlin syndrome (MGS) is an osteodysplastic syndrome that causes primordial proportional dwarfism. In addition to growth retardation, microtia (small ears) and aplastic or hypoplastic patella comprise the three core clinical findings in MGS (Table 1) [59]. The first patient was described by Meier and coworkers in 1959, with the second described in 1975 by Gorlin et al. [60]. Several other case reports soon followed under descriptive titles such as small ear syndrome or ear, patellae, short stature syndrome [61,62,63]. In 1994, the name MGS was proposed to identify patients with the triad of symptoms described above and separate them from other primordial dwarfism disorders [64]. As MGS is characterized by osteodysplasia, early reports by Gorlin hypothesized that the genetic cause lay in genes that direct bone development [65,66]. However, it was not until 2011 that the genetic cause of this syndrome was determined to be mutations in various origin licensing factors including ORC1, ORC4, ORC6, CDT1 and CDC6 (Figure 2 and Figure 3) [67,68]. Later studies have found causative mutations in CDC45, MCM5, GMNN and DONSON (Figure 4 and Figure 5; for complete list of mutations including DNA and protein change, see Supplementary Table S1) [5,69,70,71].
Table 1.
Common symptoms occurring in diseases caused by defects in DNA replication. This table compiles common symptoms of diseases discussed in this review. A more exhaustive list of symptoms can be found in Supplementary Table S2.
| Gene(s) Affected | MIM ID | Inheritance | IUGR | Short Stature | Microcephaly | Craniosynostosis | Facial Features | Skin Changes | Radial Ray Defects | A/Hypoplastic Patella | Adrenal Insufficiency | GU Abnormalities (male) | ID | DD | Hearing Loss | Immune Defects | Anorectal Malformation | Feeding Difficulties | Diarrhea | Premature Aging | Lipodystrophy | Cancer Predisposition | Other symptoms | References | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MGS | ORC1 | 224690 | AR | x | x | x | microtia microstomia full lipsret rognathia micrognathia |
x | x | x | x | [67,68,72,73,74] | |||||||||||||
| ORC4 | 613800 | AR | x | x | x | x | x | [67,68,72] | |||||||||||||||||
| ORC6 | 613803 | AR | x | x | x | x | x | x | [67,72,75] | ||||||||||||||||
| CDT1 | 613804 | AR | x | x | x | x | x | x | [67,68,72,76] | ||||||||||||||||
| CDC6 | 613805 | AR | x | x | x | x | x | [72,76] | |||||||||||||||||
| MCM5 | 617564 | AR | x | x | x | x | [70] | ||||||||||||||||||
| CDC45 | 617063 | AR | x | x | x | x | x | x | x | x | [69,77] | ||||||||||||||
| GMNN | 616835 | AD | x | x | x | x | x | x | [71] | ||||||||||||||||
| DONSON | AR | x | x | x | x | x | x | [76,78] | |||||||||||||||||
| NKD | MCM4 | 609981 | AR | x | x | x | large forehead, thin upper lip | x | x | x | x | +/− | [79,80,81,82] | ||||||||||||
| GINS1 | 617827 | AR | x | x | flat nasal bridge, round nose tip, blepharophimosis, posteriorly rotated ears, thin upper lip | E | x | +/− | [83] | ||||||||||||||||
| MCM10 | AR | UR | UR | none noted | x | [84,85] | |||||||||||||||||||
| XLPRD | POLA1 | 301220 | XLR | Blepharophimosis, flared eyebrows, upswept hair | x | x | sterile multiorgan inflammation | [86,87,88,89,90] | |||||||||||||||||
| VEODS | POLA1 | 301030 | XLR | x | x | inconsistent | x | x | +/− | [91,92] | |||||||||||||||
| PRIM1 | AR | x | x | prominent forehead, triangular face, blepharophimosis, micrognathia, small-low set ears | x | x | x | x | x | [93] | |||||||||||||||
| FILS | POLE1 | 615139 | AR | x | malar hypoplasia high forehead | L | x | [94,95,96] | |||||||||||||||||
| IMAGe | POLE1 | 618336 | AR | x | x | x | Micrognathia, long thin nose, short wide neck, small low-set, posteriorly rotated ears | other | x | x | x | x | +/− | [97] | |||||||||||
| POLE2 | AR | x | low anterior hairline, flat supraorbital ridges, downturned corners of mouth, short philtrum | x | [98] | ||||||||||||||||||||
| MDP | POLD1 | 615381 | AD | x | mandibular hypoplasia, pinched nose, microstomia, bird like facies, prominent eyes | T | x | x | x | x | [99,100,101,102,103,104,105,106,107] | ||||||||||||||
| POLD1 | AR | x | none noted | x | x | x | [108,109,110] | ||||||||||||||||||
| POLD2 | AR | x | x | none noted | x | x | x | [108] | |||||||||||||||||
| PCNA | 615919 | AR | x | none noted | T | x | x | x | x | neurodegeneration, ataxia | [111] | ||||||||||||||
| BGS | RECQL4 | 218600 | AR | x | x | x | present but variable | P | x | x | x | x | x | x | [112,113,114,115,116] | ||||||||||
| RAPA | RECQL4 | 266280 | AR | x | x | long face, long slender nose, narrow palpebral fissures, arched palate | x | x | +/− | x | x | x | [112,117,118,119] | ||||||||||||
| RTS | RECQL4 | 268400 | AR | x | x | x | present but variable | T, P | x | x | x | +/− | x | x | x | x | alopecia | [112,114,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141] | |||||||
| MCM2 | 616968 | AD | none noted | x | [142] | ||||||||||||||||||||
MIM ID, Mendelian Inheritance in Man identification number; IUGR, intrauterine growth restriction; ID, intellectual disability; DD, developmental delay; VEODS, Van Esch–O’Driscoll syndrome; RAPA, RAPADILINO; MGS, Meier-Gorlin; NKD, natural killer cell deficiency; XLPRD, X-linked pigmentary reticulate disorder; FILS, facial dysmorphism, immunodeficiency, livedo and short stature; IMAGe, intrauterine growth restriction, metaphyseal dysplasia, adrenal hypoplasia congenita and genitourinary abnormalities syndrome; MDP, mandibular hypoplasia, deafness, progeroid features; BGS, Baller–Gerold; RTS, Rothmund–Thomson;AR, autosomal recessive; AD, autosomal dominant; XLR, X-linked recessive; UR, unreported; E, eczema; L, livedo; T, telangiectasia; P, poikiloderma.
Figure 2.
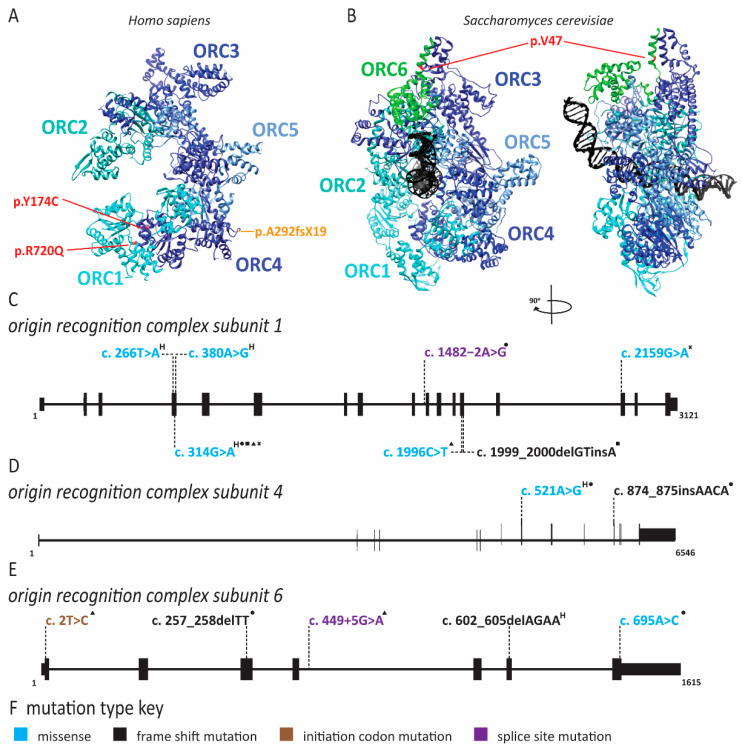
Mutations in origin recognition complex (ORC) subunits cause Meier-Gorlin syndrome. Pathogenic mutations in ORC subunits are depicted on a schematic of each gene and the crystal structure of either Homo sapiens or Saccharomyces cerevisiae proteins. Mutations that do not have corresponding amino acids in the structure or resulted in abnormal splicing are not depicted. For a complete list of mutations and associated protein changes, see Supplementary Table S1. Structures were generated with the Chimera program (http://www.cgl.ucsf.edu/chimera). Exon and intron schematics were generated with the UCSD genome browser. Reference sequences are ORC1 NM_004153.4, ORC4 NM_181742.4 and ORC6 NM_014321.4. (A) Crystal structure of human ORC subunits with mutations depicted, including missense mutations (red) and frame shift mutations (orange). The structure was generated using pdb file 5UJM. (B) Alignment of Homo sapiens and Saccharomyces cerevisiae ORC6 was completed using UniProt alignment tool (https://www.uniprot.org/align/). The amino acid corresponding to missense mutation p.Y232S (c.695A > C) is identified in red on the crystal structure of yeast ORC6. The structure was generated using pdb file 5zr1. Schematic of (C) ORC1, (D) ORC4 and (E) ORC6 genes depicted with exons as black boxes and introns as horizontal lines. Mutations are mapped to the appropriate gene regions. “H” superscript indicates a homozygous mutation. Compound heterozygous mutation pairs are indicated by superscript symbols. Each mutation combination is depicted, and certain alleles may be present in multiple combinations such as ORC1 c.314G > A. The frequency of each allele in the affected population is not indicated. (F) For each gene, the color of the mutations indicates the type of mutation according to this key.
Figure 3.
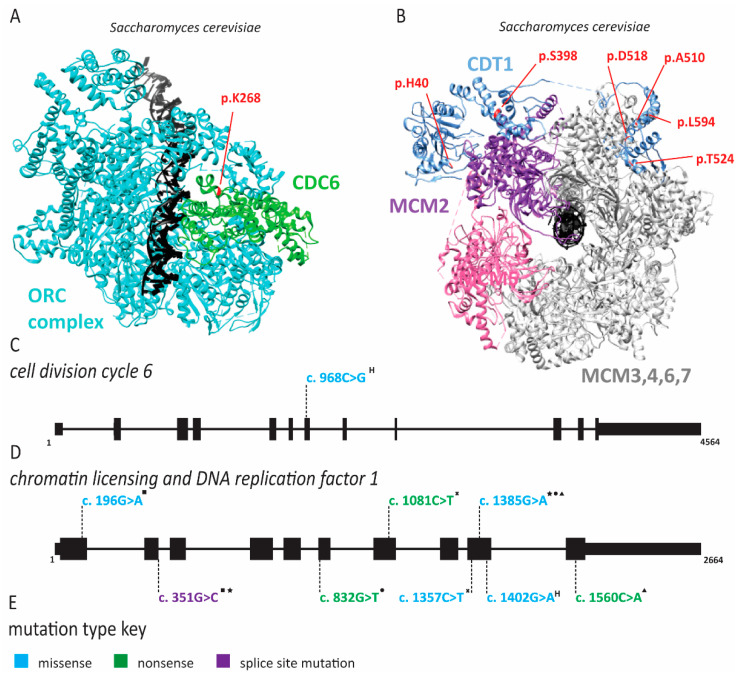
Mutations in chromatin licensing and DNA replication factor 1 (CDT1) and cell division cycle 6 (CDC6) cause Meier-Gorlin syndrome. Pathogenic mutations of CDC6 and CDT1 are depicted on a schematic of each gene and crystal structure of Saccharomyces cerevisiae proteins. Mutations that do not have corresponding amino acids in the structure or resulted in abnormal splicing are not depicted. For a complete list of mutations and associated protein changes, see Supplementary Table S1. Alignment of Homo sapiens and Saccharomyces cerevisiae genes was completed using UniProt alignment tool (https://www.uniprot.org/align/). The structure was generated using pdb file 5v8f and the Chimera program (http://www.cgl.ucsf.edu/chimera). Exon and intron schematics were generated with the UCSD genome browser. Reference sequences are CDC6 NM_001254.4 and CDT1 NM_030928.4. (A). Crystal structure of yeast CDC6-ORC-MCM2-7-CDT1 complex (MCM2-7 and CDT1 not shown) with the amino acid associated with missense mutation p.T323R (c.968C > G) is depicted. (B) Crystal structure of yeast CDC6-ORC-MCM2-7-CDT1 complex (CDC6 and ORC not shown) with amino acids corresponding to missense and nonsense mutations in the human protein identified in red on the crystal structure of yeast CDT1. Schematic of (C) CDC6 and (D) CDT1 genes depicted with exons as black boxes and introns as horizontal lines. Mutations are mapped to the appropriate gene regions. “H” superscript indicates a homozygous mutation. Compound heterozygous mutation pairs are indicated by superscript symbols. Each mutation combination is depicted, and certain alleles may be present in multiple combinations. The frequency of each allele in the affected population is not indicated. (E) For each gene, the color of the mutations indicates the type of mutation according to this key.
Figure 4.
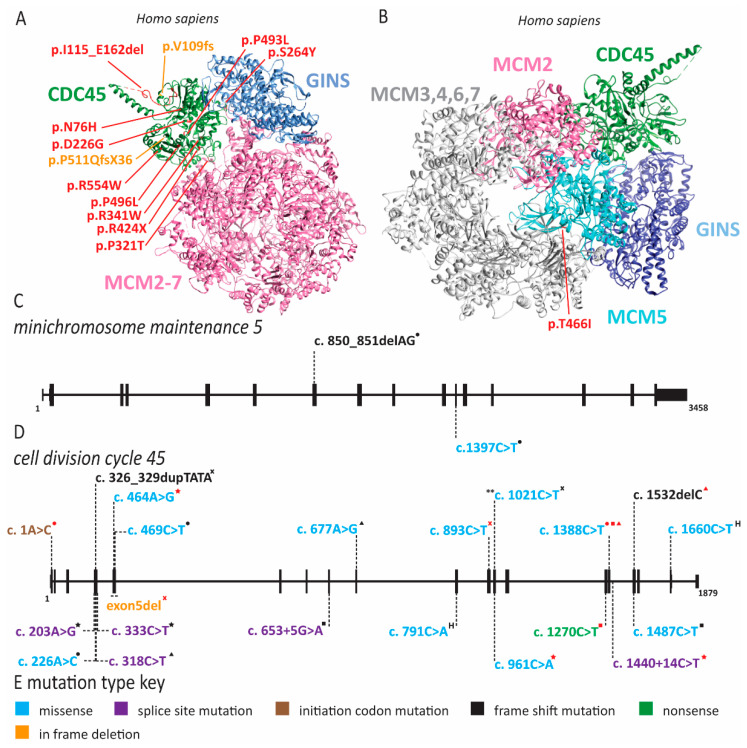
Mutations in helicase component minichromosome maintenance 5 (MCM5) and cell division cycle 45 (CDC45) cause Meier-Gorlin syndrome. Pathogenic mutations of MCM5 and CDC45 are depicted on a schematic of each gene and the crystal structure of Homo sapiens proteins. Mutations that do not have corresponding amino acids in the structure or resulted in abnormal splicing are not depicted. For a complete list of mutations and associated protein changes, see Supplementary Table S1. The structure was generated using pdb file 6xtx and the Chimera program (http://www.cgl.ucsf.edu/chimera). Exon and intron schematics were generated with the UCSD genome browser. Reference sequences are CDC45 NM_003504.4 and MCM5 NM_006739.4. (A) Crystal structure of human CDC45 with mutations depicted including missense mutations (red) and frame shift mutations (orange). Note p.115_E162del is a large deletion of exon 5, with all lost amino acids highlighted in red. (B) Crystal structure of human MCM5 with missense mutation p.T466I (c.1397C > T) depicted in red. Schematic of (C) MCM5 and (D) CDC45 genes depicted with exons as black boxes and introns as horizontal lines. Mutations are mapped to the appropriate gene regions. “H” superscript indicates a homozygous mutation. ** denotes different than reported due to different reference sequence. Compound heterozygous mutation pairs are indicated by superscript symbols. Each mutation combination is depicted, and certain alleles may be present in multiple combinations. The frequency of each allele in the affected population is not indicated. (E) For each gene, the color of the mutations indicates the type of mutation according to this key.
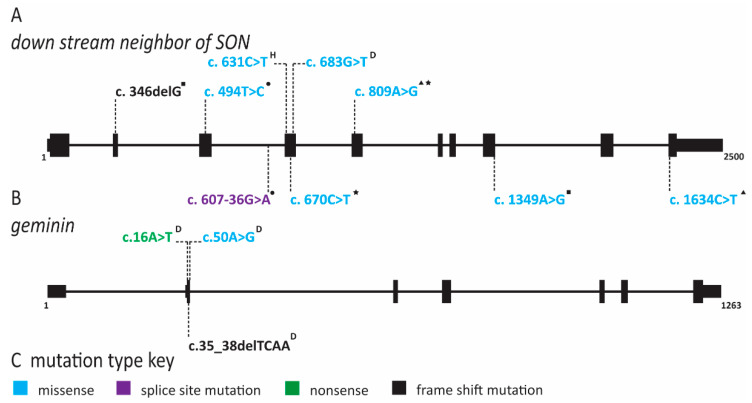
Figure 5.
Mutations in downstream neighbor of son (DONSON) and geminin (GMNN) cause Meier-Gorlin syndrome. Exon and intron schematic was generated with the UCSD genome browser. Reference sequences are DONSON NM_017613.4 and GMNN NM_015895.5. Schematic of (A) DONSON and (B) GMNN genes depicted with exons as black boxes and introns as horizontal lines. Mutations are mapped to the appropriate gene regions. “H” superscript indicates a homozygous mutation. “D” superscript indicates a heterozygous dominant mutation. Compound heterozygous mutation pairs are indicated by superscript symbols. Each mutation combination is depicted, and certain alleles may be present in multiple combinations. The frequency of each allele in the affected population is not indicated. (C) For each gene, the color of the mutations indicates the type of mutation according to this key.
Growth retardation in MGS can range from mild to severe. ORC1 and ORC4 mutations cause the most severe growth defects [72,73,143,144]. In addition to short stature, microtia and hypo/aplastic patellae, MGS patients have distinctive facial features including microstomia, full lips, retrognathia and micrognathia (Table 1) [72,75]. Intellect appears to be normal although there may be delayed motor or speech development [72]. Feeding problems are prevalent in approximately 80% of patients and respiratory tract anomalies occur in nearly half of MGS patients (Table 1 and Supplementary Table S2) [72]. Additionally, while only 7% of MGS patients have a congenital cardiac anomaly, it is advised that cardiac screening be undertaken if a diagnosis of MGS is made (Supplementary Table S2) [72]. As children with MGS grow into adulthood, they can develop conductive hearing loss due to microtia, arthritis in the knee due to absent patella and pain due to general joint hypermobility (Supplementary Table S2) [72,144]. Fertility of patients with MGS remains unclear and may be decreased in males born with cryptorchidism and in females with small uteri and polycystic ovaries [72,143]. Additionally, there is abnormal secondary sex development with sparce axillary hair in both sexes and hypoplastic mammary tissue in women (Supplementary Table S2) [144].
In 2013, Stiff and coworkers assessed immortalized fibroblasts from an MGS patient with ORC1 mutations as well as lymphoblastoid cell lines derived from patients with ORC1, ORC4, ORC6, CDT1 and CDC6 mutations to understand the etiology of MGS [145]. Epstein–Barr virus (EBV) replication, which requires highly efficient origin licensing, was reduced in these patient-derived cells [74]. In 2017, the Meng laboratory demonstrated that zebrafish models of MGS exhibited delayed S phase progression and increased apoptosis [146]. Furthermore, only some tissue types were affected, suggesting that the effects are cell-type-specific [146]. Similarly, several CDT1 mutations identified in MGS patients slowed origin licensing and delayed entry into the S phase [10]. Interestingly, one hypermorphic allele caused re-replication, replication stress and impaired proliferation [10]. Studies of fibroblasts from ORC1 patients also demonstrated delayed S phase entry and progression due to impaired origin licensing [146]. Additional biochemical studies of Drosophila ORC6 have demonstrated that mutation of Y232 resides within a highly conserved helix that facilitates interactions with ORC3 [147]. ORC6 plays an important role in recruiting ORC to DNA, and without this interaction, ORC complex binding to DNA and subsequent MCM2-7 loading is diminished [147]. Similarly, disruption of the bromo-adjacent homology domain in the N-terminus of ORC1 (as in c.266T > A, c.314G > A, and c.380A > G), which mediates its interaction with histone H4 dimethylated at lysine 20, disrupts ORC assembly on DNA [148,149].
Stiff and colleagues noted that other primordial dwarfism syndromes such as Seckel syndrome have been linked to defects in ciliogenesis [145,150]. Furthermore, Seckel syndrome (SS) can be caused by mutations in ataxia-telangiectasia and Rad3-related protein (ATR), a kinase critical for activation of the DNA damage checkpoint [151]. The related functions of MGS pathogenic variants in DNA replication and SS pathogenic variants in sensing replication stress could indicate a common etiology for these two primordial dwarfisms. When fibroblasts were depleted for ORC1, ORC4, ORC6, CDT1 and CDC6, primary cilia formation was impaired, suggesting that MGS symptoms might constitute a ciliopathy [145]. However, MGS patients do not have the cognitive impairment, abnormal neurogenesis or renal defects that are often present in primary ciliopathies [152,153]. Thus, it remains an open question as to whether a direct link exists between MGS and aberrant cilia formation. Following the original studies identifying mutations in ORC, CDC6 and CDT1 as causing MGS, additional reports revealed MCM5, CDC45, GMNN and DONSON variants in MGS patients (Figure 4 and Figure 5) [5,69,70,71,76,77,78]. One patient was identified with a compound heterozygous mutation in MCM5 resulting in a reduction in MCM5 and MCM2 on chromatin, consistent with an inability to stably load MCM2-7 onto chromatin (Figure 4 and Supplementary Table S1) [70]. Moreover, fifteen patients with biallelic CDC45 mutations, including both compound heterozygous or homozygous variants, which conferred varying degrees of protein dysfunction and depletion were described (Figure 4 and Supplementary Table S1) [69,77]. Interestingly, CDC45 MGS patients, in addition to the traditional triad of MGS symptoms, were more likely to have craniosynostosis and anorectal malformations (Table 1 and Supplementary Table S2) [69,77]. Patients carrying heterozygous GMNN mutations are afflicted by an autosomal dominant form of MGS (Figure 5 and Supplementary Table S1) [71]. All mutations produce a N-terminally truncated protein using an alternate start codon [71]. The use of this later start codon resulted in a highly stabilized protein that decreased DNA replication due to increased CDT1 inhibition [18,71,154]. It remains unclear whether mutations in DONSON represent true MGS or represent a related syndrome [5,77,153]. Patients with DONSON mutations present with primordial dwarfism, but not all have the classic triad of MGS symptoms [5,76,78]. Molecular studies of DONSON demonstrated a function in DNA replication fork stabilization rather than DNA replication initiation [5]. However, due to the lack of patient samples and appropriate models, it remains unclear whether DONSON mutations that phenotypically mimic MGS also have origin licensing defects. When taking into account all the genes found to be mutated in MGS patients, it seems clear that the disease phenotype is due to defects in DNA replication, although the connections to impaired cilia formation cannot be ruled out as a contributing factor.
2.2. MCM2 Deficiency and Familial Deafness
The Liu laboratory identified a missense variant in MCM2 that segregated in an autosomal dominant manner with sensorineural hearing loss in a large family cohort (Figure 6) [142]. This missense variant changes highly conserved R44C (c.130C > T) (Figure 6) [142]. Hearing loss in this family was variable for onset, bilateral and progressive [93]. MCM2 was expressed at low levels in various parts of the cochlea [142]. In hair cells specifically, most of the MCM2 protein was found in the cytoplasm, consistent with the non-proliferative state of these cells [142]. As previously reported, overexpression of MCM2 in HEK293 cells resulted in increased apoptosis [142]. Apoptosis further increased with overexpression of the R44C variant [142]. This suggests that accumulation of the R44C variant in the cytoplasm of hair cells may be sufficient to induce apoptosis, a known mechanism for hearing loss [142,155].
Figure 6.
Minichromosome maintenance 2 heterozygous variant is linked to familial deafness. Exon and intron schematic was generated with the UCSD genome browser. Reference sequence is MCM2 NM_004526.4. Schematic of MCM2 gene is depicted with exons as black boxes and introns as horizontal lines. Missense mutation is mapped to the appropriate gene regions. “D” superscript indicates a heterozygous dominant mutation.
2.3. Natural Killer Cell Deficiency Is Caused by Defects in Origin Licensing or Initiation Factors and Is Associated with Genome Instability
The human immune system is broadly divided into the innate and the adaptive immune system. The adaptive immune system has high specificity for pathogens and includes humoral components such as T lymphocytes (T cells), which recognize pathogens through their diverse T cell receptors, and the antibodies produced by B lymphocytes (B cells). Conversely, the innate immune system is not specific for individual pathogens and includes a third class of lymphocytes, the natural killer (NK) cells [156]. NK cells have multiple activating receptors including cluster of differentiation (CD)16, a receptor for the fragment crystallizable region (Fc) of antibodies [156]. They also express inhibitory receptors called killer immunoglobulin-like receptors (KIRs) that recognize major histocompatibility complex (MHC) class I molecules [156]. KIRs equip NK cells to target and kill virally infected or tumor cells that elude T cell recognition due to the downregulation of MHC molecules [156]. In addition to their cytotoxic activity, NK cells have important roles in immune-modulation through the secretion of cytokines such as interferon-γ and tumor necrosis factor-α [156]. NK cells develop from the same common lymphoid progenitor as B and T cells and go through a multistep process of maturation primarily in the bone marrow [156]. One model of NK cell development divides the process into five stages, with stage 5 being the fully mature cytotoxic NK cell [156,157,158,159]. Both stage 4 and 5 NK cells are present in the peripheral blood. NK cells range between 3 and 30% of all lymphocytes within the peripheral blood and stage 5 NK cells are nine times more abundant than stage 4 NK cells [160,161,162].
Natural killer cell deficiency (NKD) is a rare primary immune deficiency affecting specifically NK cells. NKD results in increased susceptibility to common pathogens and has severe effects on health and lifespan. NKD is further subdivided into functional (FNKD) and classical NKD (CNKD). FNKD is the presence of normal levels of NK cells in peripheral blood that are not able to induce cell lysis. CNKD is caused by a lack of NK cells in peripheral blood to less than 1% of total lymphocytes [163]. CNKD can be caused by pathogenic variants in DNA replication factor genes, suggesting an unexplored connection between NK cell development and DNA replication. The genes affected in individuals with CNKD are MCM4, GINS1 and MCM10, suggesting that the disease is caused by defects in origin licensing and/or firing (Figure 7) [84,160,163].
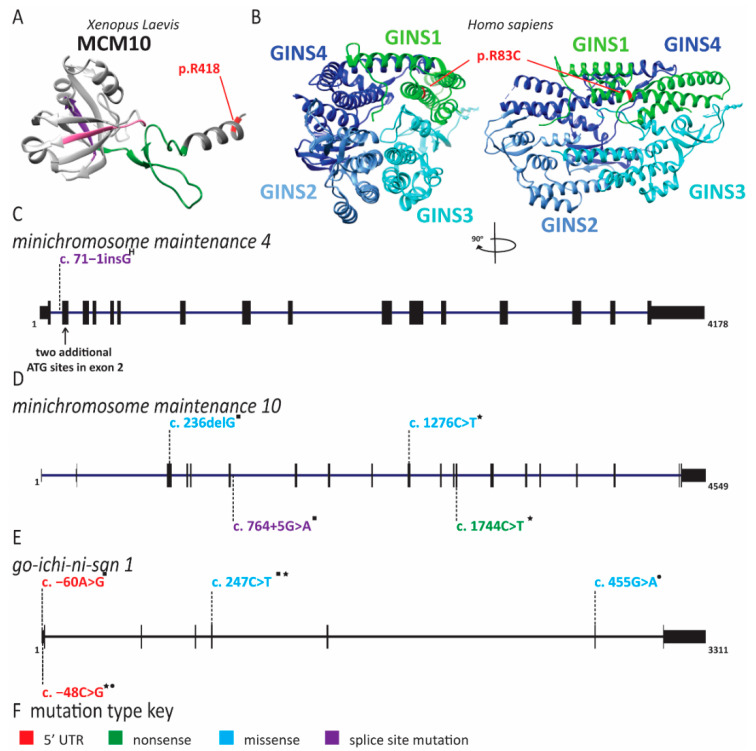
Figure 7.
Natural killer cell deficiency is caused by pathogenic variants disrupting DNA replication initiation. Pathogenic mutations in replication initiation factors are depicted on a schematic of each gene and the crystal structure of either Homo sapiens or Xenopus laevis proteins. Mutations that do not have corresponding amino acids in the structure or resulted in abnormal splicing are not depicted. For a complete list of mutations and associated protein changes, see Supplementary Table S1. Structures were generated with the Chimera program (http://www.cgl.ucsf.edu/chimera). Exon and intron schematics were generated with the UCSD genome browser. Reference sequences are MCM4 NM_005914.4, MCM10 NM_018518.5 and GINS1 NM_021067.5. (A) Alignment of Homo sapiens and Xenopus laevis MCM10 was completed using UniProt alignment tool (https://www.uniprot.org/align/). Crystal structure of the internal domain of Xenopus laevis MCM10 is shown with PCNA binding peptide box in pink, heat shock protein 10 like domain in purple, and the first zinc finger in green. The Xenopus laevis amino acid corresponding to missense mutation p.R426C (c.1276C > T) is depicted in red. The structure was generated using pdb file 3ebe. (B) Missense mutation p.R83C (c.247C > T) in GINS1 is identified in red on the crystal structure of GINS complex. The structure was generated using pdb file 2q9q. Schematic of (C) MCM4, (D) MCM10 and (E) GINS1 genes depicted with exons as black boxes and introns as horizontal lines. Mutations are mapped to the appropriate gene regions. For MCM4, two additional ATG sites that result in shorter isoforms occur in exon 2. “H” superscript indicates a homozygous mutation. Compound heterozygous mutation pairs are indicated by superscript symbols. Each mutation combination is depicted, and certain alleles may be present in multiple combinations. The frequency of each allele in the affected population is not indicated. (F) For each gene, the color of the mutations indicates the type of mutation according to this key.
The MCM4 CNKD cohort is the largest and arises in a genetically isolated Irish traveler population [79,80,81]. In addition to CNKD, affected individuals in this cohort have adrenal insufficiency, pre- and post-natal growth retardation and unique facial features (Table 1) [79,80,81,82,164]. When NK cell populations were analyzed from these patients, a dramatic lack of stage 5 NK cells was noted [80]. Whereas the amount of stage 4 cells within the peripheral blood was normal, these cells were unable to be expanded ex vivo with cytokine stimulation and underwent spontaneous apoptosis likely due to increased chromosome breakage [80]. Within the MCM4 cohort, CNKD is inherited in an autosomal recessive manner with the gene defect ablating a splice site in an early exon generating a premature stop codon (Figure 7 and Supplementary Table S1) [79,80,81]. Despite this splice site mutation, mature MCM4 mRNA can be detected at levels comparable to healthy cells [80]. Furthermore, two proteins smaller than full-length MCM4 were expressed, corresponding to two naturally occurring isoforms of MCM4 that are initiated at two separate start sites downstream of the splice site mutation and resulting premature stop codon [80]. These two MCM4 isoforms did not appear to affect MCM2-7 stability on chromatin; however, they were not sufficient to allow normal cell cycle progression in immortalized patient fibroblasts which arrested in G2/M phase. This indicates that the full-length MCM4 is necessary for normal origin firing and elongation but not for origin licensing. Patient cells also had increased DNA content, demonstrating that the N-terminal region of full-length MCM4 is required for the appropriate regulation of DNA synthesis and prevention of re-replication [80]. When treated with aphidicolin, a DNA replication inhibitor, these cells exhibited increased chromosomal breakage [80]. Similarly, cells obtained from mice with a hypomorphic MCM4 allele also had increased chromosome breaks after aphidicolin treatment [165]. Regions that undergo breaks with aphidicolin treatment often correspond to common fragile sites; however, follow-up studies to identify the chromosomal location of these breaks in MCM4 patients were not described [80,166]. The increase in genomic instability seen with a hypomorphic MCM4 allele led to an increased cancer rate in the mouse [165]. It is unclear if MCM4 pathogenic variants lead to cancer in humans as well. Some reports of cancers have been made within the MCM4 cohort, but all of them could be attributable to viral transformation secondary to NK cell depletion [167]. At the very least, this increase in DNA content and DNA breaks with replication stress indicates that these hypomorphic MCM4 proteins are unable to perform efficient DNA replication and prevent re-replication.
Unlike the MCM4 cohort, the GINS1 cohort is composed of five individuals from four unrelated families and presented with a slightly different phenotype [83]. These individuals have pre- and post-natal growth retardation and unique facial characteristics, eczema and lymphadenopathy (Table 1 and Supplementary Table S2) [83]. They do not have adrenal insufficiency [83]. Other clinical presentations that were reported include features of premature aging, hypothyroidism, protein-losing enteropathy [83], diffuse osteopenia and low amounts of CD8+ T cells [83]. All patients had a near complete lack of both stage 4 and stage 5 NK cells, as well as chronic neutropenia. Interestingly, upon stimulation, as in bacterial infection, neutrophil numbers could be restored [83]. Each patient had a compound heterozygous variant of the GINS1 gene (Figure 7 and Supplementary Table S1) [83]. Among the five patients, four unique variants, two missense mutations (c.247C > T;p.R83C and c.455G > A;p.C152Y) and two splice site mutations affecting the 5′ untranslated region (UTR) (c.−48C > G and c.−60A > G) were identified (Figure 7 an Supplementary Table S1) [83]. The 5′ UTR mutations resulted in the use of downstream start codons, causing N-terminal truncation of the protein; however, they did not cause complete aberrant splicing and each patient expressed low levels (<10%) of full-length GINS1 transcript [83]. Accordingly, formation of the GINS complex was compromised, but not ablated. The C152Y variant was able to form the GINS complex, but not the CMG complex [83]. Similar to MCM4 patient-derived cells, fibroblasts from GINS1 patients arrested in the G2/M phase of the cell cycle and displayed increased DNA content [83]. They also exhibited decreased proliferation, increased basal DNA damage and increased β-galactosidase activity, a marker for cellular senescence [83]. DNA fiber analysis revealed fewer initiation events, increased fork stalling and faster replication fork speed compared to control cells, indicative of replication stress driven by initiation defects and fork instability [83].
We recently described a new NK cell deficiency patient presenting with severe cytomegalovirus infection [84]. The patient was not reported to have growth retardation, adrenal insufficiency or dysmorphic facial features [84]. This patient had an over representation of stage 4 and a severe reduction in stage 5 NK cells with mildly decreased T and B cell counts [84]. Similar to the GINS1 cohort, this patient was a compound heterozygote with pathogenic variants in MCM10 (Figure 7 and Supplementary Table S1) [84]. One allele contained a nonsense mutation (c.1744C > T;p.R582X) that would truncate the protein prior to the nuclear localization signal, making it functionally a null allele [84]. As a truncated protein could not be detected, the transcript is likely degraded by nonsense mediated decay [84]. The second allele carries a missense mutation (c.1267C > T;p.R426C), changing a highly conserved arginine to cysteine (Figure 7 and Supplementary Table S1) [84]. Immortalized patient-derived fibroblasts exhibited increased DNA damage and abnormal cell cycle progression with an increase in cells in the S phase [84]. In model cell lines, each mutation separately resulted in shortened telomeres [85]. Strikingly, MCM10 deficiency caused not only increased inter-origin distance due to decreased origin firing, but telomere combing revealed an increased frequency of unreplicated and partially replicated telomeres [85]. Moreover, detailed karyotype analysis revealed increased chromosomal translocations, mostly originating from breaks at common fragile sites, which are known to be origin-poor [168]. Thus, telomeres and common fragile sites are particularly affected by reduced origin firing. Based on these observations, we propose that NK cell deficiency may actually constitute a telomeropathy, causing premature senescence in the NK cell lineage and providing an explanation for the lack of mature, stage 5 cells [85]. This hypothesis is supported by a case in which an individual with a regulator of telomere length 1 (RTEL1) pathogenic variant presented with NKD [169]. Genetic changes in RTEL1 have been primarily associated with Hoyeraal–Hreidarsson syndrome, a severe telomeropathy [170]. Furthermore, many other telomeropathies have low NK cell counts within the context of global cytopenia [171,172,173]. Peripheral NK cells are known to have shorter telomeres than T or B cells and a lower replicative potential than other lymphocytes [158,174,175]. Therefore, defects in MCM10 may predispose NK cells to advanced telomere erosion and premature senescence.
In addition to the NKD caused by pathogenic variants in MCM10, we also recently described a family with a history of intra-uterine fetal death due to reduced cardiac function and fetal hydrops [85]. These fetuses had biallelic pathogenic variants c.236delG and c.764 + 5G > A in MCM10 (Figure 7) [85]. One of these variants (c.236delG, p.G79EfsX6) disrupts exon 3 and is essentially a null allele [85]. The other variant reduces the splicing efficiency of exon 6, resulting in low levels of wild-type MCM10 expression [85]. Together, these two variants result in a greater reduction in MCM10 function than the compound heterozygous variants in the NKD patient [85]. On postmortem examination, all three fetuses had hypoplastic spleens and thymuses, suggesting that immune cell development was affected [85]. The cardiac dysfunction leading to fetal death was diagnosed as restrictive cardiomyopathy. Interestingly, cardiomyopathy has been associated with telomere erosion in cardiomyocytes, further supporting a role for telomere dysregulation in clinical syndromes due to MCM10 deficiency [85,176,177].
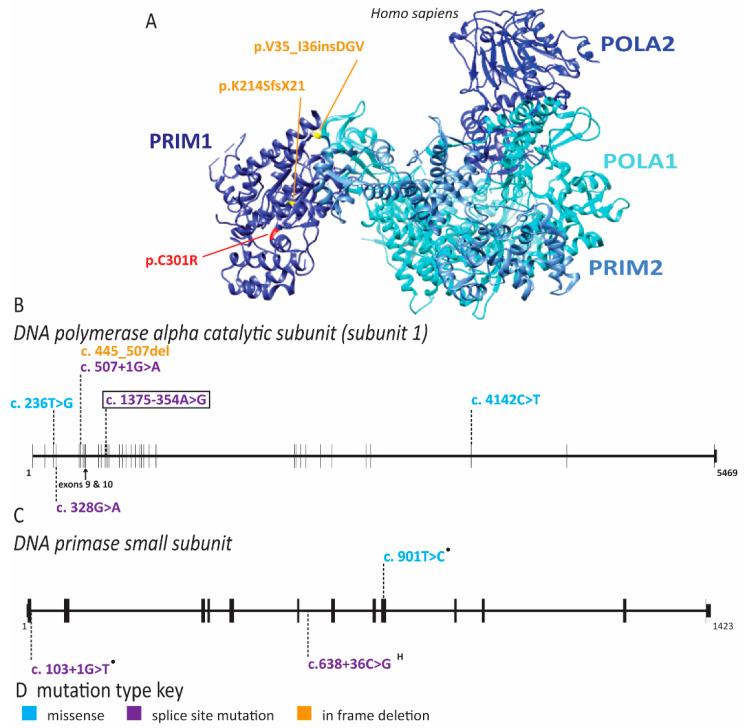
Within the context of NKD caused by replication defects, we will discuss a related disease, X-linked pigmentary reticulate disorder (XLPRD). This is another rare syndrome with recurrent infections caused by pathogenic variants in POLA1 which leads to reduced pol α expression (Figure 8) [86]. In addition to recurrent infections, affected individuals have hypogonadism, hyperpigmentation, corneal dystrophy, photophobia, unique facial features and multiorgan inflammation due to the role of pol α in modulating the interferon response [86,87,88,89,90]. Recurrent infections are due to reduced NK cell numbers and reduced cytotoxicity of peripheral NK cells, although the NK cells in these individuals do not stay consistently low enough to make the diagnosis of CNKD [90]. Interestingly, NK cells in these patients appear to also have impaired cytotoxic function, conceptually making the immune deficiency in these individuals a combination of CNKD and FNKD [90]. Surprisingly, immortalized patient fibroblasts did not have any proliferation or cell cycle defects, arguing that pol α expression remained above the necessary threshold to support normal DNA replication in vitro [90]. Whether this is true during the replicative cycles as NK cells undergo development in vivo is still an open question.
Figure 8.
Pathogenic variants in pol α cause a variety of diseases with high prevalence of immunodeficiency. Pathogenic mutations in pol α subunits are depicted on a schematic of each gene and the crystal structure of Homo sapiens proteins. Mutations that do not have corresponding amino acids in the structure or resulted in abnormal splicing are not depicted. For a complete list of mutations and associated protein changes, see Supplementary Table S1. Structures were generated with the Chimera program (http://www.cgl.ucsf.edu/chimera). Exon and intron schematics were generated with the UCSD genome browser. Reference sequences are POLA1 NM_016937.4 and PRIM1 NM_000946.3. (A) Crystal structure of human pol α subunits with mutations depicted including missense mutations (red) and frame shift mutations (orange). The structure was generated using pdb file 5exr. Schematic of (B) POLA1 and (C) PRIM1 genes depicted with exons as black boxes and introns as horizontal lines. Mutations are mapped to the appropriate gene regions. Note that POLA1 is on the X chromosome and has X-linked inheritance. Boxed alleles on POLA1 schematic occur in individuals with XLPRD while the remaining mutations occur in individuals with Van Esch-O’Driscoll disease. Exons 9 and 10 of POLA1 appear as a single line in this schematic. “H” superscript indicates a homozygous mutation. Compound heterozygous mutation pairs are indicated by superscript symbols. Each mutation combination is depicted, and certain alleles may be present in multiple combinations. The frequency of each allele in the affected population is not indicated. (D) For each gene, the color of the mutations indicates the type of mutation according to this key.
2.4. Multiple Pol α Subunits Are Linked to Human Disease
In addition to XLPRD, Van Esch-O’Driscoll disease has been linked to pathogenic variants of POLA1 in nine individuals belonging to five unrelated families (Figure 8 and Supplementary Table S1) [91,92]. This disease is characterized by intellectual disability, growth retardation, microcephaly and hypogonadism (Table 1) [91]. Additional notable features that occur in some of these patients include hypotonia, behavioral challenges, cerebral atrophy, congenital heart defect, facial dysmorphism, recurrent infections, esophageal atresia and epilepsy (Supplementary Table S2) [91,92]. All affected individuals are male, consistent with a X-linked recessive mode of inheritance [91]. Each family within this cohort has a different pathogenic variant (Figure 8 and Supplementary Table S1) [91]. As in individuals with XLPRD, these mutations can cause reduced expression of POLA1 [91]. However, substitution of highly conserved residues, such as G110R, I79S, P1381L, and deletion of amino acids 149-169 directly affect protein function (Figure 8 and Supplementary Table S1) [91]. DNA combing in lymphoblastoid cell lines from these patients revealed diminished replication initiation events, increased inter-origin distance and increased fork asymmetry, consistent with impaired origin firing and replication fork instability [91]. Moreover, cell lines from multiple families displayed increased sensitivity to the replication inhibitor hydroxyurea, consistent with the notion that defects in pol α increase sensitivity to replication stress [91]. In zebrafish, POLA1 is highly expressed in the brain both pre- and post-birth, explaining why normal brain function can be impaired in humans [91]. Pathogenic variants in Van Esch-O’Driscoll disease could have more severe effects on growth and brain development because the compound heterozygous mutations lead to exacerbated loss of function, but it is not clear why global inflammation and recurrent infections (as seen in XLPRD) are not a consistent component of the disease phenotype. More work is needed to understand how these two diseases progress during development.
Pathogenic variants in PRIM1, the catalytic subunit of DNA primase which is required for the initiation of leading and lagging strand synthesis, have been identified in four families afflicted with microcephalic dwarfism (Figure 8) [93]. In addition, these individuals have unique facial appearance, reduced subcutaneous fat, hypothyroidism, cardiac abnormalities, hepatic dysfunction indicative of chronic liver inflammation and immune dysfunction [93]. The microcephaly is accompanied by abnormal cerebral cortex structure and severe developmental delay [93]. The immune dysfunction includes agammaglobulinemia and lymphopenia, with intermittent anemia and thrombocytopenia. The severe immune dysfunction in these individuals appears to have contributed to early death in five out of six individuals [93]. The most common pathogenic variant, c.638 + 36C > G, inherited biallelically in three of the four families, results in mis-splicing of the PRIM1 transcript and subsequent nonsense mediated decay (Figure 8) [93]. As expected with decreased PRIM1 expression, patient-derived cells showed increased doubling time, reduced DNA synthesis, increased levels of DNA damage and delayed progression through the G1 phase of the cell cycle [93]. Several of the clinical features as well as molecular defects overlap with diseases resulting from pathogenic variants in other polymerase subunits (discussed below), as well as with Van Esch-O’Driscoll disease and XLPRD (Table 1). This suggests that perturbations of either lagging or leading strand synthesis can have similar effects on disease outcomes.
2.5. Pol ε Dysfunction Causes Immunodeficiency
Pol ε is composed of four subunits, POLE1, POLE2, POLE3 and POLE4. Pol ε functions as the major leading strand polymerase and in DNA repair [42]. Pathogenic variants of POLE1 can cause non-syndromic cancer predisposition (not discussed here). Additionally, mutations in POLE1 can cause intrauterine growth restriction, metaphyseal dysplasia, adrenal hypoplasia congenita and genitourinary abnormalities (IMAGe) syndrome and facial dysmorphism, immunodeficiency, livedo and short stature (FILS) syndrome [94,97,178]. Most individuals with IMAGe syndrome carry pathogenic variants in cyclin-dependent kinase inhibitor 1C (CDKN1C/p57), a negative regulator of cell proliferation [97]. Interestingly, loss-of-function variants of CDKN1C lead to an overgrowth syndrome, whereas gain-of-function variants localizing to the PCNA binding domain of CDKN1C cause IMAGe syndrome [179]. POLE1 was identified as an additional gene involved in IMAGe syndrome in 2018 by whole-genome sequencing of a cohort with microcephalic primordial dwarfism [97]. Fifteen individuals with biallelic mutations in POLE1 and features of IMAGe syndrome were identified in this study (Figure 9) [132]. Each individual carried one allele with the same intronic variant that resulted in abnormal splicing of exon 15 (Figure 9) [97]. Whereas some wild-type transcript could still be produced from this allele, the majority contained a 47 bp insertion, leading to a premature stop codon in the catalytic domain [97]. The second allele in these individuals had various loss-of-function mutations and patient-derived fibroblasts displayed impaired S phase progression [97]. Phenotypically, these individuals not only had features of IMAGe syndrome but also had immunodeficiency and common facial dysmorphism [97]. The immunodeficiency seen here was variable with low T, B or NK cell counts, but the majority suffered increased infection rates [97]. Lastly, these individuals may also have increased likelihood of cancer as two individuals in this cohort developed blood cancers [97].
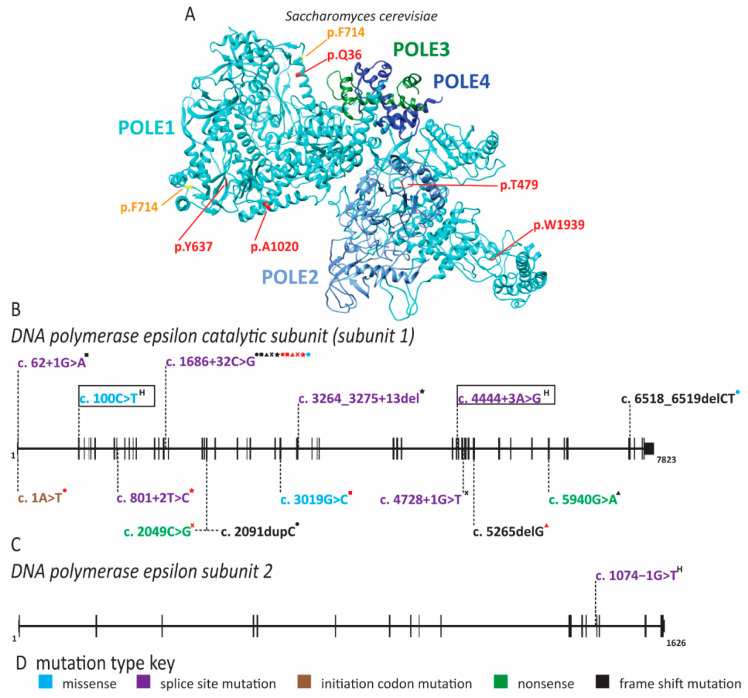
Figure 9.
Pathogenic variants in pol ε cause immunodeficiency. Pathogenic mutations in pol ε subunits are depicted on a schematic of each gene and the crystal structure of Saccharomyces cerevisiae proteins. Mutations that do not have corresponding amino acids in the structure or resulted in abnormal splicing are not depicted. For a complete list of mutations and associated protein changes, see Supplementary Table S1. Structures were generated with the Chimera program (http://www.cgl.ucsf.edu/chimera). Exon and intron schematics were generated with the UCSD genome browser. Reference sequences are POLE1 NM_006231.4 and POLE2 NM_001197331.3. (A) Alignment of Homo sapiens and Saccharomyces cerevisiae POLE1 and POLE2 was completed using UniProt alignment tool (https://www.uniprot.org/align/). Crystal structure of yeast pol ε subunits with amino acids corresponding to missense (red), nonsense mutations (red) and frame shift mutations (orange) depicted. The structure was generated using pdb file 6wjv. Schematic of (B) POLE1 and (C) POLE2 genes depicted with exons as black boxes and introns as horizontal lines. Mutations are mapped to the appropriate gene regions. Boxed alleles on POLE1 occur in individuals with FILS syndrome. “H” superscript indicates a homozygous mutation. Compound heterozygous mutation pairs are indicated by superscript symbols. Each mutation combination is depicted, and certain alleles may be present in multiple combinations such as c.1686 + 32C > G which is shared by all individuals with IMAGe syndrome. The frequency of each allele in the affected population is not indicated. (D) For each gene, the color of the mutations indicates the type of mutation according to this key.
In addition to IMAGe syndrome, reduced expression of POLE1 in a large consanguineous family caused FILS [94]. These individuals carried a mutation of intron 34, resulting in abnormal splicing and a significant reduction in the expression of full-length POLE1, as well as a corresponding decrease in POLE2 protein (Figure 9) [94]. The reduction in pol ε subunits unsurprisingly led to decreased proliferation of patient cells in vitro [94]. The de Saint Basile laboratory determined POLE1 levels in multiple tissues and identified that tissues with high expression, including chondrocytes and lymphocytes, corresponded with the clinical phenotypes [94]. Similar to the cohort of IMAGe patients, the FILS cohort also has variable immune cell phenotypes with reduced B or NK cells [94]. The most striking immune phenotype was the reduction of memory B cells and IgM and IgG levels [94]. Two additional case reports from unrelated families have been described with similar symptoms to FILS syndrome [95,96]. Surprisingly, one of these patients had the same intronic mutation as the original family but with slightly different presentation [96]. The third case report described a homozygous substitution of R34C (c.100C > T), which lies outside the exonuclease and polymerase domains [95]. These additional patients had short stature, immunodeficiency, facial dysmorphism with slight differences from the original description and poikiloderma rather than livedo. Additionally, they presented with developmental delays and cryptorchidism, as might be seen in an individual with IMAGe syndrome.
POLE1 is not the only pol ε subunit that plays a role in human disease. A patient with immunodeficiency, facial dysmorphism, short stature and autoimmunity has been reported with homozygous splice site mutations in intron 13 of POLE2 (Figure 9) [98]. This gene variant results in either the use of a cryptic splice site +3 nucleotides into exon 14, or exon 14 skipping [98]. Whereas this mutation did not appear to affect the overall expression level of POLE2, use of the cryptic splice site leads to deletion of the highly conserved S359 [98]. Patient-derived cells accumulated in the G2/M phase of the cell cycle and harbored chromosome duplications rather than rearrangements [98]. The immune phenotype in this patient appeared quite severe, with the near absence of mature B cells as well as reduced NK and T cells [98]. Thus, disruption of pol ε function causes a wide range of phenotypic outcomes, even when the defects occur in the same subunit as in IMAGe and FILS. What is particularly striking is that these symptoms overlap with diseases affecting replication origin licensing factors (MGS and skeletal anomalies), firing factors (NKD and immunodeficiency) and repair factors (RECQL4 and cancer predisposition/skin phenotypes). This is generally in agreement with the multiple roles that pol ε plays in maintaining genomic stability.
2.6. Pol δ Associated Progeroid Syndrome and Immune Deficiency
In 2010, Shastry and colleagues described a group of patients with mandibular hypoplasia, deafness, progeroid features (MDP) including lipodystrophy with the loss of subcutaneous fat and elevated visceral fat [99]. These individuals had some phenotypic overlap with mandibuloacral dysplasia (MAD). MAD is caused by pathogenic variants in LMNA, which encodes lamin A/C components of the nuclear cytoskeleton and ZMPSTE24, a zinc metalloproteinase required in the formation of mature lamin A [180]. Symptoms shared by these two syndromes are mandibular hypoplasia, bird-like face or pinched nose, short stature, joint contractures and scleroderma of the skin [99,180]. MDP patients do not have the skeletal abnormalities seen in MAD patients, nor do they have pathogenic variants of LMNA or ZMPSTE24 [99,180]. Unique features of MPD include sensorineural hearing loss in childhood, hypogonadism and undescended testes in affected males and dry eyes [99,100,180]. Additionally, MDP patients have longer survival than MAD patients, although they have multiple comorbidities consistent with a progeroid syndrome, including dyslipidemia, cardiovascular disease and hyperglycemia [99,100,101,102,103,104,105,106,107]. Interestingly, MDP is caused by a de novo monoallelic pathogenic variant in POLD1 which encodes the catalytic subunit of pol δ (Figure 10 and Supplementary Table S1) [101]. This pathogenic variant (c.1812_1814delCTC) results in the loss of S605 within motif A of POLD1 [101]. Motif A is responsible for orienting the incoming nucleotide to catalyze the phosphodiester bond and deletion of S605 ablates the polymerase activity of the enzyme [101]. The stability of this variant protein suggested that it may outcompete wild-type pol δ produced from the second normal allele in these individuals, making this a dominant negative mutation [101]. Exonuclease activity of the mutant pol δ was maintained, albeit at a slightly lower level than the wild type [101]. This is noteworthy because other pathogenic variants of pol δ affecting exonuclease activity have been associated with cancer predisposition syndromes [101]. In total, 21 individuals have been reported with MDP; 16 of these carried the S605del mutation [99,100,101,102,103,104,105,106,107]. One of the additional pathogenic variants is a R507C (c.1519C > T) substitution [104]. This amino acid is in the exonuclease domain, but not in the active site, and therefore is also not likely to increase cancer risk [104,105]. This variant is not predicted to impact polymerase activity directly, but may play a role in maintaining pol δ on chromatin during DNA elongation [105]. A third pathogenic variant is a I1070N (c.3209T > A) substitution reported in a single individual [106]. This mutation is predicted to severely impair protein folding and results in a severe clinical phenotype with fast onset of progeroid features and recurrent infections [106]. A fourth pathogenic variant, E1067K (c.3199G > A), resides in the zinc finger domain and was identified in two related individuals [107]. This variant resulted in abnormal nuclear structure, although to a lesser extent than seen with pathogenic changes in LMNA [107]. Interestingly, some MPD patients were originally diagnosed with Werner syndrome, suggesting phenotypic overlap and variable phenotypes [105]. Pol δ interacts with the Werner helicase and Werner syndrome shares many characteristics with MPD, including a bird-like face, loss of adipose tissue, joint contractures, scleroderma, hypogonadism and insulin resistance [101,105]. Werner helicase enhances pol δ activity, particularly at difficult-to-replicate secondary structures within common fragile sites, providing a rationale for the overlapping clinical phenotypes [181,182,183].
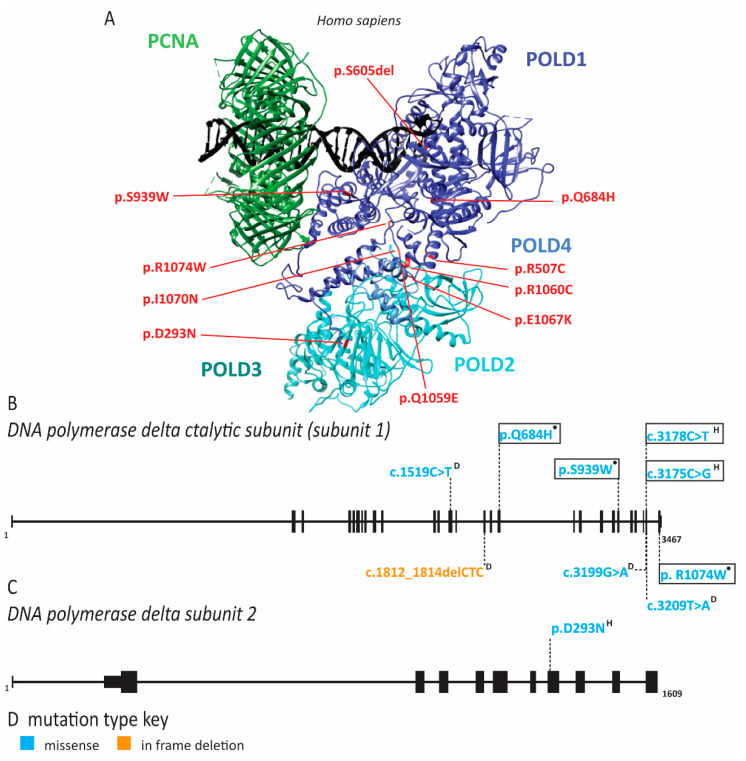
Figure 10.
Pathogenic variants in pol δ subunits cause human disease. Pathogenic mutations in pol δ subunits are depicted on a schematic of each gene and the crystal structure of Homo sapiens pol δ complex. Mutations that do not have corresponding amino acids in the structure or resulted in abnormal splicing are not depicted. For a complete list of mutations and associated protein changes, see Supplementary Table S1. Structures were generated with the Chimera program (http://www.cgl.ucsf.edu/chimera). Exon and intron schematics were generated with the UCSD genome browser. Reference sequences are POLD1 NM_001256849.1 and POLD2 NM_006230.4. (A) Crystal structure of human pol δ subunits with mutations depicted including missense and nonsense mutations depicted in red and frame shift mutations depicted in orange. The structure was generated using pdb file 6s1m. Schematic of (B) POLD1 and (C) POLD2 genes depicted with exons as black boxes and introns as horizontal lines. Mutations are mapped to the appropriate gene regions. Boxed alleles on POLD1 occur in individuals with immune deficiency. Note that the black circle symbol indicates mutations found together in an individual, p.Q684H and p.S939W were found in cis while p.R1074W was found in trans. Compound heterozygous mutation pairs are indicated by superscript symbols. Each mutation combination is depicted, and certain alleles may be present in multiple combinations. The frequency of each allele in the affected population is not indicated. (D) For each gene, the color of the mutations indicates the type of mutation according to this key.
Pathogenic variants in pol δ disrupting POLD1 and POLD2 have also been linked to immunodeficiency, similar to diseases seen with defects in other polymerase subunits (Figure 10) [90,93,94,96,97]. Affected individuals had severe lymphopenia of NK cells and CD4+ T cells, reduced CD8+ T cells, agammaglobulinemia, abnormal distribution of progenitor cells in the bone marrow or B cell lymphopenia [108,109,110]. Studies with patient-derived cells demonstrated impaired T cell development and proliferation [108,109,110]. T and NK cells exhibited increased spontaneous DNA damage and NK cells were defective in their response to X-ray irradiation [109]. DNA combing of patient fibroblasts showed reduced origin firing with a compensatory increase in fork speed [108]. Additionally, the S phase checkpoint was activated and an increase in p53 binding protein 1 (53BP1) nuclear bodies was observed in the G1 phase of the cell cycle, indicative of replication stress and under-replicated DNA [108,109]. Additional symptoms included short stature, intellectual disability and hearing loss. Although there is minor overlap of symptoms with MDP patients, it is unclear why shared features are limited. One clue may lie in the fact that pathogenic variants resulting in immunodeficiency are autosomal recessive and inherited from healthy parents, whereas those in MDP are de novo autosomal dominant mutations, suggesting that the recessive alleles have a less severe impact on pol δ function than MDP alleles.
2.7. PCNA Associated Human Disease
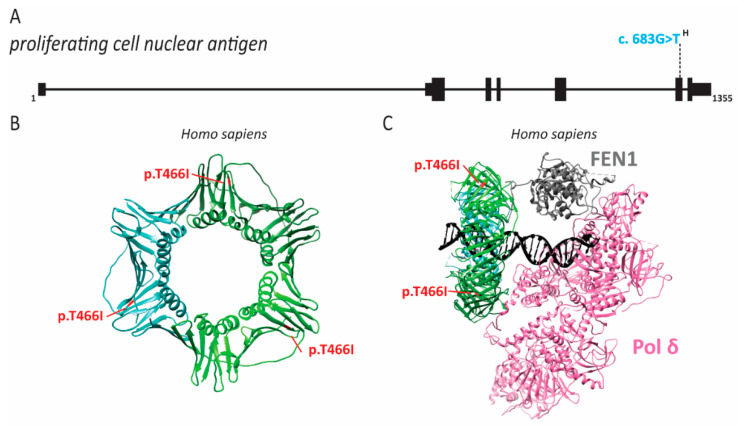
Four individuals from a large consanguineous family were identified with neurodegeneration, short stature, prelingual sensorineural hearing loss, premature aging, telangiectasias, intellectual disability, photophobia and photosensitivity [111]. The neurodegeneration appeared progressive in three of the patients at the time of study, with increasing difficulty in swallowing [111]. One patient had particularly severe loss of muscle control, resulting in the loss of speech and the use of a wheelchair [156]. Baple and colleagues identified a S228I (c.683G > T) pathogenic variant in PCNA (Figure 11) [111]. S228 is near the outer surface of PCNA, so it likely has no effect on DNA binding. Indeed, analysis of patient-derived cells assessed by BrdU incorporation demonstrated that this pathogenic variant of PCNA did not cause defects in DNA synthesis [111]. Interestingly, the patient phenotypes have similarities with xeroderma pigmentosum (XP), ataxia telangiectasia (AT) and cockayne syndrome (CS), which are all caused by defects in the DNA damage response and DNA repair [111]. Specifically, XP and CS are caused by defects in nucleotide excision repair (NER) [184]. Considering the phenotypic overlap and the critical role PCNA plays in NER, it is not surprising that these patient cells are sensitive to UV radiation [111]. As with other diseases caused by defects in NER, these individuals also seem to be predisposed to sun-induced cancers [111]. Further studies suggested that the S228I pathogenic mutant was defective in NER due to disruption of PCNA interactions with DNA ligase 1, FEN1 and XP complementation group G (XPG) [111,185]. Ligase 1 and FEN1 also play an important role in Okazaki fragment processing. A combined defect in NER and Okazaki fragment processing may explain the broader array of symptoms seen in these patients as opposed to XP and CS patients. Conversely, symptoms of these patients seemed less severe than XP, CS or AT patients, suggesting that this PCNA mutant retains some DNA repair function.
Figure 11.
PCNA pathogenic variant is on the outer surface of PCNA. Pathogenic mutation in PCNA is depicted on a schematic of the gene and crystal structure of Homo sapiens proteins. Structures were generated with the Chimera program (http://www.cgl.ucsf.edu/chimera). Exon and intron schematics were generated with the UCSD genome browser. Reference sequence is NM_002592.2. Schematic of the (A) PCNA gene is depicted with exons as black boxes and introns as horizontal lines. Missense mutation is mapped to the appropriate gene regions. “H” superscript indicates a homozygous mutation. (B) Crystal structure of human PCNA with missense mutation depicted in red. The structure was generated using pdb file 6fcm. (C) Crystal structure of human PCNA in complex with FEN1 and pol δ with missense mutation depicted in red. The structure was generated using pdb file 6tnz.
2.8. RECQL4 Dysfunction Causes Three Overlapping Syndromes
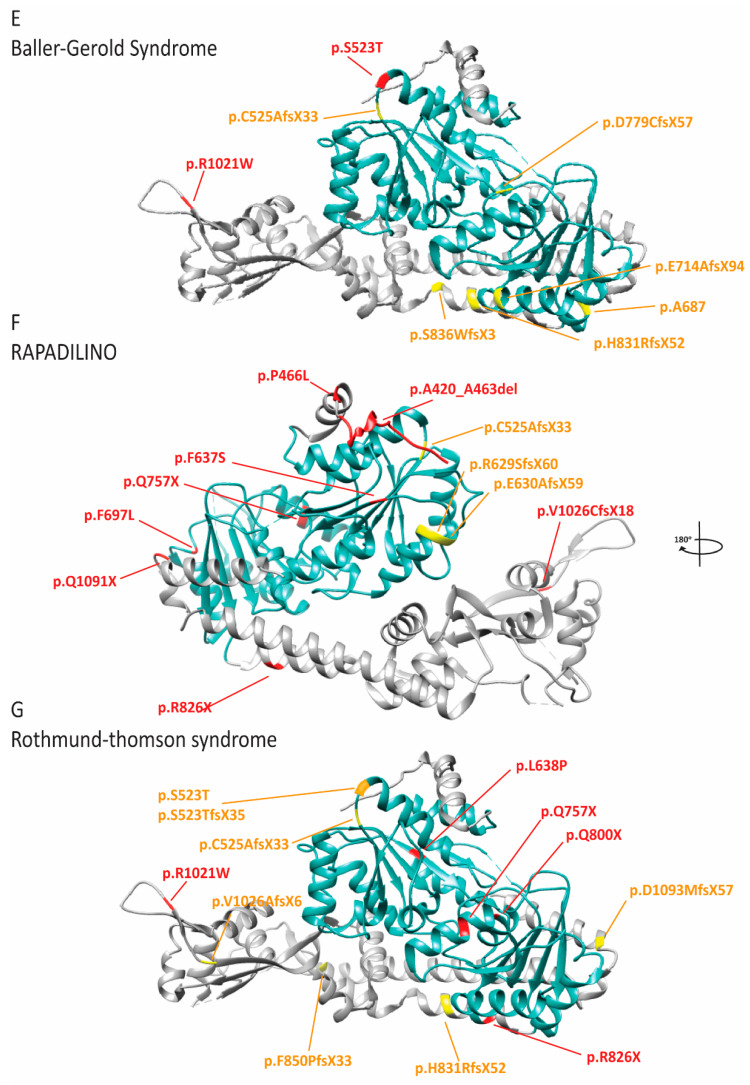
Pathogenic variants in RECQL4 have been linked to three syndromes: Rothmund–Thomson (RTS), Baller–Gerold (BGS) and RAPADILINO (RAdial hypo/aplasia, PAellae hypo/aplasia and cleft PAlate, DIarrhea and DIslocated joint, LIttle size and LImb malformation, NOse slender and NOrmal intelligence) (Figure 12 and Supplementary Table S2) [112,113,120,186,187,188,189]. All of these diseases are autosomal recessive and share growth retardation, cancer predisposition (osteosarcoma and lymphoma) and radial ray defects as symptoms [112,113,117,119,120,186,187,188,189]. RTS was the first to be described in the 19th century, but of course the gene affected in this disease was not found until more than a century later [120,187]. Kitao and coworkers investigated RECQL4 because RTS patients have chromosomal instability, growth retardation, hypogonadism and cancer predisposition similar to individuals with Bloom or Werner syndrome [120]. Bloom and Werner helicases are members of the RECQ helicase family and RECQL4 was recently identified as a new member of this family [120]. In addition to the symptoms that RTS has in common with Bloom syndrome, Werner syndrome, BGS and RAPADILINO, RTS individuals can also have photosensitivity with poikiloderma, graying hair, alopecia, hyperpigmented patches, telangiectasias, dystrophic teeth and dystrophic nails [112,113,118,121,122,187,190,191,192,193]. Many unique genetic variants have been demonstrated to be causative for RTS [112,113,117,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,187,191]. Patients afflicted with RTS usually have compound heterozygous mutations in RECQL4 with at least one nonsense mutation that causes a truncation, either deleting or disrupting the helicase domain (Figure 8 and Supplementary Table S1) [112,118,120].
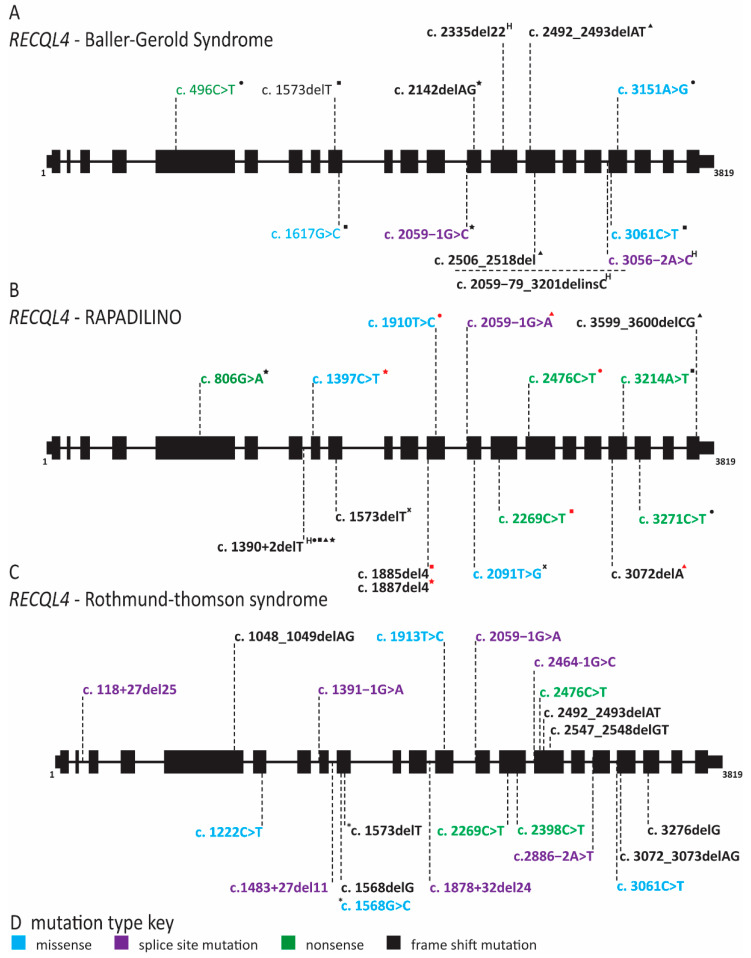
Figure 12.
Pathogenic variants in RECQL4 are associated with three different syndromes, Rothmund–Thomson (RTS), Baller–Gerold (BGS) and RAPADILINO (RAdial hy-po/aplasia, PAellae hypo/aplasia and cleft PAlate, DIarrhea and DIslocated joint, LIttle size and LImb malformation, NOse slender and NOrmal intelligence). Mutations of RECQL4 in BGS, RAPADILINO and RTS are depicted on a schematic of the gene and crystal structure of Homo sapiens RECQL4. For a complete list of mutations and associated protein changes, see Supplementary Table S1. The structure was generated using pdb file 5lst and the Chimera program (http://www.cgl.ucsf.edu/chimera). Exon and intron schematics were generated with the UCSD genome browser. Reference sequence is RECQL4 NM_004260.4. Schematic of RECQL4 gene for (A) BGS and (B) RAPADILINO is depicted with exons as black boxes and introns as horizontal lines. Mutations are mapped to the appropriate gene regions. “H” superscript indicates a homozygous mutation. Compound heterozygous mutation pairs are indicated by superscript symbols. Each mutation combination is depicted, and certain alleles may be present in multiple combinations. The frequency of each allele in the affected population is not indicated. (C) Mutations that occur in multiple individuals with RTS are depicted. “*” indicates that these mutations occur in cis, c.1573delT does occur alone in some individuals (D) For each gene, the color of the mutations indicates the type of mutation according to this key. Crystal structure of human RECQL4 with the helicase domain highlighted in blue, missense and nonsense mutations highlighted in red and frameshift mutations highlighted in orange for (E) BGS, (F) RAPADILINO and (G) RTS. For RTS, one amino acid indicated in orange on the ribbon structure has two mutations associated with it. Mutations that do not have corresponding amino acids in the structure or resulted in abnormal splicing are not depicted.
The clinical features of BGS patients show significant overlap with both RAPADILINO and RTS, including radial ray defects, growth deficiency, gastrointestinal (GI) disturbances, anal anomalies, patellar abnormalities and poikiloderma [164]. The distinguishing feature of BGS is craniosynostosis, which can lead to death in early childhood without surgery [164]. Of note, many reported BGS cases are of terminated pregnancies, limiting the description of additional clinical findings [112,113,114,194]. As we increase our understanding of the genetic cause of BGS and RTS, it may be more appropriate to consider them as a single disease with variable expressivity. In fact, the similarities between BGS and RTS are so strong that, in 2015, Piard and colleagues suggested that BGS should be considered a severe form of RTS with craniosynostosis when caused by pathogenic variants of RECQL4 [118]. As in RTS, the pathogenic variants are often inherited as compound heterozygous mutations and comprise missense, nonsense or splice site mutations (Figure 12 and Supplementary Table S1) [115,116,120,188]. It is important to note that, as with many diseases that were first described based on clinical characteristics, not all individuals with BGS and RTS have the same genetic cause. Ten percent of individuals clinically diagnosed with RTS have mutations in anaphase promoting complex subunit 1 (ANAPC1) and 30% do not have a known genetic cause [195,196]. Similarly, patients with BGS carry mutations in twist family BHLH transcription factor 1 (TWIST1), defects in which are known to cause craniosynostosis [170].
Whereas the mutation spectrum and patient population of RTS and BGS is quite broad, RAPADILINO occurs in a more homogenous Finnish population, with high prevalence of a splice site mutation in intron 7 of RECQL4 (Figure 8) [186]. This mutation results in the loss of exon 7 and an internal deletion of 44 amino acids within the N-terminal domain [186]. This region does not contain any known functional domains but was predicted to affect protein folding [186]. Subsequent studies demonstrated that the mutant is impaired for localization to the nucleus and sites of DNA damage [197,198]. Furthermore, in vitro studies with purified protein showed that the mutant lacked ATPase and ATP-dependent helicase activity [199]. RECQL4 can separate dsDNA when excess ssDNA is present with and without ATP, and surprisingly, the exon 7 mutant maintained this activity [190]. In fact, in assays without ATP, the mutant had higher activity than full-length RECQL4 [199]. Interestingly, some individuals with RAPADILINO are homozygous for the exon 7 skipping mutation, while others are compound heterozygous with a second pathogenic variant, most commonly a nonsense mutation (Figure 8 and Supplementary Table S1) [186]. A few individuals diagnosed with RAPADILINO do not carry this splice site mutation, but as poikiloderma, a distinguishing feature between RAPADILINO and RTS, does not typically appear in the first year of life, these individuals may be misdiagnosed [186,199]. Importantly, RAPADILINO patients do not have any skin or hair abnormalities as described in RTS [186,189].
Immunodeficiency has been reported in one RAPADILINO patient with decreases in T, B and NK cells [200]. Additionally, several case reports of RTS have been published reporting variable immune defects ranging from combined immune deficiency to immunoglobulin deficiency [201,202,203,204]. However, immune defects do not appear to be a consistent feature of either of these diseases. Both RTS and RAPADILINO are characterized by loss of RECQL4 helicase activity yet have discordant phenotypes. Recent studies suggested that the helicase domain is dispensable for cell viability and instead the N-terminus is required for efficient DNA replication as it has homology to S. cerevisiae Sld2, whereas the C-terminus is required for the DNA damage response [204,205]. Thus, it may be that the discordant phenotypes of RTS and RAPADILINO are due to separation-of-function mutations affecting either the N or C terminus of the protein.
3. Conclusions
Diseases caused by inherent defects in DNA replication genes exhibit a surprising diversity of symptoms. One overriding pathology seen in almost all of these diseases is growth restriction or short stature. This is consistent with the poor cell proliferation seen in many molecular studies of patient-derived cells and demonstrates the important role of efficient DNA replication in organismal growth. Other symptoms support the known functions of each protein defective in a disease. For instance, defects in pol ε, PCNA and RECQL4 lead to increased risk for cancer. In Table 1 and Supplementary Table S2, we have compiled the symptoms reported for DNA replication-associated diseases. Not only can this serve as a diagnostic resource, but it highlights similarities and differences. In the future, individuals with phenotypes overlapping to those discussed here will be identified. When the genetic cause is discovered to reside in a poorly characterized gene such as DONSON, the understanding we have about the etiology of these diseases can be leveraged to predict the molecular function of a gene product. It remains unclear why variants in proteins with interconnected function cause distinct phenotypes and display an unexpectedly high degree of tissue specificity. Another remaining question in the field is how these pathogenic variants arose. Clearly, some of these diseases that occur in homogenous populations or large consanguineous families benefit from a founder effect. However, there are common mutations that repeatedly occur in unrelated patients, the most striking example of which is POLD1 c.1686 + 32C > G in IMAGe syndrome. We suggest that it could be due to proofreading defects, failure of mismatch repair and/or residing in a mutagenesis hotspot.
An intriguing connection between MGS and ciliopathy is based on the observation that depletion of ORC1, ORC4, ORC6, CDT1 and CDC6 leads to reduced primary cilia formation [145]. The role of MCM proteins in cilia formation is also consistent with familial sensorineural hearing loss with MCM2 defects and adrenal disease in MCM4 NKD patients. Defects in origin licensing factors result in skeletal, respiratory and genitourinary symptoms. Conversely, variants in origin firing factors and polymerases are strongly associated with immune defects. Interestingly, mutations in RECQL4 have predominantly skeletal abnormalities rather than immunological effects, different from other factors involved in replication initiation. A similar picture emerges for the MCM2-7 complex. MCM4 and MCM5 are both part of the MCM2-7 double hexamer that is loaded onto DNA during origin licensing. As such, loss of these proteins would constitute an origin licensing defect. However, the MCM4 mutation associated with NKD did not alter chromatin binding of MCM2-7 but rather impaired origin firing [81,82]. Thus, it appears that two categories exist: (1) abnormal replication origin licensing resulting in abnormal body patterning such as skeletal defects and (2) abnormal replication origin firing and elongation resulting in immunodeficiency. Surprisingly, molecular studies also indicate that defects in origin licensing do not necessarily result in genomic instability, whereas defects in origin firing and elongation do [81,85,93,98,120].
One possible explanation for the different tissue manifestations may be rooted in the patterns that different cell lineages utilize to assemble and activate origins of replication. As cells differentiate from a highly proliferative pluripotent stem cell, they alter replication timing in a cell-type-dependent manner as well as origin usage [206,207,208]. Furthermore, common fragile sites show lineage specificity, indicating that different regions of the genome are sensitive to perturbation of the replication program depending on the cell type [209]. It is therefore conceivable that differences in the replication program sensitize specific cell types to depletion of origin licensing or origin firing factors [209,210,211]. For example, we recently proposed that NK cells are sensitive to MCM10 depletion due to enhanced telomere erosion [85]. Hypothetically, a reduction in MCM10 and the resulting origin firing defects might disproportionally affect chromosome ends. This would diminish origin firing in regions critical for telomere replication causing the telomere-specific replication defects that we observed in MCM10-deficient model cell lines [85]. NK cells might be particularly sensitive to telomere erosion, as they already have shorter telomeres than other highly proliferative immune cells [174,175]. Thus, the loss of efficient origin firing and a short telomere “set point” might synergize to cause premature senescence during NK cell development.
While further molecular studies are needed to understand how defects in origin licensing and origin firing result in unique phenotypes, we propose that each cell type maintains a balance between origin licensing and origin firing to efficiently replicate their DNA. Furthermore, some cell types can withstand a reduction in origin licensing and/or origin firing due to their unique replication program. Disease arises when specific cell types cannot compensate for these replication defects, resulting in genomic instability, accumulation of replication stress and loss of progenitor cells. Exploring differences in origin licensing and efficiency of origin firing in different cell types would be informative for understanding both DNA replication-associated diseases and how lineage context affects the replication program.
Acknowledgments
We thank Bielinsky laboratory members for critical reading of the manuscript. Special thanks to Ryan Baxley and Eric Hendrickson for helpful comments and advice.
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/22/2/911/s1, Table S1: Complete list of mutations in DNA replication genes, corresponding protein change, and relevant Saccharomyces cerevisiae or Xenopus laveis residues; Table S2: Extended table of phenotypes for DNA replication associated diseases.
Author Contributions
M.S. prepared the figures and co-wrote the manuscript. A.-K.B. co-wrote the manuscript. Both authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institutes of Health, grant number GM074917 and GM134681 to A.-K.B. and National Center for Advancing Translation Sciences grant number TL1R002493 and UL1TR002494 to M.S.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chandrasekaran S., Reidy T.K., Gowen J. Fundamental Aspects of DNA Replication. IntechOpen; London, UK: 2011. Regulation of DNA Replication Origin Licensing. [DOI] [Google Scholar]
- 2.Courtot L., Hoffmann J.-S., Bergoglio V. The protective role of dormant origins in response to replicative stress. Int. J. Mol. Sci. 2018;19:3569. doi: 10.3390/ijms19113569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hiratani I., Takahashi S. DNA replication timing enters the single-cell era. Genes. 2019;10:221. doi: 10.3390/genes10030221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J., Bellani M.A., James R.C., Pokharel D., Zhang Y., Reynolds J.J., McNee G.S., Jackson A.P., Stewart G.S., Seidman M.M. DONSON and FANCM associate with different replisomes distinguished by replication timing and chromatin domain. Nat. Commun. 2020;11:1–15. doi: 10.1038/s41467-020-17449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reynolds J.J., Bicknell L.S., Carroll P., Higgs M.R., Shaheen R., Murray J.E., Papadopoulos D.K., Leitch A., Murina O., Tarnauskaitė Ž., et al. Mutations in DONSON disrupt replication fork stability and cause microcephalic dwarfism. Nat. Genet. 2017;49:537–549. doi: 10.1038/ng.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bandura J.L., Beall E.L., Bell M., Silver H.R., Botchan M.R., Calvi B.R. humpty dumpty Is Required for Developmental DNA Amplification and Cell Proliferation in Drosophila. Curr. Biol. 2005;15:755–759. doi: 10.1016/j.cub.2005.02.063. [DOI] [PubMed] [Google Scholar]
- 7.Jaremko M.J., On K.F., Thomas D.R., Stillman B., Joshua-Tor L. The dynamic nature of the human Origin Recognition Complex revealed through five cryoEM structures. eLife. 2020;9:e58622. doi: 10.7554/eLife.58622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li N., Lam W.H., Zhai Y., Cheng J., Cheng E., Zhao Y., Gao N., Tye B.K. Structure of the origin recognition complex bound to DNA replication origin. Nat. Cell Biol. 2018;559:217–222. doi: 10.1038/s41586-018-0293-x. [DOI] [PubMed] [Google Scholar]
- 9.Yuan Z., Riera A., Bai L., Sun J., Nandi S., Spanos C., Chen Z.A., Barbon M., Rappsilber J., Stillman B., et al. Structural basis of Mcm2–7 replicative helicase loading by ORC–Cdc6 and Cdt1. Nat. Struct. Mol. Biol. 2017;24:316–324. doi: 10.1038/nsmb.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pozo P.N., Matson J.P., Cole Y., Kedziora K.M., Grant G.D., Temple B., Cook J.G. Cdt1 variants reveal unanticipated aspects of interactions with cyclin/CDK and MCM important for normal genome replication. Mol. Biol. Cell. 2018;29:2989–3002. doi: 10.1091/mbc.E18-04-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y., Pozo P.N., Oh S., Stone H.M., Cook J.G. Distinct and sequential re-replication barriers ensure precise genome duplication. PLoS Genet. 2020;16:e1008988. doi: 10.1371/journal.pgen.1008988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frigola J., He J., Kinkelin K., Pye V.E., Renault L., Douglas M.E., Remus D., Cherepanov P., Costa A., Diffley J.F. Cdt1 stabilizes an open MCM ring for helicase loading. Nat. Commun. 2017;8:15720. doi: 10.1038/ncomms15720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Remus D., Beuron F., Tolun G., Griffith J.D., Morris E.P., Diffley J.F. Concerted Loading of Mcm2–7 Double Hexamers around DNA during DNA Replication Origin Licensing. Cell. 2009;139:719–730. doi: 10.1016/j.cell.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evrin C., Clarke P., Zech J., Lurz R., Sun J., Uhle S., Li H., Stillman B., Speck C. A double-hexameric MCM2-7 complex is loaded onto origin DNA during licensing of eukaryotic DNA replication. Proc. Natl. Acad. Sci. USA. 2009;106:20240–20245. doi: 10.1073/pnas.0911500106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ticau S., Friedman L.J., Ivica N.A., Gelles J., Bell S.P. Single-molecule studies of origin licensing reveal mechanisms ensuring bidirectional helicase loading. Cell. 2015;161:513–525. doi: 10.1016/j.cell.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Georgescu R., Yuan Z., Bai L., Santos R.D.L.A., Sun J., Zhang D., Yurieva O., Li H., O’Donnell M.E. Structure of eukaryotic CMG helicase at a replication fork and implications to replisome architecture and origin initiation. Proc. Natl. Acad. Sci. USA. 2017;114:E697–E706. doi: 10.1073/pnas.1620500114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wohlschlegel J.A., Dwyer B.T., Dhar S.K., Cvetic C., Walter J.C., Dutta A. Inhibition of Eukaryotic DNA Replication by Geminin Binding to Cdt1. Science. 2000;290:2309–2312. doi: 10.1126/science.290.5500.2309. [DOI] [PubMed] [Google Scholar]
- 18.McGarry T.J., Kirschner M.W. Geminin, an Inhibitor of DNA Replication, Is Degraded during Mitosis. Cell. 1998;93:1043–1053. doi: 10.1016/S0092-8674(00)81209-X. [DOI] [PubMed] [Google Scholar]
- 19.Yeeles J.T.P., Deegan T.D., Janska A., Early A., Diffley J.F.X. Regulated eukaryotic DNA replication origin firing with purified proteins. Nat. Cell Biol. 2015;519:431–435. doi: 10.1038/nature14285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mueller A.C., Keaton M.A., Dutta A. DNA replication: Mammalian treslin–topbp1 interaction mirrors yeast Sld3–Dpb11. Curr. Biol. 2011;21:R638–R640. doi: 10.1016/j.cub.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez-Pulido L., Diffley J.F., Ponting C.P. Homology explains the functional similarities of Treslin/Ticrr and Sld3. Curr. Biol. 2010;20:R509–R510. doi: 10.1016/j.cub.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 22.Matsuno K., Kumano M., Kubota Y., Hashimoto Y., Takisawa H. The N-Terminal Noncatalytic Region of Xenopus RecQ4 Is Required for Chromatin Binding of DNA Polymerase α in the Initiation of DNA Replication. Mol. Cell. Biol. 2006;26:4843–4852. doi: 10.1128/MCB.02267-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Köhler K., Sanchez-Pulido L., Höfer V., Marko A., Ponting C.P., Snijders A.P., Feederle R., Schepers A., Boos D. The Cdk8/19-cyclin C transcription regulator functions in genome replication through metazoan Sld7. PLoS Biol. 2019;17:e2006767. doi: 10.1371/journal.pbio.2006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumagai A., Dunphy W.G. MTBP, the partner of Treslin, contains a novel DNA-binding domain that is essential for proper initiation of DNA replication. Mol. Biol. Cell. 2017;28:2998–3012. doi: 10.1091/mbc.e17-07-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Labib K. How do Cdc7 and cyclin-dependent kinases trigger the initiation of chromosome replication in eukaryotic cells? Genes Dev. 2010;24:1208–1219. doi: 10.1101/gad.1933010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larasati B., Duncker P. Mechanisms Governing DDK Regulation of the Initiation of DNA Replication. Genes. 2016;8:3. doi: 10.3390/genes8010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dhingra N., Bruck I., Smith S., Ning B., Kaplan D.L. Dpb11 Protein Helps Control Assembly of the Cdc45·Mcm2-7·GINS Replication Fork Helicase. J. Biol. Chem. 2015;290:7586–7601. doi: 10.1074/jbc.M115.640383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simon A.C., Sannino V., Costanzo V., Pellegrini L. Structure of human Cdc45 and implications for CMG helicase function. Nat. Commun. 2016;7:11638. doi: 10.1038/ncomms11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruck I., Kaplan D.L. GINS and Sld3 Compete with One Another for Mcm2-7 and Cdc45 Binding. J. Biol. Chem. 2011;286:14157–14167. doi: 10.1074/jbc.M111.218305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt U., Wollmann Y., Franke C., Grosse F., Saluz H.-P., Hänel F. Characterization of the interaction between the human DNA topoisomerase IIβ-binding protein 1 (TopBP1) and the cell division cycle 45 (Cdc45) protein. Biochem. J. 2007;409:169–177. doi: 10.1042/BJ20070872. [DOI] [PubMed] [Google Scholar]
- 31.Shin G., Jeong D., Kim H., Im J.-S., Lee J.-K. RecQL4 tethering on the pre-replicative complex induces unscheduled origin activation and replication stress in human cells. J. Biol. Chem. 2019;294:16255–16265. doi: 10.1074/jbc.RA119.009996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sangrithi M.N., Bernal J.A., Madine M., Philpott A., Lee J., Dunphy W.G., Venkitaraman A.R. Initiation of DNA Replication Requires the RECQL4 Protein Mutated in Rothmund-Thomson Syndrome. Cell. 2005;121:887–898. doi: 10.1016/j.cell.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 33.Sirbu B.M., McDonald W.H., Dungrawala H., Badu-Nkansah A., Kavanaugh G.M., Chen Y., Tabb D.L., Cortez D. Identification of Proteins at Active, Stalled, and Collapsed Replication Forks Using Isolation of Proteins on Nascent DNA (iPOND) Coupled with Mass Spectrometry. J. Biol. Chem. 2013;288:31458–31467. doi: 10.1074/jbc.M113.511337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noguchi Y., Yuan Z., Bai L., Schneider S., Zhao G., Stillman B., Speck C., Li H. Cryo-EM structure of Mcm2-7 double hexamer on DNA suggests a lagging-strand DNA extrusion model. Proc. Natl. Acad. Sci. USA. 2017;114:E9529–E9538. doi: 10.1073/pnas.1712537114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wasserman M.R., Schauer G.D., O’Donnell M.E., Liu S. Replication Fork Activation Is Enabled by a Single-Stranded DNA Gate in CMG Helicase. Cell. 2019;178:600–611.e16. doi: 10.1016/j.cell.2019.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petojevic T., Pesavento J.J., Costa A., Liang J., Wang Z., Berger J.M., Botchan M.R. Cdc45 (cell division cycle protein 45) guards the gate of the Eukaryote Replisome helicase stabilizing leading strand engagement. Proc. Natl. Acad. Sci. USA. 2015;112:E249–E258. doi: 10.1073/pnas.1422003112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perez-Arnaiz P., Bruck I., Colbert M.K., Kaplan D.L. An intact Mcm10 coiled-coil interaction surface is important for origin melting, helicase assembly and the recruitment of Pol-α to Mcm2–7. Nucleic Acids Res. 2017;45:7261–7275. doi: 10.1093/nar/gkx438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lõoke M., Maloney M.F., Bell S.P. Mcm10 regulates DNA replication elongation by stimulating the CMG replicative helicase. Genes Dev. 2017;31:291–305. doi: 10.1101/gad.291336.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perez-Arnaiz P., Kaplan D.L. An Mcm10 Mutant Defective in ssDNA Binding Shows Defects in DNA Replication Initiation. J. Mol. Biol. 2016;428:4608–4625. doi: 10.1016/j.jmb.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langston L.D., Mayle R., Schauer G.D., Yurieva O., Zhang D., Yao N.Y., Georgescu R.E., O’Donnell M.E. Mcm10 promotes rapid isomerization of CMG-DNA for replisome bypass of lagging strand DNA blocks. eLife. 2017;6:e29118. doi: 10.7554/eLife.29118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baxley R.M., Bielinsky A.-K. Mcm10: A Dynamic Scaffold at Eukaryotic Replication Forks. Genes. 2017;8:73. doi: 10.3390/genes8020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lujan S.A., Williams J.S., Kunkel T.A. DNA Polymerases Divide the Labor of Genome Replication. Trends Cell Biol. 2016;26:640–654. doi: 10.1016/j.tcb.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Georgescu R.E., Schauer G.D., Yao N.Y., Langston L.D., Yurieva O., Zhang D., Finkelstein J., O’Donnell M.E. Reconstitution of a eukaryotic replisome reveals suppression mechanisms that define leading/lagging strand operation. eLife. 2015;4:e04988. doi: 10.7554/eLife.04988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu W., Ukomadu C., Jha S., Senga T., Dhar S.K., Wohlschlegel J.A., Nutt L.K., Kornbluth S., Dutta A. Mcm10 and And-1/CTF4 recruit DNA polymerase to chromatin for initiation of DNA replication. Genes Dev. 2007;21:2288–2299. doi: 10.1101/gad.1585607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chattopadhyay S., Bielinsky A.-K. Human Mcm10 Regulates the Catalytic Subunit of DNA Polymerase-α and Prevents DNA Damage during Replication. Mol. Biol. Cell. 2007;18:4085–4095. doi: 10.1091/mbc.e06-12-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bowman G.D., O’Donnell M., Kuriyan J. Structural analysis of a eukaryotic sliding DNA clamp–clamp loader complex. Nat. Cell Biol. 2004;429:724–730. doi: 10.1038/nature02585. [DOI] [PubMed] [Google Scholar]
- 47.Tsurimoto T., Stillman B. Replication factors required for SV40 DNA replication in vitro. I. DNA structure-specific recognition of a primer-template junction by eukaryotic DNA polymerases and their accessory proteins. J. Biol. Chem. 1991;266:1950–1960. doi: 10.1016/S0021-9258(18)52385-1. [DOI] [PubMed] [Google Scholar]
- 48.Liu B., Hu J., Wang J., Kong D. Direct Visualization of RNA-DNA Primer Removal from Okazaki Fragments Provides Support for Flap Cleavage and Exonucleolytic Pathways in Eukaryotic Cells. J. Biol. Chem. 2017;292:4777–4788. doi: 10.1074/jbc.M116.758599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stodola J.L., Burgers P.M. Resolving individual steps of Okazaki-fragment maturation at a millisecond timescale. Nat. Struct. Mol. Biol. 2016;23:402–408. doi: 10.1038/nsmb.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stodola J.L., Burgers P.M. Advances in Experimental Medicine and Biology. Volume 1042. Springer; Singapore: 2017. Mechanism of lagging-strand dna replication in eukaryotes; pp. 117–133. [DOI] [PubMed] [Google Scholar]
- 51.Rossi M.L., Bambara R.A. Reconstituted Okazaki Fragment Processing Indicates Two Pathways of Primer Removal. J. Biol. Chem. 2006;281:26051–26061. doi: 10.1074/jbc.M604805200. [DOI] [PubMed] [Google Scholar]
- 52.Stewart J.A., Campbell J.L., Bambara R.A. DNA2 is a structure-specific nuclease, with affinity for 5′-flap intermediates. Nucleic Acids Res. 2009;38:920–930. doi: 10.1093/nar/gkp1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Howes T.R.L., Tomkinson A.E. DNA Ligase I, the Replicative DNA Ligase. Macromol. Protein Complexes Struct. Funct. 2012;62:327–341. doi: 10.1007/978-94-007-4572-8_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moreno S.P., Gambus A. Mechanisms of eukaryotic replisome disassembly. Biochem. Soc. Trans. 2020;48:823–836. doi: 10.1042/BST20190363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dewar J.M., Walter J.C. Mechanisms of DNA replication termination. Nat. Rev. Mol. Cell Biol. 2017;18:507–516. doi: 10.1038/nrm.2017.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dewar J.M., Low E., Mann M., Räschle M., Walter J.C. CRL2Lrr1promotes unloading of the vertebrate replisome from chromatin during replication termination. Genes Dev. 2017;31:275–290. doi: 10.1101/gad.291799.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moreno S.P., Bailey R., Campion N., Herron S., Gambus A. Polyubiquitylation drives replisome disassembly at the termination of DNA replication. Science. 2014;346:477–481. doi: 10.1126/science.1253585. [DOI] [PubMed] [Google Scholar]
- 58.Sedlackova H., Rask M.-B., Gupta R., Choudhary C., Somyajit K., Lukas J. Equilibrium between nascent and parental MCM proteins protects replicating genomes. Nat. Cell Biol. 2020;587:297–302. doi: 10.1038/s41586-020-2842-3. [DOI] [PubMed] [Google Scholar]
- 59.Bongers E., Van Kampen A., Van Bokhoven H., Knoers N.V.A.M., Van Bokhoven H. Human syndromes with congenital patellar anomalies and the underlying gene defects. Clin. Genet. 2005;68:302–319. doi: 10.1111/j.1399-0004.2005.00508.x. [DOI] [PubMed] [Google Scholar]
- 60.Gorlin R.J., Cervenka J., Moller K. A selected miscellany. Birth Defects Orig. Artic. Ser. 1975;11:39–50. [PubMed] [Google Scholar]
- 61.Hurst J.A., Winter R.M., Baraitser M., Optiz J.M., Reynolds J.F. Distinctive syndrome of short stature, craniosynostosis, skeletal changes, and malformed ears. Am. J. Med. Genet. 1988;29:107–115. doi: 10.1002/ajmg.1320290113. [DOI] [PubMed] [Google Scholar]
- 62.Cohen B., Temple I.K., Symons J.C., Hall C.M., Shaw D.G., Bhamra M., Jackson A.M., Pembrey M.E. Microtia and short stature: A new syndrome. J. Med. Genet. 1991;28:786–790. doi: 10.1136/jmg.28.11.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gorlin R.J. Microtia, absent patellae, short stature, micrognathia syndrome. J. Med. Genet. 1992;29:516–517. [PMC free article] [PubMed] [Google Scholar]
- 64.Boles R.G., Teebi A.S., Schwartz D., Harper J.F. Further delineation of the ear, patella, short stature syndrome (Meier-Gorlin syndrome) Clin. Dysmorphol. 1994;3:207–214. doi: 10.1097/00019605-199407000-00004. [DOI] [PubMed] [Google Scholar]
- 65.Lacombe D., Toutain A., Gorlin R.J., Oley C.A., Battin J. Clinical identification of a human equivalent to the short ear (se) murine phenotype. Annales Génétique. 1994;37:184–191. [PubMed] [Google Scholar]
- 66.Bongers E.M., Opitz J.M., Fryer A., Sarda P., Hennekam R.C., Hall B.D., Superneau D.W., Harbison M., Poss A., Van Bokhoven H., et al. Meier-Gorlin syndrome: Report of eight additional cases and review. Am. J. Med. Genet. 2001;102:115–124. doi: 10.1002/ajmg.1452. [DOI] [PubMed] [Google Scholar]
- 67.Bicknell L.S., Bongers E.M., Leitch A., Brown S., Schoots J., Harley M.E., Aftimos S., Al-Aama J.Y., Bober M.B., Brown P.A.J., et al. Mutations in the pre-replication complex cause Meier-Gorlin syndrome. Nat. Genet. 2011;43:356–359. doi: 10.1038/ng.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guernsey D.L., Matsuoka M., Jiang H., Evans S.C., MacGillivray C., Nightingale M., Perry S., Ferguson M., LeBlanc M.A., Paquette J., et al. Mutations in origin recognition complex gene ORC4 cause Meier-Gorlin syndrome. Nat. Genet. 2011;43:360–364. doi: 10.1038/ng.777. [DOI] [PubMed] [Google Scholar]
- 69.Fenwick A.L., Kliszczak M., Cooper F., Murray J., Sanchez-Pulido L., Twigg S.R., Goriely A., McGowan S.J., Miller K.A., Taylor I.B., et al. Mutations in CDC45, Encoding an Essential Component of the Pre-initiation Complex, Cause Meier-Gorlin Syndrome and Craniosynostosis. Am. J. Hum. Genet. 2016;99:125–138. doi: 10.1016/j.ajhg.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vetro A., Savasta S., Raucci A.R., Cerqua C., Sartori G., Limongelli I., Forlino A., Maruelli S., Perucca P., Vergani D., et al. MCM5: A new actor in the link between DNA replication and Meier-Gorlin syndrome. Eur. J. Hum. Genet. 2017;25:646–650. doi: 10.1038/ejhg.2017.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burrage L.C., Charng W.-L., Eldomery M.K., Willer J.R., Davis E.E., Lugtenberg D., Zhu W., LeDuc M.S., Akdemir Z.C., Azamian M., et al. De Novo GMNN Mutations Cause Autosomal-Dominant Primordial Dwarfism Associated with Meier-Gorlin Syndrome. Am. J. Hum. Genet. 2015;97:904–913. doi: 10.1016/j.ajhg.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.A De Munnik S., Bicknell L.S., Aftimos S., Al-Aama J.Y., Van Bever Y., Bober M.B., Clayton-Smith J., Edrees A.Y., Feingold M., Fryer A., et al. Meier-Gorlin syndrome genotype–phenotype studies: 35 individuals with pre-replication complex gene mutations and 10 without molecular diagnosis. Eur. J. Hum. Genet. 2012;20:598–606. doi: 10.1038/ejhg.2011.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vojtková J., Čiljaková M., Jeseňák M., Bánovčin P. Meier-Gorlin syndrome caused by ORC1 mutation associated with chromosomal breakage—Coincidental finding or new feature of known syndrome? Endokrynol. Polska. 2019;70:457–459. doi: 10.5603/EP.a2019.0032. [DOI] [PubMed] [Google Scholar]
- 74.Bicknell L.S., Walker S.A., Klingseisen A., Stiff T., Leitch A., Kerzendorfer C., Martin C.-A., Yeyati P.L., Al Sanna N., Bober M.B., et al. Mutations in ORC1, encoding the largest subunit of the origin recognition complex, cause microcephalic primordial dwarfism resembling Meier-Gorlin syndrome. Nat. Genet. 2011;43:350–355. doi: 10.1038/ng.776. [DOI] [PubMed] [Google Scholar]
- 75.Allon-Shalev S., Khayat M., Etty D.-S., Elpeleg O. Further insight into the phenotype associated with a mutation in the ORC6 gene, causing Meier-Gorlin syndrome 3. Am. J. Med. Genet. 2015;167:607–611. doi: 10.1002/ajmg.a.36906. [DOI] [PubMed] [Google Scholar]
- 76.Knapp K.M., Sullivan R., Murray J., Gimenez G., Arn P., D’Souza P., Gezdirici A., Wilson W.G., Jackson A.P., Ferreira C., et al. Linked-read genome sequencing identifies biallelic pathogenic variants in DONSON as a novel cause of Meier-Gorlin syndrome. J. Med. Genet. 2019;57:195–202. doi: 10.1136/jmedgenet-2019-106396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ting C.Y., Bhatia N.S., Lim J.Y., Goh C.-Y.J., Vasanwala R.F., Ong C.C.P., Seow W.T., Yeow V.K.-L., Ting T.W., Ng I.S.L., et al. Further delineation of CDC45-related Meier-Gorlin syndrome with craniosynostosis and review of literature. Eur. J. Med. Genet. 2020;63:103652. doi: 10.1016/j.ejmg.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 78.Karaca E., Posey J.E., Bostwick B., Liu P., Gezdirici A., Yesil G., Akdemir Z.C., Bayram Y., Harms F.L., Meinecke P., et al. Biallelic and De Novo Variants in DONSON Reveal a Clinical Spectrum of Cell Cycle-opathies with Microcephaly, Dwarfism and Skeletal Abnormalities. Am. J. Med. Genet. 2019;179:2056–2066. doi: 10.1002/ajmg.a.61315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Casey J.P., Nobbs M., Mcgettigan P.A., Lynch S., Ennis S. Recessive mutations inMCM4/PRKDC cause a novel syndrome involving a primary immunodeficiency and a disorder of DNA repair. J. Med. Genet. 2012;49:242–245. doi: 10.1136/jmedgenet-2012-100803. [DOI] [PubMed] [Google Scholar]
- 80.Gineau L., Cognet C., Kara N., Lach F.P., Dunne J., Veturi U., Picard C., Trouillet C., Eidenschenk C., Aoufouchi S., et al. Partial MCM4 deficiency in patients with growth retardation, adrenal insufficiency, and natural killer cell deficiency. J. Clin. Investig. 2012;122:821–832. doi: 10.1172/JCI61014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hughes C.R., Guasti L., Meimaridou E., Chuang C.-H., Schimenti J.C., King P.J., Costigan C., Clark A.J.L., Metherell L.A. MCM4 mutation causes adrenal failure, short stature, and natural killer cell deficiency in humans. J. Clin. Investig. 2012;122:814–820. doi: 10.1172/JCI60224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bernard F., Picard C., Cormier-Daire V., Eidenschenk C., Pinto G., Bustamante J.-C., Jouanguy E., Teillac-Hamel D., Colomb V., Funck-Brentano I., et al. A Novel Developmental and Immunodeficiency Syndrome Associated With Intrauterine Growth Retardation and a Lack of Natural Killer Cells. Pediatrics. 2003;113:136–141. doi: 10.1542/peds.113.1.136. [DOI] [PubMed] [Google Scholar]
- 83.Cottineau J., Kottemann M.C., Lach F.P., Kang Y.-H., Vély F., Deenick E.K., Lazarov T., Gineau L., Wang Y., Farina A., et al. Inherited GINS1 deficiency underlies growth retardation along with neutropenia and NK cell deficiency. J. Clin. Investig. 2017;127:1991–2006. doi: 10.1172/JCI90727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mace E., Paust S., Conte M.I., Baxley R.M., Schmit M.M., Patil S.L., Guilz N.C., Mukherjee M., Pezzi A.E., Chmielowiec J., et al. Human NK cell deficiency as a result of biallelic mutations in MCM10. J. Clin. Investig. 2020;130:10. doi: 10.1172/JCI134966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baxley R.M., Leung W., Schmit M.M., Matson J.P., Oram M.K., Wang L., Taylor J., Yin L., Hedberg J., Rogers C.B., et al. Bi-allelic MCM10 mutations cause telomere shortening with immune dysfunction and cardiomyopathy. bioRxiv. 2020:844498. doi: 10.1101/844498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Starokadomskyy P., Gemelli T., Rios J.J., Xing C., Wang R.C., Li H., Pokatayev V., Dozmorov I., Khan S., Miyata N., et al. DNA polymerase-α regulates the activation of type I interferons through cytosolic RNA:DNA synthesis. Nat. Immunol. 2016;17:495–504. doi: 10.1038/ni.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anderson R.C., Zinn A.R., Kim J., Carder K.R. X-linked Reticulate Pigmentary Disorder with Systemic Manifestations: Report of a Third Family and Literature Review. Pediatr. Dermatol. 2005;22:122–126. doi: 10.1111/j.1525-1470.2005.22206.x. [DOI] [PubMed] [Google Scholar]
- 88.Adès L.C., Rogers M., Sillence D.O. An X-Linked Reticulate Pigmentary Disorder with Systemic Manifestations: Report of a Second Family. Pediatr. Dermatol. 1993;10:344–351. doi: 10.1111/j.1525-1470.1993.tb00396.x. [DOI] [PubMed] [Google Scholar]
- 89.Pezzani L., Brena M., Callea M., Colombi M., Tadini G. X-linked reticulate pigmentary disorder with systemic manifestations: A new family and review of the literature. Am. J. Med. Genet. 2013;161:1414–1420. doi: 10.1002/ajmg.a.35882. [DOI] [PubMed] [Google Scholar]
- 90.Starokadomskyy P., Wilton K.M., Krzewski K., Lopez A.M., Sifuentes-Dominguez L., Overlee B., Chen Q., Ray A., Gil-Krzewska A., Peterson M., et al. NK cell defects in X-linked pigmentary reticulate disorder. JCI Insight. 2019;4:125688. doi: 10.1172/jci.insight.125688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Van Esch H., Colnaghi R., Freson K., Starokadomskyy P., Zankl A., Backx L., Abramowicz I., Outwin E., Rohena L., Faulkner C., et al. Defective DNA Polymerase α-Primase Leads to X-Linked Intellectual Disability Associated with Severe Growth Retardation, Microcephaly, and Hypogonadism. Am. J. Hum. Genet. 2019;104:957–967. doi: 10.1016/j.ajhg.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Van Esch H., Zanni G., Holvoet M., Borghgraef M., Chelly J., Fryns J.-P., Devriendt K. X-linked mental retardation, short stature, microcephaly and hypogonadism maps to Xp22.1-p21.3 in a Belgian family. Eur. J. Med. Genet. 2005;48:145–152. doi: 10.1016/j.ejmg.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 93.Parry D.A., Tamayo-Orrego L., Carroll P., Marsh J.A., Greene P., Murina O., Uggenti C., Leitch A., Káposzta R., Merő G., et al. PRIM1 deficiency causes a distinctive primordial dwarfism syndrome. Genes Dev. 2020;34:1520–1533. doi: 10.1101/gad.340190.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schmid J.P., Lemoine R., Nehme N.T., Cormier-Daire V., Revy P., Debeurme F., Debré M., Nitschke P., Bole-Feysot C., Legeai-Mallet L., et al. Polymerase ε1 mutation in a human syndrome with facial dysmorphism, immunodeficiency, livedo, and short stature (“FILS syndrome”) J. Exp. Med. 2012;209:2323–2330. doi: 10.1084/jem.20121303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eason C., Aleisa A., Jones J.R., Prijoles E.J., Lee L.W. Filling in the gaps on FILS syndrome: A case report and literature review. Pediatr. Dermatol. 2020;37:915–917. doi: 10.1111/pde.14274. [DOI] [PubMed] [Google Scholar]
- 96.Thiffault I., Saunders C., Jenkins J., Raje N., Canty K., Sharma M., Grote L., Welsh H.I., Farrow E., Twist G., et al. A patient with polymerase E1 deficiency (POLE1): Clinical features and overlap with DNA breakage/instability syndromes. BMC Med. Genet. 2015;16:1–8. doi: 10.1186/s12881-015-0177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Logan C.V., Murray J.E., Parry D.A., Robertson A., Bellelli R., Tarnauskaitė Ž., Challis R., Cleal L., Borel V., Fluteau A., et al. DNA Polymerase Epsilon Deficiency Causes IMAGe Syndrome with Variable Immunodeficiency. Am. J. Hum. Genet. 2018;103:1038–1044. doi: 10.1016/j.ajhg.2018.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Frugoni F., Dobbs K., Felgentreff K., Aldhekri H., Al Saud B.K., Arnaout R., Ali A.A., Abhyankar A., Alroqi F., Giliani S., et al. A novel mutation in the POLE2 gene causing combined immunodeficiency. J. Allergy Clin. Immunol. 2016;137:635–638.e1. doi: 10.1016/j.jaci.2015.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shastry S., Simha V., Godbole K., Sbraccia P., Melancon S., Yajnik C.S., Novelli G., Kroiss M., Garg A. A Novel Syndrome of Mandibular Hypoplasia, Deafness, and Progeroid Features Associated with Lipodystrophy, Undescended Testes, and Male Hypogonadism. J. Clin. Endocrinol. Metab. 2010;95:E192–E197. doi: 10.1210/jc.2010-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sasaki H., Yanagi K., Ugi S., Kobayashi K., Ohkubo K., Tajiri Y., Maegawa H., Kashiwagi A., Kaname T. Definitive diagnosis of mandibular hypoplasia, deafness, progeroid features and lipodystrophy (MDPL) syndrome caused by a recurrent de novo mutation in the POLD1 gene. Endocr. J. 2018;65:227–238. doi: 10.1507/endocrj.EJ17-0287. [DOI] [PubMed] [Google Scholar]
- 101.Weedon M.N., Ellard S., Prindle M.J., Caswell R., Allen H.L., Oram R.A., Godbole K., Yajnik C.S., Sbraccia P., Novelli G., et al. An in-frame deletion at the polymerase active site of POLD1 causes a multisystem disorder with lipodystrophy. Nat. Genet. 2013;45:947–950. doi: 10.1038/ng.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Okada A., Kohmoto T., Naruto T., Yokota I., Kotani Y., Shimada A., Miyamoto Y., Takahashi R., Goji A., Masuda K., et al. The first Japanese patient with mandibular hypoplasia, deafness, progeroid features and lipodystrophy diagnosed via POLD1 mutation detection. Hum. Genome Var. 2017;4:17031. doi: 10.1038/hgv.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang X.-W., Lu L.-Y., Xie Y., Yu X. A Chinese girl with mandibular hypoplasia, deafness, progeroid features, and lipodystrophy (MDPL) diagnosed via POLD1 mutation detection. Chin. Med. J. 2020;133:2009–2011. doi: 10.1097/CM9.0000000000000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pelosini C., Martinelli S., Ceccarini G., Magno S., Barone I., Basolo A., Fierabracci P., Vitti P., Maffei M., Santini F. Identification of a novel mutation in the polymerase delta 1 (POLD1) gene in a lipodystrophic patient affected by mandibular hypoplasia, deafness, progeroid features (MDPL) syndrome. Metabolism. 2014;63:1385–1389. doi: 10.1016/j.metabol.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 105.Lessel D., Hisama F.M., Szakszon K., Saha B., Sanjuanelo A.B., Salbert B.A., Steele P.D., Baldwin J., Brown W.T., Piussan C., et al. POLD1Germline Mutations in Patients Initially Diagnosed with Werner Syndrome. Hum. Mutat. 2015;36:1070–1079. doi: 10.1002/humu.22833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Elouej S., Beleza-Meireles A., Caswell R., Colclough K., Ellard S., Desvignes J.P., Béroud C., Lévy N., Mohammed S., De Sandre-Giovannoli A. Exome sequencing reveals a de novo POLD1 mutation causing phenotypic variability in mandibular hypoplasia, deafness, progeroid features, and lipodystrophy syndrome (MDPL) Metabolism. 2017;71:213–225. doi: 10.1016/j.metabol.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 107.Ajluni N., Meral R., Neidert A.H., Brady G.F., Buras E., McKenna B., DiPaola F., Chenevert T.L., Horowitz J.F., Buggs-Saxton C., et al. Spectrum of disease associated with partial lipodystrophy: Lessons from a trial cohort. Clin. Endocrinol. 2017;86:698–707. doi: 10.1111/cen.13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Conde C.D., Petronczki Ö.Y., Baris S., Willmann K.L., Girardi E., Salzer E., Weitzer S., Ardy R.C., Krolo A., Ijspeert H., et al. Polymerase δ deficiency causes syndromic immunodeficiency with replicative stress. J. Clin. Investig. 2019;129:4194–4206. doi: 10.1172/JCI128903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nichols-Vinueza D.X., Delmonte O.M., Bundy V., Bosticardo M., Zimmermann M.T., Dsouza N.R., Pala F., Dobbs K., Stoddard J., Niemela J.E., et al. POLD1 Deficiency Reveals a Role for POLD1 in DNA Repair and T and B Cell Development. J. Clin. Immunol. 2020:1–4. doi: 10.1007/s10875-020-00903-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cui Y., Keles S., Charbonnier L.-M., Julé A.M., Henderson L., Celik S.C., Reisli I., Shen C., Xie W.J., Schmitz-Abe K., et al. Combined immunodeficiency caused by a loss-of-function mutation in DNA polymerase delta 1. J. Allergy Clin. Immunol. 2020;145:391–401.e8. doi: 10.1016/j.jaci.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Baple E.L., Chambers H., Cross H.E., Fawcett H., Nakazawa Y., Chioza B.A., Harlalka G.V., Mansour S., Sreekantan-Nair A., Patton M.A., et al. Hypomorphic PCNA mutation underlies a human DNA repair disorder. J. Clin. Investig. 2014;124:3137–3146. doi: 10.1172/JCI74593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Siitonen H.A., Sotkasiira J., Biervliet M., Benmansour A., Capri Y., Cormier-Daire V., Crandall B., Hannula-Jouppi K., Hennekam R., Herzog D., et al. The mutation spectrum in RECQL4 diseases. Eur. J. Hum. Genet. 2008;17:151–158. doi: 10.1038/ejhg.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Van Maldergem L., Siitonen H.A., Jalkh N., Chouery E., De Roy M., Delague V., Muenke M., Jabs E.W., Cai J., Wang L.L., et al. Revisiting the craniosynostosis-radial ray hypoplasia association: Baller-Gerold syndrome caused by mutations in the RECQL4 gene. J. Med Genet. 2005;43:148–152. doi: 10.1136/jmg.2005.031781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cao D.-H., Mu K., Liu D., Sun J., Bai X., Zhang N., Qiu G., Ma X. Case Report Identification of novel compound heterozygous RECQL4 mutations and prenatal diagnosis of Baller-Gerold syndrome: A case report. Genet. Mol. Res. 2015;14:4757–4766. doi: 10.4238/2015.May.11.8. [DOI] [PubMed] [Google Scholar]
- 115.Kaneko H., Izumi R., Oda H., Ohara O., Sameshima K., Ohnishi H., Fukao T., Funato M. Nationwide survey of Baller-Gerold syndrome in Japanese population. Mol. Med. Rep. 2017;15:3222–3224. doi: 10.3892/mmr.2017.6408. [DOI] [PubMed] [Google Scholar]
- 116.Debeljak M., Zver A., Jazbec J. A patient with Baller-Gerold syndrome and midline NK/T lymphoma. Am. J. Med. Genet. 2009;149:755–759. doi: 10.1002/ajmg.a.32736. [DOI] [PubMed] [Google Scholar]
- 117.Wang L.L., Gannavarapu A., Kozinetz C.A., Levy M.L., Lewis R.A., Chintagumpala M.M., Ruiz-Maldanado R., Contreras-Ruiz J., Cunniff C., Erickson R.P., et al. Association Between Osteosarcoma and Deleterious Mutations in the RECQL4 Gene in Rothmund-Thomson Syndrome. J. Natl. Cancer Instig. 2003;95:669–674. doi: 10.1093/jnci/95.9.669. [DOI] [PubMed] [Google Scholar]
- 118.Piard J., Aral B., Vabres P., Holder-Espinasse M., Mégarbané A., Gauthier S., Capra V., Pierquin G., Callier P., Baumann C., et al. Search forReCQL4mutations in 39 patients genotyped for suspected Rothmund-Thomson/Baller-Gerold syndromes. Clin. Genet. 2014;87:244–251. doi: 10.1111/cge.12361. [DOI] [PubMed] [Google Scholar]
- 119.Cao F., Lu L., Abrams S.A., Hawthorne K.M., Tam A., Jin W., Dawson B., Shypailo R., Liu H., Lee B., et al. Generalized metabolic bone disease and fracture risk in Rothmund-Thomson syndrome. Hum. Mol. Genet. 2017;26:3046–3055. doi: 10.1093/hmg/ddx178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kitao S., Shimamoto A., Goto M., Miller R.W., Smithson W.A., Lindor N.M., Furuichi Y. Mutations in RECQL4 cause a subset of cases of Rothmund-Thomson syndrome. Nat. Genet. 1999;22:82–84. doi: 10.1038/8788. [DOI] [PubMed] [Google Scholar]
- 121.Cabral R.E.C., Queille S., Bodemer C., De Prost Y., Neto J.B.C., Sarasin A., Daya-Grosjean L. Identification of new RECQL4 mutations in Caucasian Rothmund–Thomson patients and analysis of sensitivity to a wide range of genotoxic agents. Mutat. Res. Mol. Mech. Mutagen. 2008;643:41–47. doi: 10.1016/j.mrfmmm.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 122.Kellermayer R., Siitonen H.A., Hadzsiev K., Kestilä M., Kosztolányi G. A Patient with Rothmund-Thomson Syndrome and All Features of RAPADILINO. Arch. Dermatol. 2005;141:617–620. doi: 10.1001/archderm.141.5.617. [DOI] [PubMed] [Google Scholar]
- 123.Zhang X., Geng S., Zheng Y. Rare presentation of Rothmund-Thomson syndrome with novel compound heterozygous mutations of the RECQL4 gene. Bras. Dermatol. 2020;95:538–540. doi: 10.1016/j.abd.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yadav S., Thakur S., Kohlhase J., Bhari N., Kabra M., Gupta N. Report of Two Novel Mutations in Indian Patients with Rothmund–Thomson Syndrome. J. Pediatr. Genet. 2019;8:163–167. doi: 10.1055/s-0039-1684017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang T., Chen L., She Q., Dong Y., Deng Y. Four novel RECQL4 mutations in four Chinese patients with Rothmund-Thomson syndrome and analysis of RECQL4 mRNA expression level in one typical patient. J. Dermatol. Sci. 2018;91:335–337. doi: 10.1016/j.jdermsci.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 126.Lindor N.M., Furuichi Y., Kitao S., Shimamoto A., Arndt C., Jalal S. Rothmund-Thomson syndrome due toRECQ4 helicase mutations: Report and clinical and molecular comparisons with Bloom syndrome and Werner syndrome. Am. J. Med. Genet. 2000;90:223–228. doi: 10.1002/(SICI)1096-8628(20000131)90:3<223::AID-AJMG7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 127.Wang L.L., Worley K.C., Gannavarapu A., Chintagumpala M.M., Levy M.L., Plon S.E. Intron-Size Constraint as a Mutational Mechanism in Rothmund-Thomson Syndrome. Am. J. Hum. Genet. 2002;71:165–167. doi: 10.1086/341234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Balraj P., Concannon P., Jamal R., Beghini A., Hoe T., Khoo A.S., Volpi L. An unusual mutation in RECQ4 gene leading to Rothmund–Thomson syndrome. Mutat. Res. Mol. Mech. Mutagen. 2002;508:99–105. doi: 10.1016/S0027-5107(02)00189-6. [DOI] [PubMed] [Google Scholar]
- 129.Beghini A., Castorina P., Roversi G., Modiano P., Larizza L. RNA processing defects of the helicase gene RECQL4 in a compound heterozygous Rothmund-Thomson patient. Am. J. Med. Genet. 2003;120:395–399. doi: 10.1002/ajmg.a.20154. [DOI] [PubMed] [Google Scholar]
- 130.Van Rij M.C., Grijsen M.L., Appelman-Dijkstra N.M., Hansson K., Al Ruivenkamp C., Mulder K., Van Doorn R., Oranje A.P., Kant S.G. Rothmund-Thomson syndrome and osteoma cutis in a patient previously diagnosed as COPS syndrome. Eur. J. Nucl. Med. Mol. Imaging. 2016;176:279–283. doi: 10.1007/s00431-016-2834-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gui B., Song Y., Hu X., Li H., Qin Z., Su J., Li C., Fan X., Li M., Luo J., et al. Novel pathogenic RECQL4 variants in Chinese patients with Rothmund-Thomson syndrome. Gene. 2018;654:110–115. doi: 10.1016/j.gene.2018.02.047. [DOI] [PubMed] [Google Scholar]
- 132.Salih A., Inoue S., Onwuzurike N. Rothmund-Thomson syndrome (RTS) with osteosarcoma due to RECQL4 mutation. BMJ Case Rep. 2018;2018:2017222384. doi: 10.1136/bcr-2017-222384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bhoyrul B., Lindsay H., Robinson R., Stahlschmidt J., Palmer T., Edward S., Clark S. Pili annulati in a case of Rothmund-Thomson syndrome with a novel frameshift mutation in RECQL4. J. Eur. Acad. Dermatol. Venereol. 2017;32:e221–e223. doi: 10.1111/jdv.14742. [DOI] [PubMed] [Google Scholar]
- 134.Suter A., Itin P., Heinimann K., Ahmed M., Ashraf T., Fryssira H., Kini U., Lapunzina P., Miny P., Sommerlund M., et al. Rothmund-Thomson Syndrome: Novel pathogenic mutations and frequencies of variants in the RECQL4 and USB1 (C16orf57) gene. Mol. Genet. Genom. Med. 2016;4:359–366. doi: 10.1002/mgg3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Guerrero-González G.A., Martínez-Cabriales S.A., Hernández-Juárez A.A., Lugo-Trampe J.D.J., Espinoza-González N.A., Gómez-Flores M., Ocampo-Candiani J. Rothmund-thomson syndrome: A 13-year follow-up. Case Rep. Dermatol. 2014;6:176–179. doi: 10.1159/000365625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Simon T., Kohlhase J., Wilhelm C., Kochanek M., De Carolis B., Berthold F. Multiple malignant diseases in a patient with Rothmund-Thomson syndrome with RECQL4 mutations: Case report and literature review. Am. J. Med. Genet. 2010;152:1575–1579. doi: 10.1002/ajmg.a.33427. [DOI] [PubMed] [Google Scholar]
- 137.Fradin M., Merklen-Djafri C., Perrigouard C., Aral B., Muller J., Stoetzel C., Frouin E., Flori E., Doray B., Dollfus H., et al. Long-Term Follow-Up and Molecular Characterization of a Patient with a RECQL4 Mutation Spectrum Disorder. Dermatolog. 2013;226:353–357. doi: 10.1159/000351311. [DOI] [PubMed] [Google Scholar]
- 138.Colombo E.A., Fontana L., Roversi G., Negri G., Castiglia D., Paradisi M., Zambruno G., Larizza L. Novel physiological RECQL4 alternative transcript disclosed by molecular characterisation of Rothmund–Thomson Syndrome sibs with mild phenotype. Eur. J. Hum. Genet. 2014;22:1298–1304. doi: 10.1038/ejhg.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Colombo E.A., Locatelli A., Sánchez L.C., Romeo S., Elcioglu N.H., Maystadt I., Esteve-Martínez A., Sironi A., Fontana L., Finelli P., et al. Rothmund-Thomson Syndrome: Insights from New Patients on the Genetic Variability Underpinning Clinical Presentation and Cancer Outcome. Int. J. Mol. Sci. 2018;19:1103. doi: 10.3390/ijms19041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sznajer Y., Siitonen H.A., Roversi G., Dangoisse C., Scaillon M., Ziereisen F., Tenoutasse S., Kestilä M., Larizza L. Atypical Rothmund-Thomson syndrome in a patient with compound Heterozygous Mutations in RECQL4 Gene and phenotypic features in RECQL4 syndromes. Eur. J. Nucl. Med. Mol. Imaging. 2007;167:175–181. doi: 10.1007/s00431-007-0447-6. [DOI] [PubMed] [Google Scholar]
- 141.Broom M.A., Wang L., Otta S., Knutsen A., Siegfried E., Batanian J., Kelly M., Shah M. Successful umbilical cord blood stem cell transplantation in a patient with Rothmund-Thomson syndrome and combined immunodeficiency. Clin. Genet. 2006;69:337–343. doi: 10.1111/j.1399-0004.2006.00592.x. [DOI] [PubMed] [Google Scholar]
- 142.Gao J., Wang Q., Dong C., Chen S., Qi Y., Liu Y. Whole Exome Sequencing Identified MCM2 as a Novel Causative Gene for Autosomal Dominant Nonsyndromic Deafness in a Chinese Family. PLoS ONE. 2015;10:e0133522. doi: 10.1371/journal.pone.0133522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.De Munnik S.A., Hoefsloot L.H., Roukema J., Schoots J., Knoers N.V.A.M., Brunner H.G., Jackson A.P., Bongers E.M.H.F. Meier-Gorlin syndrome. Orphanet J. Rare Dis. 2015;10:114. doi: 10.1186/s13023-015-0322-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.De Munnik S.A., Otten B.J., Schoots J., Bicknell L.S., Aftimos S., Al-Aama J.Y., Van Bever Y., Bober M.B., Borm G.F., Clayton-Smith J., et al. Meier-Gorlin syndrome: Growth and secondary sexual development of a microcephalic primordial dwarfism disorder. Am. J. Med. Genet. 2012;158:2733–2742. doi: 10.1002/ajmg.a.35681. [DOI] [PubMed] [Google Scholar]
- 145.Stiff T., Alagoz M., Alcantara D., Outwin E., Brunner H.G., Bongers E.M.H.F., O’Driscoll M., Jeggo P.A. Deficiency in Origin Licensing Proteins Impairs Cilia Formation: Implications for the Aetiology of Meier-Gorlin Syndrome. PLoS Genet. 2013;9:e1003360. doi: 10.1371/journal.pgen.1003360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yao L., Chen J., Wu X., Jia S., Meng A. Zebrafish cdc6 hypomorphic mutation causes Meier-Gorlin syndrome-like phenotype. Hum. Mol. Genet. 2017;26:4168–4180. doi: 10.1093/hmg/ddx305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bleichert F., Balasov M., Chesnokov I., Nogales E., Botchan M.R., Berger J.M. A Meier-Gorlin syndrome mutation in a conserved C-terminal helix of Orc6 impedes origin recognition complex formation. eLife. 2013;2:e00882. doi: 10.7554/eLife.00882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kuo A.J., Song J., Cheung P., Ishibe-Murakami S., Yamazoe S., Chen J.K., Patel D.J., Gozani O. The BAH domain of ORC1 links H4K20me2 to DNA replication licensing and Meier-Gorlin syndrome. Nat. Cell Biol. 2012;484:115–119. doi: 10.1038/nature10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang W., Sankaran S., Gozani O., Song J. A Meier-Gorlin Syndrome Mutation Impairs the ORC1-Nucleosome Association. ACS Chem. Biol. 2015;10:1176–1180. doi: 10.1021/cb5009684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Johnson C.A., Collis S.J. Ciliogenesis and the DNA damage response: A stressful relationship. Cilia. 2016;5:19. doi: 10.1186/s13630-016-0040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.O’Driscoll M., Ruiz-Perez V.L., Woods C.G., Jeggo P.A., Goodship J.A. Goodship, A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat. Genet. 2003;33:497–501. doi: 10.1038/ng1129. [DOI] [PubMed] [Google Scholar]
- 152.Guemez-Gamboa A., Coufal N.G., Gleeson J.G. Primary Cilia in the Developing and Mature Brain. Neuron. 2014;82:511–521. doi: 10.1016/j.neuron.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Waters A.M., Beales P.L. Ciliopathies: An expanding disease spectrum. Pediatr. Nephrol. 2011;26:1039–1056. doi: 10.1007/s00467-010-1731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yoshida K., Oyaizu N., Dutta A., Inoue I. The destruction box of human Geminin is critical for proliferation and tumor growth in human colon cancer cells. Oncogene. 2004;23:58–70. doi: 10.1038/sj.onc.1206987. [DOI] [PubMed] [Google Scholar]
- 155.Morrill S., He D.Z. Apoptosis in inner ear sensory hair cells. J. Otol. 2017;12:151–164. doi: 10.1016/j.joto.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Abel A.M., Yang C., Thakar M.S., Malarkannan S. Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front. Immunol. 2018;9:1869. doi: 10.3389/fimmu.2018.01869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shilling H.G., McQueen K.L., Cheng N.W., Shizuru J.A., Negrin R.S., Parham P. Reconstitution of NK cell receptor repertoire following HLA-matched hematopoietic cell transplantation. Blood. 2003;101:3730–3740. doi: 10.1182/blood-2002-08-2568. [DOI] [PubMed] [Google Scholar]
- 158.Chan A., Hong D.-L., Atzberger A., Kollnberger S., Filer A.D., Buckley C.D., McMichael A., Enver T., Bowness P. CD56bright Human NK Cells Differentiate into CD56dim Cells: Role of Contact with Peripheral Fibroblasts. J. Immunol. 2007;179:89–94. doi: 10.4049/jimmunol.179.1.89. [DOI] [PubMed] [Google Scholar]
- 159.Romagnani C., Juelke K., Falco M., Morandi B., D’Agostino A., Costa R., Ratto G., Forte G., Carrega P., Lui G., et al. CD56brightCD16− Killer Ig-Like Receptor− NK Cells Display Longer Telomeres and Acquire Features of CD56dim NK Cells upon Activation. J. Immunol. 2007;178:4947–4955. doi: 10.4049/jimmunol.178.8.4947. [DOI] [PubMed] [Google Scholar]
- 160.Orange J.S. Stiehm’s Immune Deficiencies. Elsevier; London, UK: 2014. Natural Killer Cell Deficiency; pp. 765–774. [Google Scholar]
- 161.Valiathan R., Deeb K., Diamante M., Ashman M., Sachdeva N., Asthana D. Reference ranges of lymphocyte subsets in healthy adults and adolescents with special mention of T cell maturation subsets in adults of South Florida. Immunobiology. 2014;219:487–496. doi: 10.1016/j.imbio.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 162.Caligiuri M.A. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Orange J.S. Natural killer cell deficiency. J. Allergy Clin. Immunol. 2013;132:515–525. doi: 10.1016/j.jaci.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Eidenschenk C., Dunne J., Jouanguy E., Fourlinnie C., Gineau L., Bacq D., McMahon C., Smith O., Casanova J.-L., Abel L., et al. A Novel Primary Immunodeficiency with Specific Natural-Killer Cell Deficiency Maps to the Centromeric Region of Chromosome 8. Am. J. Hum. Genet. 2006;78:721–727. doi: 10.1086/503269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shima N., Alcaraz A., Liachko I., Buske T.R., Andrews C.A., Munroe R.J., Hartford S.A., Tye B.K., Schimenti J.C. A viable allele of Mcm4 causes chromosome instability and mammary adenocarcinomas in mice. Nat. Genet. 2006;39:93–98. doi: 10.1038/ng1936. [DOI] [PubMed] [Google Scholar]
- 166.Glover T.W., Berger C., Coyle J., Echo B. DNA polymerase α inhibition by aphidicolin induces gaps and breaks at common fragile sites in human chromosomes. Hum. Genet. 1984;67:136–142. doi: 10.1007/BF00272988. [DOI] [PubMed] [Google Scholar]
- 167.Moon W.Y., Powis S.J. Does Natural Killer Cell Deficiency (NKD) Increase the Risk of Cancer? NKD May Increase the Risk of Some Virus Induced Cancer. Front. Immunol. 2019;10:1703. doi: 10.3389/fimmu.2019.01703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Franchitto A., Pichierri P. Replication fork recovery and regulation of common fragile sites stability. Cell. Mol. Life Sci. 2014;71:4507–4517. doi: 10.1007/s00018-014-1718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hanna S., Béziat V., Jouanguy E., Casanova J.L., Etzioni A. A homozygous mutation of RTEL1 in a child presenting with an apparently isolated natural killer cell deficiency. J. Allergy Clin. Immunol. 2015;136:1113–1114. doi: 10.1016/j.jaci.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 170.Speckmann C., Sahoo S.S., Rizzi M., Hirabayashi S., Karow A., Serwas N.K., Hoemberg M., Damatova N., Schindler D., Vannier J.-B., et al. Clinical and Molecular Heterogeneity of RTEL1 Deficiency. Front. Immunol. 2017;8:449. doi: 10.3389/fimmu.2017.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Babushok D.V., Hsu A.P., Dokal I. Stiehm’s Immune Deficiencies. Elsevier; London, UK: 2020. Bone marrow failure syndromes; pp. 411–441. [Google Scholar]
- 172.Fasth A., Forestier E., Holmberg E., Holmgren G., Nordenson I., Soderstrom T., Wahlström J. Fragility of the Centromeric Region of Chromosome 1 Associated with Combined Immunodeficiency in Siblings A Recessively Inherited Entity? Acta Paediatr. 1990;79:605–612. doi: 10.1111/j.1651-2227.1990.tb11524.x. [DOI] [PubMed] [Google Scholar]
- 173.Armando R.G., Gómez D.L.M., Maggio J., Sanmartin M.C., Gomez D.E. Telomeropathies: Etiology, diagnosis, treatment and follow-up. Ethical and legal considerations. Clin. Genet. 2019;96:3–16. doi: 10.1111/cge.13526. [DOI] [PubMed] [Google Scholar]
- 174.Ouyang Q., Baerlocher G., Vulto I., Lansdorp P.M. Telomere Length in Human Natural Killer Cell Subsets. Ann. N. Y. Acad. Sci. 2007;1106:240–252. doi: 10.1196/annals.1392.001. [DOI] [PubMed] [Google Scholar]
- 175.Fali T., Papagno L., Bayard C., Mouloud Y., Boddaert J., Sauce D., Appay V. New Insights into Lymphocyte Differentiation and Aging from Telomere Length and Telomerase Activity Measurements. J. Immunol. 2019;202:1962–1969. doi: 10.4049/jimmunol.1801475. [DOI] [PubMed] [Google Scholar]
- 176.Chang A.C.Y., Blau H.M. Short telomeres—A hallmark of heritable cardiomyopathies. Differentiation. 2018;100:31–36. doi: 10.1016/j.diff.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chang A.C.Y., Chang A.C.H., Kirillova A., Sasagawa K., Su W., Weber G., Lin J., Termglinchan V., Karakikes I., Seeger T., et al. Telomere shortening is a hallmark of genetic cardiomyopathies. Proc. Natl. Acad. Sci. USA. 2018;115:9276–9281. doi: 10.1073/pnas.1714538115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pedreira C., Savarirayan R., Zacharin M.R. IMAGe syndrome: A complex disorder affecting growth, adrenal and gonadal function, and skeletal development. J. Pediatr. 2004;144:274–277. doi: 10.1016/j.jpeds.2003.09.052. [DOI] [PubMed] [Google Scholar]
- 179.Stampone E., Caldarelli I., Zullo A., Bencivenga D., Mancini F.P., Ragione F.D., Borriello A. Genetic and Epigenetic Control of CDKN1C Expression: Importance in Cell Commitment and Differentiation, Tissue Homeostasis and Human Diseases. Int. J. Mol. Sci. 2018;19:1055. doi: 10.3390/ijms19041055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Reinier F., Zoledziewska M., Hanna D., Smith J.D., Valentíni M., Zara I., Berutti R., Sanna S., Oppo M., Cusano R., et al. Mandibular hypoplasia, deafness, progeroid features and lipodystrophy (MDPL) syndrome in the context of inherited lipodystrophies. Metab. Clin. Exp. 2015;64:1530–1540. doi: 10.1016/j.metabol.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 181.Shah S.N., Opresko P.L., Meng X., Lee M.Y.W.T., Eckert K.A. DNA structure and the Werner protein modulate human DNA polymerase delta-dependent replication dynamics within the common fragile site FRA16D. Nucleic Acids Res. 2009;38:1149–1162. doi: 10.1093/nar/gkp1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tsurimoto T., Shinozaki A., Yano M., Seki M., Enomoto T. Human Werner helicase interacting protein 1 (WRNIP1) functions as a novel modulator for DNA polymerase δ. Genes Cells. 2004;10:13–22. doi: 10.1111/j.1365-2443.2004.00812.x. [DOI] [PubMed] [Google Scholar]
- 183.Li B., Reddy S., Comai L. The Werner Syndrome Helicase Coordinates Sequential Strand Displacement and FEN1-Mediated Flap Cleavage during Polymerase δ Elongation. Mol. Cell. Biol. 2016;37:e00560-16. doi: 10.1128/MCB.00560-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Feltes B.C., Poloni J.D.F., Miyamoto K., Bonatto D. Genome Stability. Elsevier; Amsterdam, The Netherlands: 2016. Human Diseases Associated With Genome Instability; pp. 447–462. [Google Scholar]
- 185.Duffy C.M., Hilbert B.J., Kelch B.A. A Disease-Causing Variant in PCNA Disrupts a Promiscuous Protein Binding Site. J. Mol. Biol. 2016;428:1023–1040. doi: 10.1016/j.jmb.2015.11.029. [DOI] [PubMed] [Google Scholar]
- 186.Siitonen H.A., Haravuori H. Molecular defect of RAPADILINO syndrome expands the phenotype spectrum of RECQL diseases. Hum. Mol. Genet. 2003;12:2837–2844. doi: 10.1093/hmg/ddg306. [DOI] [PubMed] [Google Scholar]
- 187.Larizza L., Magnani I., Roversi G. Rothmund–Thomson syndrome and RECQL4 defect: Splitting and lumping. Cancer Lett. 2006;232:107–120. doi: 10.1016/j.canlet.2005.07.042. [DOI] [PubMed] [Google Scholar]
- 188.Dietschy T., Shevelev I., Stagljar I. The molecular role of the Rothmund-Thomson-, RAPADILINO- and Baller-Gerold-gene product, RECQL4: Recent progress. Cell. Mol. Life Sci. 2007;64:796–802. doi: 10.1007/s00018-007-6468-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kaariainen H., Ryöppy S., Norio R. RAPADILINO syndrome with radial and patellar aplasia/hypoplasia as main manifestations. Am. J. Med. Genet. 1989;33:346–351. doi: 10.1002/ajmg.1320330312. [DOI] [PubMed] [Google Scholar]
- 190.Lindor N.M., Devries E.M.G., Michels V.V., Schad C.R., Jalal S.M., Donovan K.M., Smithson W.A., Kvols L.K., Thibodeau S.N., Dewald G.W. Rothmund-Thomson syndrome in siblings: Evidence for acquired in vivo mosaicism. Clin. Genet. 1996;49:124–129. doi: 10.1111/j.1399-0004.1996.tb03270.x. [DOI] [PubMed] [Google Scholar]
- 191.Larizza L., Roversi G., Volpi L. Rothmund-Thomson syndrome. Orphanet J. Rare Dis. 2010;5:1–16. doi: 10.1186/1750-1172-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pujol L.A., Erickson R.P., Heidenreich R.A., Cunniff C. Variable presentation of Rothmund-Thomson syndrome. Am. J. Med. Genet. 2000;95:204–207. doi: 10.1002/1096-8628(20001127)95:3<204::AID-AJMG4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 193.Tong M. Rothmund-Thomson Syndrome in Fraternal Twins. Pediatr. Dermatol. 1995;12:134–137. doi: 10.1111/j.1525-1470.1995.tb00139.x. [DOI] [PubMed] [Google Scholar]
- 194.Kim J.S. Baller-Gerold Syndrome in a Premature Infant with a Mutation in the RECQL4 Gene. Neonatal Med. 2019;26:240–245. doi: 10.5385/nm.2019.26.4.240. [DOI] [Google Scholar]
- 195.Ajeawung N.F., Nguyen T.T.M., Lu L., Kucharski T.J., Rousseau J., Molidperee S., Atienza J., Gamache I., Jin W., Plon S.E., et al. Mutations in ANAPC1, Encoding a Scaffold Subunit of the Anaphase-Promoting Complex, Cause Rothmund-Thomson Syndrome Type 1. Am. J. Hum. Genet. 2019;105:625–630. doi: 10.1016/j.ajhg.2019.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J., Stephens K., Amemiya A., editors. GeneReviews [Internet] University of Washington; Seattle, DC, USA: 1993–2021. [Google Scholar]
- 197.Burks L.M., Yin J., Plon S.E. Nuclear import and retention domains in the amino terminus of RECQL4. Gene. 2007;391:26–38. doi: 10.1016/j.gene.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 198.Singh D.K., Karmakar P., Aamann M., Schurman S.H., May A., Croteau D.L., Burks L., Plon S.E., Bohr V.A. The involvement of human RECQL4 in DNA double-strand break repair. Aging Cell. 2010;9:358–371. doi: 10.1111/j.1474-9726.2010.00562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Croteau D.L., Rossi M.L., Ross J., Dawut L., Dunn C., Kulikowicz T., Bohr V.A. RAPADILINO RECQL4 Mutant Protein Lacks Helicase and ATPase Activity. Biochim. Biophys. Acta Mol. Basis Dis. 2012;1822:1727–1734. doi: 10.1016/j.bbadis.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Vollebregt M.M.G., Malfroot A., De Raedemaecker M., Van Der Burg M., Bosch J.V.D.W.T. Immunodeficiency in a Child with Rapadilino Syndrome: A Case Report and Review of the Literature. Case Rep. Immunol. 2015;2015:1–4. doi: 10.1155/2015/137368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.De Somer L., Wouters C., Morren M.-A., De Vos R., Oord J.V.D., Devriendt K., Meyts I. Granulomatous skin lesions complicating Varicella infection in a patient with Rothmund-Thomson syndrome and immune deficiency: Case report. Orphanet J. Rare Dis. 2010;5:37. doi: 10.1186/1750-1172-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ito T., Tokura Y., Moriwaki S., Yasuda K., Ohnishi A., Furukawa F., Yamaizumi K., Takigawa M. Rothmund-Thomson syndrome with herpes encephalitis. Eur. J. Dermatol. EJD. 1999;9:354–356. [PubMed] [Google Scholar]
- 203.Kubota M., Yasunaga M., Hashimoto H., Kimata H., Mikawa H., Shinya A., Nishigori C. IgG4 deficiency with Rothmund-Thomson syndrome: A case report. Eur. J. Nucl. Med. Mol. Imaging. 1993;152:406–408. doi: 10.1007/BF01955898. [DOI] [PubMed] [Google Scholar]
- 204.Abe T., Yoshimura A., Hosono Y., Tada S., Seki M., Enomoto T. The N-terminal region of RECQL4 lacking the helicase domain is both essential and sufficient for the viability of vertebrate cells. Biochim. Biophys. Acta Bioenergy. 2011;1813:473–479. doi: 10.1016/j.bbamcr.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 205.Castillo-Tandazo W., Smeets M.F., Murphy V., Liu R., Hodson C., Heierhorst J., Deans A.J., Walkley C.R. ATP-dependent helicase activity is dispensable for the physiological functions of Recql4. PLoS Genet. 2019;15:e1008266. doi: 10.1371/journal.pgen.1008266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Matson J.P., Dumitru R., Coryell P., Baxley R.M., Chen W., Twaroski K., Webber B.R., Tolar J., Bielinsky A.-K., Purvis J.E., et al. Rapid DNA replication origin licensing protects stem cell pluripotency. eLife. 2017;6:e30473. doi: 10.7554/eLife.30473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Rivera-Mulia J.C., Buckley Q., Sasaki T., Zimmerman J., Didier R.A., Nazor K., Loring J.F., Lian Z., Weissman S., Robins A.J., et al. Dynamic changes in replication timing and gene expression during lineage specification of human pluripotent stem cells. Genome Res. 2015;25:1091–1103. doi: 10.1101/gr.187989.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Besnard E., Babled A., Lapasset L., Milhavet O., Parrinello H., Dantec C., Marin J.-M., Lemaitre J.-M. Unraveling cell type–specific and reprogrammable human replication origin signatures associated with G-quadruplex consensus motifs. Nat. Struct. Mol. Biol. 2012;19:837–844. doi: 10.1038/nsmb.2339. [DOI] [PubMed] [Google Scholar]
- 209.Maccaroni K., Balzano E., Mirimao F., Giunta S., Pelliccia F. Impaired Replication Timing Promotes Tissue-Specific Expression of Common Fragile Sites. Genes. 2020;11:326. doi: 10.3390/genes11030326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Alvarez S., Díaz M., Flach J., Rodriguez-Acebes S., López-Contreras A.J., Martínez D., Cañamero M., Fernández-Capetillo O., Isern J., Passegué E., et al. Replication stress caused by low MCM expression limits fetal erythropoiesis and hematopoietic stem cell functionality. Nat. Commun. 2015;6:8548. doi: 10.1038/ncomms9548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Stevens H., Williams A.B., Michael W.M. Cell-Type Specific Responses to DNA Replication Stress in Early C. elegans Embryos. PLoS ONE. 2016;11:e0164601. doi: 10.1371/journal.pone.0164601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.