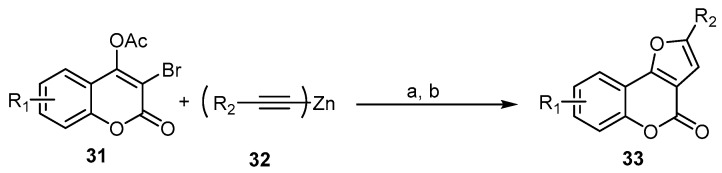Abstract
This review gives an up-to-date overview of the different ways (routes) to the synthesis of coumarin(benzopyrone)-fused, five-membered aromatic heterocycles with one heteroatom, built on the pyrone moiety. Covering 1966 to 2020.
Keywords: coumarins, benzopyrones, five-membered aromatic heterocycles, furan, pyrrole, thiophene, selenophen
1. Introduction
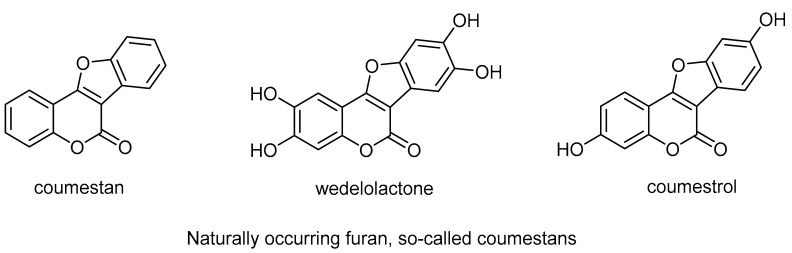
Coumarins are a family of benzopyrones (1,2-benzopyrones or 2H-[1]benzopyran-2-ones), which represent an important family of oxygen-containing heterocycles, widely distributed in nature [1,2,3,4]. Coumarins display a broad range of biological and pharmacological activities, [5,6] such as antiviral [7,8,9,10], anticancer [11,12,13], antimicrobial [14,15], and antioxidant [16,17,18] activities. On the other hand, coumarin represents an ingredient in perfumes [19], cosmetics [20], and as industrial additives [21,22]. Furthermore, coumarins play a pivotal role in science and technology as fluorescent sensors, mainly due to their interesting light-emissive characteristics, which are often responsive to the environment [23,24,25,26]. The coumarin (benzopyrane)-fused, membered aromatic heterocycles built on the α-pyrone moiety are an important scaffold. The only fused heterocycle with an α-pyrone moiety of coumarin that can be found in nature is the furan ring. One example is the naturally occurring furan 4H-furo[3,2-c]benzopyran-4-one, which provides the main core of many natural compounds of so-called coumestans. These comestans include coumestan, wedelolactone, and coumestrol. The coumestans are found in a variety of plant species that are commonly used in traditional medicine [27].
In order to enrich the limited versatility of the structures found in nature, synthesis of coumarin (benzopyrane)-fused, membered aromatic heterocycles has received considerable attention, including numerous reported routes.
This review gives an up-to-date overview of the different ways (routes) to the synthesis of benzopyrone-fused, five-membered aromatic heterocycles with one heteroatom, built on the pyrone moiety, from 1966 to 2020. Our main interest in this current work is to describe the components that have one heteroatom in an alicyclic-fused ring with the pyrone part of coumarin. The synthetic pathway of the investigated scaffold has provided systems containing oxygen, nitrogen, sulfur, and selenium in their core structure. The last heteroatom is less described in the output of the synthetic efforts. The fused heterocycles that contain more than one heteroatom will be detailed in the next part, which we intend to publish in the future.
Many strategies have been developed for the synthesis of the fused, five-membered aromatic heterocycle-benzopyran-4-ones. There are two main approaches to constructing these skeletons: five-membered, aromatic heterocycle construction, and pyrone-ring construction.
2. Synthesis of Benzopyrone-Fused, Five-Membered Aromatic Heterocycles
2.1. Five-Membered Aromatic Rings with One Heteroatom
2.1.1. Furans
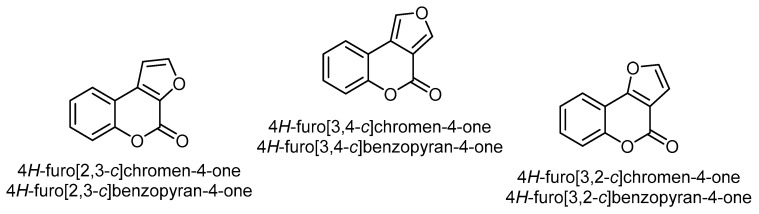
Furobenzopyrone (or furocoumarins) comprises an important class of coumarins found in a wide variety of plants, particularly in the carrot (Apiaceae/Umbelliferae), legume (Fabaceae), and citrus families (Rutaceae) [27]. The chemical structure of furobenzopyrone (furocoumarins) consists of a furan ring fused with coumarin. The fusion of the furan ring to the α-pyrone moiety of coumarin forms the core structure of the three most common isomers, viz. 4H-furo[2,3-c]chromen(benzopyran)-4-one, 4H-furo[3,4-c]chromen(benzopyran)-4-one, and 4H-furo[3,2-c]chromen (benzopyran)-4-one (Figure 1).
Figure 1.
The three most common isomers of a furan ring fused to the α-pyrone moiety of coumarin.
4H-Furo[2,3-c]benzopyran-4-one
Furan Construction
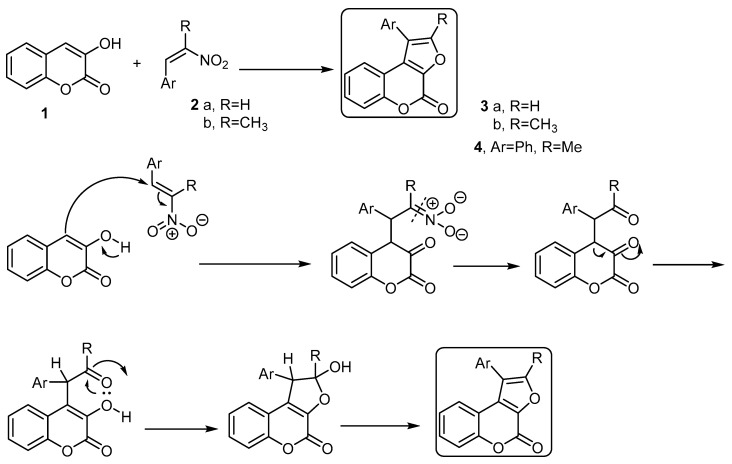
The basic building block for the formation of 4H-furo[2,3-c]benzopyran-4-one is the 3-hydroxycoumarin (1) [28,29]. Pandya and coworkers [30] developed a method to synthesize some 4H-furo[2,3-c]benzopyran-4-ones starting with 3-hydroxycoumarin using the Nef reaction. Thus, the reaction of 3-hydroxycoumarin (1) with various 2-aryl-1-nitro ethenes 2a,b, in the presence of piperidine and methanol as a solvent, followed the Nef reaction condition and afforded a series of 1-aryl-furo[2,3-c]benzopyran-4-ones 3a,b and 1-phenyl-2-methyl-furo[2,3-c]benzopyran-4-one (4), respectively (Scheme 1). The formation of these products was explained by the reaction mechanism (Scheme 1).
Scheme 1.
The Nef reaction to synthesize furo[2,3-c]benzopyran-4-ones 3a,b and 4. Reagents and conditions: MeOH, piperidine, reflux, five outputs in 55%–61% yield.
Pyrone Construction
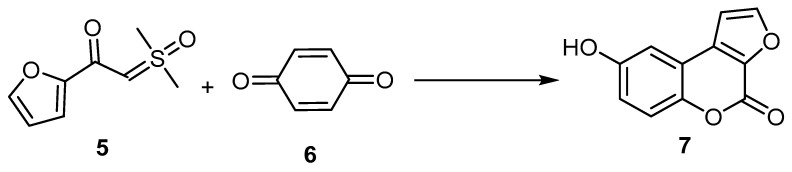
Dong et al., 2020 developed a novel and facile rhodium(III)-catalyzed process of sulfoxonium ylide (5) with hydroquinone (6). The carbonyl in the sulfoxonium ylide assisted the ortho-C–H functionalization of the sulfoxonium ylide, followed by intramolecular annulation with hydroquinone to afford 8-hydroxy-4H-furo[2,3-c]benzopyran-4-one (7) (Scheme 2) [31].
Scheme 2.
Rhodium(III)-catalyzed sequential ortho-C–H oxidative arylation/cyclization of sulfoxonium ylide to afford 4H-furo[2,3-c]benzopyran-4-one (7). Reagents and conditions: [Cp*RhCl2]2 (5 mol %), AgBF4 (20 mol %), Zn(OAc)2 (0.225 mmol), AcOH (0.3 mmol), and acetone (2 mL), 12 h, in a sealed Schlenk tube under N2 at 100 °C, 25% yield.
4H-Furo[3,4-c]benzopyran-4-one
Furan and Pyrone Construction
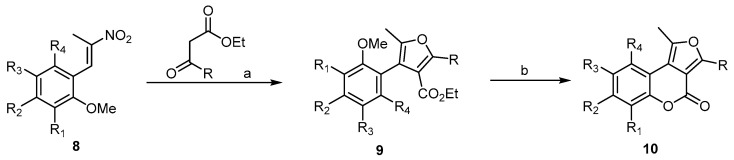
In the literature, a large number of reports described the synthesis of 4H-furo[2,3-c] and 4H-furo[3,2-c]benzopyran-4-ones, while synthesis of the 4H-furo[3,4-c]benzopyran-4-one was reported by only one study, that of Brahmbhatt and his coworkers [32]. The first 4H-furo[3,4-c]benzopyran-4-ones (10) was synthesized by the demethylation–cyclization reaction of intermediates, 3-substituted-4-ethoxycarbonyl furans 9 (Scheme 3). For the demethylation and in situ lactonization steps, several reagents were tried, of which pyridine hydrochloride and HBr in acetic acid were found to be the most promising.
Scheme 3.
Demethylation and in situ lactonization steps to prepare the first 4H-furo[3,4-c]benzopyran-4-one 10. Reagents and conditions: (a) ethyl acetoacetate or ethyl benzoylacetate, piperidine, and MeOH (the Nef reaction condition); (b) HBr, AcOH concentration, 130 °C, 4 h, 16 outputs with 50%–65% yield.
4H-Furo[3,2-c]benzopyran-4-one
Furan Construction
A wide range of research has demonstrated that 4-hydroxycoumarin is the key compound for the synthesis of 4H-furo[3,2-c]benzopyran-4-ones, which can readily react with the C=C bond of the alkene, or the C≡C bond of the alkyne [33,34,35,36,37].
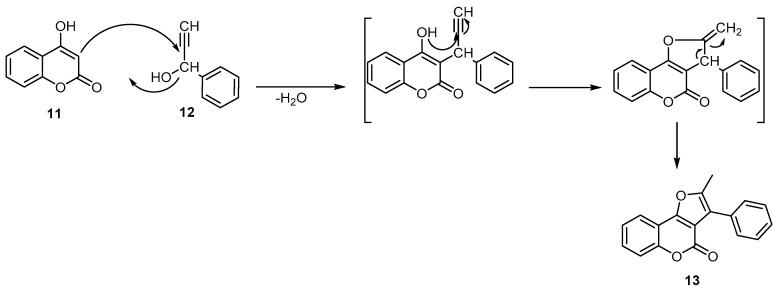
Reisch reported the condensation of 4-hydroxycoumarin (11) with 1-phenyl-2-propyn-1-ol (12) under acidic conditions (a mixture of glacial acetic and concentrated sulfuric acid) to deliver the corresponding 2-methyl-3-phenylfuro[3,2-c]benzopyran-4-one (13) (Scheme 4) [38].
Scheme 4.
Synthesis of 2-methyl-3-phenylfuro[3,2-c]benzopyran-4-one (13). Reagents and conditions: AcOH, conc. H2SO4, 110 °C, 1 h, 70% yield.
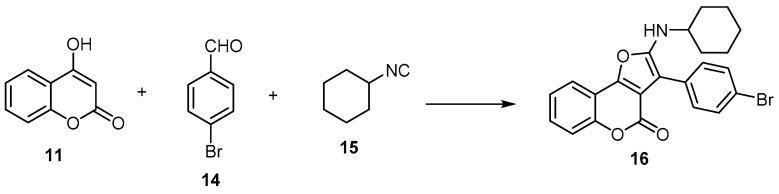
A few studies employed the aliphatic aldehydes as building blocks with 4-hydroxycoumarin (11) to synthesize 4H-furo[3,2-c]benzopyran-4-ones [25,39]. This method was ineffective as it gave a poor yield as well as a mixture of 2,3-dihydrofuran, 4H-furo[3,2-c]benzopyran-4-ones, and 4H-furo[3,2-c]benzopyran-4-ones [39]. Conversely, in the case of using the aromatic aldehyde as a building block, the 4H-furo[3,2-c]benzopyran-4-one was obtained [40]. Kadam et al. developed atom-efficient multicomponent reactions (MCRs) and step-efficient, one-pot synthesis of 3-(4-bromophenyl)-2-(cyclohexylamino)-4H-furo[3,2-c]benzopyran-4-one (16) using 4-hydroxycoumarin (11) with 4-bromobenzaldehyde (14) and cyclohexyl isocyanide (15) as an alkylene source (Scheme 5) [40].
Scheme 5.
Atom-efficient multicomponent reactions (MCRs) and step-efficient, one-pot synthesis of 4H-furo[3,2-c]benzopyran-4-one (16). Reagents and conditions: DMF or toluene, µw, 80 °C, 20 min, 97% yield.
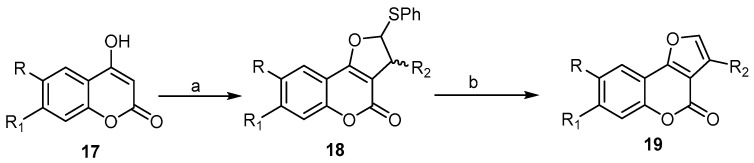
4-Hydroxycoumarin derivatives have received significant attention from researchers, as these derivatives possess 1,3-dicarbonyl systems. It allows for the easy generation of α,α′-dicarbonyl radicals, which can be readily added to the C=C bond of the alkene [41]. The first example of this reaction was described in 1998, by Lee and his coworkers. They reported an efficient way to prepare 4H-furo[3,2-c]benzopyran-4-ones 19 by Ag2CO3/celite (Fetizon’s reagent)-mediated oxidative cycloaddition of 4-hydroxycoumarin 17 to olefins, such as vinyl sulfide and phenyl propenyl sulfide. The resulting dihydrofuro[3,2-c]benzopyran-4-ones 18 was treated by sodium periodate in aqueous methanol to form the corresponding sulfoxides, which, upon refluxing with pyridine in carbon tetrachloride, directly delivered the 4H-furo[3,2-c]benzopyran-4-one 19 in good yields (Scheme 6) [41].
Scheme 6.
A facile synthesis of 4H-furo[3,2-c]benzopyran-4-ones 19 by silver(I)/celite promoted an oxidative cycloaddition reaction. Reagents and conditions: (a) CH2=CHSPh and/or CH3CH=CHSPh, Ag2CO3/celite, acetonitrile, reflux, 3 h; (b) NaIO4, MeOH, CCl4, pyridine, Al2O3, four outputs with 71%–82% yield.
Recently, different catalytic methodologies have been developed for the synthesis of 2H-chromenes, and they are based on three main approaches: catalysis with (transition) metals, metal-free Brønsted catalysis, and Lewis acid/base catalysis, which includes examples of nonenantioselective organocatalysis and enantioselective organocatalysis [42,43,44]. Alkynes have been widely employed as building blocks for this reaction in most cases.
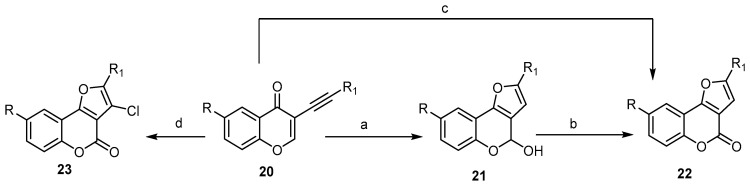
To date, different transition metal (Au, Pt, and Cu) catalyzed/mediated methodologies for benzopyrane synthesis have been reported [27,42,45,46]. Cheng and Hu described a one-pot cascade of an addition/cyclization/oxidation sequence using CuCl2 as the oxidant and CH3SO3H as the acid for regioselective synthesis of 2-substituted-4H-furo[3,2-c]benzopyran-4-ones 22 from the substituted 3-alkynyl-4H-benzopyran-4-one 20 (Scheme 7) [47]. This strategy included the CH3SO3H-acid-catalyzed construction of the furan ring, followed by oxidation of 21 with CuCl2 (Scheme 7) [47]. When the reaction was carried out in the presence of a catalytic amount of CuCl as a Lewis acid and atmospheric oxygen as an oxidative reagent, compound 22 was provided directly. On the other hand, the presence of 10% CuBr and an excess of CuCl2 as the oxidant afforded the corresponding 3-chloro-2-substituted- 4H-furo[3,2-c]benzopyran-4-ones 23 (Scheme 7) [48].
Scheme 7.
Transition metal Cu catalyzed/mediated methodologies for synthesis of the 4H-furo[3,2-c]benzopyran-4-ones 22 and 23. Reagents and conditions: (a) CH3SO3H, H2O, DMF, 90 °C, 1–3 h; (b) CuCl2, 90 °C, 20 h; (c) CuCl, O2, DMF, H2O, 90 °C, 10–20 h, 10 outputs with 37%–88% yield; (d) CuBr, CuCl2, DMF, H2O, 75 °C, 10 h, 13 outputs with 45%–81% yield.
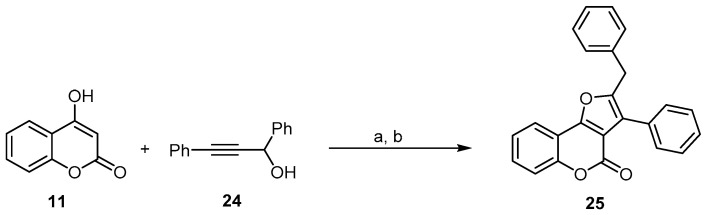
Brønsted-acid-catalyzed propargylations of several organic substrates, including 1,3-dicarbonyl compounds, with alkynols have been reported [49]. In most cases, the acid catalyst is required to promote the propargylation process efficiently. Zhou and coworkers developed a one-pot Yb(OTf)3 propargylation–cycloisomerization sequence of 4-hydroxycoumarin (11) with the propargylic alcohol (24) for the synthesis of a 2-benzyl-3- phenyl-4H-furo[3,2-c]chromen-4-one (25) skeleton using Yb(OTf)3 as a Lewis acid (Scheme 8) [50].
Scheme 8.
One-pot synthesis of 4H-furo[3,2-c]chromen-4-one (25) using a Yb(OTf)3-catalyzed propargylation and allenylation reaction. Reagents and conditions: (a) 5 mol % Yb(OTf)3, CH3NO2, dioxane, 50 °C; (b) K2CO3, 70 °C, 37% yield.
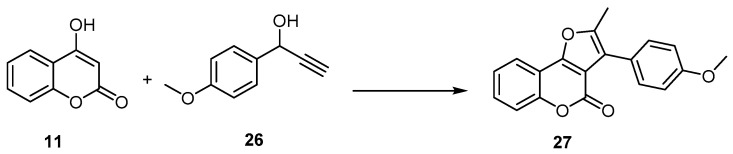
Similarly, 4H-furo[3,2-c] benzopyran-4-one formation reactions proceeded in higher yields and in a one-pot manner, employing a catalytic system composed of the 16-electron allyl–ruthenium(II) complex [Ru(η3-2-C3H4Me)(CO)(dppf)][SbF6] (dppf=1,1′-bis(diphenylphosphino)ferrocene) and trifluoroacetic acid (TFA) in the reaction of 4-hydroxycoumarin (11), with 1-(4-methoxyphenyl)-2-propyn-1-ol (26) as an example. The 4H-furo[3,2-c]benzopyran-4-one (27) was synthesized with a 72% yield (Scheme 9) [50,51,52].
Scheme 9.
The 16-electron allyl–ruthenium(II) complex in preparation of 4H-furo[3,2-c]benzopyran-4-one (27). Reagents and conditions: 16-electron allyl–ruthenium(II) complex [Ru(η3-2-C3H4Me)(CO)(dppf)][SbF6] (5 mol %), trifluoroacetic acid (TFA) (50 mol %), THF, 75 °C, 5 h, 72% yield.
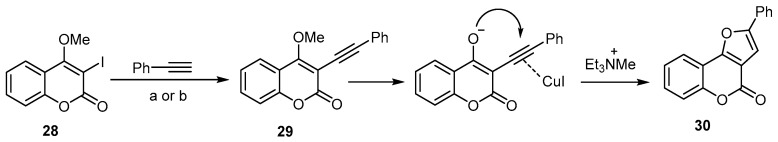
Extensive work has been done to investigate the utility of an aryl alkynyl ether as a furan substrate, instead of arylalkynol, in the synthesis of 4H-furo[3,2-c]benzopyran-4-one [29,35]. The treatment of 3-iodo-4-methoxycoumarin (28) with phenylacetylene by means of sequential Sonogashira C–C coupling conditions resulted in a high-yield formation of the 4H-furo[3,2-c]benzopyran-4-one (30) (Scheme 10) [53]. In this reaction, the triethylamine was used as a base to induce the SN2-type demethylation of the Sonogashira coupling product, followed by an intramolecular attack of the enolate onto the cuprohalide π-complex of the triple bond (Scheme 10).
Scheme 10.
Et3N-induced demethylation–annulation of an aryl alkynyl ether in the synthesis of 4H-furo[3,2-c]benzopyran-4-one (30). Reagents and conditions: (a) alkyne (3 equiv.), 8 mol % PdCl2(PPh3)2, 8 mol % CuI, Et3N/DMF, 80 °C, 48 h, 82% yield; (b) alkyne (3 equiv.), 8 mol % PdCl2(PPh3)2, 8 mol % CuI, Et3N/MeCN, 60 °C, 15 h, 70% yield.
As a follow-up to this type of reaction, a novel and rapid assembly of an interesting class of 4H-furo[3,2-c]benzopyran-4-ones, 33, was successfully achieved using a one-pot sequential coupling/cyclization strategy with 3-bromo-4-acetoxycoumarins 31 and dialkynlzincs 32 prepared in situ as reactive acetylides in transition-metal-catalyzed crosscoupling. The cascade transformation relies on palladium/copper-catalyzed alkynylation and intramolecular hydroalkoxylation (Scheme 11) [54].
Scheme 11.
A one-pot sequential coupling/cyclization strategy in the synthesis of 4H-furo[3,2-c]- benzopyran-4-ones 33. Reagents and conditions: (a) Pd(PPh3)2, CuI, THF, 60 °C; (b) K2CO3, H2O, 13 outputs with 51%–96% yield.
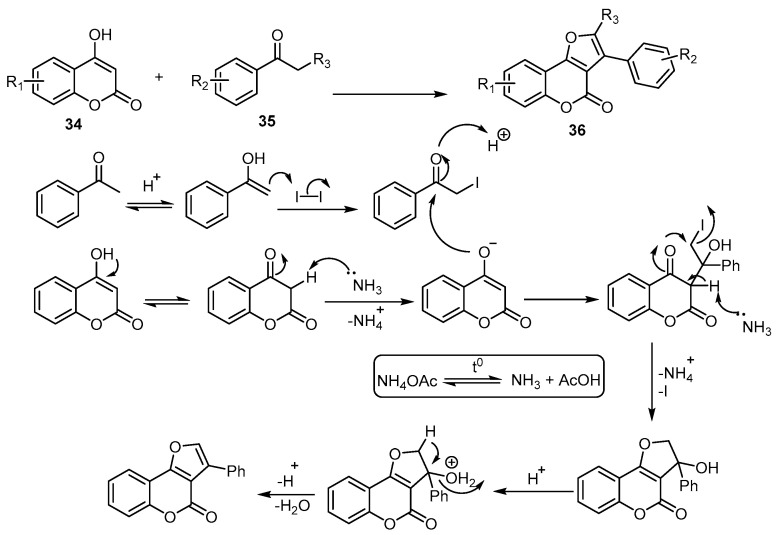
A transition-metal-free approach was developed to achieve 4-H-furo[3,2-c]benzopyran-4-ones via an iodine-promoted one-pot cyclization between 4-hydroxycoumarins 34 and acetophenones 35. The transformation spontaneously proceeded to produce (36) in the presence of NH4OAc. The possible reaction mechanism suggested for the iodine-promoted one-pot cyclization is depicted (Scheme 12) [55].
Scheme 12.
Metal-free synthesis of 4-H-furo[3,2-c]benzopyran-4-ones 36. Reagents and conditions: I2, NH4OAc, PhCl, 120 °C, 18 outputs with 28%–90% yield.
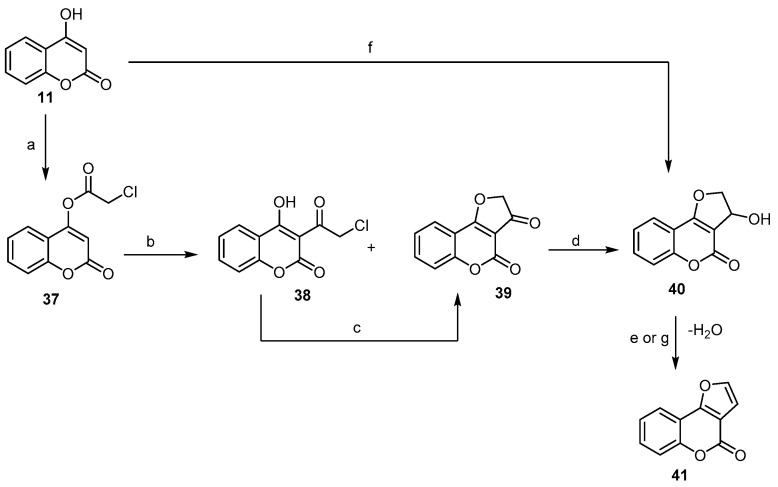
Additionally, Traven et al. [56] provided a new short way for the synthesis of 4H-furo- [3,2-c]benzopyran-4-one, employing the Fries rearrangement of 4-chloroacetoxycoumarin (37) to yield two products, namely 3-chloroacetyl4-hydroxycoumarin (38) and dihydrofuro[2,3-c]coumarin-3-one (39), in the ratio of 2:1. Compound (38), which underwent cyclization, led to the formation of (39). The latter, under reduction and dehydration conditions, afforded 4H-furo[3,2-c]chromen-4-one (41) (Scheme 13). A closely related reaction that allowed for the preparation of (41) was developed by Majumdar and Bhattacharyya [57], following a similar procedure but using chloroacetaldehyde instead of chloroactylchloride in the presence of aqueous potassium carbonate to give 3-hydroxy-2,3- dihydrofuro[3,2-c]benzopyran-4-one (40), which upon treatment with aqueous hydrochloric acid provided 4H-furo[3,2-c]benzopyran-4-one (41) with 72% yield (Scheme 13).
Scheme 13.
Regioselective synthesis of 4H-furo[3,2-c]chromen-4-one (41). Reagents and conditions: (a) ClCH2COCl, dry pyridine, 40 min, reflux, 85% yield; (b) AlCl3, 140–150 °C, 60% yield; (c) AlCl3, 140–150 °C, 30–40 min or K2CO3, acetone, 10 min, stirring, r.t., 50% yield; (d) NaBH4, 85% yield; (e) H2SO4 (30%), EtOH, heat, 30 min, 80% yield; (f) COCH2Cl, K2CO3, 73% yield; (g) HCl, 72% yield.
Pyrone Construction
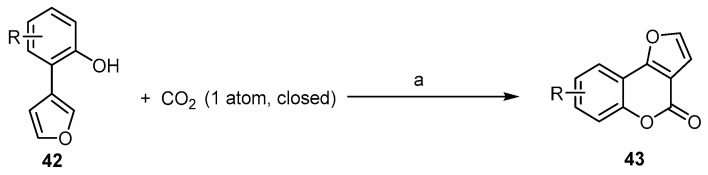
Recently, much effort has been devoted to the development of oxidative intramolecular C–O bond-forming cyclization reactions for the synthesis of bioactive benzopyranones. These methods are limited to being used with arenes building blocks [58,59,60]. Fu et al. reported a ligand-enabled, site-selective carboxylation of 2-(furan-3-yl)phenols 42 under the atmospheric pressure of CO2. It was performed through an Rh(ii)-catalyzed C–H bond activation, assisted by the ligand chelation of the phenolic hydroxyl group to afford 4H-furo[3,2-c]benzopyran-4-ones 43 (Scheme 14) [61]. This reaction indicates the role of phosphine ligands in combination with Rh2(OAc)4 in promoting the reactivity and the selectivity during C–H carboxylation. The right choice of a suitable basic catalyst is an additional critical point.
Scheme 14.
Rhodium(II)-catalyzed aryl C–H carboxylation with CO2 in the synthesis of 4H-furo[3,2-c] benzopyran-4-ones 43. Reagents and conditions: (a) Rh2(OAc)4 (1 mol %), tricyclohexylphosphine PCy3 (2 mol %), t-BuOK (4.5 equiv.), diglyme, 100 °C, 48 h, six outputs with 70%–86% yield.
2.1.2. Pyrroles
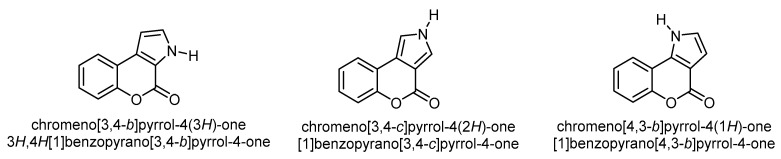
Fusion of the pyrrole ring with the pyrone ring of coumarin (benzopyrane) leads to three structural isomers, viz. chromeno[3,4-b]pyrrol-4(3H)-one, chromeno[3,4-c]pyrrol-4(2H)-one, and chromeno[4,3-b]pyrrol-4(1H)-one (Figure 2).
Figure 2.
The three common isomers of the pyrrole ring fused to the α-pyrone moiety of coumarin.
3H,4H[1]Benzopyrano[3,4-b]pyrrol-4-one
Pyrrole Construction
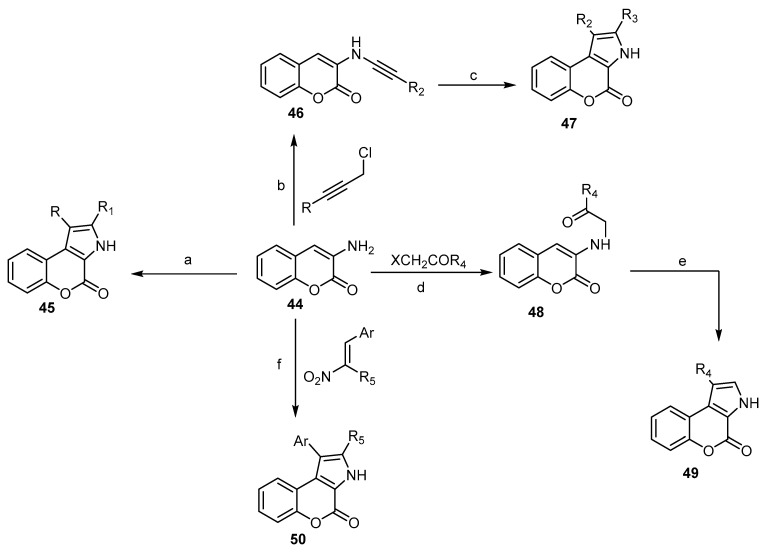
The 3-Aminocoumarin (44) is considered the starting compound for the preparation of fused 3H,4H[1]benzopyrano[3,4-b]pyrrol-4-ones. The amino group represents the key moiety of this cyclization process in the reaction with different reagents [30,62,63,64]. Compound 45 was prepared by the reaction of 3-aminocoumarin (44) with different carbonyls via Fischer indole synthesis after being diazotized and reduced to coumarin-3-yl-hydrazine [62]. The compound 1-Aryloxy-4-chlorobut-2-ynes reacted with 3-aminocoumarin (44) to afford 47 through amino-Claisen rearrangement [64]. Condensation of (44) with α-halo ketones, followed by cyclization catalyzed by TFA, led to the formation of 49 [63], while 50 was prepared under Nef conditions using 2-aryl-1- nitro ethenes [30] (Scheme 15).
Scheme 15.
Different pathways to synthesize 3H,4H[1]benzo- pyrano[3,4-b]pyrrol-4-ones 45, 47, 49, and 50 via 3-aminocoumarin. Reagents and conditions: (a) i: NaNO2, HCl, −30 °C, ii: SnCl2, −10 °C, HCl, 2 h, iii: carbonyl compounds, polyphosphoric acid, 1 h, 130 °C, seven outputs with 33%–51% yield; (b) anhydride ethyl methyl ketone, K2CO3, NaI, reflux, 24–30 h, five outputs with 51%–63% yield; (c) N,N-dimethylaniline, reflux, 6–9 h, five outputs with 90%–94% yield; (d) KI (cat.), reflux, 5 h; (e) TFA (cat.), AcOH, 12 h, reflux, four outputs with 28%–86% yield; (f) MeOH, piperidine, reflux, five outputs with 55%–61% yield.
3H,4H[1]Benzopyrano[3,4-e]pyrrol-4-one
Pyrrole Construction
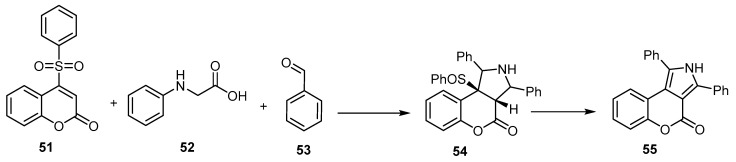
A one-pot, three-component reaction of phenylsulphinyl-2H-benzopyran-2-one (51), phenylglycine (52), and benzaldehyde (53) led to the formation of 1,3-diphenyl[l]benzopyrano[3,4-e]pyrrol-4-one (55) (Scheme 16) [65].
Scheme 16.
Synthesis of 1,3-diphenyl[l]benzopyrano[3,4-e]pyrrol-4-one (55). Reagents and conditions: DMF, stirring, 120 °C, 24 h, 51% yield.
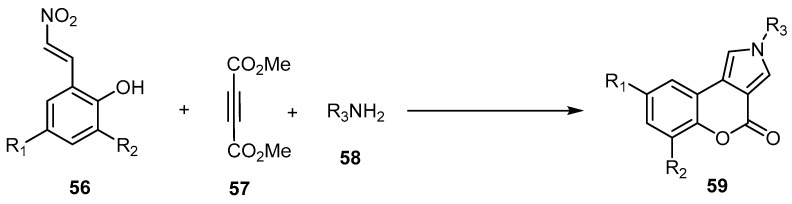
Xue et al. developed an efficient and straightforward synthetic protocol for the preparation of [1]benzopyrano[3,4-e]pyrrol-4-ones 59 through FeCl3-promoted, three-component reactions between substituted 2-(2-nitrovinyl)phenols 58, acetylene dicarboxylate (57), and amines 58 (Scheme 17) [66]. This reaction involved the sequential FeCl3-mediated nucleophilic addition of acetylenedicarboxylates, amines, and 2-(2-nitrovinyl)phenols, following intramolecular transesterifcation to form a coumarin core. This strategy offers a complementary approach to substituted pyrrolo[3,4-c]coumarin compounds, with advantages that include a variety of cheap and readily available reactants and a wide range of substrates with dense or flexible substitution patterns [66].
Scheme 17.
Synthesis of [1]benzopyrano[3,4-e]pyrrol-4-ones 59 by a FeCl3-promoted, three-component reaction. Reagents and conditions: FeCl3, toluene, 110 °C, 6 h, 17 outputs with 62%–92% yield.
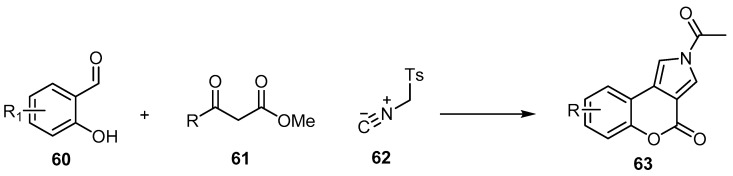
Alizadeh et al. reported a sequential three-component reaction of salicylaldehydes 60, β-keto esters 61, and p-toluenesulfonylmethyl isocyanide (TosMIC) (62) via [1,3] acyl shift to give 2-acyl[1] benzopyrano[3,4-e]pyrrol-4-ones 63 (Scheme 18) [67]. A simple workup procedure, mild reaction conditions, lack of side products, and good yields of 62%–95% are the main aspects of this method.
Scheme 18.
A sequential three-component reaction to synthesize [1]benzopyrano[3,4-e]pyrrol-4-ones 63. Reagents and conditions: TEA, piperidine, DMF, r.t., 8 h, 10 outputs with 62%–95% yield.
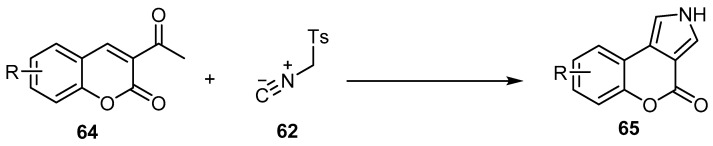
Recently, Khavasi and his coworkers investigated the reactivity, chemo-, region-, and diastreo-selectivity of p-toluenesulfonylmethyl isocyanide (TosMIC) (62) in Van Leusen-type [3 + 2] cycloaddition reactions with the 3-acetylcoumarins 64 to give [1]benzopyrano[3,4-e]pyrrol-4-ones 65 (Scheme 19) [68]. This method offers several advantages, such as being inexpensive, providing good to excellent yields, producing short reaction times, high atom economy, and ease of product isolation under catalyst-free conditions without any activation at ambient temperature.
Scheme 19.
The Van Leusen protocol for the synthesis of [1]benzopyrano[3,4-e]pyrrol-4-ones 65. Reagents and conditions: K2CO3, EtOH, 15 min, r.t, three outputs with 72%–86% yield.
[1]Benzopyrano[4,3-b]pyrrol-4-one
Pyrrole Construction
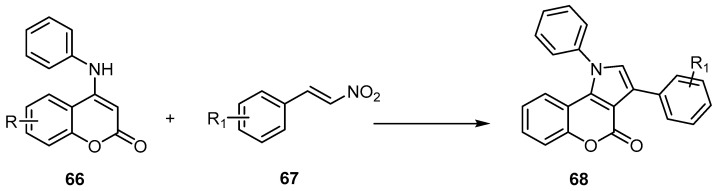
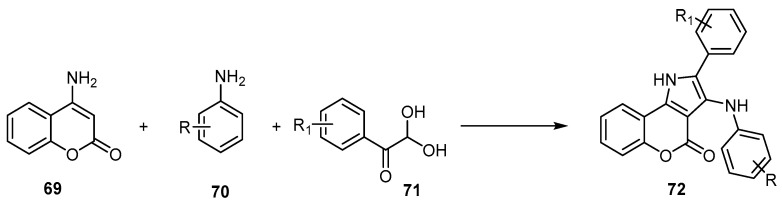
Many synthetic protocols have been reported for the synthesis of [l]benzopyrano [4,3-b]pyrrole-4(1H)-ones, including the reaction of β-nitroalkenes 67 and 4-phenylamino coumarins 66 under solvent-free conditions to afford 68 (Scheme 20) [69]. Moreover, the reaction of the 4-aminocoumarin (69), amines 70, and glyoxal monohydrates 71 in the presence of nanocrystalline CuFe2O4 [70], or KHSO4, led to the formation of [l]benzopyrano [4,3-b]pyrrole-4(1H)-ones 72 (Scheme 21) [71]. The synthesis of 71 using nanocrystalline CuFe2O4 discloses a rapid, high-yielding, green synthetic protocol for a variety of chromeno[4,3-b]pyrrol-4(1H)-one derivatives by assembling the basic building blocks in an aqueous medium using nano CuFe2O4 as the efficient, magnetically recoverable catalyst [70].
Scheme 20.
Synthetic protocol to synthesize [l]benzopyrano[4,3-b]pyrrole-4(1H)-ones 68. Reagents and conditions: TsOH.H2O, solvent-free, 24 outputs with 6%–77% yield.
Scheme 21.
Synthetic protocol to synthesize [l]benzopyrano[4,3-b]pyrrole-4(1H)-ones 72. Reagents and conditions: CuFe2O4, H2O, 70 °C, 25 outputs with 68%–94% yield; or KHSO4, toluene, reflux, 23 outputs with 37%–93% yield.
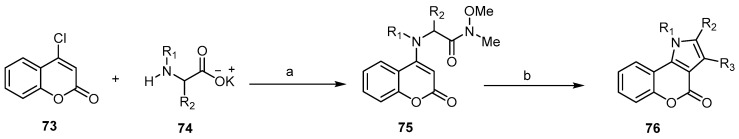
Many articles have found that the 4-chlorocoumarin is the key compound for the preparation of various [l]benzopyrano[4,3-b]pyrrol-4-ones by Knorr- or Fischer–Fink-type reactions [72,73]. Albrola et al. indicated the preparation of N(α)-(2-oxo-2H-l-benzopyran-4-yl)Weinreb-α-aminoamides 75 from 4-chlorocoumarin (73) and different α-aminoacids 74. The reaction of 75 with various organometallic compounds, followed by cyclization, led to the formation of [l]benzopyrano[4,3-b]pyrrol-4-ones 76 (Scheme 22) [74,75].
Scheme 22.
Synthesis of [l]benzopyrano[4,3-b]pyrrol-4-ones 76 from 4-chlorocoumarin. Reagents and conditions: (a) i: EtOH, TEA, HCl.N(Me)OMe, ii: CH2Cl2, TEA, DCC; (b) i: organometallic compounds (R3M), THF, N2, -H2O, ii: NaOEt, EtOH, r.t., 2 h, reflux, 1 h, nine outputs with 27%–92% yield.
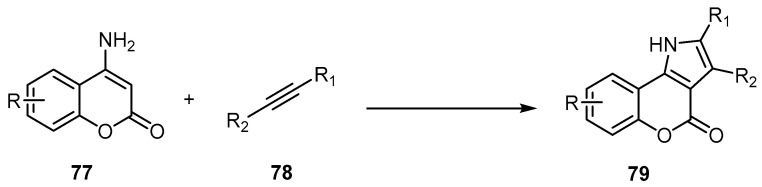
On the other hand, the 4-amino-2H-benzopyran-2-one is employed as a key compound for the preparation of [l]benzopyrano[4,3-b]pyrrol-4-one [76,77]. Peng et al. synthesized a series of [l]benzopyrano[4,3-b]pyrrol-4-ones 79 via a palladium-catalyzed oxidative annulation reaction of 4-amino-2H-benzopyran-2-ones 77, with electron-withdrawing or electron-donating groups with different alkynes 78 (Scheme 23) [76]. The method utilizes simple and readily available enamines and alkynes, and employs direct Pd(II)-catalyzed oxidative annulation to synthesize [l]benzopyrano[4,3-b]pyrrol-4-ones in high yields of 72%–99%.
Scheme 23.
The scope of 4-aminocoumarins in the preparation of [l]benzopyrano[4,3-b]pyrrol-4-ones 79. Reagents and conditions: Pd(OAc)2, oxidant, DMSO, 100 °C, 12 outputs with 72%–99% yield.
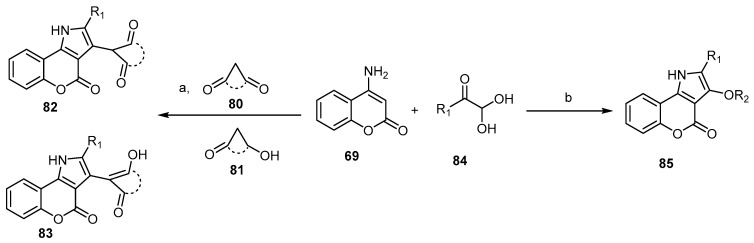
Recently, Yang et al. reported a one-pot, two-step reaction of 4-amino-2H-benzopyran-2-one (69) with arylglyoxal monohydrates 80 and p-toluenesulfonates 81 to afford a series of 3-alkoxy-substituted [l]benzopyrano[4,3-b]pyrrol-4-ones 82 and 83, respectively (Scheme 24) [78]. On the other hand, Yahyavi et al. described the Knoevenagle treatment of the arylglyoxals 84 with active methylene compounds and consequently an iodine-activated Michael-type reaction with 4-aminocoumarin (69) in a one-pot manner to afford disubstituted [l]benzopyrano[4,3-b]pyrrol-4-ones 85 (Scheme 24) [79].
Scheme 24.
Various arylglyoxals in the synthesis of [l]benzopyrano[4,3-b]pyrrol-4-ones 82, 83, and 85. Reagents and conditions: (a) i: AcOH, reflux, 40 min; ii: an appropriate alkyl p-toluenesulfonate (TsOR2), 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), toluene, reflux, 1.5 h, 14 outputs with 73%–89% yield; (b) R1=Ph, 4-CH3OC6H4, 4-CH3C6H4, 4-FC6H4, 4-ClC6H4, 4-BrC6H4, 2-thienyl; dimedone, 2-hydroxy-1,4-naphtoquinone barbituric acid, 1,3 dimethyl barbituric acid, I2, DMSO, stirring, 100 °C, 7 h, 15 outputs with 15%–80% yield.
2.1.3. Thiophenes
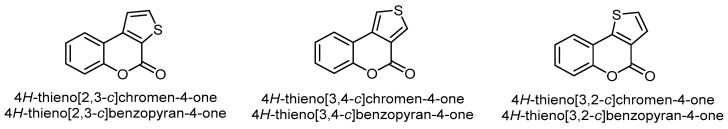
Fusion of the thiophene ring with the pyrone ring of coumarin(benzopyrane) leads to three structural isomers, viz. 4H-thieno[2,3-c]chromen(benzopyran)-4-one, 4H-thieno[3,4-c]chromen(benzopyran)-4-one, and 4H-thieno[3,2-c] chromen(benzopyran)-4-one (Figure 3)
Figure 3.
The three common isomers of the thiophene ring fused to the α-pyrone moiety of coumarin.
4H-Thieno[2,3-c]benzopyran-4-one
Thiophene Construction
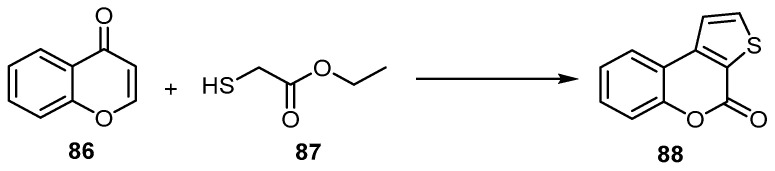
The one-pot cascade addition/condensation/intramolecular cyclization sequence of chromone (86) with ethyl 2-mercaptoacetate (87) using a complexing ligand 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) in 1,4-dioxane led to the formation of 4H-thieno[2,3-c]benzopyran-4-one (88) (Scheme 25) [80].
Scheme 25.
Synthesis of 4H-thieno[2,3-c]benzopyran-4-one (88). Reagents and conditions: 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), 1,4-dioxane, 60 °C, 12 h, N2, 95% yield.
Pyrone Construction
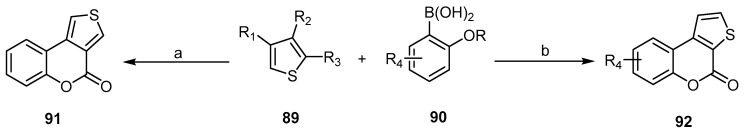
The Suzuki–Miyaura cross coupling of bromoarylcarboxylates and o-hydroxy(methoxy)arylboronic acids is one of the methods that plays an important role in the preparation of 4H-thieno[2,3-c] 91 and 4H-thieno[3,4-c]benzopyran-4-ones 92 (Scheme 26) [81,82,83].
Scheme 26.
4H-thieno[3,4-c]benzopyran-4-ones 91 and 92 via a Suzuki–Miyaura cross coupling reaction. Reagents and conditions: (a) Pd(PPh3)4 (10 mol %), Cs2CO3 (4 equiv.), DME, H2O, MW 125 °C, 15 min, 86% yield; (b) i: Pd(PPh3)4 (5 mol %), K3PO4 (1.5 equiv.), 1,4-dioxane, 90 °C, 4 h; ii: (a) BBr3, CH2Cl2, (b) KOtBu, H2O, five outputs with 75%–81% yield [83].
4H-Thieno[3,4-c]benzopyran-4-one
Thiophene Construction
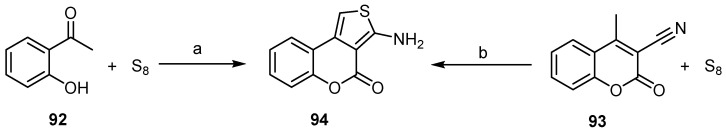
4H-thieno[3,4-c]benzopyran-4-ones (94) were meanly prepared through the Gewald reaction (Scheme 27) [84,85,86,87,88,89,90]. Low yields of 37% and 48% were observed using these methods.
Scheme 27.
The Gewald reaction to synthesize 4H-thieno[3,4-c]benzopyran-4-one (94). Reagents and conditions: (a) CNCH2COOEt, base; (b) NH3, EtOH, 48% yield [85], 37% yield [90].
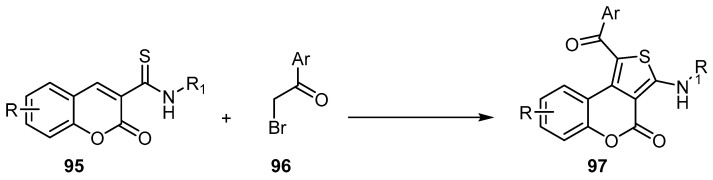
Yu et al. created a new technique for the preparation of 4H-thieno[3,4-c] [1]benzopyran-4(4H)-ones 97 by applying a chemoselective reaction of thioamides 95 with α-bromoacetophenones 96 (Scheme 28) [91].
Scheme 28.
Synthesis of 4H-thieno[3,4-c] [1]benzopyran-4(4H)-ones 97 via [4 + 1] annulations. Reagents and conditions: NaOH, diethyl azodicarboxylate (DEAD), MeCN, r.t., 20 min, 17 outputs with 68%–95% yield.
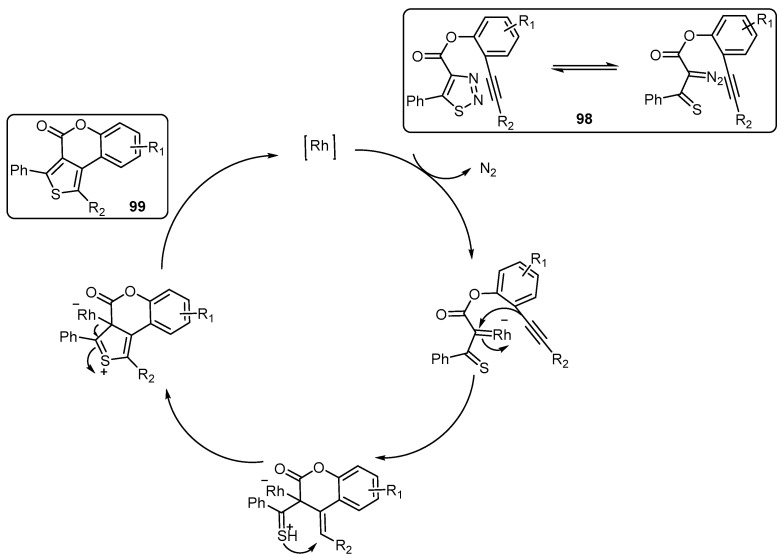
A rhodium-catalyzed intramolecular transannulation reaction of alkynyl thiadiazoles 98 provided 4H-thieno[3,4-c][1]benzopyran-4(4H)-ones 99. A plausible reaction mechanism proposed for the Rh-catalyzed intramolecular transannulation of alkynyl thiadiazoles is outlined in (Scheme 29) [92].
Scheme 29.
A plausible reaction mechanism proposed for the preparation of 4H-thieno[3,4-c][1] benzopyran-4(4H)-ones 99. Reagents and conditions: [Rh(COD)Cl]2 (5 mol %), 1,1′-Ferrocenediyl-bis(diphenylphosphine) (DPPF) (12 mol %), PhCl, 130 °C, 30 min, three outputs with 75%–90% yield.
4H-Thieno[3,2-c][1]benzopyran-4(4H)-one
Thiophene Construction
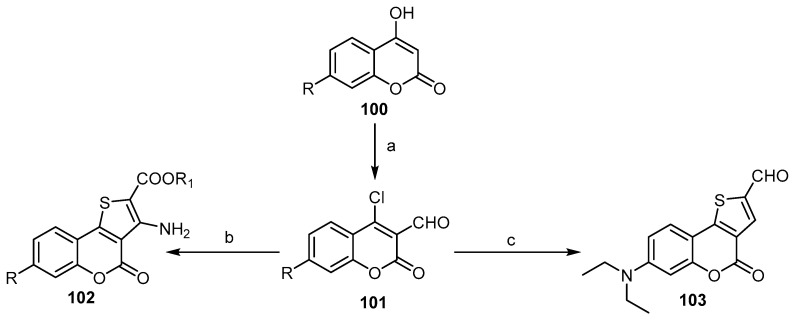
Numerous articles state the use of 4-chloro-2-H-benzopyran-3-carboxaldehydes 101 as a key compound in the preparation of a series of 4H-thieno[3,2-c][1]benzopyran-4(4H)-ones. This compound was prepared by a Vilsmeier–Haack reaction, and the cyclization process was performed through a rection with thioglycolate or dithiane to produce 102 and 103, respectively (Scheme 30) [25,26,93,94].
Scheme 30.
The synthesis of 4H-thieno[3,2-c][1]benzopyran-4(4H)-ones 102 and 103 via the cyclization of Vilsmeier–Haack products. Reagents and conditions: (a) POCl3, DMF, 60 °C, overnight; (b) SHCH2COOR1, EtOH, base, 90% yield [25], 88% yield [94]; (c) 1,4-dithiane-2,5-diole, K2CO3, acetone, stirring, 1 h, r.t., 45 °C, 3 h, 85% yield.
Pyrone Construction
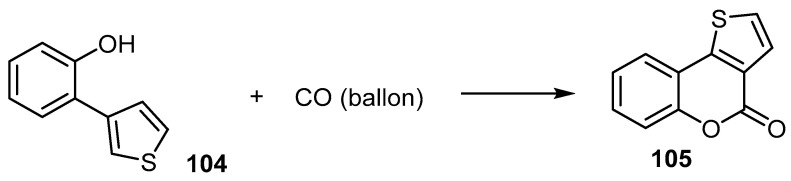
The palladium-catalyzed oxidative carbonylation of 2-(thiophen-3-yl)phenol (104) under acid-base-free and mild conditions yielded the corresponding 4H-thieno[3,2-c][1]benzopyran-4(4H)-one (105) (Scheme 31) [58].
Scheme 31.
One-pot synthesis of 4H-thieno[3,2-c][1]benzopyran-4(4H)-one (105) by CO insertion into phenol. Reagents and conditions: 10 mol % Pd(OAc)2, AgOAc, CH3CN, 80 °C, 48 h, 62% yield.
2.1.4. Selenophene
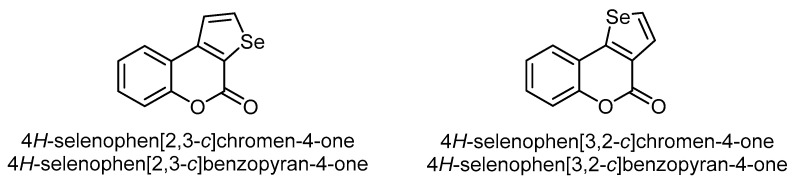
The fusion of the selenophene ring with the pyrone ring of coumarin leads to two structural isomers, viz. 4H-selenophen[2,3-c]chromen(benzopyran)-4-one and 4H-selenophen[3,2-c]chromen(benzopyran)-4-one (Figure 4).
Figure 4.
The two common isomers of the selenophene ring fused to the α-pyrone moiety of coumarin.
4H-Selenophen[2,3-c] and [3,2-c]benzopyran-4-ones
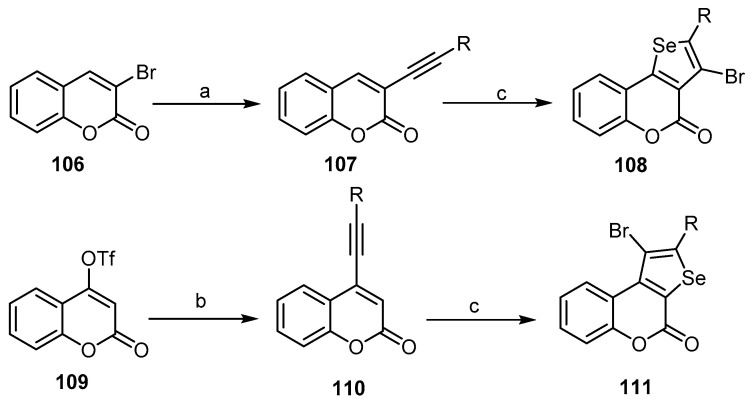
A few articles have discussed the possibility of synthesizing the fused selenophen-chromen-(benzopyran)-4-one moiety. A simple method for the synthesis of substituted 4H-selenopheno[2,3-c] benzopyran-4-ones 108 is by the treatment of 3-ethynylcoumarins 107 with selenium (IV) oxide and concentrated hydrobromic acid at room temperature [16,95]. Similarly, 4H-selenopheno-[3,2-c]benzopyran-4-ones 111 was prepared under the same conditions using 4-ethynylcoumarins 110. The alkenyl derivatives were obtained from bromocoumarin (106) and 4-(trifluoromethane-sulfonyl)coumarin (109) by Sonogashira coupling. All the reaction steps were carried out in situ from the starting materials and until the end product (Scheme 32) [16,95].
Scheme 32.
Reagents and reaction conditions: a: PdCl2 (10 mol %), Ph3P (20 mol %), CuI (10 mol %), terminal acetylene (1.5 equiv.), NMP, Et3N, 55 °C, 20 h; b: (Ph3P)4Pd (5 mol %), CuI (20 mol %), terminal acetylene (1.5 equiv.), DMF, Et3N, r.t., 20 h; c: SeO2 (2 equiv.), conc. HBr, dioxane, r.t., 24–48 h, compounds 108, four outputs with 68%–75% yield; compounds 111, four outputs with 64%–70% yield.
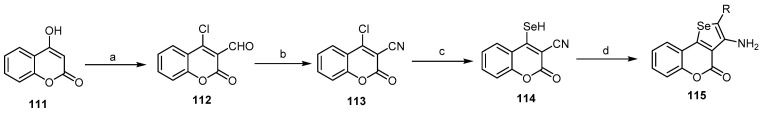
Additionally, Kirsch and his coworkers reported the synthesis of selenopheno[2,3-c]benzopyran-4-ones (115) via a multistep reaction. This reaction cascade started by Vilsmeier formylation of 4-hydroxycoumarine (111). The formylated product reacted with hydroxyl amine to afford (113) according to the reaction conditions, which subsequently transformed into 3-cyano-4-coumarinselenol (114), by refluxing with selenium and sodiumborohydride in ethanol (Scheme 33). It was the precursor of selenopheno[2,3-c]benzopyran-4-ones (115) as it was reactive towards a series of haloacids, such as chloroacetonitrile, ethyl chloroacetoacetate, and chloroacetamide [96].
Scheme 33.
Synthesis of selenopheno[2,3-c]benzopyran-4-ones (115) from 4-hydroxycoumarin. Reagents and reaction conditions: a: POCl3, DMF, 84% yield; b: NH2OH.HCl, 80% yield; c: Se, NaBH4, EtOH, 85% yield; d: ClCH2CN(R), DMF, stirring, r.t., 2 h, three outputs with 80%–88% yield.
In conclusion, since coumarins have versatile applications, the synthesis of different structures of the coumarin-based scaffold was attempted. Among all the heterocycles built on the α-pyrone moiety of coumarin, the furan ring is the only available structure in nature. Thus, it has inspired a lot of researchers to replace the oxygen atom with other heteroatoms. Wide varieties of heterocycles were constructed by a synthetic pathway to introduce furans, pyrroles, thiophenes, and selenophenes as a fused ring that is characterized by a single heteroatom to the α-pyrone moiety of coumarin. The fused heterocycles that contain more than one heteroatom will be described in the next part, which we intend to publish in the future.
Author Contributions
E.R.E.-S. and G.K. collected publications and sorted them. E.R.E.-S. analyzed the literature and wrote the first draft. A.B.A. and G.K. developed the concept of the review. E.R.E.-S., A.B.A., and G.K. wrote the final draft and corrected the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by MDPI.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Molnar M., Lončarić M., Kovač M. Green Chemistry Approaches to the Synthesis of Coumarin Derivatives. Curr. Org. Chem. 2020;24:4–43. doi: 10.2174/1385272824666200120144305. [DOI] [Google Scholar]
- 2.Matos M.J., Santana L., Uriarte E., Abreu O.A., Molina E., Yordi E.G. Coumarins—An Important Class of Phytochemicals. Phytochem. Isol. Characterisation Role Hum. Health. 2015 doi: 10.5772/59982. [DOI] [Google Scholar]
- 3.Lončarić M., Gašo-Sokač D., Jokić S., Molnar M. Recent Advances in the Synthesis of Coumarin Derivatives from Different Starting Materials. Biomolecules. 2020;10:151. doi: 10.3390/biom10010151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salem M.A., Helal M.H., Gouda M.A., Ammar Y.A., El-Gaby M.S.A., Abbas S.Y. An Overview on Synthetic Strategies to Coumarins. Synth. Commun. 2018;48:1534–1550. doi: 10.1080/00397911.2018.1455873. [DOI] [Google Scholar]
- 5.Irfan A., Rubab L., Rehman M.U., Anjum R., Ullah S., Marjana M., Qadeer S., Sana S. Coumarin Sulfonamide Derivatives: An Emerging Class of Therapeutic Agents. Heterocycl. Commun. 2020;26:46–59. doi: 10.1515/hc-2020-0008. [DOI] [Google Scholar]
- 6.Venugopala K.N., Rashmi V., Odhav B. Review on Natural Coumarin Lead Compounds for Their Pharmacological Activity. BioMed. Res. Int. 2013;2013:1–14. doi: 10.1155/2013/963248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mishra S., Pandey A., Manvati S. Coumarin: An Emerging Antiviral Agent. Heliyon. 2020;6:e03217. doi: 10.1016/j.heliyon.2020.e03217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chattopadhyay D.S.K. Pharmacological Potentiality of Coumarins as Anti-Viral Agent. Int. J. Res. Anal. Rev. 2018;5:11. [Google Scholar]
- 9.Bizzarri B.M., Botta L., Capecchi E., Celestino I., Checconi P., Palamara A.T., Nencioni L., Saladino R. Regioselective IBX-Mediated Synthesis of Coumarin Derivatives with Antioxidant and Anti-Influenza Activities. J. Nat. Prod. 2017;80:3247–3254. doi: 10.1021/acs.jnatprod.7b00665. [DOI] [PubMed] [Google Scholar]
- 10.Hassan M.Z., Osman H., Ali M.A., Ahsan M.J. Therapeutic Potential of Coumarins as Antiviral Agents. Eur. J. Med. Chem. 2016;123:236–255. doi: 10.1016/j.ejmech.2016.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menezes J.C., Diederich M. Translational Role of Natural Coumarins and Their Derivatives as Anticancer Agents. Future Med. Chem. 2019;11:1057–1082. doi: 10.4155/fmc-2018-0375. [DOI] [PubMed] [Google Scholar]
- 12.Kaur M., Kohli S., Sandhu S., Bansal Y., Bansal G. Coumarin: A Promising Scaffold for Anticancer Agents. Anticancer Agents Med. Chem. 2015;15:1032–1048. doi: 10.2174/1871520615666150101125503. [DOI] [PubMed] [Google Scholar]
- 13.Majnooni M.B., Fakhri S., Smeriglio A., Trombetta D., Croley C.R., Bhattacharyya P., Sobarzo-Sánchez E., Farzaei M.H., Bishayee A. Antiangiogenic Effects of Coumarins against Cancer: From Chemistry to Medicine. Molecules. 2019;24:4278. doi: 10.3390/molecules24234278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhagat K., Bhagat J., Gupta M.K., Singh J.V., Gulati H.K., Singh A., Kaur K., Kaur G., Sharma S., Rana A., et al. Design, Synthesis, Antimicrobial Evaluation, and Molecular Modeling Studies of Novel Indolinedione–Coumarin Molecular Hybrids. ACS Omega. 2019;4:8720–8730. doi: 10.1021/acsomega.8b02481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Majed Y.K., Kadhum A.H., Al-Amier A.A., Mohama A. Coumarins: The Antimicrobial Agents. Syst. Rev. Pharm. 2017;8:62–70. doi: 10.5530/srp.2017.1.11. [DOI] [Google Scholar]
- 16.Arsenyan P., Vasiljeva J., Shestakova I., Domracheva I., Jaschenko E., Romanchikova N., Leonchiks A., Rudevica Z., Belyakov S. Selenopheno[3,2-c]- and [2,3-c]Coumarins: Synthesis, Cytotoxicity, Angiogenesis Inhibition, and Antioxidant Properties. C. R. Chim. 2015;18:399–409. doi: 10.1016/j.crci.2014.09.007. [DOI] [Google Scholar]
- 17.Annunziata F., Pinna C., Dallavalle S., Tamborini L., Pinto A. An Overview of Coumarin as a Versatile and Readily Accessible Scaffold with Broad-Ranging Biological Activities. Int. J. Mol. Sci. 2020;21:4618. doi: 10.3390/ijms21134618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boisde P.M., Meuly W.C. Kirk-Othmer Encyclopedia of Chemical Technology. American Cancer Society; Atlanta, GA, USA: 2000. Coumarin. [Google Scholar]
- 19.Api A.M., Belmonte F., Belsito D., Biserta S., Botelho D., Bruze M., Burton G.A., Buschmann J., Cancellieri M.A., Dagli M.L., et al. RIFM Fragrance Ingredient Safety Assessment, Coumarin, CAS Registry Number 91-64-5. Food Chem. Toxicol. 2019;130:110522. doi: 10.1016/j.fct.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 20.Coumarin|Cosmetics Info. [(accessed on 18 September 2020)]; Available online: https://cosmeticsinfo.org/ingredient/coumarin.
- 21.Lončar M., Jakovljević M., Šubarić D., Pavlić M., Buzjak Služek V., Cindrić I., Molnar M. Coumarins in Food and Methods of Their Determination. Foods. 2020;9:645. doi: 10.3390/foods9050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krüger S., Winheim L., Morlock G.E. Planar Chromatographic Screening and Quantification of Coumarin in Food, Confirmed by Mass Spectrometry. Food Chem. 2018;239:1182–1191. doi: 10.1016/j.foodchem.2017.07.058. [DOI] [PubMed] [Google Scholar]
- 23.Sun X., Liu T., Sun J., Wang X. Synthesis and Application of Coumarin Fluorescence Probes. RSC Adv. 2020;10:10826–10847. doi: 10.1039/C9RA10290F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarih N.M., Ciupa A., Moss S., Myers P., Slater A.G., Abdullah Z., Tajuddin H.A., Maher S. Furo[3,2-c]Coumarin-Derived Fe3+ Selective Fluorescence Sensor: Synthesis, Fluorescence Study and Application to Water Analysis. Sci. Rep. 2020;10:7421. doi: 10.1038/s41598-020-63262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akchurin I.O., Yakhutina A.I., Bochkov A.Y., Solovjova N.P., Traven V.F. Synthesis of Novel Push-Pull Fluorescent Dyes—7-(Diethylamino)Furo[3,2-c]Coumarin and 7-(Diethylamino)Thieno[3,2-c]Coumarin Derivatives. Heterocycl. Commun. 2018;24:85–91. doi: 10.1515/hc-2017-0253. [DOI] [Google Scholar]
- 26.Shi L., Yu H., Zeng X., Yang S., Gong S., Xiang H., Zhang K., Shao G. A Novel Ratiometric Fluorescent Probe Based on Thienocoumarin and Its Application for the Selective Detection of Hypochlorite in Real Water Samples and in Vivo. New J. Chem. 2020;44:6232–6237. doi: 10.1039/D0NJ00318B. [DOI] [Google Scholar]
- 27.Cadierno V. Chapter 4—Metal-Catalyzed Routes for the Synthesis of Furocoumarins and Coumestans. In: Brahmachari G., editor. Green Synthetic Approaches for Biologically Relevant Heterocycles. Elsevier; Amsterdam, The Netherlands: 2015. pp. 77–100. [Google Scholar]
- 28.Ram R., Krupadanam S., Srimannarayana G. Synthesis of 2-Methyl-9-Oxo-9H-Furo[2,3-c]Benzopyrans and 2-Methyl-3H,4H[1]Benzopyrano[3,4-b]Pyrrol-4-Ones. Synth. Commun. 1998;28:2421–2428. doi: 10.1080/00397919808004294. [DOI] [Google Scholar]
- 29.Majumdar K.C., Khan A.T., Das D.P. Facile Regioselective Synthesis of 2-Methyl Furo [3,2-c][1] Benzopyran-4-One 2-Methyl Furo[2,3-c] [1]Benzopyran-4-One. Synth. Commun. 1989;19:917–930. doi: 10.1080/00397918908051012. [DOI] [Google Scholar]
- 30.Pandya M.K., Chhasatia M.R., Vala N.D., Parekh T.H. Synthesis of Furano[2,3-c] /Pyrrolo[2,3-c]Coumarins and Synthesis of 1(H)-[1]Benzopyrano[3,4-b][1]Benzopyrano[3′,4′-d] Furan-7(H)-Ones /1(H)-[1]Benzopyrano[3,4-b][1]Benzopyrano [3′,4′-d]Pyrrole-7(H)-Ones. J. Drug Deliv. Ther. 2019;9:32–42. doi: 10.22270/jddt.v9i4-s.3243. [DOI] [Google Scholar]
- 31.Dong Y., Yu J.-T., Sun S., Cheng J. Rh(III)-Catalyzed Sequential Ortho -C–H Oxidative Arylation/Cyclization of Sulfoxonium Ylides with Quinones toward 2-Hydroxy-Dibenzo[b,d]Pyran-6-Ones. Chem. Commun. 2020;56:6688–6691. doi: 10.1039/D0CC00176G. [DOI] [PubMed] [Google Scholar]
- 32.Brahmbhatt D.I., Gajera J.M., Patel C.N., Pandya V.P., Pandya U.R. First Synthesis of Furo[3,4-c]Coumarins. J. Heterocycl. Chem. 2006;43:1699–1702. doi: 10.1002/jhet.5570430643. [DOI] [Google Scholar]
- 33.Wang X., Nakagawa-Goto K., Kozuka M., Tokuda H., Nishino H., Lee K.-H. Cancer Preventive Agents. Part 6: Chemopreventive Potential of Furanocoumarins and Related Compounds. Pharm. Biol. 2006;44:116–120. doi: 10.1080/13880200600592178. [DOI] [Google Scholar]
- 34.Rajitha B., Geetanjali Y., Somayajulu V. Synthesis of Coumestrol Analogs as Possible Antifertility Agents. Indian J. Chem. 1986;25B:872–873. [Google Scholar]
- 35.Al-Sehemi A.G., El-Gogary S.R. Synthesis and Photooxygenation of Furo[3,2-c]Coumarin Derivatives as Antibacterial and DNA Intercalating Agent. Chin. J. Chem. 2012;30:316–320. doi: 10.1002/cjoc.201180483. [DOI] [Google Scholar]
- 36.Gorbunov Y.O., Mityanov V.S., Melekhina V.G., Krayushkin M.M. Synthesis of Novel 4H-Furo[3,2-c]Pyran-4-Ones and 4H-Furo[3,2-c]Chromen-4-Ones. Russ. Chem. Bull. 2018;67:304–307. doi: 10.1007/s11172-018-2074-y. [DOI] [Google Scholar]
- 37.Panja S.K., Ghosh J., Maiti S., Bandyopadhyay C. Synthesis of Furocoumarins and Biscoumarins by an Isocyanide-Induced Multicomponent Reaction: Isocyanide as a Masked Amine. J. Chem. Res. 2012;36:222–225. doi: 10.3184/174751912X13319228503277. [DOI] [Google Scholar]
- 38.Reisch J. Die Synthese von Hydroxy- und Furo-cumarinderivaten. Archiv. Pharm. 1966;299:798–805. doi: 10.1002/ardp.19662990912. [DOI] [PubMed] [Google Scholar]
- 39.Rahman M.-U., Khan K.-Z., Siddiqi Z.S., Zaman A. A Convenient Route for 3-Formyl-4-Hydroxycoumarin and Reactions of 3- Bromo-4-Hydroxycoumarin. J. Chem. Sect. Org. Chem. Incl. Med. Chem. Indian. 1990;29:941–943. [Google Scholar]
- 40.Kadam A., Bellinger S., Zhang W. Atom- and Step-Economic Synthesis of Biaryl-Substituted Furocoumarins, Furoquinolones and Furopyrimidines by Multicomponent Reactions and One-Pot Synthesis. Green Process. Synth. 2013;2:491–497. doi: 10.1515/gps-2013-0064. [DOI] [Google Scholar]
- 41.Lee Y.R., Kim B.S., Wang H.C. Silver(I)/Celite Promoted Oxidative Cycloaddition of 4-Hydroxycoumarin to Olefins. A Facile Synthesis of Dihydrofurocoumarins and Furocoumarins. Tetrahedron. 1998;54:12215–12222. doi: 10.1016/S0040-4020(98)00762-5. [DOI] [Google Scholar]
- 42.Majumdar N., Paul N.D., Mandal S., de Bruin B., Wulff W.D. Catalytic Synthesis of 2H-Chromenes. ACS Catal. 2015;5:2329–2366. doi: 10.1021/acscatal.5b00026. [DOI] [Google Scholar]
- 43.Nebra N., Díaz-Álvarez A.E., Díez J., Cadierno V. Expeditious Entry to Novel 2-Methylene-2,3-Dihydrofuro[3,2-c] Chromen-2-Ones from 6-Chloro-4-Hydroxychromen-2-One and Propargylic Alcohols. Molecules. 2011;16:6470–6480. doi: 10.3390/molecules16086470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raffa G., Rusch M., Balme G., Monteiro N. A Pd-Catalyzed Heteroannulation Approach to 2,3-Disubstituted Furo[3,2-c]Coumarins. Org. Lett. 2009;11:5254–5257. doi: 10.1021/ol902189q. [DOI] [PubMed] [Google Scholar]
- 45.Medina F.G., Marrero J.G., Macías-Alonso M., González M.C., Córdova-Guerrero I., Teissier García A.G., Osegueda-Robles S. Coumarin Heterocyclic Derivatives: Chemical Synthesis and Biological Activity. Nat. Prod. Rep. 2015;32:1472–1507. doi: 10.1039/C4NP00162A. [DOI] [PubMed] [Google Scholar]
- 46.Nealmongkol P., Tangdenpaisal K., Sitthimonchai S., Ruchirawat S., Thasana N. Cu(I)-Mediated Lactone Formation in Subcritical Water: A Benign Synthesis of Benzopyranones and Urolithins A–C. Tetrahedron. 2013;69:9277–9283. doi: 10.1016/j.tet.2013.08.045. [DOI] [Google Scholar]
- 47.Cheng G., Hu Y. One-Pot Synthesis of Furocoumarins through Cascade Addition–Cyclization–Oxidation. Chem. Commun. 2007:3285–3287. doi: 10.1039/b705315k. [DOI] [PubMed] [Google Scholar]
- 48.Cheng G., Hu Y. Two Efficient Cascade Reactions to Synthesize Substituted Furocoumarins. J. Org. Chem. 2008;73:4732–4735. doi: 10.1021/jo800439y. [DOI] [PubMed] [Google Scholar]
- 49.Sanz R., Miguel D., Martínez A., Álvarez-Gutiérrez J.M., Rodríguez F. Brønsted Acid Catalyzed Propargylation of 1,3-Dicarbonyl Derivatives. Synthesis of Tetrasubstituted Furans. Org. Lett. 2007;9:727–730. doi: 10.1021/ol0631298. [DOI] [PubMed] [Google Scholar]
- 50.Huang W., Wang J., Shen Q., Zhou X. Yb(OTf)3-Catalyzed Propargylation and Allenylation of 1,3-Dicarbonyl Derivatives with Propargylic Alcohols: One-Pot Synthesis of Multi-Substituted Furocoumarin. Tetrahedron. 2007;63:11636–11643. doi: 10.1016/j.tet.2007.08.114. [DOI] [Google Scholar]
- 51.Cadierno V. A Novel Propargylation/Cycloisomerization Tandem Process Catalyzed by a Ruthenium(II)/Trifluoroacetic Acid System: One-Pot Entry to Fully Substituted Furans from Readily Available Secondary Propargylic Alcohols and 1,3-Dicarbonyl Compounds. Adv. Synth. Catal. 2007;349:382–394. doi: 10.1002/adsc.200600366. [DOI] [Google Scholar]
- 52.Cadierno V., García-Garrido S.E., Gimeno J., Nebra N. Atom-Economic Transformations of Propargylic Alcohols Catalyzed by the 16-Electron Allyl-Ruthenium(II) Complex [Ru(H3-2-C3H4Me)(CO)(Dppf)][SbF6] (Dppf=1,1′-Bis(Diphenylphosphino)Ferrocene) Inorg. Chim. Acta. 2010;363:1912–1934. doi: 10.1016/j.ica.2009.05.010. [DOI] [Google Scholar]
- 53.Conreaux D., Belot S., Desbordes P., Monteiro N., Balme G. Et3N-Induced Demethylation−Annulation of 3-Alkynyl-4-Methoxy-2-Pyridones and Structurally Related Compounds in the Synthesis of Furan-Fused Heterocycles. J. Org. Chem. 2008;73:8619–8622. doi: 10.1021/jo8014038. [DOI] [PubMed] [Google Scholar]
- 54.Lei C., Li Y., Mh X. One-Pot Synthesis of Furocoumarins via Sequential Pd/Cu-Catalyzed Alkynylation and Intramolecular Hydroalkoxylation. Org. Biomol. Chem. 2010;8:3073–3077. doi: 10.1039/c004233a. [DOI] [PubMed] [Google Scholar]
- 55.Pham P.H., Nguyen Q.T.D., Tran N.K.Q., Nguyen V.H.H., Doan S.H., Ha H.Q., Truong T., Phan N.T.S. Metal-Free Synthesis of Furocoumarins: An Approach via Iodine-Promoted One-Pot Cyclization between 4-Hydroxycoumarins and Acetophenones: Metal-Free Synthesis of Furocoumarins: An Approach via Iodine-Promoted One-Pot Cyclization between 4-Hydroxycoumarins and Acetophenones. Eur. J. Org. Chem. 2018;2018:4431–4435. doi: 10.1002/ejoc.201800983. [DOI] [Google Scholar]
- 56.Traven V.F., Kravtchenko D.V., Chibisova T.A., Shorshnev S.V., Eliason R., Wakefield D.H. A New Short Way to Furocoumarins. Heterocycl. Commun. 1996;2 doi: 10.1515/HC.1996.2.4.345. [DOI] [Google Scholar]
- 57.Majumdar K.C., Bhattacharyya T. Regioselective Synthesis of Furo[3,2-c][1]Benzopyran-4-One and Furo[3,2-c]Quinolin-4-One. J. Chem. Res. 1997:244–245. doi: 10.1039/a607538j. [DOI] [Google Scholar]
- 58.Lee T.-H., Jayakumar J., Cheng C.-H., Chuang S.-C. One Pot Synthesis of Bioactive Benzopyranones through Palladium-Catalyzed C–H Activation and CO Insertion into 2-Arylphenols. Chem. Commun. 2013;49:11797. doi: 10.1039/c3cc47197g. [DOI] [PubMed] [Google Scholar]
- 59.Xiao B., Gong T.-J., Liu Z.-J., Liu J.-H., Luo D.-F., Xu J., Liu L. Synthesis of Dibenzofurans via Palladium-Catalyzed Phenol-Directed C-H Activation/C-O Cyclization. J. Am. Chem. Soc. 2011;133:9250–9253. doi: 10.1021/ja203335u. [DOI] [PubMed] [Google Scholar]
- 60.Moon Y., Kim Y., Hong H., Hong S. Synthesis of Heterocyclic-Fused Benzofurans via C–H Functionalization of Flavones and Coumarins. Chem. Commun. 2013;49:8323. doi: 10.1039/c3cc44456b. [DOI] [PubMed] [Google Scholar]
- 61.Fu L., Li S., Cai Z., Ding Y., Guo X.-Q., Zhou L.-P., Yuan D., Sun Q.-F., Li G. Ligand-Enabled Site-Selectivity in a Versatile Rhodium(Ii)-Catalysed Aryl C–H Carboxylation with CO2. Nat. Catal. 2018;1:469–478. doi: 10.1038/s41929-018-0080-y. [DOI] [Google Scholar]
- 62.Khan M.A., De Brito Morley M.L. Condensed Benzopyrans III. 3H,4H [1] Benzopyrano [3,4- b] Pyrrol-4-Ones. J. Heterocycl. Chem. 1978;15:1399–1401. doi: 10.1002/jhet.5570150830. [DOI] [Google Scholar]
- 63.Soman S.S., Thaker T.H., Rajput R.A. Novel Synthesis and Cytotoxic Activity of Some Chromeno[3,4-b]Pyrrol-4(3H)-Ones. Chem. Heterocycl. Comp. 2011;46:1514. doi: 10.1007/s10593-011-0701-8. [DOI] [Google Scholar]
- 64.Majumdar K., Chattopadhyay B. Amino-Claisen versus Oxy-Claisen Rearrangement: Regioselective Synthesis of Pyrrolocoumarin Derivatives. Synthesis. 2008;2008:921–924. doi: 10.1055/s-2008-1032195. [DOI] [Google Scholar]
- 65.Gtigga R., Vipond D. 4-phenylsulphinyl-and 4-Phenylsulphonylcoumarins as components in cycloaddition reactions. Temahedron. 1989;45:7587–7592. [Google Scholar]
- 66.Xue S., Yao J., Liu J., Wang L., Liu X., Wang C. Three-Component Reaction between Substituted 2-(2-Nitrovinyl)-Phenols, Acetylenedicarboxylate and Amines: Diversity-Oriented Synthesis of Novel Pyrrolo[3,4-c]Coumarins. RSC Adv. 2016;6:1700–1704. doi: 10.1039/C5RA23392E. [DOI] [Google Scholar]
- 67.Alizadeh A., Ghanbaripour R., Zhu L.-G. An Approach to the Synthesis of 2-Acylchromeno[3,4-c]Pyrrol-4(2H)-One Derivatives via a Sequential Three-Component Reaction. Synlett. 2013;24:2124–2126. doi: 10.1055/s-0033-1339521. [DOI] [Google Scholar]
- 68.Shaabani A., Sepahvand H., Bazgir A., Khavasi H.R. Tosylmethylisocyanide (TosMIC) [3+2] Cycloaddition Reactions: A Facile Van Leusen Protocol for the Synthesis of the New Class of Spirooxazolines, Spiropyrrolines and Chromeno[3,4-c]Pyrrols. Tetrahedron. 2018;74:7058–7067. doi: 10.1016/j.tet.2018.10.039. [DOI] [Google Scholar]
- 69.Padilha G., Iglesias B.A., Back D.F., Kaufman T.S., Silveira C.C. Synthesis of Chromeno[4,3-b]Pyrrol-4(1 H)-Ones, from β-Nitroalkenes and 4-Phenylaminocoumarins, under Solvent-Free Conditions. ChemistrySelect. 2017;2:1297–1304. doi: 10.1002/slct.201700114. [DOI] [Google Scholar]
- 70.Saha M., Pradhan K., Das A.R. Facile and Eco-Friendly Synthesis of Chromeno[4,3-b]Pyrrol-4(1H)-One Derivatives Applying Magnetically Recoverable Nano Crystalline CuFe2O 4 Involving a Domino Three-Component Reaction in Aqueous Media. RSC Adv. 2016;6:55033–55038. doi: 10.1039/C6RA06979G. [DOI] [Google Scholar]
- 71.Chen Z., Yang X., Su W. An Efficient Protocol for Multicomponent Synthesis of Functionalized Chromeno[4,3-b]Pyrrol-4(1H)-One Derivatives. Tetrahedron Lett. 2015;56:2476–2479. doi: 10.1016/j.tetlet.2015.03.095. [DOI] [Google Scholar]
- 72.Alberola A., Calvo L., González-Ortega A., Encabo A.P., Sañudo M.C. Synthesis of [1]Benzopyrano[4,3-b]Pyrrol-4(1H)-Ones from 4-Chloro-3-Formylcoumarin. Synthesis. 2001;2001:1941–1948. doi: 10.1055/s-2001-17696. [DOI] [Google Scholar]
- 73.Navarro R.A., Bleye L.C., González-Ortega A., Ruíz C.S. Synthesis of 1H-[1]Benzopyrano[4,3-b]Pyrrole and 4hthieno[3,2-c][1]Benzopyran Derivatives. Functionalisation by Aromatic Electrophilic Substitution. Heterocycles. 2001;55:2369–2386. [Google Scholar]
- 74.Alberola A., Álvaro R., Ortega A.G., Sádaba M.L., Carmen Sañudo M. Synthesis of [1]Benzopyrano[4,3-b]Pyrrol-4(1H)-Ones from N(α)-(2-Oxo-2H-1-Benzopyran-4-Yl)Weinreb α-Aminoamides. Tetrahedron. 1999;55:13211–13224. doi: 10.1016/S0040-4020(99)00802-9. [DOI] [Google Scholar]
- 75.Alberola A., Alvaro R., Andres J.M., Calvo B., González A. Synthesis of [1]Benzopyrano[4,3-b]Pyrrol-4(1H)-Ones from 4-Chlorocoumarin. Synthesis. 1994:279–281. doi: 10.1055/s-1994-25459. [DOI] [Google Scholar]
- 76.Peng S., Wang L., Huang J., Sun S., Guo H., Wang J. Palladium-Catalyzed Oxidative Annulation C-H/N-H Functionalization: Access to Substituted Pyrroles. Adv. Synth. Catal. 2013;355:2550–2557. doi: 10.1002/adsc.201300512. [DOI] [Google Scholar]
- 77.Lin C.-H., Yang D.-Y. Synthesis of Coumarin/Pyrrole-Fused Heterocycles and Their Photochemical and Redox-Switching Properties. Org. Lett. 2013;15:2802–2805. doi: 10.1021/ol401138q. [DOI] [PubMed] [Google Scholar]
- 78.Yang X., Jing L., Chen Z. An Efficient Method for One-Pot Synthesis of 3-Alkoxy-Substituted Chromeno[4,3-b]Pyrrol-4(1H)-One Derivatives. Chem. Heterocycl. Comp. 2018;54:1065–1069. doi: 10.1007/s10593-018-2393-9. [DOI] [Google Scholar]
- 79.Yahyavi H., Heravi M.M., Mahdavi M., Foroumadi A. Iodine-Catalyzed Tandem Oxidative Coupling Reaction: A One-Pot Strategy for the Synthesis of New Coumarin-Fused Pyrroles. Tetrahedron Lett. 2018;59:94–98. doi: 10.1016/j.tetlet.2017.11.055. [DOI] [Google Scholar]
- 80.Yang Y., Qi X., Liu R., He Q., Yang C. One-Pot Transition-Metal-Free Cascade Synthesis of Thieno[2,3-c]Coumarins from Chromones. RSC Adv. 2016;6:103895–103898. doi: 10.1039/C6RA21776A. [DOI] [Google Scholar]
- 81.Vishnumurthy K., Makriyannis A. Novel and Efficient One-Step Parallel Synthesis of Dibenzopyranones via Suzuki−Miyaura Cross Coupling. J. Comb. Chem. 2010;12:664–669. doi: 10.1021/cc100068a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Janosik T., Bergman J. In: Five-Membered Ring Systems: Thiophenes and Se/Te Analogues in Progress in Heterocyclic Chemistry. Gordon W.G., John A.J., editors. Volume 18 Elsevier; Amsterdam, The Netherlands: 2007. [Google Scholar]
- 83.Iaroshenko V.O., Ali S., Mkrtchyan S., Gevorgyan A., Babar T.M., Semeniuchenko V., Hassan Z., Villinger A., Langer P. Design and Synthesis of Condensed Thienocoumarins by Suzuki–Miyaura Reaction/Lactonization Tandem Protocol. Tetrahedron Lett. 2012;53:7135–7139. doi: 10.1016/j.tetlet.2012.10.096. [DOI] [Google Scholar]
- 84.Fondjo E.S., Tsemeugne J., De Dieu Tamokou J., Djintchui A.N., Kuiate J.R., Sondengam B.L. Synthesis and Antimicrobial Activities of Some Novel Thiophene Containing Azo Compounds. Heterocycl. Commun. 2013;19 doi: 10.1515/hc-2013-0096. [DOI] [Google Scholar]
- 85.Al-Mousawi S.M., El-Apasery M.A. Synthesis of Some Monoazo Disperse Dyes Derived from Aminothienochromene. Molecules. 2013;18:8837–8844. doi: 10.3390/molecules18088837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Djeukoua K.S.D., Fondjo E.S., Tamokou J.-D., Tsemeugne J., Simon P.F.W., Tsopmo A., Tchieno F.M.M., Ekom S.E., Pecheu C.N., Tonle I.K., et al. Synthesis, Characterization, Antimicrobial Activities and Electrochemical Behavior of New Phenolic Azo Dyes from Two Thienocoumarin Amines. Arkivoc. 2020;2019:416–430. doi: 10.24820/ark.5550190.p010.994. [DOI] [Google Scholar]
- 87.Gewald K. Zur Reaktion von α-Oxo-mercaptanen mit Nitrilen. Angew. Chem. 1961;73:114. doi: 10.1002/ange.19610730307. [DOI] [Google Scholar]
- 88.Hoberg H. Halogenmethyl-aluminiumverbindungen. Angew. Chem. 1961;73:114–115. doi: 10.1002/ange.19610730311. [DOI] [Google Scholar]
- 89.Gewald K. Heterocyclen aus CH-aciden Nitrilen, VII. 2-Amino-thiophene aus α-Oxo-mercaptanen und methylenaktiven Nitrilen. Chem. Ber. 1965;98:3571–3577. doi: 10.1002/cber.19650981120. [DOI] [Google Scholar]
- 90.Sopbué Fondjo E., Döpp D., Henkel G. Reactions of Some Anellated 2-Aminothiophenes with Electron Poor Acetylenes. Tetrahedron. 2006;62:7121–7131. doi: 10.1016/j.tet.2006.04.037. [DOI] [Google Scholar]
- 91.Yu L.-S.-H., Meng C.-Y., Wang J., Gao Z., Xie J.-W. Substrate-Controlled Diastereoselectivity Switch in the Formation of Dihydrothieno[3,4-c]Coumarins via [4+1] Annulations. Adv. Synth. Catal. 2019;361:526–534. doi: 10.1002/adsc.201801104. [DOI] [Google Scholar]
- 92.Kim J.E., Lee J., Yun H., Baek Y., Lee P.H. Rhodium-Catalyzed Intramolecular Transannulation Reaction of Alkynyl Thiadiazole Enabled 5,n-Fused Thiophenes. J. Org. Chem. 2017;82:1437–1447. doi: 10.1021/acs.joc.6b02614. [DOI] [PubMed] [Google Scholar]
- 93.Weibenfels M., Hantschmann A., Steinfuhrer T., Birkner E. Synthesis of 7-Substituted Ones Thieno[3,2-c]Coumarin-3-Carboxlyic Acid. Z. Chem. 1989;29:166. doi: 10.1002/zfch.19890290503. [DOI] [Google Scholar]
- 94.El-Dean A.M.K., Zaki R.M., Geies A.A., Radwan S.M., Tolba M.S. Synthesis and Antimicrobial Activity of New Heterocyclic Compounds Containing Thieno[3,2-c]Coumarin and Pyrazolo[4,3-c]Coumarin Frameworks. Russ. J. Bioorg. Chem. 2013;39:553–564. doi: 10.1134/S1068162013040079. [DOI] [PubMed] [Google Scholar]
- 95.Domracheva I., Kanepe-Lapsa I., Jackevica L., Vasiljeva J., Arsenyan P. Selenopheno Quinolinones and Coumarins Promote Cancer Cell Apoptosis by ROS Depletion and Caspase-7 Activation. Life Sci. 2017;186:92–101. doi: 10.1016/j.lfs.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 96.Abdel-Hafez S.H., Elkhateeb A., Gobouri A.A., Azab I.H.E., Kirsch G. An Efficient Route for Synthesis and Reactions of Seleno-[2, 3-c]Coumarin. Heterocycles. 2016;92:1054–1062. doi: 10.3987/COM-16-13445. [DOI] [Google Scholar]