Abstract
Purpose
The purpose of this study was to (1) develop a novel tissue-mimicking thermochromic (TMTC) phantom that permanently changes colour from white to magenta upon heating above ablative temperatures, and (2) assess its utility for specific applications in evaluating thermal therapy devices.
Materials and methods
Polyacrylamide gel mixed with thermochromic ink was custom made to produce a TMTC phantom that changes its colour upon heating above biological ablative temperatures (> 60°C). The thermal properties of the phantom were characterised, and compared to those of human tissue. In addition, utility of this phantom as a tool for the assessment of laser and microwave thermal ablation was examined.
Results
The mass density, thermal conductivity, and thermal diffusivity of the TMTC phantom were measured as 10336 ± 1.0 kg/m3, 0.590 ± 0.015 W/m•K, and 0.1456 ± 0.002 mm2/s, respectively, and found to be in agreement with reported values for human soft tissues. Heating the phantom with laser and microwave ablation devices produced clearly demarcated regions of permanent colour change geographically corresponding to regions with temperature elevations above 60°C.
Conclusion
The TMTC phantom provides direct visualisation of ablation dynamics, including ablation volume and geometry as well as peak absolute temperatures within the treated region post-ablation. This phantom can be specifically tailored for different thermal therapy modalities, such as radiofrequency, laser, microwave, or therapeutic ultrasound ablation. Such modality-specific phantoms may enable better quality assurance, device characterisation, and ablation parameter optimisation, or optimise the study of dynamic heating parameters integral to drug device combination therapies relying upon heat.
Keywords: HIFU, laser, microwave, RF, thermal phantom, thermal therapy, thermal ablation, thermochromic, tissue-mimicking
Introduction
Ablative thermal therapy (target temperature >60°C) has been widely embraced in oncology over the past two decades as a local or regional treatment for solid tumours [1]. Radiofrequency ablation (RFA) was first introduced as a regional hyperthermia therapy in the 1930s by Kirschner and Bauer to electro-coagulate Gasserian ganglia for trigeminal neuralgia [2,3]. Since then, numerous devices have been developed to deliver thermal energy in a controlled geometry, including laser, radiofrequency (RF), high intensity therapeutic ultrasound (HITU), and microwave (MW) [4–7]. However, there is an absence of cost-effective, reproducible and validated methods to adequately characterise minimally invasive delivery of thermal energy [8]. Magnetic resonance imaging (MRI) thermometry methods have been utilised to monitor thermal therapies and to characterise the delivery of heat [9–11]. MRI thermometry is not widely available, requires MRI-compatible and expensive equipment, and is thus not a broadly useful tool for thermal characterisation or standardisation.
Tissue-mimicking phantoms (TMPs) are routinely used for calibrating and testing medical equipment, quality assurance, training simulation, and for measuring and characterising energy deposition of thermal therapy applicators prior to clinical use [8,12,13]. As such, TMPs offer an alternative to ex vivo tissues whose thermal properties rapidly decay after harvest. Ex vivo tissue limitations not shared by TMPs include tissue procurement challenges, short shelf-life, non-uniformities or air within tissue, and challenges in analysis post-heating. TMPs may provide a more stable platform for characterising delivery of thermal energy by reporting absolute temperatures and defining ablated volumes, spatial dynamics, and geometries.
Thermochromism is a phenomenon in which a substance changes its colour due to a change in temperature. This change can be either permanent or reversible, and with or without intermediate stages (colours). The colour change may be produced by liquid crystals, leuco dyes, or by permanent change ink [14–19]. To date, thermochromic materials have been used in a variety of applications such as in product labelling, forehead thermometers, food quality indicators, and in novelty items such as temperature sensitive coffee mugs [20]. Heat sensitive permanent colour change inks are typically used as indicators for quality control or sterility verification, providing visual evidence of attained temperatures, and are available in a wide variety of colours and activation temperatures [20].
Recently, synthetic phantom materials that report on thermal therapy heating patterns have been formulated [21,22]. Dabbagh et al. described reusable heat sensitive tissue-mimicking phantoms composed of polyacrylamide gel and thermochromic dye [23]. This type of re-usable phantom changes colour from blue to colourless upon heating, and in reverse upon cooling. The shortcoming of this phantom is that the colour change is reversible, thus rapid colour analysis is required in order to achieve accurate absolute temperature quantification before colour reversal. Qureshi et al. described the use of a heat sensitive phantom utilising thermochromic liquid crystals for quality assurance (QA) of therapeutic ultrasound [24]. However, this QA phantom, which consists of a thin layer of thermochromic material (that produces a reversible colour change upon heating) on top of an absorbing disc, is not tissue-mimicking and does not inform on volumetric heating patterns or geographies.
A tissue-mimicking thermochromic (TMTC) phantom material that permanently changes colour upon heating can provide an inexpensive, reliable, and simple tool to assess volumetric thermal ablation patterns as well as maximum temperatures. Herein is described the development, formulation, and utility of a TMTC phantom material containing thermochromic ink that permanently changes colour from white to magenta upon heating to temperatures above 60°C. This phantom material may be used for thermal therapy quality assurance and device characterisation, and can be optimised for different thermal therapies (e.g. laser, RF, HITU, and MW) or tissue properties, by adjusting the phantom formulation.
Materials and methods
Formulation of the TMTC phantom material
All chemical reagents were purchased from Sigma Aldrich (Milwaukee, WI), unless otherwise mentioned.
This phantom material is based on tissue-mimicking polyacrylamide gel [25,26] with the addition of commercially available thermochromic ink. This ink permanently changes colour from white to magenta as temperature increases. According to the ink manufacturer, the maximum colour density is reached at around 60–65°C [27]. This property renders the phantom useful for reporting on thermoablative temperatures and the ablation geometry post-heating, since most tissues will instantly ablate at similar temperatures [28].
An aqueous solution of 40% (w/v) acrylamide/bis-acrylamide with feed ratio (acrylamide to bis-acrylamide) of 19:1 (241.0 mL) was mixed under magnetic stirring with degassed, de-ionised water (1053.0 mL). This was followed by the addition of Kromagen Magenta MB60-NH concentrate (76.6 mL, TCR Hallcrest, Glenview, IL). Finally, ammonium persulfate (APS, 2.9 g) was added to initiate polymerisation, and N,N,N',N'-tetramethylethylenediamine (2.9 mL) was added as catalyst. The final solution was immediately transferred to a plastic container of desired size and kept in a cold room (4°C) overnight. If polymerisation occurs at room temperature, the temperature of the phantom material rises due to the heat of the polymerisation process [29]. Therefore, it is important to allow the phantom material to slowly polymerise at 4°C, to avoid undesired and confounding colour changes. The resulting phantoms were tightly sealed and stored at 4°C.
Water bath heating of phantom samples to 65 °C
Using the above process, 100 mL of phantom material was prepared and transferred into two 50 mL Falcon polypropylene conical tubes (30 × 115 mm, VWR International, West Chester, PA), capped, and let cool down at 4°C. One of these phantom samples was then equilibrated to ambient temperature and heated at 65°C for 30 min using a hot water bath. Photographs of both the heated and unheated samples were taken at room temperature to illustrate the colour differences (Figure 1).
Figure 1.
Left, unheated phantom sample, and right, phantom sample heated to 65°C for 30 min using a hot water bath.
Measurement of mass density, thermal conductivity, and thermal diffusivity
Mass density of five small (~2 cm diameter) TMTC phantom samples was determined using the Archimedes principle. Briefly, the phantom was submerged in water and the density was measured in triplicate using a balance equipped with a density measurement kit (Mettler Toledo®, VWR International, Batavia, IL). In addition, thermal conductivity and diffusivity of TMTC phantom samples (10 × 5 cm) were measured in triplicate using a thermal property analyser (KD-2 Pro, Decagon Devices, Pullman, WA).
Utility of the TMTC phantom in observing microwave and laser thermal ablation
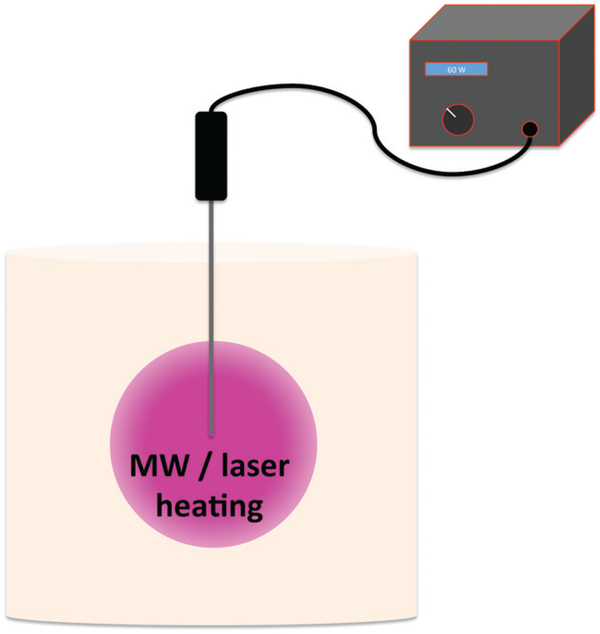
The phantom was used to assess two different thermal therapy modalities: MW and laser. Two cylindrical TMTC phantoms (volume 1 L, diameter 10 cm) were produced. Heating of the phantoms was performed using either a clinical water-cooled 1.6 mm diameter MW applicator (HS AMICA MW ablation system, Hospital Service SpA, Aprilia, Italy) with a 10 mm dielectric tip and operated at 2450 MHz, or a clinical side-firing 1.65 mm diameter laser applicator (Visualase, Medtronic, Minneapolis, MN) with a cooling catheter and 20 mm active tip. The parameters for MW and laser ablations were 60 W for 10 min and 15 W for 90 s, respectively. Figure 2 depicts the experiment set-up for both MW and laser ablation. In each phantom the applicator was advanced to the centre of the phantom, and the device operated using the above parameters.
Figure 2.
Schematic of the experiment set-up for laser and microwave heating of the tissue-mimicking thermochromic (TMTC) phantom. The thermal therapy applicator (microwave or laser) was advanced to the centre of a TMTC phantom, and the therapy device was operated with fixed parameters: 60 W and 10 min for microwave, and 15 W and 90 s for laser.
Results
Measurement of TMTC phantom tissue-mimicking thermal properties
The phantom formulation as described above produces an opaque gel phantom that permanently changes colour upon heating to temperatures above 60°C. Thermal conductivity, thermal diffusivity, and mass density of the TCTM phantom were measured to be 0.590 ± 0.015 W/m•K, 0.145 ± 0.002 mm2/s, and 1033 ± 1.0 kg/m3, respectively. These values are comparable to reported values of other tissue-mimicking phantoms and of human soft tissues [30].
Colour change of TMTC phantom after water bath heating at 65 °C
We also performed heating of a TMTC phantom sample in a hot water bath at 65°C for 30 min. Figure 1 shows the resulting colour change in comparison to an unheated phantom sample.
Heating the TMTC phantom using MW and laser
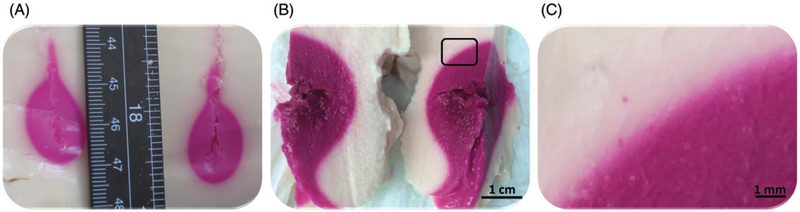
Figure 3 shows representative examples of colour change within the heated region in TMTC phantoms post-ablation. Figure 3A illustrates the resulting colour change after laser ablation, Figure 3B depicts the resulting colour change after microwave ablation, and Figure 3C showcases the rectangular region in Figure 3B magnified 7.5-fold.
Figure 3.
Photographs of cross-sections through the heated volume, as cut along the applicator track in tissue-mimicking thermochromic phantoms after laser and microwave ablation procedures. (A) Colour change after laser ablation. (B) Colour change after microwave ablation. (C) A 7.5-fold magnification of the rectangular region shown in B.
Laser ablation with 15 W for 90 s resulted in an approximately 2 × 2 × 2 cm region of colour change, while MW ablation at 60 W for 10 min resulted in an approximately 5 × 4 × 4 cm region of colour change. Here, the colour change dimensions refer to the three main axes of a spheroid, reported as the largest dimension along each axis (length along the applicator × width × depth) of the body of the tadpole-shaped heated region. For laser and MW ablations, the colour within the heated region was uniform, indicating ablative temperatures of at least 60°C achieved within the whole ablated region.
Qualitatively, the colour within the ablated region in Figure 3A and B matches with the heated phantom sample shown in Figure 1. Thus, the colour change is independent of the heating modality, and dependent primarily on the achieved temperature. The slight colour differences between Figure 3A and B may be a result of slightly different maximum temperatures, or due to different camera settings and light conditions. The thermal margins after both MW and laser heating have a relatively sharp transition on gross inspection; however, upon magnification a colour gradient from white to magenta at the thermal margin is appreciated (Figure 3C).
Discussion
A TMTC phantom material that permanently changes colour upon heating may provide an inexpensive, reliable, and simple method with which to study or optimise thermal ablation hardware, software, and algorithms, to assess relative extent of ablative thermal damage. The aim of this study was to combine a known acrylamide-based tissue-mimicking phantom material with a thermochromic ink to produce a novel TMTC phantom material that reports on the ablation zone as well as achieved temperatures. This unique ink provides a colour change that progressively becomes deeper as the temperature increases (Figure 3). The colour density reaches its saturation at around 60–65°C, making this phantom suitable for reporting on thermoablative temperatures, where cells typically undergo coagulative necrosis relatively instantaneously in this range of temperatures [28]. We also noticed that there is a gradual colour change from white to magenta at the thermal margin (Figure 3C). Therefore, it may be possible to assess temperatures below 60°C by calibrating temperature with colour change and by using, for example, a look-up table or a colour/temperature library to provide accurate characterisation of the colour change at specific temperatures. This would enable a quantitative semi-automated method of maximum temperature analysis. Thermochromic inks that provide a maximum colour change at lower temperatures may also be used to report on, for example, achieved mild hyperthermic temperatures.
However, the irreversible nature of the phantom colour change may necessitate the use of many samples, potentially resulting in high costs. Although the ink concentration in the formulation was selected in order to facilitate visualisation of the ablated regions, it may be possible to use lower concentrations of ink if the production of large TMTC phantoms becomes cost-prohibitive. For the same reason, a smaller modular TMTC phantom with ink could also be inserted within a larger phantom without thermochromic ink, to further reduce the manufacturing costs without compromising the tissue-mimicking qualities. The ink concentration may need to be readjusted if the phantom formulation is further optimised, for example for a specific thermal therapy modality.
Moreover, this thermochromic phantom could be improved in terms of its tissue-mimicking properties by embedding, for example, structures that mimic vasculature and pulsatile flow within the phantom. While still not fully comparable to living tissues, it would allow the phantom to mimic perfusion. Use of such structures within a tissue-mimicking phantom has been previously reported by Greaby et al. [31].
Finally, thermochromic ink together with an adhesive agent may also be utilised to paint bone or 3D-printed bonelike materials for visualisation of temperatures achieved on the bone surface. Such an application of a thermochromic material has been previously reported by Gelat et al., albeit using thermochromic paint with reversible colour-change properties [32]. Additionally, optimisation of this phantom material for specific thermal modalities (RFA, HITU, laser, microwave) correlated with actual thermal profiles may inform further applications of this novel research tool. For example, clinical practicalities could be addressed with this model, including clarification of the effects of convective heat loss on micro or macro scale in terms of thermal effects, or clarification of the fluid and distances required for hydrodissection to protect heat-sensitive non-target anatomy. Finally, computational thermal models could be refined, validated, or optimised without the cost and complexities of in vivo data.
Conclusion
A novel TMTC phantom material was designed and developed that permanently changes colour from white to magenta upon heating to temperatures above 60°C. This TMTC phantom material can provide direct visualisation of heated/ablated regions following thermal therapy, as it reports both the ablation geometry as well as the maximum achieved local ablative temperatures. This TMTC phantom material may have utility in various thermal therapy applications, such as in quality assurance, device characterisation, parameter optimisation, and clinical technique refinement.
Acknowledgements
We thank Fil Banovac, Ankur Kapoor, and Roxanna Juarez for their contributions to this study.
Footnotes
Disclosure statement
A.H.N., A.S.M., S.X., N.A.-J., S.M. and B.J.W. have no conflict of interest to declare. A. P. is a paid employee of Philips. The authors alone are responsible for the content and writing of the paper.
References
- 1.Wood BJ, Ramkaransingh JR, Fojo T, Walther MM, Libutti SK. Percutaneous tumor ablation with radiofrequency. Cancer 2002;94(2):443–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirschner M. Zur Elektrokoagulation des Ganglion Gasseri [On Electrocoagulation of the Gasserian Ganglion]. Zentralbl Chir 1932;47:3. [Google Scholar]
- 3.Bauer K. Unsere Technik der Koagulation des Ganglion Gasseri [Our technique of Electrocoagulation of the Gasserian Ganglion]. Zentralbl Chir 1939;14:4. [Google Scholar]
- 4.Izatt JA, Sankey ND, Partovi F, Fitzmaurice M, Rava RP, Itzkan I, et al. Ablation of calcified biological tissue using pulsed hydrogen fluoride laser radiation. IEEE J QuantElectron 1990;26(12):2261–70. [Google Scholar]
- 5.Tanaka R, Kim CH, Yamada N, Saito Y. Radiofrequency hyperthermia for malignant brain tumors: preliminary results of clinical trials. Neurosurgery 1987;21(4):478–83. [DOI] [PubMed] [Google Scholar]
- 6.McGough RJ, Kessler ML, Ebbini ES, Cain CA. Treatment planning for hyperthermia with ultrasound phased arrays. IEEE Trans Ultrason Ferroelectr Freq Control 1996;43(6):1074–84. [Google Scholar]
- 7.Ryan TP, Hoopes PJ, Taylor JH, Strohbehn JW, Roberts DW, Douple EB, et al. Experimental brain hyperthermia – techniques for heat delivery and thermometry. Int J Radiat Oncol Biol Phys 1991;20(4):739–50. [DOI] [PubMed] [Google Scholar]
- 8.Bouchard LS, Bronskill MJ. Magnetic resonance imaging of thermal coagulation effects in a phantom for calibrating thermal therapy devices. Med Phys 2000;27:1141–5. [DOI] [PubMed] [Google Scholar]
- 9.Enholm JK, Köhler MO, Quesson B, Mougenot C, Moonen CTW, Sokka SD. Improved volumetric MR-HIFU ablation by robust binary feedback control. IEEE Trans Biomed Eng 2010;57:103–13. [DOI] [PubMed] [Google Scholar]
- 10.Partanen A, Yarmolenko PS, Viitala A, Appanaboyina S, Haemmerich D, Ranjan A, et al. Mild hyperthermia with magnetic resonance-guided high-intensity focused ultrasound for applications in drug delivery. Int J Hyperthermia 2012;28:320–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNichols RJ, Kangasniemi M, Gowda A, Bankson JA, Price RE, Hazle JD. Technical developments for cerebral thermal treatment: water-cooled diffusing laser fibre tips and temperature-sensitive MRI using intersecting image planes. Int J Hyperthermia 2004;20(1):45–56. [DOI] [PubMed] [Google Scholar]
- 12.Arora D, Cooley D, Perry T, Skliar M, Roemer RB. Direct thermal dose control of constrained focused ultrasound treatments: phantom and in vivo evaluation. Phys Med Biol 2005; 50(8):1919–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonald M, Lochhead S, Chopra R, Bronskill MJ. Multi-modality tissue-mimicking phantom for thermal therapy. Phys Med Biol 2004;49:2767–78. [DOI] [PubMed] [Google Scholar]
- 14.Panak O, Drzkova M, Kaplanova M. Insight into the evaluation of colour changes of leuco dye based thermochromic systems as a function of temperature. Dyes Pigm 2015;120:279–87. [Google Scholar]
- 15.Kakade VU, Lock GD, Wilson M, Owen JM, Mayhew JE. Accurate heat transfer measurements using thermochromic liquid crystal. Part 2: Application to a rotating disc. Int J Heat Fluid Flow 2009;30(5):950–9. [Google Scholar]
- 16.Abdullah N, Abu Talib A, Jaafar AA, Salleh MAM, Chong WT. The basics and issues of thermochromic liquid crystal calibrations. Exper Thermal Fluid Sci 2010;34(8):1089–121. [Google Scholar]
- 17.Kakade VU, Lock GD, Wilson M, Owen JM, Mayhew JE. Accurate heat transfer measurements using thermochromic liquid crystal. Part 1: Calibration and characteristics of crystals. Int J Heat Fluid Flow 2009;30(5):939–49. [Google Scholar]
- 18.Newton PJ, Yan Y, Stevens NE, Evatt ST, Lock GD, Owen JM. Transient heat transfer measurements using thermochromic liquid crystal. Part 1: An improved technique. Int J Heat Fluid Flow 2003;24(1):14–22. [Google Scholar]
- 19.Christie RM, Bryant D. The application of instrumental color measurement methods to thermochromic printing inks. Surf Coat Int 1995;78:332–6. [Google Scholar]
- 20.TLCHallcrest. Handbookof thermochromic liquid crystal technology. Data sheet available from http://www.hallcrest.com/downloads/randtk_TLC_Handbook.pdf (accessed 19 January 2016).
- 21.Dabbagh A, Abdullah BJJ, Ramasindarum C, Abu KNH. Tissue-mim-icking gel phantoms for thermal therapy studies. Ultrason Imaging 2014;36(4):291–316. [DOI] [PubMed] [Google Scholar]
- 22.Butterworth I, Barrie J, Zeqiri B, Zauhar G, Parisot B. Exploiting thermochromic materials for the rapid quality assurance of physiotherapy ultrasound treatment heads. Ultrasound Med Biol 2012;38(5):767–76. [DOI] [PubMed] [Google Scholar]
- 23.Dabbagh A, Abdullah BJJ, AbuKasim NH, Ramasindarum C. Reusable heat-sensitive phantom for precise estimation of thermal profile in hyperthermia application. Int J Hyperthermia 2014;30(1):66–74. [DOI] [PubMed] [Google Scholar]
- 24.Qureshi F, Larrabee Z, Roth C, Hananel A, Eames M, Moore D, et al. Thermochromic phantom for therapeutic ultrasound daily quality assurance. J Therapeut Ultrasound 2015;3(Suppl1):72. [Google Scholar]
- 25.Bini MG, Ignesti A, Millanta L, Olmi R, Rubino N, Vanni R. The polyacrylamide as a phantom material for electromagnetic hyperthermia studies. IEEE Trans Biomed Eng 1984;31(3):317–22. [DOI] [PubMed] [Google Scholar]
- 26.Lafon C, Zderic V, Noble ML, Yuen JC, Kaczkowski PJ, Sapozhnikov OA, et al. Gel phantom for use in high-intensity focused ultrasound dosimetry. Ultrasound Med Biol 2005;31(10):1383–9. [DOI] [PubMed] [Google Scholar]
- 27.TLCHallcrest. Permanent change thermochromic ink. Data sheet available from http://www.hallcrest.com/DesktopModules/Bring2mind/DMX/Download.aspx?Command=Core_Download&EntryId=215&language=en-US&PortalId=0&TabId=163 (accessed 19 January 2016).
- 28.Goldberg SN, Gazelle GS, Halpern EF, Rittman WJ, Mueller PR, Rosenthal DI. Radiofrequency tissue ablation: importance of local temperature along the electrode tip exposure in determining lesion shape and size. Acad Radiol 1996;3(3):212–18. [DOI] [PubMed] [Google Scholar]
- 29.Orban M, Kurin-Csoergei K, Zhabotinsky AM, Epstein IR. Pattern for-mation during polymerization of acrylamide in the presence of sulfide ions. J Phys Chem B 1999;103(1):36–40. [Google Scholar]
- 30.Duck F (ed.). Physical properties of tissue: A comprehensive refer-ence book. London: Academic Press, 1990. [Google Scholar]
- 31.Greaby R, Zderic V, Vaezy S, Pulsatile flow phantom for ultrasound image-guided HIFU treatment of vascular injuries. Ultrasound Med Biol 2007;33:1269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gelat P, Sinden D, Symonds-Tayler R, Rivens I, Civale J, Ter Haar G, et al. Visualisation of the temperature rise on the surface of ribs during HIFU exposure using a phantom with a thermochromic coating. International Society for Therapeutic Ultrasound Conference, 2015, Utrecht, the Netherlands. [Google Scholar]