Abstract
Background
Melatonin is a hormone secreted by the pineal gland in a circadian rhythmic manner with peak synthesis at night. Melatonin signalling was suggested to play a critical role in metabolism during the circadian disruption.
Methods
Melatonin‐proficient (C3H‐f+/+ or WT) and melatonin receptor type 1 knockout (MT1 KO) male and female mice were phase‐advanced (6 hours) once a week for 6 weeks. Every week, we measured weight, food intake and basal glucose levels. At the end of the experiment, we sacrificed the animals and measured the blood's plasma for lipids profile (total lipids, phospholipids, triglycerides and total cholesterol), metabolic hormones profiles (ghrelin, leptin, insulin, glucagon, glucagon‐like‐peptide and resistin) and the body composition.
Results
Environmental circadian disruption (ECD) did not produce any significant effects in C3H‐f+/+, while it increased lipids profile in MT1 KO with the significant increase observed in total lipids and triglycerides. For metabolic hormones profile, ECD decreased plasma ghrelin and increased plasma insulin in MT1 KO females. Under control condition, MT1 KO females have significantly different body weight, fat mass, total lipids and total cholesterol than the control C3H‐f+/+ females.
Conclusion
Our data show that melatonin‐proficient mice are not affected by ECD. When the MT1 receptors are removed, ECD induced dyslipidaemia in males and females with females experiencing the most adverse effect. Overall, our data demonstrate that MT1 signalling is an essential modulator of lipid and metabolic homeostasis during ECD.
Keywords: environmental circadian disruption and lipids, female, male, melatonin‐proficient mice, MT1 signalling
Melatonin‐proficient mice are protected against environmental circadian disruption (ECD), while removal of melatonin receptor type 1 (MT1) signalling induces dyslipidaemia in males and females and hyperinsulinaemia in only MT1 knockout (MT1 KO) females. Our experiments suggest that MT1 KO female mice are more affected by ECD than their male counterpart, thus suggesting that ECD may have more harmful effects on females.
1. INTRODUCTION
Melatonin is a chronobiotic hormone secreted by the pineal gland in a rhythmic manner that reaches peak circulating levels during the night. 1 Once melatonin is synthesized, it exerts its function via two G‐protein‐coupled receptors named melatonin receptor type 1 (MT1) and melatonin receptor type 2 (MT2). 2 , 3 MT1 and MT2 are both expressed in the mammalian brain master clock—suprachiasmatic nuclei (SCN) of the hypothalamus, 2 , 4 , 5 and both receptors are involved in the entrainment of circadian rhythms by SCN. 6 During the circadian disruption, it is still unclear which one of the two receptors is actually responsible for the entrainment of the circadian rhythms in locomotor activity. 7 Experimental evidence suggests that MT1 is required for the melatonin‐induced phase‐shift of the circadian rhythm in locomotor activity 8 . Whereas, MT2 is implicated in the re‐entrainment of the circadian rhythm of locomotor activity. 9
Recent studies have also implicated melatonin signalling in the regulation of metabolism and glucose homeostasis. 10 , 11 , 12 Our laboratory has shown that mice lacking MT1 exhibit a marked systemic insulin resistance while MT2 knockout (KO) did not 13 ; additionally, we also found that MT1 KO mice also tend to accumulate more fat mass than the control mice. 14 We also reported that MT1 KO mice exhibit a selective leptin resistance in the arcuate nucleus. 15 Moreover, MT1 KO mice subjected to a high‐fat diet also showed a significant increase in cumulative weight gain and basal glucose levels (>200 mg/dL) after 10 weeks. 11 Interestingly in our previous study, we did not observe any phenotype in mice lacking MT2. 11 , 13
Previous studies have shown that environmental circadian disruption (ECD) has severe consequences on the mouse's overall health. In a seminal study by Davidson et al, it was reported that chronic jet‐lag (6‐hour advance for 8 weeks) increases mortality in aged mice. 16 Additional studies indicated that ECD dysregulated the innate immune system independently from sleep loss or stress 17 and led to an increase in body weight, adipocytes size and triglycerides concentration. 18 , 19 , 20 , 21 However, it is worth noting that all these experiments have been performed only in mice males on a C57/Bl6 genetic background, a melatonin‐deficient strain. 22 , 23
The aim of the present study was first to investigate the effects of ECD in melatonin‐proficient mice strain (C3H‐f+/+) and then to determine the effects of ECD in C3H‐f+/+ mice lacking MT1 receptors.
2. MATERIALS AND METHODS
2.1. Animals and housing conditions
Melatonin‐proficient mice (C3H‐f+/+ wild type), melatonin‐proficient mice lacking MT1 (C3H‐f+/+‐MT1 KO), were used in our study. 13 All mice were 3 months old at the beginning of the experiment. Mice were single caged, and water and chow (5L0D Picolab® laboratory rodent diet; LabDiet) were available ad libitum. All experimental procedures were performed under the NIH Guide on Care and Use of Laboratory Animals and were approved by the Morehouse School of Medicine Animal Care and Use Committee 24 , 25 , 26 .
2.2. Environmental circadian disruption
Control mice were maintained under the 12:12 light‐dark cycle while the ECD groups were maintained under the 6‐hour phase advance schedule described in Davidson et al 16 (Figure 1). The ECD group were maintained under ECD for 6 weeks, All mice were sacrificed and the plasma was collected at 12:00 pm on the 6th day after the last shift.
FIGURE 1.
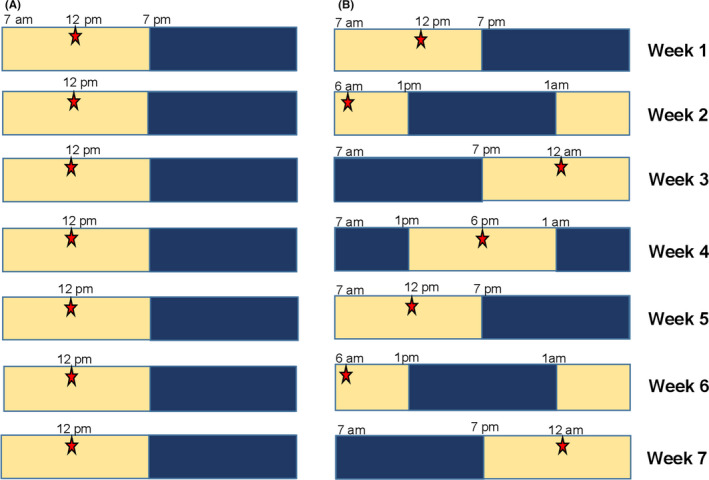
Environmental circadian disruption (ECD) light schedule. A, Control animals were under 12:12 light and dark (LD) cycle where the lights turn on at 7 am and turn off at 7 pm; for a period of 7 wk. B, ECD animals were under 12:12 light and dark cycle where the lights turn on at 7 am and turn off at 7 pm for week1; the LD was 6‐h phase advance for the following week (week 2), and the mice were given 7 d to adjust to new LD before the next phase advance; this was repeated for a total period of 7 wk. On the 6th days of the week, the mice fasted for 5 h when the light was turned on and the stars represent the time at which body parameters were measured
2.3. Body composition
At the end of the experiment, the mice were anaesthetized with isoflurane; then, we performed a cardiac puncture for the blood collection. After the blood collection, the mice were sacrificed, and the body composition analysis was performed at the University of Cincinnati National Mouse Metabolic Phenotyping Center, 27 using quantitative magnetic resonance.
2.4. Lipid analysis
Blood was collected and treated with 100 μL of 0.5 mol/L of ethylenediaminetetraacetic acid. The treated blood was centrifuged at 2000 g for 15 minutes at 4°C, and the plasma (supernatant) was frozen and stored at −80°C. Lipids profile protocol is described in detail here. 28
2.5. Metabolic hormones plasma analysis
The plasmas' level of metabolic hormones (ghrelin, leptin, insulin, glucagon, glucagon‐like‐peptide and resistin) were measured using a commercially available suspension of magnetic bead array immunoassay kit following manufacturer's specifications (BIO‐RAD Bio‐Plex Pro Mouse Diabetes 8‐Plex Assay #171f7001m).
2.6. Data analysis
Results are presented as mean ± SEM. The significance level was set at P = .05 with a power >0.8. Statistical analyses were performed using two‐way analysis of variance followed post hoc Tukey test in GraphPad Prism version 8.0.
3. RESULTS
3.1. Effects of melatonin signalling removal under normal lighting conditions
Our data indicate that under normal lighting conditions, several differences are present among the different genotypes and sex. C3H‐f+/+ females have significantly higher body weight, fat mass, total lipids and total cholesterol than MT1 KO female (Two‐way ANOVA Followed by Tukey test, P < .05) whereas C3H‐f+/+ males have significantly lower levels of phospholipids than MT1 KO males (two‐way ANOVA Followed by Tukey test, P < .05). Basal glucose levels were also slightly lower in C3H‐f+/+ males than in MT1 KO males (two‐way ANOVA followed by Tukey test, P < .05). Finally, ghrelin levels were significantly lower in C3H‐f+/+ females than in MT1 KO females (two‐way ANOVA followed by Tukey test, P < .05).
3.2. Melatonin‐proficient mice are protected from ECD physiological change
Figure 2 displays the results obtained in C3H‐f+/+ mice under the control condition and ECD. Although females showed a tendency to an increase in cumulative weight, no significant differences were observed in all the parameters investigated between males and females and between control and ECD mice (Figure 2A‐E; P > .05 in all cases). No difference among the different groups was also detected in the plasma levels of the six metabolic hormones (Figure 3A‐F, P > .05 in all cases).
FIGURE 2.
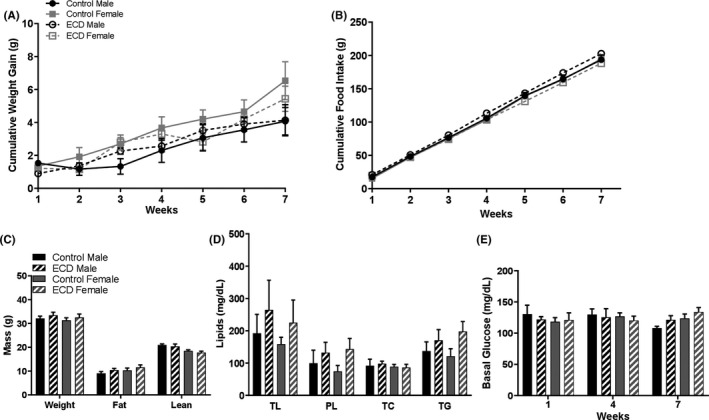
Melatonin‐proficient wild‐type mice are protected against environmental circadian disruption (ECD)‐associated dysfunctions. A, Cumulative weight gain; two‐way ANOVA repeated measured followed by Tukey post hoc test. B, Cumulative food intake; two‐way ANOVA repeated measured followed by Tukey post hoc test. C, Body composition; two‐way ANOVA and followed by Tukey post hoc test D, Lipids composition: total lipids (TL), phospholipids (PL), total cholesterol (TC), triglycerides (TG); two‐way ANOVA and followed by Tukey post hoc test. E, Fasting blood glucose; two‐way ANOVA repeated measured followed by Tukey post hoc test. Results are expressed in mean ± SEM (n = 6‐8)
FIGURE 3.
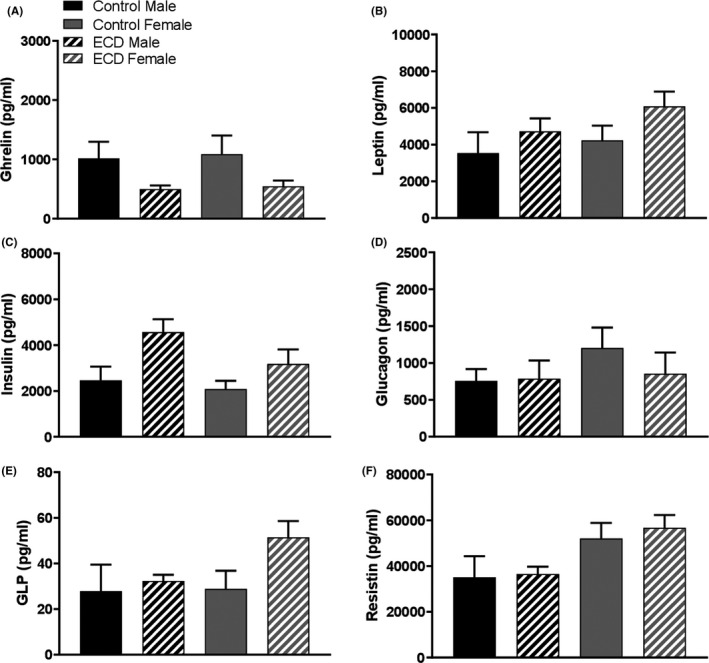
Melatonin‐proficient wild‐type mice are protected against environmental circadian disruption (ECD) dysregulation of metabolic hormones. A, Ghrelin (B) leptin (C) insulin (D) glucagon (E) glucagon‐like peptide (GLP) (F) resistin. Results are expressed in mean ± SEM (n = 6‐8; two‐way ANOVA followed by Tukey post hoc test)
3.3. Removal of MT1 signalling induces dyslipidaemia and hormonal changes in mice subjected to ECD
Figure 4 shows that the results obtained in MT1 KO mice under control and ECD conditions. No significant difference was observed in most of the parameter investigated (Figure 4A‐C,E, P > .05), but a significant increase in total lipids (almost 3 times with respect to control conditions) was observed in the female under ECD (Figure 4D; P < .05), and a significant increase in triglycerides levels was observed in both male and female mice (Figure 4E; P < .05). Under control conditions, MT1 KO females showed a significant increase in ghrelin and resistin levels with respect to the values measured in MT1 KO males (Figure 5A,F; P < .05). ECD significantly reduced the levels of ghrelin (Figure 5A; P < .05) and increased the level of Insulin (Figure 5C; P < .05) in MT1 KO females.
FIGURE 4.
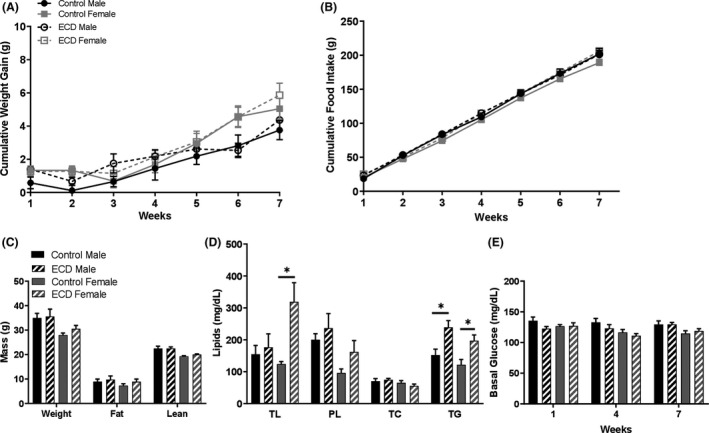
Environmental circadian disruption (ECD) induces dyslipidaemia in MT1 −/− male and female. A, Cumulative weight gain; two‐way ANOVA repeated measured followed by Tukey post hoc test. B, Cumulative food intake; two‐way ANOVA repeated measured followed by Tukey post hoc test. C, Body composition; two‐way ANOVA and followed by Tukey post hoc test. D, Lipids composition: total lipids (TL), phospholipids (PL), total cholesterol (TC), triglycerides (TG); two‐way ANOVA and followed by Tukey post hoc test,*P < .05. E, Fasting blood glucose; two‐way ANOVA repeated measured followed by Tukey post hoc test. Results are expressed in mean ± SEM (n = 6‐8)
FIGURE 5.
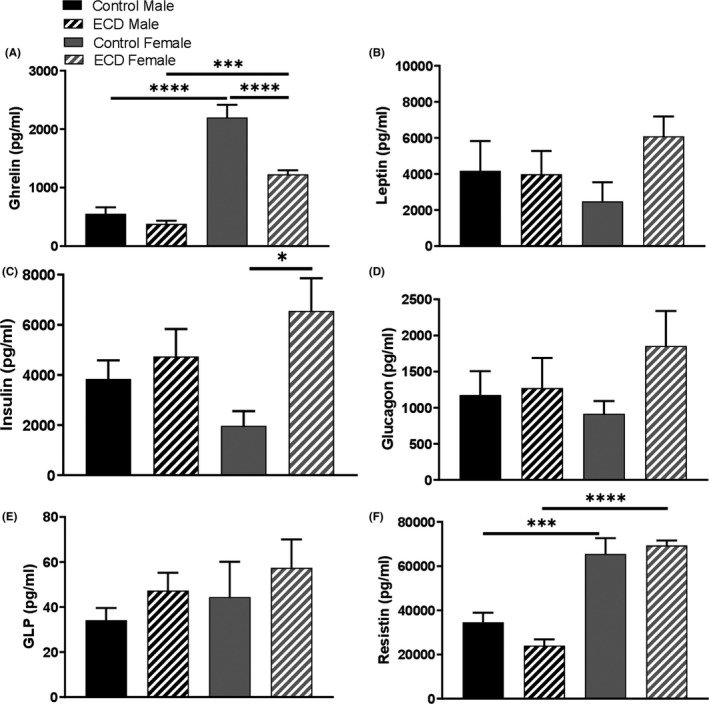
Environmental circadian disruption (ECD) decreases plasma ghrelin and increases plasma insulin in MT1 −/− female. A, Ghrelin (B) leptin (C) insulin (D) glucagon (E) glucagon‐like peptide (GLP) (F) resistin. Results are expressed in mean ± SEM (n = 6‐8; two‐way ANOVA followed by Tukey post hoc test, *P < .05, ***P < .001, ****P < .0001)
4. DISCUSSION
Disruption of melatonin synthesis and/or signalling is believed to be a co‐factor in the development of metabolic disorders in the general population 29 , 30 and in shift workers. 31 , 32 Although many studies have investigated the effects of ECD in mice, most of these studies have been performed in mice strains that do not synthesize melatonin, and no study has considered the effects of sex. In this study, we have investigated the effects of ECD in males and females' melatonin‐proficient mice model. From our research, we found that ECD did not induce any significant change in these mice, thus suggesting that melatonin signalling is exerting a protective effect against ECD. The removal of MT1 abolished the melatonin protection, and MT1 KO females seem to be more susceptible to ECD than the males.
As we have previously mentioned, our laboratory has reported that mice lacking MT1 show insulin and leptin resistance, 14 , 15 and we thus investigated whether ECD will further negatively affect the MT1 KO metabolic phenotypes. 11 , 14 , 15 Our data show that MT1 KO male and female mice showed an increase in plasma lipids with a significant increase in total lipids and triglycerides (Figure 4D). Such a result is as we expected because it has been reported that the administration of exogenous melatonin can reduce serum and liver triglycerides in diabetic male rats and mice 33 , 34 and in obese C57BL6 male mice. 35 Therefore, our data indicate that the activation of MT1 signalling exerts a protective effect on lipids concentration in mice subjected to ECD.
We also observed that ECD decreased the ghrelin level in MT1 KO with a significant decrease in MT1 KO females. Ghrelin is an orexigenic hormone secreted by the gut that binds to the growth hormone secretagogue receptor (GHS‐R). 36 The serum ghrelin level peaks during the resting phase and is mainly regulated by food intake. 37 , 38 Some studies have also reported that melatonin also decreases ghrelin levels in dogs and rats, 39 , 40 while in humans, plasma melatonin concentration correlated negatively with ghrelin, thus suggesting a possible role for melatonin in the regulation of ghrelin concentration. 41 Ghrelin is also a key regulator in glucose homeostasis, and it regulates insulin secretion via heterodimerization of G‐protein‐coupled receptor GSH‐R and somatostatin‐5 42 ; this could explain why MT1 KO females have an increase in plasma insulin after ECD. Oestrogen is reported to protect mammalian females against obesity, and circadian disruption is associated with metabolic dysfunction, 43 , 44 , 45 which might suggest that ECD has a more significant disruptive effect on oestrogen signalling in melatonin‐proficient MT1 KO female.
Our data also indicate that MT1 KO females have a higher plasma concentration of resistin when compared to MT1 KO males regardless of the treatment group. This is of interest because resistin is a hormone secreted by the white adipose whose name came from inhibiting insulin sensitivity, thus resisting insulin. 46 , 47 Loss of resistin was reported to improve glucose homeostasis, 48 and resistin was also reported to play a role in obesity via AMPKα and acetyl‐CoA carboxylase 49 , 50 , 51 . In our C3H‐f+/+ mice, there was no significant difference in plasma resistin between males and females. Thus, it appears that the removal of MT1 signalling affects the resistin level in a sex‐specific manner. Melatonin supplementation was reported to improve obesity‐induced resistin elevation. 35 , 52 , 53 Stubbins et al found that oestrogen protects female mice against obesity and impaired glucose tolerance, furthermore, they also report that oestrogen lowers the circulating resistin level. 43 Subsequent studies are needed to explore the mechanism of the effect of MT1 melatonin signalling on metabolic hormones in the female melatonin‐proficient mice. On the other note, it was observed that female shift workers experienced more stress and greater intolerance to the shift schedule than male workers. 54 , 55 Melatonin was reported to improve the subjects’ sleep quality in a simulated shift night study. 56 Thus, our findings are highlighting the need for studies that will further explore the mechanistic role of MT1 signalling in female circadian and metabolic homeostasis.
In conclusion, our results indicate that melatonin‐proficient mice are protected against ECD, while the removal of MT1 signalling induces dyslipidaemia in males and females and hyperinsulinaemia in only MT1 KO females. Similarly, it was observed that female shift workers experienced more stress and greater intolerance to the shift schedule than male workers. Our experiments also suggest that MT1 KO female mice are more affected by ECD than their male counterpart, thus suggesting that ECD may have more harmful effects on females.
CONFLICT OF INTEREST
The author(s) declare that they have no conflict of interests.
AUTHOR CONTRIBUTIONS
CT, KB, GP and GT designed the experiments, CT and GP performed the experiments, CT, KB, GP and GT analysed the data, CT wrote the first draft of manuscript that was edited and by all co‐authors.
ACKNOWLEDGMENTS
This work was supported by NIH R01‐EY026291 to G.T.; NIH T‐32 HL103104 to G.T.; NIH GM116760 to K.B.
Tchio C, Baba K, Piccione G, Tosini G. Removal of melatonin receptor type 1 signalling induces dyslipidaemia and hormonal changes in mice subjected to environmental circadian disruption. Endocrinol Diab Metab.2021;4:e00171 10.1002/edm2.171
DATA AVAILABILITY STATEMENT
The original data are available upon request from the corresponding author.
REFERENCES
- 1. von Gall C, Stehle JH, Weaver DR. Mammalian melatonin receptors: molecular biology and signal transduction. Cell Tissue Res. 2002;309:151‐162. [DOI] [PubMed] [Google Scholar]
- 2. Brydon L, Roka F, Petit L, et al. Dual signaling of human mel1a melatonin receptors via Gi2, Gi3, and Gq/11 proteins. Mol Endocrinol. 1999;13:2025‐2038. [DOI] [PubMed] [Google Scholar]
- 3. Jin X, von Gall C, Pieschl RL, et al. Targeted disruption of the mouse Mel(1b) melatonin receptor. Mol Cell Biol. 2003;23:1054‐1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dubocovich ML, Yun K, Al‐ghoul WM, Benloucif S, Masana MI. Selective MT 2 melatonin receptor antagonists block melatonin‐mediated phase advances of circadian rhythms. FASEB J. 1998;12:1211‐1220. [DOI] [PubMed] [Google Scholar]
- 5. Rivera‐Bermúdez MA, Masana MI, Brown GM, Earnest DJ, Dubocovich ML. Immortalized cells from the rat suprachiasmatic nucleus express functional melatonin receptors. Brain Res. 2004;1002:21‐27. [DOI] [PubMed] [Google Scholar]
- 6. Liu C, Weaver DR, Jin X, et al. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron. 1997;19:91‐102. [DOI] [PubMed] [Google Scholar]
- 7. Jockers R, Delagrange P, Dubocovich ML, et al. Update on melatonin receptors: IUPHAR review 20. Br J Pharmacol. 2016;173:2702‐2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dubocovich ML, Hudson RL, Sumaya IC, Masana MI, Manna E. Effect of MT1 melatonin receptor deletion on melatonin‐mediated phase shift of circadian rhythms in the C57BL/6 mouse. J Pineal Res. 2005;39:113‐120. [DOI] [PubMed] [Google Scholar]
- 9. Pfeffer M, Rauch A, Korf H‐W, von Gall C. The endogenous melatonin (MT) signal facilitates reentrainment of the circadian system to light‐induced phase advances by acting upon MT2 receptors. Chronobiol Int. 2012;29:415‐429. [DOI] [PubMed] [Google Scholar]
- 10. Cipolla‐Neto J, Amaral FG, Afeche SC, Tan DX, Reiter RJ. Melatonin, energy metabolism, and obesity: a review. J Pineal Res. 2014;56:371‐381. [DOI] [PubMed] [Google Scholar]
- 11. Owino S, Buonfiglio DDC, Tchio C, Tosini G. Melatonin signaling a key regulator of glucose homeostasis and energy metabolism. Front Endocrinol. 2019;10 10.3389/fendo.2019.00488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Szewczyk‐Golec K, Woźniak A, Reiter RJ. Inter‐relationships of the chronobiotic, melatonin, with leptin and adiponectin: implications for obesity. J Pineal Res. 2015;59:277‐291. [DOI] [PubMed] [Google Scholar]
- 13. Contreras‐Alcantara S, Baba K, Tosini G. Removal of melatonin receptor type 1 induces insulin resistance in the mouse. Obesity (Silver Spring, Md.). 2010;18:1861‐1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Owino S, Sánchez‐Bretaño A, Tchio C, et al. Nocturnal activation of melatonin receptor type 1 signaling modulates diurnal insulin sensitivity via regulation of PI3K activity. J Pineal Res. 2018;64:e12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buonfiglio D, Tchio C, Furigo I, et al. Removing melatonin receptor type 1 signaling leads to selective leptin resistance in the arcuate nucleus. J Pineal Res. 2019;67 10.1111/jpi.12580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Davidson AJ, Sellix MT, Daniel J, Yamazaki S, Menaker M, Block GD. Chronic jet‐lag increases mortality in aged mice. Curr Biol. 2006;16:R914–R916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Castanon‐Cervantes O, Wu M, Ehlen JC, et al. Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol. 2010;185:5796‐5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Casiraghi LP, Alzamendi A, Giovambattista A, Chiesa JJ, Golombek DA. Effects of chronic forced circadian desynchronization on body weight and metabolism in male mice. Physiol Rep. 2016;4:e12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci USA. 2011;108:1657–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oike H, Sakurai M, Ippoushi K, Kobori M. Time‐fixed feeding prevents obesity induced by chronic advances of light/dark cycles in mouse models of jet‐lag/shift work. Biochem Biophys Res Comm. 2015;465:556‐561. [DOI] [PubMed] [Google Scholar]
- 21. Skinner NJ, Rizwan MZ, Grattan DR, Tups A. Chronic light cycle disruption alters central insulin and leptin signaling as well as metabolic markers in male mice. Endocrinology. 2019;160:2257‐2270. [DOI] [PubMed] [Google Scholar]
- 22. Ebihara S, Marks T, Hudson DJ, Menaker M. Genetic control of melatonin synthesis in the pineal gland of the mouse. Science. 1986;231:491‐493. [DOI] [PubMed] [Google Scholar]
- 23. Goto M, Oshima I, Tomita T, Ebihara S. Melatonin content of the pineal gland in different mouse strains. J Pineal Res. 1989;7:195‐204. [DOI] [PubMed] [Google Scholar]
- 24. Guide for the care and use of laboratory animals In Guide for the Care and Use of Laboratory Animals; 1996. 10.17226/5140 [DOI] [Google Scholar]
- 25. NIH OD, OER, & OLAW . Guide Laboratory Animals for the Care and Use of Eighth Edition Committee for the Update of the Guide for the Care and Use of Laboratory Animals Institute for Laboratory Animal Research Division on Earth and Life Studies; 2011. http://www.nap.edu. Accessed May 29, 2020. [Google Scholar]
- 26. OLAW . PHS Policy on Humane Care and Use of Laboratory Animals; n.d. https://olaw.nih.gov/policies‐laws/phs‐policy.htm. Accessed May 29, 2020.
- 27. Laughlin MR, Kent Lloyd KC, Cline GW, Wasserman DH. NIH Mouse Metabolic Phenotyping Centers: the power of centralized phenotyping. Mamm Genome. 2012;23:623‐631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bertolucci C, Fazio F, Piccione G. Daily rhythms of serum lipids in dogs: influences of lighting and fasting cycles. Comp Med. 2008;58:485‐489. [PMC free article] [PubMed] [Google Scholar]
- 29. Eze I, Imboden M, Foraster M, et al. Exposure to night‐time traffic noise, melatonin‐regulating gene variants and change in glycemia in adults. Int J Environ Res Public Health. 2017;14 10.3390/ijerph14121492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Amaral FG, Cipolla‐Neto J. A brief review about melatonin, a pineal hormone. Arch Endocrinol Metab. 2018;62:472‐479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ledda C, Cinà D, Matera S, Mucci N, Bracci M, Rapisarda V. High HOMA‐IR index in healthcare shift workers. Medicina (Lithuania). 2019;55 10.3390/medicina55050186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oberlinner C, Ott MG, Nasterlack M, et al. Medical program for shift workers—impacts on chronic disease and mortality outcomes. Scand J Work Environ Health. 2009;35:309‐318. [DOI] [PubMed] [Google Scholar]
- 33. Hadjzadeh M‐A‐R, Hayatdavoudi P, Khalili M, Rajaei Z, Soukhtanloo M, Bibak B. Effects of melatonin on biochemical factors and food and water consumption in diabetic rats. Adv Biomed Res. 2014;3:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lo CC, Lin SH, Chang JS, Chien YW. Effects of melatonin on glucose homeostasis, antioxidant ability, and adipokine secretion in ICR mice with NA/STZ‐induced hyperglycemia. Nutrients. 2017;9 10.3390/nu9111187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Farias TSM, Cruz MM, Sa RCC, et al. Melatonin supplementation decreases hypertrophic obesity and inflammation induced by high‐fat diet in mice. Front Endocrinol. 2019;10 10.3389/fendo.2019.00750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pradhan G, Samson SL, Sun Y. Ghrelin: much more than a hunger hormone. Curr Opin Clin Nutr Metab Care. 2013;16:619‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bodosi B, Gardi J, Hajdu I, Szentirmai E, Obal F, Krueger JM. Rhythms of ghrelin, leptin, and sleep in rats: effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. Am J Physiol Regul Integr Comp Physiol. 2004;287:56‐65. [DOI] [PubMed] [Google Scholar]
- 38. Sánchez J, Oliver P, Picó C, Palou A. Diurnal rhythms of leptin and ghrelin in the systemic circulation and in the gastric mucosa are related to food intake in rats. Pflugers Arch. 2004;448:500‐506. [DOI] [PubMed] [Google Scholar]
- 39. Mustonen AM, Nieminen P, Hyvärinen H. Preliminary evidence that pharmacologic melatonin treatment decreases rat ghrelin levels. Endocrine. 2001;16:43‐46. [DOI] [PubMed] [Google Scholar]
- 40. Taheri P, Mogheiseh A, Shojaee Tabrizi A, Nazifi S, Salavati S, Koohi F. Changes in thyroid hormones, leptin, ghrelin and galanin following oral melatonin administration in intact and castrated dogs: a preliminary study. BMC Vet Res. 2019;15 10.1186/s12917-019-1894-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Korhonen T, Saarela S. Role of adiposity hormones in the mouse during fasting and winter‐acclimatization. Comp Biochem Physiol Part A Mol Integr Physiol. 2005;140:217‐223. [DOI] [PubMed] [Google Scholar]
- 42. Park S, Jiang H, Zhang H, Smith RG. Modification of ghrelin receptor signaling by somatostatin receptor‐5 regulates insulin release. Proc Natl Acad Sci USA. 2012;109:19003–19008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stubbins RE, Holcomb VB, Hong J, Núñez NP. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur J Nutr. 2012;51:861‐870. [DOI] [PubMed] [Google Scholar]
- 44. Yuan T, Li J, Zhao WG, et al. Effects of estrogen on insulin sensitivity and adipokines in mice. Acta Academiae Medicinae Sinicae. 2015;37:269‐273. [DOI] [PubMed] [Google Scholar]
- 45. Zhu L, Zou F, Yang Y, et al. Estrogens prevent metabolic dysfunctions induced by circadian disruptions in female mice. Endocrinology. 2015;156:2114‐2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Filková M, Haluzík M, Gay S, Šenolt L. The role of resistin as a regulator of inflammation: implications for various human pathologies. Clin Immunol. 2009;133:157‐170. [DOI] [PubMed] [Google Scholar]
- 47. Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307‐312. [DOI] [PubMed] [Google Scholar]
- 48. Qi Y, Nie Z, Lee YS, et al. Loss of resistin improves glucose homeostasis in leptin deficiency. Diabetes. 2006;55:3083‐3090. [DOI] [PubMed] [Google Scholar]
- 49. He F, Jin J‐Q, Qin Q‐Q, et al. Resistin regulates fatty acid Β oxidation by suppressing expression of peroxisome proliferator activator receptor gamma‐coactivator 1α (PGC‐1α). Cell Physiol Biochem. 2018;46:2165‐2172. [DOI] [PubMed] [Google Scholar]
- 50. Rae C, Robertson SA, Taylor JMW, Graham A. Resistin induces lipolysis and re‐esterification of triacylglycerol stores, and increases cholesteryl ester deposition, in human macrophages. FEBS Lett. 2007;581:4877‐4883. [DOI] [PubMed] [Google Scholar]
- 51. Brown RE, Wilkinson PMH, Imran SA, Wilkinson M. Resistin differentially modulates neuropeptide gene expression and AMP‐activated protein kinase activity in N‐1 hypothalamic neurons. Brain Res. 2009;1294:52‐60. [DOI] [PubMed] [Google Scholar]
- 52. Favero G, Stacchiotti A, Castrezzati S, et al. Melatonin reduces obesity and restores adipokine patterns and metabolism in obese (ob/ob) mice. Nutr Res. 2015;35:891‐900. [DOI] [PubMed] [Google Scholar]
- 53. Farias TdSMd, Paixao RID, Cruz MM, et al. Melatonin supplementation attenuates the pro‐inflammatory adipokines expression in visceral fat from obese mice induced by a high‐fat diet. Cells. 2019;8:1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kandolin I. Burnout of female and male nurses in shiftwork. Ergonomics. 1993;36:141‐147. [DOI] [PubMed] [Google Scholar]
- 55. Ogińska H, Pokorski J, Ogińska A. Gender, ageing, and shiftwork intolerance. Ergonomics. 1993;36:161‐168. [DOI] [PubMed] [Google Scholar]
- 56. Dawson D, Encel N, Lushington K. Shift work and sleep deprivation improving adaptation to simulated night shift: timed exposure to bright light versus daytime melatonin administration. Sleep. 1995; 18 https://academic.oup.com/sleep/article‐abstract/18/1/11/2749607. Accessed November 15, 2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original data are available upon request from the corresponding author.


