Abstract
Environmental enrichment for mice lags behind the standard enrichment offered to other laboratory rodents due to concerns about environmental variability and, in specific contexts, aggression. Our objective in this study was to evaluate concerns that the introduction of structural enrichment in the form of a single red acrylic mouse tunnel into murine housing may confound study findings. We measured effects on anxiety-like behaviors (elevated zero maze and open field activity), hippocampal neurogenesis, body weight gain, and physiologic markers of stress (adrenal gland weight, plasma corticosterone concentration, and neutrophil:lymphocyte ratio). Male and female C57BL/6J mice were randomly assigned to one of 2 groups: a standard-housed control group with enrichment consisting of paper nesting material, or an enriched group that received a single acrylic tunnel in addition to nesting material. All results fell within biologically normal ranges regardless of treatment, and variability (standard deviation) was not significantly different between groups for any measure. Mice in the enriched group showed modest differences during open field testing suggestive of decreased anxiety, traveling farther and depositing fewer fecal boli than standard-housed mice. Male mice in the tunnel-enriched group gained more body weight than standard-housed male mice. No significant effects by treatment were found in neurogenic or physiologic parameters. These results indicate that provision of simple structural enrichment is unlikely to have confounding effects on murine anxiety-like behaviors, neurogenesis, body weight gain, or physiologic parameters. We therefore recommend the inclusion of simple structural enrichment, such as an acrylic tunnel, to the standard environmental enrichment of social housing and nesting material for mice.
Abbreviations: BrdU, 5-bromo-2'-deoxyuridine; EE, Environmental Enrichment; EZM, Elevated Zero Maze
Mice are the most commonly used species in biomedical research,6,17,33 and as with most laboratory animals, they spend the vast majority of their lives in their home cages.32 The Guide for the Care and Use of Laboratory Animals (the Guide) describes an appropriate animal care and use program as one that, “provides environments, housing, and management that are well suited for the species or strains of animals maintained, taking into account their physical, physiological and behavioral needs.”20 Current conventional, or standardized housing for mice satisfies their basic physical needs by providing hygienic conditions, a controlled climate, and a well-balanced diet. However, their psychologic needs are often not addressed as the standard mouse cage environment affords limited opportunities to perform natural, species-typical behaviors, leading to stress that may affect physiologic parameters.7,36 Laboratory rodent housing has been designed primarily with institutional, economic and ergonomic considerations in mind, rather than animal welfare, which has resulted in relatively barren cages.6,15,36 In addition, a fairly large disparity often exists between standard enrichment provided to mice compared with standard enrichment for other laboratory rodents, such as guinea pigs and rats, which typically receive additional structural and sensory enrichment.6
Environmental enrichment (EE) for any laboratory animal species should provide species-specific resources beyond the satisfaction of basic needs (for example, food and water), and includes any environmental modification which aims to enhance an animal's innate behavioral and psychologic needs.6,7,20 The primary goal of EE is to improve an animal's psychologic wellbeing by providing sensory and motor stimulation that encourages the expression of species-typical behaviors, leading to a sense of control over the environment and an improved ability to cope with stress.2 As a result, the diverse stresses of living in a laboratory environment such as husbandry checks and laboratory procedures,15 will have less of an impact on the animal's biology and consequently, less impact on the data generated by that animal.36
Mice are highly social, nocturnal, burrowing and nesting mammals6,19 and are a thigmotactic prey species,6,7,19 preferring to seek shelter near walls or hide behind structures during exploration and foraging. Preference studies indicate that mice prefer a more structurally complex environment with nesting material over a housing environment lacking these enhancements and demonstrate motivation to work for access to such preferred environments.19,26 Given these well-documented species-typical behaviors and preferences, EE for mice that includes structural enrichment and nesting material may better support their behavioral needs.15,16,30 However, the industry standard for mouse enrichment neglects structural enhancements and is commonly limited to social housing and access to nesting material.7,19
Two main arguments are used to oppose expanding enrichment for mice. The primary argument, relating to EE in general, is the impact of EE on standardization and the potential for increased variability.8,20 However, the current standard housing environment of laboratory rodents has been criticized for neglecting psychological wellbeing and inducing abnormal behaviors, such as abnormal repetitive behaviors, or stereotypies. These stereotypies are associated with sensory and motor deprivation,6,7,13,20,36-38 which can cause stress that may alter physiologic parameters and affect brain development and behavior.36
The second argument against expanding enrichment for mice is that structural enrichment has been associated with increased aggression, particularly among male mice.14,19,26 The associated physical pain and social distress resulting from inter-animal aggression can significantly affect experimental outcomes due to alterations in immunologic and stress-induced parameters.9,14 However, the studies evaluating the effects of structural enrichment are highly variable9 and situational, with outcomes influenced by strain, sex, and the type of object introduced.7,17,19,26 Increased aggression has primarily been associated with structures, such as nest boxes, igloos, or elevated platforms,2,7,17,19,26 which can trigger territorial behavior related to control of the structure.34 Likewise, many studies evaluating the effects of EE in general evaluated highly enriched environments, with several different forms of enrichment offered either simultaneously or in rotation,29 making the interpretation of results challenging,2,19,26 and implementation in the current research setting impractical. While the effects of tunnel enrichment on reducing mouse anxiety during handling have been reported,18 we are unaware of research evaluating the effects of tunnel enrichment on standard study parameters.
To evaluate concerns that the introduction of simple structural enrichment, in addition to standard enrichment consisting of social housing and nesting material, may confound study findings, this study aims to identify whether the provision of a single red acrylic mouse tunnel induces alterations in anxiety-like behaviors, body weight gain, neurogenesis or physiologic stress levels. Aside from their demonstrated preference for a more structurally complex environment,19,26 acrylic tunnel enrichment was selected over other forms of structural enrichment due to its frequent use as enrichment among other rodent species, namely rats, as well as ease of implementation in most research settings. Our hypotheses were formulated based on the argument that the addition of simple structural enrichment into the murine housing environment would significantly alter study parameters as compared with those of mice housed with standard enrichment. Mice given an acrylic tunnel in addition to standard enrichment were hypothesized to exhibit: 1) fewer behaviors associated with anxiety, as demonstrated through standardized behavioral testing (elevated zero maze (EZM) and open field activity); 2) increased neurogenesis; 3) decreased gain in body weight; and finally, 4) decreased physiologic stress, as measured by adrenal gland weight, plasma corticosterone concentration, and neutrophil:lymphocyte ratio.
Materials and Methods
Animals.
All experimental procedures were approved by the Vanderbilt University Institutional Animal Care and Use Committee and took place in an AAALAC-accredited facility in accordance with the Guide for the Care and Use of Laboratory Animals20 and the United States Public Health Service Policy on Humane Care and Use of Laboratory Animals.25 On receipt from The Jackson Laboratory (Bar Harbor, ME), mice were maintained in a facility screened for and found free of the following pathogens: mouse hepatitis virus, mouse parvovirus, minute virus of mice, lymphocytic choriomeningitis virus, Sendai virus, pneumonia virus of mice, epizootic diarrhea of infant mice, Theiler mouse encephalomyelitis virus, mouse poxvirus, mouse adenovirus, mouse reovirus, Mycoplasma pulmonis, endoparasites (Syphacia spp. and Aspicularis spp.), and ectoparasites (Myobia musculi, Radfordia afffinis, Mycoptes musculinus, and Psorergates simplex). Throughout the study, mice were maintained under controlled conditions (ambient room temperature: 68 to 76 °F, relative humidity: 30% to 70%, 10 to 15 air changes per hour) on a standard 12:12hr light:dark cycle. Mice received a commercial pelleted laboratory rodent diet, 5L0D (Lab Diet, St Louis, MO); feed and water (reverse osmosis, autoclaved, Nashville, TN) were available ad libitum.
Study Design.
Male and female C57BL/6J mice (n = 48) were obtained from The Jackson Laboratory (Bar Harbor, ME) at 4 wk of age. On arrival, mice were individually identified via ear punch and indelible marker, weighed, and randomly assigned to one of 2 groups (n = 12 per sex per group): a standard-housed control group or an enriched group. Group sizes were selected to provide sufficient power (≥0.8) based on expected variability within the data, for both behavioral studies and markers of chronic stress,12 to detect a 20% difference between treatment groups.
The control group was housed in a standard enriched environment consisting only of corncob bedding with rolled paper nesting material (Enrich-o'Cobs, The Andersons). The enriched group was similarly housed on corncob bedding with rolled paper nesting material and, in addition, received a single red acrylic tunnel (Mouse Tunnel, Bio-Serve, Flemington, NJ).
To mitigate the potential for induced aggression among male mice due to the provision of structural enrichment, all mice were housed in same-sex groups of 3 14,31 in polycarbonate IVC cages (Allentown XJ, Allentown, NJ, 500cm2 floor space).
Cage change occurred every 2 wk, with tunnels replaced at each cage change. Water bottles were changed weekly. At cage change, mice were briefly handled to mimic noninvasive laboratory procedures, including body weight collection and refreshment of tail markings. Although the benefit of using a tunnel to reduce mouse anxiety during handling has been documented,18 all animals on this study were handled at their tail base regardless of treatment group to avoid a confounding variable when assessing the effect of the presence/absence of an acrylic tunnel as structural enrichment.
Cage-side assessments were performed at least once daily during the light cycle to identify husbandry or health concerns, including monitoring for visible signs of aggression. Although assessing aggression was not a specific aim of this study, mice were observed for clinical signs of aggression throughout the study, particularly for overt fighting behavior and/or the presence of wounding, which was used as an indirect indicator of aggression. Behavioral testing, observations, and cage changes were all performed by the same experimenter (TLO), who was aware of the treatment group.
Behavioral testing.
After 12 wk (17 wk of age), all mice underwent behavioral testing in both the EZM and the open field for locomotor activity, selected in consultation with a qualified neurobehavioral expert (FH), due to their ability to measure anxiety-like behaviors.22,27,28 Both tests were performed during the light phase of the light:dark cycle and were completed before 1200 h on 2 consecutive days to control for time of day effects. Testing order was randomized by cage, with all mice from the same cage tested on the same day. Mice performed one trial per behavioral test, such that a mouse underwent testing in the EZM on day one, received 24 h of rest, and was tested in the open field on the subsequent day. Mice were acclimated to the testing room for at least 30 min before the start of each test. After training to proficiency, a single experimenter (TLO) administered all behavioral tests.
Elevated zero maze.
The apparatus consisted of an elevated (0.61m above the floor) annular platform, constructed of opaque, white plastic, with 2 opposite enclosed quadrants, measuring 5 cm × 30 cm, W x H, and 2 opposite open quadrants measuring 5 cm × 0.5 cm, W x H (Stoelting, Wood Dale, IL). Room lighting was measured at 265 to 280 lux in the open quadrants and 118 to 125 lux in the closed. Mice were placed in the center of an open quadrant and allowed 5-min of free exploration. Movement in the maze was automatically recorded, via video camera, from above using AnyMaze (Stoelting, Wood Dale, IL). The total distance traveled and time spent in open and closed quadrants were digitally measured, with entrance into a quadrant defined as 80% of the area of the mouse having entered a new quadrant. The percentage of time spent in open quadrants and total distance traveled were analyzed. At the end of the test, mice were returned to their home cages, and the maze wiped clean using 70% ethanol between each mouse to minimize olfactory signals left by the previous subject.
Open field activity.
The apparatus consisted of an empty, clear Plexiglas open-field arena with a solid white floor, measuring 27.5 cm × 27.5 cm × 20 cm, L x W x H (Med Associates, St Albans, VT), housed in a sound-attenuating case. Lighting in individual chambers was not measured. Mice were placed in the center of the arena and allowed 60-min of free exploration. Movement in the arena was detected via the breaking of infrared beams and was automatically recorded (Med Associates, St. Albans, VT). The total distance traveled and time spent in the central and surrounding zones were digitally measured. The central zone comprised approximately 48% of the total floor space. The percentage of time spent in the central zone and the total distance traveled were analyzed. At the end of the test, mice were returned to their home cages and the number of fecal boli in the field was counted as an additional index of anxiety.27 The arena was wiped clean using 70% ethanol between each mouse to minimize olfactory signals left by the previous subject.
Administration of BrdU.
Approximately 12 h prior to euthanasia, a randomly assigned subset of mice (n = 15) received a single intraperitoneal injection in the lower right abdomen with 5-bromo-2’-deoxyuridine (BrdU) (Sigma–Aldrich, Milwaukee, WI, 243.5 mg/kg, 10 mg/mL), diluted in sterile phosphate buffered saline (PBS; Fisher Scientific, Suwanee, GA) to allow assessment of neurogenesis.35,39 The entire procedure of catching, restraining, injecting, and returning each mouse to its cage required a maximum time of 2 min.
Tissue collection and brain histology.
Twenty to 24 h after the last behavioral test, mice were euthanized by carbon dioxide asphyxiation in accordance with the AVMA Guidelines for the Euthanasia of Animals.1 Euthanasia was completed within a 2 h period to control for time of day effects. As CO2 euthanasia has been shown to have minimal effect on other mice housed in the same room,10 all animals were held behind an opaque barrier within the same room during the procedure. Cage order was randomized for euthanasia and after body weight measurement and euthanasia, whole blood was collected via cardiac puncture and preserved with EDTA (1.3 mL tubes, Fisher Scientific, Suwanee, GA) for hematology analysis and corticosterone measurement. All blood samples were obtained in the first 3 h of the light phase to control for time of day effects. Adrenal glands were dissected and weighed as a pair for each mouse.
Immunohistochemistry.
Tissues from BrdU-treated mice were fixed in 10% neutral buffered formalin and routinely processed and embedded. Serial 6 µm coronal brain sections were made through the dentate gyri onto hydrophobic adhesive slides. Three nonserial coronal sections separated by at least 12µm were selected for each animal. Immunohistochemical staining was performed on a Leica Bond-Max autostainer (Leica Biosystems, Buffalo Grove, IL). All steps besides dehydration, clearing, and cover-slipping were performed on the Bond-Max. Slides were deparaffinized. Heat-induced antigen retrieval was performed using Epitope Retrieval 2 solution (Leica Biosystems) for 10 min. Slides were incubated with BrdU primary antibody (H2724, Accurate, Westbury, NY) at a 1:2000 dilution for 60 min and then incubated in a rabbit antirat secondary antibody (BA-4001, Vector Laboratories, Burlingame, CA) at a 1:2000 dilution for 15 min. The Bond Polymer Refine detection system (Leica Biosystems) was used for visualization. Slides were then dehydrated, cleared, and cover slipped.
Analysis of Neurogenesis.
A board-certified veterinary anatomic pathologist (LEH) conducted interpretation under masked conditions. Intestinal sections from the subset of mice selected for analysis of neurogenesis were first assessed for appropriate BrdU immunolabeling before analysis of brain sections to confirm success of the antemortem intraperitoneal injections. Slide scanning of BrdU-immunolabeled brain sections was performed on the Pannoramic 250 Flash III digital scanner (3DHISTECH, Budapest, Hungary). Quantification of BrdU positive cells in the dentate gyri was performed in QuPath, an open-source digital pathology platform,3 using manual region of interest delineation and the positive cell detection feature. Data were collected per brain section (n = 3/mouse) to calculate individual mouse and group-wide means. Image analysis data are reported as both percent BrdU positive cells per total number of cells analyzed, as well as number of BrdU positive cells per µm2.
Hematology and corticosterone assays.
Complete blood cell counts were performed on an automated analyzer (Forcyte Hematology Analyzer, Oxford Science, Oxford, CT). EDTA-preserved whole blood was spun down, and plasma submitted to the Vanderbilt University Medical Center Hormone Assay and Analytical Services Core for corticosterone measurement by using an ImmuChem 125I-corticosterone double-antibody radioimmunoassay (MP Biomedicals, LLC, Orangeburg, NY).
Statistical analyses.
Statistical test selection, analysis and interpretation was performed in consultation with qualified biostatisticians and a qualified expert in the neurobehavioral field (FH). Mann–Whitney U tests were selected a priori to assess effect of treatment across all measures (Table 1).When no sex difference was identified, male and female data were combined for analysis to yield a more powerful test. Two-way ANOVA was used to identify interactions between sex and treatment as well as differences between sexes for all continuous variables except neurogenesis. Because sex differences in markers of neurogenesis were not expected in these young adult mice,23 sexes were combined for neurogenesis analysis. Body weight gain for each mouse was calculated by taking the difference between baseline body weight (4 wk old) and weight at the end of the study (17 wk old). Two-way ANOVA was also used posthoc to cross-validate analyses when evaluating effect of treatment, and comparable results were found. Therefore, only results from the a priori statistical plan are reported here. A post hoc comparison of variance between groups for each study measure was performed using the Brown–Forsythe test. All analyses were performed using GraphPad Prism 8 (version 8.4.1, GraphPad, San Diego, CA).
Table 1.
Evaluation for effect of treatment across all measures, with results reported from the a priori statistical approach (Mann–Whitney U) alongside post-hoc parametric testing (2-way ANOVA). Normality was assessed using the Shapiro-Wilk test. Comparable results were found across all measures except for total distance during open field activity assessment, for which Mann–Whitney U found a significant effect of treatment but ANOVA did not. The data for this measure were not normally distributed, so assumptions of ANOVA are not met.
| Mann–Whitney U | Normal distribution | Two-way ANOVA | |||
| Study measure | U-statistic | P-value | Yes/No | F (DFn, DFd) | P-value |
| Elevated zero maze | |||||
| Percent time in open quadrants | 248.5 | 0.42 | Yes | F (1, 44) = 1.068 | 0.31 |
| Total distance traveled (cm) | No | F (1, 44) = 2.655 | 0.11 | ||
| Males | 41 | 0.08 | |||
| Females | 51 | 0.24 | |||
| Open field activity | |||||
| Percent time in central zone | Yes | F (1, 44) = 0.1518 | 0.70 | ||
| Males | 66 | 0.76 | |||
| Females | 57 | 0.41 | |||
| Total distance traveled (cm) | 157 | 0.01 | No | F (1, 44) = 1.284 | 0.26 |
| Fecal boli (no. pellets) | 170.5 | 0.01 | Yes | F (1, 43) = 5.183 | 0.03 |
| Neurogenesis | |||||
| Percent of total cells BrdU-positive | 14.5 | 0.13 | Yes | F (1, 11) = 2.164 | 0.17 |
| BrdU-positive cells by area (no. cells) | 18 | 0.27 | Yes | F (1, 11) = 2.183 | 0.17 |
| Body weight gain (g/course of study) | No | F (1, 43) = 8.929 | 0.005 | ||
| Males | 19 | 0.0014 | |||
| Females | 64 | 0.93 | |||
| Adrenal gland weight (mg) | No | F (1, 43) = 0.01101 | 0.76 | ||
| Males | 67 | 0.78 | |||
| Females | 61.5 | 0.79 | |||
| Plasma corticosterone concentration (ng/mL) | No | F (1, 43) = 0.002178 | 0.96 | ||
| Males | 68 | 0.84 | |||
| Females | 51 | 0.38 | |||
| Neutrophil:lymphocyte ratio | 242.5 | 0.48 | Yes | F (1, 43) = 0.5352 | 0.47 |
Results
Aside from anticipated minor bouts of aggression immediately after cage change, evidence of mild overt aggression was noted only once, by the presence of superficial abrasions on the tail of a single, enriched male mouse 10 wk into study; treatment was not required, and abrasions were fully resolved by the next cage change. No other health concerns were identified. After euthanasia, one female mouse was found to have an imperforate vagina; this mouse was excluded from body weight and physiologic stress measure analyses due to significant mucometra.
Behavioral testing.
Elevated zero maze.
During EZM testing, there was no interaction between sex and treatment (F(1,44) = 0.65, P = 0.42) and no difference between male and female mice regarding the percentage of time spent in the open quadrants (F(1,44) = 1.23, P = 0.27) as determined by 2-way ANOVA. Therefore, sexes were combined for Mann-Whitney U analysis by treatment. The 2 treatment groups did not differ in the percentage of time spent in the open quadrants (U = 248.5, P = 0.42; Figure 1 A). Total distance traveled during EZM testing showed no interaction between sex and treatment when evaluated by 2-way ANOVA (F(1,44)=0.01, P = 0.92). However, a significant difference was detected by sex, with females traveling farther than males (F(1,44) = 6.78, P = 0.01). Sexes were therefore analyzed separately with Mann–Whitney U tests to examine effect of treatment; however, a difference by treatment was not identified for either sex (females: U = 51, P = 0.24; males: U = 41, P = 0.08; Figure 1 B).
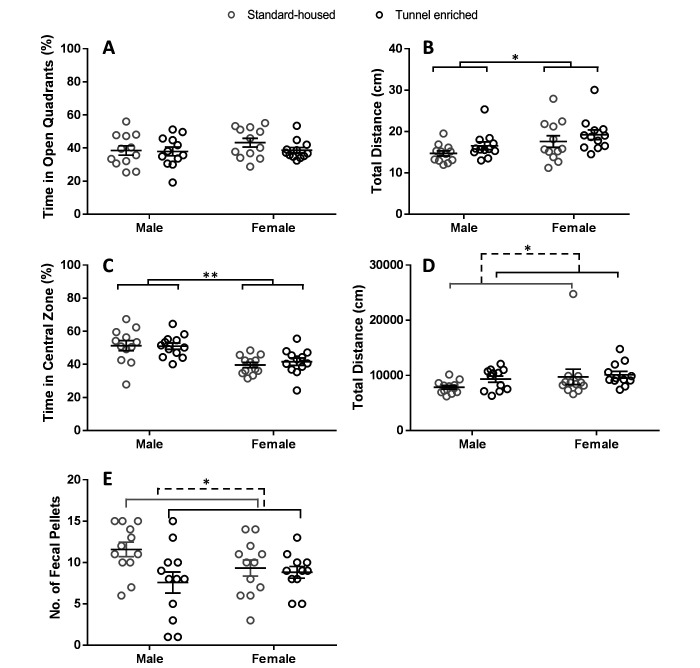
Figure 1.
Behavioral test results (means ± SEM; n = 12 per sex per treatment). Elevated Zero Maze (A) and (B). (A) There was no effect of tunnel enrichment on percent time spent in the open quadrants during the elevated zero maze behavioral test (P = 0.42) as determined by Mann–Whitney U test. (B) There was not a statistically significant effect of tunnel enrichment on total distance traveled during the elevated zero maze behavioral test for female (P = 0.24) or male mice as determined by Mann–Whitney U test. Females traveled farther than males (*P ≤ 0.01) as determined by 2-way ANOVA. Open Field Activity (C), (D), and (E). (C) There was no effect of tunnel enrichment on percent time spent in the central zone during the open field activity behavioral test for female (P = 0.41) or male mice (P = 0.76) as determined by Mann–Whitney U test. Male mice spent a significantly larger percentage of time in the central zone compared with female mice (**P < 0.0001) as determined by 2-way ANOVA. (D) Enriched mice traveled 9.6% farther than standard-housed mice (*P ≤ 0.01) during the open field activity behavioral test as determined by Mann–Whitney U test. (E) Enriched mice deposited 25% fewer fecal boli than standard-housed mice (*P ≤ 0.01) during the open field activity behavioral test as determined by Mann–Whitney U test.
Open field activity.
No interaction between sex and treatment (F(1,44) = 0.26, P = 0.62) was detected regarding the percentage of time spent in the central zone during open field testing. However, male mice spent approximately 10% more time in the central zone than did female mice (F(1,44) = 21.63, P < 0.0001) as determined by 2-way ANOVA. Sexes were therefore analyzed separately with Mann–Whitney U tests, with no difference by treatment identified for either sex (females: U = 57, P = 0.41; males: U = 66, P = 0.76; Figure 1 C). With regard to the total distance traveled during open field testing, no interaction was detected between sex and treatment (F(1,44) = 0.44, P = 0.51) and no significant differences were present between male and female mice (F(1,44) = 2.61, P = 0.11) as determined by 2-way ANOVA. Sexes were therefore combined for Mann-Whitney U analysis to examine effect of treatment. A statistically significant difference by treatment was detected for total distance traveled, with enriched mice traveling an average of 9.6% farther than standard-housed mice (U = 157, P = 0.0063; Figure 1 D).
Fecal boli numbers showed no interaction between sex and treatment (F(1,44) = 3.09, P = 0.09) and no difference between male and female mice (F(1,43) = 0.26, P = 0.61) as determined by 2-way ANOVA. Sexes were therefore combined for Mann–Whitney U analysis of the effect of treatment. The analysis detected a statistically significant difference by treatment, with enriched mice depositing 25% fewer fecal boli than standard-housed mice (U = 170.5, P = 0.01; Figure 1 E).
Neurogenesis.
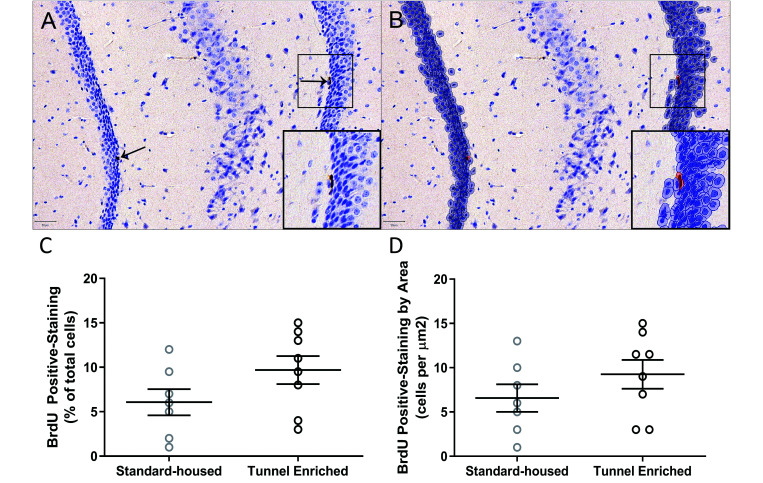
BrdU immunohistochemistry of the dentate gyri was quantitatively analyzed using QuPath region of interest delineation and positive cell detection (Figure 2 A and B). There was no interaction between sex and treatment (F(1,11) = 0.06, P = 0.82) and no difference between male and female mice (F(1,11) = 0.02, P = 0.90) for the percent of BrdU positive cells per total number of cells, nor was there an interaction between sex and treatment (F(1,11) = 1.86, P = 0.20) or a difference between male and female mice (F(1,11) = 1.046, P = 0.33) for the total number of BrdU positive cells per µm2. There was no significant difference by treatment for either the percent of BrdU positive cells per total number of cells analyzed (U = 14.5, P = 0.13, Figure 2 C) or the total number of BrdU positive cells per µm2 (U = 18, P = 0.27, Figure 2 D).
Figure 2.
Representative quantitative BrdU immunohistochemistry (scale bars = 50µm) and results (n = 15; 3 brain sections per mouse; means ± SEM). (A) Few BrdU immunolabeled nuclei were present in the subgranular zone and migrating into the granular cell layer of the dentate gyrus (arrows). (B) Following region of interest delineation and positive cell detection analysis in QuPath, a mockup image quantifying total number of negative (blue) and positive (red) cells was created. There was not a statistically significant effect of tunnel enrichment on either the (C) percent of BrdU positive cells per total number of cells analyzed (P = 0.13) or (D) the total number of BrdU positive cells per µm2 (P = 0.27) as determined by Mann–Whitney U test.
Body weight gain.
A significant interaction was detected between sex and treatment for body weight gain over the course of the study (F(1,43) = 7.479, P = 0.009, 9.1% of variation). As expected,34 male mice gained more weight than female mice (F(1,43) = 22.08, P = < 0.0001) as determined by 2-way ANOVA; sexes were therefore analyzed separately with Mann–Whitney U tests to examine effect of treatment. While no difference by treatment was identified for female mice (U = 64, P = 0.93), enriched male mice gained 3.7 ± 1.0 grams more over the course of the study than did standard-housed male mice (U = 19, P = 0.0014; Figure 3).
Figure 3.
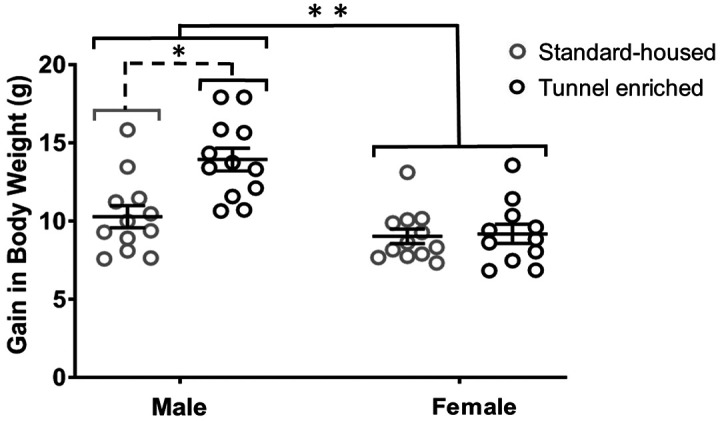
While there was not an effect of tunnel enrichment on change in body weight (means ± SEM, n = 12 per sex per treatment) over the course of the study for female mice (P = 0.93), male mice with tunnel enrichment gained an average of 3.7 grams more over the course of the study compared with standard-housed male mice (*P = 0.0014) as determined by Mann–Whitney U test. As expected, male mice gained more weight than female mice (**P = less than 0.0001) as determined by 2-way ANOVA.
Measures of stress.
No interactions were detected between sex and treatment for adrenal gland weight (F(1,43) = 0.09, P = 0.76), plasma corticosterone (F(1,43) = 0.64, P = 0.43), or neutrophil:lymphocyte ratio (F(1,43) = 0.28, P = 0.60) as determined by 2-way ANOVA. Anticipated4,12,30,34 differences by sex were noted on 2-way ANOVA, with females exhibiting heavier adrenal glands (F(1,43) = 4.59, P = 0.04) and higher levels of plasma corticosterone (F(1,43) = 5.15, P = 0.03) compared with males, but no difference between sexes for neutrophil:lymphocyte ratio (F(1,43) = 0.92, P = 0.34). Sexes were therefore analyzed separately by Mann–Whitney U to examine effect of treatment on adrenal gland weight and plasma corticosterone, while sexes were combined for Mann–Whitney U analysis to examine effect of treatment on neutrophil:lymphocyte ratio. None of the endpoints measured to evaluate stress were significantly different between treatment groups, including adrenal gland weight (females: U = 61.5, P = 0.79; males: U = 67, P = 0.78; Table 2), plasma corticosterone (females: U = 51, P = 0.38; males: U = 68, P = 0.84; Table 2), and neutrophil:lymphocyte ratio (U = 242.5, P = 0.48; Table 2).
Table 2.
Tunnel enrichment had no effect on physiologic stress parameters (means ± SEM). While expected sex differences were present, there were no significant differences between treatment groups as determined by Mann-Whitney U test.
| Standard-housed | Enriched | |||
| Males | Females | Males | Females | |
| Adrenal gland weighta (mg) | 5.17 ± 0.37 | 6.67 ± 0.68 | 5.42 ± 0.5 | 6.55 ± 0.85 |
| Plasma corticosterone concentrationa (ng/mL) | 180.6 ± 42.21 | 239.7 ± 45.92 | 150.5 ± 28.11 | 287 ± 40.67 |
| Neutrophil:lymphocyte ratio | 6.8 ± 0.7 | 6.54 ± 0.66 | 6.68 ± 0.42 | 5.78 ± 0.59 |
Sex differences as determined by 2-way ANOVA (P < 0.05)
Variance.
Variance between groups was assessed for each study measure using the Brown–Forsythe test to evaluate equality of variance. None of the study measures had significantly different variances between standard-housed and enriched mice (Table 3).
Table 3.
Variance was not different between groups for any measure as determined by the Brown-Forsythe test of equality of variance. Where sex differences were identified, standard deviations for each sex are reported.
| Standard deviation | Brown-Forsythe Test | ||||
| Study measure | Standard-housed | Enriched | F (DFn, DFd) | P value | |
| Elevated zero maze | |||||
| Percent time in open quadrants | 9.52 | 7.56 | 2.181 (3, 44) | 0.10 | |
| Total distance traveled (cm) | 1.235 (3, 44) | 0.31 | |||
| Males | 2.17 | 3.19 | |||
| Females | 4.85 | 4.03 | |||
| Open field activity | |||||
| Percent time in central zone | 0.7751 (3, 44) | 0.51 | |||
| Males | 10.53 | 6.75 | |||
| Females | 5.50 | 7.75 | |||
| Total distance traveled (cm) | 3564 | 2013 | 0.4502 (3, 44) | 0.72 | |
| Fecal boli (no. pellets) | 3.35 | 3.77 | 0.4953 (3, 44) | 0.69 | |
| Neurogenesis | |||||
| Percent of total cells BrdU-positive | 0.04 | 0.06 | 1.562 (3, 11) | 0.25 | |
| BrdU-positive cells by area (no. cells) | 2.03 | 2.37 | 0.5478 (3, 11) | 0.66 | |
| Body weight gain (g/course of study) | 0.7100 (3, 43) | 0.55 | |||
| Males | 1.41 | 2.03 | |||
| Females | 1.45 | 1.65 | |||
| Adrenal gland weight (mg) | 2.093 (3, 43) | 0.12 | |||
| Males | 1.27 | 1.73 | |||
| Females | 2.35 | 2.81 | |||
| Plasma corticosterone concentration (ng/mL) | 1.644 (3, 43) | 0.19 | |||
| Males | 146.20 | 97.38 | |||
| Females | 159.10 | 139.50 | |||
| Neutrophil:lymphocyte ratio | 2.31 | 1.74 | 1.321 (3, 43) | 0.28 | |
Discussion
Our results indicate that provision of a single red acrylic tunnel is unlikely to induce significant confounding effects on murine anxiety-like behaviors, neurogenesis, body weight gain, or physiologic parameters. The primary arguments against expanding EE for mice are the potential impact on standardization and the potential for increased variability.8,20 Standardized housing was initially developed with the main objective of reducing variability,6 and expanding EE for mice could potentially reduce the precision and replicability of animal experiments.38 However, with few exceptions,5 this premise is not typically put forward as cause to withhold the provision of EE in other laboratory animal species. Further, the current standard housing environment of laboratory rodents has been criticized for inducing abnormal behaviors and poor wellbeing6-8,36–38 due to sensory and motor deprivation20,37,38 and resulting in stress that may alter physiologic parameters.13 In addition, chronic interference with motivated behaviors can lead to functional changes in the nervous system.5,6,13,26,36,37 Mice reared and housed under these standard cage conditions may not be appropriate models for research that depends on the normal function of the endocrine and/or nervous systems.6,35 The Guide states, “[e]nvironments that fail to meet the animals’ needs may result in abnormal brain development, physiologic dysfunction, and behavioral disorders that may compromise both animal well-being and scientific validity,” and that, “[t]he primary enclosure or space may need to be enriched to prevent such effects.”20 With the hope of extending additional EE to our most commonly used laboratory species, our goal for this study was therefore to evaluate concerns that the introduction of structural enrichment may confound study findings, by evaluating anxiety-like behaviors, neurogenesis, body weight gain, and physiologic stress levels, and observing for signs of increased aggression.
All behavioral results fell within biologically normal ranges regardless of treatment, suggesting that with inclusion of appropriate control groups, housing experimental mice with simple acrylic tunnel enrichment is unlikely to confound behavioral measures of anxiety. Mice in the enriched group showed modest differences suggestive of decreased anxiety during open field testing, traveling 9.6% farther (Figure 1 D) and depositing 25% fewer fecal boli (Figure 1 E) than did standard-housed mice. Anxiety-like behaviors in rodents are thought to resemble behaviors of anxiety in humans.24 The main indicator of anxiety in both the EZM and open field tests is the degree of thigmotaxis, or the amount of time spent in open compared with closed areas,22,27 whereas total distance traveled indicates a mouse's general activity level.22 Mice are a thigmotaxic prey species, preferring to seek shelter near walls or hide behind structures, particularly when anxiety levels are high. As such, when placed in an open space, as during open field testing, mice may freeze in place and are expected to preferentially stay in the surrounding zones near the walls of the field, rather than in the open central zone.27 Similarly, mice with higher anxiety are expected to exhibit more defecation and therefore larger numbers of fecal boli.27 We found no difference between treatment groups when evaluating the percentage of time spent in the central zone of the open field activity test, or in the open quadrants of the EZM. However, greater exploratory activity throughout all zones (central and surrounding), in addition to the smaller number of fecal boli deposited during open field testing by the enriched mice suggests that they were less anxious about exploring the novel environment. Sex differences were detected during behavioral testing, such that males exhibited a greater willingness to explore the more anxiogenic central zone during open field testing. In contrast, females were more active in the EZM. Although significant differences were noted during open field activity testing, these differences were not consistently observed across all behavioral measures or between tests. Further, because the 2 treatment groups showed no differences in time spent in thigmotaxis (the main indicator of anxiety), provision of acrylic tunnel enrichment is unlikely to confound behavioral assessments of anxiety. Our data suggest that C57BL/6J mice are less anxious after provision of tunnel enrichment, therefore supporting the addition of this simple structural enrichment to standard murine housing.
Neurogenesis in the dentate gyri of enriched mice was not significantly altered after 12 wk of tunnel enrichment in addition to standard EE. Several studies have demonstrated that depending on the degree and form of environmental complexity, EE can affect both the number of individual neurons as well as overall learning capacity in several species, including mice.5-7,19,36 However, the majority of studies evaluating the effects of EE have been performed in highly enriched environments, offering multiple combinations of varying enrichment options simultaneously or in rotation.19 The variation in study designs using these enrichment schemes, in relation to sex, strain, type and duration of enrichment offered, precludes a true evaluation of the effects of individual components.19,26 As such, their results should not be construed as representative of structural enrichment as a whole.19 In addition, while certain cage structures, such as running wheels,11 influence neurogenesis due to voluntary exercise, our study found no significant difference between standard-housed and enriched mice.
Preference studies indicate that mice prefer a more structurally complex environment and one containing nesting material over unenriched housing, and they will work for access to such environments.19,26 Collectively, these results suggest that long term provision of simple structural enrichment in the form of an acrylic tunnel is unlikely to confound the evaluation of neurogenesis in the dentate gyri. Data were acquired by examining 3 brain sections per mouse to obtain an individual mean, with sexes combined for final analysis. Data are reported as both percent BrdU positive cells per total number of cells analyzed and the number of BrdU positive cells per µm2.
Although we hypothesized tunnel-enriched mice would have a decreased gain in body weight, we found that enriched male mice gained more body weight over the course of the study as compared with standard-housed male mice (Figure 3). While no difference by treatment was identified among female mice, the presence of tunnel enrichment may have resulted in an improved ability to thermoregulate, resulting in heavier body weights. A similar finding was reported by one group who found heavier body weights in mice provided nesting material as compared to those without, even though the heavier animals were consuming less food.30 Acrylic tunnel enrichment had no significant effect on physiologic stress measurements and is therefore unlikely to have a confounding effect on the measured parameters. Sex differences4,30,34 were identified (Table 2), with female mice exhibiting heavier adrenal gland weights and higher concentrations of plasma corticosterone than male mice, whereas males demonstrated more gain in body weight than female mice. Although serial measurement of corticosterone levels would have provided data specific to the impact of tunnel enrichment over time, for this study, a single measurement of plasma corticosterone combined with additional indicators of stress and anxiety, including neutrophil:lymphocyte ratio, was used to compare physiologic stress between treatments at a single time point at the end of the study.
Variance (standard deviation) was not significantly different between groups for any measure, refuting the argument that expanding EE in mice through the provision of a single acrylic mouse tunnel introduces confounding variability. Rather, given the well-documented species-typical preferences for structural enrichment,19,26 tunnel enrichment is likely to create more reliable research results due to improved psychologic wellbeing. In addition, clear and complete reporting of housing parameters, including EE, as suggested in the ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments), should eliminate concerns related to experimental reproducibility.21
When housed in groups of 3 from 4 wk of age, acrylic tunnel enrichment did not increase aggression beyond what is typically expected. Clinical signs of overt aggression were noted only once throughout our study, in a single, enriched male mouse as inferred by the presence of superficial abrasions on the tail 10 wk into study. Treatment was not required, and abrasions were fully resolved by the next cage change. Increased cage complexity in the form of structural enrichment has been criticized due to the potential for induced aggression, particularly among male mice.15,19,26 The drive for this aggression has been attributed to the idea that rigid shelters prompt the dominant mice to monopolize access of the valued resource.14 Given this potential for induced aggression, investigators may refrain from offering structural enrichment due to the potential impact of aggressive behaviors on experimental outcomes and overall wellbeing.9 However, studies evaluating the effects of structural enrichment are highly varied and situational.9 Aggression has primarily been associated with shelters such as nest boxes, igloos, or elevated platforms2,7,17,19,26 which provide ambush sites or ‘choke points’ that encourage territorial aggression over control of the structure.34 In the wild, aggression is typically mitigated once a subordinate mouse has fled the territory or broken the line of sight;14 providing these options can be challenging in the current standard laboratory housing environment lacking structural enrichment. While a low level of aggression is expected among socially housed laboratory mice,14,33,38 increasing the opportunity for avoidance or termination of agonistic interactions may be considered a refinement. Given that mice are a thigmotactic prey species, appropriate structuring of the cage environment is likely more beneficial than provision of a larger floor area.5 Equipping cages with elongated structures, such as a red-tinted acrylic tunnel, may offer an effective means of compartmentalization of the cage, providing the ability to retreat or hide to avoid aggression. The low incidence of aggression noted in our study, particularly among enriched male mice, may be related to the low housing density.14,31 The housing density evaluated in this study (3 mice per cage) was selected based on current recommendations for mitigating aggression in mice;14,31 however, this does not necessarily represent typical housing density, given current minimum floorspace guidelines.20
Areas for future research include assessment of simple tunnel enrichment under higher-density housing (for example, 4 or 5 mice per cage), particularly in light of documented effects of structural enrichment on aggression.14,19,26 Our study also evaluated only one age cohort in one commonly used strain, C57BL/6J mice, after 12 wk of housing with or without acrylic tunnel enrichment; subsequent studies evaluating age-, strain-, and duration-specific effects are warranted. Assessing the potential positive impact of this added enrichment (for example, mouse preference for and use of the tunnel, resilience when stressed, muscle coordination, cognitive ability, etc.) was beyond the scope of this study and represent several areas for additional research. Lastly, the current standard housing environment of laboratory rodents has received criticism for inducing abnormal behaviors and poor wellbeing due to sensory and motor deprivation,6,7,13,20,36-38 future research evaluating the incidence of stereotypic behaviors would further inform widespread implementation of simple tunnel enrichment.
In summary, our goal was to determine whether the addition of simple structural enrichment to the standard mouse cage environment could confound study results. While some behavioral test results were statistically different between treatments (total distance traveled, fecal boli deposition), all behavioral results fell within biologically normal ranges, such that these differences were neither clinically or practically significant. Neurogenesis in the dentate gyri of enriched mice was not altered, and acrylic tunnel enrichment had no effect on any of the physiologic stress measures, including adrenal gland weight, plasma corticosterone concentration and neutrophil:lymphocyte ratio. Enriched male mice gained more body weight over the course of the study than did standard-housed male mice, which may be due to an improved ability to thermoregulate when housed with a tunnel. Finally, while investigators may refrain from offering structural enrichment due to the potential for induced aggression, our study found no increase in aggression beyond what is typically expected. Although no one-size-fits-all program may suffice for EE of all laboratory mice, the results of this study indicate that provision of acrylic tunnel enrichment is unlikely to confound experimental measures of stress and anxiety. Therefore, the authors recommend inclusion of simple structural enrichment, in addition to social housing and nesting material, as standard environmental enrichment for mice.
Acknowledgments
The authors thank the Vanderbilt University Medical Center Division of Comparative Medicine for funding support, Dr John Allison in the Vanderbilt University Neurobehavioral Core for technical expertise and training on behavioral testing, Dr William Dupont in the Vanderbilt University Medical Center Department of Biostatistics and Dr Shanna Arnold in the Department of Pathology, Microbiology, and Immunology for consultation on sample size and study design, statistical test selection, analysis, and interpretation. In addition, we acknowledge the contributions of our mice. The Translational Pathology Shared Resource is supported by NCI/NIH Cancer Center Support Grant 5P30 CA68485-19 and the Vanderbilt Mouse Metabolic Phenotyping Center Grant 2 U24 DK059637-16, in addition, we thank Dr Kelli Boyd and Miranda Wilkes for their technical expertise and assistance with tissue collection. The Hormone Assay and Analytical Services Core is supported by NIH grants DK059637 and DK020593. The Mouse Behavioral Core is supported by U54HD083211.
References
- 1.American Veterinary Medical Association (AVMA) . 2013. AVMA guidelines for the euthanasia of animals. Schaumburg (IL): AVMA. [Google Scholar]
- 2.André V, Gau C, Scheideler A, Aguilar-Pimentel JA, Amarie OV, Becker L, Garrett L, Hans W, Holter SM, Janik D, Moreth K, Neff F, Ostereicher M, Racz I, Rathkolb B, Rozman J, Bekeredjian R, Graw J, Klingenspor M, Klopstock T, Ollert M, Schmidt-Weber C, Wolf E, Wurst W, Gailus-Durner V, Brielmeier M, Fuchs H, Hrabe de Angelis M. 2018. Laboratory mouse housing conditions can be improved using common environmental enrichment without compromising data. PLoS Biol 16:1–24. 10.1371/journal.pbio.2005019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bankhead P, Loughrey MB, Fernandez JA, Dombrowski Y, McArt DG, Dunne PD, McQuaid S, Gray RT, Murray LJ, Coleman HG, James JA, Salto-Tellez M, Hamilton PW. 2017. QuPath: Open source software for digital pathology image analysis. Sci Rep 7:1–7. 10.1038/s41598-017-17204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barthold SW, Griffey SM, Percy DH. 2016. Pathology of laboratory rodents and rabbits 4th ed. Hoboken (NJ):Wiley. [Google Scholar]
- 5.Baumans V. 2005. Environmental enrichment for laboratory rodents and rabbits: requirements of rodents, rabbits, and research. ILAR J 46:162–170. 10.1093/ilar.46.2.162. [DOI] [PubMed] [Google Scholar]
- 6.Baumans V, Van Loo PL. 2013. How to improve housing conditions of laboratory animals: the possibilities of environmental refinement. Vet J 195:24–32. 10.1016/j.tvjl.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 7.Bayne K. 2018. Environmental enrichment and mouse models: Current perspectives. Animal Model Exp Med 1:82–90. 10.1002/ame2.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bayne K, Würbel H. 2014. The impact of environmental enrichment on the outcome variability and scientific validity of laboratory animal studies. Rev Sci Tech 33:273–280. 10.20506/rst.33.1.2282. [DOI] [PubMed] [Google Scholar]
- 9.Blankenberger WB, Weber EM, Chu DK, Geronimo JT, Theil J, Gaskill BN, Pritchett-Corning K, Albertelli MA, Garner JP, Ahloy-Dallaire J. 2018. Breaking up is hard to do: Does splitting cages of mice reduce aggression? Appl Anim Behav Sci 206:94–101. 10.1016/j.applanim.2018.06.003. [DOI] [Google Scholar]
- 10.Boivin GP, Bottomley MA, Grobe N. 2016. Responses of male C57BL/6N mice to observing the euthanasia of other mice. J Am Assoc Lab Anim Sci 55:406–411. [PMC free article] [PubMed] [Google Scholar]
- 11.Ehninger D, Kempermann G. 2003. Regional effects of wheel running and environmental enrichment on cell genesis and microglia proliferation in the adult murine neocortex. Cereb Cortex 13:845–851. 10.1093/cercor/13.8.845. [DOI] [PubMed] [Google Scholar]
- 12.Everds NE, Synder PW, Bailey KL, Bolon B, Creasy DM, Foley GL, Rosol TJ, Sellers T. 2013. Interpreting stress responses during routine toxicity studies: a review of the biology, impact, and assessment. Toxicol Pathol 41:560–614. 10.1177/0192623312466452. [DOI] [PubMed] [Google Scholar]
- 13.Garner JP. 2005. Stereotypies and other abnormal repetitive behaviors: potential impact on validity, reliability, and replicability of scientific outcomes. ILAR J 46:106–117. 10.1093/ilar.46.2.106. [DOI] [PubMed] [Google Scholar]
- 14.Gaskill B. [Internet]. 2014. Aggression in laboratory mice: Potential influences and how to manage it. The Enrichment Record. Winter:22 –25. [Cited 21 March 2018]. Available at: https://www.research.uky.edu/uploads/aggression-lab-mice [Google Scholar]
- 15.Gaskill BN, Garner JP. 2017. Stressed out: providing laboratory animals with behavioral control to reduce the physiological effects of stress. Lab Anim (NY) 46:142–145. 10.1038/laban.1218. [DOI] [PubMed] [Google Scholar]
- 16.Gaskill BN, Karas AZ, Garner JP, Pritchett-Corning KR. 2013. Nest building as an indicator of health and welfare in laboratory mice. J Vis Exp 82:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giles JM, Whitaker JW, Moy SS, Fletcher CA. 2018. Effect of environmental enrichment on aggression in BALB/cJ and BALB/cByJ mice monitored by using an automated system. J Am Assoc Lab Anim Sci 57:236–243. 10.30802/AALAS-JAALAS-17-000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gouveia K, Hurst JL. 2013. Reducing mouse anxiety during handling: effect of experience with handling tunnels. PLoS One 8:1–8. 10.1371/journal.pone.0066401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hutchinson E, Avery A, Vandewoude S. 2005. Environmental enrichment for laboratory rodents. ILAR J 46:148–161. 10.1093/ilar.46.2.148. [DOI] [PubMed] [Google Scholar]
- 20.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 21.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. 2012. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Vet Clin Pathol 41:27–31. 10.1111/j.1939-165X.2012.00418.x. [DOI] [PubMed] [Google Scholar]
- 22.Komada M, Takao K, Miyakawa T. 2008. Elevated plus maze for mice. J Vis Exp 22:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lagace DC, Fischer SJ, Eisch AJ. 2007. Gender and endogenous levels of estradiol do not influence adult hippocampal neurogenesis in mice. Hippocampus 17:175–180. 10.1002/hipo.20265. [DOI] [PubMed] [Google Scholar]
- 24.Lezak KR, Missig G, Carlezon WA., Jr 2017. Behavioral methods to study anxiety in rodents. Dialogues Clin Neurosci 19:181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Institutes of Health, Office of Laboratory Animal Welfare. [Internet]. 2015. PHS policy on humane care and use of laboratory animals. [Cited 12 November 2017]. Available at: https://olaw.nih.gov/policies-laws/phs-policy.htm [Google Scholar]
- 26.Olsson IA, Dahlborn K. 2002. Improving housing conditions for laboratory mice: a review of “environmental enrichment”. Lab Anim 36:243–270. 10.1258/002367702320162379. [DOI] [PubMed] [Google Scholar]
- 27.Seibenhener ML, Wooten MC. 2015. Use of the open field maze to measure locomotor and anxiety-like behavior in mice. J Vis Exp 96:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. 1994. Behavioural and phamacological characterisation of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacology (Berl) 116:56–64. 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- 29.Slater AM, Cao L. 2015. A protocol for housing mice in an enriched environment. J Vis Exp 100:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van de Weerd HA, Van Loo PL, Van Zutphen LF, Koolhaas JM, Baumans V. 1997. Nesting material as environmental enrichment has no adverse effects on behavior and physiology of laboratory mice. Physiol Behav 62:1019–1028. 10.1016/S0031-9384(97)00232-1. [DOI] [PubMed] [Google Scholar]
- 31.Van Loo PL, Mol JA, Koolhaas JM, Van Zutphen BF, Baumans V. 2001. Modulation of aggression in male mice: influence of group size and cage size. Physiol Behav 72:675–683. 10.1016/S0031-9384(01)00425-5. [DOI] [PubMed] [Google Scholar]
- 32.Van Loo PL, Van Zutphen LF, Baumans V. 2003. Male management: Coping with aggression problems in male laboratory mice. Lab Anim 37:300–313. 10.1258/002367703322389870. [DOI] [PubMed] [Google Scholar]
- 33.Weber EM, Dallaire JA, Gaskill BN, Pritchett-Corning KR, Garner JP. 2017. Aggression in group-housed laboratory mice: why can't we solve the problem? Lab Anim (NY) 46:157–161. 10.1038/laban.1219. [DOI] [PubMed] [Google Scholar]
- 34.Whary MT, Baumgarth N, Fox JG, Barthold SW. 2015. Biology and diseases of mice, p 43–150. In: Fox JG, Anderson LC, Otto G, Pritchett-Corning KR, Whary MT, editors. Laboratory animal medicine 3rd ed. San Diego (CA): Elsevier. [Google Scholar]
- 35.Wojtowicz JM, Kee N. 2006. BrdU assay for neurogenesis in rodents. Nat Protoc 1:1399–1405. 10.1038/nprot.2006.224. [DOI] [PubMed] [Google Scholar]
- 36.Würbel H. 2001. Ideal homes? Housing effects on rodent brain and behaviour. Trends Neurosci 24:207–211. 10.1016/S0166-2236(00)01718-5. [DOI] [PubMed] [Google Scholar]
- 37.Würbel H. 2007. Environmental enrichment does not disrupt standardisation of animal experiments. ALTEX 24:70–73. [PubMed] [Google Scholar]
- 38.Würbel H, Garner J P. 2007. Refinement of rodent research through environmental enrichment and systematic randomization. National Centre for the Replacement, Refinement and Reduction of Animals in Research. NC3Rs 1–9. [Cited 12 November 2017]. Available at: www.nc3rs.org.uk. [Google Scholar]
- 39.Zeng C, Pan F, Jones LA, Lim MM, Griffin EA, Sheline YI, Mintun MA, Holtzman DM, Mach RH. 2010. Evaluation of 5-ethynyl-2 ‘-deoxyuridine staining as a sensitive and reliable method for studying cell proliferation in the adult nervous system. Brain Res 1319:21–32. 10.1016/j.brainres.2009.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]