Abstract
Highly immunodeficient NSG mice (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) are commonly used as a models in preclinical studies for patient-derived engraftment. However, despite the frequency of their use, reference values for their clinical pathology markers have not been determined. In accordance with the American Society of Veterinary Clinical Pathology (ASVCP) recommendations, we established de novo reference values for hematologic and biochemical variables and evaluated bone marrow cytology and histology in forty 9-wk-old male and female NSG mice. Hematologic analyses were performed using 2 separate analyzers (IDEXX ProCyte Dx, Sysmex XT-2000iV) and biochemical values were measured using a Scil VetScan2. The primary hematologic characteristic seen in NSG mice was a very low white blood cell (WBC) count (below 1.6 109/L). Lymphocyte and monocyte counts were respectively over- and under-estimated by the analyzers, as compared with manual counts, likely due to misidentification of the very low concentrations of these cell types by the analyzers. This analytical bias highlights the need for confirmatory microscopic observation of blood smears from these mice for WBC differential identification. Results for all other hematology and biochemistry variables were similar to those previously reported in inbred mice, except for MPV and an unexpectedly high glucose concentration (11.5 to 19.0 mmol/L), potentially due to the nonfasting status of the animals. The differential bone marrow cell count and Myeloid:Erythroid ratio (median 1.76) were also established. Megakaryocyte and adipocyte count differed significantly between the femoral diaphysis and metaphysis and between genders. These results provide a reliable resource of baseline data for hematologic variables for researchers monitoring graft rejection studies in NSG mice.
Abbreviations: ASVCP, American society of veterinary clinical pathology; CLSI, clinical and laboratory standards institute; HFR, reticulocyte high-fluorescence ratio; HGB, hemoglobin concentration; IRF, immature reticulocyte fraction; LFR, reticulocyte low-fluorescence ratio; MFR, reticulocyte medium-fluorescence ratio; NSG, NOD scid gamma; PCT, plateletcrit; PDW, platelet distribution width; PLT-O, platelet count by optical measurement; PLT-I, platelet count by impedance measurement; RBC-I, RBC count by impedance measurement; RBC-O, RBC count by optical measurement; RDW-CV, RDW by coefficient of variation; RDW-SD, RDW by standard deviation; RET, Reticulocyte; RET-HGB, reticulocyte hemoglobin content; RI, reference interval
The development of severely immunodeficient mice strains has permitted successful engraftment of human hematopoietic cells and tissues to produce “humanized mice”. These murine models allow in vivo studies of human hematopoietic stem cell development and function. Likewise, they are of particular interest in preclinical studies of numerous human cancers, infectious or immune diseases.51 The NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mouse, developed by L. Shultz at the Jackson Laboratory in 2005, is currently one of the most widely used animal models for patient-derived engraftment.34,46,47,51 Some biologic characteristics of the NSG mouse have been reported, particularly their low peripheral leukocyte counts, lack of mature T, B and NK cells, severe aplasia of thymus and lymph nodes and the absence of splenic white pulp.47
While additional physiologic data, including mean values of hematology and serum biochemistry variables, for male and female NSG mice are available on the Jackson laboratory website,49 these data did not meet the recommended international criteria for establishing reference values because little information was available about preanalytics, analytics and statistics.10,15,17 Reliable reference values are vital to researchers evaluating the potential effect of various treatments or drugs, even if the interpretation of clinical pathology data is based on a statistical comparison of results from controls and treated animals. However, if baseline reference values for hematologic and biochemical variables are not considered, this comparison is only a crude use of the data. Moreover, the mixed genetic background of the NSG mouse and its biologic specificity precludes the use of data for other mouse strains. De novo reference values thus needed for the NSG mouse so that users of this strain can improve their interpretation of clinical pathology data.
The nonparametric method is recommended for establishing de novo reference limits and their confidence intervals if at least 120 reference individuals are available.15,17 However, when “a laboratory has statistical and computing competence, […] procedures are encouraged that do not require 120 individuals”.10 In such cases, the American Society of Veterinary Clinical Pathology (ASVCP) RI guidelines15 recommend using a robust (distribution independent) or parametric (if Gaussian distribution can be established) method to provide the reference limits and methods such as bootstrap for their confidence intervals.7 In human medicine, Clinical and Laboratory Standards Institute (CLSI) also acknowledges that alternative methods can be used when only smaller numbers of subjects are available.10 The determination of RIs requires control and strict standardization of the preanalytical and analytical procedures to reduce their possible effects on intra- or interindividual variability.15
To our knowledge, no data are available that characterize the cytology and histology of the bone marrow of the NSG mouse, despite its frequent use as a model for research in human immune function, stem cell biology, infectious disease, diabetes, and oncology including hematologic malignancies.51 The aim of this study was therefore to establish this critical reference data for hematology, plasma biochemistry, bone marrow cytology, and bone histology, and whenever possible, to estimate the corresponding RIs according to international recommendations.10,15
Materials and Methods
The experimental protocol was designed according to the European recommendations for laboratory animal welfare and protection (Directive 2010/63/UE) and to the Institutional Animal Care and User Ethical Committee of the Centre Régional d'Exploration Fonctionnelle et de Ressources Expérimentales (CREFRE) (Approval#14301 2018032718073351V7). Experiments were performed at the CREFRE in Toulouse (France).
Animals.
Adult 9-wk-old NSG male and female mice were produced at the CREFRE Production service using breeders obtained from the Charles River Laboratory. The mice were housed 5 per polycarbonate cage in positive ventilated racks (IVC Sealsafe Plus GM500 Rack, Tecniplast, France) with strict light, temperature, humidity, pressure, and air renewal conditions in a Specific and Opportunistic Pathogen Free (SOPF) breeding unit (Tecniplast, France). Cages were washed in a tunnel washing machine, and autoclaved before entering the breeding unit, which was kept under a positive pressure barrier of 10Pa with an airflow of 75 air changes per hour (75 ACH). Environmental conditions were a 12:12h light-dark cycle, ambient temperature of 21± 3 °C and an average of 46% humidity. All mice received an irradiated mouse maintenance complete diet, (S9955- S410 Spezialdiä10 GmbH, DE-59494 Soest, Germany) exposed to a total energy of 12.8 Mj/kg. Drinking water was filtered at 0.2 µm and then passed through a UV filter before landing in the bottle filler and was available ad libitum.
Health monitoring.
The breeding unit mice underwent frequent health screening, alternating between PCR Rodent Infectious Agent panels test and sentinels testing, looking for pathogen and opportunist agents in the FELASA list. These tests were performed by Charles River Diagnostic Service (CR RADS Wilmington,USA).
For the purposes of our study, the animals were transferred in their home cage in groups of 5 from the breeding unit to the experimental unit next door, which also has a SOPF status. To limit the stress on the animals, and the consequent alteration of their biochemical values, mice were brought into the experimental unit 5 at a time to be sampled and subsequently euthanized. A group of 5 mice was sampled and euthanized within 1 h after arrival in the experimental unit. All blood samplings were performed between 8 h and 13 h. Three operators were dedicated to the animal use (anesthesia, sampling and euthanasia) (CLL), blood sample management (CT) and bone marrow collection (NBA) respectively.
Specimen collection.
Specimen collection was performed under inhalant anesthesia. Anesthesia was initially induced by gently moving the mouse from its cage to a chamber filled with 3% isoflurane (Vetflurane, Virbac, France), where it remained for 2 to 3 min. Anesthesia was then maintained by inhalation of 2.5% isoflurane from a dedicated mask. An absence of movement, the abolition of the withdrawal reflex and slowed rhythm of thoracoabdominal breathing were used to determine the plane of anesthesia was sufficient to start the laparotomy. A significant increase in respiratory rate during the procedure resulted in the immediate cessation any painful stimuli and an increase in the percentage of isoflurane being administered in steps of 0.5% until the breath frequency was reduced and regular. The painful procedure was resumed once the respiration rate was judged satisfactory.
Once the skin and muscle layers of abdominal cavity were incised, the intestinal loops were gently pulled apart on the right side of the abdomen, and a 25-gauge needle attached to a 1mL syringe was carefully inserted in the caudal vena cava. A minimum of 400 µL blood volume was drawn into the syringe, and the needle removed from the vein only when aspiration became too difficult. Venous blood had to be aspirated at a rate sufficient to avoid clotting but slow enough to prevent vein collapse. Animals were euthanized immediately after blood sampling by cervical dislocation, without regaining consciousness. An average delay of 40 s to a maximum of 50 s occurred between the beginning of the laparotomy and euthanasia.
Immediately after sampling, the blood specimen was equally divided into two 200 µL K3-EDTA and Li-heparin-coated-microtubes (Microvette, Sarstedt, Nümbrecht, Germany). Two blood smears were obtained from the EDTA microtube, and the heparin microtube was centrifuged at 7000 x g for 10 min (iFuge M08VT, Neuation, Gujarat, India). Without delay after centrifugation, the supernatant was collected into a safe lock tube (Eppendorf AG, Hamburg, Germany) after checking for the absence of hemolysis by visual examination of the plasma color. EDTA and plasma specimens were immediately refrigerated at 4 °C until analyzed at the Laboratoire Central de Biologie Médicale de l'Ecole Nationale Vétérinaire de Toulouse within 2 h of sampling. After air-drying, blood smears were stained using a May Grünwald-Giemsa automatic stainer (Aerospray Haematology Slide Stainer Cytocentrifuge 7150; Wescor) and stored in an air-tight box until microscopic evaluation.
Bone marrow histology and cytology specimens were prepared after dissection of the hind limb long bones: femurs and tibias were dislocated and the muscles, fat, and connective tissues were trimmed. The right femur was placed in a 10% neutral buffered formalin container for histologic evaluation. For cytologic evaluation, the left femur was split longitudinally, a small plug of marrow was gently extracted with a fine paintbrush, moistened with fetal calf serum and ‘‘painted’’ across the length of 2 slides using 3 to 5 gentle strokes as previously described.4 After air-drying, one slide per animal was stained with May Grünwald-Giemsa as above, and the other with Perl's Prussian blue reaction for iron detection (HemaPerls Kit, RAL Diagnostics, Martillac, France).
Hematology.
Before analysis, EDTA specimens were placed on a rotary agitator for 20 min (Speci Mixメ, Oxford), which allowed specimens to be both homogenized and warmed to room temperature. Careful pipetting was performed to exclude any macroscopically visible clot. CBCs were obtained on undiluted specimens with the IDEXX ProCyte Dx analyzer (software v.00-25-18) and the Sysmex XT-2000iV (software v.00 to 13) with the corresponding mouse settings: RBC count by impedance and optical measurements (RBC-I and RBC-O), hemoglobin concentration (HGB), Hct, MCV, MCH, MCHC, RDW by standard deviation and coefficient of variation (RDW-SD and RDW-CV), RBC hemoglobin content (RBC-HGB), reticulocyte (RET) count and percentage, immature reticulocyte fraction (IRF), reticulocyte low-, medium-, and high-fluorescence ratios (LFR, MFR, and HFR, respectively), reticulocyte hemoglobin content (RET-HGB), WBC count, neutrophil, lymphocyte, monocyte and eosinophil counts, platelet count by optical and impedance (PLT-O and PLT-I) measurements, MPV, platelet distribution width (PDW), and plateletcrit (PCT). Quality controls of the analyzers were performed with the corresponding manufacturer's control solutions (e-check, XS, L1 and L2 IDEXX ProCyte Dx Quality Control, Westbrook, USA and Sysmex XT-2000iV e-check XE L1, L2 and L3, Villepinte, France).
All microscopic cell counts were performed by the same clinical pathology board-certified veterinarian (CT) under light microscopy (E200 microscope, Nikon, Kobé, Japan) using ×20, ×40, and ×100-oil objective lens. A 100-leukocyte differential count was performed under oil immersion (1,000×), the percentages of each cell type were determined, and the corresponding cell counts were calculated from the mean WBC counts of the 2 analyzers.
Plasma biochemistry.
Biochemical analyses of plasma specimens were performed with a VetScan VS2 chemistry analyzer and reagents (Scil animal care company, Altdorf, France) and included: sodium, potassium, calcium, phosphate, glucose, urea, creatinine, bilirubin, total proteins, albumin, globulins, ALT, ALP and α-amylase. Quality control of the analyzer was performed using the manufacturer's control solution (Abaxis Chemistry Control Level 2).
Bone marrow cytology.
May Grünwald-Giemsa stained bone marrow films were used for semiquantitative assessment of cell integrity at low magnification (×100). Cytologic evaluation was performed by the clinical pathologist (CT). Five hundred cells were counted at high magnification (×1000) and classified as myeloid, erythroid and megakaryocyte lineages, lymphocytes, plasma cells, macrophages, and mast cells Myeloid:Erythroid (M:E) ratio and cell percentages were subsequently calculated. Iron positive inclusions on Perl's stained bone marrow smears were estimated using the following scoring system: 0 = no inclusion, 1 = inclusions visualized at magnification ≥ ×1000 but not at ×100, 1.5 = inclusions visualized at magnification ×100 on some fields, 2 = inclusions visualized at magnification ×100 on all fields, 3 = inclusions visualized at magnification ×100 on all fields with several inclusions per field, 4 = so many inclusions that the fields were darkened.
Bone marrow histology.
After fixation for 72 h, the right femur was decalcified in ethylenediaminetetracetic acid (EDTA 0.27 M, pH 7.4) at 37 °C for 48 h and thereafter processed for paraffin embedding. One 3 to 4 µm-thick femoral section was stained with hematoxylin-eosin (HE). Histologic evaluation was performed by the same histologist (NBA) under light microscopy (50i Eclipse microscope, Nikon, Kobé, Japan). The first low magnification screening (x20 and ×40) included an assessment of bone architecture and a semiquantitative estimate of overall cellularity (nucleated cells compared with adipocytes) in the femoral medullar cavity. Megakaryocytes, adipocytes were counted from 10 fields at ×400 magnification in the diaphysis and metaphysis.
Statistics.
Effects of sex were tested by analysis of variance (ANOVA). For hematological data, the effects of the analyzer were also tested, and when the results were not significantly different, the mean was used to establish the reference values.
For all variables and subgroups, possible outliers were identified by visual inspection of dot plots and Tukey test. Outliers were mostly retained for the calculations, except when their distance from the nearest value was very large, and were included in the figures. RIs were determined with the Reference value advisor freeware18 using the nonparametric method when n ≥ 40, and when < 40 by the parametric method on non-transformed or Box-Cox transformed data when the distributions were not significantly different from Gaussian; in the other cases they were determined by the robust method. Confidence intervals of limits were determined by the bootstrap method.
Results
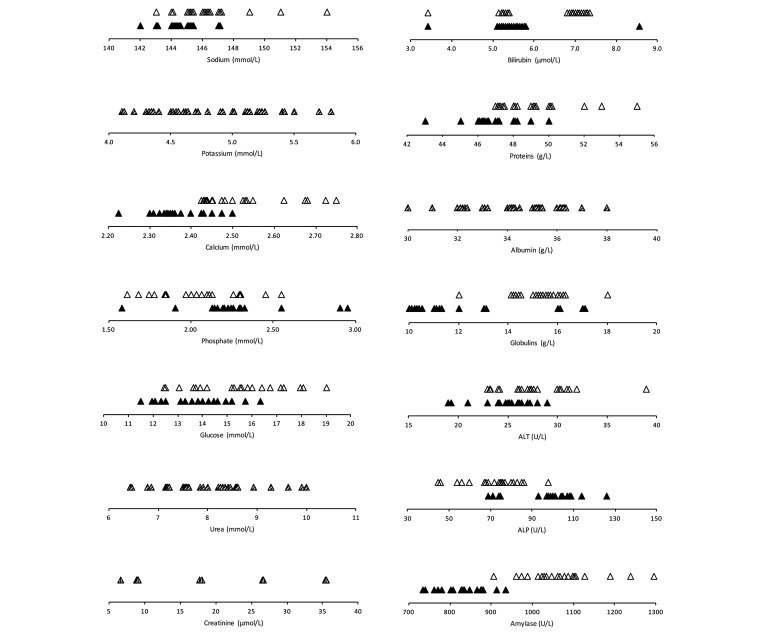
Quality control results with precision, bias, and observed total error (TEobs) were presented in Supplementary Tables S1 and S2. The TEobs of most measurements were lower than or close to the allowable total error (TEa), if the latter was available for animal clinical pathology.20 Hematology and biochemistry results obtained for forty, 9 wk-old NSG mice are fully depicted in Figures 1 and 2. respectively. Descriptive statistics (median, minimum-maximum interval) are presented for RBC and PLT variables and total WBC counts (Table 1), WBC differential counts (Table 2), biochemical analytes (Table 3) and for bone marrow cytologic variables (Table 4). Most distributions did not differ significantly from Gaussian, and possible outliers were retained, except for 2 reticulocyte values (count and percentage), 3 WBC counts (and the resulting differential counts), 1 ALT and 3 phosphate variables. For comparison purposes, corresponding median and mean values3,39 from adult C57BL/6 mice were added in the Tables. When no technical error occurred, and when no outlier had to be deleted, a maximum of 40 values could be obtained for determination of a nonparametric reference interval and to compute the 90% CIs of the limits. Parametric methods were necessary for smaller reference sample groups, for which normality tests are weak31 and calculations whether standard or robust and with or without transformation can be misleading depending on the assumptions made about normality or transformation of values. Calculated RIs are reported as Supplementary Tables (S3 through S7).
Figure 1.
Dot plots of hematology reference values in NSG mice (white, black, hatched for males, females, males + females when not different) with the Sysmex XT-2000iV (Δ) and Idexx ProCyte Dx (○) analyzers and microscopic observation (□) (◊ when not different).
Figure 2.
Dot plots of biochemistry reference values in NSG mice (white, black, hatched for males, females, males + females when not different) with the Scil VetScan VS2.
Table 1.
Median and mean concentrations of RBC, reticulocyte, total WBC and PLT counts in NSG male and female mice measured with a IDEXX ProCyte Dx and a Sysmex XT-2000iV analyzer (data for C57BL6 mice are given for comparison).
| ANOVA p |
|||||||
| Variable (unit) | n | A | Analyzer | Gender | Median (range) | Mean (SD) | C57 BL/6J39 Median (range) |
| RBC-I (1012/L) | 20m+20f | P/S | 0.074 | <0.001 | 8.26 (7.02–9.09) | 8.27 (0.38) | 9.11 (7.75–9.61) |
| 20m | 8.38 (8.13–9.09) | 8.47 (0.30) | |||||
| 20f | 8.09 (7.02–8.54) | 8.07 (0.34) | |||||
| RBC-O (1012/L) | 20m+20f | P | <0.001 | <0.001 | 8.40 (7.10–9.20) | 8.40 (0.41) | 9.545 (8.25–10.33) |
| 20m | 8.56 (8.13–9.20) | 8.61 (0.35) | |||||
| 20f | 8.25 (7.10–8.84) | 8.20 (0.36) | |||||
| 20m+20f | S | 7.90 (6.77–8.67) | 7.90 (0.35) | ||||
| 20m | 8.15 (7.53–8.67) | 8.07 (0.30) | |||||
| 20f | 7.73 (6.77–8.23) | 7.72 (0.30) | |||||
| HGB (g/L) | 20m+20f | P/S | 0.496 | <0.001 | 135.8 (121.0–149.5) | 135.9 (5.5) | 131.0 (122–139) |
| 20m | 137.0 (131.5–149.5) | 137.9 (5.0) | |||||
| 20f | 135.0 (121.0–141.5) | 133.6 (5.2) | |||||
| Hct (L/L) | 20m+20f | P | <0.001 | <0.001 | 0.446 (0.393–0.497) | 0.446 (0.022) | 0.4255 (0.38–0.45) |
| 20m | 0.459 (0.429–0.497 | 0.460 (0.018) | |||||
| 20f | 0.437 (0.393–0.463) | 0.435 (0.018) | |||||
| 20m+20f | S | 0.421 (0.378–0.459) | 0.419 (0.019) | ||||
| 20m | 0.435 (0.409–0.459) | 0.433 (0.013) | |||||
| 20f | 0.406 (0.378–0.427) | 0.406 (0.014) | |||||
| MCV (fL) | 20m+20f | P | <0.001 | 0.011 | 53.7 (51.6–56.5) | 53.7 (1.1) | 46.5 (46.0–49.8) |
| 20m | 54.1 (51.8–56.5) | 54.0 (1.2) | |||||
| 20f | 53.5 (51.6–55.8) | 53.5 (0.9) | |||||
| 20m+20f | S | 51.0 (49.2–54.1) | 51.1 (1.1) | ||||
| 20m | 51.6 (49.7–54.0) | 51.5 (1.1) | |||||
| 20f | 50.7 (49.2–54.1) | 50.7 (1.0) | |||||
| MCH (pg) | 20m+20f | P | 0.005 | <0.001 | 16.3 (15.9–17.2) | 16.4 (0.3) | 14.5 (14.1–16.0) |
| 20m | 16.3 (15.9–16.9) | 16.3 (0.2) | |||||
| 20f | 16.4 (16.1–17.2) | 16.6 (0.3) | |||||
| 20m+20f | S | 16.5 (16.0–17.3) | 16.5 (0.3) | ||||
| 20m | 16.3 (16.0–16.8) | 16.4 (0.2) | |||||
| 20f | 16.6 (16.3–17.3) | 16.7 (0.3) | |||||
| MCHC (g/L) | 20m+20f | P | <0.001 | <0.001 | 305.0 (290-315) | 304.6 (5.1) | 311 (304–327) |
| 20m | 301.5 (290–310) | 301.9 (4.7) | |||||
| 20f | 307.0 (299–315) | 307.3 (4.2) | |||||
| 20m+20f | S | 324.0 (306–334) | 323.4 (7.4) | ||||
| 20m | 316.5 (306–327) | 317.7 (5.3) | |||||
| 20f | 330.0 (320–334) | 329.2(3.9) | |||||
| RDW-SD (fL) | 20m+20f | P | <0.001 | 0.652 | 30.4 (28.4–34.6) | 30.5 (1.1) | 29.7 (27.4–31.0) |
| 20m+20f | S | 29.6 (27.8–33.8) | 29.7 (1.0) | ||||
| RDW-CV (%) | 20m+20f | P | <0.001 | 0.535 | 21.4 (20.6–22.4) | 21.5 (0.5) | 24.3 (20.1–25.2) |
| 20m+20f | S | 18.8 (18.2-20.0) | 18.9 (0.4) | ||||
| RBC-HGB (pg) | 20m+20f | P | <0.001 | 0.003 | 18.0 (17.7–18.9) | 18.1 (0.3) | |
| 20m | 18.0 (17.8–18.3) | 18.0 (0.2) | |||||
| 20f | 18.2 (17.7–18.9) | 18.2 (0.3) | |||||
| 20m+20f | S | 17.2 (16.7–18.0) | 17.2 (0.3) | ||||
| 20m | 17.2 (16.7–17.4) | 17.1 (0.2) | |||||
| 20f | 17.2 (16.9–18.0) | 17.3 (0.3) | |||||
| RET (109/L) | 19ma+20f | P/S | 0.444 | 0.127 | 469 (296–673) | 483 (76) | 307.5 (241.8–368.1) |
| RET (%) | 20m+19fb | P/S | 0.360 | 0.022 | 5.70 (3.81–8.25) | 5.82 (0.90) | 3.41 (2.97–3.83) |
| 20m | 5.62 (4.94–6.18) | 5.63 (0.35) | |||||
| 19fb | 5.80 (3.81–8.25) | 6.02 (1.22) | |||||
| IRF(%) | 20m+20f | P | <0.001 | 0.846 | 61.1 (54.0–70.1) | 61.0 (3.5) | 52.9 (50.4–56.9) |
| 20m+20f | S | 45.2 (37.9–55.8) | 45.4 (3.4) | ||||
| LRF(%) | 20m+20f | P | <0.001 | 0.139 | 39.0 (29.9–46.0) | 39.0 (3.5) | 47.2 (43.1–49.6) |
| 20m+20f | S | 20.1 (17.3–22.9) | 20.3 (1.5) | ||||
| MRF(%) | 20m+20f | P | <0.001 | 0.325 | 17.0 (12.3–20.0) | 16.7 (1.7) | 20.0 (16.9–23.2) |
| 20m+20f | S | 43.2 (24.8–43.6) | 34.4 (4.0) | ||||
| HRF(%) | 20m+20f | P | <0.001 | 0.371 | 44.3 (34.0–55.5) | 44.3 (4.2) | 33.35 (27.7–37.3) |
| 20m+20f | S | 54.9 (44.2–62.1) | 54.7 (3.4) | ||||
| Ret-HGB(pg) | 20m+20f | P | <0.001 | <0.001 | 18.8 (18.3–20.2) | 18.9 (0.4) | |
| 20m | 18.7 (18.3–18.9) | 18.7 (0.2) | |||||
| 20f | 19.0 (18.5–20.2) | 19.1 (0.5) | |||||
| 20m+20f | S | 20.0 (19.4–21.6) | 20.1 (0.5) | ||||
| 20m | 19.9 (19.4–20.1) | 19.8 (0.2) | |||||
| 20f | 20.1 (19.6–21.6) | 20.3 (0.5) | |||||
| WBC (109/L) | 20m+17fc | P/S | 0.881 | <0.001 | 1.11 (0.42–2.60) | 1.23 (0.5) | 6.0 (4.0–10.9) |
| 20m | 0.93 (0.42–1.24) | 0.9 (0.2) | |||||
| 17fc | 1.56 (0.88–2.60) | 1.59 (0.49) | |||||
| PLT-I (109/L) | 17m+19f | P | <0.001 | 0.007 | 979 (798–1189) | 974 (93) | 1006 (853–1951) |
| 17m | 1001 (907–1189) | 1015 (74) | |||||
| 19f | 959 (798–1097) | 937 (95) | |||||
| 17m+19f | S | 0.115 | 1733 (1247–1990) | 1733 (167) | |||
| PLT-O (109/L) | 17m+19f | P | <0.001 | <0.001 | 1212 (930–1392) | 1213 (125) | 1107 (878–2283) |
| 17m | 1258 (1146–1392) | 1273 (71) | |||||
| 19f | 1092 (930–1255) | 1101 (105) | |||||
| 20m+20f | S | <0.001 | 1576 (1090–1845) | 1532 (191) | |||
| 17m | 1658 (1090–1845) | 1629 (170) | |||||
| 19f | 1410 (1199–1733) | 1435 (163) | |||||
| MPV (fL) | 17m+19f | P | <0.001 | 0.339 | 6.1 (5.8–6.4) | 6.1 (0.2) | 5.7 (5.3–6.1) |
| 20m+20f | S | 0.004 | 6.6 (6.3–7.0) | 6.6 (0.2) | |||
| 17m | 6.6 (6.3–6.8) | 6.5 (0.2) | |||||
| 19f | 6.7 (6.5–7) | 6.7 (0.1) | |||||
| PCT (mL/L) | 17m+19f | P | <0.001 | 0.007 | 6.0 (4.8–7.3) | 6.0 (0.6) | 5.7 (4.8–11.0) |
| 17m | 6.3 (5.4–7.3) | 6.2 (0.5) | |||||
| 19f | 5.7 (4.8–6.8) | 5.8 (0.6) | |||||
| 20m+20f | S | 0.377 | 11.6 (8.4–13.3) | 11.5 (1.1) | |||
| PDW (fL) | 17m+19f | P/S | 0.092 | 0.340 | 6.8 (6.5–7.4) | 6.8 (0.2) | 6.5 (6.2–7.2) |
Corresponding reference intervals are given as supplementary tables
P: ProCyte Dx analyzer; S: XT-2000iV analyzer; m: males; f: females; SD: standard deviation; outliers deleted: a: 965 109/L; b:13.8%; c: 4.89; 4.35; 3.57 109/L
Table 2.
Median and mean concentrations of WBC DIFF counts in NSG male and female mice measured with a IDEXX ProCyte Dx analyzer and by a microscopic count. (data for C57/BL6 mice are given for comparison)
| ANOVA p |
|||||||
| Variable (unit) | n | Method | Method | Gender | Median (range) | Mean (SD) | C57 BL/6J39 Median (range) |
| Neutrophils (109/L) | 17m+16fa | P/M | 0.177 | <0.001 | 0.81 (0.44–2.05) | 0.97 (0.45) | 0.76 (0.38–2.92) |
| 17m | 0.65 (0.44–0.89) | 0.65 (0.14) | |||||
| 16fa | 1.30 (0.62–2.05) | 1.30 (0.42) | |||||
| Lymphocytes (109/L) | 17m+16fb | P | <0.001 | 0.014 | 0.30 (0.14–0.57) | 0.30 (0.10) | 5.14 (2.27–9.76) |
| 17m | 0.26 (0.14–0.35) | 0.26 (0.07) | |||||
| 16fb | 0.35 (0.17–0.57) | 0.34 (0.11) | |||||
| 17m+16fc | M | 0.844 | 0.02 (0.00–0.12) | 0.03 (0.03) | |||
| Monocytes (109/L) | 17m+16fd | P | <0.001 | 0.132 | 0.01 (0.00–0.005) | 0.02 (0.01) | 0.02 (0.01–0.09) |
| 17m+16fe | M | 0.731 | 0.17 (0.06–0.42) | 0.19 (0.09) | |||
| Eosinophils (109/L) | 17m+16ff | P/M | 0.196 | 0.052 | 0.02 (0.01–0.05) | 0.02 (0.01) | 0.11 (0.05–0.22) |
P: ProCyte Dx analyzer; M: Microscopic count; m: males; f: females; SD: standard deviation; outliers deleted: a: 4.49; 2.98; 3.85; b: 0.53; 0.45; 0.41; c: 0.05; 0.18; 0.00; d: 0.1; 0.03; 0.07; e: 0.1; 0.18; 0.26; f: 0.02; 0.03; 0.02. Corresponding reference intervals are given as supplementary tables.
Table 3.
Median and mean concentrations of biochemical analytes measured by the VetScan VS2 analyzer in NSG male and female mice.
| Variable (unit) | n | ANOVA P Gender | Median (range) | Mean (SD) | C57 BL/6J 3 Median (range) | |
| Sodium (mmol/L) | 20m+17f | 0.007 | 145.0 (142–154) | 145.5 (2.2) | ||
| 20m | 146.0 (143–154) | 146.4 (2.5) | 155 (149–165) | |||
| 17f | 144.0 (142–147) | 144.4 (1.3) | 158 (147–163) | |||
| Potassium (mmol/L) | 19m§+17f | 0.074 | 4.8 (3.7–6.3) | 4.8 (0.5) | 4.30 (3–6.2) (m) | |
| 4.60 (3.5–5.1)(f) | ||||||
| Calcium (mmol/L) | 20m+17f | <0.001 | 2.43 (2.23–2.75) | 2.43 (0.12) | ||
| 20m | 2.49 (2.43–2.75) | 2.52 (0.11) | 2.04 (1.87–2.34) | |||
| 17f | 2.35 (2.23–2.50) | 2.36 (0.07) | 2.06 (1.78–2.30) | |||
| Phosphate (mmol/L) | 20m+14fa | 0.022 | 2.1 (1.6–2.5) | 2.1 (0.2) | ||
| 20m | 2.0 (1.6–2.5) | 2.0 (0.3) | ||||
| 14fa | 2.2 (1.9–2.5) | 2.2 (0.1) | ||||
| Glucose (mmol/L) | 20m+17f | 0.003 | 14.4 (11.5–19.0) | 14.6 (1.9) | ||
| 20m | 15.5 (12.4–19.0) | 15.4 (1.9) | 8.3 (2.6–16.3) | |||
| 17f | 13.6 (11.5–16.3) | 13.7 (1.4) | 9.9 (5.7–16.9) | |||
| Urea (mmol/L) | 20m+17f | 0.107 | 7.8 (6.4–10.7) | 8.0 (1.0) | 9 (2.8–13.3)(m) | |
| 8.7 (5.7–11.7) (f) | ||||||
| Creatinine (µmol/L) | 20m+17f | 0.278 | <10 (<10–35) | — | 17 (10–29) (m) | |
| 15 (11–28) (f) | ||||||
| Bilirubin (µmol/L) | 19m§+17f | 0.012 | 5 (3–9) | 6 (1) | ||
| 19m§ | 7 (3–7) | 6 (1) | 5.1 (<1.7–8.6) | |||
| 17f | 5 (3v9) | 5 (3-9) | 6.8 (1.7–15.4) | |||
| Proteins (g/L) | 20m+17f | 0.001 | 48.0 (43–55) | 48.0 (2.3) | ||
| 20m | 49.0 (47–55) | 49.1 (2.2) | 40 (20–60) | |||
| 17f | 46.0 (43–50) | 46.7 (1.6) | 40 (30–50) | |||
| Albumin (g/L) | 20m+17f | 0.538 | 34.0 (30–39) | 34.2 (2.0) | ||
| Globulins (g/L) | 20m+17f | <0.001 | 14.0 (10–18) | 13.7 (2.4) | ||
| 20m | 15.0 (12–18) | 15.0 (1.2) | ||||
| 17f | 11.0 (10–17) | 12.2 (2.6) | ||||
| ALT (U/L) | 19mb+17f | 26.0 (19–32) | 25.9 (3.1) | |||
| 19mb | 0.011 | 27.0 (23–32) | 27.1 (2.9) | 21 (13–39) | ||
| 17f | 25.0 (19–29) | 24.6 (2.8) | 22 (12–44) | |||
| ALP (U/L) | 20m+17f | <0.001 | 80.0 (45–126) | 83.1(19.6) | ||
| 20m | 74.5 (45–98) | 71.2 (13.6) | 153 (63-221) | |||
| 17f | 100.0 (69–126) | 97.1 (16.1) | 129 (44–235) | |||
| Amylase (U/L) | 20m+17f | <0.001 | 961 (735–1297) | 960 (146) | ||
| 20m | 1065 (906–1297) | 1073 (93) | 1942 (1501–3084) | |||
| 17f | 832 (735–936) | 829 (59) | 1940 (1157–2762) |
m: males; f: females; SD: standard deviation; Outliers deleted a: 1.6; 2.9; 2.9 mmol/L; b: 39 U/L; §: Value missing due to technical errors.
Corresponding reference intervals are provided as supplementary tables.
Table 4.
Median and mean cell percentages, ratio and indexes for bone marrow smear in NSG male (n= 20) and female (n= 20) mice. SD: standard deviation; M:E: Ratio Myeloid:Erythroid; MMI: Myeloid Maturation Index; EMI: Erythroid Maturation Index.
| Cell type | ANOVA P Gender | Median (range) | Mean (SD) |
| Myeloblast | 0.661 | 1.2 (0.2–1.4) | 1.17 (0.08) |
| Promyelocyte | 0.179 | 2.9 (1.4–5.6) | 3.12 (1.09) |
| Myelocyte | 0.352 | 5.8 (3.6–10.8) | 5.88 (1.41) |
| Metamyelocyte | 0.129 | 7.9 (5.2–11.8) | 8.30 (2.20) |
| Band neutrophil | 0.592 | 18.4 (12.8–26.8) | 18.23 (2.90) |
| Segmented neutrophil | 0.919 | 26.1 (17.2–37.4) | 26.40 (4.86) |
| Eosinophil | 0.619 | 0.8 (0.0–2.2) | 0.90 (0.44) |
| Basophil§ | 0.159 | 0.0 (0.0–0.2) | 0.03 (0.07) |
| Rubriblast | 0.126 | 0.6 (0.2–1.4) | 0.65 (0.33) |
| Prorubricyte | 0.794 | 2.2 (0.4–4.0) | 2.16 (0.83) |
| Rubricyte | 0.855 | 13.4 (6.4–18.4) | 19.80 (4.16) |
| Metarubricyte | 0.111 | 19.4 (9.4–29.2) | 15.4 (1.9) |
| Lymphocyte | 0.179 | 0.2 (0.0–1.2) | 0.17 (0.23) |
| Plasma cell§ | 0 | 0 | |
| Macrophage | 0.609 | 0.2 (0.0–1.2) | 0.29 (0.30) |
| Mast cell§ | 0 | 0 | |
| M:E ratio | 0.368 | 1.76 (1.12–4.66) | 1.92 (0.68) |
| MMI | 0.305 | 0.18 (0.13–0.31) | 0.19 (0.04) |
| EMI | 0.154 | 0.09 (0.03–0.14) | 0.09 (0.03) |
: 35, 40 and 39 zero values for basophils, plasma cells and mast cells respectively.
Corresponding reference intervals are given as supplementary tables.
Hematology.
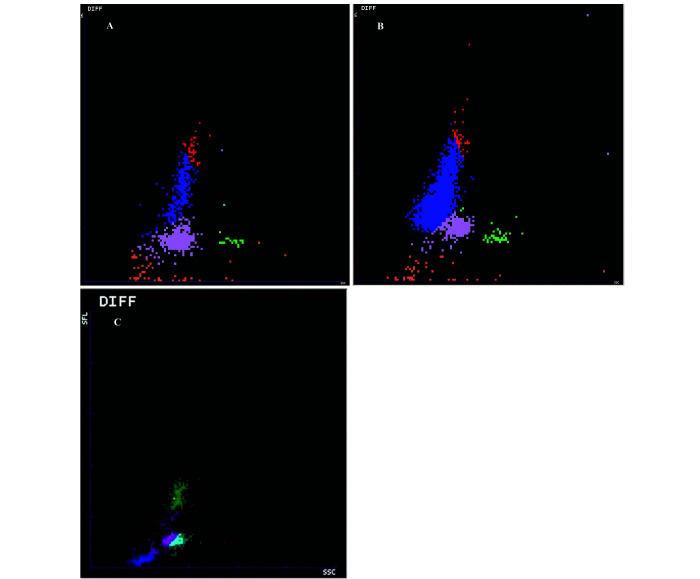
Some results (4 ProCyte Dx WBC and PLT counts) were not validated due to analytical flags. Most results differed according to the analyzer and/or to the gender of the animals. Except for impedance measurement of RBC, reticulocytes count and percentage and total WBC counts, hematologic results were significantly different (P < 0.001) between the ProCyte Dx and the Sysmex XT 2000iV analyzers. Typical WBC differential scattergrams of NSG mouse analyzed with the ProCyte Dx and the XT2000iV are presented in Figure 3. With regard to differential leukocyte counts, we chose not to report XT-2000iV DIFF WBC counts because this analyzer produced systematically abnormal DIFF scattergrams (fused neutrophils and lymphocytes clouds, Figure 3 C) and numerous analytical flags warning of low reliability of the differential WBC numerical data. Conversely, ProCyte Dx produced DIFF scattergrams with small but well delineated clouds that could be reliably identified (Figure 3 A). Therefore, DIFF WBC counts comparisons were made between ProCyte Dx and blood smear analysis (Table 2). The main findings were a statistically significant effect of the method (analytical compared with manual; P < 0.001) on lymphocytes and monocytes, with mean counts being 10 fold lower and higher on blood smear evaluation respectively. Moreover, lymphocyte and eosinophil counts were very low in NSG mice, approximatively 10 and 20% of corresponding C57BL/6J analytical counts, whereas the neutrophil count did not differ from that currently reported in other murine strains.37 Sex-related differences were observed either with one or both analyzers in most of the hematologic variables, with females having usually slightly lower values except for WBC count, which was 1.8 fold higher than in male (Table 1). Neutrophil counts for females were twice as high as for males (Table 2).
Figure 3.
ProCyte Dx hematology typical white blood cell differential (DIFF) scattergrams of a NSG mouse (A) and C57BL/6 mouse (B) blood specimens. Lymphocytes (blue dots); monocytes (red dots), neutrophils (purple dots), eosinophils (green dots). XT2000iV DIFF scattergram obtained from NSG mouse blood specimen (C). In (A), lymphocytes cluster is significantly reduced compared with (B), while it is not identifiable in (C) where theoretical lymphocyte population (pink dots) is not clearly separated from neutrophil (light blue dots) and monocyte (green dots) populations.
Biochemistry.
We could not obtain biochemical panels for 3 female specimens due to preanalytical errors or the bilirubin and potassium results for one male due to a hemolysis flag of the analyzer. For creatinine, 12 values of between 8 and 10 µmol/L, and 9 values below the detection threshold of the analyzer precluded accurate calculation of the inferior limit of the reference interval. Ten of 14 biochemical variables showed weak but statistically significant gender effects (Table 3). Most of the NSG values were similar to the C57BL/6J values except for glucose concentration (14.6 ± 1.9 mmol/L), which was 80% higher in NSG mouse. Potassium and inorganic phosphate showed unexplained high biases and good precision.
Bone marrow evaluation.
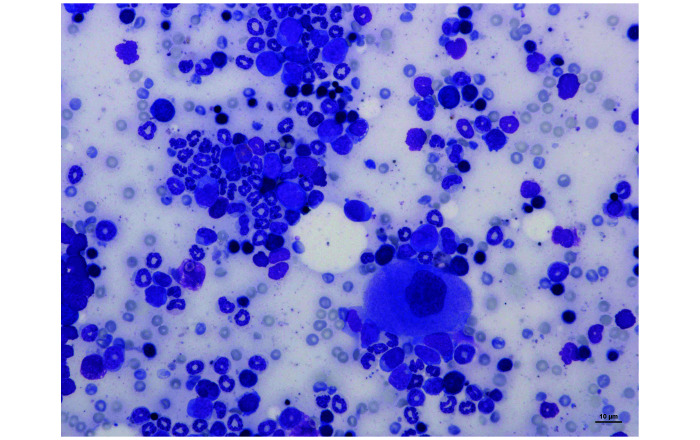
The results of myelograms realized on 40 NSG mice are presented in Table 4. A gender effect was not observed for any of the variables evaluated. The median and mean of M:E ratio were 1.76 and 1.92 respectively. An illustration of NSG bone marrow cells from a May Grünwald-Giemsa stained bone marrow film is shown in Figure 4.
Figure 4.
Femoral bone marrow smear from a NSG mouse showing a megakaryocyte (the largest cell), erythroid precursors (the darkest cells) and myeloid precursors (the lightest cells) on a bloody background. Magnification ×200, May-Grünwald Giemsa
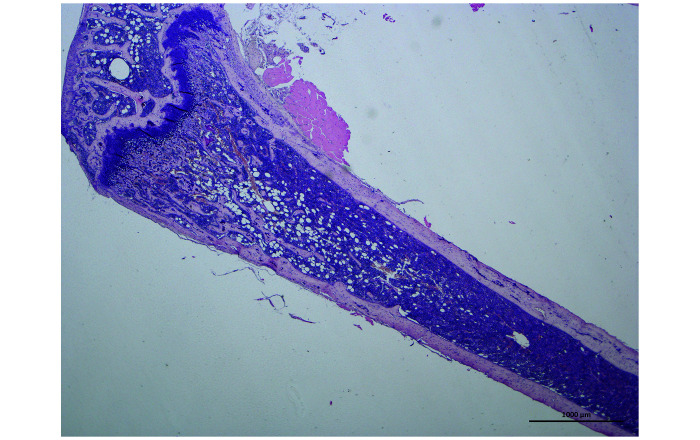
Histologic evaluation of the femoral bone marrow revealed normal bone architecture, with an overall cellularity of 90–100% in the medullary space (Figure 5). In male and female mice, a significant effect of bone location (diaphysis compared with metaphysis; P < 0.001) was observed on the distribution of cell types, with more numerous megakaryocytes and adipocytes in diaphysis and metaphysis, respectively. Moreover, except for metaphyseal adipocytes, bone marrow cell counts were significantly lower (P < 0.05) in female mice (Table 5).
Figure 5.
Longitudinal section from a NSG mouse femur showing bone marrow cellularity in the metaphysis and the diaphysis. The adipocytes (optically empty areas) tend to be more distally located. Magnification ×20, Hemalun-Eosin
Table 5.
Median and mean megakaryocytes and adipocytes counts in diaphysis and metaphysis of femoral bone marrow from NSG male and female mice. D: diaphysis; M: metaphysis; m: males; f: females; SD: standard deviation
| Femoral location |
Gender |
Median (range) |
Mean (SD) |
|||
| Cell type | P | P | ||||
| Megakaryocytes | <0.001 | D | <0.001 | 20m+20f | 98.0 (32–157) | 95.3 (26.4) |
| 20m | 108.0 (32–157) | 109.5 (25.7) | ||||
| 20f | 80.0 (60–128) | 81.0 (18.5) | ||||
| M | <0.001 | 20m+20f | 57.0 (13–94) | 52.5 (20.3) | ||
| 20m | 61.5 (45–94) | 65.9 (12.1) | ||||
| 20f | 33.0 (13–90) | 38.4 (17.6) | ||||
| Adipocytes | <0.001 | D | 0.002 | 20m+20f | 9.0 (0–27) | 9.8 (7.3) |
| 20m | 12.5 (0–27) | 13.3 (7.7) | ||||
| 20f | 5.0 (0–17) | 6.4 (5.2) | ||||
| M | 0.653 | 20m+20f | 78.0 (9–224) | 88.9 (54.5) | ||
Corresponding reference intervals are given as supplementary tables
Discussion
As expected, given its highly immunodeficient nature, the main characteristic of NSG from other strains was the very low WBC count. We found a mean WBC count of 1.23 109/L, whereas it ranges from 2 to 10 109/L in various other inbred mouse strains8,9,21,27,28,33,39,52 and from 0.9 to 1.9 109/L in CB17-SCID male mice,32 the latter having the same genetic background as the NSG mouse.46 The WBC counts reported in our study for males (0.9 109/L) are in accordance with the data available online for NSG mice of same age and gender.49 However, the higher female WBC count and the resulting sex-related significant difference we observed are in contradiction with those previous data. Although the previous study used mice of the same strain, they were not from the same breeder, which may explain the discrepancy observed with our finding of higher WBC counts in females.49
With regard to differential WBC populations, analysis of blood smears showed that, although the hematology ProCyte Dx analyzer had been validated in C57BL/6J mice,30 NSG lymphocytes and monocytes were over- and under-estimated respectively when comparing automated to manual counts. Comparison of NSG and C57BL/6J ProCyte Dx DIFF scattergrams (Figures 3 A and 3 B), strongly suggested that the low number of leukocytes may have led to the misidentification of different cell types by the analyzer. Despite being small, the other clusters were nonetheless individualized and thus identifiable, in contrast to results obtained using the XT 2000iV, in which clouds were contiguous or fused, making interpretation impossible (Figure 3 C). These observations, combined with the presence of numerous WBC DIFF flags generated by the analyzer software, supported our decision to exclude the XT 2000iV differential leukocyte counting from reference intervals calculations. This result highlights the need to systematically evaluate the blood film concurrent with DIFF scattergrams, which potentially could be used as a screening tool to detect CBC abnormalities. The lymphocyte counts reported in the Jackson laboratory database49 are 2 to 3 times higher than the monocyte counts, depending on the sex and age of the animal, without reference to blood smear observation. We did not have any information about their analytical conditions and suspect that leukocyte type discrimination was, as for us, challenging and probably based on hematology analyzer results only, without parallel blood film evaluation.
Unlike WBC, most of the sex-related significant difference in hematologic variables were not clinically relevant, in our opinion. Mean platelet volumes were similar between males and females, but were 30% above those reported online in NSG.49 Storage of specimens for a prolonged period of time is the main preanalytical cause of increased MPV.42 However, our analyses were performed within 2 h of sampling, and this duration of storage is unlikely to have notably affected platelet volume. Covariables such as age, gender or mouse strain that could significantly affect MPV27 were similar in both studies. In addition, the mouse platelet size is reported to exhibit great variability, ranging from 4 to 6 fL.14,39 The MPV is a measured variable, thus dependent on the analyzer. This dependency was confirmed in the present study by the difference between ProCyte Dx and XT-2000iV results, as in previous studies in mice1 and humans.29 The discrepancy in MPV between the present study and online data in NSG mice49 might, therefore, reflect individual as well as analytical variability.
Bone marrow lymphocyte count was previously reported to range from 11.8% to 32.4% in 10 to 12 mo-old C57BL/6J mice.53 In the present study, the median percentage of lymphocytes in bone marrow was extremely low (0.2%) compared with routinely used inbred mice, in which lymphocytes represent one-fifth to one-third of bone marrow cells.39,53 This result appeared consistent for the NSG strain and is in accordance with a previous study on bone marrow CB17 SCID male mice.32 Moreover, this low marrow lymphocyte percentage supported the low blood lymphocyte count even though the correlation between bone marrow and blood lymphocyte counts is reported to be weak in most species.40
In most studies, bone marrow evaluation is focused on cell immunophenotyping by flow cytometry of femoral bone marrow liquid suspensions.23,43 Moreover morphologic assessment of bone marrow tissue sections provides information about marrow architecture including cellularity and morphologic features,13,50 and a cytologic examination of the bone marrow allows detailed quantitative cellular evaluation of early hematopoietic precursors of granulocytic, erythroid and lymphoid lineages and calculation of the myeloid:erythroid (M:E) ratio.40 In the present study, the bone marrow histologic assessment revealed high overall cellularity, which is usually described in mice.36,37 However, the higher adipocyte and megakaryocyte counts, observed respectively in the metaphysis and diaphysis, has not been previously described. Although the regulation and function of marrow adipose tissue remain unclear, the accumulation of adipocytes in bone marrow was reportedly related to different conditions such as aging, diabetes, or anorexia.35,44 Nevertheless, as previous authors report marrow adipose tissue distribution to be strain-dependent, whether our finding is specific to NSG or a feature encountered in other species remains unknown. The M:E ratio, which represents a useful parameter to evaluate abnormal peripheral blood cell counts and changes in bone marrow cellularity, ranged from 1.12 to 4.66 in NSG mice, which was close to 10 to 12 mo-old inbred C57BL/6J mice (0.9 to 4.7)53 and to 9-wk-old male CB17-SCID mice (2.28 to 5.75).32 Lastly, bone marrow cytology remains challenging and requires high-quality smears and a clinical pathology specialist trained in myelogram evaluation. A specific distinction between lymphocytes and erythroid or myeloid series would necessitate immunocytochemistry using mouse-specific lymphocyte antibodies. Quantitative bone marrow differential counts in laboratory animals were reported to improve the precision and timeless of hematopoietic safety evaluations in preclinical safety studies.12
Although enzyme activities could not be validly compared due to the difference of analytical methods employed, the results for most biochemistry variables were similar to those previously reported in inbred mice,37,39 including the sex-related differences we observed for most of the variables and that are reported in mice3,8,27,33,38,45,54 and rats.3,19,22 We nonetheless observed an unexpectedly high glucose concentration for both genders, far above previously reported values in NSG mice of unknown fasting status49 but closer to those of nonfasted male C57BL/6N mice sampled in the morning.25 Glucose concentration is reported to increase after food intake, isoflurane anesthesia,8,48 and stressful events such as unaccustomed handling or cage transportation.48 In the present study, preanalytical conditions were designed to minimize animal disturbance and to maximize results quality and transferability: we restricted transportation and handling to gentle displacement of the animals from their cage to the induction box so these are unlikely causes of the observed high plasma glucose concentration. As we used isoflurane anesthesia in unfasted animals, both these factors might explain the discrepancy between our values and previous ones obtained under unreported and maybe different preanalytical conditions. General anesthesia by isoflurane inhalation was used because it is safer, allows precise adjustments, and has less impact on the liver, cardiac and kidney functions than injectable mixtures.11,16 Moreover, it has no significant effects on complete blood cell count, whereas it alters the concentrations of some biochemical analytes, notably increasing glucose.8,11 Although a 12-h overnight fasting is reported to be adequate for most biochemical analytes in animals,6 we decided not to fast the mice before sampling because peak mouse activity, feeding, and metabolism occur during the dark period26 and because fasting leads to hormonal, biochemical, and behavioral changes that can vary between strains.26 The CBC count is not reported to be affected by fasting in mice, unlike biochemical analytes such as amylase, creatinine and particularly glucose.8,26 Effects of anesthesia and fasting may have be different in each animal. However, as expected in animals belonging to the same strain,3 the values we obtained were homogeneous with low interindividual variability for most variables, except total and differential WBC counts. For the latter, the interindividual CVg (CVg = SD/Mean) was high due to the very low number of cells counted. For the other variables, CVg were similar or lower than reported in laboratory beagles,5,24 cats2 or humans.41
Our study was designed based on CLSI10 and ASVCP15 recommendations, which endorse the usual minimum of 120 reference subjects recommended as being “the best means” for determining reference limits and their 90% confidence intervals (CIs) by using the nonparametric method. CLSI also acknowledges that alternative methods can be used, when only smaller numbers are available, but they are reluctant at using them below n = 80, “except in the most extreme instances”.10 This was the case in the present study; we chose to use much smaller reference sample groups because it would have been unethical to euthanize a large number of animals when RIs, even if they cannot be precise, can be computed from small samples.17 When n ≤ 20, our opinion is that estimated RIs are less representative than the median, the minimum-maximum interval, and the dot plots for each group of partition, as already reported in an example in the canine species.17 This is why the calculated RIs are only reported as supplementary material.
Given the genetic characteristics of NSG mice, researchers need access to a reliable database regarding the hematology, biochemistry, and bone marrow of this mouse. The establishment of reference values for each sex, even based on a small sample size, is therefore a necessary preliminary step in the production and exploitation of reliable scientific results. In addition to the determination of reference values, our work also stresses the analytical bias that might be generated by the absence of microscopic observation of blood smears in WBC differential population identification and quantification.
Supplementary Material
Table S1. Results of QC of hematology analysis using the Procyte Dx and the XT 2000iV analyzers
Table S2. Results of QC of biochemical analysis using the Vetscan VS2 analyzer.
Table S3. Reference Intervals for RBC, reticulocyte, total WBC and PLT counts in NSG male and female mice measured with an IDEXX ProCyte Dx and a Sysmex XT-2000iV analyzers.
Table S4. Reference Intervals for WBC DIFF counts in NSG male and female mice measured with a IDEXX ProCyte Dx analyzer (P) and by a microscopic count (M).
Table S5. Reference Intervals for biochemical analytes measured by the VetScan VS2 analyzer in NSG male and female mice.
Table S6. Reference Intervals for cell percentages, ratio and indexes for bone marrow aspiration in NSG male (n = 20) and female (n = 20) mice.
Table S7. Reference Intervals for megakaryocytes and adipocytes counts in diaphysis and metaphysis of femoral bone marrow of NSG male (n = 20) and female (n = 20) mice.
References
- 1.Aurbach K, Spindler M, Haining EJ, Bender M, Pleines I. 2019. Blood collection, platelet isolation and measurement of platelet count and size in mice-a practical guide. Platelets 30:698–707. 10.1080/09537104.2018.1528345. [DOI] [PubMed] [Google Scholar]
- 2.Baral RM, Dhand NK, Freeman KP, Krockenberger MB, Govendir M. 2014. Biological variation and reference change values of feline plasma biochemistry analytes. J Feline Med Surg 16:317–325. 10.1177/1098612X13508770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boehm O, Zur B, Koch A, Tran N, Freyenhagen R, Hartmann M, Zacharowski K. 2007. Clinical chemistry reference database for Wistar rats and C57/BL6 mice. Biol Chem 388:547–554. 10.1515/BC.2007.061. [DOI] [PubMed] [Google Scholar]
- 4.Bolliger AP. 2004. Cytologic evaluation of bone marrow in rats: indications, methods, and normal morphology. Vet Clin Pathol 33:58–67. 10.1111/j.1939-165X.2004.tb00351.x. [DOI] [PubMed] [Google Scholar]
- 5.Bourgès-Abella NH, Gury TD, Geffré A, Concordet D, Thibault-Duprey KC, Dauchy A, Trumel C. 2015. Reference intervals, intraindividual and interindividual variability, and reference change values for hematologic variables in laboratory beagles. J Am Assoc Lab Anim Sci 54:17–24. [PMC free article] [PubMed] [Google Scholar]
- 6.Braun JP, Bourges-Abella N, Geffré A, Concordet D, Trumel C. 2014. The preanalytic phase in veterinary clinical pathology. Vet Clin Pathol 44:8–25. 10.1111/vcp.12206. [DOI] [PubMed] [Google Scholar]
- 7.Braun JP, Concordet D, Geffré A, Bourges Abella N, Trumel C. 2013. Confidence intervals of reference limits in small reference sample groups. Vet Clin Pathol 42:395–398. 10.1111/vcp.12065. [DOI] [PubMed] [Google Scholar]
- 8.Champy MF, Selloum M, Piard L, Zeitler V, Caradec C, Chambon P, Auwerx J. 2004. Mouse functional genomics requires standardization of mouse handling and housing conditions. Mamm Genome 15:768–783. 10.1007/s00335-004-2393-1. [DOI] [PubMed] [Google Scholar]
- 9.Champy MF, Selloum M, Zeitler V, Caradec C, Jung B, Rousseau S, Pouilly L, Sorg T, Auwerx J. 2008. Genetic background determines metabolic phenotypes in the mouse. Mamm Genome 19:318–331. 10.1007/s00335-008-9107-z. [DOI] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. 2010. EP28-A3c: Defining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline, 3rd ed. Wayne (PA): Clinical and Laboratory Standards Institute (CLSI). [Google Scholar]
- 11.Constantinides C, Mean R, Janssen BJ. 2011. Effects of isoflurane anesthesia on the cardiovascular function of the C57BL/6 mouse. ILAR J 52:e21–e31. [PMC free article] [PubMed] [Google Scholar]
- 12.Criswell KA, Bock JH, Johnson K, Criswell RA, Giovanelli RP. 2018. Validation of Sysmex XT-2000iV analyzer-generated quantitative bone marrow differential counts in cynomolgus monkeys, Beagle dogs, and CD-1 mice. Vet Clin Pathol 47:539–555. 10.1111/vcp.12672. [DOI] [PubMed] [Google Scholar]
- 13.Elmore SA. 2006. Enhanced histopathology of the bone marrow. Toxicol Pathol 34:666–686. https://doi.org/10.1080/ 01926230600939971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Everds NE. 2007. Hematology of the laboratory mouse, p 133–170. In: Fox JG, Barthold SW, Davisson MT, Newcomer CE, Quimby FW, Smith AL, editors. The mouse in biomedical research 2nd ed, vol 3, Normative biology, husbandry, and models. Burlington (MA): Academic Press. [Google Scholar]
- 15.Friedrichs KR, Harr KE, Freeman KP, Szladovits B, Walton RM, Barnhart KF, Blanco-Chavez J. 2012. ASVCP reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics. Vet Clin Pathol 41:441–453. 10.1111/vcp.12006. [DOI] [PubMed] [Google Scholar]
- 16.Gargiulo S, Greco A, Gramanzini M, Esposito S, Affuso A, Brunetti A, Vesce G. 2012. Mice anesthesia, analgesia, and care, Part II: anesthetic considerations in preclinical imaging studies. ILAR J 53:E70–E81. 10.1093/ilar.53.1.70. [DOI] [PubMed] [Google Scholar]
- 17.Geffré A, Braun JP, Trumel C, Concordet D. 2009. Estimation of reference intervals from small samples: an example using canine plasma creatinine. Vet Clin Pathol 38:477–484. [DOI] [PubMed] [Google Scholar]
- 18.Geffré A, Concordet D, Braun JP, Trumel C. 2011. Reference value advisor: a new freeware set of macroinstructions to calculate reference intervals with Microsoft Excel. Vet Clin Pathol 40:107–112. 10.1111/j.1939-165X.2011.00287.x. [DOI] [PubMed] [Google Scholar]
- 19.Gustavsson C, Yassin K, Wahlstrom E, Cheung L, Lindberg J, Brismar K, Ostenson CG, Norstedt G, Tollet-Egnell P. 2010. Sex-different hepaticglycogen content and glucose output in rats. BMC Biochem 11:1–17. 10.1186/1471-2091-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harr KE, Flatland B, Nabity M, Freeman KP. 2013. ASVCP guidelines: allowable total error guidelines for biochemistry. Vet Clin Pathol 42:424–436. 10.1111/vcp.12101. [DOI] [PubMed] [Google Scholar]
- 21.Harrison SD, Jr, Burdeshaw JA, Crosby RG, Cusic AM, Denine EP. 1978. Hematology and clinical chemistry reference values for C57BL/6 X DBA/2 F1 mice. Cancer Res 38:2636–2639. [PubMed] [Google Scholar]
- 22.He Q, Su G, Liu K, Zhang F, Jiang Y, Gao J, Liu L, Jiang Z, Jin M, Xie H. 2017. Sex-specific reference intervals of hematologic and biochemical analytes in Sprague-Dawley rats using the nonparametric rank percentile method. PLoS One 12:1–18. 10.1371/journal.pone.0189837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Her Z, Yong KSM, Paramasivam K, Tan WWS, Chan XY, Tan SY, Liu M, Fan Y, Linn YC, Hui KM, Surana U, Chen Q. 2017. An improved pre-clinical patient-derived liquid xenograft mouse model for acute myeloid leukemia. J Hematol Oncol 10:1–14. 10.1186/s13045-017-0532-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen AL, Iversen L, Petersen TK. 1998. Study on biological variability of haematological components in dogs. Comparative Haematology International 8:202–204. 10.1007/BF02752849. [DOI] [Google Scholar]
- 25.Jensen TL, Kiersgaard MK, Mikkelsen LF, Sørensen DB. 2019. Fasting of male mice—Effects of time point of initiation and duration on clinical chemistry parameters and animal welfare. Lab Anim 53:587–597. 10.1177/0023677218824373. [DOI] [PubMed] [Google Scholar]
- 26.Jensen TL, Kiersgaard MK, Sørensen DB, Mikkelsen LF. 2013. Fasting of mice: a review. Lab Anim 47:225–240. 10.1177/0023677213501659. [DOI] [PubMed] [Google Scholar]
- 27.Kile BT, Mason-Garrison CL, Justice MJ. 2003. Sex and strain-related differences in the peripheral blood cell values of inbred mouse strains. Mamm Genome 14:81–85. 10.1007/s00335-002-2160-0. [DOI] [PubMed] [Google Scholar]
- 28.Klempt M, Rathkolb B, Fuchs E, de Angelis MH, Wolf E, Aigner B. 2006. Genotype-specific environmental impact on the variance of blood values in inbred and F1 hybrid mice. Mamm Genome 17:93–102. 10.1007/s00335-005-0119-7. [DOI] [PubMed] [Google Scholar]
- 29.Latger-Cannard V, Hoarau M, Salignac S, Baumgart D, Nurden P, Lecompte T. 2012. Mean platelet volume: comparison of three analyzers towards standardization of platelet morphological phenotype. Int J Lab Hematol 34:300–310. 10.1111/j.1751-553X.2011.01396.x. [DOI] [PubMed] [Google Scholar]
- 30.Layssol-Lamour C, Lavabre T, Braun JP, Trumel C, Bourges-Abella N. 2019. The effects of storage at 4°C and 20°C on the hemograms of C57BL/6 mice and Wistar rats using the IDEXX ProCyte Dx and blood smear evaluations. Vet Clin Pathol 48:652–667. 10.1111/vcp.12784. [DOI] [PubMed] [Google Scholar]
- 31.Linnet K. 1987. Two-stage transformation systems for normalization of reference distributions evaluated. Clin Chem 33:381–386. 10.1093/clinchem/33.3.381. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto K, Inukai S, Isaka T, Aruga N, Nakamura S, Nagasawa T, Naito J. 1995. Cell counts in peripheral blood and bone marrow of male C.B-17 scid/scid mice. Lab Anim 29:218–222. 10.1258/002367795780740230. [DOI] [PubMed] [Google Scholar]
- 33.Mazzaccara C, Labruna G, Cito G, Scarfo M, De Felice M, Pastore L, Sacchetti L. 2008. Age-related reference intervals of the main biochemical and hematological parameters in C57BL/6J, 129SV/EV and C3H/HeJ mouse strains. PLoS One 3:1–7. 10.1371/journal.pone.0003772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDaniel Mims B, Grisham MB. 2018. Humanizing the mouse immune system to study splanchnic organ inflammation. J Physiol 596:3915–3927. 10.1113/JP275325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Messick J. 2006. Blood and bone marrow, p 61–78. In: Eurell JA, Frappier BL, editors. Dellmann's texbook of veterinary histology, 6th ed. Ames (IA): Blackwell publishing. [Google Scholar]
- 36.Moore DM. 2000. Hematology of the mouse (Mus musculus), p 1219–1224. In: Feldman BF, Zinkl JG, Jain NC, editors. Schalm's veterinary hematology, 5th ed. Philadelphia (PA): Lippincott Williams and Wilkins. [Google Scholar]
- 37.O'Connell KE, Mikkola AM, Stepanek AM, Vernet A, Hall CD, Sun CC, Yildirim E, Staropoli JF, Lee JT, Brown DE. 2015. Practical murine hematopathology: a comparative review and implications for research. Comp Med 65:96–113. [PMC free article] [PubMed] [Google Scholar]
- 38.Otto GP, Rathkolb B, Oestereicher MA, Lengger CJ, Moerth C, Micklich K, Fuchs H, Gailus-Durner V, Wolf E, Hrabe de Angelis M. 2016. Clinical Chemistry Reference Intervals for C57BL/6J, C57BL/6N, and C3HeB/FeJ Mice (Mus musculus). J Am Assoc Lab Anim Sci 55:375–386. [PMC free article] [PubMed] [Google Scholar]
- 39.Provencher Bolliger A, Everds NE, Zimmerman KL, Moore DM, Smith SA, Barnhart KF. 2010. Hematology of laboratory animals, p 852–887. Chapter 110. In: Weiss DJ, Wardrop KJ, editors. Schalm's Veterinary Hematology, 6th ed. Ames (IA): Wiley-Blackwell. [Google Scholar]
- 40.Reagan WJ, Irizarry-Rovira A, Poitout-Belissent F, Bolliger AP, Ramaiah SK, Travlos G, Walker D, Bounous D, Walter GBone Marrow Working Group of ASVCP/STP. 2011. Best practices for evaluation of bone marrow in nonclinical toxicity studies. Vet Clin Pathol 40:119–134. 10.1111/j.1939-165X.2011.00323.x. [DOI] [PubMed] [Google Scholar]
- 41.Ricos C, Alvarez V, Cava F, Garcia-Lario JV, Hernandez A, Jimenez CV, Minchinela J, Perich C, Simon M. [Internet]. 2014. Desirable biological variation database specifications. [Cited 10 April 2019]. Available at: https://www.westgard.com/biodatabase1.htm.
- 42.Russell KE. 2010. Platelet kinetics and laboratory evaluation of thrombocytopenia, p. 576–585. In: Weiss DJ, Wardrop KJ, editors. Schalm's Veterinary Hematology, 6th ed. Ames (IA): Wiley-Blackwell. [Google Scholar]
- 43.Saland E, Boutzen H, Castellano R, Pouyet L, Griessinger E, Larrue C, de Toni F, Scotland S, David M, Danet-Desnoyers G, Vergez F, Barreira Y, Collette Y, Recher C, Sarry JE. 2015. A robust and rapid xenograft model to assess efficacy of chemotherapeutic agents for human acute myeloid leukemia. Blood Cancer J 5:1–7. 10.1038/bcj.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scheller EL, Doucette CR, Learman BS, Cawthorn WP, Khandaker S, Schell B, Wu B, Ding SY, Bredella MA, Fazeli PK, Khoury B, Jepsen KJ, Pilch PF, Klibanski A, Rosen CJ, MacDougald OA. 2015. Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat Commun 6:1–2. https://doi.org/10.1038/ncomms8808. Erratum in Correction: Corrigendum: Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. 2016. Nat Commun 7:13775. https://doi.org/ 10.1038/ncomms13775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schnell MA, Hardy C, Hawley M, Propert KJ, Wilson JM. 2002. Effect of blood collection technique in mice on clinical pathology parameters. Hum Gene Ther 13:155–161. 10.1089/10430340152712700. [DOI] [PubMed] [Google Scholar]
- 46.Shultz LD, Goodwin N, Ishikawa F, Hosur V, Lyons BL, Greiner DL. 2014. Human cancer growth and therapy in immunodeficient mouse models. Cold Spring Harb Protoc 2014:694–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, Greiner DL, Handgretinger R. 2005. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol 174:6477–6489. 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 48.Tabata H, Kitamura T, Nagamatsu N. 1998. Comparison of effects of restraint, cage transportation, anaesthesia and repeated bleeding on plasma glucose levels between mice and rats. Lab Anim 32:143–148. 10.1258/002367798780599983. [DOI] [PubMed] [Google Scholar]
- 49.The Jackson Laboratory. [Internet]. 2019. Physiological data summary—NOD.Cg-Prkdc scid IL2rgtm1Wjl/SzJ (005557). [Cited 20 December 2019]. Available at: http://jackson.jax.org/rs/444-BUH-304/images/physiological_data_005557.pdf
- 50.Travlos GS. 2006. Histopathology of bone marrow. Toxicol Pathol 34:566–598. 10.1080/01926230600964706. [DOI] [PubMed] [Google Scholar]
- 51.Walsh NC, Kenney LL, Jangalwe S, Aryee KE, Greiner DL, Brehm MA, Shultz LD. 2017. Humanized mouse models of clinical disease. Annu Rev Pathol 12:187–215. 10.1146/annurev-pathol-052016-100332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.White JR, Gong H, Colaizy TT, Moreland JG, Flaherty H, McElroy SJ. 2015. Evaluation of hematologic variables in newborn C57/BL6 mice up to day 35. Vet Clin Pathol 45:87–95. 10.1111/vcp.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang M, Büsche G, Ganser A, Li Z. 2013. Cytological characterization of murine bone marrow and spleen hematopoietic compartments for improved assessment of toxicity in preclinical gene marking models. Ann Hematol 92:595–604. 10.1007/s00277-012-1655-3. [DOI] [PubMed] [Google Scholar]
- 54.Zhou X, Hansson GK. 2004. Effect of sex and age on serum biochemical reference ranges in C57BL/6J mice. Comp Med 54:176–178. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Results of QC of hematology analysis using the Procyte Dx and the XT 2000iV analyzers
Table S2. Results of QC of biochemical analysis using the Vetscan VS2 analyzer.
Table S3. Reference Intervals for RBC, reticulocyte, total WBC and PLT counts in NSG male and female mice measured with an IDEXX ProCyte Dx and a Sysmex XT-2000iV analyzers.
Table S4. Reference Intervals for WBC DIFF counts in NSG male and female mice measured with a IDEXX ProCyte Dx analyzer (P) and by a microscopic count (M).
Table S5. Reference Intervals for biochemical analytes measured by the VetScan VS2 analyzer in NSG male and female mice.
Table S6. Reference Intervals for cell percentages, ratio and indexes for bone marrow aspiration in NSG male (n = 20) and female (n = 20) mice.
Table S7. Reference Intervals for megakaryocytes and adipocytes counts in diaphysis and metaphysis of femoral bone marrow of NSG male (n = 20) and female (n = 20) mice.