Abstract
Measurement of intraocular pressure (IOP) is a standard procedure in ophthalmic research in animals, specifically in glaucoma research, and the control of IOP is essential during certain veterinary ophthalmic surgeries. We evaluated the effect of isoflurane on IOP in the clinically healthy laboratory rabbits and tested a way to minimize the alteration of IOP during isoflurane anesthesia. After measurement of the baseline IOP in each eye of 9 awake New Zealand white rabbits, animals were anesthetized by using either: (1) isoflurane without premedication, (2) a combination of ketamine and xylazine, or (3) isoflurane inhalation after an injection of ketamine–xylazine premedication. Isoflurane led to a sustained increase in IOP of approximately 12 mm Hg. In contrast, ketamine and xylazine decreased IOP by nearly 5 mm Hg (all values compared with baseline measurements in awake, unrestrained animals). The observed decrease in IOP after ketamine–xylazine anesthesia is consistent with anesthetic effects generally seen during anesthesia in other studies. The increased IOP after isoflurane anesthesia in rabbits in this study was an unexpected result that appears to be specific to this combination of anesthetic and animal species. Premedication with ketamine–xylazine diminished the effect of isoflurane inhalation on IOP. These results should be considered in the design of ophthalmic research studies using rabbits and in intraocular surgery where IOP stability is desired.
Abbreviation: IOP, intraocular pressure
The measurement of intraocular pressure (IOP) is an important procedure to ensure ocular health in both clinical and research settings. IOP values in the normal range indicate a low risk of glaucoma and severe uveitis, and reliable IOP measurement is a critical component of glaucoma studies, ocular toxicity tests, and other preclinical studies. Elevated IOP is a significant risk factor for glaucoma, which is the leading cause of irreversible blindness in the world. An estimated 75 million people are projected to have this disease in 2020.32 Current glaucoma treatments focus on lowering IOP to delay or stop vision loss. Research into the pathophysiology of glaucoma relies extensively on animal models in which IOP is elevated, leading to ocular changes that mimic glaucomatous optic neuropathy. A critical aspect of such models is the measurement of IOP during experimental studies, which often are conducted on anesthetized laboratory animals.
Anesthesia and associated drugs are well known to affect IOP in both humans10,26 and animals,11,15,33 thus potentially complicating the interpretation of IOP measurements in experimental studies. Isoflurane is an inhaled anesthetic used for general anesthesia. It is considered a primary choice as an inhaled anesthetic for animals because it allows rapid recovery from anesthesia and near real-time control of the plane of anesthesia. In addition, combined administration of ketamine and xylazine is widely used for inducing and maintaining anesthesia both in veterinary clinics and in research.28 This combination of medicine is easy to administer and provides a stable plane of anesthesia. Generally, depressants of the CNS reduce IOP. However, a dissociative anesthetic, ketamine,10,15,33 increases IOP when administered alone and thus may be contraindicated for ophthalmic surgeries where low or normal IOP is desirable.10 Xylazine is a selective α2-agonist that reduces IOP by suppressing sympathetic neuronal function which causes a reduction in aqueous flow.6 Combining xylazine with ketamine causes the ketamine-induced elevation of IOP to be reduced and even reversed.7,16
Although New Zealand white rabbits are a very widely used laboratory animal in ocular research and pharmacologic testing, the effects of isoflurane on IOP in this species have not been reported. In the current study, we found that isoflurane increases IOP in clinically healthy laboratory rabbits without premedication and that premedication with a mixture of ketamine and xylazine counteracts the increased IOP that occurs during isoflurane inhalation.
Materials and Methods
Because the tonometer (TonoVet, iCare, Espoo, Finland) that we used in this study was designed for use in dogs and cats, we first performed a calibration procedure by using 5 fresh, ex vivo rabbit eyes according to a modified method from a previous study.22,31 A 25-gauge needle was inserted through the sclera 2 to 3 mm posterior to the limbus, angled such that the tip passed anterior to the iris and entered into the anterior chamber. We connected a needle to a reservoir containing a balanced salt solution that could be manipulated to set IOP. Tonometric measurements were collected as IOP was set at levels from 5 to 20 mm Hg in increments of 5 mm Hg; the resulting data were used for calculating a calibration curve.
Next, we evaluated both eyes in 9 SPF New Zealand white rabbits (Oryctolagus cuniculi; Charles River Breeding Laboratories, Wilmington, MA; male and female; weight, 3.0 to 3.5 kg). All procedures were approved by the Georgia Institute of Technology IACUC and followed the ARVO statement for the Use of Animals in Ophthalmic and Vision Research.2 Rabbits were housed in an animal facility at Georgia Institute of Technology in compliance with the Guide for the Care and Use of Laboratory Animals.17 These animals had not been involved in a previous study nor had they received prior treatments that could interfere with IOP measurement. The animals were housed in individual cages at a controlled temperature of 19.0 ± 0.8 °C and humidity of 6 ± 9% on a 12:12-h light:dark cycle. All rabbits were given ad libitum access to food and water for preventing dehydration effects on IOP.5 Food and water uptake, and urine and stool production were observed daily. After the rabbits completed a 7-d acclimation period, all eyes were confirmed to be clinically normal by routine ophthalmic examination before starting the experiment. All tonometric measurements in this study were performed by an experienced veterinarian (JJC) without assistance. All IOPs were measured in awake rabbits by using a hand-held rebound tonometer (TonoVet, iCare) operated in the ‘d’ mode, with 5 consecutive measurements being averaged to obtain a single value. Topical anesthetic was not used during IOP measurement.
Baseline IOP in all eyes was obtained by averaging a single measurement every hour over 12 h (from 0900) daily for 5 d (that is, 60 measurements per rabbit). During the baseline measurement, no sedative agents or restraints were used. Each rabbit was carefully removed from the cage by using a towel and placed on a table in a quiet room where the animal was normally housed.
As soon as the rabbit was crouching without moving on the table, the investigator's thumb and index finger were applied to the cheek and forehead, respectively. The other 3 fingers were used for fixing the head of the rabbit, and the tonometer was applied to the central cornea by using the other hand. After baseline IOP measurements, rabbits were separated into 3 groups of 3 animals each and anesthetized as follows. All IOP measurements under anesthesia, for all groups, were obtained with the animal in a sternal recumbent position. All procedures involving anesthesia were performed between 0900 and 1200, and all IOP measurements were performed between 0900 and 2100. Corneal hydration was maintained by applying 2 drops of ocular lubricant (Refresh Tears, Allergan, Irvine, CA) before the loss of the Purkinje images.
Rabbits in the first group (1 male, 2 females; weight, 3.3 ± 0.1 kg) received only inhaled isoflurane. After placement of the animal in an induction chamber (model 90100, Bickford, Wales Center, NY), 5% isoflurane (Isothesia, Henry Schein, Melville, NY) was delivered by using an anesthesia machine and isoflurane vaporizer (Isotec4, Surgivet, Dublin, OH), with an oxygen flow rate of 400 mL/min maintained at all times, unless otherwise specified. After removal of the rabbit from the induction chamber, a nose cone was promptly applied, and the concentration of the isoflurane was gradually decreased from 5% to 3% over 7.5 min. The isoflurane concentration was then kept at 3% for 1 h to maintain anesthesia, after which the nose cone was removed, and the rabbit was allowed to recover. The rabbit's IOP was measured before induction; at 7.5, 15, 30, 45, and 75 min after induction; and at 3, 5, 10, 15, 30 min, and 1, 2, and 3 h after removing the nose cone.
Rabbits in the second group (1 male, 2 females; weight, 3.3 ± 0.1 kg) received a subcutaneous injection of a mixture of ketamine (25 mg/kg, Ketathesia, Henry Schein) and xylazine (5 mg/kg, AnaSed Injection, Akorn, Lake Forest, IL). The IOP value was measured before injection; at 15 and 30 min after injection, and then hourly for 8 h.
Animals in the third group (3 females; weight, 3.3 ± 0.1 kg) received both injectable premedication and inhalational isoflurane maintenance in a protocol designed to avoid significant IOP changes. First, rabbits received subcutaneous premedication consisting of a mixture of ketamine (10 mg/kg) and xylazine (2 mg/kg). After 7.5 min, a nose cone was applied to deliver isoflurane, with the concentration increasing gradually from 1% to 3% over 7.5 min, after which the concentration of anesthesia was maintained at 3% for 1 h. Then the nose cone was removed. The IOP was measured before and at 7.5, 15, 30, 45, and 75 min after injection; at 3, 5, 10, 15, and 30 min after completing the anesthesia procedure; and then hourly for 6 h.
The data obtained from the tonometer were converted to the actual IOP by using the relationship obtained from the calibration study, namely y = 0.8784x – 1.26 (R2 =0.98; x, reservoir pressure in mm Hg; y, tonometric measurement in mm Hg; Figure 1). Data are presented as mean values ± 1 SD. Repeated-measures ANOVA was performed to determine statistical significance of differences between postanesthetic and baseline IOP by using SPSS 22.0 for Windows (SPSS, Chicago, IL). The threshold for statistical significance was taken as a P value of less than 0.05.
Figure 1.
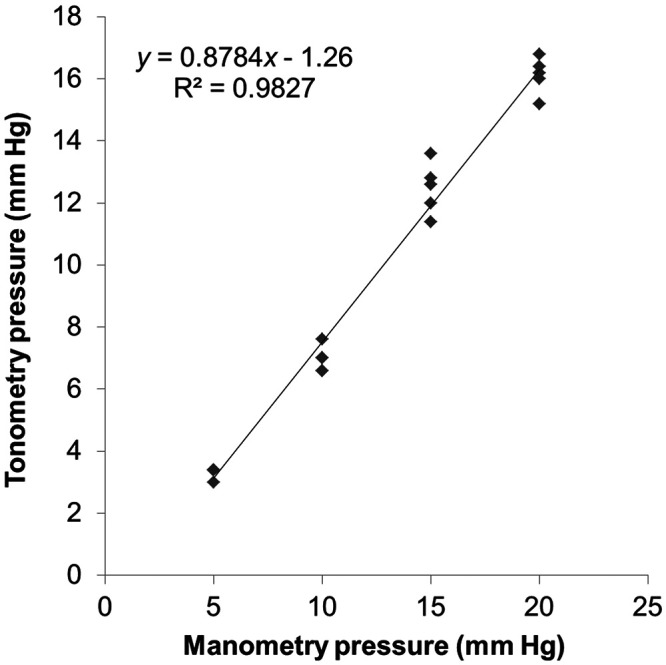
Calibration curve for the TonoVet rebound tonometer by using direct manometry. IOP values by rebound tonometry showed a strong linear correction with the manometrically set pressure. Five enucleated rabbit eyes were used for calibration. Each data point presents a measurement, with some points overlapping. The linear regression line is shown as a solid line.
Results
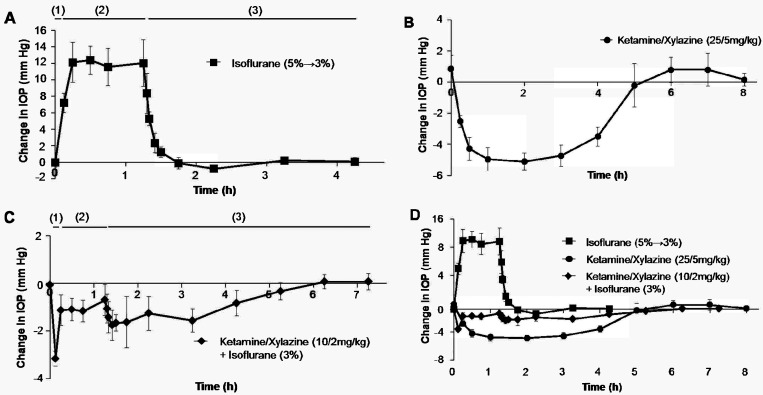
The baseline IOP was 14.4 ± 1.0 mm Hg in rabbits anesthetized with isoflurane only, 13.9 ± 0.9 in those given subcutaneous ketamine and xylazine only, and 13.1 ± 1.0 mm Hg in animals that received ketamine–xylazine premedication followed by isoflurane anesthesia (overall average, 13.8 ± 1.1 mm Hg). Rabbits that received inhaled isoflurane only showed an almost immediate increase (P < 0.001) in IOP of 12.0 ± 2.2 mm Hg over baseline during the maintenance period; this increased IOP quickly (within 30 min) reversed to baseline levels after isoflurane was discontinued (Figure 2 A). In the second group, the ketamine–xylazine mixture decreased (P < 0.001) IOP for 4 h, with a peak reduction of 5.1 ± 0.6 mm Hg occurring 2 h after injection; IOP then slowly recovered and returned to baseline within 5 h (Figure 2 B). In rabbits that received injectable premedication and then inhalant isoflurane, IOP dropped (P < 0.001) rapidly by 3.5 ± 0.3 mm Hg by 7.5 min after ketamine–xylazine injection; this decrease was reversed (P = 0.045) to a value of approximately 1 mm Hg below baseline IOP on initiation of isoflurane administration. After isoflurane administration was stopped, IOP decreased again and then gradually recovered to baseline over 4 h (Figure 2 C).
Figure 2.
Effect of general anesthetics on the intraocular pressure in healthy rabbit eyes. (A) Isoflurane was delivered to the rabbit by using an induction chamber (5%) and a nose cone (3%) for induction and maintenance, respectively. (B) A combination of ketamine and xylazine (25 and 5 mg/kg, respectively) was injected subcutaneously. (C) A combination of ketamine and xylazine (10 and 2 mg/kg, respectively) was administrated subcutaneously as a premedication, and isoflurane (3%) administered by nose cone was used for maintenance. (D) The summary of all data from panels A through C are shown on a single plot. The numbers indicate the stage of anesthesia: (1) induction, (2) maintenance, and (3) recovery. Results are presented as mean ± 1 SD from 6 rabbit eyes per group.
Discussion
We conclude from our current data that isoflurane anesthesia can significantly increase IOP in the eyes of normal laboratory rabbits and that ketamine–xylazine anesthesia can significantly decrease IOP in these animals. Furthermore, these IOP changes can be minimized by using a combination of ketamine–xylazine premedication, followed by isoflurane anesthesia.
The isoflurane-induced increase in IOP in rabbits is opposite to that reported in prior studies in rodents, in which IOP decreases of 45.3% occurred in normal mice,11 and decreases of approximately 11 mm Hg were seen in glaucomatous rats.19 This IOP-lowering effect of isoflurane has been suggested to result from hemodynamic changes.4,21 The different effects we observed in rabbits as compared with prior work in rodents may reflect differences in species as well as in anesthetic procedures, depth of anesthesia, or preexisting glaucomatous factors.
Anesthesia involving isoflurane has been reported to lower IOP, but in those studies,1,4,20,21 isoflurane administration was preceded by premedication (that is, as in group 3 of our study), with the exception of research on normotensive nonhuman primates.18,21,29 Those studies were conducted in normal dogs premedicated with xylazine and thiopental,20 pigs premedicated with thiopental,4 human children premedicated with pentobarbital,1 and glaucomatous NHP premedicated with ketamine.21 These findings are generally consistent with our observations in rabbits exposed to both isoflurane and ketamine–xylazine premedication, in which IOP increases caused by isoflurane were counteracted by premedication with the other agents. However, in normotensive NHP, the effect of premedication (ketamine or ketamine–xylazine) and isoflurane inhalation on IOP was insignificant throughout the procedure.18,21,29 Premedication with ketamine and dexmedetomidine decreased IOP in normotensive NHP, but the effect on IOP due to isoflurane was not significant.18 This pattern of changes has been reported only in NHP, suggesting species specificity. We were unable to find literature reports on IOP changes in rabbits anesthetized with isoflurane with or without premedication, rodents anesthetized with isoflurane and premedication, or other animals anesthetized with isoflurane alone.
Identifying the mechanism of IOP elevation due to isoflurane was well beyond the scope of our study. Inhaled anesthetics could increase IOP by affecting the midbrain, altering blood pressure and, therefore IOP, or by changing intra- and extraocular muscle tone.26 Trichloroethylene, another inhaled anesthetic, was reported to increase IOP by increasing central venous pressure,30 and we speculate this mechanism may also be the case for isoflurane. Alternatively, obstruction of blood flow by the nose cone could explain our findings. However, the cone used in this study was fitted with a very soft rubber gasket and thus was unlikely to compress facial blood vessels; furthermore, rabbits have no significant superficial veins in the face that might increase IOP by compression.8
Elevation of IOP is usually undesirable during intraocular surgeries because it increases the risk for severe consequences such as vitreous prolapse or retinal detachment.10 By adding premedication (that is, a combination of ketamine and xylazine), we found that the IOP elevation due to isoflurane was preventable, thus likely reducing the risk of surgical complications in rabbits. Other premedications could also be considered for this role. However, the combination of diazepam or midazolam with ketamine as premedication is unlikely to decrease IOP or to do in a short time, as was shown in dogs14,15 and sheep.13 Alternatively, the effect of isoflurane to elevate IOP could be used to create a transient ocular hypertension model in the rabbit if a well-equipped monitoring and control system could use isoflurane could cause high IOP for a prolonged time. However, the interpretation of any results from such a model would have to account for any additional effects (that is, other than increasing IOP) of extended anesthesia.
An acute stress response due to handling, tonometry, and restraint can increase IOP in awake animals.12,18,24,25,34 To address this issue, we carefully designed this study to minimize such stress. All animals were handled by a well-trained veterinarian, who treated the rabbits gently at all times and who measured IOP in these animals many times to accustom them to the procedure. The housing environment was well-controlled, and tonometry was performed in a quiet room where the rabbits were normally housed, without extraneous personnel present. The rebound tonometer was used without a restraint box or topical anesthesia, which can cause stress and alter IOP;3,25 this procedure was feasible for the New Zealand white rabbits we used due to their docile and calm nature. Finally, repeated measurements (60 per animal) were taken to establish a baseline IOP, and these sessions served as a training regimen to acclimate animals to IOP measurement and thus avoid stress-induced ocular hypertension. These baseline IOP values were consistent over time (variation of only around 1 mm Hg), further suggesting that IOP measurements were well tolerated; otherwise, we would have expected to see them decline over time as the animals become acclimated to the procedure.
The average baseline IOP for the rabbits in this study (13.8 ± 1.1 mm Hg) was consistent with prior studies, which reported baseline IOP values between 12.2 to 13.5 mm Hg9,23,27 without anesthesia but lower than earlier reports of IOP (15 to 23 mm Hg) in albino rabbits.35
In summary, we found that the widely used inhalation anesthetic agent isoflurane significantly increased IOP in laboratory rabbits and that this alteration of IOP could be minimized by using a preinjection of ketamine and xylazine. These findings will be of use to investigators using rabbits in ophthalmic research, including glaucoma, ocular toxicology, and preclinical studies and potentially during ocular surgery in companion rabbits.
Acknowledgments
This work was supported by a grant from the National Eye Institute (R01 EY025286) and the Georgia Research Alliance (CRE). We thank Jeff Kiel and Brian Samuels for helpful discussions and Donna Bondy for administrative support.
References
- 1.Ausinsch B, Graves SA, Munson ES, Levy NS. 1975. Intraocular pressures in children during isoflurane and halothane anesthesia. Anesthesiology 42:167–172. 10.1097/00000542-197502000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Association for Research in Vision and Ophthalmology. [Internet]. 2016. Statement for the use of animals in ophthalmic and vision research. [Cited 21 August 2019]. Available at: www.arvo.org
- 3.Baudouin C, Gastaud P. 1994. Influence of topical anesthesia on tonometric values of intraocular pressure. Ophthalmologica 208:309–313. 10.1159/000310527. [DOI] [PubMed] [Google Scholar]
- 4.Buehner E, Pietsch UC, Bringmann A, Foja C, Wiedemann P, Uhlmann S. 2011. Effects of propofol and isoflurane anesthesia on the intraocular pressure and hemodynamics of pigs. Ophthalmic Res 45:42–46. 10.1159/000317060. [DOI] [PubMed] [Google Scholar]
- 5.Bunch TJ, Tian B, Seeman JL, Gabelt BT, Lin TL, Kaufman PL. 2008. Effect of daily prolonged ketamine anesthesia on intraocular pressure in monkeys. Curr Eye Res 33:946–953. 10.1080/02713680802447121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burke JA, Potter DE. 1986. The ocular effects of xylazine in rabbits, cats, and monkeys. J Ocul Pharmacol 2:9–21. 10.1089/jop.1986.2.9. [DOI] [PubMed] [Google Scholar]
- 7.Camras LJ, Sufficool KE, Camras CB, Fan S, Liu H, Toris CB. 2010. Duration of anesthesia affects intraocular pressure, but not outflow facility in mice. Curr Eye Res 35:819–827. https://doi.org/10.3109/02713683.2010.494241. [DOI] [PubMed] [Google Scholar]
- 8.Capello V, Gracis M. 2005. Anatomy of the skull and teeth (rabbit), p 8–17. Chapter 2. In: Capello V, editor. Rabbit and rodent dentistry handbook, vol 1. Lake Worth (FL): Wiley [Google Scholar]
- 9.Chiang B, Kim YC, Doty AC, Grossniklaus HE, Schwendeman SP, Prausnitz MR. 2016. Sustained reduction of intraocular pressure by supraciliary delivery of brimonidine-loaded poly(lactic acid) microspheres for the treatment of glaucoma. J Control Release 228:48–57. 10.1016/j.jconrel.2016.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham AJ, Barry P. 1986. Intraocular pressure–physiology and implications for anaesthetic management. Can Anaesth Soc J 33:195–208. 10.1007/BF03010831. [DOI] [PubMed] [Google Scholar]
- 11.Ding C, Wang P, Tian N. 2011. Effect of general anesthetics on IOP in elevated IOP mouse model. Exp Eye Res 92:512–520. 10.1016/j.exer.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinslage S, McLaren J, Brubaker R. 1998. Intraocular pressure in rabbits by telemetry II: effects of animal handling and drugs. Invest Ophthalmol Vis Sci 39:2485–2489. [PubMed] [Google Scholar]
- 13.Gatson BJ, Pablo L, Plummer CE, Granone TD. 2015. Effects of premedication with sustained-release buprenorphine hydrochloride and anesthetic induction with ketamine hydrochloride or propofol in combination with diazepam on intraocular pressure in healthy sheep. Am J Vet Res 76:771–779. 10.2460/ajvr.76.9.771. [DOI] [PubMed] [Google Scholar]
- 14.Ghaffari MS, Rezaei MA, Mirani AH, Khorami N. 2010. The effects of ketamine-midazolam anesthesia on intraocular pressure in clinically normal dogs. Vet Ophthalmol 13:91–93. 10.1111/j.1463-5224.2010.00762.x. [DOI] [PubMed] [Google Scholar]
- 15.Hofmeister EH, Mosunic CB, Torres BT, Ralph AG, Moore PA, Read MR. 2006. Effects of ketamine, diazepam, and their combination on intraocular pressures in clinically normal dogs. Am J Vet Res 67:1136–1139. 10.2460/ajvr.67.7.1136. [DOI] [PubMed] [Google Scholar]
- 16.Holve DL, Gum GG, Pritt SL. 2013. Effect of sedation with xylazine and ketamine on intraocular pressure in New Zealand white rabbits. J Am Assoc Lab Anim Sci 52:488–490. [PMC free article] [PubMed] [Google Scholar]
- 17.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 18.Jasien JV, Girkin CA, Downs JC. 2019. Effect of anesthesia on intraocular pressure measured with continuous wireless telemetry in nonhuman primates. Invest Ophthalmol Vis Sci 60:3830–3834. 10.1167/iovs.19-27758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jia L, Cepurna WO, Johnson EC, Morrison JC. 2000. Effect of general anesthetics on IOP in rats with experimental aqueous outflow obstruction. Invest Ophthalmol Vis Sci 41:3415–3419. [PubMed] [Google Scholar]
- 20.Kılıç S, Ünsaldi S. 2009. Effects of isoflurane and enflurane on ocular parameters in dogs. YYU Vet Fak Derg 20:1–3. [Google Scholar]
- 21.Kim J, Sapp HL, Jr, Plummer CE, Brooks DE, Kim D, Kim MS. 2012. IOP change undergoing anesthesia in rhesus macaques (Macaca mulatta) with laser-induced ocular hypertension. J Vet Med Sci 74:1359–1361. 10.1292/jvms.12-0059. [DOI] [PubMed] [Google Scholar]
- 22.Kim YC, Edelhauser HF, Prausnitz MR. 2014. Targeted delivery of antiglaucoma drugs to the supraciliary space using microneedles. Invest Ophthalmol Vis Sci 55:7387–7397. 10.1167/iovs.14-14651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma D, Chen CB, Liang J, Lu Z, Chen H, Zhang M. 2016. Repeatability, reproducibility and agreement of intraocular pressure measurement in rabbits by the TonoVet and Tono-Pen. Sci Rep 6:1–7. 10.1038/srep35187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Millar JC, Pang IH. 2015. Non-continuous measurement of intraocular pressure in laboratory animals. Exp Eye Res 141:74–90. 10.1016/j.exer.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 25.Miyazaki Y, Matsuo T, Kurabayashi Y. 2000. Immobilization stress induces elevation of intraocular pressure in rabbits. Ophthalmic Res 32:270–277. 10.1159/000055625. [DOI] [PubMed] [Google Scholar]
- 26.Murphy DF. 1985. Anesthesia and intraocular pressure. Anesth Analg 64:520–530. 10.1213/00000539-198505000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Paschalis EI, Cade F, Melki S, Pasquale LR, Dohlman CH, Ciolino JB. 2014. Reliable intraocular pressure measurement using automated radio-wave telemetry. Clin Ophthalmol 8:177–185. 10.2147/OPTH.S54753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajaei SM, Mood MA, Paryani MR, Williams DL. 2017. Effects of diurnal variation and anesthetic agents on intraocular pressure in Syrian hamsters (Mesocricetus auratus). Am J Vet Res 78:85–89. 10.2460/ajvr.78.1.85. [DOI] [PubMed] [Google Scholar]
- 29.Raposo AC, Ofri R, Schaffer DP, Gomes Júnior DC, Libório FA, Martins Filho EF, Oriá AP. 2015. Evaluation of ophthalmic and hemodynamic parameters in capuchin monkeys (Sapajus sp.) submitted to dissociative anesthetic protocols. J Med Primatol 44:381–389. 10.1111/jmp.12200. [DOI] [PubMed] [Google Scholar]
- 30.Schreuder M, Linssen GH. 1972. Intra-ocular pressure and anaesthesia. Direct measurements by needling the anterior chamber in the monkey. Anaesthesia 27:165–170. 10.1111/j.1365-2044.1972.tb08192.x. [DOI] [PubMed] [Google Scholar]
- 31.Stockslager MA, Samuels BC, Allingham RR, Klesmith ZA, Schwaner SA, Forest CR, Ethier CR. 2016. System for rapid, precise modulation of intraocular pressure, toward minimally-invasive in vivo measurement of intracranial pressure. PLoS One 11:1–15. 10.1371/journal.pone.0147020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. 2014. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 121:2081–2090. 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 33.Trim CM, Colbern GT, Martin CL. 1985. Effect of xylazine and ketamine on intraocular pressure in horses. Vet Rec 117:442–443. 10.1136/vr.117.17.442. [DOI] [PubMed] [Google Scholar]
- 34.Turner DC, Miranda M, Morris JS, Girkin CA, Downs JC. 2019. Acute stress increases intraocular pressure in nonhuman primates. Ophthalmol Glaucoma 2:210–214. 10.1016/j.ogla.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vareilles P, Conquet P, Le Douarec JC. 1977. A method for the routine intraocular pressure (IOP) measurement in the rabbit: range of IOP variations in this species. Exp Eye Res 24:369–375. 10.1016/0014-4835(77)90149-X. [DOI] [PubMed] [Google Scholar]