Abstract
Ammonia control is an important characteristic of rodent bedding materials. Among natural bedding materials, corncob bedding provides excellent ammonia control but contains estrogenic compounds and is ingested by mice. By comparison, processed cellulose bedding products are biologically inert and harbor fewer bacteria but historically have shown low absorbency or poor ammonia control. New cellulose products have been developed to address these shortcomings. Over a 2-wk period, we evaluated intracage ammonia levels in mouse IVC using 4 bedding types: shaved aspen, corncob, virgin pelleted cellulose, and refined virgin diced cellulose. Ammonia levels were measured by using 3 methods: colored reagent tubes, colorimetric paper strips, and a photoionization detector. Corncob, pelleted cellulose, and diced cellulose showed better ammonia control than aspen as early as 4 d after cage changing and throughout the 2-wk measurement period. In addition, pelleted and diced cellulose products resulted in lower ammonia levels than corncob at the end of the 14-d cage-change interval. Our data indicate that pelleted or refined diced cellulose are viable alternatives to natural bedding products in IVC to limit the risk of exposure of mice to high ammonia levels.
Abbreviation: PID, photoionization detector
Control of intracage ammonia is a vital component of mouse bedding. Increased ammonia levels have been associated with impaired respiratory physiology, immune function, and hepatic microsomal enzyme activity5 as well as with increased severity of Mycoplasma pulmonis infection.3,19 Ammonia levels are also an important consideration in microenvironmental air quality and bedding change frequency.9
Mouse bedding materials have traditionally consisted of processed plant material, recycled or synthetic paper, and cellulose products. Hardwood beddings such as shaved aspen provide a natural material that is light and soft and allows for burrowing and nest building, thus aiding in thermoregulation.8 However, wood beddings generally display poor fluid absorbency4 and ammonia control.16 In comparison, pelleted corncob is often selected for its availability, high absorbency, ability to minimize ammonia levels, and low cost. In terms of ammonia control, corncob has consistently been shown to perform better than wood6,16 or cellulose-based bedding products11. Therefore, corncob is often recommended for use in static cages or with extended cage-change intervals.7
Despite the benefits of corncob bedding, concerns arise regarding its potential biologic effects. Standard processing involves primarily the pelletizing of corncob material and does not eliminate microorganisms. Therefore, irradiation or autoclaving are needed to avoid contamination from bacteria,25 viruses,18 fungi17 and parasites.12 Corncob bedding contains high levels of estrogenic compounds.20 These compounds may induce endocrine disruption with implications for breast and prostatic cancer models13 or alter rodents’ behavior.21 These findings may have broad implications for studies involving behavioral testing or estrogen-dependent pathways. Furthermore, mice may ingest corncob bedding, which contains digestible material and can affect feed-conversion efficiency in high-fat diet studies.1
Processed cellulose products are generally softer than corncob, have good absorbency compared with unrefined wood products, and are devoid of digestible material or estrogenic compounds.13 Standard processing of cellulose products usually involves high temperatures and chemical extractants that result in reduced endotoxin content and coliform counts compared with natural bedding products.14 However, prior studies reported that, compared with corncob, cellulose bedding provided poor long-term ammonia control.11
Some attempts have been made to manufacture cellulose bedding with improved ammonia control characteristics. Strategies have included pelleting, which provides added absorbency,2 and refined pulping techniques targeted to reduce ammonia levels.15 To assess these new bedding products, we used a crossover design to compare the ammonia levels in cages containing aspen shavings, corncob, virgin pelleted cellulose, or refined virgin diced cellulose. Several methods have previously been used to assess intracage ammonia levels. The 3 primary modalities have been colorimetric paper, color-based reagents, and photoionization devices (PID).14 We applied all of these strategies to the same group of animals to provide a more robust collection of data, increase confidence in results, and reduce the number of animals needed to repeat experiments with different modalities. We hypothesized that the novel cellulose materials would perform better than aspen and corncob with regard to microenvironmental ammonia control.
Materials and Methods
All activities were approved by the Baylor College of Medicine IACUC and performed in accordance with the Guide for the Care and Use of Laboratory Animals.9 Findings are reported in accordance with the ARRIVE guidelines.10
Animals and caging.
All animals were adult male outbred Crl:CD1(ICR) mice. Mice were bred from an established inhouse colony with foundation stock originating from Charles River Laboratories (Wilmington, MA). All mice were weighed 3 d prior to beginning the experiment.
Facilities used for housing included a dirty-bedding sentinel program, in which sentinels consistently tested negative for mouse hepatitis virus, mouse rotavirus, lymphocytic choriomeningitis virus, ectromelia virus, mouse parvovirus, minute virus of mice, pneumonia virus of mice, reovirus type 3, Sendai virus, Theiler mouse encephalomyelitis virus, mouse adenovirus, K virus, polyomavirus, mouse cytomegalovirus, mouse thymic virus, Haantan virus, and Mycoplasma pulmonis. In addition, sentinels were negative for parasites by fecal flotation, anal tape test, and fur-mite examination.
Mice were housed in polycarbonate cages (7.5 in. × 11.75 in. × 5 in.; Allentown Caging, Allentown, NJ). Cages were all equipped with polycarbonate lids including polyester microisolation filter inserts. Cage lids were left in place throughout the measurement period. Cages were individually ventilated in an IVC rack (Micro-VENT rack, model MA0JV140DD, Allentown). Rack air supply was 100% HEPA-filtered outside air with dampers calibrated to 60 air changes hourly per cage. Light cycle was automated for a 12:12-h light:dark cycle. Mice were given standard irradiated feed (LabDiet 5V5R, PMI Nutrition International, St Louis, MO) and filtered water containing 0.5 to 0.6 ppm chlorine dioxide (Quiptrol 3000, Quip Laboratories, Wilmington, DE) at point of use ad libitum. The holding area was supplied with 100% HEPA-filtered outside air at 10 to 15 air changes hourly. Macroenvironmental temperature and humidity remained relatively stable throughout the study (68.9 to 70.0 °F [20.5 to 21.1 °C] and 41% to 54%, respectively).
Twenty cages were used, with 4 mice per cage. Mice were divided into 4 groups of 5 cages each. To preclude variability between groups, a crossover design was used in which each group was exposed to each bedding type for a period of 2 wk. Groups were assigned randomly at the beginning of the study and then rotated every 2 wk. At the time of rotation, mice were placed into clean cages, with each group moved to a different bedding material, followed by ammonia measurements. This crossover design yielded a total of 20 data points daily for each bedding material.
Bedding materials.
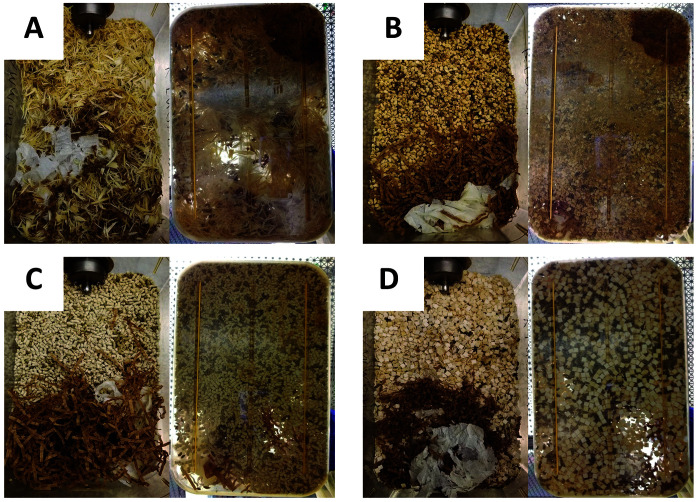
Four bedding materials were evaluated in this study: kiln-dried aspen shavings (Northeastern Products, Warrensburg, NY; Figure 1 A); 1/4-in. corncob (Bed-o’ Cobs, The Andersons, Maumee, OH; Figure 1 B); 1/8-in. pelleted cellulose (BioFresh, Ferndale, WA; Figure 1 C); and a refined virgin diced cellulose product (Alpha-Dri 2.0, Shepherd Specialty Papers, Watertown, TN; Figure 1 D). The diced cellulose product is refined by using iron or copper during the pulping process, which is later removed from the final product.15 Because autoclaving has been shown to affect intracage humidity and temperature,23 cages were not subject to autoclaving, and all of the bedding was irradiated. Each cage was filled to a target volume, consistent with our institution's standard practices. Before study initiation, 5 cages of each bedding type were bedded to the standard level. Next, the bedding was weighed, and we calculated the average weight used for each cage. This amount was used throughout the remainder of the study. Because the bedding types differed in density, the weights used were proportional to their density: 33 g of aspen shavings, 180 g of corncob, 140 g of pelleted cellulose, and 155 g of diced cellulose.
Figure 1.
Bedding types. Bedding types are pictured in bedded cages. The 4 bedding materials used were (A) shaved aspen, (B) 1/4-in. corncob, (C) 1/8-in. pelleted cellulose, and (D) refined virgin diced cellulose.
Ammonia measurements.
On Monday morning (day 0), all cages were changed. Ammonia levels were measured immediately after cage change and on Monday, Wednesday, and Friday of the next 2 wk, providing measurements on days 0, 2, 4, 7, 9, 11, and 14 after cage change.
Ammonia levels were measured in each cage by using each of 3 measuring devices. Colorimetric paper (Hydrion Test Papers, Micro Essential Lab, Brooklyn, NY) was used by inserting paper strips (approximately 1.0 cm × 1.0 cm) through the water bottle port in the cage top, with the strip held approximately 1 in. from the cage floor (Figure 2 A). Strips were wetted with deionized distilled water just prior to placement and left in place for 15 s, based on manufacturer's recommendations. Readings were made based on the color scale provided by the manufacturer. Reagent tubes and the PID probe were placed through predrilled holes 1 in. from the cage bottom. To minimize introduction of outside air during measurement, the holes were sized to closely approximate the diameter of the measurement devices. Between measurements, holes were covered with autoclave tape. Reagent tubes (Gastec model 3La, NextTeq, Tampa, FL) were inserted approximately 4 in. into the cage (Figure 2 B) while attached to a sampling pump (Gastec Sampling Pump model GV-100S, NextTeq). The sampling pump was used according to the manufacturer recommendations, and the color change was read according to the scale on the side of each reagent tube. The PID (X-am model 8000 equipped with ammonia detector chip only, Dräger Safety, Lubeck, Germany) was connected to a hydrophobic filter and 6-ft gas collection tubing, which was attached via luer lock to a blunted 16-gauge, 1.5-in. needle. The full length of the needle was inserted through the same predrilled hole as for the reagent tubes. The device was used according to manufacturer recommendations to obtain ammonia levels (Figure 2 C). At the beginning of the study, the PID was calibrated by using known standards (compressed ammonia at 50 ppm and compressed oxygen) and then zeroed to room air on each measurement day, according to the manufacturer's recommendations. Measurement outputs for each device were given in parts per million. When ammonia concentrations exceeded 50 ppm by at least 2 measuring devices, the cages were removed to avoid excessive distress or illness to the animals. Using this endpoint criterion also allowed crossover between treatments without the potential confounding effects of cumulative ammonia-related pathology. After cages were removed, the latest ammonia measurement was used for the remainder of that 2-wk period to avoid artificially decreasing the average for that group.
Figure 2.
Intracage ammonia measurement techniques. (A) Colorimetric strips were torn into approximately 1-cm segments and inserted through the cage's water bottle port by using hemostats. (B) Colored reagent tubes attached to a sampling pump were inserted 4 in. into cages, through predrilled holes located 1 in. from the cage bottom. (C) The photoionisation detector was connected to a hydrophobic filter, and a gas sampling line attached to a blunted 16-gauge, 1.5-in. needle. The blunted needle probe was inserted fully into cages, through predrilled holes.
Statistics.
Statistical significance was preset at P values of less than 0.05. Differences in ammonia levels were analyzed by using Kruskal–Wallis and posthoc Mann–Whitney tests for individual group comparisons. In addition, cages were evaluated for the time needed to exceed the threshold values of 25 and 50 ppm. Values were assessed by survival analysis using a log-rank Mantel–Cox test. A nonsurvival event was defined as a value exceeding the given threshold according to at least 2 of the 3 measurement methods. Analysis was performed separately for 25- and 50-ppm thresholds. Cages served as experimental units for all analyses. Data analysis was performed by using Prism 8.3.0 for Windows (GraphPad Software, La Jolla, CA). Ammonia values are presented graphically as mean ± standard error of the mean.
Results
Individual mouse weights ranged from 36 to 57 g. Group weight averages ranged from 42.7 to 45.4 g, with no significant difference between groups, as determined by ANOVA (P = 0.27).
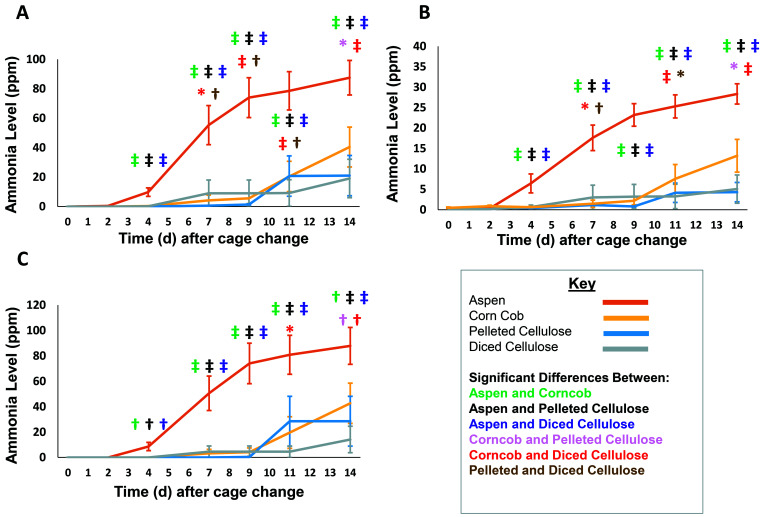
Kruskal–Wallis tests showed significant differences among bedding types in ammonia levels measured by all devices beginning at day 4 (P < 0.0001). Ammonia concentrations in cages containing aspen bedding were significantly higher than all other bedding types at all measured time points beginning at day 4 (days 4, 7, 9, 11, and 14) when measured by using reagent tubes, colorimetric strips, and PID (P < 0.01 for PID at day 4, P < 0.001 for all other comparisons; Figure 3). At day 7, cages containing diced cellulose had small but significantly higher increases in intracage ammonia levels than did cages containing corncob or pelleted cellulose when measured by using reagent tubes and colorimetric strips (diced cellulose compared with corncob, P < 0.05; diced cellulose compared with pelleted cellulose, P<0.01; Figure 3 A and B). At day 9, ammonia remained higher in cages containing diced cellulose than in those containing corncob (P < 0.001) or pelleted cellulose (P < 0.01) but only when measured by using reagent tubes. On day 11, a significant difference was found between cages containing pelleted cellulose and diced cellulose when measured by using reagent tubes (P < 0.01) and colorimetric strips (P < 0.05) but not PID. These differences were not detected at the conclusion of the cage-change cycle (day 14).
Figure 3.
Intracage ammonia levels (mean ± SEM) associated with 4 bedding types over time as measured by using (A) reagent tubes, (B) colorimetric strips, and (C) a photoionization detector (n = 20 measurements per group per time point). Significant (*, P < 0.05; †, P < 0.01; ‡, P < 0.001) differences between beddings (green, aspen and corncob; black, aspen and pelleted cellulose; purple, aspen and diced cellulose; pink, corncob and pelleted cellulose; red, corncob and diced cellulose; and brown, pelleted and diced cellulose) are indicated.
The ammonia levels in cages containing corncob bedding were significantly higher than those containing diced cellulose beginning at day 11 and continuing to the end of the cage change cycle (day 14) when measured by reagent tubes (P < 0.001; Figure 3 A), colorimetric strips (P < 0.001; Figure 3 B), and PID (P < 0.05 d 11; P < 0.01 d 14; Figure 3 C). On day 14, cages containing corncob bedding had significantly higher ammonia levels than those containing pelleted cellulose when measured by using reagent tubes (P < 0.05), colorimetric strips (P < 0.05), and PID (P < 0.01).
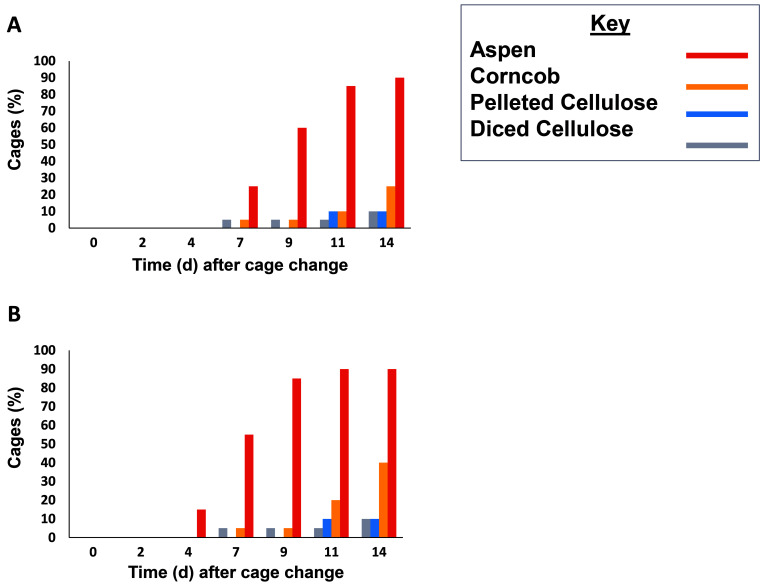
In addition to evaluating average ammonia concentrations, we also evaluated cages for the time taken to reach the thresholds of 50 ppm and 25 ppm ammonia. The 50-ppm threshold was exceeded in 85% of aspen cages, 10% of corncob and pelleted cellulose cages, and 5% of diced cellulose cages (Figure 4 A). This value was used as a welfare endpoint, and mice in these cages were removed from the study. By the end of the study, the 25-ppm threshold was exceeded in 90% of aspen cages, 40% of corncob cages, and 10% of pelleted cellulose and diced cellulose cages (Figure 4 B). Most of the cages that exceeded 25 ppm at the end of the study also exceeded 50 ppm at that time. Comparison of survival curves by log-rank Mantel–Cox testing revealed that all aspen cages reached 25 ppm sooner than all other bedding types (P < 0.0001 for all comparisons). In addition, corncob cages reached 25 ppm sooner than either pelleted cellulose (P = 0.035) or diced cellulose (P = 0.034). Aspen cages also reached the 50 ppm threshold sooner than other bedding types (P < 0.0001 for all comparisons). Survival curves were not statistically different when comparing the 50 ppm thresholds for corncob to those of the cellulose products (P = 0.23 for both comparisons).
Figure 4.
Proportion (%) of cages that reached threshold levels of either (A) 50 ppm or (B) 25 ppm ammonia during the 2-wk measurement period. A cage was considered to exceed a threshold when above-threshold readings were obtained from at least 2 measurement devices. Aspen cages exceeded all thresholds earlier than other materials. Above-threshold readings in corncob cages were delayed until near the end of the measurement period. In contrast, the novel cellulose materials largely prevented above-threshold measurements, compared with other bedding types.
Discussion
Our results showed that corncob, pelleted cellulose, and refined diced cellulose consistently provided better ammonia control in IVC than did aspen shavings. The novel cellulose products performed better than corncob by the end of the 2-wk study period, in contrast with a prior report using a traditional cellulose product.11 The current study used novel cellulose beddings designed to provide better ammonia control, including a pelleted cellulose with porous pellets and a diced cellulose made using a refined pulping process. Despite statistically significant differences between the 2 cellulose beddings used (days 7, 9, and 11 for reagent tubes and days 7 and 11 for colorimetric strips), the differences were of low magnitude and did not support an overall conclusion in favor of either product.
Ammonia levels are an important factor in assessing cage change interval. The Occupational Safety and Health Administration has established a permissible ammonia exposure limit of 50 ppm as an 8-h time-weighted average. The National Institute for Occupational Safety and Health and the American Conference of Governmental Industrial Hygienists have established an exposure limit of 25 ppm as an 8-h time-weighted average.22 Although these exposure limits were established for the human workplace, they form the only regulatory basis for evaluating ammonia exposure in any species and are often used as a measure of maximal desired levels for changing of rodent cages. According to these thresholds, our data suggest that cages with aspen bedding require changing more frequently than weekly, at which point most cages will have surpassed 25 ppm. Cages with corncob bedding showed lower ammonia levels, but a high proportion of cages still exceeded the thresholds of 25 ppm and 50 ppm ammonia by 2 wk (40% and 25%, respectively).
In contrast, only 10% of the cages with the novel cellulose products exceeded 25 ppm ammonia by 2 wk. Cages with these novel cellulose materials may therefore be better suited to extended cage change intervals. The current study used 4 large male mice per cage to take advantage of increased biomass and marking behavior to maximize ammonia levels. A longer cage change interval might be appropriate for cages with less biomass or a lower housing density.
Three ammonia measurement methods were used in this study. The study was not designed to compare the devices, and the use of different locations for the 3 devices precludes quantitative comparison of the measurement methods. Instead, the 3 devices allowed measurement at 3 separate locations throughout the cage, representing 3 independent experiments. This approach allowed us to assess whether effects were consistent among devices. As a result, the minor, inconsistent differences detected between corncob, pelleted cellulose, and diced cellulose before 14 d after cage change were considered not to have practical relevance.
Although devices could not be compared quantitatively, all were easy to use and required approximately the same amount of time per measurement (just over 1 min). Reagent tubes gave precise, reliable measurements and were our preferred method. The tubes had an upper limit of 200 ppm, beyond which higher scale tubes could have been used. Because of the levels measured here, this limit was not a problem for our study. The PID gave similar values to reagent tubes but was sometimes difficult to interpret when the unit was unable to settle on a stable value. This was especially true with high ammonia levels. The PID also failed to register low values (< 10 ppm) and had an upper limit of 300 ppm, which was adequate for the current study. Colorimetric strips were inexpensive, but interpretation of color change was subjective, with some inconsistency across the strip. Strips gave consistently lower values than reagent tubes or the PID, often by 4-fold. Given that devices were used at different locations, differences in ammonia levels and device performance could have been a consequence of cage location rather than the devices themselves.
Bedding properties might also affect the frequency of “as needed” spot changes. Each soiled cage was photographed at day 14 or at the time of removal from the study, with representative day-14 examples presented in Figure 5. Cages showed visually similar accumulation of fecal material. Aspen and corncob cages showed urine spots when viewed from the bottom of the cage. Pelleted cellulose showed primarily fecal accumulation, whereas diced cellulose showed fecal accumulation and yellow discoloration. We did not observe the same correlation between urine spot characteristics and ammonia levels that have been reported previously for corncob cages;24 however our observations were subjective, and the experimental setting differed from the prior report. Aspen and corncob cages did show more urine spots with time, which could be a useful visual aide for making “as-needed” spot changes. When cellulose beddings are used, other criteria must be used to evaluate cage soil level, although spot changes may be required less frequently due to improved ammonia control.
Figure 5.
Soiled cages after 2 wk of housing mice. These representative images of (A) shaved aspen, (B) corncob, (C) pelleted cellulose, and (D) refined diced cellulose were obtained at day 14 after cage change from above (left panel) and below (right panel) each cage. Cages showed visually similar accumulation of fecal material. Aspen and corncob cages show urine spots when viewed from the bottom of the cage, but cellulose products did not. Pelleted cellulose showed primarily fecal accumulation, whereas diced cellulose showed fecal accumulation and yellow discoloration.
According to our results, either pelleted virgin cellulose or refined virgin diced cellulose can be viable alternatives to corncob bedding when the use of corncob may interfere with research data, without the need for more frequent cage changing. In addition, these novel bedding types appear suitable for general use with regard to successfully controlling intracage ammonia levels during a 2-wk cage-change interval in IVC.
Acknowledgments
We thank the staff of the Center for Comparative Medicine—including Robinson Deleon, Rachel Rodriguez, Jermario Reynolds, Destinie Miranda, Alondra Pruneda, and Mallory Lenio—for their care of the animals and facilities in this study. Nicholas M Tataryn thanks mentors of the Tri-Institutional Laboratory Animal Medicine Training Program, including Neil Lipman, for their guidance and support. Shepherd Specialty Papers provided the AlphaDri 2.0 bedding material as well as half of the reagent tubes used for this study.
References
- 1.Ambery AG, Tackett L, Penque BA, Hickman DL, Elmendorf JS. 2014. Effect of Corncob bedding on feed conversion efficiency in a high-fat diet-induced prediabetic model in C57Bl/6J mice. J Am Assoc Lab Anim Sci 53:449–451. [PMC free article] [PubMed] [Google Scholar]
- 2.Biofresh. [Internet]. 2020. Performance bedding product page. [Cited 28 June 2020]. Available from: http://biofreshvet.com/products/. [Google Scholar]
- 3.Broderson JR, Lindsey JR, Crawford JE. 1976. The role of environmental ammonia in respiratory mycoplasmosis of rats. Am J Pathol 85:115–130. [PMC free article] [PubMed] [Google Scholar]
- 4.Burn CC, Mason GJ. 2005. Absorbencies of six different rodent beddings: commercially advertised absorbencies are potentially misleading. Lab Anim 39:68–74. 10.1258/0023677052886592. [DOI] [PubMed] [Google Scholar]
- 5.Faith RE, Huerkamp MJ. 2009. Environmental considerations for research animals, p 63 Chapter 7. In: Hessler JR, Lehner NDM, editors. Planning and designing research animal facilities. San Diego (CA): Elsevier. [Google Scholar]
- 6.Ferrecchia CE, Jensen K, Van Andel R. 2014. Intracage ammonia levels in static and individually ventilated cages housing C57BL/6 mice on 4 bedding substrates. J Am Assoc Lab Anim Sci 53:146–151. [PMC free article] [PubMed] [Google Scholar]
- 7.Fox JG, Anderson LC, Otto GM, Pritchett-Corning KR, Whary MT, editors. 2015. Laboratory animal medicine, 3rd ed San Diego (CA): Academic Press; 10.1016/B978-0-12-409527-4.00040-7. [DOI] [Google Scholar]
- 8.Gordon CJ. 2004. Effect of cage bedding on temperature regulation and metabolism of group-housed female mice. Comp Med 54:63–68. [PubMed] [Google Scholar]
- 9.Institute for Laboratory Animal Research. 2010. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 10.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. 2010. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8:1–5. 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koontz JM, Kumsher DM, Kelly R, 3rd, Stallings JD. 2016. Effect of 2 bedding materials on ammonia levels in individually ventilated cages. J Am Assoc Lab Anim Sci 55:25–28. [PMC free article] [PubMed] [Google Scholar]
- 12.Leblanc M, Berry K, Graciano S, Becker B, Reuter JD. 2014. False-positive results after environmental pinworm PCR testing due to Rhabditid nematodes in Corncob bedding. J Am Assoc Lab Anim Sci 53:717–724. [PMC free article] [PubMed] [Google Scholar]
- 13.Markaverich B, Mani S, Alejandro MA, Mitchell A, Markaverich D, Brown T, Velez-Trippe C, Murchison C, O'Malley B, Faith R. 2002. A novel endocrine-disrupting agent in corn with mitogenic activity in human breast and prostatic cancer cells. Environ Health Perspect 110:169–177. 10.1289/ehp.02110169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrow RB, Wiler RJ. 2019. Ammonia measurement in the IVC microenvironment. J Am Assoc Lab Anim Sci 58:184–189. 10.30802/AALAS-JAALAS-18-000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nonni AJ, Courchene CE, Slone CM, Abitz PR. [Internet]. 2014. Modified cellulose from chemical kraft fiber and methods of making and using the same. US008778136 B2. US United States Patent and Trademark Office, 15 July 2014. [Cited 02 February 2020]. Available at: https://pubchem.ncbi.nlm.nih.gov/patent/US2019024308 [Google Scholar]
- 16.Perkins SE, Lipman NS. 1995. Characterization and quantification of microenvironmental contaminants in isolator cages with a variety of contact beddings. Contemp Top Lab Anim Sci 34:93–98. [PubMed] [Google Scholar]
- 17.Royals MA, Getzy DM, VandeWoude S. 1999. High fungal spore load in corncob bedding associated with fungal-induced rhinitis in two rats. Contemp Top Lab Anim Sci 38:64–66. [PubMed] [Google Scholar]
- 18.Sanchez R, Johnson D, Terzi MC, Izuka M, Allen ED, O'Donnoghue M, Mendonca A, Yachera K, DeTolla L. 2017. Sanitized corncob bedding: destruction of murine parvovirus. Lab Anim (NY) 46:95–96. 10.1038/laban.1226. [DOI] [PubMed] [Google Scholar]
- 19.Schoeb TR, Davidson MK, Lindsey JR. 1982. Intracage ammonia promotes growth of Mycoplasma pulmonis in the respiratory tract of rats. Infect Immun 38:212–217. 10.1128/IAI.38.1.212-217.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thigpen JE, Setchell KD, Kissling GE, Locklear J, Caviness GF, Whiteside T, Belcher SM, Brown NM, Collins BJ, Lih FB, Tomer KB, Padilla-Banks E, Camacho L, Adsit FG, Grant M. 2013. The estrogenic content of rodent diets, bedding, cages, and water bottles and its effect on bisphenol A studies. J Am Assoc Lab Anim Sci 52:130–141. [PMC free article] [PubMed] [Google Scholar]
- 21.Trainor BC, Takahashi EY, Campi KL, Florez SA, Greenberg GD, Laman-Maharg A, Laredo SA, Orr VN, Silva AL, Steinman MQ. 2013. Sex differences in stress-induced social withdrawal: independence from adult gonadal hormones and inhibition of female phenotype by corncob bedding. Horm Behav 63:543–550. 10.1016/j.yhbeh.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.US Centers for Disease Control and Prevention. [Internet]. 2014. Table of IDLH Values: Ammonia. [Cited 5 April 2020]. Available at: https://www.cdc.gov/niosh/idlh/7664417.html. [Google Scholar]
- 23.Ward GM, Cole K, Faerber J, Hankenson FC. 2009. Humidity and cage and bedding temperatures in unoccupied static mouse caging after steam sterilization. J Am Assoc Lab Anim Sci 48:774–779. [PMC free article] [PubMed] [Google Scholar]
- 24.Washington IM, Payton ME. 2016. Ammonia levels and urine-spot characteristics as cage-change indicators for high-density individually ventilated mouse cages. J Am Assoc Lab Anim Sci 55:260–267. [PMC free article] [PubMed] [Google Scholar]
- 25.Whiteside TE, Thigpen JE, Kissling GE, Grant MG, Forsythe D. 2010. Endotoxin, coliform, and dust levels in various types of rodent bedding. J Am Assoc Lab Anim Sci 49:184–189. [PMC free article] [PubMed] [Google Scholar]