Abstract
OBJECTIVES:
To develop a method of identifying children with medical complexity (CMC) from the National Survey of Children’s Health (NSCH) 2016–2017 combined data set, to compare this approach to existing CMC identification strategies, and to describe sociodemographic characteristics of our CMC sample.
METHODS:
Using survey items pertinent to the medical complexity domains in the style by Cohen et al (chronic health conditions, health service needs, health care use, and functional limitations), we created a schema to categorize children as CMC by applying a 95th percentile cutoff for survey item positivity. We applied existing CMC identification techniques to the NSCH. We used 2-proportion z tests to compare the classification output of our CMC identification method to those of existing approaches. We used χ2 analyses to examine relationships between child and family characteristics, comparing CMC with children with special health care needs (CSHCN) and children with no special health care needs.
RESULTS:
Among the 71 811 children in the sample, 1.5% were classified as CMC by our method, representing almost 1.2 million children (weighted) in the United States in 2016–2017. CSHCN and children with no special health care needs represented 17.2% (weighted n = 12.6 million) and 81.2% (weighted n = 59.6 million) of the sample, respectively. Our approach classified a significantly smaller number of CSHCN as CMC than existing CMC identification methods, which classified 3.9% to 13.2% of the 2016–2017 NSCH sample as more complex (P < .001). CMC status was significantly associated with male sex, minority race or ethnicity, and experiencing socioeconomic adversity (all P < .001).
CONCLUSIONS:
This method enables standardized identification of CMC from NSCH data sets, thus allowing for an examination of CMC health outcomes, pertinent to pediatric hospitalist medicine, contained in the survey.
Children with medical complexity (CMC), as defined by Cohen et al,1 are characterized by chronic health conditions, significant health service needs, major functional limitations, and high health care use. Although this definitional framework is widely accepted, methods to identify CMC differ greatly, producing varied estimates of CMC prevalence ranging from 0.4% to 1.9%.2–4 Popular tools relying on administrative data have been criticized for not fully capturing needs and functional limitations.5,6 Other strategies using nationally representative data were developed for the discontinued National Survey of Children with Special Health Care Needs, but those data are now >10 years old.3,4,7–11
The redesigned National Survey of Children’s Health (NSCH) is an opportunity to update estimates and characterization of the CMC population. Although the NSCH contains elements of the National Survey of Children with Special Health Care Needs, CMC identification methods described by Kuo et al3 and Coller et al9 cannot be applied to the NSCH because they rely on survey items that were discarded or altered significantly. Techniques developed by Bramlett et al8 and Bethell et al,7 which use numeric thresholds or combinations of children with special health care needs (CSHCN) screener components, including the current approach used by the Data Resource Center for Child and Adolescent Health (DRCCAH), have not been closely examined when applied to the NSCH data set.12
Therefore, we developed a method, informed by existing strategies, to identify CMC from the NSCH that operationalizes the holistic definition of medical complexity presented by Cohen et al.1,3,9 We compare the classification output of this method to previously described approaches when applied to the NSCH 2016–2017 data set.7,8,13 We then characterize sociodemographics of our CMC population and compare with previous descriptions in the literature.
Methods
This is a secondary analysis of the NSCH combined 2016–2017 data set. The NSCH underwent significant changes in 2016.13 Conducted annually by the US Census Bureau, 1 child per randomly selected household is chosen to produce national-level data on the health of American children.14,15 The overall weighted response rate was 40.7% for 2016 and 37.4% for 2017.13 This study received exempt status from the university’s institutional review board.
Using the CSHCN screener questionnaire contained in the NSCH, we differentiated between children with no special health care needs (non-SHCN) and CSHCN (children experiencing ≥1 health consequence because of a condition lasting ≥12 months).16 Informed by existing strategies and collective clinical experiences, a multidisciplinary team, including clinicians trained in pediatric rehabilitation medicine, general pediatrics, and pediatric and adult palliative care, constructed diagnoses- and consequences-based CMC inclusion criteria using NSCH survey items. We considered and sorted all survey items, through group consensus, into 1 of 5 categories based on the domains of medical complexity defined by Cohen et al1: chronic health conditions, health service needs, functional limitations, health care use, or item deemed nonpertinent (Table 1).3,7–9 For each domain, the numeric cutoff of survey items to be considered positive was greater than or equal to that domain’s 95th percentile.17,18 CSHCN who met inclusion criteria in all 4 medical complexity domains were classified as CMC.
TABLE 1.
Medical Complexity Domains and Pertinent NSCH Items
| Domain | Pertinent Survey Items | Descriptive Statistics | Domain Threshold |
|---|---|---|---|
| Health care use | ≥2 ED visits; ≥2 preventive check-ups; child used more medical care than most children because of a medical condition lasting ≥12 mo | Mean: 0.4;median: 0;75th percentile: 1; 95th percentile: 2; 99th percentile: 2 | ≥2 (6.8% of the sample) |
| Health service needs | Prescription medications; physical, occupational, speech, and/or developmental therapy; specialist care; vision care requiring an eye specialist; home health care; care coordination; mental health care; goes to ED or hospital when sick | Mean: 1.3; median: 1; 75th percentile: 2; 95th percentile: 4; 99th percentile: 5 | ≥4 (5.9% of the sample) |
| Functional limitations | Health conditions affect ability to do things; limited ability to do things most children can do because of a health condition lasting ≥12 mo | Mean: 0.1; median: 0; 75th percentile: 0; 95th percentile: 1; 99th percentile: 2 | ≥1 (6.2% of sample) |
| Complex chronic conditions (positive if either symptoms or conditions subdomain criteria are met) | 13.1% of sample met criteria | ||
| Symptoms (difficulty with the following) | Breathing, swallowing, digestion or intestines, chronic physical pain, using hands, coordination or moving around, gum bleeding, deafness or problems hearing, blindness or problems seeing | Mean: 0.3;median: 0;75th percentile: 0;95th percentile: 2;99th percentile: 3 | ≥2 |
| Conditions (current and/or severe) | Allergies, arthritis, asthma, blood disorder, brain injury, cerebral palsy, cystic fibrosis, diabetes, Down syndrome, epilepsy, genetic condition, heart condition, headaches, Tourette’s syndrome, anxiety, depression, behavior problems, developmental delay, intellectual or leaning disability, speech disorder, autism spectrum disorder, substance abuse, other | Conditions currently present: mean: 0.8; median: 0; 75th percentile: 1; 95th percentile: 4; 99th percentile: 7.Severe conditions: mean: 0.1;median: 0; 75th percentile: 1; 95th percentile: 1; 99th percentile: 2 | ≥4 conditions currently present or ≥1 condition described as severe |
ED, emergency department.
We also applied existing techniques to categorize complexity status using combinations of CSHCN screener components. Using the quantitative strategy by Bramlett et al,8 we identified children who screened positive for ≥4 criteria. Using an approach described by Bethell et al,7,16 we identified children who met the activity limitations criterion plus at least 1 additional criterion. Lastly, we used the DRCCAH’s current approach to identify more complex CSHCN as children who met the prescription medication criterion plus at least 1 additional criterion.12
To compare the child, family, and household characteristics of CMC identified by our method with those of CSHCN and non-SHCN, we examined child age, sex, race or ethnicity, insurance type, caregiver employment status, caregiver educational attainment, primary household language, household income, and family structure.
We compared proportions of children classified as CMC using our identification method by survey year (2016 vs 2017) to examine internal consistency. We summarized child and family characteristics by medical complexity status using descriptive statistics and χ2 analysis. We then compared proportions of children classified as CMC by our method with proportions classified by existing methods using 2-proportion z tests. The correlation between our schema and existing techniques was assessed by using χ2 tests of association with Cramer’s V postestimation (V > 0.5 signified a high strength of association).19 All statistical analyses were conducted using appropriate person-level weights provided by survey administrators to generate national estimates, and all statistical analyses accounted for multiple imputation of missing values for household income by using Stata version 15.1.13
Results
In Table 1, we summarize descriptive statistics and numeric cutoffs for each domain of our CMC inclusion criteria. For example, the health service needs domain includes 8 criteria items. Because the 95th percentile of the number of survey items met was 4, any child responding affirmatively to ≥4 items had a positive result for this domain.
Among the 71 811 children in the sample, 1.5% (n = 1071; 95% confidence interval [CI]: 1.4%–1.6%) were classified as CMC. Using survey weights to generate national estimates, we determined this represented 1.6% of US children in 2016–2017 (weighted; ∼1.2 million children). CSHCN and non-SHCN represented 17.2% (12.6 million) and 81.2% (59.6 million) of the pediatric population, respectively. The proportion of children classified as CMC did not differ significantly by survey year (2016 vs 2017: 1.4% [95% CI: 1.3%–1.6%] vs 1.6% [95% CI: 1.4%–1.8%]) (P = .161).
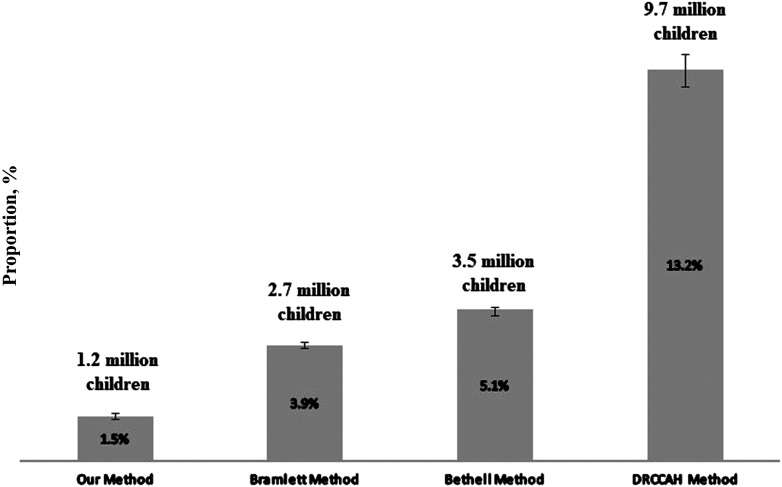
In all existing approaches, significantly larger proportions of children were assigned to the “more complex” category than in our identification method (all P < .001) (Fig 1). Children meeting ≥4 CSHCN screener criteria (Bramlett et al8) accounted for 3.9% (95% CI: 3.8%–4.0%) of the sample (2.7 million children). Children meeting the CSHCN screener activity limitation criterion and at least 1 additional criterion (Bethell et al7) represented 5.1% (95% CI: 4.9%–5.2%) of the sample (3.5 million children). The DRCCAH’s current approach classified 13.2% (95% CI: 12.6%–13.7%) of the sample as more complex (9.7 million children).
FIGURE 1.
Proportions of children classified as medically complex. Our CMC identification method classified significantly smaller proportions of the NSCH 2016–2017 sample as medically complex than methods described by Bramlett et al8 (≥4 CSHCN screener criteria), Bethell et al7 (CSHCN screener activity limitations criterion plus 1 additional criterion), and the DRCCAH (CSHCN screener prescription medication criterion plus 1 additional criterion). National estimates of CMC by using survey weight are displayed above each approach. Error bars indicate 95% CIs.
Our method’s classification output was significantly and strongly correlated to the classification of these approaches. Of the 1071 children our method classified as CMC, 85.8% met criteria for method by Bramlett et al8 (n = 919; P < .001; Cramer’s V = 0.582), 90.9% were CSHCN with activity limitations by using the method by Bethell et al7 (n = 974; P < .001; Cramer’s V = 0.581), and 90.7% met the DRCCAH’s “more complex” CSHCN criteria (n = 971; P < .001; Cramer’s V = 0.646).
Compared to non-SHCN and CSHCN status, medical complexity status was significantly associated with male sex, minority race or ethnicity, and having public insurance (all P < .001) (Table 2). It was also significantly associated with living in primarily English-speaking, poorer, and single-parent households and having unemployed parental caregivers with lower educational attainment (all P < .001).
TABLE 2.
Child and Family Characteristics Overall and by Medical Complexity Status (Total Sample N = 73 387 211)
| Characteristic | Overall, Weighted % | Non-SHCN (n = 59c624c982 [81.2%]), Weighted % | CSHCN (n = 12c606c103 [17.2%]), Weighted % | CMC (n = 1c156c126 [1.6%]), Weighted % | Pa |
|---|---|---|---|---|---|
| Age, y | |||||
| 0–5 | 32.3 | 35.6 | 18.1 | 16.0 | <0.001 |
| 6–11 | 33.9 | 32.9 | 38.3 | 35.9 | |
| 12–17 | 33.8 | 31.5 | 43.6 | 48.1 | |
| Sex | |||||
| Male | 51.1 | 49.5 | 57.2 | 66.9 | <0.001 |
| Female | 48.9 | 50.5 | 42.8 | 33.1 | |
| Race | |||||
| White | 51.4 | 51.5 | 51.7 | 45.9 | <0.001 |
| Black | 13.1 | 12.0 | 17.7 | 19.3 | |
| Hispanic | 24.7 | 25.3 | 21.8 | 27.1 | |
| Other | 10.8 | 11.2 | 8.9 | 7.6 | |
| Insurance type | |||||
| Public | 31.3 | 29.3 | 38.4 | 52.7 | <0.001 |
| Private | 57.1 | 59.4 | 49.8 | 22.0 | |
| Mix of public and private | 4.6 | 3.9 | 6.7 | 20.1 | |
| Unknown type | 0.8 | 0.8 | 0.9 | 0.5 | |
| Uninsured | 6.2 | 6.6 | 4.1 | 4.3 | |
| Family income, % FPL | |||||
| ≥400 | 30.2 | 30.9 | 27.9 | 15.7 | <0.001 |
| 200–399 | 6.8 | 27.2 | 25.7 | 21.2 | |
| 100–199 | 21.8 | 21.7 | 22.3 | 26.2 | |
| <100 | 21.2 | 20.2 | 24.1 | 36.9 | |
| Parental employment | |||||
| ≥1 parent employed | 96.3 | 96.4 | 96.0 | 90.0 | <0.001 |
| Unemployed | 3.7 | 3.6 | 4.0 | 10.0 | |
| Highest household education level | |||||
| College or higher | 50.4 | 51.3 | 47.6 | 35.3 | <0.001 |
| Some college | 23.8 | 23.2 | 25.6 | 31.3 | |
| High school or equivalent | 20.9 | 20.6 | 21.7 | 27.4 | |
| Less than high school | 4.9 | 4.9 | 5.1 | 6.1 | |
| Household family structure | |||||
| 2 parents, married | 67.1 | 69.6 | 56.9 | 49.7 | <0.001 |
| 2 parents, not married | 8.9 | 8.8 | 9.2 | 12.7 | |
| Single parent | 16.3 | 14.6 | 23.4 | 24.4 | |
| Other family type | 7.7 | 6.9 | 10.6 | 13.2 | |
| Primary household language | |||||
| English | 85.6 | 84.3 | 91.2 | 88.9 | <0.001 |
| Non-English | 14.4 | 15.7 | 8.8 | 11.1 |
All data were weighted for survey to generate national estimates. FPL, federal poverty level.
χ2 analysis.
Discussion
We describe a novel method, taking a multidimensional view of medical complexity, to identify CMC from the combined 2016–2017 NSCH.1 We estimated the national prevalence of medical complexity to be 1.6%. We redemonstrate that medical complexity status is associated with socioeconomic adversity.11,20,21
Unlike existing approaches, which all rely exclusively on the CSHCN screener, our CMC identification method does not discount children’s health service needs or functional limitations. Similar to the multidimensional model of CMC as children with functional limitations requiring extensive health care use (estimated CMC prevalence of 2%–3%) by Coller et al,9 our method identifies children whose health conditions impact most, if not all, aspects of their well-being. Although we were unable to adapt this technique to the NSCH for comparison, our criteria items map onto similar domains: difficulty with physical, cognitive, or mental health function; activity limitations; health care use; and health needs. We believe this explains why the proportion of CSCHN our schema identified as medically complex is similar to that identified by Coller et al9 but significantly smaller than those identified by existing techniques by using the CSHCN screener in the NSCH.
The similarities between the sociodemographics of our CMC sample and previous descriptions of the CMC population also suggest our method captured the expected population.3,9 Similar to previous reports, we found CMC status was significantly associated with male sex, Black race and Hispanic ethnicity, and receiving public insurance.9,11,22–24 We also redemonstrate that higher proportions of CMC live in poorer, single-parent households with unemployed parental caregivers with lower educational attainment.11,22
Our identification method provides an additional strategy for stratifying children in the NSCH on the basis of medical complexity. This approach identifies children meeting all aspects of the complexity construct by Cohen et al1 and would benefit from intensive care coordination interventions.25 Existing techniques can still be applied to the NSCH to identify other meaningful subgroups of CSHCN, such as children primarily experiencing emotional or behavioral conditions.7
The NSCH was not designed to focus on CMC, so additional information pertinent to the domains of medical complexity by Cohen et al1 might be absent. The cross-sectional design of the NSCH cannot account for the fluctuating nature of medical complexity. The survey format is dependent on parent report; hence, it is vulnerable to recall and sampling bias.8,13 Although the decision to use the 95th percentile as the cutoff for each complexity domain is somewhat arbitrary, this is often the case when initially selecting percentile standards in relation to any health outcome measure.26 Validation of this cutoff will require further data collection and analysis. Because we were unable to link to data sets containing billing and/or expenditure information (because of the deidentified nature of the NSCH), we could not compare our method to other CMC identification tools that use administrative data sets.13
Conclusions
We developed a method to identify CMC from the redesigned NSCH. This approach can be reapplied to the NSCH for ongoing assessments of CMC health outcomes pertinent to pediatric hospitalists and for comparing outcomes between CMC and other pediatric populations. Validation of our approach among patients whose medical complexity can be confirmed is a potential next step.
Footnotes
This work was presented in part at the annual meeting of the Pediatric Academic Society Meeting; April 24–May 1, 2019; Baltimore, MD.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the institutions with which the authors are affiliated or the National Institutes of Health.
All authors were involved in the conception and design, collection, analysis, and interpretation of data, and writing; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Dr Yu was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (TL1 TR001858). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Cohen E, Kuo DZ, Agrawal R, et al. Children with medical complexity: an emerging population for clinical and research initiatives. Pediatrics. 2011;127(3):529–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen E, Berry JG, Camacho X, Anderson G, Wodchis W, Guttmann A. Patterns and costs of health care use of children with medical complexity. Pediatrics. 2012;130(6). Available at: www.pediatrics.org/cgi/content/full/130/6/e1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuo DZ, Cohen E, Agrawal R, Berry JG, Casey PH. A national profile of caregiver challenges among more medically complex children with special health care needs. Arch Pediatr Adolesc Med. 2011;165(11):1020–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neff JM, Sharp VL, Muldoon J, Graham J, Myers K. Profile of medical charges for children by health status group and severity level in a Washington State Health Plan. Health Serv Res. 2004;39(1):73–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simon TD, Cawthon ML, Stanford S, et al. ; Center of Excellence on Quality of Care Measures for Children with Complex Needs (COE4CCN) Medical Complexity Working Group. Pediatric medical complexity algorithm: a new method to stratify children by medical complexity. Pediatrics. 2014;133(6). Available at: www.pediatrics.org/cgi/content/full/133/6/e1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bethell CD, Blumberg SJ, Stein REK, Strickland B, Robertson J, Newacheck PW. Taking stock of the CSHCN screener: a review of common questions and current reflections. Acad Pediatr. 2015;15(2):165–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bramlett MD, Read D, Bethell C, Blumberg SJ. Differentiating subgroups of children with special health care needs by health status and complexity of health care needs. Matern Child Health J. 2009;13(2):151–163 [DOI] [PubMed] [Google Scholar]
- 9.Coller RJ, Lerner CF, Eickhoff JC, et al. Medical complexity among children with special health care needs: a two-dimensional view. Health Serv Res. 2016;51(4):1644–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burns KH, Casey PH, Lyle RE, Bird TM, Fussell JJ, Robbins JM. Increasing prevalence of medically complex children in US hospitals. Pediatrics. 2010;126(4):638–646 [DOI] [PubMed] [Google Scholar]
- 11.Kuo DZ, Goudie A, Cohen E, et al. Inequities in health care needs for children with medical complexity. Health Aff (Millwood). 2014;33(12):2190–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Data Resource Center for Child and Adolescent Health. 2016–17 National Survey of Children’s Health Stata constructed data set. 2018. Available at: https://www.childhealthdata.org/help/dataset. Accessed March 14, 2019
- 13.US Census Bureau. 2017 National Survey of Children’s Health: Methodology Report. Washington, DC: US Census Bureau; 2018 [Google Scholar]
- 14.Child and Adolescent Health Measurement Initiative. Title V Maternal and Child Health Services Block Grant Measures Content Map, 2017 National Survey of Children’s Health. Rockville, MD: Health Resources and Services Administration; 2018 [Google Scholar]
- 15.Health Resources and Services Administration. The 2016-17 National Survey of Children’s Health (NSCH) Combined Data Set: Fast Facts. Rockville, MD: Health Resources and Services Administration; 2018 [Google Scholar]
- 16.Bethell CD, Read D, Stein REK, Blumberg SJ, Wells N, Newacheck PW. Identifying children with special health care needs: development and evaluation of a short screening instrument. Ambul Pediatr. 2002;2(1):38–48 [DOI] [PubMed] [Google Scholar]
- 17.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA. 2004;291(23):2847–2850 [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Physical Status: the Use of and Interpretation of Anthropometry, Report of a WHO Expert Committee. Geneva, Switzerland: World Health Organization; 1995 [PubMed] [Google Scholar]
- 19.Akoglu H. User’s guide to correlation coefficients. Turk J Emerg Med. 2018;18(3):91–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berry JG, Hall M, Neff J, et al. Children with medical complexity and Medicaid: spending and cost savings. Health Aff (Millwood). 2014;33(12):2199–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berry JG, Agrawal RK, Cohen E, Kuo D. The Landscape of Medical Care for Children with Medical Complexity. Lenexa, KS: Children's Hospital Association; 2013 [Google Scholar]
- 22.Aboneh EA, Chui MA. Care coordination, medical complexity, and unmet need for prescription medications among children with special health care needs. Res Social Adm Pharm. 2017;13(3):524–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cady RG, Belew JL. Parent perspective on care coordination services for their child with medical complexity. Children (Basel). 2017;4(6):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin JL, Cohen E, Sanders LM. Shared decision making among children with medical complexity: results from a population-based survey. J Pediatr. 2018;192:216–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuo DZ, Houtrow AJ; Council on Children With Disabilities. Recognition and management of medical complexity. Pediatrics. 2016;138(6):e20163021. [DOI] [PubMed] [Google Scholar]
- 26.Zhu W. “17% at or above the 95th percentile”—what is wrong with this statement? J Sport Health Sci. 2012;1(2):67–69 [Google Scholar]